Abstract
Reproductive interference can shape regional distribution patterns in closely related species, if prezygotic isolation barriers are weak. The study of such interaction could be more challenging in nuptial gift‐giving species due to the direct nutritional effects on both sexes of both species during copulation. We mapped the distribution of two sister bush‐cricket species, Pholidoptera aptera and Pholidoptera transsylvanica, at the northern margin of their overlapping ranges in Europe, and with a behavioral experiment, we tested the possibility of heterospecific mating. We found a very rare coexistence of species locally (0.5%, n = 391 sites) with mostly mutually exclusive distribution patterns, resulting in a mosaic pattern of sympatry, whereas they occupied the same climate niche in forest‐dominated mountain landscape. Over 14 days of a mating experiment with seven mixed groups of conspecifics and heterospecifics (n = 56 individuals in total), the number of received spermatophores per female was 3–6 in P. aptera and 1–7 in P. transsylvanica. In total, we found 8.1% of heterospecific copulations (n = 99 transferred spermatophores with genetic identification of the donor species), while we also confirmed successful transfer of heterospecific sperms into a female's reproductive system. Because bush‐cricket females also obtain required nutrition from a heterospecific spermatophylax what should increase their fitness and fecundity, we suggest that their flexibility to mate with heterospecifics is beneficial and drives reproductive interference. This may substantially limit the reproductive success of the less frequent species (P. transsylvanica), coupled with eventual detrimental effects from hybridization, and result in the competitive exclusion of that species from their areas of coexistence.
Keywords: coevolution, cross‐mating, home ranges, hybridization, Orthoptera, precopulatory behavior
We found a very rare coexistence of Pholidoptera aptera and Pholidoptera transsylvanica with mostly mutually exclusive distribution patterns, whereas they occupied the same climate niche in forest‐dominated mountain landscape. Experimental trials revealed 8% of heterospecific copulations, while successful transfer of heterospecific sperms into a female's reproductive system was also confirmed. Because females also obtain required nutrition from a heterospecific spermatophylax, we suggest that this reproductive interference may result in the competitive exclusion of the inferior species from their areas of coexistence.

1. INTRODUCTION
The study of interactions between closely related taxa which occupy a sympatric range is important for understanding the evolutionary adaptations and isolation mechanisms that lead to their coexistence or divergence (Chesson, 2000; Hewitt, 2001). A specific spatial niche, habitat or behavior, often limits species interactions and prevents their local coexistence (Kádár, Fazekas, Sárospataki, & Lövei, 2015; Michalko, Košulič, Hula, & Surovcová, 2016; Pellissier et al., 2012; Schirmel & Fartmann, 2013). However, when species interact more closely, resource competition and especially reproductive interference (heterospecific mating) may hamper their coexistence even more, resulting in a mosaic pattern of sympatry (Gröning, Lücke, Finger, & Hochkirch, 2007; Hochkirch, Gröning, & Bücker, 2007). To learn how regional distribution patterns of such species are shaped, we should be interested in both the barriers that prevent coexistence and the factors that result in the competitive exclusion (demographic displacement) of the inferior species (Grether, Losin, Anderson, & Okamoto, 2009; Gröning & Hochkirch, 2008; Kyogoku, 2015; Wellenreuther, Larson, & Svensson, 2012).
Heterospecific mating, ranging from mating attempts to hybridization between sister species, has been demonstrated in a wide range of animal taxa (Gröning & Hochkirch, 2008), and it is also known in different genera of Orthoptera (e.g., Allonemobius, Gregory & Howard, 1993; Pterophylla, Barrientos‐Lozano, 1998; Orchelimum, Shapiro, 2000; Tetrix, Hochkirch, Deppermann, & Gröning, 2006, Gröning et al., 2007, Chorthippus, Vedenina, Kulygina, & Panyutin, 2007; Poecilimon, Lehmann, Siozios, Bourtzis, Reinhold, & Lehmann, 2011; Aglaothorax, Cole, 2016; Phaneroptera, own unpublished data). In Orthoptera, such occasional events suggest utilization of similar signal channels during mate recognition (Hochkirch et al., 2006) and/or the similar morphology of their sexual organs, which are used as stimulatory devices during copulatory courtship and for spermatophore transfer (Wulff, Kamp, Santos, Baumbach, & Lehmann, 2017; Wulff, Lehmann, Hipsley, & Lehmann, 2015). Copulation between related species shows no obvious behavioral differences to conspecific mating, as spermatophores, including sperms, are successfully transferred (Lehmann et al., 2011). If there is a weak genetic basis for postzygotic reproductive isolation, then potential interference and hybridization may have detrimental effects on the reproductive output of at least one of the species (Gregory & Howard, 1993; Gröning & Hochkirch, 2008; Shapiro, 2000). On the other hand, because the spermatophores of bush‐cricket males include a proteinaceous courtship meal (the spermatophylax) that is served to females during copulation, this interaction should also include direct nutritional effects on both sexes of both species (Vahed, 1998). Heterospecific nuptial gifts can be energetically beneficial for females, increasing their fitness and fecundity (Brown, 1997; Fedorka & Mousseau, 2002; Voigt, Kretzschmar, Speakman, & Lehmann, 2008), but they should be unprofitable for males, which expended resources to manufacture them (Costa‐Schmidt & Machado, 2012; Lehmann, 2012; Simmons, 1990; Vahed, 2007). Thus, reproductive interference between competing nuptial gift‐giving species may be even more entangled and interesting also from the view of the evolution of their distributional ranges (Tregenza, 2002).
The Alpine and the Transylvanian dark bush‐crickets, Pholidoptera aptera (Fabricius 1793) and Pholidoptera transsylvanica (Fischer, 1853), body length 17–25 and 19–30 mm, respectively (Figure 1), are related, morphologically similar, ground‐living and flightless Orthoptera species with European distribution (Chobanov et al., 2016; Harz, 1969; Hochkirch et al., 2016). The Transylvanian dark bush‐cricket is a species of European importance by the Habitats Directive of the EU (Krištín & Kaňuch, 2013), and both these large predatory bush‐crickets belong to species of preserved habitats where their behavior is still difficult to study due to cryptic life and low abundance (e.g., up to 5–15 males/ha; own data). The species extent of occurrence suggests a possible overlap of their ranges, mostly in the Carpathian Mountains (Chobanov et al., 2016; Hochkirch et al., 2016; Figure S1). However, there is almost no evidence on their coexistence at the same sites. In Slovakia, at the northern margin of their ranges, only two old records of coexistence were known until now (Chládek, 2003). Both species are considered to be dwellers at forest edges, in open forests, forest meadows, shrubs, or abandoned tall grasslands (Jordán, Báldi, Orci, Rácz, & Varga, 2003; Krištín & Kaňuch, 2013; Löffler & Fartmann, 2017; Nagy, Rácz, & Varga, 1999; Rácz, 1998).
Figure 1.

(a) A male (bottom) of Pholidoptera aptera attaches to the female's genital opening a spermatophylax (white mass) during mating experiment. (b) A male (top) and female (bottom) of Pholidoptera transsylvanica in Slovakia. Photographs by A. Krištín
We could hypothesize that there is weak premating isolation between these two related Pholidoptera species, and their rare coexistence results from the selection of different niches, which prevents hybridization and shapes a mosaic pattern of sympatry (Cole, 2016; Gröning et al., 2007). However, if the two species prefer a similar landscape type and climate niche but do not co‐occur as evidence suggests, we alternatively hypothesize that reproductive interference instead, characterized by poaching on nuptial gifts and incorrect sperm investment, may have led to the demographic displacement of species (Kyogoku, 2015). In the first instance, we mapped the occurrence of both species in Slovakia to obtain detailed information on their distribution and environment. Subsequently, using behavioral trials, we tested the possibility of heterospecific mating and whether this behavior also involved the successful transfer of sperms and consumption of the alien nuptial gift. In the present study, we thus aimed to obtain a better understanding of how P. aptera and P. transsylvanica current distribution patterns provide evidence for a role of reproductive interference and to learn whether there is a prezygotic isolating barrier between these two closely related bush‐crickets.
2. MATERIAL AND METHODS
2.1. Species mapping
Distribution maps of P. aptera and P. transsylvanica in Slovakia (Western Carpathians) were based primarily on our own field work during 1994–2018 (~90% of records) and supplemented by collated data from relevant published sources since the 19th century (Appendix S1). Field collections were conducted from June to September (in average 50 days of field work per year with the help of contributions from more than 80 coworkers) and aimed to sample most of the area during different seasons. For our spatial sampling, we visited 99% of the 10 × 10 km squares of the Universal Transverse Mercator coordinate system that cover the target area and mapped ~1,700 potential sites (http://www.orthoptera.sk). The presence of the study species was recorded mostly by acoustic registration of species‐specific calls of stridulating males, with subsequent catching of individuals using entomological hand nets and their morphological determination (Harz, 1969). We geo‐referenced 331 and 60 occurrence sites of P. aptera and P. transsylvanica, respectively (Figure 2).
Figure 2.
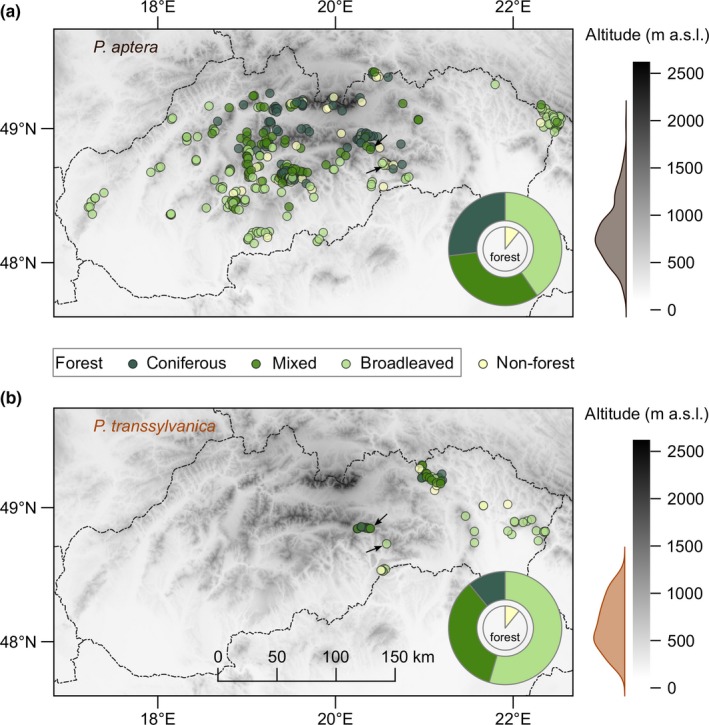
Distributions of Pholidoptera aptera (a) and Pholidoptera transsylvanica (b) in Slovakia. The dominant habitat of the surrounding landscape around sites within a 1,000 m radius is color‐labelled according to CORINE land cover inventory data with overall proportions in pie charts. Altitudes of sites are summarized in density plots. Arrows point to the two sites of current species coexistence
2.2. Environmental analyses
The CORINE Land Cover 2012 inventory (European Environment Agency, https://land.copernicus.eu) providing consistent information on land cover was used to characterize the dominant habitat type of the surrounding landscape within a 1,000 m radius around geo‐referenced occurrence sites of P. aptera and P. transsylvanica (Figure 2). The selected radius was used due to the potential seasonal dispersal range for these large species, according to observed daily movement distances of similar or even smaller ground‐dispersing bush‐crickets (Benedek, Nagy, Rácz, Jordán, & Varga, 2011; Berggren, 2004; Diekötter et al., 2007; Kindvall, 1999; Lorch, Sword, Gwynne, & Anderson, 2005). A principal component analysis (PCA) of climate variables extracted for each site was used to define the climate niches of the species. To reduce colinearity between the 16 climate variables from WorldClim database (https://www.worldclim.org; Hijmans, Cameron, Parra, Jones, & Jarvis, 2005), we retained seven variables with a Pearson's pairwise correlation coefficient of less than |0.8|: annual mean temperature, mean diurnal range, isothermality, precipitation of driest quarter, precipitation of coldest quarter, precipitation of driest month, and precipitation seasonality.
Geo‐spatial analyses for landscape characterization were processed in the QGIS 3.4 software (https://qgis.org). Difference tests of the habitats proportions (Pearson's chi‐square test), sites altitudes (Mann–Whitney U test), and PCA of climate niches were performed using the default packages of the R 3.4.4 environment for statistical computing (R Core Team, 2018).
2.3. Behavioral experiment
Behavioral sequence of mating in the study species begins with attraction of a female by male's stridulation. Then, the male may be accepted or refused depending on the female's preferences (Gwynne, 2001). Prior to copulation, either a male approaches under the female's abdomen or the female climbs on the male's back, while during the copulation the male grasps the female's ovipositor with its cerci or legs. After copulation, the male attaches to the female's genital opening a spermatophylax (Figure 1). The acoustic characteristics of P. aptera and P. transsylvanica are similar and vary mainly in echeme repetition rate (Orci, 2002). To obtain evidence of how sexual activity of the two species overlaps in time, we recorded stridulation of multiple captive males during four 45 min sessions at the same time in calm and unclouded summer weather. Acoustic recordings were made using an Edirol R‐09HR (Roland, Inc.) digital recorder (sampling frequency of 96 kHz and 16‐bit amplitude resolution). Oscillographic and spectrographic analyses in the software Audacity 2.2.1 confirmed that during the highest measured activity of males at 16:00 and 20:00, the species almost fully overlapped, because both stridulated persistently (Figure S2).
The hypothesis that reproductive interference may have led to the demographic displacement of the inferior species was tested in a mating experiment with mixed groups of conspecifics and heterospecifics. In early June 2017, bush‐crickets in nymphal stages were collected at two sites in southeastern Slovakia where the absence of coexistence was confirmed (P. aptera, 48.585°N, 20.408°E; P. transsylvanica, 48.540°N, 20.536°E). Virginal individuals were housed in glass containers 40 × 20 × 20 cm in size with wire netting on top (maximum 4 individuals per one container) and reared for 2 weeks until they fully developed and sexual maturity was ensured. The containers were equipped with a broad‐spectrum 25 W daylight lamp concentrated at a wavelength of 700–900 nm UV‐A with a neodymium sleeve Day Glo (Exo‐Terra, Inc.) to contribute to the insects' physiological well‐being. Prior to our experiment, using a set of adult individuals (18 P. aptera and 17 P. transsylvanica) in room conditions, we successfully verified that these two species are willing to heterospecific mating in the absence of conspecific mate (10 heterospecific copulations were observed). The behavioral experiment, with mixed mating groups and which lasted 14 days (16–30 June 2017), was then run under open‐air conditions (300 m a.s.l.; average daily temperature 20.3°C, range 13.7–23.1°C; average relative humidity 67%, range 58%–96%) with individuals housed in collapsible insect cages, 55 cm in each dimension, made from white polyester netting, which provided natural temperature and ventilation for the bush‐crickets. Each mating group comprised two unmated adult males and females of each species. In total, 56 individuals were divided into seven mating groups. Rearing cages were checked continuously every 4 hr (i.e., six times per day) to record every possible copulation event. This period was found by previous observations to be the routine time that they needed to finish eating the spermatophylax meal after copulation, and it is similar to other such tettigonids (Gwynne, 2001). Females were individually labelled on the top of their shield with a nontoxic permanent marker, and the date and time of copulations were recorded. When we found a female with a spermatophore or its remnants, up to ~50 mm3 of spermatophylax volume (<10% of the total volume) was cut off with sterilized surgical scissors and stored in 96% ethanol for DNA extraction of the respective male donor of this nuptial gift. We aimed to leave most of the spermatophylax for females to freely consume to minimize the effect on their next choice. Difference in the proportion of conspecific to heterospecific copulations between species was tested using the Pearson's chi‐square test. Differences in the length of the refractory period after conspecific and heterospecific copulations were tested using the Mann–Whitney U test.
During the whole experiment, bush‐crickets were fed with ground dry cat food, pollen, oat flakes, and fresh leaves of European dewberries. The interiors of the containers and cages were sprayed with water every day (usually in the morning) to provide moisture and water for drinking. Besides bunches of dewberries, individuals could also hide under egg cartons to avoid direct light and reduce stress in captivity. At the end of the experiment, females were euthanized and under a binocular enhancer we dissected spermatodoses from their spermathecas. These spermatophore‐like structures, which are formed to envelope the male ejaculate after copulation, remain within the spermatheca for the duration of the female's life. Using this method, we could verify whether heterospecific sperms were successfully transferred into the female reproductive system, as each spermatodose represents one copulation (Gwynne, 2001; Vahed, 2003).
2.4. PCR‐based identification of spermatophores
Identification of the spermatophores of the two Pholidoptera species was performed using multiplex PCR and agarose gel electrophoresis. DNA extraction from spermatophylax mass was conducted according to the salting‐out protocol (Aljanabi & Martinez, 1997) modified by added RNaseA (Hornett & Wheat, 2012). In addition to forward Jerry (C1‐J‐2183) and reverse Pat (TL2‐N‐3014) primers (Simon et al., 1994), which amplify a fragment of the COI mtDNA gene, another external forward primer PHO‐F was designed on the basis of homology of known Pholidoptera sequences deposited in GenBank (access. no. KY554963–64). Fragments of 750–850 bp amplified with external primers PHO‐F/Pat or Jerry/Pat, respectively, were screened for sequences of 20–23 nucleotides characterized by a maximum of one substitution within species but differing by at least four mutations from the other species (GC content 40%–50%). At these regions, we designed internal forward primers Papt‐F for P. aptera and Ptra‐F for P. transsylvanica (Table 1), which should produce shorter species‐specific PCR products of 550–650 bp (Figure 3). We prepared three combinations of primers. The first combination (test #1) used 0.4 μM of external forward primer (Jerry), 0.6 μM of external reverse primer (Pat), and 0.2 μM of each internal forward primers Papt‐F and Ptra‐F and 1 × PCRBIO HS Taq Mix master mix (PCR Biosystems) in a 12‐μl reaction volume, including 3 μl of DNA template (5–10 ng/μl), while other combinations used 0.2 μM of external forward primer (PHO‐F), 0.4 μM of Pat and 0.2 μM of Papt‐F (test #2) or 0.2 μM of PHO‐F, 0.4 μM of Pat, and 0.2 μM of Ptra‐F (test #3). COI fragments were amplified in a PCR thermocycler (Biometra TAdvanced) in the following steps: initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 30 s, annealing at 47°C for 30 s and extension at 72°C for 90 s, and a final elongation step at 72°C for 5 min. Bands of PCR products were separated on 1.5% agarose gel and visualized by HydraGreen DNA dye. The specificity and efficacy of this rapid and easy discriminating molecular method were verified using the set of adult bush‐crickets. Correct determination of each spermatophore species identity was always secured by test #1 and test #3, respectively, which yielded distinct pattern of bands of the gel (Figure 3).
Table 1.
Primers used in this study (e—external, i—internal/f—forward, r—reverse)
Figure 3.

Amplification of COI mtDNA fragments of Pholidoptera aptera (apt) and Pholidoptera transsylvanica (tra) spermatophores on 1.5% agarose gel. DNA was amplified using the external (bold) and the species‐specific internal (italic) primers combined in three different determination tests
3. RESULTS
3.1. Distribution, landscape type, and climate niche
Surveying the occurrence of bush‐crickets in Slovakia, we found a mostly nonoverlapping mosaic distribution pattern in the two Pholidoptera species. The range of P. aptera covered most of mountains in the Western Carpathians, whereas the occurrence of P. transsylvanica was delimited to the mountain regions of eastern Slovakia, apart from the easternmost area, which was inhabited by the other species. The recent coexistence of the species was found at two sites, which only represents 0.5% of all 391 sites (Figure 2). Regarding landscape type, mostly forests surrounded both P. aptera and P. transsylvanica sites, 88% and 87%, respectively (Figure 2), while the proportions of dominant forest habitats did not differ significantly between these species (χ 2 = 7.79, df = 6, p = .25). The moderately higher representation of coniferous (mostly Norway spruce) forests around the P. aptera sites is likely related to the regional topography of the region where it occurs. Comparing to regions of P. transsylvanica, occurrence sites of P. aptera were located in higher altitudes (Z = 3.42, p < .001). The first PCA axis explained 83.5% and the second 13.5% of the climatic data variance. The climate niche of P. transsylvanica was in complete overlap with P. aptera (Figure 4), and its relative breadth was most likely related to the size of distributional area sampled (Figure 2).
Figure 4.

Biplot of Pholidoptera aptera and Pholidoptera transsylvanica climatic niches constructed from seven climatic variables using PCA. For the sake of clarity, only three variables are presented (temperature—annual mean temperature, precipitation—precipitation of the driest and the coldest quarter, and seasonality—precipitation seasonality)
3.2. Heterospecific mating
The mating experiment with mixed groups of conspecifics and heterospecifics provided evidence about possible heterospecific mating choice in the two Pholidoptera species. During 14 days in open‐air cages, the average number of received spermatophores was 4.1 (range 3–6) in P. aptera and 2.9 (range 1–7) in P. transsylvanica females, respectively. In total, 8.1% of copulations were heterospecific (total number of copulations = 99), while females of P. aptera admitted 10.3% (n = 58) and P. transsylvanica 4.9% (n = 41) of alien spermatophores. Although copulation of a P. transsylvanica male with a P. aptera female in the mixed mating groups was slightly more frequent than in the reverse case (Figure 5), proportions of different type of copulations did not differ between species (χ 2 = 0.37, df = 1, p = .54). Each female which took part in heterospecific copulation also copulated with conspecifics. Two heterospecific copulations were found in only a single P. aptera female, which had five copulations in total. Heterospecific copulations occurred randomly regarding the order of mating events of individual females. We also did not find a significant difference (Z = 0.01, p = .99) in the length of refractory period that occurred after conspecific (median 3, range 0–6 days) and heterospecific copulation (median 3, range 2–3 days).
Figure 5.

Results of the behavioral experiment. Relative proportion (mean ± SE) of mating frequency between conspecifics and heterospecifics in mixed mating groups (n = 7 groups of two males and two females of each Pholidoptera aptera and Pholidoptera transsylvanica) as determined by DNA of transferred spermatophores (n = 99)
Along with conspecific, we found also heterospecific spermatodoses in the females' spermathecas, which confirmed that heterospecific sperms were successfully transferred into the female's reproductive system during copulation. The number of spermatodoses was identical to the number of recorded spermatophores in all females.
4. DISCUSSION
By merging field‐based evidence with experimental testing, we suggested reproductive interference in two related bush‐crickets, P. aptera and P. transsylvanica, where analyzed climatic and environmental factors likely did not play a significant role in the shaping of their geographical distributions in Central Europe. Instead, we found that the two species do not differ in either the landscape type or climate niche they occupy, but that they can engage in heterospecific mating if they come into contact.
The observed distribution pattern suggests that the species have colonized the study area at the northern margin of their ranges from different refugia, which favors the imperfect premating isolation between them (Gröning & Hochkirch, 2008; Tregenza, 2002). The range of P. aptera is typical for postglacial recolonization of Central Europe from the Balkans, whereas the restricted distribution of P. transsylvanica in Carpathian Mountains has obviously a relict character (Kenyeres, Rácz, & Varga, 2009). We also found that the two species only coexist in two sites in Western Carpathians in Slovakia, supporting species‐specific colonization histories but also a contemporary contact zone with a mosaic distribution pattern (Figure 2). Although there is no evidence about the utilization of different resources (e.g., prey) by these two species, premating isolation in this zone could be facilitated by spatial or temporal barriers to gene flow, which arise as by‐products of ecological divergence (Eroukhmanoff, Hargeby, & Svensson, 2011; Kádár et al., 2015). There could also be some habitat differentiation at the local scale, as indicated by the larger distance of P. transsylvanica from the forest edge toward a warmer microclimate than in P. aptera (Nagy et al., 1999 and own unpublished data), leading to a possible shift in the phenological development of the eggs (missing data) between species (earlier hatching of nymphs is expected in P. transsylvanica). However, none of these mechanisms can physically separate individuals and thus prevent reproductive interference at common sites (Jang & Gerhardt, 2006).
The females of P. transsylvanica in our experiment admitted twice as less alien spermatophores than P. aptera, and we can speculate how much of this proportion is responsible for the demographic displacement of one of the species. If the proportion of heterospecific mating is similar under field conditions, from the long‐term perspective we believe that permanent poaching on heterospecific spermatophores—which is in a way a form of resource competition—may substantially limit the reproductive success of the less frequent P. transsylvanica in the area (Hochkirch et al., 2007). In fact, because one of the older records by Chládek (2003) is no longer a coexistence site and P. transsylvanica is the species that was displaced, this could be evidence of exclusion by reproductive interference. However, the effect of temporary habitat change should be considered in that case also.
Although males can control the protein composition of the spermatophylax, both qualitatively and quantitatively, according to traits of the receiving female (Jarrige, Body, Giron, Greenfield, & Goubault, 2015), preliminary evidence on the protein diversity of the spermatophylax suggests that males provide basic nutrients in nuptial gifts to deter females from removing the spermatophore until successful sperm transfer has happened, rather than to chemically manipulate female postcopulation behavior (Lehmann et al., 2017). At least, we have no evidence that heterospecific mating would somehow affect the length of the refractory period. Bush‐cricket females have larger parental investment than males, and their mate choice is therefore also influenced by direct nutritional benefits (Gwynne, 1990; Lehmann, 2012). Occasional mating with heterospecific males should depend on their size, because if they are larger than conspecifics they should provide larger nuptial gifts (Costa‐Schmidt & Machado, 2012; Dorková, Naďo, Jarčuška, & Kaňuch, 2019). We, therefore, suggest that in the case of interspecific mating interactions between P. aptera and P. transsylvanica the female's choice of large males is what drives reproductive interference. Consequently, P. aptera females may benefit more from interspecific mating than P. transsylvanica females, which may increase their relative fitness. Moreover, larger P. transsylvanica males can outperform smaller P. aptera competitors, and also P. transsylvanica females can easily reject a forced heterospecific copulation attempt from P. aptera males (Ribeiro & Spielman, 1986). However, a measure of the net costs of heterospecific mating for females is clearly needed. A little is also known about inter‐ and intra‐specific variation in the shape of males' titillators (Harz, 1969). Since these sclerotized genital appendices are used in female stimulation and spermatophore transfer, it would be also interesting to determine the possible role of this sexual trait in heterospecific female mate choice (Vahed, 2015; Wulff et al., 2015). The costs of heterospecific mating are particularly high if postmating barriers are complete (e.g., gametic incompatibility or zygotic mortality), and there is no chance of siring viable hybrids (Gröning & Hochkirch, 2008). As we confirmed the successful transfer of heterospecific sperms into females' spermathecas, further research is needed to investigate whether these two species are fully reproductively isolated, especially at sites where they coexist.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
P.K. and A.K. conceived the idea and designed the study. A.K. performed most of the field work; M.D., A.K., and B.J. conducted experiment; M.D. carried out the molecular work. B.J. and P.K. performed analyses. P.K. drafted the manuscript. All authors contributed to writing the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
This study was funded by the Slovak Scientific Grant Agency VEGA (grants no. 2/0097/16, 2/0076/19). We are grateful to three anonymous reviewers for valuable comments and suggestions which helped to improve a previous version of the manuscript.
Dorková M, Krištín A, Jarčuška B, Kaňuch P. The mosaic distribution pattern of two sister bush‐cricket species and the possible role of reproductive interference. Ecol Evol. 2020;10:2570–2578. 10.1002/ece3.6086
DATA AVAILABILITY STATEMENT
Geographical coordinates of the distributional data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vmcvdncpk.
REFERENCES
- Aljanabi, S. M. , & Martinez, I. (1997). Universal and rapid salt‐extraction of high quality genomic DNA for PCR‐based techniques. Nucleic Acids Research, 25, 4692–4693. 10.1093/nar/25.22.4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos‐Lozano, L. (1998). Mate choice and hybridization experiments between allopatric populations of Pterophylla beltrani Bolívar and Bolívar and P. robertsi Hebard (Orthoptera: Tettigoniidae: Pseudophyllinae). Journal of Orthoptera Research, 7, 41–49. 10.2307/3503491 [DOI] [Google Scholar]
- Benedek, Z. , Nagy, A. , Rácz, I. A. , Jordán, F. , & Varga, Z. (2011). Landscape metrics as indicators: Quantifying habitat network changes of a bush‐cricket Pholidoptera transsylvanica in Hungary. Ecological Indicators, 11, 930–933. 10.1016/j.ecolind.2010.11.007 [DOI] [Google Scholar]
- Berggren, Å. (2004). Impact of grazing on individual male movement in Roesel's bush‐cricket Metrioptera roeseli: One possible clue to species range expansion. Journal of Insect Behavior, 17, 419–429. 10.1023/B:JOIR.0000042531.27859.ac [DOI] [Google Scholar]
- Brown, W. D. (1997). Courtship feeding in tree crickets increases insemination and female reproductive life span. Animal Behaviour, 54, 1369–1382. 10.1006/anbe.1997.0541 [DOI] [PubMed] [Google Scholar]
- Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics, 31, 343–366. 10.1146/annurev.ecolsys.31.1.343 [DOI] [Google Scholar]
- Chládek, F. (2003). Zweiter Beitrag zur Kenntnis der Geradflügler (Orthoptera s. l., Insecta) in der Slowakei. Tetrix, 10, 58–60. [Google Scholar]
- Chobanov, D. P. , Hochkirch, A. , Iorgu, I. S. , Ivkovic, S. , Krištín, A. , Lemonnier‐Darcemont, M. , … Willemse, L. P. M. (2016). Pholidoptera transsylvanica The IUCN red list of threatened species 2016: e.T62148044A70272060 10.2305/IUCN.UK.2016-3.RLTS.T62148044A70272060.en [DOI] [Google Scholar]
- Cole, J. A. (2016). Reinforcement and a cline in mating behaviour evolve in response to secondary contact and hybridization in shield‐back katydids (Orthoptera: Tettigoniidae). Journal of Evolutionary Biology, 29, 1652–1666. 10.1111/jeb.12900 [DOI] [PubMed] [Google Scholar]
- Costa‐Schmidt, L. E. , & Machado, G. (2012). Reproductive interference between two sibling species of gift‐giving spiders. Animal Behaviour, 84, 1201–1211. 10.1016/j.anbehav.2012.08.026 [DOI] [Google Scholar]
- Diekötter, T. , Speelmans, M. , Dusoulier, F. , Van Wingerden, W. K. R. E. , Malfait, J. P. , Crist, T. O. , … Dietz, H. (2007). Effects of landscape structure on movement patterns of the flightless bush cricket Pholidoptera griseoaptera . Environmental Entomology, 36, 90–98. [DOI] [PubMed] [Google Scholar]
- Dorková, M. , Naďo, L. , Jarčuška, B. , & Kaňuch, P. (2019). Size‐dependent mating pattern in a nuptial gift‐giving insect. Ecology and Evolution, 9, 454–462. 10.1002/ece3.4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroukhmanoff, F. , Hargeby, A. , & Svensson, E. I. (2011). The role of different reproductive barriers during phenotypic divergence of isopod ecotypes. Evolution, 65, 2631–2640. 10.1111/j.1558-5646.2011.01327.x [DOI] [PubMed] [Google Scholar]
- Fedorka, K. M. , & Mousseau, T. A. (2002). Material and genetic benefits of female multiple mating and polyandry. Animal Behaviour, 64, 361–367. 10.1006/anbe.2002.3052 [DOI] [Google Scholar]
- Gregory, P. G. , & Howard, D. J. (1993). Laboratory hybridization studies of Allonemobius fasciatus and A. socius (Orthoptera: Gryllidae). Annals of the Entomological Society of America, 86, 694–701. 10.1093/aesa/86.6.694 [DOI] [Google Scholar]
- Grether, G. F. , Losin, N. , Anderson, C. N. , & Okamoto, K. (2009). The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biological Reviews, 84, 617–635. 10.1111/j.1469-185X.2009.00089.x [DOI] [PubMed] [Google Scholar]
- Gröning, J. , & Hochkirch, A. (2008). Reproductive interference between animal species. Quarterly Review of Biology, 83, 257–282. 10.1086/590510 [DOI] [PubMed] [Google Scholar]
- Gröning, J. , Lücke, N. , Finger, A. , & Hochkirch, A. (2007). Reproductive interference in two ground‐hopper species: Testing hypotheses of coexistence in the field. Oikos, 116, 1449–1460. 10.1111/j.0030-1299.2007.15850.x [DOI] [Google Scholar]
- Gwynne, D. T. (1990). Testing parental investment and the control of sexual selection in katydids: The operational sex ratio. The American Naturalist, 136, 474–484. 10.1086/285108 [DOI] [Google Scholar]
- Gwynne, D. T. (2001). Katydids and bush‐crickets. Reproductive behaviour and evolution of Tettigoniidae. Ithaca, NY and London, UK: Cornell University Press. [Google Scholar]
- Harz, K. (1969). Die Orthopteren Europas I. Series entomologica (vol. 5). Hague, the Netherlands: Dr. W. Junk BV. [Google Scholar]
- Hewitt, G. M. (2001). Speciation, hybrid zones and phylogeography – Or seeing genes in space and time. Molecular Ecology, 10, 537–549. 10.1046/j.1365-294x.2001.01202.x [DOI] [PubMed] [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- Hochkirch, A. , Deppermann, J. , & Gröning, J. (2006). Visual communication behaviour as a mechanism behind reproductive interference in three pygmy grasshoppers (Genus Tetrix, Tetrigidae, Orthoptera). Journal of Insect Behavior, 19, 559–571. 10.1007/s10905-006-9043-2 [DOI] [Google Scholar]
- Hochkirch, A. , Gröning, J. , & Bücker, A. (2007). Sympatry with the devil: Reproductive interference could hamper species coexistence. Journal of Animal Ecology, 76, 633–642. 10.1111/j.1365-2656.2007.01241.x [DOI] [PubMed] [Google Scholar]
- Hochkirch, A. , Zuna‐Kratky, T. , Puskas, G. , Ivkovic, S. , Monnerat, C. , Chobanov, D. P. , … Szovenyi, G. (2016). Pholidoptera aptera The IUCN red list of threatened species 2016: e.T47712216A74621887 10.2305/IUCN.UK.2016-3.RLTS.T47712216A74621887.en [DOI] [Google Scholar]
- Hornett, E. A. , & Wheat, C. W. (2012). Quantitative RNA‐seq analysis in non‐model species: Assessing transcriptome assemblies as a scaffold and the utility of evolutionary divergent genomic reference species. BMC Genomics, 13, 361 10.1186/1471-2164-13-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, Y. , & Gerhardt, H. C. (2006). Divergence in the calling songs between sympatric and allopatric populations of the southern wood cricket Gryllus fultoni (Orthoptera: Gryllidae). Journal of Evolutionary Biology, 19, 459–472. 10.1111/j.1420-9101.2005.01014.x [DOI] [PubMed] [Google Scholar]
- Jarrige, A. , Body, M. , Giron, D. , Greenfield, M. D. , & Goubault, M. (2015). Amino acid composition of the bushcricket spermatophore and the function of courtship feeding: Variable composition suggests a dynamic role of the nuptial gift. Physiology & Behavior, 151, 463–468. 10.1016/j.physbeh.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Jordán, F. , Báldi, A. , Orci, K.‐M. , Rácz, I. , & Varga, Z. (2003). Characterizing the importance of habitat patches and corridors in maintaining the landscape connectivity of a Pholidoptera transsylvanica (Orthoptera) metapopulation. Landscape Ecology, 18, 83–92. [Google Scholar]
- Kádár, F. , Fazekas, J. P. , Sárospataki, M. , & Lövei, G. L. (2015). Seasonal dynamics, age structure and reproduction of four Carabus species (Coleoptera: Carabidae) living in forested landscapes in Hungary. Acta Zoologica Hungarica, 61, 57–72. 10.17109/AZH.61.1.57.2015 [DOI] [Google Scholar]
- Kenyeres, Z. , Rácz, I. A. , & Varga, Z. (2009). Endemism hot spots, core areas and disjunctions in European Orthoptera. Acta Zoologica Cracoviensia, 52B, 189–211. 10.3409/azc.52b_1-2.189-211 [DOI] [Google Scholar]
- Kindvall, O. (1999). Dispersal in a metapopulation of the bush cricket, Metrioptera bicolor (Orthoptera: Tettigoniidae). Animal Ecology, 68, 172–185. [Google Scholar]
- Krištín, A. , & Kaňuch, P. (2013). A review of distribution and ecology of three Orthoptera species of European importance with contributions from their recent north‐western range. North‐western Journal of Zoology, 9, 185–190. [Google Scholar]
- Kyogoku, D. (2015). Reproductive interference: Ecological and evolutionary consequences of interspecific promiscuity. Population Ecology, 57, 253–260. 10.1007/s10144-015-0486-1 [DOI] [Google Scholar]
- Lehmann, G. U. C. (2012). Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Frontiers in Zoology, 9, 19 10.1186/1742-9994-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, G. U. C. , Lehmann, K. , Neumann, B. , Lehmann, A. W. , Scheler, C. , & Jungblut, P. R. (2017). Protein analysis of the spermatophore reveals diverse compositions in both the ampulla and the spermatophylax in a bushcricket. Physiological Entomology, 43, 1–9. 10.1111/phen.12218 [DOI] [Google Scholar]
- Lehmann, G. U. C. , Siozios, S. , Bourtzis, K. , Reinhold, K. , & Lehmann, A. W. (2011). Thelytokous parthenogenesis and the heterogeneous decay of mating behaviours in a bushcricket (Orthopterida). Journal of Zoological Systematics and Evolutionary Research, 49, 102–109. 10.1111/j.1439-0469.2010.00588.x [DOI] [Google Scholar]
- Löffler, F. , & Fartmann, T. (2017). Effects of landscape and habitat quality on Orthoptera assemblages of pre‐alpine calcareous grasslands. Agriculture, Ecosystems & Environment, 248, 71–81. 10.1016/j.agee.2017.07.029 [DOI] [Google Scholar]
- Lorch, P. D. , Sword, G. A. , Gwynne, D. T. , & Anderson, G. L. (2005). Radiotelemetry reveals differences in individual movement patterns between outbreak and non‐outbreak Mormon cricket populations. Ecological Entomology, 30, 548–555. 10.1111/j.0307-6946.2005.00725.x [DOI] [Google Scholar]
- Michalko, R. , Košulič, O. , Hula, V. , & Surovcová, K. (2016). Niche differentiation of two sibling Wolf spider species, Pardosa lugubris and Pardosa alacris, along a canopy openness gradient. Journal of Arachnology, 44, 46–51. 10.1636/M15-46.1 [DOI] [Google Scholar]
- Nagy, B. , Rácz, I. , & Varga, Z. (1999). The Orthopteroid insects of the Aggtelek Karst Region (NE Hungary) referring to zoogeography and nature conservation In Mahunka S. (Ed.), The fauna of the Aggtelek National Park (pp. 83–102). Budapest, Hungary: Akademia. [Google Scholar]
- Orci, K. M. (2002). On the bioacoustics and morphology of some species‐groups of Orthoptera. Ph.D. thesis, University of Debrecen. [Google Scholar]
- Pellissier, L. , Fiedler, K. , Ndribe, C. , Dubuis, A. , Pradervand, J. N. , Guisan, A. , & Rasmann, S. (2012). Shifts in species richness, herbivore specialization, and plant resistance along elevation gradients. Ecology and Evolution, 2, 1818–1825. 10.1002/ece3.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.r-project.org [Google Scholar]
- Rácz, I. (1998). Biogeographical survey of the Orthoptera fauna in Central part of the Carpathian Basin (Hungary): Fauna types and community types. Articulata, 13, 53–69. [Google Scholar]
- Ribeiro, J. M. C. , & Spielman, A. (1986). The satyr effect: A model predicting parapatry and species extinction. The American Naturalist, 128, 513–528. 10.1086/284584 [DOI] [Google Scholar]
- Schirmel, J. , & Fartmann, T. (2013). Coexistence of two related bush‐cricket species (Orthoptera: Tettigonia caudata, T. viridissima) in an agricultural landscape. Biologia, 68, 510–516. 10.2478/s11756-013-0177-3 [DOI] [Google Scholar]
- Shapiro, L. H. (2000). Reproductive costs to heterospecific mating between two hybridizing katydids (Orthoptera: Tettigoniidae). Annals of the Entomological Society of America, 93, 440–446. 10.1603/0013-8746(2000)093[0440:RCTHMB]2.0.CO;2 [DOI] [Google Scholar]
- Simmons, L. W. (1990). Nuptial feeding in tettigoniids: Male costs and the rates of fecundity increase. Behavioral Ecology and Sociobiology, 27, 43–47. 10.1007/BF00183312 [DOI] [Google Scholar]
- Simon, C. , Frati, F. , Beckenbach, A. , Crespi, B. , Liu, H. , & Flook, P. (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 87, 651–701. 10.1093/aesa/87.6.651 [DOI] [Google Scholar]
- Tregenza, T. (2002). Divergence and reproductive isolation in the early stages of speciation. Genetica, 116, 291–300. [PubMed] [Google Scholar]
- Vahed, K. (1998). The function of nuptial feeding in insects: A review of empirical studies. Biological Reviews, 73, 43–78. 10.1017/S0006323197005112 [DOI] [Google Scholar]
- Vahed, K. (2003). Structure of spermatodoses in shield‐back bushcrickets (Tettigoniidae, Tettigoniinae). Journal of Morphology, 257, 45–52. 10.1002/jmor.10110 [DOI] [PubMed] [Google Scholar]
- Vahed, K. (2007). Comparative evidence for a cost to males of manipulating females in bushcrickets. Behavioral Ecology, 18, 499–506. 10.1093/beheco/arm021 [DOI] [Google Scholar]
- Vahed, K. (2015). Cryptic female choice in crickets and relatives (Orthoptera: Ensifera) In Peretti A. V., & Aisenberg A. (Eds.), Cryptic female choice in arthropods: Patterns, mechanisms and prospects (pp. 285–324). Berlin, Germany: Springer. [Google Scholar]
- Vedenina, V. Y. , Kulygina, N. K. , & Panyutin, A. K. (2007). Isolation mechanisms in closely related grasshopper species Chorthippus albomarginatus and Ch. oschei (Orthoptera: Acrididae). Entomological Review, 87, 263–272. 10.1134/S0013873807030037 [DOI] [Google Scholar]
- Voigt, C. C. , Kretzschmar, A. S. , Speakman, J. R. , & Lehmann, G. U. C. (2008). Female bushcrickets fuel their metabolism with male nuptial gifts. Biology Letters, 4, 476–478. 10.1098/rsbl.2008.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenreuther, M. , Larson, K. W. , & Svensson, E. I. (2012). Climatic niche divergence or conservatism? Environmental niches and range limits in ecologically similar damselflies. Ecology, 93, 1353–1366. 10.1890/11-1181.1 [DOI] [PubMed] [Google Scholar]
- Wulff, N. C. , Lehmann, A. W. , Hipsley, C. A. , & Lehmann, G. U. C. (2015). Copulatory courtship by bushcricket genital titillators revealed by functional morphology, μCT scanning for 3D reconstruction and female sense structures. Arthropod Structure & Development, 44, 388–397. 10.1016/j.asd.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Wulff, N. C. , van de Kamp, T. , dos Santos, R. T. , Baumbach, T. , & Lehmann, G. U. C. (2017). Copulatory courtship by internal genitalia in bushcrickets. Scientific Reports, 7, 42345 10.1038/srep42345 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Geographical coordinates of the distributional data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vmcvdncpk.


