 Oral lyophilized powder of Gynura divaricata, an edible medicinal plant, can alleviate both hyperglycaemia and hepatic insulin resistance in type 2 diabetic mice.
Oral lyophilized powder of Gynura divaricata, an edible medicinal plant, can alleviate both hyperglycaemia and hepatic insulin resistance in type 2 diabetic mice.
Abstract
Diabetes mellitus, one of the fastest growing epidemics worldwide, has become a serious health problem in modern society. Gynura divaricata (GD), an edible medicinal plant, has been shown to have hypoglycaemic effects. The molecular mechanisms by which GD improves hepatic insulin resistance (IR) in mice with type 2 diabetes (T2D) remain largely unknown. The aerial parts of GD were prepared in a lyophilized powder, which was added into the diet of T2D mice for 4 weeks. GD could result in an obvious decrease in fasting blood glucose and insulin levels in T2D mice. Meanwhile, the underlying mechanisms involved in the insulin-signalling pathway, glucose metabolism, lipid metabolism and inflammatory reaction in the liver tissue were also investigated by western blot, which indicated that GD further ameliorated hepatic IR by activating the PI3K/p-AKT pathway, decreasing the levels of hepatic phosphoenolpyruvate carboxykinase and glucose-6-phosphatase and increasing the levels of glucokinase and peroxisome proliferator-activated receptor-γ in the livers of T2D mice. GD has the potential to alleviate both hyperglycaemia and hepatic IR in T2D mice. Therefore, GD might be a promising functional food or medicine for T2D treatment.
1. Introduction
Diabetes mellitus is a frequently occurring, chronic metabolic disease caused by inherited and/or acquired deficiency in insulin production by the pancreas, or by ineffectiveness of the insulin that the pancreas produces.1 Such a deficiency causes an increased blood glucose level, which in turn damages many bodily systems. At present, diabetes mellitus is a common metabolic syndrome affecting more than 170 million people around the world,2 among whom more than 90% are type 2 diabetes mellitus (DM) patients.3 Type 2 DM, also known as non-insulin dependent diabetes mellitus, is one of the fastest growing epidemics worldwide and it is characterised by IR and impaired insulin secretion due to β-cell dysfunction.4,5 IR, which occurs mainly in peripheral tissue and the liver, is the main cause of hyperglycaemia in DM.6 Although several synthetic drugs are available for the treatment of diabetes, adverse effects and drug resistance are of great concern. Therefore, there is an urgent need to continue working to prevent and control this pathology. As a promising alternative, researchers are seeking natural products such as traditional Chinese medicinal herbs to prevent or treat diabetes because of their high pharmacological activity, low toxicity and rare side effects.
Gynura divaricata (L.) DC. (GD), a traditional Chinese medicinal herb, is called “Bai Bei San Qi” in China.7,8 It is one of the most famous traditional Chinese medicinal herbs and is usually used for the treatment of bronchitis, pulmonary tuberculosis, kink cough rheumatism, pertussis, sore eye, toothache, rheumatic arthralgia and diabetes.9,10 It has been planted widely in the south of China.11 In addition, GD is an edible plant, which was approved by the Minister of Health of the People's Republic of China in 2010.12 GD's roots, stems and leaves can all be used as a medicine, so it has good health protection efficacy and can be used widely in both the medicine and food industries. This edible plant contains many natural components, including polysaccharides, flavonoids, phenolic compounds, terpenoids, alkaloids, fatty acids and cerebrosides.8,12–17 It has a long history as a treatment for diabetes in folk medicine. The ethanol extract of GD's aerial parts was reported to show hypoglycaemic activity in vivo, with the flavonoid compounds as the active constituents.18,19 Investigations have also demonstrated that both the extract and polysaccharide from GD cause anti-hyperglycaemic activity in T2D mice and rats.20,21 Recently in many areas of China, the fresh leaf of GD has been commonly used as a tea that could significantly decrease the blood glucose of diabetic patients.12 It has also been reported that many constituents with antiproliferation properties exist in this plant.8,14 Moreover, our latest studies also demonstrated the good hypoglycaemic activity of the lyophilised powder of GD,22 which successfully showed that GD has the potential to be an effective medicine for the treatment of diabetes. However, the pharmacological mechanism of GD has not been thoroughly studied. Therefore, in the present study, we evaluated the GD-mediated therapeutic effects on streptozotocin (STZ)-induced T2D mice on a high-fat diet (HFD). Furthermore, the underlying mechanisms involved in the insulin-signalling pathway, glucose metabolism, lipid metabolism of peroxisome proliferator-activated receptor-γ (PPAR-γ) expression and inflammatory reactions in the liver tissue were also investigated.
2. Materials and methods
2.1. Materials and chemicals
GD was obtained from Silk Biotechnology Lab, Soochow University (Suzhou, P. R. China). Fresh aerial parts of GD were collected, washed and lyophilised into powders for further research. Other required materials are outlined in the following sections.
2.2. Detection of the main component of the GD methanol extract
Two hundred fifty milligrams of GD powder was added to a 10 ml of 70% methanol aqueous solution, which was treated at 75 °C by ultrasonic assisted extraction for 1.5 h. Then, the supernatant was filtered through a 0.22 μm nylon membrane for the high performance liquid chromatography (HPLC) analysis. The HPLC-DAD system contained a coupled HPLC system (SHIMADZU Inc., LC-20A, Japan). A J&K CHEMICAL HPLC-C18 column (2.1 69 × 150 mm, 5 μm) was used for chromatographic separation, and solvents for the mobile phase were acetonitrile (A) and 0.4% glacial acetic acid (B). The gradient elution was 0–30 min, linear gradient 95–70% B; 30–40 min, linear gradient 70% B. The column temperature was maintained at 40 °C. The flow rate was 1.0 mL min–1 and the injection volume was 20 μL. Peaks were detected with the DAD at 323 nm. Mass spectrometry analysis was performed using a TSQ quantum ultra-triple-quadrupole mass spectrometer (Thermo Fisher Scientific Inc. Waltham, MA, USA), which is equipped with an electrospray ionisation (ESI) interface in negative mode. The following are the parameters of the mass spectrometer: sheath gas flow rate at 40 (arbitrary units); auxiliary gas flow rate at 10 (arbitrary units); spray voltage at 2500 V; vaporiser temperature at 350 °C; capillary temperature at 350 °C. Helium was used as the collision gas for collision-induced dissociation (CID).
2.3. Induction of T2D mice & experimental design
Healthy male ICR mice (3 weeks old) that were Specific Pathogen Free (SPF) were purchased from the Experimental Animal Centre, Soochow University (Suzhou, Jiangsu Province). Five mice per cage were housed under standard conditions (18–22 °C, humidity 50–80%, with a cycle of 12 h–12 h light/dark). After 3 days of acclimatisation, the mice were randomly divided into 5 groups: 1 non-diabetic group (ND, normal mice), 1 untreated diabetic group (model mice) and 3 GD-treated diabetic groups at doses of 1%, 5% and 10%. All mice (except the mice in the non-diabetic group) were fed with an HFD composed of 18% lard, 20% sugar, 3% egg yolk and 59% basal diet. The mice in the non-diabetic group received normal chow. After 4 weeks of HFD feeding, the mice were fasted for 12 h and injected once with low-dose STZ (Sigma, USA) at 100 mg kg–1. One week after the STZ injection, blood samples of mice were withdrawn from the tail vein and fasting blood glucose (FBG) was measured. T2D mice with FBG levels ≥11.1 mmol l–1 were considered for study. Subsequently, mice in the non-diabetic group and untreated diabetic group were treated with a normal diet. The three GD-treated diabetic groups received different doses of the GD diet daily (the diets containing 1%, 5% and 10% GD, respectively. 15 mice per group). The physical and mental states of the mice were observed and recorded every day. After 4 weeks of GD treatment, mice were fasted overnight and then sacrificed. The eyeballs of the mice were extracted, and blood was drawn. Their livers and pancreas were excised, weighed and stored at –80 °C for further use. Animal experiments are all abided by the rules of the international animal welfare committee requirements and regulations. All animal experimental protocols used in this study were approved by the Animal Ethics Committee at Soochow University (201504A028).
2.3.1. Body weight and the liver index
During 4 weeks of GD treatment, the average weight of each group of mice was measured once a week. The liver index was calculated using the formula: liver index = (liver weight/body weight) × 100.
2.3.2. FBG and serum insulin level detection
During 4 weeks of GD treatment, the FBG levels were measured using a ONETOUCH Blood Glucose Meter (LifeScan, Inc., Milpitas, CA, USA) once a week after fasting overnight. At the end of the study, the mice were fasted for 10 h and then sacrificed. A blood sample was collected from each mouse and serum was separated from the samples by centrifugation at 4 °C and stored at –80 °C. The mouse serum insulin level was measured using a mouse enzyme-linked immunosorbent assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All procedures were performed in accordance with the manufacturer's instructions.
2.3.3. HOMA-IR and ISI
Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated using the following formula:23,24 [FBG (mmol L–1) × fasting plasma insulin (mIU L–1)]/22.5. At present, HOMA-IR has been widely used to evaluate IR in patients with diabetes. The insulin sensitivity index (ISI) was calculated according to the following formula: ln[1/(FBG × fasting plasma insulin)].25
2.3.4. Serum enzyme detection
The serum enzyme levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were determined using commercial test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
2.3.5. GSP and blood lipid detection
Glycosylated serum protein (GSP) was measured using a commercial kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Plasma triacylglycerol (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels were determined for assessing the blood lipid level using the BS-800 Chemistry Analyser (Mindray Medical International Ltd, Shenzhen, China).
2.3.6. Liver histopathology
The liver samples were sectioned and stained using haematoxylin and eosin (H&E), and then evaluated for histophysiological changes under an optical microscope (U-III Multi-point Sensor System, Nikon, Tokyo, Japan).
2.3.7. Antioxidant capacity of the liver
Antioxidant enzymes including glutathione peroxidase (GSH-PX) and total superoxide dismutase (T-SOD) activities in the liver were tested using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The malondialdehyde (MDA) level in the liver was measured using a commercial kit (Nanjing Jiancheng Bio, China). The levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) in the liver were determined using an 8-OHdG enzyme-linked immunosorbent assay kit (Nanjing Jiancheng Bio, China).
2.3.8. Western blot
To detect the expression levels of insulin receptor, insulin receptor substrate (IRS), phosphatidylinositol 3-kinase (PI3K), phosphorylated protein kinase B (p-AKT, Ser473), phosphorylated glycogen synthase kinase 3β (p-GSK3β, Ser9), glycogen synthase kinase 3β (GSK3β), glycogen synthase (GS), glucose transport protein 4 (GLUT4), phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6pase), glucokinase (GK), PPARγ, tumour necrosis factor-α (TNF-α) and nuclear factor-kappa B (NF-κB), equal amounts of protein were separated with 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Shanghai, China). The membranes were first blocked with 5% skimmed milk for 2 h followed by overnight incubation with the corresponding primary antibody at 4 °C. After being washed, the membranes were incubated with an appropriate secondary antibody for 2 h at room temperature. The desired blots were visualised with enhanced chemiluminescence (ECL) detection. Protein expression levels were normalised using GAPDH as the internal standard.
2.4. Statistics
The data were expressed as the mean ± standard deviation (±SD). Differences between two sets of data were evaluated using one-way ANOVA with the Origin 7.5 software program. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Identification of the main bioactive components of the GD methanol extract
Four active components in GD were identified by HPLC-MS/MS and reported last year by our group.26 These compounds were identified as chlorogenic acid (3-caffeoylquinic acid), 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid, respectively. The amounts of chlorogenic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid in a 70% methanol extract of GD are 5.57 ± 0.35 mg g–1, 0.67 ± 0.08 mg g–1, 10.29 ± 0.74 mg g–1, and 6.84 ± 0.14 mg g–1, respectively. It is known that these four compounds are the products of one or two caffeic acid groups bonded to quinic acid at different positions. After oral administration, the animals will first digest them into quinic acid and caffeic acid in the body.
3.2. Body weight and the liver index
The body weights of the mice in different groups and changes during the experiment are presented in Table 1. The body weights of the normal mice increased during the 4 weeks. Untreated diabetic mice were always significantly lower than those of the non-diabetic mice (P < 0.001). The body weights of the mice in the untreated diabetic group varied slightly in both body weight and liver index, while the weights in the three GD-treated diabetic groups decreased slightly during the first week and then increased slowly. In fact, the trend of weight body changes is similar to that of the untreated diabetic mice. However, the highest dose group in three GD-treated groups weighs individually higher than the untreated diabetic group (P < 0.05). The liver indices in all diabetic groups were higher than those of the non-diabetic group. The liver index of the untreated diabetic mice was significantly higher than that of the non-diabetic group (P < 0.001). After oral GD administration, the liver index of the highest dose group was evidently lower than that of the untreated diabetic group (P < 0.05). In general, the three doses of GD-treated groups slightly reduced the liver index in a dose-dependent manner.
Table 1. Effect of GD on the body weight of mice.
| Groups | 0 week | 1 week | 2 weeks | 3 weeks | 4 weeks | Liver index |
| ND | 42.64 ± 3.17 | 44.20 ± 3.09 | 45.48 ± 3.64 | 47.05 ± 3.76 | 49.11 ± 3.78 | 4.32 ± 0.32 |
| UG | 37.20 ± 3.34*** | 36.84 ± 3.25*** | 38.50 ± 3.56*** | 38.04 ± 3.76*** | 40.01 ± 3.95 | 6.09 ± 0.58** |
| 1% GD | 38.51 ± 2.96 | 37.22 ± 2.41 | 37.87 ± 2.28 | 38.03 ± 2.48 | 38.66 ± 2.46 | 5.95 ± 0.43 |
| 5% GD | 38.33 ± 3.98 | 36.51 ± 3.06 | 38.01 ± 4.32 | 38.11 ± 4.60 | 38.58 ± 3.43 | 5.73 ± 0.55 |
| 10% GD | 40.33 ± 4.00# | 39.81 ± 4.05# | 41.67 ± 4.44# | 41.73 ± 3.65# | 41.99 ± 3.90 | 5.49 ± 0.89# |
3.3. FBG and the serum insulin level
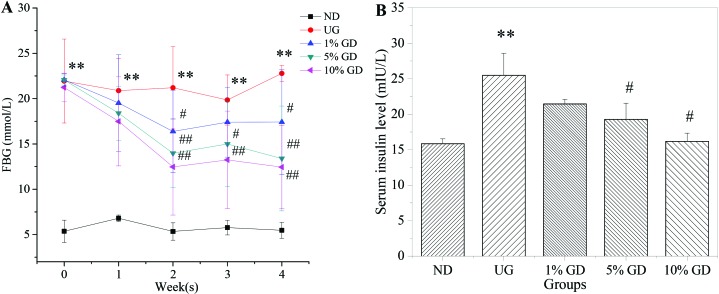
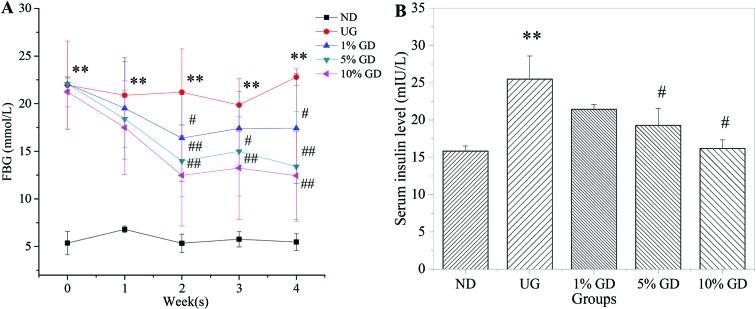
DM was characterised by IR and a higher insulin concentration in serum in the HFD- and STZ-induced diabetic mice.27 Furthermore, IR is often accompanied by compensatory hyperinsulinaemia and hyperglycaemia.28Fig. 1A shows that the FBG level was decreased significantly in GD-treated diabetic groups in a dose-dependent manner after 4 weeks of GD treatment. Fig. 1B shows that the serum insulin level in the untreated diabetic group abnormally increased, which subsequently triggered IR. GD could cause an obvious reduction in the serum insulin level compared with the untreated diabetic group. These results proved that GD could efficiently alleviate the FBG level and ameliorate the IR caused by DM.
Fig. 1. Effects of GD on the FBG and serum insulin levels. (A) FBG levels of mice in different groups and changes during the 4 weeks of GD treatment. (B) The serum insulin levels were measured on mice in the fasting state after 4 weeks of GD treatment. ND, non-diabetic group; UG, untreated diabetic group; 1% GD, 1% GD-treated diabetic group; 5% GD, 5% GD-treated diabetic group; 10% GD, 10% GD-treated diabetic group. Data are expressed as means ± SD. **P < 0.01, versus ND; #P < 0.05 and ##p < 0.01, respectively, versus UG.
3.4. HOMA-IR and the ISI
As shown in Table 2, we observed a significant increase in HOMA-IR (P < 0.001) and an obvious decrease in the ISI (P < 0.001) in the untreated diabetic group compared to the non-diabetic group. Notably, oral administration of GD led to a substantial reduction in the HOMA-IR index, which in turn increased the ISI progressively. These results suggested that hepatic IR was ameliorated in T2D mice after GD oral administration, indicating that GD can enhance the insulin sensitivity of DM.
Table 2. Effect of GD on the HOMA-IR and ISI of mice.
| Groups | HOMA-IR | ISI |
| ND | 2.99 ± 0.29 | –4.21 ± 0.10 |
| UG | 29.56 ± 3.47*** | –6.49 ± 0.13*** |
| 1% GD | 18.09 ± 3.85## | –5.99 ± 0.22# |
| 5% GD | 10.72 ± 2.55### | –5.47 ± 0.23## |
| 10% GD | 4.39 ± 0.62### | –4.59 ± 0.15### |
3.5. Effects of oral GD on the serum activities of ALT, AST and ALP in diabetic mice
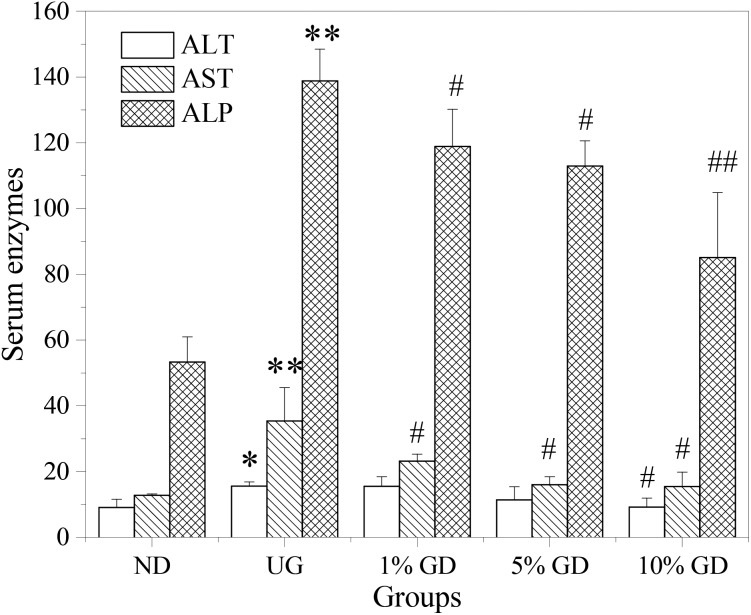
In order to explore liver function in DM, the activities of hepatic functional enzymes in the serum were determined (Fig. 2). A notable elevation of ALT, AST and ALP activities was found in the serum of the untreated diabetic group (p < 0.05, p < 0.01 and p < 0.01, respectively) compared to the non-diabetic group. Following GD administration, the levels of these enzymes were effectively reversed in a dose-dependent manner. Three treatment groups had a tendency to decrease regardless of the dose of GD. Among them, AST and ALP decreased obviously; ALP in the high-dose GD group reached a significant level (p < 0.01). From the results in the figure, we could know that GD could partially eliminate the abnormal enzyme activity caused by diabetes.
Fig. 2. Effects of GD on serum enzymes. ND, non-diabetic group (normal mice); UG, untreated diabetic group; 1% GD, 1% GD-treated diabetic group; 5% GD, 5% GD-treated diabetic group; 10% GD, 10% GD-treated diabetic group. Data are expressed as means ± SD. *p < 0.05 and **p < 0.01, respectively, versus ND; #p < 0.05 and ##p < 0.01, respectively, versus UG.
3.6. Effects of oral GD on GSP and blood lipids
GSP, which is related to the blood glucose concentration, can effectively reflect the average blood glucose level of diabetic patients in the past 1 to 2 weeks. Table 4 shows that the serum GSP levels were significantly higher in the untreated diabetic group than those in the non-diabetic group (P < 0.01). GD treatment significantly reversed the serum GSP levels in the 5% GD-treated diabetic group (P < 0.05) and the 10% GD-treated diabetic group (P < 0.01).
Table 4. Effect of GD on oxidative stress in the liver of mice.
| Groups | GSH-PX (U per mg prot) | T-SOD (U per mg prot) | MDA (nmol per mg prot) | 8-OHdG (ng per mg prot) |
| ND | 1109.85 ± 174.90 | 105.49 ± 9.54 | 0.74 ± 0.05 | 0.05 ± 0.02 |
| UG | 439.26 ± 106.62** | 88.26 ± 0.95* | 0.97 ± 0.11* | 0.17 ± 0.05* |
| 1% GD | 679.56 ± 104.54# | 95.33 ± 3.49# | 0.95 ± 0.04 | 0.12 ± 0.03 |
| 5% GD | 757.88 ± 21.44# | 102.68 ± 0.55## | 0.82 ± 0.03 | 0.08 ± 0.01# |
| 10% GD | 967.30 ± 192.04## | 105.31 ± 10.22# | 0.78 ± 0.10# | 0.07 ± 0.02# |
Patients with diabetes and prediabetes are often at an increased risk of cardiovascular disease and dyslipidaemia.29 It is generally known that patients with DM tend to be more dyslipidaemic than the general population, and blood lipid is a crucial and essential determinant of cardiovascular risk in type 2 diabetes patients.26 As shown in Table 3, TG, TC and LDL-C levels in the untreated diabetic group were much higher than those of the non-diabetic group, whereas the HDL-C level was significantly lower than that in the non-diabetic group (P < 0.01). Table 3 demonstrates that GD had the ability to decrease the TG, TC and LDL-C levels and enhance the HDL-C level in the serum of the GD-treated diabetic group, and the levels of blood lipids in the 10% GD-treated diabetic group were close to those of the non-diabetic group after 4 weeks of GD treatment. Although TG and TC levels tended to decrease in the GD-treated groups, the differences were not statistically significant when compared with the untreated diabetic mice. At present, increasing the HDL-C level remains a promising area of research on prevention of cardiovascular risks especially in high-risk individuals such as type 2 diabetic people.30 The results showed that GD could obviously increase the level of HDL-C, which indicated that GD was a promising lipid-decreasing medicine.
Table 3. Effect of GD on GSP and blood lipids.
| Groups | GSP (mmol L–1) | TG (mmol L–1) | TC (mmol L–1) | HDL-C (mmol L–1) | LDL-C (mmol L–1) |
| ND | 2.01 ± 0.49 | 1.78 ± 0.45 | 2.82 ± 0.42 | 3.05 ± 0.04 | 0.24 ± 0.05 |
| UG | 3.71 ± 0.22** | 2.04 ± 0.25 | 3.12 ± 0.13 | 2.13 ± 0.08** | 0.39 ± 0.02** |
| 1% GD | 3.05 ± 0.69 | 1.86 ± 0.25 | 2.99 ± 0.39 | 2.3 ± 0.26 | 0.31 ± 0.04# |
| 5% GD | 2.90 ± 0.31# | 1.68 ± 0.04 | 2.95 ± 0.54 | 2.40 ± 0.09## | 0.29 ± 0.07 |
| 10% GD | 2.58 ± 0.10## | 1.79 ± 0.16 | 2.85 ± 0.27 | 2.83 ± 0.91 | 0.28 ± 0.06# |
3.7. Histopathological evaluation of the liver
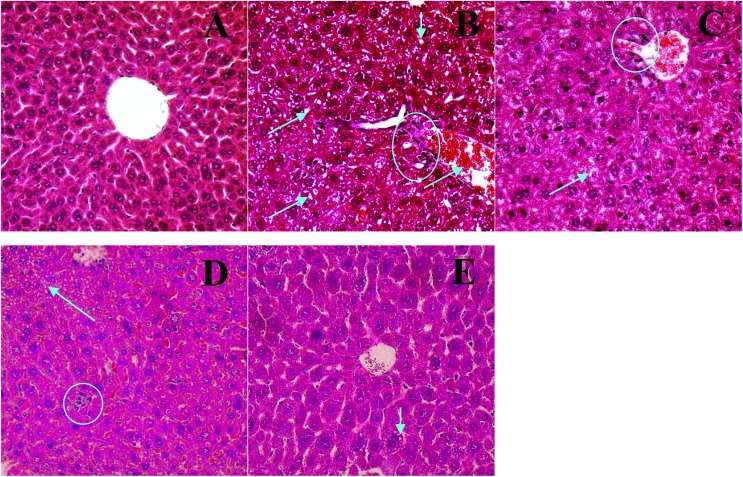
As shown by a histopathological examination of liver tissues (Fig. 3), there were no abnormal pathological changes in the liver tissues of normal mice (Fig. 3A). In contrast, the liver tissue was damaged obviously with potential inflammatory cell infiltration, and fat and lipoprotein deposition in the untreated diabetic mice (Fig. 4B). However, a histological analysis of liver tissue from the diabetic mice showed significant improvements in a dose-dependent manner after 4 weeks of GD treatment. Specifically, there were only a few small fat droplets in the 10% GD-treated group (Fig. 3E), and the appearance was also similar to that in the non-diabetic group.
Fig. 3. Histopathological examination of the liver in diabetic mice (HE stain, ×400). (A) Non-diabetic group (normal mice); (B) untreated diabetic group; (C) 1% GD-treated diabetic group; (D) 5% GD-treated diabetic group; (E) 10% GD-treated diabetic group. Arrows indicate fat accumulation and circles indicate inflammatory cell infiltration.
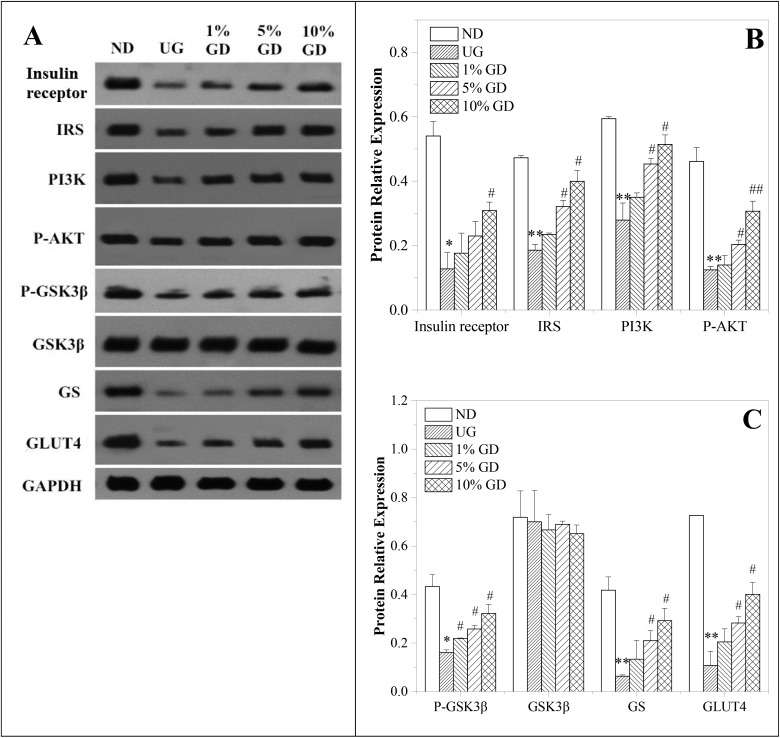
Fig. 4. Effects of the GD diet on the key proteins of the liver insulin signalling pathway of mice. (A) Western blot analysis of insulin receptor, IRS, PI3K, p-AKT, p-GSK3β, GSK3β, GS and GLUT4 protein expressions. (B) Quantitative analysis of insulin receptor, IRS, PI3K and p-AKT protein expressions. (C) Quantitative analysis of p-GSK3β, GSK3β, GS and GLUT4 protein expressions. ND, non-diabetic group; UG, untreated diabetic group; 1% GD, 1% GD-treated diabetic group; 5% GD, 5% GD-treated diabetic group; 10% GD, 10% GD-treated diabetic group. Data are expressed as means ± SD. *P < 0.05 and **P < 0.01, respectively, versus ND; #P < 0.05 and ##P < 0.01, respectively, versus UG.
3.8. Antioxidative capability of the liver
As shown in Table 4, liver GSH-PX and T-SOD activities in the untreated diabetic group were significantly lower than those in the non-diabetic group (P < 0.01 and P < 0.05, respectively). As expected, the GD diet led to obvious improvements in the activities of GSH-PX and T-SOD compared to the untreated diabetic mice. Notably, MDA and 8-OHdG levels in the untreated diabetic group were significantly increased compared to the normal mice (P < 0.05). The 10% GD diet induced a significant decrease in the MDA and 8-OHdG levels compared to those in the untreated diabetic mice (P < 0.05). In summary, GD significantly enhanced liver GSH-PX and T-SOD activities and reduced liver MDA and 8-OHdG levels in a dose-dependent manner when compared with the untreated diabetic group.
3.9. Oral GD improves insulin signalling in the liver
It is well known that an impaired insulin signalling pathway in the liver is characterised by an alteration of key proteins of the route during pre-diabetes.31 In this experiment, we studied in detail whether a GD diet modulates insulin signalling in the liver by western blot. Among the insulin signalling pathways associated with the improvement in IR, the PI3K/p-AKT pathway is a pivotal pathway of glucose uptake and glucose transport systems.26 From the results displayed in Fig. 4A and B, there were significant differences in the content of insulin receptor, IRS, PI3K and p-AKT between the non-diabetic group and the untreated diabetic group. After three doses of GD treatment, the levels of the above-mentioned proteins were notably increased compared with those of the untreated diabetic group.
GSK3 is a rate-limiting enzyme in glycogen synthesis. As shown in Fig. 4A and C, p-GSK3β, the most abundant isoform in the liver, decreased in the untreated diabetic group when compared to the non-diabetic group and the three diabetic groups receiving different dosages of GD treatment. In line with these results, mice in the untreated diabetic group presented decreased GS levels that were increased to achieve similar values to those of the non-diabetic group after 4 weeks of a GD diet (Fig. 4A and C). The liver content of total GSK3β was not modified among groups. In addition, a decrease in the GLUT4 content in the untreated diabetic group was found, whereas three dosages of GD treatment enlarged the GLUT4 content in a dose-dependent manner (Fig. 4A and C). Together, these findings suggest that the GD diet prevented the blockage of the insulin signalling cascade observed in TD2 mice by modulating the main proteins of the insulin pathway, contributing to the glucose homeostasis.
3.10. Glucose metabolism in the liver
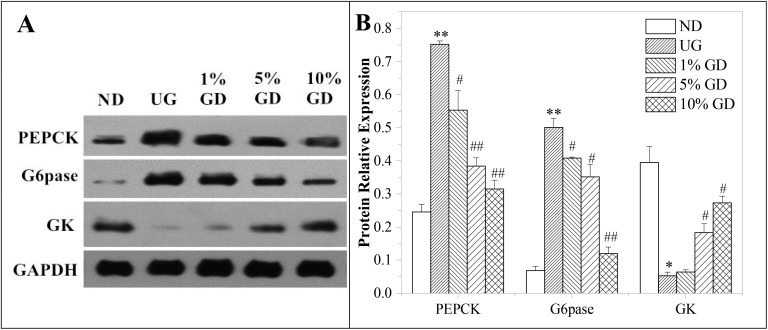
To further study the glucose homeostasis, the key proteins involved in hepatic glucose metabolism were evaluated by western blot. Hepatic PEPCK levels increased in the untreated diabetic group significantly when compared to the non-diabetic group (P < 0.01), and this effect was gradually repressed in mice receiving one of the three different dosages of the GD diet. Indeed, mice in the 10% GD-treated diabetic group showed comparable PEPCK levels to those of the non-diabetic group (Fig. 5A and B). The enzyme system G6pase is known to play a major role in the homeostatic regulation of blood glucose, which increased in the untreated diabetic group significantly when compared to the non-diabetic group (P < 0.01), and the G6pase levels decreased in a dose-dependent manner in the 3 groups of GD-treated diabetic mice. Additionally, the GK level decreased significantly in the untreated diabetic group compared with the non-diabetic group (P < 0.05), which reversed in a dose-dependent manner after 4 weeks of GD treatment. Overall, these results suggested that a GD diet might help to preserve the hepatic functionality and modulate the glucose homeostasis in diabetic mice.
Fig. 5. Effects of the GD diet on the key proteins of hepatic glucose metabolism of mice. (A) Western blot analysis of PEPCK, G6pase and GK protein expressions. (B) Quantitative analysis of PEPCK, G6pase and GK protein expressions. ND, non-diabetic group; UG, untreated diabetic group; 1% GD, 1% GD-treated diabetic group; 5% GD, 5% GD-treated diabetic group; 10% GD, 10% GD-treated diabetic group. Data are expressed as means ± SD. *P < 0.05 and **P < 0.01, respectively, versus ND; #P < 0.05 and ##P < 0.01, respectively, versus UG.
3.11. Effect of oral GD on lipid metabolism and inflammation in the liver
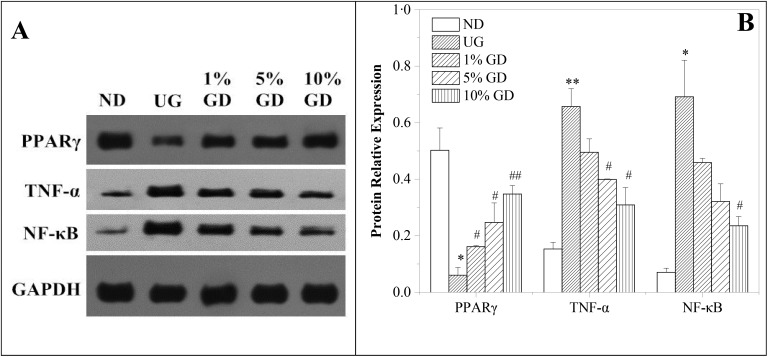
The nuclear receptor transcription factor PPARγ, which plays a crucial role in regulating lipid and glucose homeostasis,32 was analyzed by western blot. The data in Fig. 6A and B reveal that the expression of hepatic PPARγ in the untreated diabetic group was less than that of the non-diabetic group (P < 0.05). Additionally, compared with the untreated diabetic group, the expression of PPARγ in the GD-treatment groups was significantly upregulated in the liver tissue. We detected a significantly increased PPARγ expression in the 10% GD-treated diabetic group (P < 0.01).
Fig. 6. Effects of the GD diet on PPARγ, TNF-α & NF-κB protein expressions in the liver tissue of mice. (A) Western blot analysis of PPARγ, TNF-α and NF-κB protein expressions. (B) Quantitative analysis of PPARγ, TNF-α and NF-κB protein expressions. ND, non-diabetic group (normal group); UG, untreated diabetic group; 1% GD, 1% GD-treated diabetic group; 5% GD, 5% GD-treated diabetic group; 10% GD, 10% GD-treated diabetic group. Data are expressed as means ± SD. *P < 0.05 and **P < 0.01, respectively, versus ND; #P < 0.05 and ##P < 0.01, respectively, versus UG.
Our data in Fig. 6A and B also show that the expression of inflammatory factors (TNF-α and NF-κB) was significantly increased in the untreated diabetic group, while it was evidently blocked by GD administrations in the GD-treatment groups.
4. Discussion
GD is consumed as a medicinal and edible plant with rich nutrients and special healthcare functions. The roots, stems and leaves can also be used as a medicine for the treatment of diabetes, bronchitis, pulmonary tuberculosis, mastitis and other diseases. In the present study, an HPLC chromatogram of the GD methanol extract showed that GD is rich in chlorogenic acid and its derivatives (Fig. 1). Chlorogenic acid, a phenolic compound found ubiquitously in plants, is widely recognised to be an antioxidant,33 which is a scavenger of reactive oxygen species.34 Moreover, many studies have suggested that chlorogenic acid has hypoglycaemic effects.35–37 Importantly, chlorogenic acid was identified as a specific inhibitor of glucose-6-phosphatase in the liver, which may be useful for the reduction of inappropriately high rates of hepatic glucose output often found in non-insulin-dependent diabetes.38,39 In addition, as chlorogenic acid derivatives, 3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid isolated from GD had a considerable inhibitory effect on Protein Tyrosine Phosphatase 1B.13 3,4-Dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid isolated from this plant exhibited significant inhibitory activities against yeast α-glucosidase.13 The three chlorogenic acid derivatives were also reported to be active principles related to the hypoglycemic effect.40 Therefore, our results suggested that chlorogenic acid and its derivatives, the major constituents of the GD methanol extract, are possibly responsible for its hypoglycaemic activity.
IR is a crucial factor in the development and progression of diabetes,30 a state in which the natural hormone insulin becomes less effective at lowering blood sugars, which causes plasma with high insulin and high sugar levels. Hyperinsulinaemia is a marker of IR.36 In the present study, we observed that GD treatment lowered serum insulin levels, suggesting that GD could improve IR and enhance insulin sensitivity in type 2 diabetics. This was further supported by HOMA-IR and the ISI, two indexes used to quantify IR and insulin sensitivity, respectively.
As mentioned earlier, diabetes mellitus is often accompanied by lipid abnormalities,41 which may account for a series of complications including high blood pressure, atherosclerosis and coronary heart disease.42 Thus, the ideal drugs for diabetes treatment should have a favourable effect on lipid metabolism. At present, several studies of fat accumulation and steatosis in the livers of animals fed with an HFD indicated that IR is associated with fat deposition in the liver.43 Specifically, excessive fat accumulation can cause reduced insulin sensitivity of liver cells.44 In the present study, we found some hepatic steatosis in the untreated diabetic mice. However, alleviation of this condition was dose-dependent for the 3 GD-treated diabetic groups, indicating that GD may improve hepatic lipid metabolism and prevent lipid accumulation to retard the progression of diabetes.
Oxidative stress is elevated in patients with type 2 diabetes, and this may be the common pathway for IR and metabolic syndrome in type 2 diabetes.45 The liver is the main target of insulin and the key organ for glucose metabolism, and is also the main organ involved in detoxifying processes. Therefore, the liver is frequently exposed to damage from oxidative stress, which is an easy pathway for inducing abnormal glucose metabolism.46 It is widely believed that T-SOD and GSH-PX are the important enzymes for scavenging reactive oxygen species (ROS) in an organism and protecting the liver tissue against oxidative stress injury. In addition, the contents of MDA and 8-OHdG in the liver reflect the degree of hepatic lipid peroxidation and hepatocyte injury, respectively. Our data found that GD could improve T-SOD and GSH-PX activities in the livers of diabetic mice and lower the MDA and 8-OHdG contents, noting that GD treatment had beneficial effects on diabetes by directly scavenging ROS.
Hepatic IR is characterised by impaired insulin signalling.47 Thus, the modulation of insulin receptor and its downstream substrate IRS, which is essential for activating downstream pathways, is damaged. It is well known that the PI3K/p-AKT pathway, which plays a critical role in insulin signal transduction,48 can be activated in response to IRS. Also, GSK3β is a critical substrate of the PI3K/p-AKT signalling pathway in the regulation of glycogen synthesis, and its activity can be inhibited by phosphorylation at Ser9. Thus, the untreated diabetic mice showed decreased hepatic insulin receptor, IRS, PI3K, P-AKT and p-GSK3β levels. Subsequently, these effects were abolished when mice were fed the GD diet. The hepatic glucose transporter GLUT4 is the final effector molecule in the hepatic insulin signalling pathway. GLUT4 expression was significantly reduced in the diabetic mice, and GD effectively upregulated the hepatic GLUT4 expression. Our results implied that GD activated insulin-mediated PI3K/p-Akt-dependent glucose transport through the activation of GLUT4.
Hepatic insulin signalling plays roles in the activation of glycogen synthesis for energy storage and suppression of hepatic glucose output by inhibiting PEPCK and G6pase. In addition, GK plays a major role in controlling glucose utilisation in the liver. In the current study, the untreated diabetic mice showed increased levels of PEPCK and G6pase compared to the normal mice. GD administration diminished the expression levels and activities of the gluconeogenic enzymes PEPCK and G6pase, and upregulated GK in the liver of DM. Therefore, all of these results suggest that GD might improve hyperglycaemia by reducing PEPCK and G6pase, and enhancing GK in diabetic mice.
Recently, many modulators of PPARγ contained in foods and nutritional sources have been identified, and they may play roles in the prevention and treatment of metabolic disorders such as hyperglycaemia and hyperlipidaemia.49 PPARγ is a key transcription factor involved in lipid metabolism. It is also the target for drugs used to treat type 2 diabetes which is downregulated during tissue IR.50 Our study showed that GD treatment ameliorated the serum lipid content in DM. This activity might be related to the upregulation of PPAR-γ expression and the subsequent acceleration of lipogenesis and lipoprotein transport in the liver, thereby reducing lipotoxicity.51,52 In the present study, the expression of PPARγ in the GD-treatment groups was significantly upregulated in the liver tissue. This result is also in agreement with some previous reports.51 Moreover, recent studies have indicated that the increased expression of PPARγ also has significant therapeutic effects on lipid metabolic disorders.50,53 Therefore, we proposed that GD may play roles in the treatment of metabolic disorders such as hyperlipidemia and hyperglycemia by modulating PPARγ in DM. Chronic inflammation is widely found in metabolic syndrome, including diabetes. The expression of various inflammatory factors was increased in the livers of untreated diabetic mice, such as TNF-α and NF-κB. These alterations suggest that inflammatory factors participate in the pathogenesis of diabetes. Hepatic inflammation was also evidenced by the increase in the transaminase (ALT and AST) activities in the serum. The increased activities of hepatic transaminases of the untreated diabetic mice can be interpreted due to the liver cell destruction. Apparently, the upregulation of TNF-α and NF-κB was effectively reversed in the liver tissue by the GD intervention. Its mechanism may be related to the inhibition of PPARγ on NF-κB and TNF-α-mediated inflammatory reactions.
5. Conclusion
In summary, a GD diet improves liver function and alleviates IR in T2D mice. Impairment of PI3K/p-Akt signalling in the hepatic tissues may play a critical role in hepatic dysfunction. This study provided novel mechanistic insight into the preventive and therapeutic effects of GD on hepatic IR in type 2 diabetes. To our knowledge, this is the first systematic demonstration that a GD diet ameliorates the hepatic dysfunction that takes place in type 2 diabetes. Thus, our study provides a foundation for the further use of GD as a therapeutic agent against type 2 diabetes and insulin resistance.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the earmarked fund (CARS-18-ZJ0502) from the China Agriculture Research System (CARS) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, P. R. China.
References
- Ramar M., Manikandan B., Raman T. Eur. J. Pharmacol. 2012;690(3):226–235. doi: 10.1016/j.ejphar.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Harold B., Marjana T. C. J. Clin. Invest. 2007;117(5):1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Wu K., Mao X. J. Ethnopharmacol. 2010;127(1):32–37. doi: 10.1016/j.jep.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Gandhi G. R., Stalin A., Balakrishna K. Biochim. Biophys. Acta, Gen. Subj. 2013;1830(1):2243–2255. doi: 10.1016/j.bbagen.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Bharti S., Goyal S. Phytother. Res. 2011;25(10):1457–1465. doi: 10.1002/ptr.3442. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Hull R. L., Utzschneider K. M. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Chen L., Wang J. J., Zhang G. G. Nat. Prod. Res. 2009;23(14):1330–1336. doi: 10.1080/14786410902836677. [DOI] [PubMed] [Google Scholar]
- Chen L., Wang J. J., Song H. T. Chin. Chem. Lett. 2009;20(9):1091–1093. [Google Scholar]
- College Jiangsu New Medial, The Dictionary of Traditional Chinese Medicines, Shanghai Peoples’ Press, Shanghai, 1979, p. 1502. [Google Scholar]
- Jiesheng Y. Chem. Pap. 2009;63(5):506–511. [Google Scholar]
- Jie M., Wan C., Yu Y. Asian – J. Chem. 2011;23(9):3964–3968. [Google Scholar]
- Chen J., Mangelinckx S., Ma L. Fitoterapia. 2014;99:1–6. doi: 10.1016/j.fitote.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Chen S. C., Hong L. L., Chang C. Y. J. Chin. Pharm. Sci. 2003;55(2):109–119. [Google Scholar]
- Wang J. J., Chen L., Song H. T. China Pharm. 2009;12(2):146–149. [Google Scholar]
- Hu Y., Li W. L., Lin H. W. Chin. J. Nat. Med. 2006;4:156–158. [Google Scholar]
- Roeder E., Eckert A., Wiedenfeld H. Planta Med. 1996;62(4):386–386. doi: 10.1055/s-2006-957921. [DOI] [PubMed] [Google Scholar]
- Wan C., Yu Y., Zhou S. Pharmacogn. Mag. 2011;7(26):101–108. doi: 10.4103/0973-1296.80666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akowuah G. A., Sadikun A., Mariam A. Pharm. Biol. 2002;40(6):405–410. [Google Scholar]
- Zhang X. F., Tan B. K. H. Singapore Med. J. 2000;41(1):9–13. [PubMed] [Google Scholar]
- Li W. L., Ren B. R., Zhuo M. Am. J. Chin. Med. 2009;37(05):961–966. doi: 10.1142/S0192415X09007430. [DOI] [PubMed] [Google Scholar]
- Deng Y., Chen Y., Zhang W. Br. J. Nutr. 2011;106(9):1323–1329. doi: 10.1017/S0007114511001693. [DOI] [PubMed] [Google Scholar]
- Xu B. Q., Yang P., Zhang Y. Q. Food Nutr. Res. 2015;59:29652. doi: 10.3402/fnr.v59.29652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Hanley A. J. G., Williams K., Stern M. P. Diabetes Care. 2002;25(7):1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- Li R., Liang T., Xu L. Food Chem. Toxicol. 2013;51:419–425. doi: 10.1016/j.fct.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Yin X. L., Xu B. Q., Zhang Y. Q. Nutr. Metab. 2018;15(73):1–14. doi: 10.1186/s12986-018-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chen H., Wang J. Int. J. Biol. Macromol. 2015;81:967–974. doi: 10.1016/j.ijbiomac.2015.09.037. [DOI] [PubMed] [Google Scholar]
- Nakagomi A., Saiki Y., Kosugi M. Int. J. Cardiol. 2013;167(5):2222–2227. doi: 10.1016/j.ijcard.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Hassan A., Tajuddin N., Shaikh A. Cardiol. Ther. 2015;4(1):83–93. doi: 10.1007/s40119-014-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarossa G., Renzi A., D'Erasmo L. Diabetes Res. Clin. Pract. 2014;104(1):e26–e28. doi: 10.1016/j.diabres.2013.12.061. [DOI] [PubMed] [Google Scholar]
- Klover P. J., Mooney R. A. Int. J. Biochem. Cell Biol. 2004;36(5):753–758. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Evans J. L., Lin J. J., Goldfine I. D. Curr. Diabetes Rev. 2005;1:299–307. doi: 10.2174/157339905774574365. [DOI] [PubMed] [Google Scholar]
- Sotillo D. V. Rodriguez de, Hadley M. J. Nutr. Biochem. 2002;13:717–726. doi: 10.1016/s0955-2863(02)00231-0. [DOI] [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Tagawa S. Biochim. Biophys. Acta. 2002;1335:335–342. doi: 10.1016/s0304-4165(96)00151-1. [DOI] [PubMed] [Google Scholar]
- Park J. S., Yang J. S., Hwang B. Y., Yoo B. K., Han K. Biomol. Ther. 2009;17:256–262. [Google Scholar]
- Ong K. W., Hsu A., Tan B. K. H. Biochem. Pharmacol. 2013;85:1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Meng S., Cao J., Feng Q., Peng J., Hu Y. J. Evidence-Based Complementary Altern. Med. 2013:801457. doi: 10.1155/2013/801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerle H., Burger H. J., Below P., Schubert G., Rippel R., Schindler P. W., Paulus E., Herling A. W. J. Med. Chem. 1997;40:137–145. doi: 10.1021/jm9607360. [DOI] [PubMed] [Google Scholar]
- Arion W. J., Canfielda W. K., Ramosa F. C., Schindlerb P. W., Burgerb H. J., Hemmerleb H., Schubertb G., Belowb P., Herlingb A. W. Arch. Biochem. Biophys. 1997;339:315–322. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- Genta S. B., Wilfredo M. C., Mercado M. I., Grau A., Catalán C. A., Sánchez S. S. Chem.-Biol. Interact. 2010;185:143–152. doi: 10.1016/j.cbi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Fuliang H. U. Pharmacol. Res. 2005;51:147–152. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Gopumadhavan S., Muralidhar T. S., Anturlikar S. D., Sujatha M. B. Indian J. Exp. Biol. 1995;33:798–800. [PubMed] [Google Scholar]
- Huang T. H. W. Toxicol. Appl. Pharmacol. 2006;210:225–235. doi: 10.1016/j.taap.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Steneberg P., Rubins N., Bartoov-Shifman R., Walker M. D., Edlund H. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Meigs J. B., Larson M. G., Fox C. S. Diabetes Care. 2007;30(10):2529–2535. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- Ren C., Zhang Y., Cui W. Int. J. Biol. Macromol. 2015;72:951–959. doi: 10.1016/j.ijbiomac.2014.09.060. [DOI] [PubMed] [Google Scholar]
- Cordero-Herrera I., Martín M. Á., Escrivá F. J. Nutr. Biochem. 2015;26(7):704–712. doi: 10.1016/j.jnutbio.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Leibiger B., Moede T., Uhles S. FASEB J. 2010;24(6):1824–1837. doi: 10.1096/fj.09-148072. [DOI] [PubMed] [Google Scholar]
- Patel R. P., Barnes S. PPAR Res. 2010:153252. doi: 10.1155/2010/153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzky J. Science. 2003;302:406–407. doi: 10.1126/science.1091172. [DOI] [PubMed] [Google Scholar]
- Gu M., Zhang Y., Fan S., Ding X., Ji G., Huang C. PLoS One. 2013;8:e81724. doi: 10.1371/journal.pone.0081724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Gong W. Q., Lu L. Int. Immunopharmacol. 2017;42:176–184. doi: 10.1016/j.intimp.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Ortuño Sahagún D., Marquez-Aguirre A. L., Quintero-Fabian S. PPAR Res. 2012:318613. doi: 10.1155/2012/318613. [DOI] [PMC free article] [PubMed] [Google Scholar]