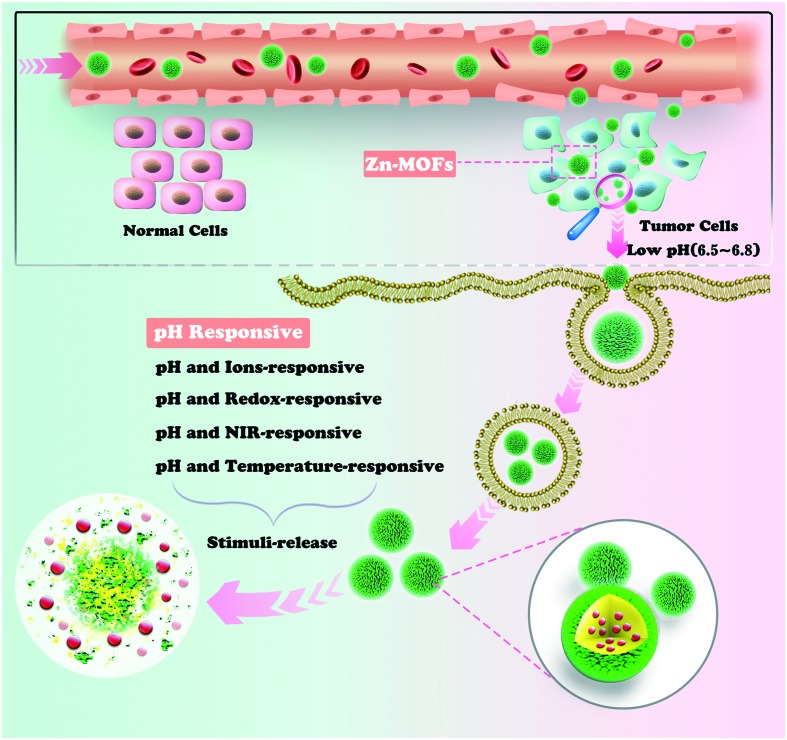
In this review article, we discuss the diverse stimuli achieved upon outside activation from single pH-stimulus-responsive or/and multiple pH-stimuli-responsive viewpoints in the body.
In this review article, we discuss the diverse stimuli achieved upon outside activation from single pH-stimulus-responsive or/and multiple pH-stimuli-responsive viewpoints in the body.
Abstract
The high storage capacities and excellent biocompatibilities of zinc(ii) metal–organic frameworks (Zn-MOFs) have made them outstanding candidates as drug delivery carriers. Recent studies on the pH-responsive processes based on carrier–drug interactions have proven them to be the most efficient and effective way to control the release profiles of drugs. To satisfy the ever-growing demand in cancer therapy, great efforts are being devoted to the development of methods to precisely control drug release and achieve targeted use of an active substance at the right time and place. In this review article, we discuss the diverse stimuli based on Zn-MOFs carriers that have been achieved upon external activation from single pH-stimulus-responsive or/and multiple pH-stimuli-responsive viewpoints. Also, the perspectives and future challenges in this type of carrier system are discussed.
1. Introduction
Nanoscale metal–organic frameworks (NMOFs), as a new class of porous organic–inorganic crystalline hybrid materials, are governed by the self-assembly of metal atoms and organic struts, and thus have attracted tremendous attention due to their special properties.1–14 In particular, NMOFs have been rapidly explored in drug delivery and cancer theranostic systems during the past few years, which indicates that NMOFs are one of the most promising candidates for biological application.15–17
The bio-applications of NMOFs, especially serving as ideal nanocarrier platforms for drug delivery systems (DDSs), have received extensive attention. NMOFs possess many unique properties over conventional nanocarriers (e.g., mesoporous silica, liposomes and polymers) as follows.15 (i) NMOFs with diverse compositions, morphologies, and highly porous structures exhibit desirable drug loading capacities and stimuli-responsive controllable drug release.18,19 (ii) NMOFs can be easily functionalized by in situ synthetic modification or post-synthetic modification, and numerous targeted functional bio-molecules and imaging agents can been capsulated in their frameworks, which enable them to overcome the drawbacks of the direct administration of drugs and inhibit the associated side effects resulting from the nonspecific drug distribution.20 (iii) The design ability of NMOFs allows them to be designed to form desired architectures by selecting biocompatible metals and organic ligands, which can decrease the toxicity of the NMOFs toward normal cells and increase their biocompatibility.21–24 In the last two decades, several types of NMOFs have been designed and synthesized, such as MIL, UIO and ZIF-based, and NMOFs are widely explored and applied in the field of biomedicine, which show fascinating controllable drug release. As is well-known, pH-responsive release is the key factor to triggering drug release from a nanocarrier.25,26 As an endogenous stimulus, the pH response is sensitive to small pH variations, and encapsulated drugs can release automatically when the carriers pass through the acidic tumor microenvironment (pH 5.5–6.8).27–29 Thus, it is desirable to select appropriate pH-responsive drug carriers to improve the therapeutic effect. In this regard, Zn-based NMOFs are extensively investigated for drug delivery and are expected to become ideal carriers for DDSs owing to their favorable biocompatibilities and their unique structures.30 In fact, acidic conditions result in the protonation of the coordinate bonds of Zn-based NMOFs, and then the encapsulated drugs can be released to the target tissues.30 However, the complexity of the human body environment limits the ability to precisely deliver drugs in the body using single stimulus-responsive Zn-NMOF drug carriers. To overcome the above drawback, multiple stimuli-responsive Zn-based carrier systems have been used as a better alternative to improve the delivering capacities and chemotherapeutic efficiencies. In this review, we focus on the subfield of pH-responsive carriers based on Zn-based MOFs in the last 20 years (Fig. 1). Zn-NMOF pH-responsive DDSs are summarized and their related releasing drug mechanisms will be discussed. Finally, the outlook of future research in this field will be presented.
Fig. 1. Schematic illustration of Zn-NMOF-based pH-responsive system for drug delivery.
2. Single pH-responsive Zn-MOFs drug@Zn(ii)-based MOFs (drug = 5-FU; DOX and other drugs)
2.1. 5-FU
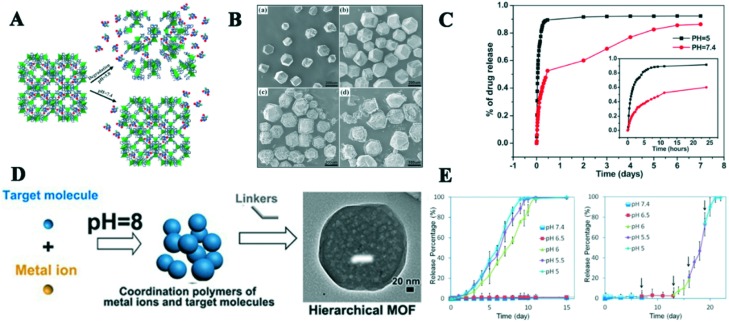
The zeolitic imidazolate framework-8 (ZIF-8), which is formed by Zn2+ and 2-methylimidazolate, is the most widely investigated MOF as a pH-responsive DDS due to its large pores (11.6 Å), high drug loading capacity and acid sensitivity. Sun and co-workers, for the first time, reported the drug delivery system of 5-FU@ZIF-8 (5-FU = 5-fluorouracil).31 This DDS showed a high drug capacity of 66 wt% and a pH-responsive controlled drug release process. In the drug release experiment, 5-FU was released faster in phosphate-buffer saline (PBS) pH 5.0 than at pH 7.4, and more than 45% of the drug was released within one hour at pH 5.0, but only 17% at pH 7.4 (Fig. 2A–C). This work initiated the use of pH-responsive NMOFs as DDS to deliver anticancer drugs and offer a valuable way to study pH-responsive NMOFs. However, from a biological perspective, the size and functionalization of ZIF-8 should be considered because its size and morphology will affect the intake rate of cancer cells and endow ZIF-8 with specific functions to improve therapeutic efficiency. Then, Chowdhuri et al. successfully constructed a multifunctional nanoscale core–shell structured nanocomposite, UCNPs@ZIF-8/FA (UCNP = upconversion nanoparticles and FA = folic acid), in one step.32 This DDS exhibited the functions of targeting, imaging and pH-responsive drug release, and its drug loading capacity was up to 685 mg g–1. In the in vitro drug release experiment, the 5-FU release rate of UCNPs@ZIF-8/FA reached up to 71% and 82% at pH 5.5 after 12 and 24 h, respectively; however, only 35% at pH 7.4 was observed because ZIF-8 easily decomposed under the acidic environment. Meanwhile, the result of folate receptor-mediated endocytosis showed that 5-FU-loaded UCNP@ZIF-8/FA was more fleetly taken up by HeLa cells via fluorescence microscopy compared to the free FA-mediated material. This study offered a way to synthesize multifunctional upconversion NMOFs with targeted imaging and delivery of anticancer drugs. In addition, polyaniline-decorated ZIF-8 nanoparticles (nPANI@nZIF-8) were reported.33 The utilization of nPANI with strong near infrared (NIR) adsorption was grafted into the surface of ZIF-8, providing the function of photothermal therapy (PTT) system under an NIR laser (λ = 980 nm). The drug amounts of approximately 58%, 68% and 80% were released from nPANI@nZIF-8 at pH 7.2, pH 5.2, and pH 5.2 under NIR laser irradiation, respectively. Meanwhile, the in vitro cell cytotoxicity experiments indicated that the cell viability of the MCF-7 cells decreased significantly by approximately 33% when nPANI@nZIF-8/5-FU was added under NIR laser condition compared to single ZIF-8/5-FU. Besides the classic ZIF-8 series, biocompatible ligands and Zn2+ ions have been employed to synthesize pH-responsive NMOFs. For example, Cai and his group synthesized a new nanoscale Zn-based MOF through the mixed ligand 3,5-benzenetricarboxylic acid and melamine,34 which exhibited a moderate 5-FU loading rate (34.32 wt%) and pH-responsive drug release behavior.
Fig. 2. A) Schematic illustration of encapsulated 5-Fu released from ZIF-8 at pH 7.4 and 5.0. B) Scanning electron microscopy (SEM) images of ZIF-8 immersed in different pH solutions at 37 °C for (a) 0 day (as-prepared), (b) 7 days in pH 7.4 buffer, (c) 5 min in pH 5.0 buffer and (d) 15 min in pH 5.0 buffer. C) 5-FU release curve from ZIF-8. D) Synthesis and loading target molecules of ZIF-8 via a one-pot method. E) pH-Responsive release curves of DOX from DOX@ZIF-8particles: (a) typical release system at different pH values and (b) stepped release system. Reprinted, copyright from RSC, 2012 and ACS, 2014.
2.2. DOX
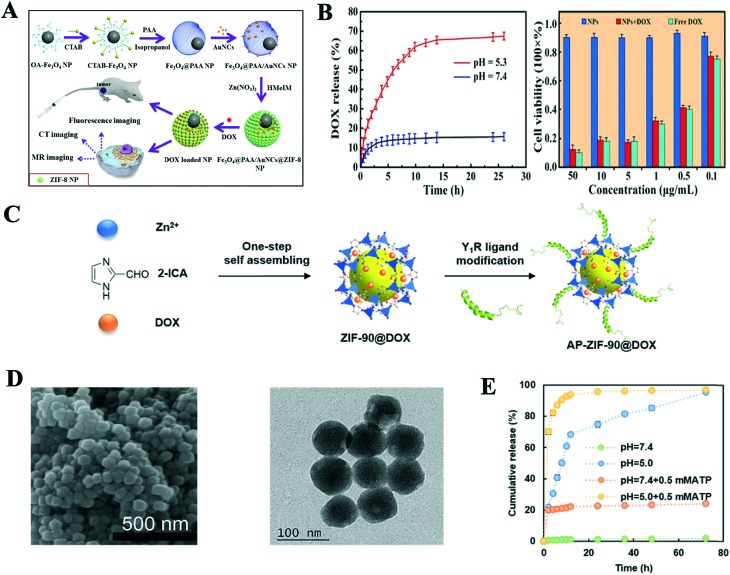
Doxorubicin (DOX), a common clinical anticancer drug, has been intensively investigated as model drug due to its unique chemical structure. Zheng and co-workers reported a novel approach to synthesize uniform nanoscale DOX@ZIF-8 via a one-pot process (Fig. 2D).22 The DOX loading capacity of ZIF-8 was up to 20%. It was amazing that DOX@ZIF-8 was stable without releasing DOX even at 60 °C and PBS solution (pH 7.4) after 7 days, while approximately 90% of DOX was released from the ZIF-8 carrier at pH 5.5 because its structure was decomposed in the acidic environment (Fig. 2E). The efficacy of DOX@ZIF-8 toward breast cancer cell lines was higher than free DOX according to the MTT assay. To realize a high DOX loading capacity and excellent pH-responsive drug release of ZIF-8, Ren et al. successfully synthesized PAA@ZIF-8 (PAA = polyacrylic acid) using a facile and simple approach.35 Electrostatic interactions between the negatively charged PAA and positively charged DOX and the weak coordination interaction between Zn2+ and DOX were observed, which induced an ultrahigh DOX loading capacity (1.9 g DOX g–1 NPs) in the PAA@ZIF-8 nanoparticles (NPs). The drug release behavior of the NPs showed that the DOX release amount of ZIF-8 was higher in acidic buffer solution (pH 5.5) than at neutral pH 7.4 in the cytoplasm via confocal laser scanning microscopy. The MTT assay results showed low toxicity in the live cells, which indicated the NPs are potential biocompatible DDS in biomedicine. Another example of a pH-responsive BSA/DOX@ZIF-8 (BAS = bovine serum albumin) was reported by Liang's group.36 The core@shell nanostructure was synthesized using BSA/DOX and ZIF-8 via one-pot encapsulation process. The activity of BSA was protected by the core–shell nanostructure, which improved the efficacy of DOX and will reduce the side effects of drugs. Also, the introduction of positive charges on the surfaces of BAS/DOX will improve its cellular uptake. Similarly, the drug release experiments showed only 8% drug was released at pH 7.4 after 24 h, while nearly all the drugs were released from the NPs at pH 5.5 in the same time. Therefore, the core–shell ZIF-8 material showed a pH-responsive feature to control the premature release of DOX under physiological conditions. The biocompatibility and cytotoxicity of BAS/DOX@ZIF-8 were obviously better than that of the pure DOX, BAS/DOX and ZIF-8, and these results can be assigned to their synergistic effect. This new core–shell DDS will provide an interesting platform with pH-responsive release and synergistic effect in antitumor therapy. To prevent premature release drug and improve therapeutic efficacy of drugs at the tumor site, Zheng et al. also synthesized a pH-responsive ZnO-DOX/ZIF-8 DDS with a core–shell structure using a one-pot strategy.37 The DDS displayed an excellent pH-responsive drug release property, where only 20% of DOX was released from DDS at pH 7.4, while over 84% was released in buffer of pH 5.5. The reasons for this were mainly that the ZIF-8 shell decomposed under the acidic environment and ZnO was slowly dissolved. ZnO-DOX/ZIF-8 had a better uptake by HeLa cells via endocytosis and exhibited higher toxicity than the free DOX and ZnO@ZIF-8 because the synergistic effect was generated by the DOX and reactive oxygen species (ROS). These results indicate the ZnO-DOX@ZIF-8 carrier is a promising pH-responsive drug delivery system in cancer therapy. Interestingly, Dong and co-workers reported new aptamer-modified DOX@ZIF-8 NPs, which were used for intracellular-environment endo/lysosomal escape and pH-responsive release.38 The aptamer (AS1411) grafted on the surface of ZIF-8 could enhance the uptake property of tumor cells and reduce the side effect of DOX. The modified-NPs also presented a good pH-responsive release drug behavior, where not more than 5% of DOX was released from the NPs after 24 h in PBS (pH 7.4), while 50% drug was discharged under acidic conditions (pH 5.0). The fluorescence images of the NPs incubated with HeLa cells were stronger than HEK 293T cells according to fluorescence microscopy, and flow cytometry showed that the increase in the fluorescence intensity of the HeLa cells was 6 times, indicating this system has excellent targeting effectiveness. Multifunctional core–shell NPs with targeted delivery, pH-responsive drug release, imaging and magnetic properties were designed and synthesized.27 The in vitro drug release study showed 22.5% and 26.72% of DOX were released from the NPs after 12 and 24 h at pH 7.4, while 47.92% and 55.1% of the drug were released in the same time at pH 5.5, respectively. This can be attributed to the fact that the pH-responsive chitosan material can control the drug release for a long period and the decomposition effect of IRMOF-3 under acidic medium. Based on the paramagnetic feature of Fe3O4, the NPs possessed magnetic resonance imaging (MRI) function and their normalized saturation magnetization value was 51 emu g–1, which facilitated a magnetic-guided drug delivery system. To overcome the multi-drug resistance and improve efficacy of DOX, Zhang et al. firstly designed and constructed a co-delivery, pH-responsive and targeted multifunctional drug carrier with DOX/verapamil hydrochloride (VER)@ZIF-8 methoxy poly(ethylene glycol)-folate (PEG-FA).39 The DOX loading content of the carrier was up to 40.9%, but its therapeutic efficiencies were higher than that of free DOX and other DOX@ZIF-8 in vivo and in vitro because VER serves as P-glycoprotein inhibitor to overcome the multi-drug resistance and targeted property of FA. Meanwhile, the pH-responsive release experiments showed obvious sustained release behaviors between DOX and VER. Combining imaging and chemotherapy, Bian and coworkers constructed a multifunctional theranostic drug delivery system.40 A new system of Fe3O4@PAA/gold nanoclusters (AuNCs)/ZIF-8 was synthesized via a facile and mild method, which can improve the magnetic resonance (MR), X-ray computed tomography (CT) and fluorescence tri-modal imaging and ultrahigh DOX loading capacity (Fig. 3A). The loading efficiency of DOX was up to 1.54 g g–1. The in vitro drug release experiments showed that more than 68% of loaded drug was released from the system after 26.5 h at pH 5.3 compared with only 15% in pH 7.4 at same time because of the protonation of the carboxyl groups from DOX and dissolution of ZIF-8, which indicates the dual pH-responsive behavior of this carrier (Fig. 3B). Both the in vitro and in vivo cytotoxicity studies reflected the Fe3O4@PAA/AuNCs/ZIF-8 NPs were biocompatible and had good antitumor efficacy. On account of the tri-modal imaging of Fe3O4@PAA and AuNCs, the multifunctional system has a great potential for diagnostics, chemotherapy and dual pH-responsive drug delivery.
Fig. 3. A) Schematic illustration of the preparation of Fe3O4@PAA/AuNCs/ZIF-8 for simultaneous tri-modal cancer imaging and chemotherapy. B) DOX release curve of Fe3O4@PAA/AuNCs/ZIF-8 at pH 5.3 and 7.4, and in vitro cytotoxicity results of Fe3O4@PAA/AuNCs/ZIF-8 NPs, DOX-loaded Fe3O4@PAA/AuNCs/ZIF-8 NPs and free DOX against HepG-2 cells at different concentrations. C) Schematic illustration of the preparation of AP-ZIF-90@DOX. D) SEM and TEM images of AP-ZIF-90@DOX. E) DOX release profiles of AP-ZIF-90@DOX at different pH values and ATP concentrations. Reprinted with permission. Copyright RSC, 2015 and Elsevier, 2019.
ZIF-90, as the one of classic ZIFs, is comprised of zinc ions and imidazole-2-carboxaldehyde (2-ICA), and shows good biocompatibility with cells and pH-responsive drug releasing behavior. Interestingly, Zhang et al. successfully modified the surface of ZIF-90 via Schiff base reaction between the aldehyde group of 2-ICA and amino group of DOX, and loaded 5-FU into ZIF-90 to construct a co-delivery nanoscale drug carrier.41 The drug loading results showed that the loading amounts of drugs were 36.35% and 13.5% for 5-FU and DOX, respectively. The drug releasing behavior of ZIF-90 also presented pH-stimulus responsivity, and 5-FU@ZIF-90-DOX exhibited the release of 44% 5-FU and 20% of DOX in PBS with pH 7.4 within 25 h, but 95% and 91% of the drug at pH 5.5 after 15 h and 25 h, respectively. Recently, Jiang and his group synthesized a targeted and pH-responsive nano-carrier utilizing the Y1 receptor ligand [Asn,6 Pro34]-NPY (AP) to conjugate on surface of ZIF-90 via a Mannich reaction and loading DOX into the pores of ZIF-90 (Fig. 3C and D). The carrier specifically recognized and treated triple negative breast cancer cells.42 The loading DOX experiment showed that the drug capacity was 12.6% via the one-pot encapsulation. It has been reported that the coordination bonding of adenosine triphosphate (ATP) and Zn2+ is much stronger than that of the imidazole and Zn2+, and AP-ZIF-90-DOX presented dual ATP and pH-responsive drug releasing behavior. The in vitro release experiments exhibited that only 1.7% DOX was released from AP-ZIF-90@DOX at pH 7.4, but about 19.8% with the addition of 0.5 mM ATP. Under the conditions of pH 5.0 with 0.5 mM ATP, greater than 21.7% DOX was released after 2 h, which resulted in the collapse of ZIF-90 under the acidic and ATP conditions (Fig. 3E). The in vitro and in vivo cytotoxicity indicated AP-ZIF-90@DOX had excellent biocompatibility with the mouse model over 30 days. To improve therapy efficacy and reduce side effects, Xie et al. successfully constructed an O2-loaded pH-responsive multifunctional UC@mSiO2-RB@ZIF-90-O2-DOX-PEGFA nanocarrier (UC = NaYF4:Yb/Er@NaYbF4:Nd@NaGdF4, RB = Rose Bengal).43 The core–shell structured UC not only had dual upconversion/MR imaging function, but also activated the photosensitizer (RB) in mSiO2 to produce ROS for PDT under laser irradiation. ZIF-90 as an O2 reservoir offers oxygen radicals to alleviate tumor hypoxia and improve PDT efficacy, and presents pH-responsive DOX releasing behavior in the tumor. Meanwhile, NH2-PEG-modified folic acid (PEGFA) was covalently conjugated on the surface of ZIF-90 to increase its targeted delivery and improve the therapy efficacy. The pH-responsive and DOX release experiments showed the released amount of DOX was in the range of 79.8% to 7.1% as the pH value increased from 5.5 to 7.3 after 17 h. Based on the synergetic effects of O2-loaded PDT and DOX, UC@mSiO2-RB@ZIF-O2-DOX-PEGFA exhibited an excellent anti-tumor therapeutic effect according to the in vitro and vivo experiments.
2.3. Other drugs
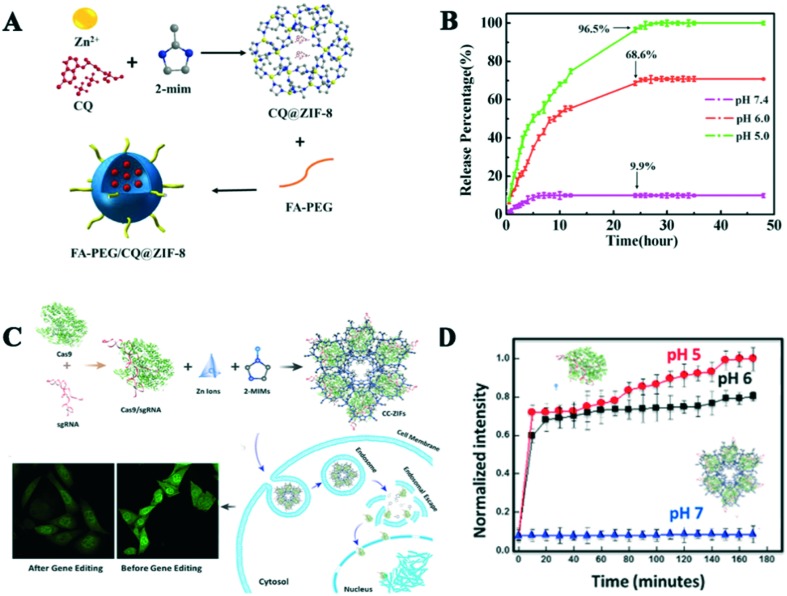
Besides loading 5-FU and DOX, pH-responsive Zn-based MOFs can also load other drugs, some proteins, enzymes, etc. For example, Zheng et al. utilized nano-ZIF-8 to encapsulate curcumin (CCM) using a simple and rapid route.44 CCM@ZIF-8 possessed 12.7 wt% loading capacity and pH-responsive releasing behavior in the tumor acidic environment.35 It showed a slow release phenomenon, where only 15.6% of CCM was released from ZIF-8 in pH 7.4 after 48 h, while it was 43.4% in pH 5.0 in the same time. Shi et al. reported chloroquine diphosphate (CQ) as an autophagy inhibitor, which was successfully encapsulated in ZIF-8 with an 18 wt% loading ratio, and then methoxy poly(ethylene glycol)-folate (FA-PEG) was decorated on the surface of CQ@ZIF-8 to enhance its targeting ability to HeLa cells and improve its therapeutic efficacy (Fig. 4A).45 The release assays showed the releasing amount of CQ decreased with an increase in the pH value. Specifically, 9.9% of the loaded CQ was released from the drug carrier at pH 7.4 after 24 h; however, more than 68.6% and 96.5% release at pH 6.0 and 5.0 were observed, respectively (Fig. 4B). The cellular uptake result exhibited that FA-PEG/CQ@ZIF-8 was more easily taken up by HeLa cells compared with CQ@ZIF-8 due to the overexpression folate receptors in HeLa cells. FA-PEG/CQ@ZIF-8 obviously inhibited the formation of autophagosomes and the process of autophagy flux in HeLa cells by releasing CQ. Zhang and coworkers prepared a multifunctional drug carrier via a versatile prodrug strategy for encapsulating cytarabine–indocyanine green (Ara-IR820) in ZIF-8, which could implement fluorescence imaging, PTT and chemotherapy treatments.46 Ara-IR820@ZIF-8 was functionalized with HA to improve its tumor-targeted ability, and the drug loading content was up to 39.8%. Based on the pH-sensitivity of ZIF-8 and the protection of the HA shell, the in vitro drug releasing feature showed that beyond 38% drug was released from the drug carrier at pH 5.0 in first 4 h and the accumulative release amount reached 99.1% after 36 h. Under laser irradiation, HA/Ara-IR820@ZIF-8 presented an excellent anti-tumor efficacy via combined PTT and chemotherapy compared with the free Ara, Ara-IR820@ZIF-8 and IR-820. Thus, thus strategy offers a new way to solve the drug loading drawback of MOFs and enlarge the biomedical applications of MOFs.
Fig. 4. A) Schematic process of the synthesis of FA-PEG/CQ@ZIF-8. B) CQ release profiles of FA-PEG/CQ@ZIF-8 at different pH. C) Schematic diagram of the synthesis of CC-ZIF-8 and its endosomal escape, and CLSM images of the cells before and after treatment with CC-ZIF-8. D) sgRNA and Cas9 release profiles of CC-ZIF-8 at different pH. Reprinted with permission from RSC, 2018 and ACS, 2018.
Some carriers have been designed to load drugs, proteins, antibiotics, enzymes, etc.47–54 Cytochrome c (Cyt c) was successfully encapsulated in ZIF-8 via a co-precipitation route.55 The Cyt c/ZIF-8 composite not only had a high protein loading rate, but maintained the activity of the protein, which showed 10 times peroxidase activity than free Cyt c. It was slowly released from ZIF-8 with the decomposition of ZIF-8 in acidic conditions. Similarly, Cheng et al. reported an example of biomimetic nanoparticles constructed using an extracellular vesicle (EV) membrane and therapeutic protein gelonin@ZIF-8.56 The NPs not only exhibited excellent biocompatibility, but also increased the therapeutic efficacy of gelonin by 14-fold compared to that of the free protein through selectively targeting the tumor site. The protein release behavior also showed pH-sensitivity. Thus, biomimetic NPs based on ZIF-8 offer a new idea and tool to deliver protein to intracellular. Inspired by the above ideas, Zhang et al. successfully utilized ZIF-8 to encapsulated ovalbumin (OVA) vaccine, which was further modified by cytosine–phosphate–guanine oligodeoxynucleotides (CpG ODNs). These NPs can enhance the systemic immune and memory response.57 Meanwhile, Alsaiari et al. reported the first example of engineered single-guide RNA (sgRNA)/protein (Cas9) encapsulated by ZIF-8 to promote fast endosomal escape and enhance nuclei delivery (Fig. 4C and D).58 Some other insulin/glucose oxidase (GOx) and VEGF aptamers were successfully loaded into ZIF-8 NPs, which act as autonomous, sense-and-treat vehicles for controlling diabetes and macular diseases.59
3. Multiple stimuli-responsive Zn-MOFs
To satisfy the ever-growing demand in controllable drug release, multiple stimuli-responsive strategies based on pH-responsive systems have been explored in detail, which will achieve more precise cancer therapies and improved drug therapeutic efficiencies. Herein, we list some mixed and multiple stimuli.
3.1. pH- and temperature-responsive
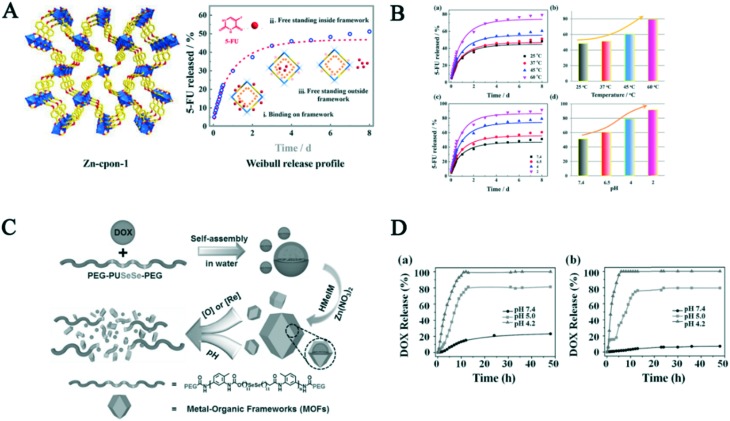
A chemically stable biocompatible MOF, formed by semirigid 5-(4′-carboxyphenoxy)nicotinic acid (H2cpon) and Zn2+, was synthesized using the template strategy.60 Amazingly, free post-modification resulted in pH and temperature responsive programmable drug release behavior (Fig. 5A). The amount of drug released increased with a decrease in pH, and the release kinetics of 5-FU was fitted with the Weibull distribution model. Meanwhile, the drug release amount was similar with the pH-responsive release trend. A small amount of drug was released at pH 7.4 at 37 °C in the initial stage, while approximately 68.3% of 5-FU was observed at 60 °C after 70 h (Fig. 5B). Additionally, a porous MOF (Zn-TBDA), formed by Zn2+ and 4′-(1H-tetrazol-5-yl)-[1,10-biphenyl]-3,5-dicarboxylic acid, was synthesized.61 About 12.59% drug MTX could be loaded into the Zn-TBDA NMOF via in situ encapsulation. The drug release behavior was studied at the various pH values and temperatures, which showed dual pH- and temperature-responsive release behavior. The amount of MTX released increased with a decline in pH or increase in temperature, and about 100% of drug could be released from Zn-TBDA at pH 6.5 and 42 °C after 48 h, while only 43% was detected at pH 7.4 and 37 °C, indicating this delivery system is a smart drug carrier.
Fig. 5. A) 3D framework of Zn-cpon-1 and schematic illustration of its release process. B) (a) 5-FU released from Zn-cpon-1 in PBS at different temperatures. (b) Comparison of the release rate in different temperatures. (c) 5-FU released from Zn-cpon-1 in PBS with a change in pH. (d) Comparison of the release rate in different pH conditions. C) Schematic illustration of the synthetic procedures and DOX release of P@ZIF-8. D) Release behavior of DOX in a) 1 × 10–3 M GSH and b) 1 × 10–3 M H2O2 with the pH-dependent changes. Reprinted copyright from ACS, 2018 and Wiley, 2017.19,60 .
3.2. pH- and redox-responsive
A dual pH- and redox-responsive drug delivery system consisting of selenium-containing polymer was designed and prepared.19 Presumably 19.1% DOX can be encapsulated by ZIF-8 through weak interactions (Fig. 5C). The dual responsive release drug behavior was demonstrated by in vitro experiments. Firstly, 21.1% of DOX was released from the carrier at the glutathione (GSH) concentration of 10–3 M and pH 7.4 after 24 h, which was caused by the blocking effect of the ZIF-8 shell. However, due to the acid sensitivity of ZIF-8, nearly 81.2% and 100% drug was released at pH 5.0 and 4.2 from the carrier, respectively. The release trend in H2O2 and different pH values was similar that with a change in GSH conditions. Similarly, the release drug trends at different redox agent concentrations were consistent with the pH-responsive release. The DOX release results without the redox agents showed only 5.1%, 1.6% and 14.5% of drug was observed at the pH 7.4, 5.0 and 4.2 after 48 h, respectively (Fig. 5D). These results indicate that this carrier is a promising candidate for dual-controllable drug release in biomaterials.
3.3. pH- and NIR-responsive
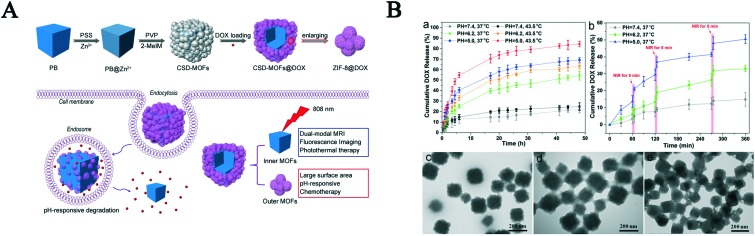
Li et al. constructed a core–shell nanostructure formed by AuNRs and ZIF-8, which was used to the near-infrared-induced synergistic chemo-photothermal therapy.62 Due to the strong NIR adsorption and pH-sensibility of the AuNRs NP, the nanostructure possessed dual-responsive DOX release property under acidic conditions and NIR irradiation. The drug release results showed the cumulative release amount approached 60% at pH 5.5 after 12 h, which was higher than that at pH 7.4 (17%). To demonstrate the synergistic effect of NIR irradiation and pH-sensibility on the controlled DOX release, a drug-loaded carrier experiment was performed. The cumulative release amount showed that approximately 95% of DOX was observed at pH 5.5 and under NIR laser. Thus, this core–shell nanostructure possessed an excellent therapeutic efficacy with cancer treatment via the synergistic effect of combined chemo-photothermal cancer therapy. A similar dual-responsive function nanocarrier was constructed using CoFe2O4, PDA and ZIF-8, which was used to deliver CPT and DOX for the multidrug chemotherapy and PTT synergistic treatment of cancer.63 Song and co-workers reported an example of a pH-responsive and light-triggered drug carrier containing 2-nitrobenzaldehyde (o-NBA, pH-jump reagent) and rifampicin (RFP, antibacterial agent).54 Under 365 nm irradiation, o-NBA as a gatekeeper was triggered to release acid in situ, which further induced the decomposition of ZIF-8 to release antibiotic RFP. The released zinc ions strengthened the efficiency of the drug and facilitated wound healing to produce a switchable and synergistic antibacterial effect. To integrate diagnosis and treatment, Wang et al. constructed core–shell dual NMOFs (CSD-MOFs) via a versatile stepwise approach (Fig. 6A).64 The CSD-MOFs possessed multi-functions with MRI and FOI, and pH- and NIR property. Due to its particular dual core–shell structure, CSD-MOF exhibited a high DOX loading content (85.73%). It released about 20%, 45% and 60% of drug at pH 7.4, 6.2 and 5.0 after 30 h at 37 °C, respectively. Once the temperature was increased to 43.5 °C, the drug cumulative release amount was not obviously changed. However, DOX was quickly released from the carrier under NIR laser irradiation (1.6 W cm–2, 5 min) at different points. More than 10%, 25% and 40% of drug was released at pH 7.4, 6.2 and 5.0 with laser irradiation within only 240 min, respectively (Fig. 6B). These results indicate these dual-responsive release properties are beneficial to improve drug efficacy and minimize size effects. In vitro and in vivo experiments demonstrated CSD-MOFs@DOX + NIR was 7.16 and 5.07 times more effective compared to a simple system or therapeutic treatment. Thus, the current results indicate that CSD-MOFs are a promising efficient multi-modal imaging guided synergistic chemo-thermal therapy nanoplatform. Hereafter, other carriers possessing NIR-responsive release feature have been designed and constructed.65 All the above studies offer a new idea for synergistic thermos-chemotherapy.
Fig. 6. A) Schematic illustration of the synthetic procedure and therapeutic effect of CSD-MOFs. B) (a) DOX release from CSD-MOFs@DOX at different pH and temperature values. (b) NIR-triggered release of DOX. (c–e) TEM images of DOX@CSD-MOFs after drug release at pH = 7.4, 6.2 and 5.0, respectively. Reprinted copyright from Ivyspring, 2017.64.
3.4. pH- and ion-responsive
The synthesis of pH-responsive nanoparticle MOFs for controlled drug-release by i-motif or triplex gating units has been.66a Kahn et al. constructed DNA-modified nanocarriers formed by Zn2+ and two different ligands with amino terephthalic acid and 4,4′,4′′-benzene-1,3,5-triyl-tribenzoic acid (BTB), which were used to load Rhodamine 6G (Rh6G).66b By modifying i-motif, triplex DNA and K+ stabilized G-quadruplex on the surface of Zn-MOF, the carrier showed pH- and K+-responsive release drug behavior. The loaded Rh6G was promoted to be released from the system when the pH and K+ ions were stimulated. The i-motif units were dissociated to unlock and release Rh6G at pH 7.4, while they locked the MOFs at pH 5.5. Meanwhile Rh6G was also released from Zn-MOFs because the K+-stabilized G-quadruplex and G-quadruplex locking units were dissociated in the presence of K+ ions. Thus, this model carrier offers a new idea to develop multi-responsive DNA-modified MOFs. The loading capacity and release rate of drugs on Zn-MOFs are summarized in Table 1.
Table 1. pH-Responsive drug delivery systems based on Zn-MOFs.
| Stimuli | Drug | MOFs | Rate (wt%) | Release rate | Ref. |
| Simple pH-responsive | 5-FU | ZIF-8 | 66 | 85% (pH 5.0) in 12 h | 31 |
| ZIF-8 + LP | 54 | 80% (acetate buffer 5.0) in 10 h | 67 | ||
| ZIF-8@FA-CHI-5-FAM | 51 | 100% (pH 5.0) in 21 h | 68 | ||
| UCNP@ZIF-8/FA | 68.5 | 82% (pH 5.5) in 24 h | 32 | ||
| nPANI@nZIF-8 | 68 | 68% (pH 5.2) in 6 h | 33 | ||
| Zn3[(BTC)2(Me)-(H2O)2](MeOH)13 | 34.32 | 70.1% (pH 5.0) in 20 h | 34 | ||
| APT-Mn-ZIF-90 | 67.93 | 80% (pH 5.5) in 5 h | 69 | ||
| DOX | ZIF-8 | 20 | 95% (pH 5.0) in 2 days | 22 | |
| PAA@ZIF-8 | 190 | 75.9% (pH 5.5) in 60 h | 35 | ||
| BSA/DOX@ZIF-8 | 10 | 80% (pH 5.0) in 14 h | 36 | ||
| ZnO-DOX/ZIF-8 | 20 | 70% (pH 5.5) in 5 h | 37 | ||
| HMS/ZIF-8 | 44 | 95% (pH 4) in 10 h | 70 | ||
| ZIF-8-PAAS | 385 | 60% (pH 5.0) in 3 h | 71 | ||
| DOX@ZIF-8@AS1411 | 10 | 80% (pH 5.0) in 20 h | 38 | ||
| DOX@ZIF-HA | 8.92 | 70% (pH 5.0) in 50 h | 72 | ||
| Fe3O4@OCMC@IRMOF-3/FA | 163 | 55.1% (pH 5.5) in 24 h | 27 | ||
| PEG-FA/(DOX + VER)@ZIF-8 | 40.9 | 41.61% (pH 5.0) in 132 h | 39 | ||
| Fe3O4@PAA/AuNCs/ZIF-8 | 154 | 68% (pH 5.3) in 26.5 h | 40 | ||
| ZIF-8@ZrO2@IL | 28.28 | 22.5% (pH 5.5) in 72 h | 73 | ||
| ZIF-90 | 13.5 | 20% (pH 7.4) in 4 h | 41 | ||
| AP-ZIF-90 | 12.6 | 70.2% (pH 5.0 + 0.5 mM ATP) in 12 h | 42 | ||
| UC@mSiO2-RB@ZIF-90-O2-PEGFA | 6 | 75% (pH 5.5) in 200 min | 43 | ||
| Fe3O4@C@ZIF-8 | 73 | 90% (pH 5.5) in 140 h | 74 | ||
| PLNPs@ZIF-8 | 90 | 49% (pH 4.0) in 7 days | 75 | ||
| Others | |||||
| CCM | ZIF-8 | 12.7 | 43.4% (pH 5.0) in 48 h | 44 | |
| CQ | FA-PEG/ZIF-8 | 18 | 96.5% (pH 5.0) in 24 h | 45 | |
| PEGCG | ZIF-8 | 19.9 | 90% (pH 6.0) in 10 h | 76 | |
| CRISPR/Cas9 | ZIF-8 | 1.2 | 100% (pH 5.0) in 3 h | 58 | |
| Gelonin | ZIF-8 | 41 | 90% (pH 6.0) in 12 h | 56 | |
| MA | ZIF-8 | 19.8 | 100% (pH 5.0) in 35 h | 77 | |
| OVA | ZNPs | 30.6 | 80% (pH 5.0) in 5 h | 57 | |
| ZnPc | ZIF-8 | 29.5 | 90% (pH 5.0) in 20 h | 78 | |
| Ceftazidime | ZIF-8 | 10.9 | 80% (pH 5.0) in 2 days | 49 | |
| 2I-BodipyPhNO2 | ZIF-90 | 28.8 | 39.2% (pH 5.0) in 24 h | 79 | |
| PseD | MAF-4 | 16.1 | 47.8% (pH 5.0) in 24 h | 80 | |
| Multiple stimuli-responsive | |||||
| pH and temperature | 5-FU | 5-Fu@Zn-copn-1 | 44.75 | — | 60 |
| MTX | Zn-TBDA/MTX | 12.59 | 100% (pH 6.5 + 42 °C) in 40 h | 61 | |
| pH and NIR | 5-FU | mCNFs | 31 | 80% (pH 5.0 + NIR) in 250 min | 81 |
| QT | FA-BSA/CuS@ZIF-8 | 24 | 60% (pH 5.0 + NIR) in 48 h | 82 | |
| ICG | ZIF-8 | 10.2 | — | 83 | |
| DOX | Pd@Au@ZIF-8 | 3.93 | 62% (pH 4.0 + NIR) in 300 min | 84 | |
| AuNR@ZIF-8 | 35.8 | 95% (pH 5.5 + NIR) in 12 h | 62 | ||
| LA-AuNR/ZIF-8 | 30 | 83% (pH 5.3 + NIR) in 25 h | 85 | ||
| CoFe2O4@PDA@ZIF-8 | 98 | 40% (pH 5.0 + NIR) in 12 h | 63 | ||
| PDA-PCM@ZIF-8 | 37.86 | 78% (pH 7.4 + NIR) in 48 h | 65 | ||
| CSD-MOF | 85.73 | 50% (pH 5.0 + NIR) in 300 min | 64 | ||
| H-ZIF-8/PDA-CD | 42.0 | 75% (pH 5.3 + NIR) in 8 h | 86 | ||
| pH and ions | Rh6G | DNA/MOF | — | — | 66 |
| pH and redox | DOX | P@ZIF-8 | 19.1 | 100% (pH 4.2 + H2O2) in 10 h | 19 |
| ZDOS | 41.2 | 80% (pH 5.0 + DTT) in 50 h | 87 | ||
4. Other biomedical applications
Due to their unique structural features, pH-responsive character and biocompatibility, Zn-MOFs have also great potential in other biomedical applications, such as imaging and sensing. MRI has been widely investigated in biomedical imaging owing to its great imaging property, and the post-synthesized NPs of ZIF-8, ZIF-90 and IRMOF-3 can be usually used as MRI contrast agents.88–92 For example, Lin et al. constructed an Fe3O4-based responsive T2–T1 switching MRI contrast agent by utilizing the pH-responsive ZIF-8 to encapsulate Fe3O4 NPs.93 The agent presented good MRI imaging properties according to the in vitro and in vivo experiments. Using 19F to mark the ZIF-8 ligand of 2-methylimidazolate, Guo et al. also fabricated an 19F-based MRI agent based on ZIF-8, which showed stimuli-responsive imaging with high penetration depth and low background.94 Interestingly, three 2D porous organic nanosheets containing tetraphenyl-ethylene units with aggregation-induced emission (AIE) were synthesized and encapsulated by ZIF-8 for in vitro live-cell imaging.95 Similarly, Zn-MOFs have been introduced as biosensors to for detecting gases, biomolecules and cancer markers due to their host–guest chemistry property.96–99 An et al. designed and synthesized a bio-MOF-1, which was further decorated with lanthanide cations, and the full system served as a photostable O2 sensor for biosample analysis.100 A biosensor constructed using ferrocene and ZIF-8 was used to detect amyloid-beta oligomer (AβO), which is the main neuropathological hallmark of Alzheimer's disease (AD).101 Meanwhile, a novel bimetallic ZnZr-MOF architecture as an aptasensor was also fabricated and used to detect the cancer marker protein tyrosine kinase-7 (PTK7).102 The results showed its high selectivity, good stability, reproducibility, and acceptability in human serum. On account of their facile synthesis, excellent pore structure and biocompatibility, Zn-MOFs are also promising carriers in domain of imaging and sensors, not only in drug delivery.
5. The relationship between the structures and pH-responsive strategies
According to the literature, the strategies of incorporating therapeutics into MOFs is mainly summarized as four ways, including noncovalent encapsulation, conjugation to ligands, and therapeutics as linkers, and coordination to SBUs. Firstly, for Zn-MOFs, especially the ZIF series of MOFs, noncovalent encapsulation was the most investigated loading drug method, and the loading forms are classified as post-synthesis and one-pot synthesis.103 Traditionally, Zn-MOFs are immersed in drug solutions under stirring condition to realize drug loading via physical adsorption. Also, their loading mechanism is as follows. (i) Small-sized cargoes are entrapped in the pores of Zn-MOFs via π–π interaction and hydrogen bond of host–guest molecules, such as loading the classical drugs 5-FU and DOX.22,31 (ii) Electrostatic interaction between the charged Zn-MOFs and guests can successfully load cargoes, for example, negative bio-MOF-1 adsorbed the cationic agent procainamide. However, the size of the cargoes is usually bigger than the pore size of Zn-MOFs or host–guest electrostatic interactions may also influence the category of loading cargo.104 To solve the size-dependent and charge limitation, the one-pot synthesis of cargo-loaded Zn-MOFs was proposed. Especially for ZIF-8 and ZIF-90, with their simple and facile synthetic methods, they can easily encapsulate all types of cargoes into their inner pores such as drugs, proteins, enzymes and dyes, which are almost unlimited size, charge or polarity, and efficiently deliver cargoes without premature release. This is an important reason why ZIF-8 and -90 as carriers are the most and widely investigated in biomedical applications. Secondly, the strategy of conjugation to ligands is commonly the loading cargo method. Cargoes are covalently bonded with the ligands of Zn-MOFs through ether bonds, amide bonds, imine bonds and click chemistry,105–111 taking advantage of preventing premature release, stimulating release, targeting delivery and increasing biostability under the tumor microenvironment (TME). For example, FA-COOH was modified on the surface of IRMOF-3-NH2via amide reaction to increase the targeting ability of IRMOF-3 NPs,112 DOX-NH2 was covalently bonded with ZIF-90-CHO via Schiff base reaction to synthesize a codelivery carrier, and carboxylatopillar[5]arene (CP5) was linked with UMCM-1-NH2 to improve its stability and construct a stimuli-responsive system.113 For the last two loading methods, a only few examples were applied in Zn-MOFs biomedical applications. It is mainly an investigational direction utilizing biocompatible ligands (adenine, amino acid, protein, and TCPP) to synthesize Bio-Zn-MOF-loaded cargoes for therapeutics.114–116 The above approaches not only improve the biocompatibility of MOFs, but also enhance their therapeutic effectiveness. However, it is still a challenge to more precisely deliver drugs to tumor tissues and to improve therapeutic effects from a structural veiwpoint.117–130
6. Conclusions
Nanoscale MOFs-based DDSs have been extensively investigated for controlled release, targeted delivery and gene delivery. These smart DDSs can release drugs in response to external stimuli, especially pH gradient stimuli. Currently, to satisfy the ever-growing demand in controllable drug release, different strategies have been developed to implement the stimuli concept, which will be a major advantage with respect to future clinical applications. However, the drawbacks of pH-responsive DDSs remain and should be addressed in the future. Firstly, the degradation, stability and toxicity of NMOFs should be considered as important issues in their design optimization. The second issue is that the installation of stimulus-responsive functional groups on the surface of NPs usually requires complicated synthetic steps, which may result the leakage of the cargo. Therefore, the performances of the nanosystem and major technical difficulties have led to the birth of key technologies, which are urgently needed due to current clinical integration demand. The pH-responsive DDSs based on NMOF-based carriers are beginning to be mastered, underlining the infancy of this research field, but also leaving plenty of room for new creative insights.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This research was partially funded by the Public Research and Capacity Building Projects of Department of Guangdong Province grant number (2017A010103022), Natural Science Foundation of Guangdong Province (2017A030313079), Featured Innovation Project of Guangdong Province (2017KTSCX083 and 2018KTSCX083) and Special Funds for Scientific Technological Innovation of Undergraduates in Guangdong Province (pdjh2019b0215, pdjh2019b0222, pdjh2019b0219, pdjh2019b0221), Shenzhen Peacock Plan (Research and Development and Industrialization of in vitro Diagnostic Nanomaterials for Tumors (KQTD2016022920325195)), Science Foundation of Guangdong Medical University (GDMUM201821, ZYDM040, ZYDM041, ZYDM016, ZYDM017), and “Group type” Special Supporting Project for Educational Talents in Universities (4SG19045G, 4SG19057G and G619080438), Funds for PHD researchers of Guangdong Medical University in 2019.
References
- Qiu S., Zhu G. Coord. Chem. Rev. 2009;253:2891–2911. [Google Scholar]
- Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M. Science. 2013;341:1230444–1230457. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- (a).Eddaoudi M., Sava D. F., Eubank J. F., Adil K., Guillerm V. Chem. Soc. Rev. 2015;44:228–249. doi: 10.1039/c4cs00230j. [DOI] [PubMed] [Google Scholar]
- (a).Yue Y., Fulvio P. F., Dai S. Acc. Chem. Res. 2015;48:3044–3052. doi: 10.1021/acs.accounts.5b00349. [DOI] [PubMed] [Google Scholar]
- (a).Cui Y., Li B., He H., Zhou W., Chen B., Qian G. Acc. Chem. Res. 2016;49:483–493. doi: 10.1021/acs.accounts.5b00530. [DOI] [PubMed] [Google Scholar]
- (a) Li J. R., Sculley J., Zhou H. C. Chem. Rev. 2012;112:869–932. doi: 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]; (b) Zhao Y., Deng D. S., Ma L. F., Ji B. M., Wang L. Y. Chem. Commun. 2013;49:10299–10301. doi: 10.1039/c3cc45310c. [DOI] [PubMed] [Google Scholar]
- (a).He Y., Zhou W., Qian G., Chen B. Chem. Soc. Rev. 2014;43:5657–5678. doi: 10.1039/c4cs00032c. [DOI] [PubMed] [Google Scholar]
- (a) Jiao L., Wang Y., Jiang H. L., Xu Q. Adv. Mater. 2018;30:1703663–1703682. doi: 10.1002/adma.201703663. [DOI] [PubMed] [Google Scholar]; (b) Wang H., Meng W., Wu J., Ding J., Hou H., Fan Y. Coord. Chem. Rev. 2016;307:130–146. [Google Scholar]; (c) Luo Z. D., Fan S. R., Gu C. Y., Liu W. C., Li B. H., Liu J. Q. Curr. Med. Chem. 2019;26:3341–3369. doi: 10.2174/0929867325666180214123500. [DOI] [PubMed] [Google Scholar]; (d) Wang Y. M., Zhou C. Q., Chen J. X., Lin Y. L., Zeng W., Kuang B. C., Fu W. L., Chen W. H. Med. Chem. Commun. 2013;4:1400–1404. [Google Scholar]
- (a) Horcajada P., Gref R., Baati T., Allan P. K., Maurin G., Couvreur P., Ferey G., Morris R. E., Serre C. Chem. Rev. 2012;112:1232–1268. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]; (b) Yang B. C., Shen M., Liu J. Q., Ren F. Pharm. Res. 2017;34:2440–2450. doi: 10.1007/s11095-017-2253-9. [DOI] [PubMed] [Google Scholar]
- (a) Hu Z., Deibert B. J., Li J. Chem. Soc. Rev. 2014;43:5815–5840. doi: 10.1039/c4cs00010b. [DOI] [PubMed] [Google Scholar]; (b) Li X. Q., Zhou Z., Zhang C. C., Zheng Y. H., Gao J. W., Wang Q. M. Sens. Actuators, B. 2018;276:95–100. [Google Scholar]; (c) Zhou Z., Han M. L., Fu H. R., Ma L. F., Luo F., Li D. S. Dalton Trans. 2018;47:5359–5365. doi: 10.1039/c8dt00594j. [DOI] [PubMed] [Google Scholar]
- (a) Ramaswamy P., Wong N. E., Shimizu G. K. Chem. Soc. Rev. 2014;43:5913–5932. doi: 10.1039/c4cs00093e. [DOI] [PubMed] [Google Scholar]; (b) Yang X. G., Ma L. F., Yan D. P. Chem. Sci. 2019;10:4567–4572. doi: 10.1039/c9sc00162j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Zhang T., Lin W. Chem. Soc. Rev. 2014;43:5982–5993. doi: 10.1039/c4cs00103f. [DOI] [PubMed] [Google Scholar]; (b) Zhou Z., Gu J. P., Qiao X. G., Wu H. X., Fu H. R., Li H. Y., Ma L. F. Sens. Actuators, B. 2019;282:437–442. [Google Scholar]
- Horcajada P., Gref R., Baati T., Allan P. K., Maurin G., Couvreur P., Ferey G., Morris R. E., Serre C. Chem. Rev. 2012;112:1232–1268. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- Wu M. X., Yang Y. W. Adv. Mater. 2017;29:1606134–1606154. [Google Scholar]
- (a) Horcajada P., Chalati T., Serre C., Gillet B., Sebrie C., Baati T., Eubank J. F., Heurtaux D., Clayette P., Kreuz C., Chang J. S., Hwang Y. K., Marsaud V., Bories P. N., Cynober L., Gil S., Ferey G., Couvreur P., Gref R. Nat. Mater. 2010;9:172–178. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]; (b) Han Y. Y., Liu W. C., Huang J. J., Qiu S. W., Zhong H. R., Liu D., Liu J. Q. Pharmaceutics. 2018;10:271–293. doi: 10.3390/pharmaceutics10040271. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Guan M., Chen Y. M., Wei Y., Song H., Cao C., Cheng H., Li Y., Huo K. F., Fu J. J. Int. J. Nanomed. 2019;14:2903–2914. doi: 10.2147/IJN.S202625. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li X. X., Xiao H., Lin C. W., Sun W. T., Wu T., Wang J., Chen B., Chen X., Cheng D. Int. J. Nanomed. 2019;14:649–665. doi: 10.2147/IJN.S189819. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xue Y. X., Hong X. F., Gao J., Shen R. Z., Ye Z. C. Int. J. Nanomed. 2019;14:483–498. doi: 10.2147/IJN.S184396. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shen X., Pan Y., Sun Z. H., Liu D., Xu H. J., Yu Q., Tribedi M., Kumar A., Liu J. Q. Mini-Rev. Med. Chem. 2019 doi: 10.2174/1389557519666190722164247. [DOI] [PubMed] [Google Scholar]
- Chen D., Yang D., Dougherty C. A., Lu W., Wu H., He X., Cai T., Van Dort M. E., Ross B. D., Hong H. ACS Nano. 2017;11:4315–4327. doi: 10.1021/acsnano.7b01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a).Wu M. X., Gao J., Wang F., Yang J., Song N., Jin X., Mi P., Tian J., Luo J., Liang F., Yang Y. W. Small. 2018;14:1704440–1704445. doi: 10.1002/smll.201704440. [DOI] [PubMed] [Google Scholar]
- (a) Wuttke S., Lismont M., Escudero A., Rungtaweevoranit B., Parak W. J. Biomaterials. 2017;123:172–183. doi: 10.1016/j.biomaterials.2017.01.025. [DOI] [PubMed] [Google Scholar]; (b) Pan C. Q., Liu Y. Q., Zhou M. Y., Wang W. S., Shi M., Xing M., Liao W. J. Int. J. Nanomed. 2018;13:1119–1137. doi: 10.2147/IJN.S147464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wang L., Li F., Zhang W., Huang W., Huo F., Xu H. Adv. Funct. Mater. 2017;27:1605465–1605472. [Google Scholar]
- Freund R., Lachelt U., Gruber T., Ruhle B., Wuttke S. ACS Nano. 2018;12:2094–2105. doi: 10.1021/acsnano.8b00932. [DOI] [PubMed] [Google Scholar]
- Huxford R. C., Della Rocca J., Lin W. Curr. Opin. Chem. Biol. 2010;14:262–268. doi: 10.1016/j.cbpa.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Zhang Y., Liu L., Wan W., Guo P., Nystrom A. M., Zou X. J. Am. Chem. Soc. 2016;138:962–968. doi: 10.1021/jacs.5b11720. [DOI] [PubMed] [Google Scholar]
- Baati T., Njim L., Neffati F., Kerkeni A., Bouttemi M., Gref R., Najjar M. F., Zakhama A., Couvreur P., Serre C., Horcajada P. Chem. Sci. 2013;4:1597. [Google Scholar]
- Wuttke S., Zimpel A., Bein T., Braig S., Stoiber K., Vollmar A., Muller D., Haastert-Talini K., Schaeske J., Stiesch M., Zahn G., Mohmeyer A., Behrens P., Eickelberg O., Bolukbas D. A., Meiners S. Adv. Healthcare Mater. 2017;6:1600818–1600828. doi: 10.1002/adhm.201600818. [DOI] [PubMed] [Google Scholar]
- Cinay G. E., Erkoc P., Alipour M., Hashimoto Y., Sasaki Y., Akiyoshi K., Kizilel S. ACS Biomater. Sci. Eng. 2017;3:370–380. doi: 10.1021/acsbiomaterials.6b00670. [DOI] [PubMed] [Google Scholar]
- Li B., Meng Z., Li Q., Huang X., Kang Z., Dong H., Chen J., Sun J., Dong Y., Li J., Jia X., Sessler J. L., Meng Q., Li C. Chem. Sci. 2017;8:4458–4464. doi: 10.1039/c7sc01438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Chowdhuri A. R., Singh T., Ghosh S. K., Sahu S. K. ACS Appl. Mater. Interfaces. 2016;8:16573–16583. doi: 10.1021/acsami.6b03988. [DOI] [PubMed] [Google Scholar]; (b) Ren F., Chen R. D., Wang Y., Sun Y. B., Jiang Y. D., Li G. F. Pharm. Res. 2011;28:897–906. doi: 10.1007/s11095-010-0346-9. [DOI] [PubMed] [Google Scholar]; (c) Li Z., Chen W. H. Mini-Rev. Med. Chem. 2017;17:1398–1405. doi: 10.2174/1389557517666170206152330. [DOI] [PubMed] [Google Scholar]
- (a) Maher S., Santos A., Kumeria T., Kaur G., Lambert M., Forward P., Evdokiou A., Losic D. J. Mater. Chem. B. 2017;5:4097–4109. doi: 10.1039/c7tb00588a. [DOI] [PubMed] [Google Scholar]; (b) Luo Z. D., Fan S. R., Gu C. Y., Li W. C. B. H., Liu J. Q. Curr. Med. Chem. 2019;26:3341–3369. doi: 10.2174/0929867325666180214123500. [DOI] [PubMed] [Google Scholar]
- (a) Abazari R., Mahjoub A. R., Ataei F., Morsali A., Carpenter-Warren C. L., Mehdizadeh K., Slawin A. M. Z. Inorg. Chem. 2018;57:13364–13379. doi: 10.1021/acs.inorgchem.8b01955. [DOI] [PubMed] [Google Scholar]; (b) Wang Y. M., Zhou C. Q., Chen J. X., Lin Y. L., Zeng W., Kuang B. C., Fu W. L., Chen W. H. Med. Chem. Commun. 2013;4:1400–1404. [Google Scholar]
- Gao C., Zheng H., Xing L., Shu M., Che S. Chem. Mater. 2010;22:5437–5444. [Google Scholar]
- Sun C. Y., Qin C., Wang X. L., Yang G. S., Shao K. Z., Lan Y. Q., Su Z. M., Huang P., Wang C. G., Wang E. B. Dalton Trans. 2012;41:6906–6909. doi: 10.1039/c2dt30357d. [DOI] [PubMed] [Google Scholar]
- Chowdhuri A. R., Laha D., Pal S., Karmakar P., Sahu S. K. Dalton Trans. 2016;45:18120–18132. doi: 10.1039/c6dt03237k. [DOI] [PubMed] [Google Scholar]
- Silva J. S. F., Silva J. Y. R., de Sa G. F., Araujo S. S., Filho M. A. G., Ronconi C. M., Santos T. C., Junior S. A. ACS Omega. 2018;3:12147–12157. doi: 10.1021/acsomega.8b01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Sheng Z., Wang J. Z. Anorg. Allg. Chem. 2018;644:877–882. [Google Scholar]
- Ren H., Zhang L., An J., Wang T., Li L., Si X., He L., Wu X., Wang C., Su Z. Chem. Commun. 2014;50:1000–1002. doi: 10.1039/c3cc47666a. [DOI] [PubMed] [Google Scholar]
- Liang Z., Yang Z., Yuan H., Wang C., Qi J., Liu K., Cao R., Zheng H. Dalton Trans. 2018;47:10223–10228. doi: 10.1039/c8dt01789a. [DOI] [PubMed] [Google Scholar]
- Zheng C., Wang Y., Phua S. Z. F., Lim W. Q., Zhao Y. ACS Biomater. Sci. Eng. 2017;3:2223–2229. doi: 10.1021/acsbiomaterials.7b00435. [DOI] [PubMed] [Google Scholar]
- Dong K., Wang Z., Zhang Y., Ren J., Qu X. ACS Appl. Mater. Interfaces. 2018;10:31998–32005. doi: 10.1021/acsami.8b11972. [DOI] [PubMed] [Google Scholar]
- Zhang H., Jiang W., Liu R., Zhang J., Zhang D., Li Z., Luan Y. ACS Appl. Mater. Interfaces. 2017;9:19687–19697. doi: 10.1021/acsami.7b05142. [DOI] [PubMed] [Google Scholar]
- Bian R., Wang T., Zhang L., Li L., Wang C. Biomater. Sci. 2015;3:1270–1278. doi: 10.1039/c5bm00186b. [DOI] [PubMed] [Google Scholar]
- Zhang F. M., Dong H., Zhang X., Sun X. J., Liu M., Yang D. D., Liu X., Wei J. Z. ACS Appl. Mater. Interfaces. 2017;9:27332–27337. doi: 10.1021/acsami.7b08451. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Wang Y., Sun L., Yuan B., Tian Y., Xiang L., Li Y., Li Y., Li J., Wu A. Biomaterials. 2019;197:41–50. doi: 10.1016/j.biomaterials.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Xie Z., Cai X., Sun C., Liang S., Shao S., Huang S., Cheng Z., Pang M., Xing B., Kheraif A. A. A., Lin J. Chem. Mater. 2018;31:483–490. [Google Scholar]
- Zheng M., Liu S., Guan X., Xie Z. ACS Appl. Mater. Interfaces. 2015;7:22181–22187. doi: 10.1021/acsami.5b04315. [DOI] [PubMed] [Google Scholar]
- Shi Z., Chen X., Zhang L., Ding S., Wang X., Lei Q., Fang W. Biomater. Sci. 2018;6:2582–2590. doi: 10.1039/c8bm00625c. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Q., Liu R., Zhang X., Li Z., Luan Y. Adv. Funct. Mater. 2018;28:1802830–1802839. [Google Scholar]
- (a) Feng Y., Wang H., Zhang S., Zhao Y., Gao J., Zheng Y., Zhao P., Zhang Z., Zaworotko M. J., Cheng P., Ma S., Chen Y. Adv. Mater. 2019;31:1805148–1805154. doi: 10.1002/adma.201805148. [DOI] [PubMed] [Google Scholar]; (b) Chen W. H., Luo G.-F., Vazquez-Gonzalez M., Cazelles R., Sohn Y. S., Nechushtai R., Mandel Y., Willner I. ACS Nano. 2018;12:7539–7545. doi: 10.1021/acsnano.8b03417. [DOI] [PubMed] [Google Scholar]
- Ranji-Burachaloo H., Reyhani A., Gurr P. A., Dunstan D. E., Qiao G. G. Nanoscale. 2019;11:5705–5716. doi: 10.1039/c8nr09107b. [DOI] [PubMed] [Google Scholar]
- Sava Gallis D. F., Butler K. S., Agola J. O., Pearce C. J., McBride A. A. ACS Appl. Mater. Interfaces. 2019;11:7782–7791. doi: 10.1021/acsami.8b21698. [DOI] [PubMed] [Google Scholar]
- Yang X., Tang Q., Jiang Y., Zhang M., Wang M., Mao L. J. Am. Chem. Soc. 2019;141:3782–3786. doi: 10.1021/jacs.8b11996. [DOI] [PubMed] [Google Scholar]
- Zhong X., Zhang Y., Tan L., Zheng T., Hou Y., Hong X., Du G., Chen X., Zhang Y., Sun X. J. Controlled Release. 2019;300:81–92. doi: 10.1016/j.jconrel.2019.02.035. [DOI] [PubMed] [Google Scholar]
- Du Y., Gao J., Zhou L., Ma L., He Y., Zheng X., Huang Z., Jiang Y. Adv. Sci. 2019;6:1801684–1801689. doi: 10.1002/advs.201801684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu L., Huang L., Zhang W., Wang R., Yue T., Sun J., Li G., Wang J. Nanoscale. 2019;11:9468–9477. doi: 10.1039/c9nr01284b. [DOI] [PubMed] [Google Scholar]
- Song Z., Wu Y., Cao Q., Wang H., Wang X., Han H. Adv. Funct. Mater. 2018;28:1800011. [Google Scholar]
- Lyu F., Zhang Y., Zare R. N., Ge J., Liu Z. Nano Lett. 2014;14:5761–5765. doi: 10.1021/nl5026419. [DOI] [PubMed] [Google Scholar]
- Cheng G., Li W., Ha L., Han X., Hao S., Wan Y., Wang Z., Dong F., Zou X., Mao Y., Zheng S. Y. J. Am. Chem. Soc. 2018;140:7282–7291. doi: 10.1021/jacs.8b03584. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang F., Ju E., Liu Z., Chen Z., Ren J., Qu X. Adv. Funct. Mater. 2016;26:6454–6461. [Google Scholar]
- Alsaiari S. K., Patil S., Alyami M., Alamoudi K. O., Aleisa F. A., Merzaban J. S., Li M., Khashab N. M. J. Am. Chem. Soc. 2018;140:143–146. doi: 10.1021/jacs.7b11754. [DOI] [PubMed] [Google Scholar]
- Duan Y., Ye F., Huang Y., Qin Y., He C., Zhao S. Chem. Commun. 2018;54:5377–5380. doi: 10.1039/c8cc02708k. [DOI] [PubMed] [Google Scholar]
- Xing K., Fan R., Wang F., Nie H., Du X., Gai S., Wang P., Yang Y. ACS Appl. Mater. Interfaces. 2018;10:22746–22756. doi: 10.1021/acsami.8b06270. [DOI] [PubMed] [Google Scholar]
- Lin W., Hu Q., Jiang K., Cui Y., Yang Y., Qian G. Microporous Mesoporous Mater. 2017;249:55–60. [Google Scholar]
- Li Y., Jin J., Wang D., Lv J., Hou K., Liu Y., Chen C., Tang Z. Nano Res. 2018;11:3294–3305. [Google Scholar]
- Yang J. C., Chen Y., Li Y. H., Yin X. B. ACS Appl. Mater. Interfaces. 2017;9:22278–22288. doi: 10.1021/acsami.7b06105. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhou J., Shi R., Wu H., Chen R., Duan B., Xia G., Xu P., Wang H., Zhou S., Wang C., Wang H., Guo Z., Chen Q. Theranostics. 2017;7:4605–4617. doi: 10.7150/thno.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Niu M., Chen X., Tan L., Fu C., Ren X., Ren J., Li L., Xu K., Zhong H., Meng X. Biomaterials. 2018;162:132–143. doi: 10.1016/j.biomaterials.2018.02.022. [DOI] [PubMed] [Google Scholar]
- (a) Chen W. H., Yu X., Cecconello A., Sohn Y. S., Nechushtai R., Willner I. Chem. Sci. 2017;8:5769–5780. doi: 10.1039/c7sc01765k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kahn J. S., Freage L., Enkin N., Garcia M. A., Willner I. Adv. Mater. 2017;29:1602782–1602787. doi: 10.1002/adma.201602782. [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Sutar P., Yadav P., Eswaramoorthy M., Maji T. K. Inorg. Chem. 2018;57:14480–14483. doi: 10.1021/acs.inorgchem.8b02545. [DOI] [PubMed] [Google Scholar]
- Gao X., Hai X., Baigude H., Guan W., Liu Z. Sci. Rep. 2016;6:37705–37714. doi: 10.1038/srep37705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Yuan B., Qiu N., Wang Y., Sun L., Wei Z., Li Y., Zheng J., Jin Y., Li Y., Du S., Li J., Wu A. Nano-Micro Lett. 2019;11:61–77. doi: 10.1007/s40820-019-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Yang Z., Wang Y., Chen Y., Yuan H., Chen H., Xu X., Gao X., Liang Z., Sun Y., Li J. R., Zheng H., Cao R. ChemMedChem. 2018;13:400–405. doi: 10.1002/cmdc.201800019. [DOI] [PubMed] [Google Scholar]
- Yan L., Chen X., Wang Z., Zhang X., Zhu X., Zhou M., Chen W., Huang L., Roy V. A. L., Yu P. K. N., Zhu G., Zhang W. ACS Appl. Mater. Interfaces. 2017;9:32990–33000. doi: 10.1021/acsami.7b10064. [DOI] [PubMed] [Google Scholar]
- Shu F., Lv D., Song X.-L., Huang B., Wang C., Yu Y., Zhao S.-C. RSC Adv. 2018;8:6581–6589. doi: 10.1039/c7ra12969f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Wu Q., Tan L., Huang Z., Fu C., Ren X., Xia N., Chen Z., Ma X., Lan X., Zhang Q., Meng X. ACS Appl. Mater. Interfaces. 2019;11:10520–10531. doi: 10.1021/acsami.8b22177. [DOI] [PubMed] [Google Scholar]
- He M., Zhou J., Chen J., Zheng F., Wang D., Shi R., Guo Z., Wang H., Chen Q. J. Mater. Chem. B. 2015;3:9033–9042. doi: 10.1039/c5tb01830g. [DOI] [PubMed] [Google Scholar]
- Zhao H., Shu G., Zhu J., Fu Y., Gu Z., Yang D. Biomaterials. 2019;217:119332–119339. doi: 10.1016/j.biomaterials.2019.119332. [DOI] [PubMed] [Google Scholar]
- Chen X., Shi Z., Tong R., Ding S., Wang X., Wu J., Lei Q., Fang W. ACS Biomater. Sci. Eng. 2018;4:4183–4192. doi: 10.1021/acsbiomaterials.8b00840. [DOI] [PubMed] [Google Scholar]
- Chen X., Tong R., Shi Z., Yang B., Liu H., Ding S., Wang X., Lei Q., Wu J., Fang W. ACS Appl. Mater. Interfaces. 2018;10:2328–2337. doi: 10.1021/acsami.7b16522. [DOI] [PubMed] [Google Scholar]
- Song M.-R., Li D.-Y., Nian F.-Y., Xue J.-P., Chen J.-J. J. Mater. Sci. 2017;53:2351–2361. [Google Scholar]
- Guan Q., Zhou L. L., Li Y. A., Dong Y. B. Inorg. Chem. 2018;57:10137–10145. doi: 10.1021/acs.inorgchem.8b01316. [DOI] [PubMed] [Google Scholar]
- Sang C., Ma L., Luo D., Liu H., Li D., Chen T. Chem. Eng. J. 2019;371:301–305. [Google Scholar]
- Wei X., Zhang L., Li S., Chen X., Zhang M., Wang C., Wang T., Li L. New J. Chem. 2018;42:923–929. [Google Scholar]
- Jiang W., Zhang H., Wu J., Zhai G., Li Z., Luan Y., Garg S. ACS Appl. Mater. Interfaces. 2018;10:34513–34523. doi: 10.1021/acsami.8b13487. [DOI] [PubMed] [Google Scholar]
- Wang T., Li S., Zou Z., Hai L., Yang X., Jia X., Zhang A., He D., He X., Wang K. J. Mater. Chem. B. 2018;6:3914–3921. doi: 10.1039/c8tb00351c. [DOI] [PubMed] [Google Scholar]
- Yang X., Li L., He D., Hai L., Tang J., Li H., He X., Wang K. J. Mater. Chem. B. 2017;5:4648–4659. doi: 10.1039/c7tb00715a. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang Q., Liu C., Han B. Biomater. Sci. 2019;7:1696–1704. doi: 10.1039/c8bm01591k. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang L., Liang X., Wang T., Chen X., Liu C., Li L., Wang C. Chem. Eng. J. 2019;378:122175–122210. [Google Scholar]
- Ren S. Z., Zhu D., Zhu X. H., Wang B., Yang Y. S., Sun W. X., Wang X. M., Lv P. C., Wang Z. C., Zhu H. L. ACS Appl. Mater. Interfaces. 2019;11:20678–20688. doi: 10.1021/acsami.9b04236. [DOI] [PubMed] [Google Scholar]
- Bahrani S., Hashemi S. A., Mousavi S. M., Azhdari R. Drug Metab. Rev. 2019;51:356–377. doi: 10.1080/03602532.2019.1632887. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. A. J. Biomed. Mater. Res., Part A. 2017;105:1184–1194. doi: 10.1002/jbm.a.35995. [DOI] [PubMed] [Google Scholar]
- Ma Y., Xu G., Wei F., Cen Y., Xu X., Shi M., Cheng X., Chai Y., Sohail M., Hu Q. ACS Appl. Mater. Interfaces. 2018;10:20801–20805. doi: 10.1021/acsami.8b05643. [DOI] [PubMed] [Google Scholar]
- Zhang J., He M., Nie C., He M., Pan Q., Liu C., Hu Y., Yi J., Chen T., Chu X. Anal. Chem. 2019;91:9049–9057. doi: 10.1021/acs.analchem.9b01343. [DOI] [PubMed] [Google Scholar]
- Terzopoulou A., Hoop M., Chen X. Z., Hirt A. M., Charilaou M., Shen Y., Mushtaq F., Del Pino A. P., Logofatu C., Simonelli L., de Mello A. J., Doonan C. J., Sort J., Nelson B. J., Pane S., Puigmarti-Luis J. Angew. Chem., Int. Ed. 2019;58:13550–13555. doi: 10.1002/anie.201907389. [DOI] [PubMed] [Google Scholar]
- Lin J., Xin P., An L., Xu Y., Tao C., Tian Q., Zhou Z., Hu B., Yang S. Chem. Commun. 2019;55:478–481. doi: 10.1039/c8cc08943d. [DOI] [PubMed] [Google Scholar]
- Guo C., Xu S., Arshad A., Wang L. Chem. Commun. 2018;54:9853–9856. doi: 10.1039/c8cc06129g. [DOI] [PubMed] [Google Scholar]
- Dong J., Qiao Z., Pan Y., Peh S. B., Yuan Y. D., Wang Y., Zhai L., Yuan H., Cheng Y., Liang H., Liu B., Zhao D. Chem. Mater. 2019;31:4897–4912. [Google Scholar]
- Cattaneo D., Warrender S. J., Duncan M. J., Kelsall C. J., Doherty M. K., Whitfield P. D., Megson I. L., Morris R. E. RSC Adv. 2016;6:14059–14067. doi: 10.1039/c5ra24023a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. W., Liu S. J., Hu T. L., Bu X. H. Dalton Trans. 2014;43:11470–11473. doi: 10.1039/c4dt00894d. [DOI] [PubMed] [Google Scholar]
- Wang C., Tian L., Zhu W., Wang S., Wang P., Liang Y., Zhang W., Zhao H., Li G. ACS Appl. Mater. Interfaces. 2017;9:20076–20085. doi: 10.1021/acsami.7b04172. [DOI] [PubMed] [Google Scholar]
- Yang Y., Shen K., Lin J.-z., Zhou Y., Liu Q.-y., Hang C., Abdelhamid H. N., Zhang Z.-q., Chen H. RSC Adv. 2016;6:45475–45481. [Google Scholar]
- An J., Shade C. M., Chengelis-Czegan D. A., Petoud S., Rosi N. L. J. Am. Chem. Soc. 2011;133:1220–1223. doi: 10.1021/ja109103t. [DOI] [PubMed] [Google Scholar]
- Qin J., Cho M., Lee Y. ACS Appl. Mater. Interfaces. 2019;11:11743–11748. doi: 10.1021/acsami.8b21425. [DOI] [PubMed] [Google Scholar]
- Zhou N., Su F., Guo C., He L., Jia Z., Wang M., Jia Q., Zhang Z., Lu S. Biosens. Bioelectron. 2019;123:51–58. doi: 10.1016/j.bios.2018.09.079. [DOI] [PubMed] [Google Scholar]
- Lu K., Aung T., Guo N., Weichselbaum R., Lin W. Adv. Mater. 2018;30:1707634. doi: 10.1002/adma.201707634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J., Geib S.-J., Rosi N.-L. J. Am. Chem. Soc. 2009;131:8376–8377. doi: 10.1021/ja902972w. [DOI] [PubMed] [Google Scholar]
- Goto Y., Sato H., Shinkai S., Sada K. J. Am. Chem. Soc. 2008;130:14354–14355. doi: 10.1021/ja7114053. [DOI] [PubMed] [Google Scholar]
- Taylor-Pashow K. M. L., Rocca J. D., Xie Z., Tran S., Lin W. B. J. Am. Chem. Soc. 2009;131:14261–14263. doi: 10.1021/ja906198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Q., Cohen S. M. J. Am. Chem. Soc. 2007;129:12368–12369. doi: 10.1021/ja074366o. [DOI] [PubMed] [Google Scholar]
- Britt D., Lee C., Uribe-Romo F. J., Furukawa H., Yaghi O. M. Inorg. Chem. 2010;49:6387–6389. doi: 10.1021/ic100652x. [DOI] [PubMed] [Google Scholar]
- Costa J. S., Gamez P., Black C. A., Roubeau O., Teat S. J., Reedijk J. Eur. J. Inorg. Chem. 2008;10:1551–1554. [Google Scholar]
- Gadzikwa T., Farha O. K., Mulfort K. L., Hupp J. T., Nguyen S. T. Chem. Commun. 2019;25:3720–3722. doi: 10.1039/b823392f. [DOI] [PubMed] [Google Scholar]
- Liu C., Li T., Rosi N. L. J. Am. Chem. Soc. 2012;134:18886–18888. doi: 10.1021/ja307713q. [DOI] [PubMed] [Google Scholar]
- Ray Chowdhuri A., Bhattacharya D., Sahu S. K. Dalton Trans. 2016;45:2963–2973. doi: 10.1039/c5dt03736k. [DOI] [PubMed] [Google Scholar]
- Tan L. L., Li H., Qiu Y. C., Chen D. X., Wang X., Pan R. Y., Wang Y., Zhang S. X., Wang B., Yang Y. W. Chem. Sci. 2015;6:1640–1644. doi: 10.1039/c4sc03749a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Li T., An J. Chemistry. 2015;21:17010–17015. doi: 10.1002/chem.201501560. [DOI] [PubMed] [Google Scholar]
- Sontz P. A., Bailey J. B., Ahn S., Tezcan F. A. J. Am. Chem. Soc. 2015;137:11598–11601. doi: 10.1021/jacs.5b07463. [DOI] [PubMed] [Google Scholar]
- Lei X., Tay S. W., Ong P. J., Hong L. J. Ind. Eng. Chem. 2019;78:410–420. [Google Scholar]
- Bahrani S., Hashemi S. A., Mousavi S. M., Azhdari R. Drug Metab. Rev. 2019;7:1–22. doi: 10.1080/03602532.2019.1632887. [DOI] [PubMed] [Google Scholar]
- Deng J., Wang K., Wang M., Yu P., Mao L. J. Am. Chem. Soc. 2017;139:5877–5882. doi: 10.1021/jacs.7b01229. [DOI] [PubMed] [Google Scholar]
- Li S., Wang K., Shi Y., Cui Y., Chen B., He B., Dai W., Zhang H., Wang X., Zhong C., Wu H., Yang Q., Zhang Q. Adv. Funct. Mater. 2016;26:2715–2727. [Google Scholar]
- Ruyra A., Yazdi A., Espin J., Carne-Sanchez A., Roher N., Lorenzo J., Imaz I., Maspoch D. Chem. 2015;21:2508–2518. doi: 10.1002/chem.201405380. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. A. J. Biomed. Mater. Res., Part A. 2017;105:1184–1194. doi: 10.1002/jbm.a.35995. [DOI] [PubMed] [Google Scholar]
- Lian X., Fang Y., Joseph E., Wang Q., Li J., Banerjee S., Lollar C., Wang X., Zhou H. C. Chem. Soc. Rev. 2017;46:3386–3401. doi: 10.1039/c7cs00058h. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Young A. P., Tsung C. K. Small. 2017;13:1700880–1700893. doi: 10.1002/smll.201700880. [DOI] [PubMed] [Google Scholar]
- Bailey J. B., Zhang L., Chiong J. A., Ahn S., Tezcan F. A. J. Am. Chem. Soc. 2017;139:8160–8166. doi: 10.1021/jacs.7b01202. [DOI] [PubMed] [Google Scholar]
- Samanta D., Roy S., Sasmal R., Saha N. D., Pradeep K. R., Viswanatha R., Agasti S. S., Maji T. K. Angew. Chem., Int. Ed. 2019;58:5008–5012. doi: 10.1002/anie.201900692. [DOI] [PubMed] [Google Scholar]
- Zhang J., He M., Nie C., He M., Pan Q., Liu C., Hu Y., Yi J., Chen T., Chu X. Anal. Chem. 2019;91:9049–9057. doi: 10.1021/acs.analchem.9b01343. [DOI] [PubMed] [Google Scholar]
- Yen C. I., Liu S. M., Lo W. S., Wu J. W., Liu Y. H., Chein R. J., Yang R., Wu K. C., Hwu J. R., Ma N., Shieh F. K. Chemistry. 2016;22:2925–2929. doi: 10.1002/chem.201505005. [DOI] [PubMed] [Google Scholar]
- Cavaco L. M., Hasman H., Aarestrup F. M. Vet. Microbiol. 2011;150:344–348. doi: 10.1016/j.vetmic.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Lucena G. N., Alves R. C., Abuçafy M. P., Chiavacci L. A., da Silva I. C., Pavan F. R., Frem R. C. G. J. Solid State Chem. 2018;260:67–72. [Google Scholar]
- Zhang L., Wang Z. Z., Zhang Y., Cao F. F., Dong K., Ren J. S., Qu X. G. ACS Nano. 2018;12:10201–10211. doi: 10.1021/acsnano.8b05200. [DOI] [PubMed] [Google Scholar]