Abstract
The newly emerging NDM-5 confers increased antibiotic resistance and attracts extensive global attention, but the prevalence, dissemination mechanism, and clinical significance of NDM-5 among clinical Escherichia coli (E. coli) infections have not been thoroughly characterized to date. In the present study, 109 unique carbapenem-resistant E. coli (CR-EC) isolates were collected in Southwest China, from 2013 to 2017, among which 41 (37.61%) CR-EC isolates were identified as NDM-5-producers, with most isolates carrying the IncF-type plasmids. Molecular epidemiological studies revealed ST167 being the most common sequence type (ST). Moreover, we described the first report of a clinical CR-EC isolate co-harboring blaKPC–2 and blaNDM–5, which showed a higher level of resistance to carbapenems. In addition, blaNDM–5 plasmid transformation and conjugation indicated that blaNDM–5 itself did confer resistance to carbapenems. Complete sequencing of the blaNDM–5-harboring IncF plasmid revealed highly conserved regions (bleMBL-trpF-tat) and some transposons around blaNDM–5. Our findings revealed a new potential threat of NDM-5-postive CR-EC in mainland China and emphasized an urgent need to control their further spread.
Keywords: carbapenem resistant, Escherichia coli, NDM-5, transformation, conjugation
Introduction
In recent years, accumulating researches have shed light on the dissemination of carbapenem-resistant Escherichia coli (E. coli) (CR-EC) worldwide, mainly due to the acquisition of carbapenemase-related genes located on several mobile resistance elements (Janvier et al., 2013; Cheng et al., 2016; Giufre et al., 2018).
Among the major geographically widespread carbapenemases, blaNDM has gained further worldwide attention for its high-level resistance to many clinically available β-lactams and the incredible horizontal transfer between different isolates. Since the first report of NDM-positive isolate in 2008 (Yong et al., 2009), 24 variants of NDM enzymes have been identified to date (Wu et al., 2019). NDM-5, which differed from NDM-1 by increased carbapenemase activity and substitutions at positions 88 (Val→Leu) and 154 (Met→Leu), was first identified in a CR-EC strain in United Kingdom (Hornsey et al., 2011).
Notably, one of the major reasons for the rapid emergence and spread of NDM-5 is its location on different incompatibility typing plasmids (Feng et al., 2018; Wang et al., 2018). Various plasmid incompatibility typing groups carrying blaNDM–5 have been reported worldwide with the most prevalent being IncFIA/B and IncX3 in Korea (Baek et al., 2019), IncFIA and IncFIB in Egypt (Gamal et al., 2016), IncFIA and IncFK in India (Ahmad et al., 2018), and IncFII in the United States (Rojas et al., 2017). Previous studies in China have shown several sporadic cases of clinical infections linked to ST167-type E. coli carrying the blaNDM–5 gene, which was mainly located on the IncX3 plasmid (Chen et al., 2014; Yang et al., 2014; Li et al., 2018; Sun et al., 2019). Unexpectedly, our initial study revealed that the epidemic incompatibility type of NDM-5 plasmid in our region is IncF, representing a new potential threat of carbapenem resistance Enterobacteriaceae (CRE) in China.
More importantly, the coproduction of NDM-5 and other carbapenemases in a single isolate is worthy of special concern, since it has been demonstrated to confer a higher-level resistance to carbapenems (Gamal et al., 2016; Rojas et al., 2017). To the best of our knowledge, this is the first report of clinical E. coli strains simultaneously producing NDM-5 and KPC-2 carbapenemases.
Therefore, the present study was initiated: (i) to describe the prevalence of clinical CR-EC isolates collected successively for approximately 5 years, (ii) to identify the resistance mechanisms among these CR-EC strains, and (iii) to explore the genetic context of blaNDM–5 to further elucidate the mechanisms involved in antibiotic resistance gene transferring.
Materials and Methods
Bacterial Strains
This retrospective study was performed in the First Affiliated Hospital of Chongqing Medical University and associated two branch hospitals in Southwestern China. 109 non-repetitive nosocomial CR-EC strains were collected between 2013 and 2017. All the isolates were identified at the species level by the VITEK MS (bioMérieux, Hazelwood, MO, United States) automated system, and routine antimicrobial susceptibility testing was performed by using the VITEK2 compact (bioMérieux, Inc., Durham, NC, United States) system. According to the breakpoint recommendations by the Clinical and Laboratory Standards Institute, 2017 (CLSI-2017), isolates which were non-susceptible to at least one of the carbapenems by the broth microdilution method, with the criteria of minimum inhibitory concentration (MIC) of ≥2 μg/mL for ertapenem (ETP), ≥4 μg/mL for imipenem (IPM), or ≥4 μg/mL for meropenem (MEM), were included in the study.
Antibiotics and in vitro Susceptibility Testing
All the isolates were tested for antibiotic susceptibilities to ceftazidime (CAZ), ceftriaxone (CRO), cefepime (FEP), gentamicin (GM), tobramycin (TOB), ciprofloxacin (CIP), and levofloxacin (LEV) by using AST GN13 cards on the VITEK2 compact system. MICs for ETP, IPM, MEM, colistin (CS), Aztreonam (ATM), and tigecycline (TGC) were determined using the broth microdilution method. MIC results were interpreted according to CLSI-2017 breakpoint recommendations. Non-carbapenemase-producing E. coli strain ATCC 25922 was served as a quality-control strain.
DNA Amplification and Analysis
Total DNA was extracted by boiling centrifugation method (Green and Sambrook, 2017). Briefly, single colonies were picked from overnight culture of each E. coli isolate, resuspended in 200 μl of sterile distilled water, and boiled at 100°C for 10 min. After centrifugation at 15,000 × g for 15 min, supernatants were collected and stored at −20 C (Rahman et al., 2014). The potential presence of carbapenemase genes, including blaKPC, blaNDM, blaVIM, blaIMP, blaOXA–23, blaOXA–24, and blaOXA–48, were detected by polymerase chain reaction (PCR),and all the variants of these carbapenemase genes were confirmed by sequencing (Wang et al., 2018). Furthermore, ESBL genes, such as blaCTXM, blaTME, blaSHV, and blaOXA–1, AmpC genes, such as blaACC, blaFOX, blaMOX, blaDHA, blaCIT, and blaEBC, porin genes, such as ompF and ompC were also determined by using primers as described previously (Supplementary Table S1). All the amplified PCR products were gel purified and sequenced by the Sanger method, while the plasmids were sequenced by the next-generation high throughput sequencing on the Illumina Hiseq 2000 (Illumina Inc., San Diego, CA, United States) platform.
Conjugation, Transformation, and Plasmid Analysis
To assess whether the carbapenemase-producing genes were located on the plasmids, conjugation with E. coli EC600 and transformation with E. coli DH5α were repeatedly conducted. For conjugative assays, the rifampin-resistant E. coli EC600 strain was use as the recipient, and the transconjugants were selected on Mueller-Hinton agar plates supplemented with a combination of 32 μg/ml ampicillin and 512 μg/ml rifampin. For transformation assays, the transformants were selected on Mueller-Hinton agar plates containing 2 μg/ml MEM (Jia et al., 2018). Both the transconjugants and transformants were tested for antimicrobial susceptibilities by the VITEK2 compact system, and MICs for ETP, IPM, MEM, colistin (CS), and tigecycline (TGC) were further determined by the broth microdilution method. The presence of resistance determinants were confirmed by PCR.
Plasmids carrying blaNDM were extracted from transconjugants using a QIAGEN Plasmid Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. To investigate the genetic context around the blaNDM–5 gene, we first chose the plasmid pNDM5-1001 which was extracted from transconjugant and harbored a blaNDM–5 gene for sequencing. The whole plasmid sequence was obtained on the HiSeq 2000 platform (Illumina Inc., San Diego, CA, United States) generating 400 bp paired-end reads. Then, the derived reads were trimmed and assembled using SOAP de novo v2.041, and gaps were closed through PCR and Sanger sequencing. Sequence analysis was done by both the ORF Finder2 and the BLAST functions3. The genomes of the plasmids were annotated by GeneMarkS4. The circular map of the pNDM5-1001 plasmid was generated using the Snap Gene server5.
A comparison of pNDM5-1001 and four other related plasmids pGUE_NDM (accession number JQ364967.1), pNDM5-020007 (accession number CP025626.1), pNDM-5-IT (accession number MG649062.1), and pNDM-BJ01 (accession number JQ001791.1) was performed with Mauve6.
Incompatibility typing of the blaNDM plasmid was performed by PCR-based replicon typing (Carattoli et al., 2005).
Genetic Environments of blaNDM-Carrying Plasmids
The genetic environment of blaNDM was established by PCR as described previously (Zhang et al., 2016). Primers were designed based on the reported blaNDM flanking sequences to determine the genetic background of the blaNDM-harboring strains (Supplementary Tables S2, S3). PCR experiments were performed using the same thermocycling conditions for all strains as follows: one cycle of 94°C for 5 min; followed by 35 cycles of 94°C for 35 s, 56°C for 45 s, and 72°C for 1 min; and a final cycle at 72°C for 10 min. The amplified products were sequenced and compared with the similar sequences deposited in the BLAST database, and the DNA sequences obtained were compared with those available in the NCBI GenBank database.
Multi-Locus Sequence Typing (MLST) and Pulsed-Field Gel Electrophoresis (PFGE)
Multi-locus sequence typing (MLST) was performed by the amplifications of the internal fragments of seven housekeeping genes of carbapenemase-producing E. coli isolates (adk, fumC, icd, purA, gyrB, recA, and mdh) according to the database7. The clonal relationships of the isolates harboring the blaNDM–5 gene were further determined by pulsed-field gel electrophoresis (PFGE). Briefly, genomic DNA of the blaNDM–5-positive E. coli isolates was prepared in agarose blocks and digested with restriction enzyme XbaI. DNA fragments were separated using a CHEF II D-Mapper XA PFGE system (Bio-Rad, Hercules, California, CA, United States) with running conditions as described previously (Jain et al., 2018).
Ethical Considerations
The data and samples analyzed in the present study were obtained in accordance with the standards and approved by the Chongqing Medical University Institutional Review Board and Biomedical Ethics Committee. For this study, samples were collected at the microbiology laboratory of our hospital, with no contact with the patients. This study was retrospective and there was no patient identification performed during data collection. Therefore, the ethics committee determined that informed consent was not required.
Statistical Analysis
All analyses were performed using SPSS v.25.0 software (SPSS Inc., Chicago, IL, United States). Univariate analyses were performed separately for each of the variables. All variables with a P value of ≤0.05 in the univariate analyses were considered for inclusion in the multivariate logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the strength of any association. Categorical variables were calculated using a chi-square test or Fisher’s exact test as appropriate. Continuous variables were calculated using Student t test (normally distributed variables) and Wilcoxon rank-sum test (non-normally distributed variables) as appropriate. For all calculations, statistical significance was defined at P < 0.05 for 2-tailed tests.
Results
General Characteristics and Antimicrobial Susceptibility of CR-EC Isolates
As shown in Table 1, a total of 109 non-repetitive CR-EC strains were isolated from 2013 to 2017 in our hospitals. These non-duplicated isolates were mainly isolated from urine, followed by the respiratory tract samples, drainage, secretion, blood, and other sources including bile and ascites. For the antimicrobial susceptibility of these isolates, our study showed the highest non-susceptible rate to ETP (97.3%), with 57.8% and 55.9% non-susceptible rates to imipenem and meropenem, respectively. Almost all the CR-EC isolates showed resistances to cephalosporins, especially to CRO, CAZ and FEP. Although NDM does not normally show activity against the monobactams such as aztreonam, CR-EC isolates showed high resistances to aztreonam, about 88.9%, probably due to the high prevalence of blaCTX–M among these isolates (Table 1). Moreover, over half of the CR-EC strains exhibited resistance to aminoglycoside and gentamycin. However, these CR-EC isolates were mostly susceptible to tigecycline and colistin. Compared with carbapenemase-negative isolates, our research revealed that a significantly greater proportion of carbapenemase-positive E. coli isolates were resistant to most antibiotics, including ETP, imipenem, meropenem, FEP, CIP, and LEV. Notably, 86.2% (94/109) of the CR-EC isolates were identified to be multi-drug resistant (MDR) as they were resistant to three or more classes of antimicrobial agents.
TABLE 1.
Antimicrobial susceptibility of CR-EC isolates with or without carbapenemases.
| Antimicrobial agents | Total (N = 109) |
Carbapenemase positive (N = 50) |
Carbapenemase negative (N = 59) |
P value | ||||||
| R (%) | R | MIC50 | MIC90 | Range | R | MIC50 | MIC90 | Range | ||
| Ertapenem | 106 (97.3) | 50 (100.0) | 32 | 256 | 2–512 | 56 (94.9) | 4 | 64 | 1–512 | < 0.001 |
| Imipenem | 63 (57.8) | 50 (100.0) | 32 | 128 | 4–512 | 13 (22.0) | 1 | 8 | 0.25–32 | < 0.001 |
| Meropenem | 61 (55.9) | 48 (96.0) | 8 | 54 | 0.25–256 | 13 (22.0) | 1 | 8 | 0.25–8 | < 0.001 |
| Ceftriaxone | 109 (100.0) | 50 (100.0) | 128 | 512 | 64–512 | 59 (100.0) | 64 | 128 | 16–512 | – |
| Ceftazidime | 102 (93.6) | 50 (100.0) | 128 | 512 | 64–512 | 52 (88.2) | 64 | 128 | 1–256 | < 0.001 |
| Cefepime | 101 (92.7) | 49 (98.0) | 128 | 512 | 8–512 | 52 (88.1) | 64 | 128 | 1–256 | < 0.001 |
| Aztreonam | 97 (88.9) | 48 (96.0) | 128 | 512 | 8–512 | 49 (83.0) | 64 | 256 | 2–256 | < 0.001 |
| Ciprofloxacin | 85 (77.9) | 41 (82.0) | 16 | 32 | 0.25–32 | 44 (74.6) | 4 | 8 | 0.25–16 | < 0.001 |
| Levofloxacin | 76 (69.7) | 37 (74.0) | 16 | 64 | 0.25–64 | 39 (66.1) | 8 | 8 | 0.25–32 | < 0.001 |
| Gentamycin | 70 (64.2) | 34 (68.0) | 32 | 128 | 1–512 | 36 (61.0) | 16 | 16 | 1–32 | 0.02 |
| Tobramycin | 51 (46.8) | 24 (48.0) | 8 | 128 | 1–512 | 27 (45.8) | 8 | 16 | 1–32 | 0.735 |
| Colistin | 22 (20.2) | 12 (24.0) | 1 | 4 | 1–8 | 10 (16.9) | 1 | 8 | 1–8 | 0.988 |
| Tigecycline | 0 (0.00) | 0 (0.00) | 1 | 4 | 0.25–4 | 0 (0.0) | 0.25 | 1 | 0.25–2 | – |
Data are number resistant (% of resistance rates). P-value for comparisons of the resistance rates of carbapenemase-positive and carbapenemase-negative groups. Bold face indicates values that are significant (P < 0.05). R, resistance.
Genotypic Distribution of the CR-EC Isolates
Among the 109 CR-EC isolates, 50 (45.9%) strains were demonstrated to be carbapenemase-producers, and most notably, all the 50 strains were positive for NDM, among which 41 (82.0%) of them were NDM-5-positive, 8 (16.0%) were NDM-1-positive, and 1 (2.0%) was NDM-9-positive. Notably, there was one isolate co-harboring blaNDM–5 and blaKPC–2. In addition to the production of carbapenemases, 76.2% (83/109) of the isolates were demonstrated to be ESBLs-positive, and blaCTX–M was the most prevalent ESBLs gene in the CR-EC isolates, with blaCTX–M–1 being the most prevalent (42.2%, 46/109) subtype, followed by blaCTX–M–9 (22.9%, 25/109) and blaCTX–M–15 (21.1%, 23/109) subtypes. Notably, all the 50 NDM-positive isolates were demonstrated to be co-harboring ESBLs. Besides blaCTX–M, blaTEM (exclusively blaTEM–1) also showed a high prevalence (32.1%, 35/109). However, no AmpC positive strains were identified. In addition, loss of expression of OmpC and OmpF were not common in CR-EC isolates, only accounting for 24.8% (27/109) and 19.3% (21/109) of these isolates. Notably, 45.9% (50/109) of the isolates were demonstrated to be co-harboring carbapenemases and ESBLs; and 11.0% (12/109) of the isolates co-produced carbapenemases and ESBLs with OMPs loss (Table 2). Most of the patients carrying NDM-producers were from ICU (28%, 14/50), the hepatological surgery department (12%, 6/50), and the gastrointestinal surgery department (8%, 4/50). Patients who didn’t carry NDM-producers were mostly from the hepatological surgery department (23.7%, 14/59), the urinary surgery department (11.86%, 7/59), and the gastrointestinal surgery department (10.16%, 6/59). It was interesting that isolates carrying the blaNDM–5 gene were mostly from sputum (10/50, 20.0%), urine (8/50, 16.0%), and blood (6/50, 12.0%), nevertheless, NDM negative isolates were mostly from urine (20/59, 33.8%), bile (7/59, 11.8%), and sputum (7/59, 11.8%).
TABLE 2.
Presence of antibiotic resistance genes in clinical carbapenemase-positive and carbapenemase-negative CR-EC isolates.
| Carbapenem resistance mechanisms | Total (109) | Carbapenemase positive (50) | Carbapenemase negative (59) | |
| Carbapenemase type(s) | 50(45.9%) | |||
| NDM- positive | NDM-1 | 8(7.3%) | 8(16.0%) | NA |
| NDM-5 | 41(37.6%) | 41(82.0%) | ||
| NDM-9 | 1(0.9%) | 1(2.0%) | ||
| KPC- positive | KPC-2 | 1(0.9%) | 1(2.0%) | NA |
| NDM-5, KPC-2 | 1(0.9%) | 1(2.0%) | NA | |
| ESBL type(s) | 83(76.2%) | |||
| CTXM- positive | CTXM-1 | 46(42.2%) | 24(48.0%) | 22 (37.3%) |
| CTXM-9 | 25(22.9%) | 16(32.0%) | 9 (15.3%) | |
| CTXM-15 | 23(21.1%) | 16(32.0%) | 7 (11.9%) | |
| TEM- positive | TEM-1 | 35(32.1%) | 29(58.0%) | 6 (10.2%) |
| OXA-1-positive | OXA-1 | 23(21.1%) | 8(16.0%) | 15 (25.4%) |
| OMPs loss | 32(29.4%) | |||
| OMPs | OmpC | 27(24.8%) | 12(24.0%) | 15 (25.4%) |
| OmpF | 21(19.3%) | 11(22.0%) | 10 (16.9%) | |
| OmpC, OmpF | 16(14.7%) | 4(8.0%) | 12 (20.3%) | |
| Carbapenemase, ESBL type | 50(45.9%) | 50(100.0%) | NA | |
| Carbapenemase, ESBL type, OMPs loss | 12(11.0%) | 12(24.0%) | NA | |
| Carbapenemase, OMPs loss | 12(11.0%) | 12(24.0%) | NA | |
| ESBL type, OMPs loss | 27(24.8%) | 12(24.0%) | 15 (25.4%) | |
NA, not applicable.
Characteristics of the Isolated CR-EC Strains Carrying blaNDM–5
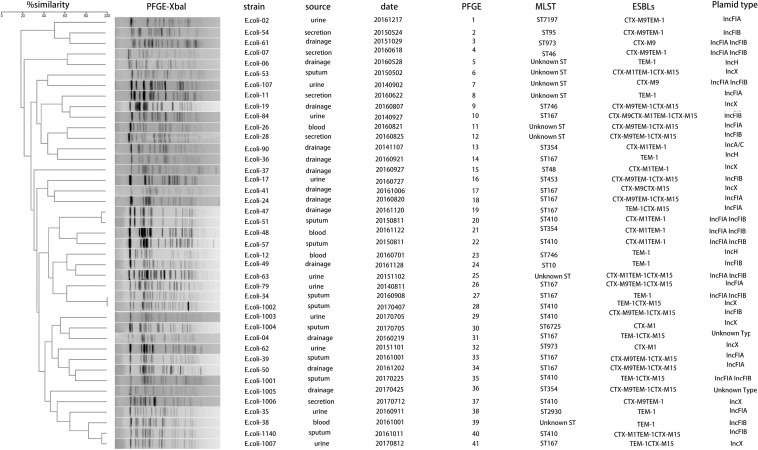
The blaNDM–5-carrying CR-EC isolates were found to be more resistant to carbapenems than CR-EC isolates carrying other blaNDM subtypes. While PCR assays demonstrated that blaNDM–5 was successfully transferred by both conjugation and transformation experiments in all the CR-EC isolates carrying the blaNDM–5 gene, multiple attempts to co-transfer or co-conjugate blaKPC–2 and blaNDM–5 failed, and no transconjugants or transformants co-harboring blaKPC–2 and blaNDM–5 were detected. Notably, all of the transconjugants and transformants acquired multi-drug resistance phenotypes similar to those of the donors except for reduced MICs to carbapenems and cephalosporins, and still remained susceptible to colistin and tigecycline. According to the plasmid replicon typing results, plasmid types of the transconjugants and transformants harboring blaNDM were IncF (33/50, 66.0%), IncX (11/50, 22.0%), IncH (3/50, 6.0%), and IncA/C (1/50, 2.0%). For chromosomal characteristics, the most prevalent sequence types (STs) was ST167 (12/50, 24.0%), followed by ST410 (5/50, 10.0%) and ST354 (3/50, 6.0%). Some rare ST types, including ST973 and ST746, were also witnessed, only accounting for 4.0% of the isolates, respectively. Notably, 16.0% of the CR-EC isolates still remain unknown ST types. PFGE was performed on all the 41 blaNDM–5-positive isolates, and all but 2 (E. coli-34 and E. coli-1002) of these strains showed a different PFGE band pattern (Table 3 and Figure 1).
TABLE 3.
Profiles of plasmids and corresponding average carbapenem MICs in 50 blaNDM-positive E. coli clinical isolates.
| Characteristics | NDM-5 | NDM-1 | NDM-9 | |
| Average MICs (μg/ml) | ETP | 84.79 | 31.75 | 8 |
| IPM | 55.10 | 31 | 8 | |
| MEM | 27.67 | 8.13 | 4 | |
| MLST | ST167 (10) | ST167 (1) | ST167 (1) | |
| ST410 (5) | ST361 (1) | |||
| ST354 (3) | ST4538 (1) | |||
| ST973 (2) | ST453 (1) | |||
| ST746 (2) | Unknown ST (4) | |||
| Unknown ST (8) Other types (11) | ||||
| Replicon type | IncF (26) | IncF (6) | IncF (1) | |
| IncX (9) | IncX (2) | |||
| IncA/C (1) | ||||
| IncH (3) UnknownType (2) | ||||
ETP, ertapenem; IPM, imipenem; MEM, meropenem.
FIGURE 1.
Dendrogram analysis and molecular epidemiology of NDM-5 positive Escherichia coli isolates. The dendrogram is based on the similarity of PFGE patterns from 41 blaNDM–5 positive clinical E. coli isolates. The right columns illustrate results of source, date, PFGE, MLST, ESBLs, and plasmid types.
Sequence Analysis of Plasmid Containing blaNDM–5
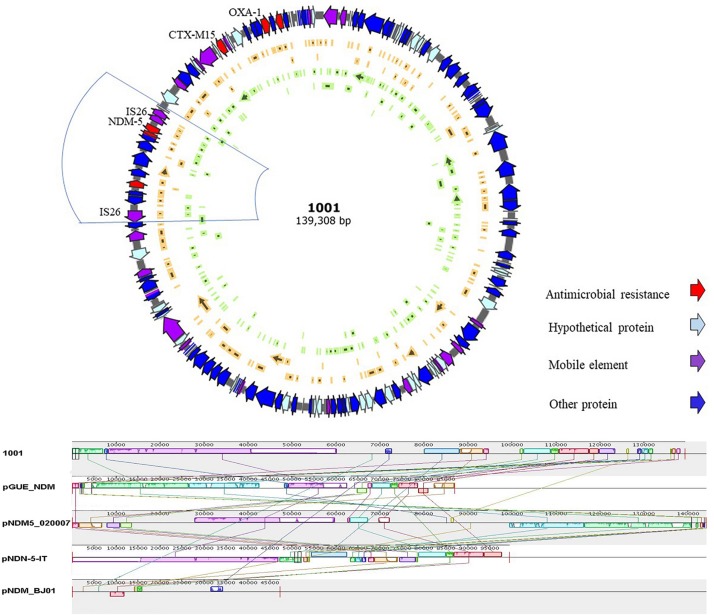
High throughput sequencing analysis was conducted on E. coli-1001, a strain co-harboring blaKPC–2 and blaNDM–5 genes. The 139-kb pNDM-5-1001 plasmid was identified to be of the IncF replicon type: containing FIA and FIB replicons, and also co-harboring blaCTX–M15, blaOXA–1, aminoglycoside resistance genes [aac(6’)-I, aac(6’)-II], and tetracycline resistance gene (tet). Within the pNDM-5-1001 plasmid, blaNDM–5 was bracketed by two insertion sequences IS26, containing the ISCR1 element and some other resistance genes (Figure 2). Further sequence alignments based on BLAST revealed that the plasmid sequence showed the most similar nucleotide sequences to those of the following previously reported plasmids: pGUE-NDM (accession number JQ364967.1) from an E. coli strain, pNDM5-020007 (accession number CP025626.1) from an E. coli strain, and pNDM-5-IT (GenBank accession No. MG649062.1) from an E. coli strain, but less similar to pNDM-BJ01 (GenBank accession No. JQ001791) from an Acinetobacter lwoffii (A. lwoffii) strain, which is the first NDM-expressing plasmid known to have been fully sequenced in China. The identical regions, including the replicon, stabilization elements, toxin-antitoxin systems (sopA and sopB) and virulence factors (arcA, arcB, arc, and arcD), were highly conserved (Figure 2).
FIGURE 2.
Schematic map of plasmid pNDM-5-1001 and comparative analysis of plasmid pNDM-5-1001 with other three blaNDM–5-carrying plasmids and pNDM-BJ01. Reference sequences: pNDM-BJ01 from an Acinetobacter lwoffii strain (GenBank accession No. JQ001791), pNDM-5-IT from an E. coli strain (GenBank accession No. MG649062.1), pGUE_NDM from an E. coli strain (GenBank accession number JQ364967.1), and pNDM5-020007 from an E. coli strain (GenBank accession number CP025626.1).
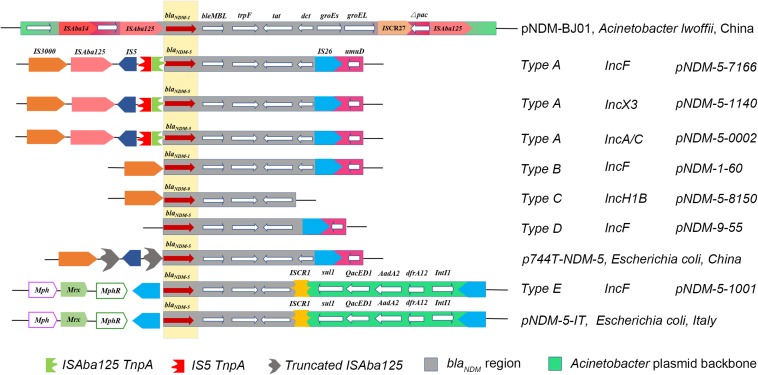
For genetic environments, 50 NDM-positive isolates were divided into five different types (A to E) based on the analysis of genetic structures, among which type A was the most common (n = 25), followed by type E (n = 9), type B (n = 8), type D (n = 5), and type C (n = 3). For type A, the flanking genetic structure of the blaNDM–5 gene was composed of an IS3000 and incomplete ISAba125 interrupted by IS5 located upstream, and the genes bleMBL (bleomycin resistance gene), trpF (phosphoribosylanthranilate isomerase), tat (DsbC superfamily protein), dct, IS26, and umD. Type B had a complete deletion of IS3000 and ISAba125; type C and type D only had IS3000 and IS26, respectively. Type E, another prevalent type which was different from type A, harbored another insertion sequence (ISCR1), followed by the sul gene (sulfonamide-resistant dihydropteroate synthase), the QacED1 (quaternary ammonium compound resistance protein), the AadA2 (aminoglycoside nucleotidyltransferase), the dfrA12 (dihydrofolate reductase), and the IntI1 (class 1 integron integrase) genes downstream, the MphR (Macrolide 2′-phosphotransferase), the Mrx (major facilitator family protein transporter), and the Mph [Mph(A) family macrolide 2′-phosphotransferase] upstream (Figure 3). As for the blaKPC–2 gene, we did not extract a KPC-2-expressing plasmid in this isolate. Moreover, multiple transformation and transconjugation experiments by the blaKPC–2-carrying bacteria failed. Thus, we speculated that the blaKPC–2 gene was most likely to be located on the chromosome.
FIGURE 3.
Comparison of the genetic elements surrounding the blaNDM–5 gene identified in this study with the other similar sequences. Reference sequences: pNDM-BJ01 (GenBank accession No. JQ001791) from an A. lwoffii strain, p744T-NDM5 (GenBank accession No. MF547511.1) from an E. coli strain, and pNDM-5-IT (GenBank accession No. MG649062.1) from an E. coli strain.
Discussion
In the present study, 37.61% (41/109) of the CR-EC isolates were identified to be NDM-5-producers, with most isolates carrying the IncF-type plasmids and ST167 being the most common ST type. Moreover, a clinical carbapenem-resistant E. coli isolate co-harboring blaKPC–2 and blaNDM–5 was reported for the first time, with the characterizations of its genetic environment. Our findings revealed a new potential threat of NDM-5-postive CR-EC in mainland China, emphasizing an urgent need to control their further spread.
Some conclusions of this study were noteworthy.
First, our results revealed that the main carbapenem resistance mechanism of the CR-EC isolates collected in our hospital could be attributed to the productions of carbapenemases and ESBL/AmpC enzymes combined with porin deficiencies. Notably, while NDM-1 subtype was reported to be the most prevalent from previous studies in China (Tuem et al., 2018; Markovska et al., 2019), NDM-5 was observed to be prevalent among the CR-EC isolates. Most importantly, our study revealed that the most prevalent epidemic incompatibility type of the blaNDM–5-carrying plasmids in our region is IncF (such as IncFIA, IncFIB, and IncFII), which was substantially different from those reported by the other NDM-5 studies (IncA/C and IncX3-type) (Huang et al., 2016; Li et al., 2018), indicating that a new molecular epidemiological CR-EC will be prevalent in China. In addition, a more recent study from eastern China have shown several blaNDM–5-positive E. coli infections linked to ST167-type CR-EC (Sun et al., 2019), which was also located on the IncX3 plasmid. Moreover, comparisions of the susceptibility results revealed that the E. coli isolates carrying the blaNDM–5 gene were more resistant than those with blaNDM–1 or blaNDM–9.
Second, we reported for the first time a clinical E. coli strain co-producing NDM-5 and KPC-2, which was isolated from sputum of a patient with respiratory infection. This isolate showed high-level resistances to many antibiotics, but remained susceptible to both tigecycline and colistin. Moreover, while the blaNDM–5 gene was demonstrated to be located on separate IncF-type plasmids, the blaKPC–2 gene failed to transfer into a recipient isolate with repeated experiments. To further determine the location of the blaKPC–2 gene, we performed high-throughput plasmid sequencing and did not find the blaKPC–2 gene in plasmid sequencing results, indicating that the blaKPC–2 gene might be located on the chromosome.
Third, we found five different types of blaNDM gene environments. Of the greatest interest was that all the NDM-producers all carried the highly conserved regions (blaNDM-bleMBL-trpF-tat) surrounding the blaNDM gene, suggesting that these four genes were important elements that produced resistances and were not lost during NDM-5 transmission. To the best of our knowledge, this is also the first report of Type C in China, which showed a similar genetic context to pNDM-5-IT from Italy. However, none of these patients in our study had a history of traveling abroad. As previous researches have demonstrated that the rolling circle replication of ISCR1 element could produce the upstream adjacent antibiotic resistance genes, which could be rescued through recombination between homologous fragments (Li et al., 2014; Cheng et al., 2016), we speculated that the ISCR1 element in type C could be an important gene assisting blaNDM spread via rolling circle transposition.
Conclusion
In summary, the present study revealed a new emergence of NDM-5-producing CR-EC in a teaching hospital in Chongqing, China, described the first report of a blaKPC–2 and blaNDM–5-coharboring E. coli isolate, and indicated for the first time that the IncF-type plasmids may contribute to the prevalence of blaNDM–5, highlighting an urgent need to develop effective measures to prevent and control the further spread of blaNDM–5 in China.
Data Availability Statement
The sequences described in this paper have been submitted to GenBank with the following accession numbers: No. MH985166 (Escherichia coli p-NDM-5-1140), MH985167 (Escherichia coli p-NDM-5-1001), MH985168 (Escherichia coli p-NDM-5-7166), MH985169 (Escherichia coli p-NDM-5-8150), MH985170 (Escherichia coli p-NDM-9-55), and MH985171 (Escherichia coli p-NDM-1-60).
Author Contributions
SH and HZ designed the study. HZ, XJ, SL, HL, and XW performed the experiments. HZ and XJ analyzed the data. HZ, XJ, and SH wrote this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This study was supported in part by the National Natural Science Foundation of China (Grant Nos. 81772239 and 31500749), the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant Nos. KJ1500235 and KJ1702022), and the Medical Research Program of Chongqing Health and Family Planning Commission (Nos. 2018MSXM009 and 2016MSXM001).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00334/full#supplementary-material
References
- Ahmad N., Khalid S., Ali S. M., Khan A. U. (2018). Occurrence of blaNDM variants among Enterobacteriaceae from a neonatal intensive care unit in a Northern India Hospital. Front. Microbiol. 9:407. 10.3389/fmicb.2018.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J. Y., Cho S. Y., Kim S. H., Kang C. I., Peck K. R., Song J. H., et al. (2019). Plasmid analysis of Escherichia coli isolates from South Korea co-producing NDM-5 and OXA-181 carbapenemases. Plasmid 104:102417. 10.1016/j.plasmid.2019.102417 [DOI] [PubMed] [Google Scholar]
- Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K. L., Threlfall E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63 219–228. 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Chen C. J., Wu T. L., Lu P. L., Chen Y. T., Fung C. P., Chuang Y. C., et al. (2014). Closely related NDM-1-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. PLoS One 9:e104899 10.1371/journal.pone.0104899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Sun J., Zheng F., Lu W., Yang Q., Rui Y. (2016). New structures simultaneously harboring class 1 integron and ISCR1-linked resistance genes in multidrug-resistant Gram-negative bacteria. BMC Microbiol. 16:71. 10.1186/s12866-016-0683-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Liu L., Mcnally A., Zong Z. (2018). Coexistence of Two blaNDM-5 genes on an IncF plasmid as revealed by nanopore sequencing. Antimicrob. Agents Chemother. 62:e00110-18. 10.1128/AAC.00110-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamal D., Fernandez-Martinez M., El-Defrawy I., Ocampo-Sosa A. A., Martinez-Martinez L. (2016). First identification of NDM-5 associated with OXA-181 in Escherichia coli from Egypt. Emerg. Microb. Infect. 5:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giufre M., Errico G., Accogli M., Monaco M., Villa L., Distasi M. A., et al. (2018). Emergence of NDM-5-producing Escherichia coli sequence type 167 clone in Italy. Int. J. Antimicrob. Agents 52 76–81. 10.1016/j.ijantimicag.2018.02.020 [DOI] [PubMed] [Google Scholar]
- Green M. R., Sambrook J. (2017). Isolating DNA from gram-negative bacteria. Cold Spring Harb. Protoc. 2017 83–85. 10.1101/pdb.prot093369 [DOI] [PubMed] [Google Scholar]
- Hornsey M., Phee L., Wareham D. W. (2011). A novel variant, NDM-5, of the New Delhi metallo-beta-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 55 5952–5954. 10.1128/AAC.05108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Yu X., Xie M., Wang X., Liao K., Xue W., et al. (2016). Widespread dissemination of carbapenem-resistant Escherichia coli sequence type 167 strains harboring blaNDM-5 in clinical Settings in China. Antimicrob. Agents Chemother. 60 4364–4368. 10.1128/AAC.00859-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P., Sudhanthirakodi S., Chowdhury G., Joshi S., Anandan S., Ray U., et al. (2018). Antimicrobial resistance, plasmid, virulence, multilocus sequence typing and pulsed-field gel electrophoresis profiles of Salmonella enterica serovar Typhimurium clinical and environmental isolates from India. PLoS One 13:e0207954. 10.1371/journal.pone.0207954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier F., Jeannot K., Tesse S., Robert-Nicoud M., Delacour H., Rapp C., et al. (2013). Molecular characterization of blaNDM-1 in a sequence type 235 Pseudomonas aeruginosa isolate from France. Antimicrob. Agents Chemother. 57 3408–3411. 10.1128/AAC.02334-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Dai W., Ma W., Yan J., He J., Li S., et al. (2018). Carbapenem-resistant E. cloacae in Southwest China: molecular analysis of resistance and risk factors for infections caused by NDM-1-producers. Front. Microbiol. 9:658 10.3389/fmicb.2018.00658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lan R., Xiong Y., Ye C., Yuan M., Liu X., et al. (2014). Sequential isolation in a patient of Raoultella planticola and Escherichia coli bearing a novel ISCR1 element carrying blaNDM-1. PLoS One 9:e89893. 10.1371/journal.pone.0089893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Fu Y., Shen M., Huang D., Du X., Hu Q., et al. (2018). Dissemination of blaNDM-5 gene via an IncX3-type plasmid among non-clonal Escherichia coli in China. Antimicrob. Resist. Infect. Control 7:59. 10.1186/s13756-018-0349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovska R., Stoeva T., Boyanova L., Stankova P., Schneider I., Keuleyan E., et al. (2019). Multicentre investigation of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in bulgarian hospitals - Interregional spread of ST11 NDM-1-producing K. pneumoniae. Infect. Genet. Evol. 69 61–67. 10.1016/j.meegid.2019.01.013 [DOI] [PubMed] [Google Scholar]
- Rahman M., Shukla S. K., Prasad K. N., Ovejero C. M., Pati B. K., Tripathi A., et al. (2014). Prevalence and molecular characterisation of New Delhi metallo-beta-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacteriaceae from India. Int. J. Antimicrob. Agents 44 30–37. 10.1016/j.ijantimicag.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Rojas L. J., Hujer A. M., Rudin S. D., Wright M. S., Domitrovic T. N., Marshall S. H., et al. (2017). NDM-5 and OXA-181 Beta-lactamases, a significant threat continues to spread in the americas. Antimicrob. Agents Chemother. 61:e00454-17. 10.1128/AAC.00454-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Xia W., Liu G., Huang X., Tang C., Liu C., et al. (2019). Characterization Of bla NDM-5-positive Escherichia coli prevalent in a University Hospital In Eastern China. Infect. Drug Resist. 24 3029–3038. 10.2147/IDR.S225546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuem K. B., Gebre A. K., Atey T. M., Bitew H., Yimer E. M., Berhe D. F. (2018). Drug resistance patterns of Escherichia coli in ethiopia: a meta-analysis. Biomed. Res. Int. 2018:4536905. 10.1155/2018/4536905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tong M. K., Chow K. H., Cheng V. C., Tse C. W., Wu A. K., et al. (2018). Occurrence of highly conjugative IncX3 epidemic plasmid carrying blaNDM in Enterobacteriaceae isolates in geographically widespread areas. Front. Microbiol. 9:2272. 10.3389/fmicb.2018.02272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Feng Y., Tang G., Qiao F., Mcnally A., Zong Z. (2019). NDM metallo-beta-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 32:e00115-18. 10.1128/CMR.00115-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Xie Y., Feng P., Zong Z. (2014). blaNDM-5 carried by an IncX3 plasmid in Escherichia coli sequence type 167. Antimicrob. Agents Chemother. 58 7548–7552. 10.1128/AAC.03911-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong D., Toleman M. A., Giske C. G., Cho H. S., Sundman K., Lee K., et al. (2009). Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53 5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Xie L., Wang X., Han L., Guo X., Ni Y., et al. (2016). Further spread of bla NDM-5 in Enterobacteriaceae via IncX3 plasmids in shanghai, China. Front. Microbiol. 7:424. 10.3389/fmicb.2016.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences described in this paper have been submitted to GenBank with the following accession numbers: No. MH985166 (Escherichia coli p-NDM-5-1140), MH985167 (Escherichia coli p-NDM-5-1001), MH985168 (Escherichia coli p-NDM-5-7166), MH985169 (Escherichia coli p-NDM-5-8150), MH985170 (Escherichia coli p-NDM-9-55), and MH985171 (Escherichia coli p-NDM-1-60).