Abstract
INTRODUCTION
Hypertension affects up to 5% of children worldwide and predicts later cardiovascular morbidity. Associations of short sleep and hypertension have been frequently reported in adults but less consistently in children. This study aims to examine the role of late bedtimes, a marker of short sleep duration, and potentially misaligned circadian rhythms, on incident elevated blood pressure (BP) in a large cohort of Mexican children.
METHODS
Participants included 2,033 adolescents recruited from public schools in Morelos, Mexico, free from elevated BP (<90th sex, age, and height-standardized percentile). Fourteen months later, all adolescents had a second BP assessment. We abstracted baseline habitual bedtimes from questionnaires to evaluate the association between bedtime and elevated BP incidence (≥90th percentile). Risk ratios and 95% confidence intervals were estimated with discrete-time mixed survival models, adjusting for potential confounders and accounting for clustering by school.
RESULTS
Participants were 12.5 (SD = 0.6) years old at baseline. At the follow-up visit 10% of adolescents had developed elevated BP. Compared to participants with a habitual weekday bedtime between 9 and 10 pm, those with a weekday bedtime 11 pm or later had a 1.87 times higher risk of developing elevated BP over the follow-up period (95% CI = 1.09, 2.21), after accounting for confounders. Participants with earlier weekday bedtimes also had a higher risk of elevated BP (RR = 1.96; 95% CI = 1.27, 3.01). The associations persisted after accounting for wake time.
CONCLUSION
These data showed a U-shaped association between weekday bedtime and elevated/high BP risk among Mexican adolescents.
Keywords: adolescents, blood pressure, circadian rhythm, hypertension, sleep, sleep timing
Worldwide, hypertension affects up to 5% of children, but its prevalence is much higher in low income countries and obese children.1,2 Elevated blood pressure (BP) diagnosis in childhood is based on age-, sex-, and height-specific BP percentiles3 and is defined with systolic blood pressure (SBP) or diastolic blood pressure (DBP) above the 90th percentile on three consecutive occasions. Notably, elevated BP during childhood is associated with increased risk for cardiovascular morbidity later in life.4
Poor sleep is a known risk factor for childhood obesity,5 but the role of sleep on elevated BP among youth remains unclear. Consistent with adult reports,6 multiple studies among pediatric populations have observed associations between short sleep duration and hypertension.7 Yet, a lack of consistency exists regarding possible U-shaped or sex-specific associations between sleep and hypertension in adolescents.8 Many prior studies have been limited by their cross-sectional design, which does not allow to disentangle the directionality of the relationship between poor sleep and hypertension. Another limitation of prior cross-sectional studies is the single assessment of BP. Finally, sleep duration has been the most examined sleep characteristic in relation to adolescent hypertension, while other sleep measures—such as sleep timing—have been rarely considered. Delayed bedtimes may reflect misaligned circadian rhythms (when sleep/wake behavior is not in line with internal body rhythms), which have been suggested as independent predictors of high BP among adults.9,10 This study examined the role of bedtimes on adolescent incident hypertension in a cohort of n = 2,033 Mexican children free of hypertension (<90th percentile) at baseline. We hypothesized that later bedtimes at baseline would be associated with higher incidence of elevated/high BP 14 months years later. Substantial changes in BP over a 14-month period are plausible during the pubertal transition, when changes in BP are heightened.11
METHODS
Study population
The study population is composed of secondary school students from public schools in Morelos, Mexico who agreed to participate in a study on lifestyle determinants of hypertension.12 Briefly, a random sample of 11 schools were selected to participate in the study and 2,893 students took part in the baseline visit (92.5% participation rate). During the baseline visit, BP was measured in addition to the administration of surveys on lifestyle habits and sociodemographic characteristics. A second follow-up visit, which occurred on average 14 months later (possible range 11–19 months), collected additional measurements of BP. The study was approved by the Institutional Review Boards at the National Institute of Public Health in Mexico, and informed consent was received from each participant’s parent/guardian in addition to assent from the participant.
Primary exposure: Bedtimes
Participants were asked to report their habitual bedtime during weekdays (options included <8 pm, between 8 and 9 pm, between 9 and 10 pm, between 10 and 11 pm, or after 11 pm) and weekends (options included <8 pm, between 8 and 9 pm, between 9 and 10 pm, between 10 and 11 pm, between 11 pm and 12 am, between 12 am and 1 am, or after 1 am). Weekday bedtimes were recoded into four categories: before 9 pm, between 9 and 10 pm, between 10 and 11 pm, or after 11 pm. Weekend bedtimes were recoded into five categories (more categories than weekdays due to greater response options for weekend bedtimes): before 9 pm, between 9 and 10 pm, between 10 and 11 pm, between 11 pm and 12 am, or after 12 am. To examine bedtime consistency, a weekend–weekday difference variable was calculated such that participants whose weekend bedtime was <2 hours different (i.e., changed by <2 categories) were considered to have a “stable” bedtime, while those with a ≥2 hours difference were considered to have a “substantially different” bedtime. Because the outer bed time and wake time categories were loosely defined, an accurate estimated sleep duration for the purposes of descriptive statistics could not be calculated.
Primary outcome: Incident hypertension
Trained nurses measured BP using digital blood pressure monitors. Measurements were taken in a seated position from the right arm after a resting period of 5 minutes. Two readings were taken 4–5 days apart in the morning and averaged during both the baseline visit and the follow-up visit. BP was categorized into age-, sex-, and height-specific percentiles based on the current standards from the American Academy of Pediatrics3 using a macro package for R hosted on a Shiny server (https://apps.cpeg-gcep.net/BPz_cpeg/). According to these standards, the 90th to <95th percentiles represent “elevated blood pressure”, while 95th and above represent “stage 1” and “stage 2” (≥95th percentile + 12 mm Hg). For the present analysis, BP was dichotomized, with adolescents in the ≥90th percentile considered as having elevated/high BP and those with <90th percentile as normal.
Covariates
Information on potential confounders was collected by questionnaires at baseline. These confounders included age, sex, pubertal status, maternal and paternal education, physical activity, screen time, alcohol and smoking habits, depression, and adiposity. Weight and height were measured by trained nurses during the baseline and follow-up visits using calibrated equipment (Tanita Solar Scale, Tanita Corporation). Baseline BMI-for-age z-scores were calculated using CDC growth charts,13 rather than the WHO reference, to be consistent with the fact that we used US blood pressure standards. Baseline BMI z-scores were categorized into <1 (normal or underweight), 1 to <2 (overweight), and 2 or greater (obese). Adolescents were grouped into four age categories: 9.5 to <12 years; 12 to <14 years; 14 to <16 years; and 16 to 18 years. For pubertal status, girls were asked whether they had started menstruating (menarche) and boys were asked whether they had experienced the first ejaculation (spermarche). Maternal and paternal education were categorized into ≤11 years, 12 years (completed high school), and >12 years (post high school education). Although both maternal and paternal education were examined in descriptive analyses, maternal education was selected over paternal education for the multivariable analysis due to lower missingness. Physical activity (hours/week) and screen time (hours/day) were measured with a standard questionnaire validated in the Mexican population,14 and were categorized into quartiles. For alcohol and smoking, adolescents reported whether or not they had ever smoked and whether or not they had an alcoholic drink within the past 12 months. Depression symptoms were measured with the Zung Depression Questionnaire,15 which contains 20 questions answered on a scale from 1 to 4 and summed for a score with a range of 20–80. The continuous depression scores were categorized into levels of low (<50), minimal (between 50 and 60), and moderate/severe (≥60).
Statistical analysis
Adolescents who did not have information on weekday bedtimes, baseline, and follow-up BP, or who met the criteria for risk of hypertension (≥90th BP percentile) at the baseline visit were excluded from all analyses (sample sizes of exclusions reported in results). We first evaluated associations between baseline bedtimes and confounders by examining the proportion of adolescents within categories of confounders according to bedtimes. P values for difference in proportions were calculated with the use of chi-square tests. To account for the multilevel study design of clustered students within schools, we used a discrete time survival mixed-effect model with a random intercept. Likelihood ratio tests that compared models with a random intercept for schools to those without confirmed the necessity of accounting for the school-level variance. The discrete-time model was used instead of a standard survival model because we were not able to ascertain the precise timing of the change in BP status, only that it occurred over the timeframe of the study. The incidence of hypertension was considered as the outcome for the models, and the exposures were (each in separate models): weekday bedtimes, weekend bedtimes, and difference in bedtimes. The statistical approach for each exposure was stepwise: first, we fitted a model with only bedtime categories as the predictor and a random effect for schools. Then, we added to this model demographic covariates: sex, age, and maternal education. Finally, we fitted a fully adjusted model that further accounted for screen time, depression, and alcohol consumption. Sensitivity analyses were conducted to evaluate the potential for selection bias in two ways. First, we compared the analytic sample to the full baseline sample by the distributions of confounding variables. Second, to examine the impact of missing covariates in the fully adjusted models on the robustness of the effects, we compared bivariate analysis (cross-tabulations of bedtimes and incident elevated BP) in the full analytic sample to the analytic sample with complete covariate information. Data analysis was performed using Stata version 14 (StataCorp LLC, College Station, TX).
RESULTS
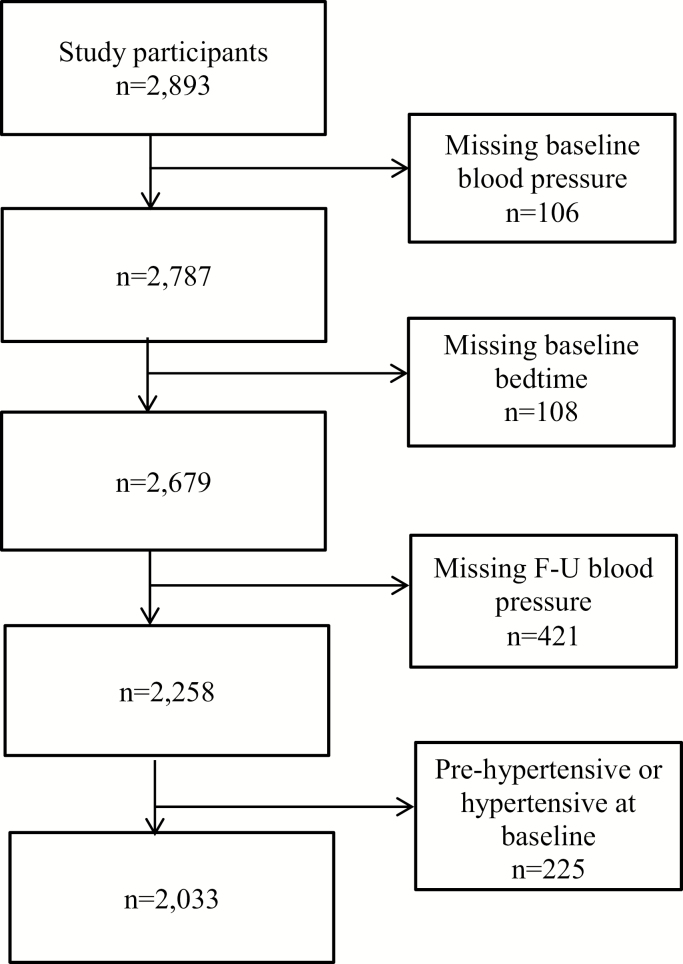
Of the 2,893 secondary school adolescents who participated in the baseline visit, we excluded those with missing BP (n = 106 at baseline and 421 at follow-up) or bedtime data (n = 108) (Figure 1). Compared to participants with missing values, those with complete information were slightly younger, more likely to be female, had fathers with higher education, had more moderate levels of screen time, had earlier bedtimes, and were less likely to have tried smoking or to have consumed alcohol.
Figure 1.
Flow chart of analytic sample exclusions.
To calculate risk of developing elevated BP over the follow-up period, adolescents with elevated BP at baseline (n = 225) were excluded, resulting in an analytic sample of 2,033 adolescents. In the analytic sample, almost half were female (48%) and most (95%) were younger than 15 years at baseline. Differences in bedtime distributions were noted by sex, paternal education, screen-time, and alcohol consumption. (Table 1). One-third of the adolescents had an inconsistent bedtime (≥2 hours difference in bedtimes) from weekends to weekdays; most of these differences (85%) were from delayed rather than earlier bedtimes on weekends compared with weekdays.
Table 1.
Associations between baseline sociodemographic and lifestyle characteristics and weekday bedtime category among 2,033 adolescents without high blood pressure at baseline
| Baseline sociodemographic and lifestyle characteristics | N a | Before 9 pm, % | Between 9 pm and 10 pm, % | Between 10 pm and 11 pm, % | After 11 pm, % |
|---|---|---|---|---|---|
| Weekday wake time | |||||
| Before 8 am | 548 | 70.80 | 75.46 | 64.21 | 51.30 |
| Between 8 and 9 am | 656 | 15.69 | 12.50 | 19.00 | 23.04 |
| Between 9 and 10 am | 542 | 2.55 | 5.79 | 5.72 | 10.43 |
| After 10 am | 230 | 10.95 | 6.25 | 11.07 | 15.22 |
| P | <0.0001 | ||||
| Sex | |||||
| Male | 1,063 | 46.63 | 44.00 | 49.82 | 55.98 |
| Female | 970 | 53.37 | 56.00 | 50.18 | 44.02 |
| P | 0.01 | ||||
| Age group, years | |||||
| 9.5 to <12 | 1,094 | 59.40 | 53.93 | 50.89 | 47.01 |
| 12 to <14 | 818 | 35.28 | 41.04 | 41.79 | 46.15 |
| 14 to <16 | 109 | 4.96 | 4.44 | 6.61 | 5.98 |
| 16 to 18 | 12 | 0.35 | 0.59 | 0.71 | 0.85 |
| P | 0.06 | ||||
| Menarche status (girls) | |||||
| Had not experienced | 322 | 35.82 | 31.13 | 28.90 | 34.04 |
| Had experienced | 680 | 64.18 | 68.87 | 71.10 | 65.96 |
| P | 0.34 | ||||
| Spermarche (boys) | |||||
| Had not experienced | 1,006 | 56.33 | 49.92 | 50.09 | 58.06 |
| Had experienced | 904 | 43.67 | 50.08 | 49.91 | 41.94 |
| P | 0.300 | ||||
| Maternal education, years | |||||
| ≤11 (secondary or primary) | 1,409 | 79.92 | 76.38 | 75.76 | 75.36 |
| 12 years (completed high school) | 204 | 12.40 | 10.42 | 10.51 | 11.85 |
| >12 years | 215 | 7.68 | 13.19 | 13.74 | 12.80 |
| P | 0.06 | ||||
| Paternal education, years | |||||
| ≤11 years (some high school) | 1,344 | 81.43 | 75.99 | 75.74 | 75.65 |
| 12 years (completed high school) | 202 | 11.22 | 9.95 | 14.47 | 10.88 |
| >12 years | 190 | 7.35 | 14.07 | 9.79 | 13.47 |
| P | 0.004 | ||||
| Physical activity, quartiles | |||||
| Q1, 0–8 hours/week | 567 | 28.66 | 30.60 | 29.86 | 34.86 |
| Q2, 9–11 hours/week | 389 | 19.96 | 21.14 | 22.20 | 18.81 |
| Q3, 12–16 hours/week | 448 | 25.30 | 25.24 | 21.81 | 22.48 |
| Q4, 17–42 hours/week | 463 | 26.09 | 23.03 | 26.13 | 23.85 |
| P value | 0.67 | ||||
| Screen time, quartiles | |||||
| Q1, None | 218 | 17.69 | 7.19 | 9.36 | 9.65 |
| Q2, <1 hour/day | 389 | 22.74 | 18.50 | 17.61 | 20.18 |
| Q3, 1 to 2 hours/day | 531 | 25.27 | 28.90 | 27.34 | 23.25 |
| Q4, 2 to 3 hours/day | 419 | 18.6 | 24.0 | 21.8 | 17.5 |
| 5, 4, or more hours/day | 424 | 15.7 | 21.4 | 23.9 | 29.4 |
| P | <0.0001 | ||||
| Consumed alcohol in the past year | |||||
| No | 1,808 | 94.63 | 93.83 | 91.99 | 87.44 |
| Yes | 140 | 5.37 | 6.17 | 8.01 | 12.56 |
| P | 0.003 | ||||
| Ever smoked cigarettes | |||||
| No | 1,889 | 95.99 | 96.07 | 94.64 | 93.48 |
| Yes | 92 | 4.01 | 3.93 | 5.36 | 6.52 |
| P | 0.29 | ||||
| Baseline BMI-Z scoresb | |||||
| Normal/underweight (<1 BMI-Z) | 1,403 | 69.86 | 71.26 | 66.35 | 62.09 |
| Overweight (≥1 to <2 BMI-Z) | 410 | 21.10 | 18.81 | 21.25 | 19.23 |
| Obese (≥2 BMI-Z) | 220 | 9.04 | 9.93 | 12.50 | 13.68 |
| P | 0.12 | ||||
| Measure of depressionc | |||||
| Normal (<50) | 1,828 | 90.39 | 92.58 | 88.21 | 86.70 |
| Mild (≥50 to <60) | 163 | 8.01 | 5.49 | 10.00 | 10.73 |
| Moderate/severe (≥60) | 38 | 1.60 | 1.93 | 1.79 | 2.58 |
| P | 0.06 |
aTotal sample sizes for some individual confounders are <2,033 due to missing information.
bBased on CDC reference.
cFrom Zung questionnaire.
Incident elevated/high BP was observed in 201 adolescents (10%). Of these, 66% were classified as “elevated,” 31% were in “stage 1 hypertension,” and 3% were “stage 2 hypertension.” In bivariate analyses, adolescents with incident elevated/high BP were mostly represented in the earliest or latest baseline bedtime categories during weekdays or weekends (Figure 2). Higher incidence of elevated/high BP was observed among adolescents with a ≥2-hour difference between their weekdays and weekends bedtime.
Figure 2.
Bivariate associations between bedtimes and elevated BP incidence.
Regression-based weekday bedtime results
In comparison to weekday bedtime between 9 and 10 pm, bedtime before 9pm was associated with 1.96 times higher incident elevated/high BP, after adjustment for age, sex, maternal education, screen time, depression, and alcohol consumption (95% CI = [1.27, 3.01]). Weekday bedtimes between 10 and 11 pm, or after 11 pm were associated with 1.81 and 1.87 times higher incident elevated/high BP relative to bedtime between 9 and 10 pm, after controlling for demographic, health, and behavioral characteristics (Table 2; [1.16, 2.80] and [1.09, 3.21], respectively). Estimates did not substantially change upon addition of wake time categories, which was considered as a potential mediator (Supplementary Table). Similarly, adding estimated sleep duration to the models resulted in only slightly attenuated estimates for the later bedtime categories (indicating potential partial mediation). In sex-stratified models, associations appeared stronger among females compared with males (Table 3), yet there were no statistically significant interactions.
Table 2.
Associations between baseline bedtimes and development of prehypertension and hypertension over follow-up
| N | Developed elevated/high BP, risk ratio (95% CI) crude modela | Developed elevated/high BP, adjusted risk ratio (95% CI), model 1b | Developed elevated/high BP, adjusted risk ratio (95% CI), model 2c,d | |
|---|---|---|---|---|
| Weekday bedtime | ||||
| <9 pm | 564 | 1.77 (1.20, 2.62)* | 1.75 (1.18, 2.58)* | 1.96 (1.27, 3.01)* |
| Between 9 pm and 10 pm | 675 | Reference | Reference | Reference |
| Between 10 pm and 11 pm | 560 | 1.82 (1.23, 2.69)* | 1.73 (1.16, 2.56)* | 1.81 (1.16, 2.80)* |
| 11 pm or later | 234 | 2.16 (1.35, 3.46)* | 1.96 (1.23, 3.14)* | 1.87 (1.09, 3.21)* |
| Weekend bedtime | ||||
| <9 pm | 298 | 1.41 (0.88, 2.26) | 1.33 (0.83, 2.12) | 1.53 (0.91, 2.59) |
| Between 9 pm and 10 pm | 409 | Reference | Reference | Reference |
| Between 10 pm and 11 pm | 534 | 1.06 (0.68, 1.65) | 1.01 (0.66, 1.57) | 1.10 (0.67, 1.83) |
| Between 11 pm and 12 pm | 498 | 1.16 (0.74, 1.81) | 1.06 (0.68, 1.67) | 1.28 (0.77, 2.12) |
| 12 pm or later | 266 | 1.61 (1.00, 2.60) | 1.40 (0.87, 2.28) | 1.57 (0.90, 2.72) |
| Difference in weekday– weekend bedtimes | ||||
| Minimal difference | 1,407 | Reference | Reference | Reference |
| >2 hours difference (earlier or later) | 598 | 1.13 (0.84, 1.53) | 1.08 (0.80, 1.47) | 1.07 (0.76, 1.50) |
*P < 0.05.
aFrom a discrete time survival mixed-effect model with development of hypertension risk (90th blood pressure percentile or higher) as the outcome and categorical bedtimes as the predictor (each bedtime variable run as its own model). Clustering by school was included as a random intercept.
bAdjusted for age and sex.
cAdjusted for age, sex, maternal education, screen time, depression, and alcohol consumption.
dSample sizes are slightly smaller due to missing covariates.
Table 3.
Sex-stratified associations between baseline bedtimes and development of prehypertension and hypertension over follow-up
| N | Boys: developed elevated/high BP, adjusted risk ratio (95% CI), model 23,4 | Girls: developed elevated/high BP, adjusted risk ratio (95% CI), model 23,4 | |
|---|---|---|---|
| Weekday bedtime | |||
| <9 pm | 564 | 1.58 (0.96, 2.60) | 4.64 (1.86, 11.57)* |
| Between 9 pm and 10 pm | 675 | Reference | Reference |
| Between 10 pm and 11 pm | 560 | 1.22 (0.73, 2.05) | 5.15 (2.03, 13.07)* |
| 11 pm or later | 234 | 1.18 (0.62, 2.25) | 6.10 (2.09, 17.76)* |
| Weekend bedtime | |||
| <9 pm | 298 | 1.54 (0.76, 3.10) | 1.46 (0.66, 3.25) |
| Between 9 pm and 10 pm | 409 | Reference | Reference |
| Between 10 pm and 11 pm | 534 | 1.29 (0.67, 2.48) | 0.79 (0.34, 1.82) |
| Between 11 pm and 12 pm | 498 | 1.41 (0.74, 2.67) | 0.97 (0.41, 2.35) |
| 12 pm or later | 266 | 1.23 (0.59, 2.53) | 2.78 (1.20, 6.44)* |
| Difference in weekday–weekend bedtimes | |||
| Minimal difference | 1,407 | Reference | Reference |
| >2 hours difference (earlier or later) | 598 | 1.25 (0.84, 1.86) | 0.81 (0.41, 1.58) |
*P < 0.05.
aFrom a discrete time survival mixed-effect model with development of hypertension risk (90th blood pressure percentile or higher) as the outcome and categorical bedtimes as the predictor (each bedtime variable run as its own model). Clustering by school was included as a random intercept.
bAdjusted for age and sex.
cAdjusted for age, sex, maternal education, screen time, depression, and alcohol consumption.
dSample sizes are slightly smaller due to missing covariates.
Regression-based weekend bedtime results
In contrast to weekday bedtimes, there were no statistically significant differences in incidence of elevated/high BP across the different bedtime categories during the weekend based on survival models.
Regression-based weekday–weekend bedtime difference results
Neither unadjusted nor adjusted analyses showed statistically significant differences in incidence of elevated/high BP among adolescents with consistent bedtimes on weekdays and weekends vs. those who had at least 2-hour difference in their weekday and weekend bedtime.
DISCUSSION
In this longitudinal investigation of BP among a large cohort of Mexican adolescents, there was evidence of U-shaped associations between weekday bedtimes and development of elevated/high BP. Adolescents with later or earlier bedtimes had nearly a 2-fold higher risk of developing elevated/high BP over the 14-month follow-up period, compared with adolescents who reported bedtimes between 9 and 10 pm on weekdays.
Although previous findings on sleep duration and BP in pediatric populations are fairly heterogeneous,7 recent meta-analyses have shown associations between short sleep duration and higher BP.8,16–18 Whether an association exists between long sleep duration and hypertension has remained controversial. A study of Portuguese adolescents19 associated long sleep duration to higher SBP among girls. Similarly, a nationally representative Korean study reported a 1.79 times higher odds of hypertension among those who slept >10 hours,20 although this association was not statistically significant. Other pediatric studies have found no association between long sleep duration and hypertension.21,22 One of the main limitations of prior reports on short sleep or long sleep duration is their cross-sectional design. The two existing studies with a longitudinal design are the Portuguese report23 and a US study in children aged 6 years, which found that a decrease in sleep duration over a 5-year period was related to higher SBP.24
Reports on sex-specific associations between sleep and hypertension have also been mixed. Whereas findings have been observed primarily among adolescent girls in certain populations (e.g., Portugal19), a recent meta-analysis concluded that the relationship with short sleep duration was stronger among adolescent boys.8 We noted a stronger relationship among females, although we did not find a statistically significant interaction between sex and bedtimes.
The causal nature of the U-shaped associations between sleep duration and cardiometabolic health is a source of debate.25,26 Specifically, whether longer sleep durations (or earlier bedtimes) are causally related to higher BP or are markers of underlying morbidity or lifestyle choices is unknown. For example, earlier bedtimes could be related to an underlying medical condition or sleep disorders (e.g., obstructive sleep apnea) that result in daytime sleepiness.27 These conditions could, in turn, increase susceptibility to hypertension. Earlier bedtimes in this population may also be an indicator of lower socioeconomic status, since households with lower SES during the time of the study had reduced access to internet, cell phones, and television28; predictors of late bedtimes in adolescents.29 Indeed, lower maternal and paternal education in this sample were associated with earlier bedtimes. Although we accounted for maternal education as an SES marker, residual confounding may still exist. One potential causal pathway between earlier bedtime and hypertension could be attributed to higher sedentary time that in turn increases BP. Regardless of the causal nature of the associations, our results suggest that early bedtime (relative to age-specific norms) could serve as a clinical indicator of possible future hypertension risk in adolescents.
Currently lacking from the adolescent literature is an examination on whether sleep timing may be an independent risk factor for hypertension aside from sleep duration. We found that associations between bedtime and risk of hypertension were unaltered upon addition of wake time. If sleep duration were an important mediator between bedtime and incident elevated/high BP, we would expect the risk to be attenuated upon addition of wake time to the model. In contrast, our findings suggest that sleep duration may not be an important mediator in the association between bedtime and BP and that instead there is a specific role of sleep timing. Indeed, studies among adults have shown that circadian misalignment, for example, a mismatch between sleep and wake patterns and internal circadian rhythms, is associated with higher BP independently of sleep duration.30
This study found that one-third of the adolescents displayed “social jetlag,” that is, weekend bedtimes >2 hours different than their weekday bedtimes. As expected, most of these differences represent later bedtimes on weekends compared with weekdays, rather than earlier bedtimes on weekends compared with weekdays. Inconsistent rhythms further exacerbate circadian misalignment and have been related to higher BP in adults.31 Nonetheless, we did not find statistically significant associations between change in bedtime and incidence of elevated/high BP, although the association was in the expected direction.
The main strength of this investigation was the longitudinal study design, which established the temporal sequence between bedtime and incidence of hypertension risk. The large sample size and objective markers of hypertension represent additional strengths. With the vast array of lifestyle behaviors and sociodemographic characteristics, we were able to account for several important confounders. Finally, this community sample of Mexican adolescents had a high participation rate, reducing the threat of selection bias. Nonetheless, there are limitations to consider. First, loss to follow-up at the second time point and missing information on some covariates may introduce selection bias. To evaluate the potential bias due to missing covariates, we compared the bivariate analysis results (i.e., Figure 2) from the full sample to those from the complete-case sample, i.e., without missing covariates. We found that the percentage of adolescents with elevated BP according to bedtimes was highly consistent across both samples. This finding provided evidence that selection bias due to missing covariates likely did not substantially impact the reported associations. A second limitation is that the self-reported bedtimes may carry measurement error and recall bias, and because these are categorical variables, the direction of the bias is uncertain.32 Third, although we considered bedtimes as a potential proxy for circadian misalignment (mismatch between behavior and endogenous circadian rhythms), we did not have information on endogenous circadian rhythms (e.g., dim light melatonin onset). The fact that we did not have three BP readings during each visit meant that the BP classifications in this study were not based on standard clinical protocols. This could have resulted in over-classification of children with elevated/high BP. Nonetheless, using a more stringent method of classifying hypertension (each of the two BP measurements had to be elevated rather than the average of the two measurements being elevated) did not alter the findings. Finally, this study included Mexican adolescents, and thus may have limited generalizability to the US and other populations.
In conclusion, this longitudinal study of Mexican adolescents identified U-shaped associations between weekday bedtime and 14-month incidence of hypertension. Determining and promoting age-appropriate bedtimes may be a salient public health strategy for preventing development of hypertension among adolescents.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The results from this study were obtained due to the financial support granted by Mexico’s National Council on Science and Technology (CONACYT, SALUD-2003-C01-059). Dr Jansen reports funding from National Heart Lung and Blood Institute (T32HL110952) over the course of the study.
REFERENCES
- 1. Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: a systematic review and meta-analysis. Lancet Public Health 2017. doi: 10.1016/S2468-2667(17)30123-8. [DOI] [PubMed] [Google Scholar]
- 2. Chiolero A, Madeleine G, Gabriel A, Burnier M, Paccaud F, Bovet P. Prevalence of elevated blood pressure and association with overweight in children of a rapidly developing country. J Hum Hypertens 2007;21:120–127. [DOI] [PubMed] [Google Scholar]
- 3. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, De Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simase M, Thaker VV, Urbina EM, Simasek M, Okechukwu K. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140. [DOI] [PubMed] [Google Scholar]
- 4. Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Circulation 2011; 124:1673–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jansen EC, Dunietz GL, Chervin RD, Baylin A, Baek J, Banker M, Song PXK, Cantoral A, Tellez Rojo MM, Peterson KE. Adiposity in adolescents: the interplay of sleep duration and sleep variability. J Pediatr 2018; 203:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 2012; 35:1012–1018. [DOI] [PubMed] [Google Scholar]
- 7. Matricciani L, Paquet C, Galland B, Short M, Olds T. Children’s sleep and health: a meta-review. Sleep Med Rev 2019; 46:136–150. [DOI] [PubMed] [Google Scholar]
- 8. Jiang W, Hu C, Li F, Hua X, Zhang X. Association between sleep duration and high blood pressure in adolescents: a systematic review and meta-analysis. Ann Hum Biol 2018;45:457–462. [DOI] [PubMed] [Google Scholar]
- 9. Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens 2014; 27:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep–wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med 2007; 8:668–680. [DOI] [PubMed] [Google Scholar]
- 11. Shankar RR, Eckert GJ, Saha C, Tu W, Pratt JH. The change in blood pressure during pubertal growth. J Clin Endocrinol Metab 2005; 90:163–167. [DOI] [PubMed] [Google Scholar]
- 12. Sánchez-Zamorano LM, Burguete-García AI, Flores-Sánchez G, Salmerón-Castro J, Lazcano-Ponce EC, Diaz-Benitez CE. Conducta no saludable asociada con el desarrollo de presión arterial elevada en adolescentes. Cad Saude Publica 2017;33:1–9. [DOI] [PubMed] [Google Scholar]
- 13. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 2000;( 314):1–27. [PubMed] [Google Scholar]
- 14. Hernández B, Gortmaker SL, Laird NM, Colditz GA, Parra-Cabrera S, Peterson KE. Validity and reproducibility of a questionnaire on physical activity and non-activity for school children in Mexico City. Salud Publica Mex 2000; 42:315–323. [PubMed] [Google Scholar]
- 15. Zung WWK. A self-rating depression scale. Arch Gen Psychiatry 1965; 12:63–70. [DOI] [PubMed] [Google Scholar]
- 16. Quist JS, Sjödin A, Chaput JP, Hjorth MF. Sleep and cardiometabolic risk in children and adolescents. Sleep Med Rev 2016; 29:76–100. [DOI] [PubMed] [Google Scholar]
- 17. Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep Med 2016; 18:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaput J-P, Gray CE, Poitras VJ, Carson V, Gruber R, Olds T, Weiss SK, Connor Gorber S, Kho ME, Sampson M, Belanger K, Eryuzlu S, Callender L, Tremblay MS. Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Appl Physiol Nutr Metab 2016; 17. [DOI] [PubMed] [Google Scholar]
- 19. Paciencia I, Araujo J, Ramos E. Sleep duration and blood pressure: a longitudinal analysis from early to late adolescence. J Sleep Res 2016;25(6):702–708. [DOI] [PubMed] [Google Scholar]
- 20. Lee JA, Park HS. Relation between sleep duration, overweight, and metabolic syndrome in Korean adolescents. Nutr Metab Cardiovasc Dis 2014; 24:65–71. [DOI] [PubMed] [Google Scholar]
- 21. Azadbakht L, Kelishadi R, Khodarahmi M, Qorbani M, Heshmat R, Motlagh ME, Taslimi M, Ardalan G. The association of sleep duration and cardiometabolic risk factors in a national sample of children and adolescents: the CASPIAN III study. Nutrition 2013;29:1133–1141. [DOI] [PubMed] [Google Scholar]
- 22. Guo X, Zheng L, Li Y, Yu S, Liu S, Zhou X, Zhang X, Sun Z, Wang R, Sun Y. Association between sleep duration and hypertension among Chinese children and adolescents. Clin Cardiol 2011;34:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paciência I, Barros H, Araújo J, Ramos E. Association between sleep duration and blood pressure in adolescents. Hypertens Res 2013;36:747–752. [DOI] [PubMed] [Google Scholar]
- 24. Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr 2012; 161:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stamatakis KA, Punjabi NM. Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev 2007; 11:337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep 2006. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 27. Tsukada E, Kitamura S, Enomoto M, Moriwaki A, Kamio Y, Asada T, Arai T, Mishima K. Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness. PLoS One 2018; 13:e0204409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopez Romo H. AMAI Advances: Distribution of Socioeconomic Levels in Urban Mexico. Mexican Association of Marketing Research and Public Opinion Agencies: Mexico City, Mexico, 2006. [Google Scholar]
- 29. Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med 2010; 11:735–742. [DOI] [PubMed] [Google Scholar]
- 30. Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer FAJL. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms 2017; 32:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, Nyenhuis SM, Ramos AR, Shah NA, Sotres-Alvarez D, Patel SR, Zee PC. Sleep timing, stability, and BP in the Sueño Ancillary Study of the Hispanic Community Health Study/Study of Latinos. Chest 2019; 155:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rothman KJ, Greenland S, Lash TL.. Modern epidemiology, 3rd edn. Lippincott Williams & Wilkins: Philadelphia, PA, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.