Abstract
Failed oak regeneration is widely reported in temperate forests and has been linked in part to changed disturbance regimes and land‐use. We investigated if the North American fire–oak hypothesis could be applicable to temperate European oaks (Quercus robur, Quercus petraea) using a replicated field experiment with contrasting canopy openness, protection against ungulate browsing (fencing/no fencing), and low‐intensity surface fire (burn/no burn). Survival, relative height growth (RGRH), browsing damage on naturally regenerated oaks (≤300 cm tall), and changes in competing woody vegetation were monitored over three years. Greater light availability in canopy gaps increased oak RGRH (p = .034) and tended to increase survival (p = .092). There was also a trend that protection from browsing positively affected RGRH (p = .058) and survival (p = .059). Burning reduced survival (p < .001), nonetheless, survival rates were relatively high across treatment combinations at the end of the experiment (54%–92%). Most oaks receiving fire were top‐killed and survived by producing new sprouts; therefore, RGRH in burned plots became strongly negative the first year. Thereafter, RGRH was greater in burned plots (p = .002). Burning altered the patterns of ungulate browsing frequency on oaks. Overall, browsing frequency was greater during winter; however, in recently burned plots summer browsing was prominent. Burning did not change relative density of oaks, but it had a clear effect on competing woody vegetation as it reduced the number of individuals (p < .001) and their heights (p < .001). Our results suggest that young, temperate European oaks may respond similarly to fire as their North American congeners. However, disturbance from a single low‐intensity fire may not be sufficient to ensure a persistent competitive advantage—multiple fires and canopy thinning to increase light availability may be needed. Further research investigating long‐term fire effects on oaks of various ages, species‐specific response of competitors and implications for biodiversity conservation is needed.
Keywords: browsing, burn, disturbance, fire–oak hypothesis, light, Quercus robur/petraea, temperate
We investigated if the North American fire–oak hypothesis could be applicable to temperate European oaks. Although a low‐intensity fire reduced oak seedling survival, survival rates were relatively high across treatment combinations (54%–92%) and relative height growth was greater in burned plots after 2 years. Burning did not change relative density of oaks, but it had a clear effect on competing woody vegetation as it reduced the number of individuals and their heights, especially for conifers.

1. INTRODUCTION
Present forest composition is the result of historical disturbance regimes, anthropogenic land‐use, climate, and local site conditions. For centuries, natural disturbance and traditional land‐use practices maintained relatively open canopies in many northern temperate forests (Abrams, 1992; Kirby & Watkins, 2015; Vera, 2000). During the 20th century, however, secondary succession following abandonment of past land‐use practices or introduction of modern high‐production forestry changed forest composition and structure, often creating dense forests (Kirby & Watkins, 2015; Nowacki & Abrams, 2008).
Oaks (Quercus L.) are typical components of northern temperate ecosystems in which they provide multiple ecosystem services (Johnson, Shifley, Rogers, Dey, & Kabrick, 2018; Löf et al., 2016; Mölder, Meyer, & Nagel, 2019). Due to their disproportionate importance for biodiversity they are foundational species (Dayton, 1972; Ellison et al., 2005), creating crucial habitats for saproxylic invertebrates (Jonsell, Weslien, & Ehnström, 1998), lichens, fungi (Ranius, Eliasson, & Johansson, 2008), and birds (Rodewald & Abrams, 2002). In many areas, however, a combination of changing climate and human activities has favored other woody species over oaks, and thereby, lessened oak abundance relative to previous centuries (Lindbladh & Foster, 2010; McEwan, Dyer, & Pederson, 2011). Furthermore, oak forests face natural regeneration challenges throughout much of their range, which has been linked in part to intensification of forest‐use, increasing ungulate populations, efficient fire suppression, and a lack of sustained management in oak habitats of cultural origin (Bergmeier, Petermann, & Schröder, 2010; Crow, 1988; Dey et al., 2019; Petersson, Milberg, et al., 2019; Shaw, 1968; Watt, 1919).
Though their large acorns allow for seedling establishment in relatively dark understory conditions, oaks are often considered light demanding as they require ample irradiance for survival and growth once energy reserves of the cotyledons are exhausted (Annighöfer, Beckschäfer, Vor, & Ammer, 2015; Johnson et al., 2018). In addition to sufficient light, successful oak regeneration hinges on a variety of other factors that often influence each other, including competing vegetation, ungulate browsing, and site conditions (Annighöfer et al., 2015; Harmer, 2001; Jensen & Löf, 2017; Lorimer, Chapman, & Lambert, 1994). Though knowledge is lacking regarding the combined influence of different disturbance types on suitable environmental conditions, traditional land‐use systems that included grazing regimes, utilization of low‐intensity fire, and cutting of shade‐tolerant trees, likely promoted oak regeneration (Bobiec et al., 2019; Bobiec, Reif, & Öllerer, 2018; Vera, 2000).
In eastern North America, failed oak regeneration has been linked in part to changed disturbance regimes such as fire suppression during the last century (Abrams, 1992; Brose, Schuler, Lear, & Berst, 2001; Dey et al., 2019). This led to development of the fire–oak hypothesis, which states that fire has been an integral part of temperate oak ecosystems and that oaks have ecological traits (e.g., high sprouting capacity and thick bark) that make them better adapted to survive and benefit from periodic fires compared to other hardwood species (Abrams, 1992; Arthur, Alexander, Dey, Schweitzer, & Loftis, 2012; Brose et al., 2001; Nowacki & Abrams, 2008). Thus, prescribed burns along with stand thinning to increase light availability are used extensively in the United States to restore and regenerate oak ecosystems (Brose, Dey, & Waldrop, 2014; Dey & Hartman, 2005). Depending on stage of life and fire regimes, oaks can be classified as fire resisters and endurers (Rowe, 1983). Because of the complexities of fire regime effects on survival and mode of regeneration that complicate classification by Rowe (1983), we, hereafter, refer to oaks as being fire adapted.
In Europe, modern‐day forest fires have mainly been associated with the boreal and Mediterranean zones, and studies of fire effects on oak regeneration have almost entirely been conducted in Mediterranean forests with varying results (e.g., Catry, Moreira, Cardillo, & Pausas, 2012; Monteiro‐Henriques & Fernandes, 2018). However, reconstructions of past forest conditions suggest that fire may have had an important role in the dynamics of certain temperate European forest types as well (Bradshaw & Lindbladh, 2005; Niklasson, Lindbladh, & Björkman, 2002; Niklasson et al., 2010; Tinner, Conedera, Ammann, & Lotter, 2005). In western Ukraine, woodland fires may effectively prevent understory development of hazel (Corylus avellana L.), hornbeam (Carpinus betulus L.), and beech (Fagus sylvatica L.), thereby favoring pedunculate oak (Quercus robur L.) reproduction (Bobiec et al., 2019; Ziobro et al., 2016). Moreover, Adámek, Hadincová, and Wild (2016) found that survival rates of sessile oak [Quercus petraea (Matt.) Liebl.] trees following fire in central Europe was notably high and almost unaffected by fire intensity. A recent greenhouse experiment revealed that seedlings of Q. robur responded similarly to the fire adapted North American white oak (Q. alba L.) when they experienced shoot destruction typically sustained during fire (Petersson, Löf, Jensen, Chastain, & Gardiner, 2019). Together, these findings suggest that European temperate oak species (Q. robur and Q. petraea) may respond to fire in a similar fashion as their North American congeners.
Against this background, we investigated how the combined effects of different disturbances influence natural oak regeneration, and if the North American fire–oak hypothesis could be applicable during the regeneration phase of temperate European oak forests. A replicated field experiment using naturally regenerated oaks and a randomized block design with split–split plots including contrasting canopy openness, protection against browsing ungulates, and low‐intensity surface fire was established in southern Sweden. We hypothesized that: (a) greater light availability would increase tolerance of natural oak reproduction to disturbance; (b) protection against browsing would improve oak growth and survival; and, (c) oaks would survive a low‐intensity surface fire by producing new sprouts. Further, we evaluated the response of competing woody vegetation following the low‐intensity fire.
2. MATERIAL AND METHODS
2.1. Study sites and species
The experiment was conducted in five oak‐dominated forests, 45–95 m above sea level, in southern Sweden (Figure 1; Table 1). Southern Sweden consists of a mosaic landscape of forests, farmlands, and lakes. Due to active forest management favoring conifers, the dominant tree species in the region are Norway spruce (Picea abies L. Karst) and Scots pine (Pinus sylvestris L.). Two ecologically and morphologically similar oak species are native to the region, Q. robur and Q. petraea. The species naturally hybridize and are sometimes taxonomically treated as two subspecies under Q. robur (Roloff, Bärtels, & Schulz, 2008). In this study, they were treated as one species. At the start of the experiment, all study sites had naturally regenerated oak seedlings and saplings in the understory (Table S1).
Figure 1.
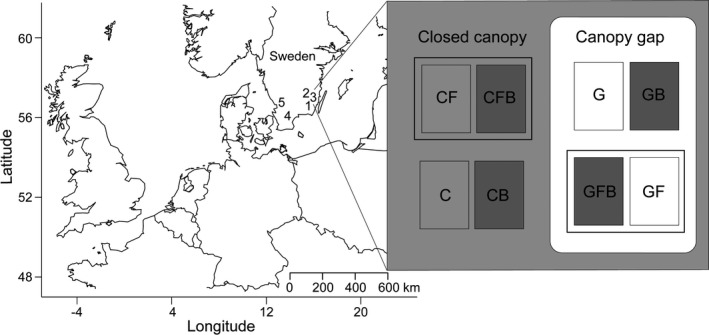
Experimental design including location of the five sites (blocks) in southern Sweden: (1) Abbetorp; (2) Barnebo; (3) Hornsö; (4) Sösdala; and, (5) Sperlingsholm. The enlarged figure shows one block with eight split–split plots, that is, treatment combinations: closed canopy (C), closed canopy and fence (CF), closed canopy, fence, and burn (CFB), closed canopy and burn (CB), canopy gap (G), canopy gap and fence (GF), canopy gap, fence, and burn (GFB) and canopy gap and burn (GB)
Table 1.
Characteristics of the five study sites
| Sites | Species composition | Heighta (m) | Basal areaa (m2/ha) | PACL (%) | Moose densityb (animals/km2) | Deer densityb (animals/km2) | ||
|---|---|---|---|---|---|---|---|---|
| Closed canopy | Canopy gap | Closed canopy | Canopy gap | |||||
| 1. Abbetorp | Quercus robur/petraea, Corylus avellana, Tilia cordata | 20 | 22 | 5 | 22 ± 1 | 35 ± 2 | 0.5–1.2 | 1.0–5.3 |
| 2. Barnebo | Q. robur/petraea, Picea abies, Pinus sylvestris | 21 | 27 | 16 | 23 ± 1 | 39 ± 1 | 0.4–1.5 | 3.2–4.2 |
| 3. Hornsö | Q. robur/petraea, Acer platanoides, P. abies | 17 | 29 | 21 | 20 ± 1 | 38 ± 2 | 0.5–0.7 | 5.2–8.3 |
| 4. Sösdala | Q. robur/petraea, Sorbus aucuparia, Betula pendula | 19 | 28 | 6 | 19 ± 1 | 39 ± 2 | 0.0–0.9 | 5.5–15.0 |
| 5. Sperlingsholm |
Q. robur/petraea, P. sylvestris, P. abies |
21 | 24 | 9 | 19 ± 1 | 45 ± 1 | 0.0–0.1 | 2.5–4.5 |
The three most frequent tree species (listed with respect to their abundance), mean height and basal area for all trees >10 cm dbh in August 2018; percentage of the above canopy light (PACL) in June averaged across the three study years; densities of moose and three deer species during the winter of 2016/2017 and 2017/2018.
Measurements conducted in a circular plot with a 10 m radius.
Densities are estimated based on pellet counts, see Appendix S1.
Four browsing ungulate species of the family Cervidae occur in southern Sweden: moose (Alces alces L.), roe deer (Capreolus capreolus L.), red deer (Cervus elaphus L.), and fallow deer (Dama dama L.). Moose and roe deer are common and likely responsible for most browsing damage in this study. Also, two species of hare (Lepus timidus L. and L. europaeus Pallas) may cause browsing damage.
2.2. Experimental design and treatments
We used a randomized block design with split–split plots and five blocks, that is, sites (Figure 1). The main treatment was canopy manipulation to create different light levels (closed canopy or canopy gap), with protection against browsing (no fence or fence) nested within the canopy treatment, and a low‐intensity surface fire (no burn or burn) nested within the fence treatment. This created eight treatment combinations: closed canopy (C), closed canopy and fence (CF), closed canopy, fence, and burn (CFB), closed canopy and burn (CB), canopy gap (G), canopy gap and fence (GF), canopy gap, fence, and burn (GFB), and canopy gap and burn (GB). The size of a split–split treatment plot was 25 m2, with a distance of 25–188 m and 7–42 m between canopy‐ and fence‐treatments, respectively.
In April 2016, cutting to create canopy gaps of about 400 m2 was performed at each site, and a 2‐m steel wire fence was erected around two adjacent, randomly selected plots in each canopy treatment. Cutting was conducted with a chainsaw to remove all large canopy trees in the canopy gaps, but saplings, seedlings, and shrubs were not cut. The fence excluded all ungulates and hares, but provided free access to rodents (mesh size 5 × 5 cm from 0–0.8 m, 16 × 20 cm from 0.8–2.0 m). Two plots in each canopy treatment were thereafter randomly selected for the burn treatment, one inside and one outside the fence. All oak recruits, defined as seedlings, saplings, or sprouts, within browsing height ≤ 300 cm tall (Nichols, Cromsigt, & Spong, 2015) in treatment plots were marked with a uniquely numbered aluminum tag. In plots with > 100 oak recruits, a random subset of at least 50 were chosen for measurements. In total, 2,357 oak recruits were measured through the duration of the experiment (Table S1).
Burning was performed between 29 September and 7 October 2016, following an unusually warm and dry late summer and autumn (Table S2). Delaying the burn to these dates allowed understory vegetation time to acclimate to canopy manipulation and fencing for one growing season prior to burning. To ensure similar burn treatment across sites and plots, we used a propane fired blowtorch (121960L, Kemper) to simulate a low‐intensity surface fire. Plots were systematically burned from one side to the other so that herbaceous vegetation and the top of the fine litter layer was burned (Table S3), but larger pieces of litter and shrubs were charred. Flame heights were visually estimated to be <0.4 m at all sites. It took about one hour to treat one plot and all four plots per site were treated during the same day. Environmental conditions and litter depth were recorded before and after each burn (Table S3).
2.3. Measurements
Height (±1 cm) and basal diameter (±1 mm) of all oak recruits were recorded at the start of the experiment in April 2016 (Table S1), and in late August 2016, 2017, and 2018. There were no significant difference in height nor basal diameter between treatments at the start of the experiment (Table S4). In each measurement period, we noted if an oak was alive and the number of stems per individual. For oaks with multiple stems, height and basal diameter were recorded for the tallest stem.
Browsing damage, that is, herbivore removal of twigs, shoots, or buds, was recorded in each year in both April and August 2016–2018. In April 2017, that is, the spring following burning, browsing damage could not be recorded in burn plots as most oaks were top‐killed by the fire and, therefore, had no shoots available for browsers. When possible, the herbivore (hare or ungulate) responsible for damage was determined following Kullberg and Bergström (2001). We defined browsing frequency as the proportion of browsed oaks per treatment plot. Fences remained intact throughout the experiment, and browsing damage did not occur inside fences. Densities of moose and the three deer species were estimated using pellet counts (Table 1, Appendix S1) (Eberhardt & Van Etten, 1956; Månsson, Andrén, & Sand, 2011).
In August of each year in 2016–2018, competing woody vegetation ≤300 cm tall was recorded in four circular 2 m2 subplots randomly placed in each treatment plot. Height (±1 cm) and number of individuals per species were recorded, as well as the number of oaks per subplot. These measurements included seedlings that established during the course of the experiment. As oaks dominated the understory at most sites, woody vegetation was combined into species groups: conifers, broadleaves excluding oaks, and oaks.
In late June for each year of 2016–2018, light availability at 160 cm above ground level was estimated using hemispherical imagery (Table 1). One photograph was taken in the middle of each treatment plot on an overcast day (Nikon Coolpix 8800VR, fisheye lens LC‐ER2). The camera lens was oriented perpendicular to the forest floor, and magnetic north was referenced in the image. Images were thresholded in the blue color plane, and percentage of above canopy light reaching the camera was calculated using Gap Light Analyzer software (Frazer, Canham, & Lertzman, 1999).
2.4. Calculations and statistical analysis
To account for height variation in oak recruits at the start of the experiment and any size‐related differences in growth rates, we calculated relative height growth rate per year (RGRH) for each oak following Hunt (1982):
where H 2 and H 1 were oak height the year of interest and the previous year, respectively, t 2 and t 1 were the year of interest and the previous year (in this work t 2− t 1 was always one year). We then calculated average RGRH per treatment plot. Data from the last year of the experiment, that is, August 2018, were used in the statistical analyses. We analyzed RGRH using a linear mixed‐effects model with the package “lme4” (Bates, Maechler, Bolker, & Walker, 2015), with the following factors (treatments) and their interactions as fixed effects: canopy openness, fence, and burn (all binary). To account for the hierarchical design of the experiment, we included nested (site/light/fence) random effects in the model. Residual and random effect distributions were examined graphically. A possible outlier was identified, however, when excluded it did not affect model results.
Survival was analyzed using a generalized linear mixed‐effects model with binomial error distribution, using a similar model structure (i.e., fixed and random effects) as described for the RGRH model. To investigate whether initial plant size affected survival after burning, an additional survival model using the fixed effects canopy openness, fence (both binary), and initial basal diameter (continuous), and only including the burn treatment, was analyzed. We tested for overdispersion and residual distribution using “testDispersion” and “testUniformity” functions from the DHARMa package (Hartig, 2019).
We analyzed the change of competing woody vegetation, that is, number of plants, using a generalized linear mixed‐effects model with Poisson error distribution, using the following factors as fixed effects: canopy openness, fence, burn (all binary), and species group (three levels). We included nested (site/light/fence/burn) random effects and the initial number of plants per species group as an offset variable. A postanalysis of deviance (Wald χ 2 Type III) revealed no significance of light or fence as predictor variables, hence we simplified the model using only burn and species group as predictors. This was supported by Akaike's Information Criterion (AIC). Overdispersion and residual distribution were tested as described above. Relative density of oaks, that is, number of oaks divided by total number of plants, and height of competing vegetation (log‐transformed) were analyzed using linear mixed‐effects models as described for the RGRH model.
As ungulate browsing pressure may vary over time, due to, for example, fluctuating ungulate population densities and season, data from the three measurement occasions after burning (August 2017, April 2018, August 2018) were modeled separately to analyze browsing frequency on oak. We used generalized linear mixed‐effects models with binomial error distribution, as described for the survival model, only including the treatment without fences and only ungulate browsing (not hare).
All analyses were performed using R version 3.5.0 (R Core Team, 2017). For all models, AIC was used to identify whether interactions between fixed effects were justified. Significance was determined at an alpha level of 0.05.
3. RESULTS
For oak survival and growth, as well as the development of competing woody vegetation, the statistical analyses presented here are based on data from the last year of the experiment (August 2018).
3.1. Survival
Survival of naturally regenerated oak recruits declined in all treatment combinations over the course of the experiment, ranging between 54% (CB treatment) and 92% (GF treatment) at the end of the experiment (Figure 2a). No interactions between effects were identified through AIC testing. Though not significant at α < 0.05, canopy openness (χ 2 = 2.83, df = 1, p = .092) and protection against browsing (χ 2 = 3.57, df = 1, p = .059) tended to increase survival. Burning, however, had a significant negative effect on survival (χ 2 = 141.43, df = 1, p < .001). The majority of oak recruits affected by fire were top‐killed and survived by producing new sprouts the following year; 88%–96% of surviving oaks affected by burning were resprouts at the end of the experiment. Furthermore, analysis of survival among oaks receiving the burn treatment revealed greater survival for recruits with larger initial stem basal diameter (χ 2 = 40.02, df = 1, p < .001), and for recruits growing in a canopy gap (χ 2 = 4.56, df = 1, p = .033), while protection against browsing had no significant effect (χ 2 = 2.51, df = 1, p = .114).
Figure 2.
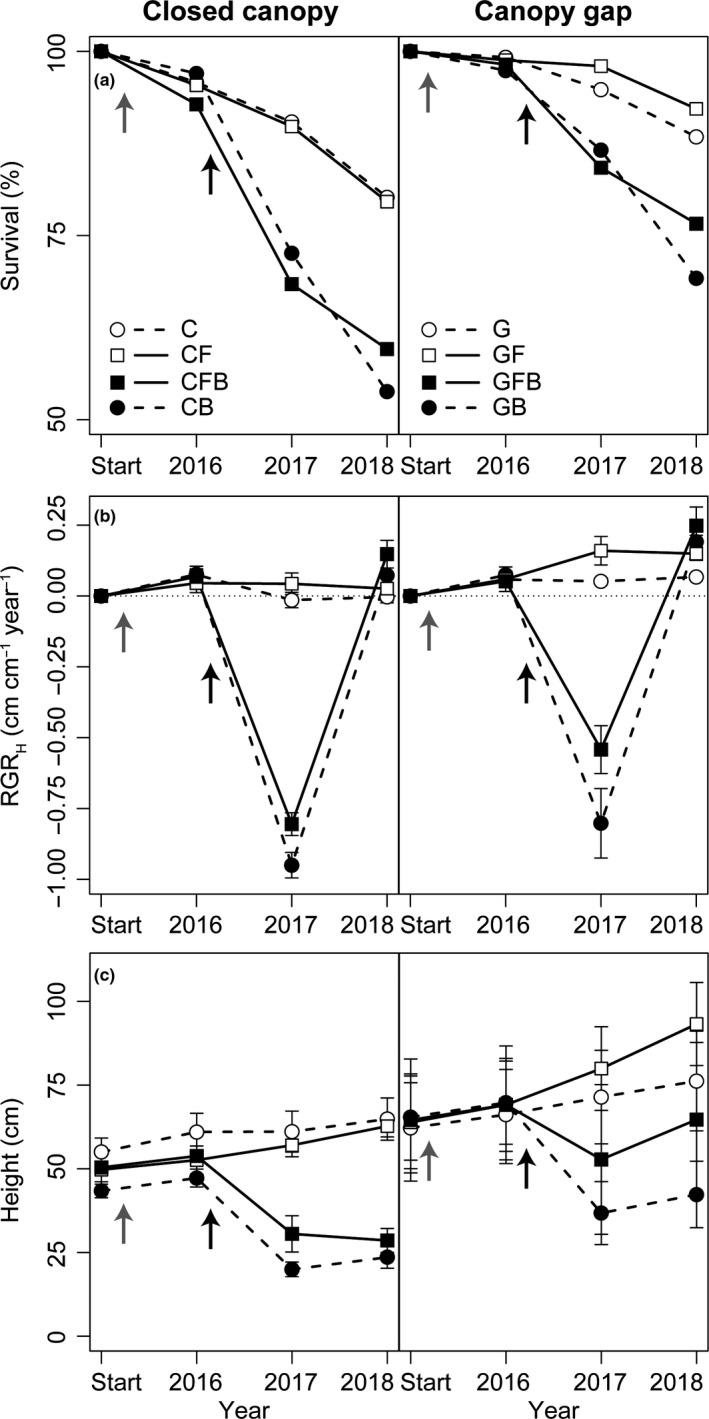
Development of naturally regenerated oaks as (a) survival, (b) relative height growth (RGRH), and (c) absolute height under eight treatment combinations [closed canopy (C), closed canopy and fence (CF), closed canopy, fence, and burn (CFB), closed canopy and burn (CB), canopy gap (G), canopy gap and fence (GF), canopy gap, fence, and burn (GFB), and canopy gap and burn (GB)]. Time of canopy gap creation and fencing is indicated with a gray arrow, and time of burn with a black arrow
3.2. Growth
As most oaks recruits that survived burning sprouted after top‐kill, their relatively small shoots resulted in a strongly negative RGRH one year after burning (Figure 2b). On average, oaks that sprouted produced 2.6 ± 0.1 shoots. RGRH was greatest under canopy gaps (F 1,4 = 10.10, p = .034), and, though not statistically significant, there was a trend that protection from browsing had a positive effect (F 1,9 = 4.71, p = .058). Furthermore, RGRH was greater for oaks receiving the burn treatment than those that did not receive burning (F 1,19 = 13.65, p = .002). AIC testing supported no interactions between treatments. Despite their greater RGRH, height of oak recruits subjected to burning remained lower through the experiment than those that were not burned (Figure 2c). By the end of the study, oaks had not grown above browsing height regardless of treatment.
3.3. Competing woody vegetation
Competing woody vegetation (number) was reduced by the burn treatment (χ 2 = 21.98, df = 1, p < .001). Moreover, differences were observed between species groups (χ 2 = 32.00, df = 2, p < .001). Burning reduced conifers from 750 to 125 plants/ha after two years, broadleaves (excluding oaks) from 9,812 to 5,500 plants/ha, and oaks from 51,652 to 38,250 plants/ha (Table S5). However, the relative density of oaks was not affected by treatment factors (Table S6).
Height of competing vegetation (broadleaves and conifers combined) was not affected by canopy openness (F 1,4 = 0.20, p = .681; Table 2). There was a trend, though not significant at α < 0.05, suggesting protection against browsing increased competitor height (F 1,9 = 4.71, p = .058). Concurrently, competitor height was lowest where plots were burned (F 1,19 = 23.36, p < .001).
Table 2.
Height of competing woody vegetation in August 2016 and 2018 (mean ± SE, n = 5). Heights in 2018 also include individuals that were established after measurements in 2016
| Treatment | Height (cm) | |
|---|---|---|
| 2016 | 2018 | |
| Closed canopy (C) | 95 ± 22 | 105 ± 26 |
| Closed canopy and fence (CF) | 51 ± 12 | 71 ± 13 |
| Closed canopy, fence, and burn (CFB) | 76 ± 17 | 45 ± 3 |
| Closed canopy and burn (CB) | 94 ± 19 | 37 ± 5 |
| Canopy gap (G) | 40 ± 4 | 55 ± 9 |
| Canopy gap and fence (GF) | 73 ± 12 | 117 ± 10 |
| Canopy gap, fence, and burn (GFB) | 71 ± 15 | 60 ± 7 |
| Canopy gap and burn (GB) | 58 ± 19 | 39 ± 5 |
3.4. Browsing frequency
The majority of browsing damage was caused by ungulates; hares were responsible for < 5% of all observed browsing damage. Overall, ungulate browsing frequency on oak recruits was greater during winter than summer (Figure 3). Burning, which was applied in the autumn of 2016, temporarily influenced the pattern of browsing frequency. In August 2017 (one year after burning), browsing frequency was greatest in the burned plots (Figure 3, Table 3), presumably a response to the new sprouts that emerged in the summer of 2017. Thereafter, browsing frequency was greatest in nonburned plots (Figure 3, Table 3). Except for the winter 2017/2018, browsing frequency was unaffected by canopy openness. In that winter, an interaction between the canopy and burn treatments suggested that browsing frequency was lowest in the CB treatment.
Figure 3.
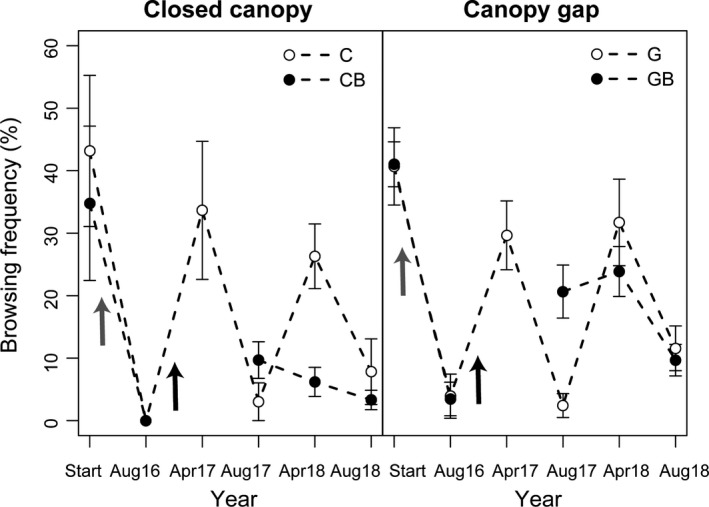
Ungulate browsing frequency (proportion of browsed oaks per plot) on naturally regenerated oaks in four treatment combinations [closed canopy (C), closed canopy and burn (CB), canopy gap (G), and canopy gap and burn (GB)]. Measurements in April correspond to preceding winter browsing, measurements in August correspond to preceding summer browsing. Time of canopy gap creation is indicated with a gray arrow, and time of burn with a black arrow
Table 3.
Analysis of deviance table based on a generalized linear mixed‐effects model (binomial) explaining ungulate browsing frequency on naturally regenerated oak recruits among treatments and their interactions (excluding fence treatment). Browsing frequency measured in August corresponds to preceding summer browsing, measurements in April corresponds to preceding winter browsing
| Factor | χ 2 | df | p |
|---|---|---|---|
| Aug 2017 | |||
| Canopy openness | 0.97 | 1 | .325 |
| Burn | 37.02 | 1 | <.001 |
| Canopy openness: burn | 3.19 | 1 | .074 |
| Apr 2018 | |||
| Canopy openness | 8.07 | 1 | .005 |
| Burn | 30.14 | 1 | <.001 |
| Canopy openness: burn | 10.32 | 1 | .001 |
| Aug 2018 | |||
| Canopy openness | 3.67 | 1 | .056 |
| Burn | 5.63 | 1 | .018 |
| Canopy openness: burn | 3.58 | 1 | .058 |
Type III Wald χ 2 tests.
4. DISCUSSION
We investigated if the fire–oak hypothesis (Abrams, 1992; Arthur et al., 2012) is applicable to European temperate oaks by establishing a field experiment with contrasting canopy openness, protection against wild ungulate browsers, and a low‐intensity fire. The experimental design allowed us to examine potential combined effects of these disturbance‐related factors. At the end of the experiment, oak survival was high across treatment combinations, and relative height growth rate (RGRH) was greatest where burning was applied. Furthermore, the relatively higher light availability within canopy gaps increased RGRH and oak survival where fire was prescribed. Burning also clearly effected competing woody vegetation by reducing the number of individuals, though it did not change relative density of oaks. These results, which are similar to reports from North American studies (e.g., Brose, Dey, Phillips, & Waldrop, 2013; McEwan et al., 2011), could indicate a common ecological mechanism impactful to the dynamics of oak regeneration in temperate forests across the Atlantic.
Though fire reduced survival of naturally regenerated oaks, survival rates were still relatively high across treatments. This high survival was largely dependent on new shoot production following top‐kill. Top‐kill initially decreased RGRH, but by the end of the experiment sprouting resulted in a vigorous RGRH. One reason many North American oaks are considered fire adapted is the relatively greater sprouting capacity they exhibit over their competitors (Abrams, 1992; Brose et al., 2014). The high sprouting capacity and increased growth rate of Q. robur/petraea observed in this field experiment supports the hypothesis that natural regeneration of temperate European oaks could be promoted by low‐intensity fire. However, long‐term observations are needed to determine if the positive fire effects noted through this research will persist over time.
As expected, oak RGRH was greater in canopy gaps than under closed canopies. However, oak survival was not significantly affected by canopy openness, except where burning was applied. Q. robur and Q. petraea require a minimum of 15%–20% of full light for sustained growth (Löf, Karlsson, Sonesson, Welander, & Collet, 2007; von Lüpke, 1998). This corresponds to the available light beneath closed canopies in this study and can probably explain the high survival rates of the oaks growing there. Nevertheless, the observation that survival after prescribed burning was improved in canopy gaps suggests that oak resilience to disturbance (which is conferred by sprouting) is enhanced by light. North American studies have shown that prescribed burning offers the greatest to oak regeneration when conducted in combination with overstory treatments that increase light availability (e.g., Brose et al., 2013; Hutchinson, Long, Rebbeck, Sutherland, & Yaussy, 2012). Improving light availability the year prior to burning in this experiment likely favored development of belowground carbohydrate reserves by oaks, which supported sprouting and rapid growth following the prescribed fire (Kabeya & Sakai, 2005; Welander & Ottosson, 1998). Furthermore, the observation of survival being greatest for oaks with the largest stem diameters is consistent with previous research (Dey & Hartman, 2005).
Though oaks are preferentially browsed by ungulates (Bergqvist, Wallgren, Jernelid, & Bergström, 2018), they are considered browsing tolerant because they can survive moderate browsing for extended periods (Harmer, 2001). However, browsing can severely limit height growth and high browsing pressure can prevent oak and other palatable tree species from advancing to the overstory (Churski, Bubnicki, Jedrzejewska, Kuijper, & Cromsigt, 2016; Rooney & Waller, 2003). We observed a trend indicating that protection from wild ungulate browsers positively affected oak recruit survival and RGRH. Considering the relatively short duration of our experiment, it was not surprising that protection against browsing had a somewhat limited impact. Additionally, there was considerable variation in browsing animal density between study sites and years, so it is possible that this variation masked positive effects of protection against browsers in this study.
According to the fire–oak hypothesis, many oaks have traits that confer a competitive advantage over other tree species following periodic fires (Abrams, 1992; Arthur et al., 2012). Testing the fire–oak hypothesis on temperate European oaks should therefore also include an assessment of fire effects on competing vegetation. In this study, woody vegetation was assessed as species groups since oak seedlings and saplings dominated the understory on most sites. This limits our ability to distinguish species‐specific responses of oak competitors. Furthermore, it was not possible to distinguish sprouts from newly established seedlings. Nevertheless, we observed clear fire effects on competing woody vegetation as prescribed burning reduced the number of individuals, especially the number of conifers (P. abies and P. sylvestris). This was expected because these conifers lack the ability to sprout when top‐killed (Leonardsson & Götmark, 2015). It is, however, worth noting that there were fewer conifers present on the study sites as compared to broadleaves (Table S5). The height of competing vegetation was also lowered in burned plots, but competing vegetation remained roughly the same height or slightly taller than oak reproduction at the end of the experiment.
Our review of literature from the eastern United States indicated that a single, dormant season prescribed fire is often not sufficient to favor oak regeneration (McEwan et al., 2011). Rather multiple fires have been more successful (Dey & Hartman, 2005; Hutchinson et al., 2012). This corresponds to Ziobro et al. (2016) and Bobiec et al. (2019), who reported that reoccurring grass burning promoted Q. robur regeneration by reducing competing shade‐tolerant species in Ukrainian woodlands. We, therefore, suggest that additional experiments involving multiple burning events are needed to determine whether reoccurring fire provides Q. robur and Q. petraea competitive advantage. The relatively small burn treatments in this study allowed us to experimentally examine potential combined effects of multiple disturbance‐related factors. Nevertheless, natural wildfires or prescribed burns generally impact larger areas, and the varying fire intensity of fire over larger areas can often result in pockets of unburnt vegetation (e.g., Lampainen, Kuuluvainen, Wallenius, Karjalainen, & Vanha‐Majamaa, 2004). Future studies investigating the effects of larger fire events on oak regeneration would, therefore, be of interest. Furthermore, the timing of a fire is known to affect how vegetation responds after fire. North American studies have demonstrated that growing season fires have greater impact than do dormant season fires because vegetation is physiologically active (Brose et al., 2014). Though burning was conducted at the very end of the growing season in this study, it is likely that the timing of the burns we applied impacted plants that were physiologically active.
Fire changed the pattern of ungulate browsing frequency. While the peak in browsing frequency was during the winter, summer browsing was also prominent where plots were recently burned. A similar observation has been reported for the United States (e.g., Andruk, Schwope, & Fowler, 2014; Collins & Carson, 2003). Considering the relatively small treatment plots of this study, it seems unlikely the increased browsing frequency was caused by a decrease in availability of alternative forage. Rather it may have resulted from changed foliar nutrient concentrations and/or foliar defensive chemistry that increased palatability of postfire–oak sprouts (Reich, Abrams, Ellsworth, Kruger, & Tabone, 1990; Rieske, 2002). This could also explain why browsing frequency was lower in the burn treatments compared to the nonburned treatments two years after the fire, as both Reich et al. (1990) and Rieske (2002) found increases in foliar nutrients resulting from prescribed burning diminished over time.
Considering most north temperate ecosystems have experienced dramatic increases in ungulate populations over the last few decades (Milner et al., 2006; Rooney & Waller, 2003) the interaction between fire and browsing frequency, we observed is particularly notable. It suggests that positive effects of prescribed fire on oak regeneration could be diminished in forests subject to high browsing pressure. In other words, oaks may readily survive moderate browsing pressure (Harmer, 2001), but browsing in combination with fire is likely to be more detrimental to survival of oak recruits. Indeed, Nuttle, Royo, Adams, and Carson (2013) found that high browsing pressure reduced the benefits of fire and canopy gap creation on tree diversity in forest understories.
4.1. Conclusion and management implications
Our results indicate that it is vital to increase light availability to promote naturally regenerated oak recruit survival and growth, especially when disturbances such as browsing and/or prescribed fire damage oak shoots. In areas of high conservation concern, this could be accomplished by removing canopy trees of less importance for biodiversity than oaks (cf. Leonardsson, Löf, & Götmark, 2015). Protection from ungulate browsing had a limited impact in this experiment; however, considering ungulate preference for oak browse, it is likely their impact will be maintained or even increase over time. This work also demonstrated relatively high survival and invigorated growth of Q. robur/petraea sprouts following a low‐intensity surface fire.
Altogether, our results indicate that a low‐intensity surface fire combined with some form of canopy cutting to increase light availability may serve as a first step for promoting natural regeneration of Q. robur and Q. petraea in temperate European forests. In particular, broadleaved forests of conservation concern in north temperate Europe, such as those examined in this study, often develop a significant regeneration pool of the shade‐tolerant P. abies (Götmark, Berglund, & Wiklander, 2005). The reduction of conifer reproduction that we observed through burning supports the application of low‐intensity fire as an efficient management tool in such situations. Fire would likely also be efficient in situations where shade‐tolerant F. sylvatica and C. betulus constitute the main competitors, which is often the case in central temperate Europe (Bobiec et al., 2019; Ziobro et al., 2016). However, further research is needed in several areas before we can recommend prescribed burns in oak‐dominated forests. Long‐term vitality of oak regeneration and overstory oaks following fire needs to be evaluated, preferably within different light environments and in areas with varying browsing pressure to further elucidate combined effects. Also, an understanding of the possible benefits of multiple fires, as well as evaluation of fire frequency and seasonality, is needed. Furthermore, the species‐specific response of competing woody vegetation following fire needs further investigation to determine whether oaks gain a competitive advantage. Finally, as oak‐dominated forests are of great importance for conservation of biodiversity, the effects of fire on species requiring oak habitats needs thorough examination.
AUTHOR CONTRIBUTIONS
All authors contributed to conceive the ideas and designed methodology; LP collected and analyzed the data; LP and ML led writing of the manuscript. All authors contributed critically to drafts and gave final approval for publication.
CONFLICT OF INTETEST
None declared.
Supporting information
ACKNOWLEDGMENTS
The Foundation Oscar and Lili Lamms Memory as well as Erik and Ebba Larssons and Thure Rignells Foundation supported this research. We thank private landowners and Sveaskog for providing field sites, Max Jensen, Per Nordin, and Alessandra Salvalaggio for field assistance. The authors declare no conflict of interest.
Petersson LK, Dey DC, Felton AM, Gardiner ES, Löf M. Influence of canopy openness, ungulate exclosure, and low‐intensity fire for improved oak regeneration in temperate Europe. Ecol Evol. 2020;10:2626–2637. 10.1002/ece3.6092
DATA AVAILABILITY STATEMENT
Data are available at Dryad Digital Repository: https://doi.org/10.5061/dryad.cz8w9gj0h.
REFERENCES
- Abrams, M. D. (1992). Fire and the development of oak forests. BioScience, 42, 346–353. 10.2307/1311781 [DOI] [Google Scholar]
- Adámek, M. , Hadincová, V. , & Wild, J. (2016). Long‐term effect of wildfires on temperate Pinus sylvestris forests: Vegetation dynamics and ecosystem resilience. Forest Ecology and Management, 380, 285–295. 10.1016/j.foreco.2016.08.051 [DOI] [Google Scholar]
- Andruk, C. M. , Schwope, C. , & Fowler, N. L. (2014). The joint effects of fire and herbivory on hardwood regeneration in central Texas woodlands. Forest Ecology and Management, 334, 193–200. 10.1016/j.foreco.2014.08.037 [DOI] [Google Scholar]
- Annighöfer, P. , Beckschäfer, P. , Vor, T. , & Ammer, C. . (2015) Regeneration patterns of European oak species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in dependence of environment and neighborhood. PLoS ONE, 10, e0134935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, M. A. , Alexander, H. D. , Dey, D. C. , Schweitzer, C. J. , & Loftis, D. L. (2012). Refining the oak‐fire hypothesis for management of oak‐dominated forests of the eastern United States. Journal of Forestry, 110, 257–266. 10.5849/jof.11-080 [DOI] [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Bergmeier, E. , Petermann, J. , & Schröder, E. (2010). Geobotanical survey of wood‐pasture habitats in Europe: Diversity, threats and conservation. Biodiversity and Conservation, 19, 2995–3014. 10.1007/s10531-010-9872-3 [DOI] [Google Scholar]
- Bergqvist, G. , Wallgren, M. , Jernelid, H. , & Bergström, R. (2018). Forage availability and moose winter browsing in forest landscapes. Forest Ecology and Management, 419–420, 170–178. 10.1016/j.foreco.2018.03.049 [DOI] [Google Scholar]
- Bobiec, A. , Podlaski, R. , Ortyl, B. , Korol, M. , Havryliuk, S. , Öllerer, K. , … Angelstam, P. (2019). Top‐down segregated policies undermine the maintenance of traditional wooded landscapes: Evidence from oaks at the European Union's eastern border. Landscape and Urban Planning, 189, 247–259. 10.1016/j.landurbplan.2019.04.026 [DOI] [Google Scholar]
- Bobiec, A. , Reif, A. , & Öllerer, K. (2018). Seeing the oakscape beyond the forest: A landscape approach to the oak regeneration in Europe. Landscape Ecology, 33, 513–528. 10.1007/s10980-018-0619-y [DOI] [Google Scholar]
- Bradshaw, R. H. W. , & Lindbladh, M. (2005). Regional spread and stand‐scale establishment of Fagus sylvatica and Picea abies in Scandinavia. Ecology, 86, 1679–1686. 10.1890/03-0785 [DOI] [Google Scholar]
- Brose, P. H. , Dey, D. C. , Phillips, R. J. , & Waldrop, T. A. (2013). A meta‐analysis of the fire‐oak hypothesis: Does prescribed burning promote oak reproduction in eastern North America? Forest Science, 59, 322–334. 10.5849/forsci.12-039 [DOI] [Google Scholar]
- Brose, P. , Dey, D. , & Waldrop, T. (2014). The fire‐oak literature of eastern North America: Synthesis and guidelines. U.S. Forest Service. General Technical Report NRS‐135.
- Brose, P. , Schuler, T. , van Lear, D. , & Berst, J. (2001). Bringing fire back: The changing regimes of the Appalachian mixed‐oak forests. Journal of Forestry, 99, 30–35. [Google Scholar]
- Catry, F. X. , Moreira, F. , Cardillo, E. , & Pausas, J. G. (2012). Post‐fire management of cork oak forests In Moreira F., Arianoutsou M., Corona P., &de las Heras J. (Eds.), Post‐fire management and restoration of southern European forests (pp. 195-222). Berlin, Germany: Springer. [Google Scholar]
- Churski, M. , Bubnicki, J. W. , Jedrzejewska, B. , Kuijper, D. P. J. , & Cromsigt, J. P. G. M. (2016). Brown world forests: Increased ungulate browsing keeps temperate trees in recruitment bottlenecks in resource hotspots. New Phytologist, 214, 158–168. 10.1111/nph.14345 [DOI] [PubMed] [Google Scholar]
- Collins, R. J. , & Carson, W. P. (2003) The fire and oak hypothesis: Incorporating the influence of deer browsing and canopy gaps. Thirteenth Central Hardwood Forest Conference, pp. 44–63. USDA Forest Service General Technical Report NC‐234, Minnesota, USA.
- Core Team, R. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Crow, T. R. (1988). Reproductive mode and mechanisms for self‐replacement of northern red oak (Quercus rubra) ‐ A review. Forest Science, 34, 19–40. [Google Scholar]
- Dayton, P. K. (1972). Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica In Parker B. (Ed.), Proceedings of the colloquium on conservation problems in Antarctica. Lawrence, KS: Allen Press. [Google Scholar]
- Dey, D. , & Hartman, G. (2005). Returning fire to Ozark Highland forest ecosystems: Effects on advance regeneration. Forest Ecology and Management, 217, 37–53. 10.1016/j.foreco.2005.05.002 [DOI] [Google Scholar]
- Dey, D. C. , Knapp, B. O. , Battaglia, M. A. , Deal, R. L. , Hart, J. L. , O'Hara, K. L. , … Schuler, T. M. (2019). Barriers to natural regeneration in temperate forests across the USA. New Forests, 50(1), 11–40. 10.1007/s11056-018-09694-6 [DOI] [Google Scholar]
- Eberhardt, L. , & Van Etten, R. C. (1956). Evaluation of the pellet group count as a deer census method. The Journal of Wildlife Management, 20, 70–74. 10.2307/3797250 [DOI] [Google Scholar]
- Ellison, A. M. , Bank, M. S. , Clinton, B. D. , Colburn, E. A. , Elliott, K. , Ford, C. R. , … Webster, J. R. (2005). Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment, 3, 479–486. 10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2 [DOI] [Google Scholar]
- Frazer, G. W. , Canham, C. D. , & Lertzman, K. P. (1999). Gap Light Analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true‐colour fisheye photographs. Users manual and program documentation.Millbrook, New York, NY.
- Götmark, F. , Berglund, Å. , & Wiklander, K. (2005). Browsing damage on broadleaved trees in semi‐natural temperate forest in Sweden, with a focus on oak regeneration. Scandinavian Journal of Forest Research, 20, 223–234. 10.1080/02827580510008383 [DOI] [Google Scholar]
- Harmer, R. (2001). The effect of plant competition and simulated summer browsing by deer on tree regeneration. Journal of Applied Ecology, 38, 1094–1103. 10.1046/j.1365-2664.2001.00664.x [DOI] [Google Scholar]
- Hartig, F. (2019). DHARMa: Residual diagnostics for hierarchical (multi‐level/mixed) regression models. R package version 0.2.4.
- Hunt, R. (1982). Plant growth curves. The functional approach to plant growth analysis. London, UK: Edward Arnold. [Google Scholar]
- Hutchinson, T. F. , Long, R. P. , Rebbeck, J. , Sutherland, E. K. , & Yaussy, D. A. (2012). Repeated prescribed fires alter gap‐phase regeneration in mixed‐oak forests. Canadian Journal of Forest Research, 42, 303–314. 10.1139/x11-184 [DOI] [Google Scholar]
- Jensen, A. M. , & Löf, M. (2017). Effects of interspecific competition from surrounding vegetation on mortality, growth and stem development in young oaks (Quercus robur). Forest Ecology and Management, 392, 176–183. 10.1016/j.foreco.2017.03.009 [DOI] [Google Scholar]
- Johnson, P. S. , Shifley, S. R. , Rogers, R. , Dey, D. C. , & Kabrick, J. M. (2018). The ecology and silviculture of oaks (3rd ed.). Wallingford, UK: CABI. [Google Scholar]
- Jonsell, M. , Weslien, J. , & Ehnström, B. (1998). Substrate requirements of red‐listed saproxylic invertebrates in Sweden. Biodiversity & Conservation, 7, 749–764. 10.1023/A:1008888319031 [DOI] [Google Scholar]
- Kabeya, D. , & Sakai, S. (2005). The relative importance of carbohydrate and nitrogen for the resprouting ability of Quercus crispula seedlings. Annals of Botany, 96, 479–488. 10.1093/aob/mci200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, K. J. , & Watkins, C. (2015). Europe's changing woods and forests: From wildwood to managed landscapes. Wallingford, UK: CABI. [Google Scholar]
- Kullberg, Y. , & Bergström, R. (2001). Winter browsing by large herbivores on planted deciduous seedlings in Southern Sweden. Scandinavian Journal of Forest Research, 16, 371–378. 10.1080/02827580117385 [DOI] [Google Scholar]
- Lampainen, J. , Kuuluvainen, T. , Wallenius, T. H. , Karjalainen, L. , & Vanha‐Majamaa, I. (2004). Long‐term forest structure and regeneration after wildfire in Russian Karelia. Journal of Vegetation Science, 15, 245–256. [Google Scholar]
- Leonardsson, J. , & Götmark, F. (2015). Differential survival and growth of stumps in 14 woody species after conservation thinning in mixed oak‐rich temperate forests. European Journal of Forest Research, 134, 199–209. 10.1007/s10342-014-0843-1 [DOI] [Google Scholar]
- Leonardsson, J. , Löf, M. , & Götmark, F. (2015). Exclosures can favour natural regeneration of oak after conservation‐oriented thinning in mixed forests in Sweden: A 10‐year study. Forest Ecology and Management, 354, 1–9. 10.1016/j.foreco.2015.07.004 [DOI] [Google Scholar]
- Lindbladh, M. , & Foster, D. R. (2010). Dynamics of longlived foundation species: The history of Quercus in southern Scandinavia. Journal of Ecology, 98, 1330–1345. 10.1111/j.1365-2745.2010.01733.x [DOI] [Google Scholar]
- Löf, M. , Brunet, J. , Filyushkina, A. , Lindbladh, M. , Skovsgaard, J. P. , & Felton, A. (2016). Management of oak forests: Striking a balance between timber production, biodiversity and cultural services. International Journal of Biodiversity Science, Ecosystem Services & Management, 12, 59–73. 10.1080/21513732.2015.1120780 [DOI] [Google Scholar]
- Löf, M. , Karlsson, M. , Sonesson, K. , Welander, T. N. , & Collet, C. (2007). Growth and mortality in underplanted tree seedlings in response to variations in canopy closure of Norway spruce stands. Forestry, 80, 371–383. 10.1093/forestry/cpm022 [DOI] [Google Scholar]
- Lorimer, C. G. , Chapman, J. W. , & Lambert, W. D. (1994). Tall understorey vegetation as a factor in the poor development of oak seedlings beneath mature stands. Journal of Ecology, 82, 227–237. 10.2307/2261291 [DOI] [Google Scholar]
- Månsson, J. , Andrén, H. , & Sand, H. (2011). Can pellet counts be used to accurately describe winter habitat selection by moose Alces alces? European Journal of Wildlife Research, 57, 1017–1023. 10.1007/s10344-011-0512-3 [DOI] [Google Scholar]
- McEwan, R. W. , Dyer, J. M. , & Pederson, N. (2011). Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography, 34, 244–256. 10.1111/j.1600-0587.2010.06390.x [DOI] [Google Scholar]
- Milner, J. M. , Bonenfant, C. , Mysterud, A. , Gaillard, J.‐M. , Csányi, S. , & Stenseth, N. C. (2006). Temporal and spatial development of red deer harvesting in Europe: Biological and cultural factors. Journal of Applied Ecology, 43, 721–734. 10.1111/j.1365-2664.2006.01183.x [DOI] [Google Scholar]
- Mölder, A. , Meyer, P. , & Nagel, R.‐V. (2019). Integrative management to sustain biodiversity and ecological continuity in Central European temperate oak (Quercus robur, Q. petraea) forests: An overview. Forest Ecology and Management, 437, 324–339. 10.1016/j.foreco.2019.01.006 [DOI] [Google Scholar]
- Monteiro‐Henriques, T. , & Fernandes, P. M. (2018). Regeneration of native forest species in mainland Portugal: Identifying main drivers. Forests, 9, 694 10.3390/f9110694 [DOI] [Google Scholar]
- Nichols, R. V. , Cromsigt, J. P. G. M. , & Spong, G. (2015). DNA left on browsed twigs uncovers bite‐scale resource use patterns in European ungulates. Oecologia, 178, 275–284. 10.1007/s00442-014-3196-z [DOI] [PubMed] [Google Scholar]
- Niklasson, M. , Lindbladh, M. , & Björkman, L. (2002). A long‐term record of Quercus decline, logging and fires in a southern Swedish Fagus‐Picea forest. Journal of Vegetation Science, 13, 765–774. [Google Scholar]
- Niklasson, M. , Zin, E. , Zielonka, T. , Feijen, M. , Korczyk, A. F. , Churski, M. , … Brzeziecki, B. (2010). A 350‐year tree‐ring fire record from Białowieża Primeval Forest, Poland: Implications for Central European lowland fire history. Journal of Ecology and Environment, 98, 1319–1329. [Google Scholar]
- Nowacki, G. J. , & Abrams, M. D. (2008). The demise of fire and the mesophication of forests in the eastern United States. BioScience, 58, 123–138. [Google Scholar]
- Nuttle, T. , Royo, A. A. , Adams, M. B. , & Carson, W. P. (2013). Historic disturbance regimes promote tree diversity only under low browsing regimes in eastern deciduous forest. Ecological Monographs, 83, 3–17. 10.1890/11-2263.1 [DOI] [Google Scholar]
- Petersson, L. K. , Löf, M. , Jensen, A. M. , Chastain, D. R. , & Gardiner, E. S. (2019). Sprouts of shoot‐clipped oak (Quercus alba and Q. robur) germinants show morphological and photosynthetic acclimation to contrasting light environments. New Forests, 10.1007/s11056-019-09762-5 [DOI] [Google Scholar]
- Petersson, L. K. , Milberg, P. , Bergstedt, J. , Dahlgren, J. , Felton, A. M. , Götmark, F. , … Löf, M. (2019). Changing land use and increasing abundance of deer cause natural regeneration failure of oaks: Six decades of landscape‐scale evidence. Forest Ecology and Management, 444, 299–307. 10.1016/j.foreco.2019.04.037 [DOI] [Google Scholar]
- Ranius, T. , Eliasson, P. , & Johansson, P. (2008). Large‐scale occurrence patterns of red‐listed lichens and fungi on old oaks are influenced both by current and historical habitat density. Biodiversity and Conservation, 17, 2371–2381. 10.1007/s10531-008-9387-3 [DOI] [Google Scholar]
- Reich, P. B. , Abrams, M. D. , Ellsworth, D. S. , Kruger, E. L. , & Tabone, T. J. (1990). Fire affects ecophysiology and community dynamics of central Wisconsin oak forest regeneration. Ecology, 71, 2179–2190. 10.2307/1938631 [DOI] [Google Scholar]
- Rieske, L. K. (2002). Wildfire alters oak growth, foliar chemistry, and herbivory. Forest Ecology and Management, 168, 91–99. 10.1016/S0378-1127(01)00731-9 [DOI] [Google Scholar]
- Rodewald, A. D. , & Abrams, M. D. (2002). Floristic and avian community structures: Implications for regional changes in eastern forest composition. Forest Science, 48, 267–272. [Google Scholar]
- Roloff, A. , Bärtels, A. , & Schulz, B. (2008). Flora der Gehölze. Bestimmung, Eigenschaften und Verwendung (3rd ed.). Stuttgart, Germany: Ulmer. [Google Scholar]
- Rooney, T. P. , & Waller, D. M. (2003). Direct and indirect effects of white‐tailed deer in forest ecosystems. Forest Ecology and Management, 181, 165–176. 10.1016/S0378-1127(03)00130-0 [DOI] [Google Scholar]
- Rowe, L. S. (1983). Concepts of fire effects on plant individuals and species In Wein R. W., & MacLean D. A. (Eds.), The role of fire in northern circumpolar ecosystems (pp. 135–151). New York, NY: John Wiley & Sons. [Google Scholar]
- Shaw, M. W. (1968). Factors affecting the natural regeneration of Sessile oak (Quercus petraea) in North Wales: II. Acorn losses and germination under field conditions. Journal of Ecology, 56, 647–660. 10.2307/2258097 [DOI] [Google Scholar]
- Spitzer, R. , Churski, M. , Felton, A. , Heurich, M. , Kuijper, D. P. J. , Landman, M. , … Cromsigt, J. P. G. M. (2019). Doubting dung: eDNA reveals high rates of misidentification in diverse European ungulate communities. European Journal of Wildlife Research, 65, 28 10.1007/s10344-019-1264-8 [DOI] [Google Scholar]
- Tinner, W. , Conedera, M. , Ammann, B. , & Lotter, A. F. (2005). Fire ecology north and south of the Alps since the last ice age. The Holocene, 15, 1214–1226. 10.1191/0959683605hl892rp [DOI] [Google Scholar]
- Vera, F. W. M. (2000). Grazing ecology and fire history. Wallingford, UK: CABI Publishing. [Google Scholar]
- von Lüpke, B. (1998). Silvicultural methods of oak regeneration with special respect to shade tolerant mixed species. Forest Ecology and Management, 106, 19–26. 10.1016/S0378-1127(97)00235-1 [DOI] [Google Scholar]
- Watt, A. S. (1919). On the causes of failure of natural regeneration in British oakwoods. Journal of Ecology, 7, 173–203. 10.2307/2255275 [DOI] [Google Scholar]
- Welander, N. T. , & Ottosson, B. (1998). The influence of shading on growth and morphology in seedlings of Quercus robur L. and Fagus sylvatica L. Forest Ecology and Management, 107, 117–126. 10.1016/S0378-1127(97)00326-5 [DOI] [Google Scholar]
- Ziobro, J. , Koziarz, M. , Havrylyuk, S. , Korol, M. , Ortyl, B. , Wolanski, P. , & Bobiec, A. (2016). Spring grass burning: An alleged driver of successful oak regeneration in sub‐Carpathian marginal woods. A Case Study. Prace Geograficzne, 146, 67–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at Dryad Digital Repository: https://doi.org/10.5061/dryad.cz8w9gj0h.


