Background:
The likelihood of neurologically favorable survival declines with prolonged resuscitation. However, the ability of extracorporeal cardiopulmonary resuscitation (ECPR) to modulate this decline is unknown. Our aim was to examine the effects of resuscitation duration on survival and metabolic profile in patients who undergo ECPR for refractory ventricular fibrillation/ventricular tachycardia out-of-hospital cardiac arrest.
Methods:
We retrospectively evaluated survival in 160 consecutive adults with refractory ventricular fibrillation/ventricular tachycardia out-of-hospital cardiac arrest treated with the University of Minnesota (UMN) ECPR protocol (transport with ongoing cardiopulmonary resuscitation [CPR] to the cardiac catheterization laboratory for ECPR) compared with 654 adults who had received standard CPR in the amiodarone arm of the ALPS trial (Amiodarone, Lidocaine, or Placebo Study). We evaluated the metabolic changes and rate of survival in relation to duration of CPR in UMN-ECPR patients.
Results:
Neurologically favorable survival was significantly higher in UMN-ECPR patients versus ALPS patients (33% versus 23%; P=0.01) overall. The mean duration of CPR was also significantly longer for UMN-ECPR patients versus ALPS patients (60 minutes versus 35 minutes; P<0.001). Analysis of the effect of CPR duration on neurologically favorable survival demonstrated significantly higher neurologically favorable survival for UMN-ECPR patients compared with ALPS patients at each CPR duration interval <60 minutes; however, longer CPR duration was associated with a progressive decline in neurologically favorable survival in both groups. All UMN-ECPR patients with 20 to 29 minutes of CPR (8 of 8) survived with neurologically favorable status compared with 24% (24 of 102) of ALPS patients with the same duration of CPR. There were no neurologically favorable survivors in the ALPS cohort with CPR ≥40 minutes, whereas neurologically favorable survival was 25% (9 of 36) for UMN-ECPR patients with 50 to 59 minutes of CPR and 19% with ≥60 minutes of CPR. Relative risk of mortality or poor neurological function was significantly reduced in UMN-ECPR patients with CPR duration ≥60 minutes. Significant metabolic changes included decline in pH, increased lactic acid and arterial partial pressure of carbon dioxide, and thickened left ventricular wall with prolonged professional CPR.
Conclusions:
ECPR was associated with improved neurologically favorable survival at all CPR durations <60 minutes despite severe progressive metabolic derangement. However, CPR duration remains a critical determinate of survival.
Keywords: cardiopulmonary resuscitation; death, sudden, cardiac; extracorporeal membrane oxygenation; heart arrest; ventricular fibrillation
Clinical Perspective.
What Is New?
Extracorporeal cardiopulmonary resuscitation (ECPR) is associated with improved survival for patients with shockable out-of-hospital cardiac arrest compared with standard cardiopulmonary resuscitation (CPR) at all durations of professional CPR <60 minutes.
Although still improved compared with patients receiving only standard CPR, patients receiving ECPR have a reduced likelihood of neurologically favorable survival with prolonged duration of professional CPR, accounting for a 25% increase in mortality with every 10 minutes of CPR beyond 30 minutes.
The progressive metabolic derangement that develops during prolonged CPR is associated with reduced neurologically favorable survival.
What Are the Clinical Implications?
Healthcare systems using ECPR for out-of-hospital cardiac arrest should optimize prehospital and in-hospital processes to minimize time to ECPR.
ECPR may allow successful treatment of patients receiving prolonged professional CPR up to 60 to 98 minutes.
Further research is necessary to identify strategies to increase CPR efficiency, to improve perfusion, and to decrease metabolic demand such that the progressive metabolic derangement associated with prolonged CPR can be delayed and the effective time to ECPR can be increased.
Editorial, see p 887
Out-of-hospital cardiac arrest (OHCA) affects ≈360 000 people in the United States annually.1,2 One-third of these patients present to emergency medical services (EMS) with a shockable rhythm (ventricular fibrillation [VF]/ventricular tachycardia [VT]).3,4 Although VF/VT is a favorable prognostic marker for patients with cardiac arrest overall,5–7 50% of patients with VF/VT OHCA are refractory to treatment, failing to achieve sustained return of spontaneous circulation (ROSC) after 3 defibrillation attempts and administration of 300 mg amiodarone.8–10 Neurologically favorable survival is achieved for 29% of patients with VF/VT OHCA overall11 but only 8% to 15% of patients with refractory VF/VT OHCA.12–14
The likelihood of neurologically favorable survival for patients with refractory VF/VT declines with longer duration of standard cardiopulmonary resuscitation (CPR), dropping below 5% if ROSC is not achieved within 30 to 45 minutes of standard advanced cardiac life support.15–18 Extracorporeal CPR (ECPR), using extracorporeal membrane oxygenation (ECMO) for hemodynamic support, has recently been shown to enhance survival for patients with refractory VF/VT OHCA, with survival rates as high as 40% to 50%.10,19–22 In these studies, mean CPR duration before initiation of ECMO was ≈1 hour.
CPR duration has been incorporated into algorithms for the termination of resuscitation.23 However, clear guidelines for the appropriate duration of resuscitation efforts remain elusive, particularly in the setting of ECPR. The metabolic effects of prolonged CPR and the association with survival have not been well described. This study aims to describe the effect of prolonged CPR on the development of metabolic derangements and survival in patients with refractory VF/VT OHCA who receive ECPR.
Methods
Data related to the UMN-ECPR cohort are available from the corresponding author on reasonable request. The ALPS (Amiodarone, Lidocaine, or Placebo Study) data set can be requested from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Centre (https://biolincc.nhlbi.nih.gov). The authors will provide additional methodological details as required.
Study Design
Two groups of patients with refractory VF/VT OHCA were analyzed: (1) a cohort of 160 consecutive patients treated with the University of Minnesota refractory VF/VT ECPR (UMN-ECPR) protocol between December 2015 and February 201912,14 and (2) a comparison cohort of patients who received standard CPR in the amiodarone arm of ALPS in OHCA.13 The Institutional Review Board at the University of Minnesota approved this study (No. 1703M11301) with waiver of informed consent.
UMN-ECPR Protocol
Details of the UMN-ECPR protocol were published previously.12,14 Briefly, patients underwent prehospital screening by paramedics and were rapidly transported to the University of Minnesota cardiac catheterization laboratory (CCL) if they met the following criteria: (1) 18 to 75 years of age, (2) OHCA of presumed cardiac origin, (3) initial rhythm of VF/VT, (4) received 3 direct current shocks for VF/VT without ROSC or shock resulting in ongoing pulseless electrical activity or asystole, (5) received amiodarone 300 mg, (6) body habitus accommodating a Lund University Cardiac Arrest System automated CPR device, and (7) estimated transfer time to the CCL of <30 minutes.14 Patients were transported with mechanical CPR and ongoing advanced cardiac life support directly to the CCL, where arterial blood gas and arterial lactic acid levels were immediately assessed. Patients failing to meet ≥1 of the following criteria for resuscitation continuation were declared dead on CCL arrival: (1) end-tidal CO2 ≥10 mm Hg, (2) arterial partial pressure of oxygen (Pao2) ≥50 mm Hg or O2 saturation ≥85%, or (3) lactic acid ≤18 mmol/L. Patients meeting these criteria underwent continued resuscitation with emergent ultrasound-guided percutaneous cannulation for peripheral venoarterial ECMO performed by interventional cardiologists. Patients who had achieved ROSC en route with sufficient hemodynamic stability received an intra-aortic balloon pump (Maquet Cardiovascular, Wayne, NJ) instead of ECMO. Coronary angiography was then performed, and percutaneous coronary intervention was provided as indicated. All patients underwent therapeutic hypothermia to 34°C via an intravascular cooling catheter (Thermogard XP, Zoll, Chelmsford, MA); if bleeding complications were present, 36°C was used. If an organized cardiac rhythm could not be established after 90 minutes of stabilized hemodynamics and treatment of reversible cardiac arrest causes in the CCL, the patient was declared dead. Patients with an organized rhythm were admitted to the cardiac intensive care unit.19
Transthoracic echocardiography was performed within 24 hours after hospital admission for all patients admitted to the cardiac intensive care unit. Left ventricular lateral wall thickness was assessed as a surrogate for ischemic injury because ischemia induces progressive thickening of the ventricle.24,25 Images of the left ventricular lateral wall in the parasternal long-axis, parasternal short-axis, and apical 4-chamber views were collected. The lateral wall thickness was measured with standard methods in accordance with American Society of Echocardiography guidelines in all 3 views, with the mean used for analysis. The echocardiography reader was blinded to the clinical outcome and characteristics of the patients.
ALPS in OHCA
ALPS was a randomized, double-blind, multicenter clinical trial comparing parenteral administration of amiodarone, lidocaine, and saline placebo added to standard care in adults (age >18 years) who had nontraumatic OHCA, shock-refractory VF, or pulseless VT after at least 1 shock and vascular access.13 The cohort used in this analysis consisted of all patients 18 to 75 years of age enrolled in the amiodarone arm for whom data on duration of CPR and outcome were available. Patients with OHCA were treated in accordance with local EMS protocols in compliance with American Heart Association guidelines for advanced cardiac life support.
Data Classification, Management, and Statistical Analysis
Demographic data and OHCA characteristics were recorded for both UMN-ECPR and ALPS patients. Neurologically favorable survival was defined as a Cerebral Performance Category (CPC) score of 1 to 2 for the UMN-ECPR cohort or a modified Rankin Scale (mRS) score of 0 to 3 for the ALPS cohort. CPC score was assessed at the time of hospital discharge and the 6-month clinic follow-up for the UMN-ECPR cohort. Survivors from the ALPS cohort underwent mRS determination at the time of discharge; follow-up mRS score was not available for the ALPS cohort. Clinical data were placed in a REDCap database hosted at the University of Minnesota. Clinical data were exported to Excel for biostatistical analysis.
Data are presented as mean±SEM for continuous variables and as frequency (percentage) for categorical variables. Continuous variables across 3 groups were compared by use of 2-way ANOVA with multiple comparisons performed with the Tukey multiple-comparison test. For 2-group comparisons, ttests were used for continuous variables. We used χ2 tests to compare categorical variables across groups with relative risk reduction calculated for mortality and poor neurological outcome between groups. Univariate and multivariable linear regression models were used to compare unadjusted and adjusted survival probabilities between studies at different CPR duration intervals. Logistic regression models were used to study unadjusted and adjusted associations of survival with study. The same set of confounders were used for adjustment in multivariable models: age, sex, race (white versus black versus other), witnessed arrest, bystander CPR, and public location. A value of P<0.05 was considered statistically significant. Statistical analysis was performed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA) and Stata Version 16 (StataCorp LLC, College Station, TX, 2019).
Results
Patients
Of 160 patients transported with the UMN-ECPR protocol, 133 (83%) met resuscitation criteria, receiving full CCL treatment (Figure 1). Of these, 52 survived to discharge with neurologically favorable status, representing 33% of all transported patients and 39% of those meeting resuscitation criteria. All patients with neurologically favorable survival to hospital discharge were alive with favorable neurological function at 6 months. Significantly fewer patients in the ALPS cohort survived with neurologically favorable status: 148 of 654 patients (23%; P<0.001). This resulted in an overall relative risk reduction for death or poor neurological outcome of 13% (95% CI, 2–22; P<0.001). The cohorts were similar in terms of demographic characteristics, rates of witnessed arrest, bystander CPR, and arrest in a public location (Table 1). The difference in age between the groups reached statistical significance, with 57 years of age for UMN-ECPR patients versus 59 years of age for ALPS patients (P=0.033). ALPS patients had a significantly shorter mean duration of professional CPR performed (P<0.001). In the ALPS cohort, 34% (221 of 654) had CPR for <20 minutes, whereas all UMN-ECPR patients had CPR for ≥20 minutes (Figure 2A).
Figure 1.
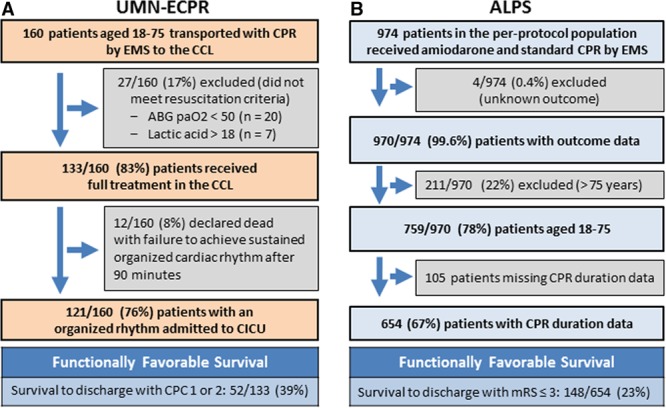
Patient flow diagrams for the University of Minnesota Extracorporeal Cardiopulmonary Resuscitation (UMN-ECPR) and ALPS (Amiodarone, Lidocaine, or Placebo Study) cohorts. A, Patients transported for the UMN-ECPR protocol. B, Patient selection for the ALPS cohort. ABG indicates arterial blood gas; CCL, cardiac catheterization laboratory; CICU, cardiac intensive care unit; CPC, Cerebral Performance Category; CPR, cardiopulmonary resuscitation; EMS, emergency medical services; mRS, modified Rankin Scale; and Pao2, arterial partial pressure of oxygen.
Table 1.
Patient and Cardiac Arrest Characteristics
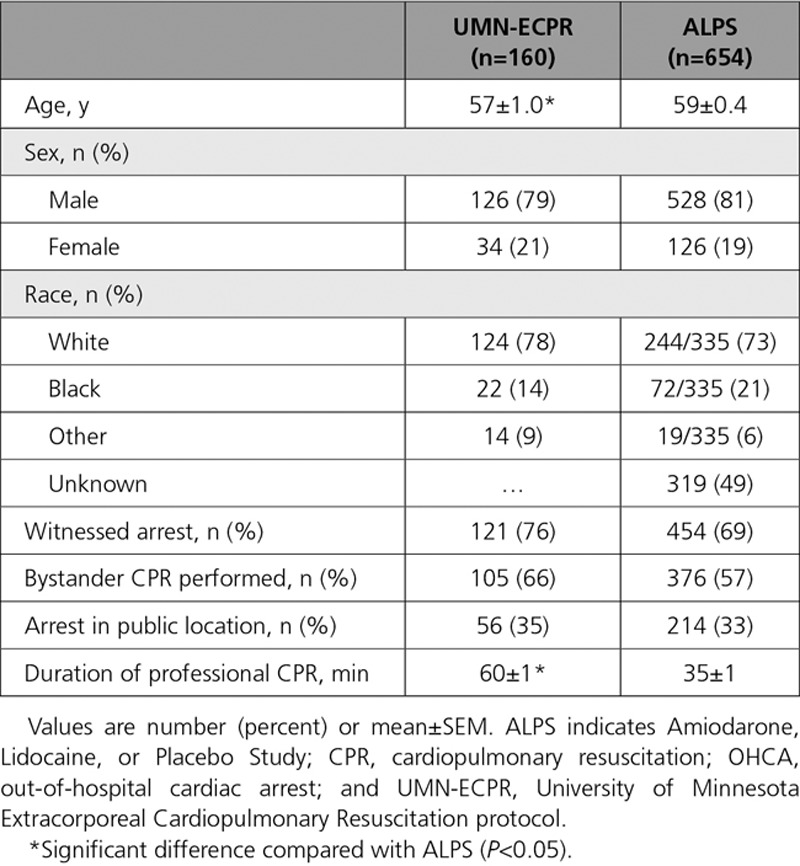
Figure 2.
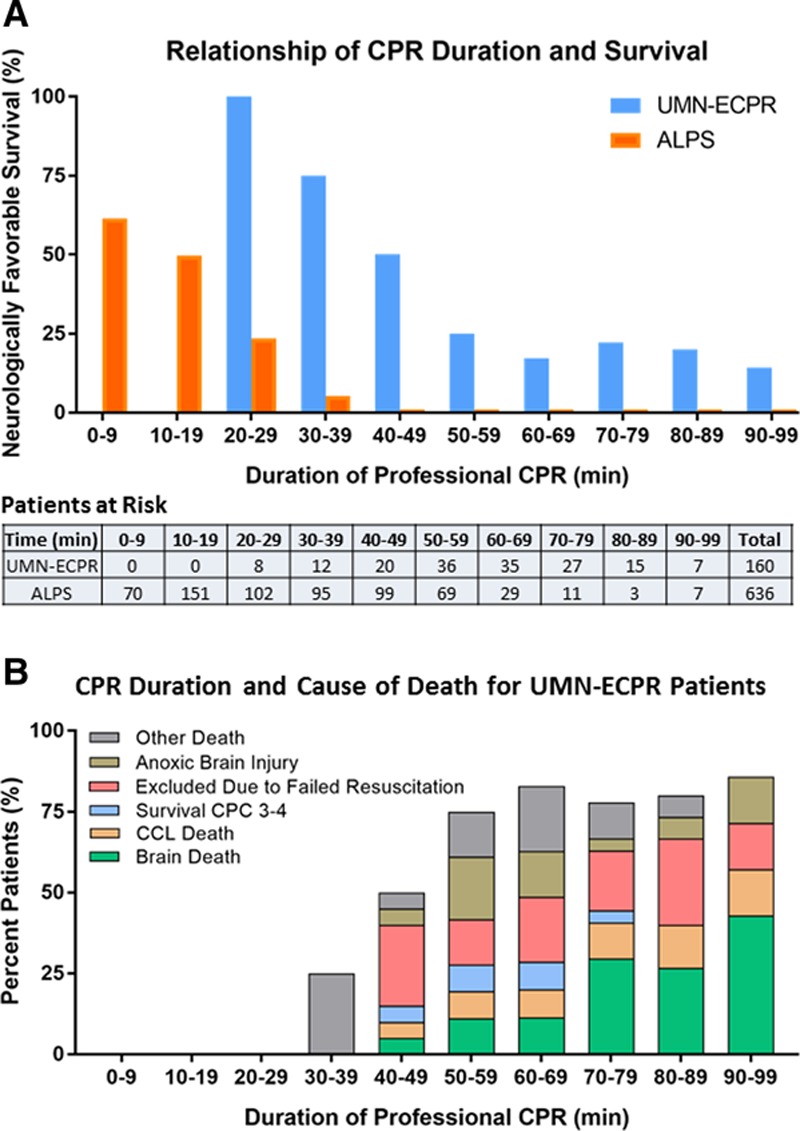
Neurologically favorable survival and cause of death related to duration of professional cardiopulmonary resuscitation (CPR). A, Neurologically favorable survival related to duration of professional CPR in the University of Minnesota Extracorporeal Cardiopulmonary Resuscitation (UMN-ECPR) and ALPS (Amiodarone, Lidocaine, or Placebo Study) cohorts. The Patients at Risk table shows the number of patients receiving CPR of each duration. B, Cause of death in the UMN-ECPR cohort related to duration of CPR. Data are shown as percent of the overall patient cohort. CCL indicates cardiac catheterization laboratory; and CPC, Cerebral Performance Category.
Patient and OHCA Characteristics
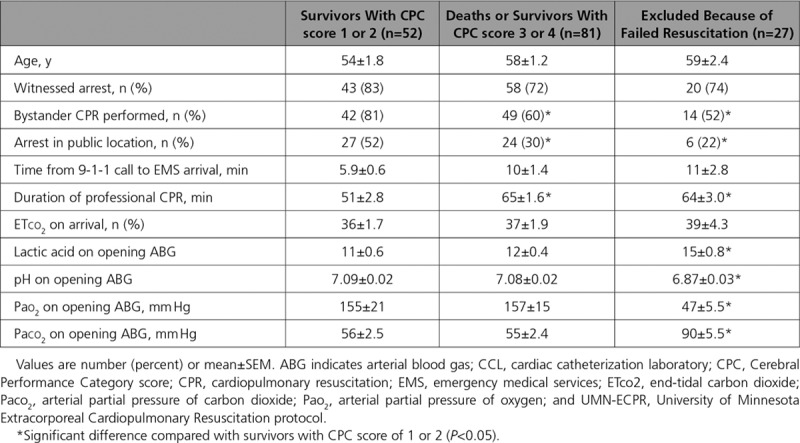
Neurologically favorable survival (CPC score 1–2) in UMN-ECPR patients was not significantly related to age, likelihood of witnessed OHCA, time from 9-1-1 call to EMS arrival, or end-tidal CO2 (Table 2). Patients with neurologically favorable survival were more likely to have received bystander CPR and to have the OHCA in a public location compared with patients who died or survived with a CPC score of 3 to 4 (P=0.024 and P=0.017, respectively) or patients who failed to meet resuscitation criteria (P=0.015 and P=0.022, respectively). Neurologically favorable survivors also had shorter duration of professional CPR compared with patients who died or survived with a CPC score of 3 to 4 (P<0.001) or patients who failed to meet resuscitation criteria (P=0.003). Neurologically favorable survival was associated with significantly lower lactic acid (P<0.001), higher pH (P<0.001), higher arterial partial pressure of oxygen (Pao2; P<0.001), and lower arterial partial pressure of carbon dioxide (Paco2; P=0.004) values on arrival to the CCL compared with patients who failed to meet resuscitation criteria. Although low Pao2 was a criterion for discontinuing resuscitation efforts, the additional abnormality in Paco2 demonstrated the lack of oxygenation and ventilation in these patients, suggesting airway management failure during prolonged resuscitation.
Table 2.
UMN-ECPR Cohort: Arrest Characteristics and Laboratory Results on Arrival to the CCL in Relation to Patient Outcome
CPR Duration
In patients with ≥20 minutes of CPR, neurologically favorable survival was significantly higher (P<0.001) in UMN-ECPR patients compared with ALPS patients at all CPR durations <60 minutes, with a rate of 100% (8 of 8) for UMN-ECPR versus 24% (24 of 102) for ALPS patients receiving 20 to 29 minutes of CPR (Figure 2A). Neurologically favorable survival declined by 1.9%/min in ALPS patients and 2.5%/min up to 60 minutes in UMN-ECPR patients. Of the 34% (218 of 636) of ALPS patients who underwent CPR for ≥40 minutes, none survived with neurologically favorable status, whereas neurologically favorable survival was 25% (9 of 36) in UMN-ECPR patients with 50 to 59 minutes of CPR and 19% with ≥60 minutes. The relative risk reduction for death or poor neurological function was 29% (95% CI, 18–41; P<0.001) for patients receiving between 20 and 59 minutes of CPR and 19% (95% CI, 10–27; P<0.001) for patients receiving ≥60 minutes of CPR.
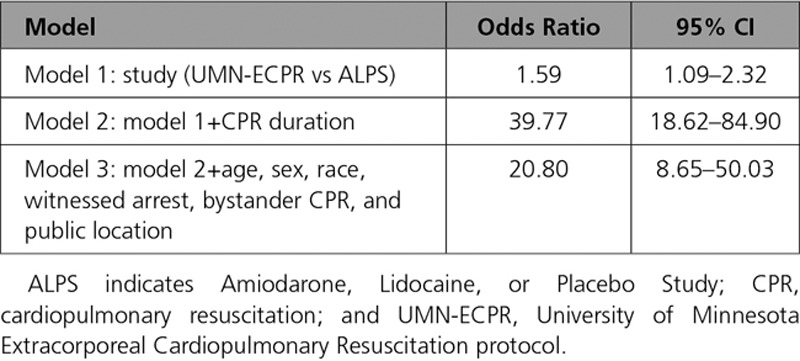
Multivariable regression incorporating all available patient and arrest characteristics (ie, age, sex, race, witnessed arrest, bystander CPR, and public location) confirmed increased neurologically favorable survival in UMN-ECPR patients at all CPR durations <60 minutes. Logistic regression using models that included adjustment for CPR duration alone or all patient and arrest characteristics plus CPR duration revealed significantly higher neurologically favorable survival in UMN-ECPR patients versus ALPS patients, with odds ratios of 39.77 (95% CI 18.62–84.90; P<0.001) and 20.80 (95% CI 8.65–50.03; P<0.001), respectively (Table 3).
Table 3.
Survival Adjusted for Patient and Arrest Characteristics
In the UMN-ECPR cohort, longer duration of CPR was associated with higher risk of brain death (23% [19 of 84] of patients with ≥60 minutes of CPR; P=0.004) and failure to achieve an organized cardiac rhythm in the CCL (11% [9 of 84] of patients with ≥60 minutes of CPR; P<0.001; Figure 2B). The risk of survival with a CPC score of 3 to 4 increased to 9% (3 of 35) at 60 to 69 minutes and then decreased with longer CPR duration. Death caused by anoxic brain injury and failure to meet resuscitation continuation criteria occurred sporadically without a clear trend.
Metabolic Changes During Prolonged Resuscitation
The metabolic changes during prolonged resuscitation observed in all 160 patients in the UMN-ECPR cohort are shown in Figure 3. Arterial pH decreased from 7.21±0.02 in patients with a CPR duration of 20 to 29 minutes to 6.93±0.05 in those with 90 to 99 minutes of CPR (P<0.001). Mean arterial lactic acid increased from 7.35±0.9 mmol/L in patients with 20 to 29 minutes of CPR to 14.1±1.6 mmol/L at 90 to 99 minutes (P<0.001). Mean Paco2 increased from 47±4.3 mm Hg at 20 to 29 minutes to 68±5.4 mm Hg at 90 to 99 minutes (P=0.042). The largest changes in arterial pH, arterial lactic acid, and Paco2 occurred between patients with 30 to 39 minutes and those with 40 to 49 minutes of CPR. No trend in Pao2 levels was observed in relation to duration of CPR.
Figure 3.
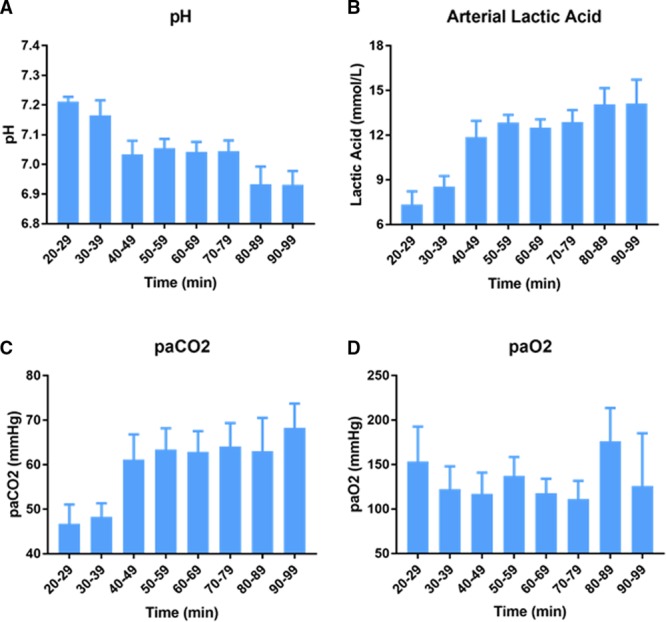
Metabolic effects of prolonged professional cardiopulmonary resuscitation (CPR). Arterial blood gas and arterial lactic acid levels in the University of Minnesota Extracorporeal Cardiopulmonary Resuscitation cohort before extracorporeal membrane oxygenation (n=160). A, pH, (B) arterial lactic acid, (C) arterial partial pressure of carbon dioxide (Paco2), and (D) arterial partial pressure of oxygen (Pao2) are shown. Results are mean±SEM.
Transthoracic echocardiography, performed in 121 patients admitted to the cardiac intensive care unit, showed significantly increased left ventricular lateral wall thickness with prolonged CPR (P=0.004; Figure 4A). Patients who died or survived with a CPC score of 3 to 4 had a significantly thicker left ventricular lateral wall compared with neurologically intact survivors (1.40±0.03 cm versus 1.27±0.04 cm; P=0.006; Figure 4B).
Figure 4.
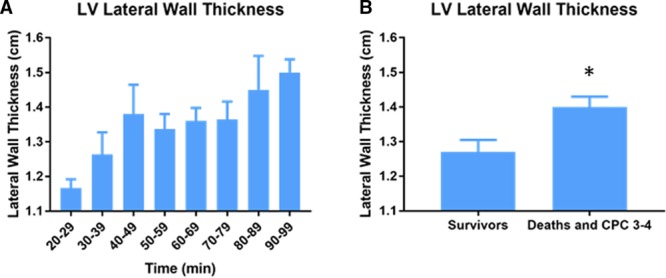
Association of left ventricular lateral wall thickness with professional cardiopulmonary resuscitation (CPR) duration and patient outcome. Patients were assessed by transthoracic echocardiography within 24 hours of admission to the cardiac intensive care unit (n=121). A, Duration of professional CPR in relation to thickness of the left ventricular (LV) lateral wall. B, LV lateral wall thickness in neurologically intact survivors vs patients who died or survived with Cerebral Performance Category (CPC) score of 3 to 4. Results are mean±SEM. *P<0.05.
Discussion
ECPR normalizes perfusion for patients with refractory VF/VT cardiac arrest independently of cardiac rhythm at the time of initiation. By stabilizing the hemodynamic state, ECPR halts the accumulation of ischemic injury without the need for ROSC, provides time to correct severe metabolic derangement developed during prolonged CPR, and allows the reversal of underlying pathogeneses that may be perpetuating the refractory VF. Provided together by the UMN-ECPR protocol, these stabilization capabilities are associated with improved neurologically favorable survival in patients with refractory cardiac arrest.12,21,22
The UMN-ECPR cohort demonstrated 100% neurologically favorable survival for patients receiving CPR for 20 to 29 minutes before the initiation of ECMO compared with 24% for standard CPR. In contrast to standard CPR alone, survival remained possible even after 98 minutes of CPR when ECPR was used. Nevertheless, the ischemic injury accrued before ECMO initiation is critical. UMN-ECPR patients incurred a 25% decline in neurologically favorable survival with every 10 minutes of delay beyond 29 minutes of CPR. Previous studies have also suggested an association between duration of CPR and survival with ECPR.26–28 Wengenmayer et al26 analyzed a mixed cohort of patients with in-hospital cardiac arrest and OHCA treated with ECPR resulting in 67% survival in patients with 6 to 20 minutes of CPR and 10% survival in patients receiving 45 to 60 minutes of CPR. Using this cohort, they developed a logarithmic probability model estimating a 4% to 6% decline in survival with every 10 minutes of delay between 22 and 64 minutes of CPR.
ECPR may enhance survival after prolonged standard CPR, but care must be taken to avoid harm to those who would have survived with standard CPR alone. Recent studies of patients with OHCA receiving standard CPR alone demonstrated that 99% of patients with neurologically favorable survival had achieved ROSC within a maximum of 28 to 39 minutes of professional CPR.15,17,18,29 The ALPS cohort in our study was consistent with these results. Most ECPR systems require that patients are transported to a hospital setting for ECMO insertion; however, transport may decrease the efficiency of resuscitation, potentially preventing survival in some patients. Using the PRIMED database (Prehospital Resuscitation Using an Impedance Valve and Early Versus Delayed Trial), Reynolds et al15,18 investigated the balance between advanced therapies and risk of transport in patients meeting ECPR criteria gathered from observational studies, including age of 18 to 65 years, witnessed cardiac arrest, CPR started within 10 minutes of EMS activation, and absence of asystole as the initial cardiac rhythm at the time of EMS arrival. They found that 90% of neurologically favorable survivors achieved ROSC within 21 minutes and that the likelihood of neurologically favorable survival with CPR duration beyond 20 minutes was 8.4%. They therefore proposed 21 minutes of standard resuscitation before transport for ECPR. The UMN-ECPR protocol asks paramedics to transport immediately on establishment of refractory cardiac arrest, typically resulting in 15 to 20 minutes of professional CPR before transport. To be eligible, all patients have to accommodate mechanical CPR with a Lund University Cardiac Arrest System so that high-quality CPR can be maintained during transport.
Despite the use of mechanical CPR before ECMO, UMN-ECPR patients who underwent CPR for ≥40 minutes demonstrated severe metabolic deterioration with decreasing arterial pH, increasing arterial lactic acid and Paco2 values, and increasing left ventricular wall thickening. At best, standard CPR provides 15% to 25% of normal cardiac output.30–32 Prolonged hypoperfusion results in increasing lactic acid levels and worsening acidemia with multiple cardiac effects, including cardiomyocyte hyperpolarization, decreased cardiac myofilament calcium sensitivity, decreased surface localization of adrenoreceptors, and prolongation and increased amplitude of cardiomyocyte calcium transients.33–35 Lactic acidemia also affects vascular smooth muscle cells, eventually leading to vasodilation and hypotension.36,37
Although the cause of the sudden worsening in metabolic deterioration at 40 minutes remains unclear, progressive decline in CPR efficiency may play a role. The reduced cardiac perfusion during CPR and the resulting cumulative ischemic injury to cardiomyocytes may perpetuate diastolic dysfunction, thereby reducing the efficiency of cardiac filling during CPR. The resulting decrease in systemic perfusion may then accelerate the worsening metabolic profile and cellular injury. The accelerated worsening after 40 minutes of CPR is consistent with previously published studies on the depletion of high-energy phosphates such as ATP in cardiomyocytes during times of ischemia.38,39 Loss of ATP induces progressive structural pathology and progression to diastolic contracture in severe cases.
Patients often have an uncontrolled airway for the first 10 to 15 minutes of OHCA, and aspiration is common.19 A mix of supraglottic airways and endotracheal intubation was used in the UMN-ECPR cohort. In 20 patients, Pao2 values were <50 mm Hg on arrival at the CCL, leading to cessation of further resuscitation. This was unrelated to duration of CPR. In contrast, Paco2 levels increased substantially after 40 minutes of CPR, likely contributing to the acidemia represented by the decline in pH and suggesting that standard airway management and ventilation strategies were insufficient for complete ventilation during prolonged CPR. The association between duration of CPR and brain death and failure to achieve an organized rhythm in the CCL further suggests that the metabolic derangement is progressive during the course of CPR. Lactic acid may simply be a marker of the injury experienced by all of the organ systems, or it may potentiate the dysfunction experienced during refractory cardiac arrest and during the immediate post-OHCA syndrome.
The time dependence of ECPR has important implications for the development of ECPR programs in the community. Our metabolic and outcome data indicate an optimal time to ECMO of ≈30 minutes for OHCA with current prehospital resuscitation techniques. However, the survival benefit of ECPR extended beyond 60 minutes in the UMN-ECPR cohort. Therefore, ECPR programs should aim to maximize the patient population reached within 30 minutes while not necessarily excluding patients with longer duration of resuscitation. The diagnosis of refractory VF typically requires 15 to 20 minutes of on-scene CPR, limiting the population who can be reached by a single ECPR center. The limited reach of any single ECPR center is illustrated by this UMN-ECPR cohort, with only 5% of patients reaching the University of Minnesota within 30 minutes of professional CPR, whereas 48% of patients reached the University of Minnesota within 60 minutes. Therefore decentralization of ECMO resources may be required with strategic allocation of resources throughout the community. These data do not evaluate the importance of colocalizing ECMO initiation capabilities and the critical care resources needed for recovery, leaving open the potential to centralize the resource-intense multidisciplinary postresuscitation care while reaching a larger population with decentralized ECMO cannulation resources.
Future optimization of prehospital care may also enhance ECPR-associated survival. Prehospital CPR strategies that enhance the perfusion achieved by CPR or reduce the metabolic demand of the patient may extend the effective professional CPR time, thereby delaying the onset of metabolic derangement. Prehospital initiation of ECPR may also provide a means for rapid stabilization. A recent analysis of the Sudden Death Expertise Center registry, including all patients >18 years of age with OHCA in Paris and the surrounding area, demonstrated that use of prehospital ECPR was associated with improved survival despite a lack of demonstrable survival benefit in the ECPR cohort overall.28 The benefit of prehospital ECPR is most likely related to the more rapid deployment of ECPR; however, other differences in procedural or patient characteristics cannot be excluded. The lack of an overall ECPR-associated survival benefit may be related to multiple factors, including patient selection (eg, all presenting rhythms were included with only 27% having shockable rhythms), severely prolonged CPR duration with 39% of ECPR patients receiving CPR for >90 minutes, reduced use of coronary angiography and percutaneous coronary intervention (56% and 30% of ECPR patients, respectively), and potential selection bias in a system in which both conventional CPR and ECPR options were available.
This study has several limitations. The UMN-ECPR cohort is derived from a single center. Although data were collected prospectively from consecutive patients, selection bias and local differences in treatment or patient population cannot be excluded. This was not a randomized study, and it is subject to the limitations of any observational study, including selection, information, and confounding bias. In addition, the observational nature of this study limits the ability to draw conclusions about the causal impact of ECPR in these patients. Because only patients with refractory VF were included in this study, its generalizability to nonshockable cardiac arrest rhythms is limited. Although this is the largest published refractory VF/VT study population in the United States, the sample size may not have the statistical power to detect intergroup differences. Comparisons between the UMN-ECPR and ALPS cohorts are also limited by the use of different outcome measures (CPC score in the UMN-ECPR cohort and mRS score in the ALPS cohort). However, previous studies have demonstrated a fair relationship between CPC score and mRS score in cardiac arrest cohorts.40,41
Conclusions
Compared with standard CPR alone, the University of Minnesota refractory VF/VT ECPR protocol for OHCA is associated with significantly increased neurologically favorable survival, even with prolonged CPR preceding the initiation of ECPR. However, the duration of CPR remains critical because survival in the ECPR group decreases rapidly with continued CPR. The cause of this decrease remains unclear, but the increased left ventricular wall thickening and worsening metabolic profile suggest that the incomplete perfusion provided by CPR leads to cumulative cellular injury. Therefore, to maximize survival, ECPR programs and their associated prehospital systems of care must minimize time to ECPR. Further investigation is needed to establish methods of minimizing metabolic deterioration in the prehospital setting, potentially by maintaining or improving CPR efficiency, improving perfusion, or decreasing metabolic demand.
Sources of Funding
None.
Disclosures
None.
Footnotes
Sources of Funding, see page 885
Guest Editor for this article was Greet Van den Berghe, MD, PhD.
References
- 1.Becker LB, Aufderheide TP, Graham R. Strategies to improve survival from cardiac arrest: a report from the Institute of Medicine. JAMA. 2015;314:223–224. doi: 10.1001/jama.2015.8454. doi: 10.1001/jama.2015.8454. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, Olinger ML, Holcomb RG, Tupper DE, Yannopoulos D, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aufderheide TP, Nichol G, Rea TD, Brown SP, Leroux BG, Pepe PE, Kudenchuk PJ, Christenson J, Daya MR, Dorian P, et al. Resuscitation Outcomes Consortium (ROC) Investigators. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med. 2011;365:798–806. doi: 10.1056/NEJMoa1010821. doi: 10.1056/NEJMoa1010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumas F, Bougouin W, Geri G, Lamhaut L, Rosencher J, Pene F, Chiche JD, Varenne O, Carli P, Jouven X, et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: insights from the PROCAT II registry. JACC Cardiovasc Interv. 2016;9:1011–1018. doi: 10.1016/j.jcin.2016.02.001. doi: 10.1016/j.jcin.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Spaite DW, Bobrow BJ, Stolz U, Berg RA, Sanders AB, Kern KB, Chikani V, Humble W, Mullins T, Stapczynski JS, et al. Arizona Cardiac Receiving Center Consortium. Statewide regionalization of postarrest care for out-of-hospital cardiac arrest: association with survival and neurologic outcome. Ann Emerg Med. 2014;64:496–506.e1. doi: 10.1016/j.annemergmed.2014.05.028. doi: 10.1016/j.annemergmed.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Rea TD, Cook AJ, Stiell IG, Powell J, Bigham B, Callaway CW, Chugh S, Aufderheide TP, Morrison L, Terndrup TE, et al. Resuscitation Outcomes Consortium Investigators. Predicting survival after out-of-hospital cardiac arrest: role of the Utstein data elements. Ann Emerg Med. 2010;55:249–257. doi: 10.1016/j.annemergmed.2009.09.018. doi: 10.1016/j.annemergmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Cheskes S, Schmicker RH, Rea T, Powell J, Drennan IR, Kudenchuk P, Vaillancourt C, Conway W, Stiell I, Stub D, et al. Resuscitation Outcomes Consortium Investigators. Chest compression fraction: a time dependent variable of survival in shockable out-of-hospital cardiac arrest. Resuscitation. 2015;97:129–135. doi: 10.1016/j.resuscitation.2015.07.003. doi: 10.1016/j.resuscitation.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Stiell IG, Nichol G, Leroux BG, Rea TD, Ornato JP, Powell J, Christenson J, Callaway CW, Kudenchuk PJ, Aufderheide TP, et al. ROC Investigators. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med. 2011;365:787–797. doi: 10.1056/NEJMoa1010076. doi: 10.1056/NEJMoa1010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yannopoulos D, Bartos JA, Aufderheide TP, Callaway CW, Deo R, Garcia S, Halperin HR, Kern KB, Kudenchuk PJ, Neumar RW, et al. American Heart Association Emergency Cardiovascular Care Committee. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139:e530–e552. doi: 10.1161/CIR.0000000000000630. doi: 10.1161/CIR.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 11.Daya MR, Schmicker RH, Zive DM, Rea TD, Nichol G, Buick JE, Brooks S, Christenson J, MacPhee R, Craig A, et al. Resuscitation Outcomes Consortium Investigators. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC). Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yannopoulos D, Bartos JA, Martin C, Raveendran G, Missov E, Conterato M, Frascone RJ, Trembley A, Sipprell K, John R, et al. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5:e003732. doi: 10.1161/JAHA.116.003732. doi: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, et al. Resuscitation Outcomes Consortium Investigators. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 14.Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, John R, Connett J, Benditt DG, Lurie KG, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. doi: 10.1016/j.jacc.2017.06.059. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JC, Grunau BE, Rittenberger JC, Sawyer KN, Kurz MC, Callaway CW. Association between duration of resuscitation and favorable outcome after out-of-hospital cardiac arrest: implications for prolonging or terminating resuscitation. Circulation. 2016;134:2084–2094. doi: 10.1161/CIRCULATIONAHA.116.023309. doi: 10.1161/CIRCULATIONAHA.116.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunau B, Reynolds JC, Scheuermeyer FX, Stenstrom R, Pennington S, Cheung C, Li J, Habibi M, Ramanathan K, Barbic D, et al. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: informing minimum durations of resuscitation. Resuscitation. 2016;101:50–56. doi: 10.1016/j.resuscitation.2016.01.021. doi: 10.1016/j.resuscitation.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Grunau B, Puyat J, Wong H, Scheuermeyer FX, Reynolds JC, Kawano T, Singer J, Dick W, Christenson J. Gains of continuing resuscitation in refractory out-of-hospital cardiac arrest: a model-based analysis to identify deaths due to intra-arrest prognostication. Prehosp Emerg Care. 2018;22:198–207. doi: 10.1080/10903127.2017.1356412. doi: 10.1080/10903127.2017.1356412. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds JC, Grunau BE, Elmer J, Rittenberger JC, Sawyer KN, Kurz MC, Singer B, Proudfoot A, Callaway CW. Prevalence, natural history, and time-dependent outcomes of a multi-center North American cohort of out-of-hospital cardiac arrest extracorporeal CPR candidates. Resuscitation. 2017;117:24–31. doi: 10.1016/j.resuscitation.2017.05.024. doi: 10.1016/j.resuscitation.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Bartos JA, Carlson K, Carlson C, Raveendran G, John R, Aufderheide TP, Yannopoulos D. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. doi: 10.1016/j.resuscitation.2018.08.030. doi: 10.1016/j.resuscitation.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Kim HJ, Lee HY, Ahn HS, Lee SW. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: a meta-analysis. Resuscitation. 2016;103:106–116. doi: 10.1016/j.resuscitation.2016.01.019. doi: 10.1016/j.resuscitation.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Lamhaut L, Hutin A, Puymirat E, Jouan J, Raphalen JH, Jouffroy R, Jaffry M, Dagron C, An K, Dumas F, et al. A pre-hospital extracorporeal cardio pulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi: 10.1016/j.resuscitation.2017.04.014. doi: 10.1016/j.resuscitation.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Stub D, Bernard S, Pellegrino V, Smith K, Walker T, Sheldrake J, Hockings L, Shaw J, Duffy SJ, Burrell A, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Grunau B, Taylor J, Scheuermeyer FX, Stenstrom R, Dick W, Kawano T, Barbic D, Drennan I, Christenson J. External validation of the universal termination of resuscitation rule for out-of-hospital cardiac arrest in British Columbia. Ann Emerg Med. 2017;70:374–381.e1. doi: 10.1016/j.annemergmed.2017.01.030. doi: 10.1016/j.annemergmed.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Cooley DA, Reul GJ, Wukasch DC. Ischemic contracture of the heart: “stone heart.”. Am J Cardiol. 1972;29:575–577. doi: 10.1016/0002-9149(72)90454-7. doi: 10.1016/0002-9149(72)90454-7. [DOI] [PubMed] [Google Scholar]
- 25.Sorrell VL, Paleru V, Altbach MI, Hilwig RW, Kern KB, Gaballa M, Ewy GA, Berg RA. Mild hypothermia delays the development of stone heart from untreated sustained ventricular fibrillation: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2011;13:17. doi: 10.1186/1532-429X-13-17. doi: 10.1186/1532-429X-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wengenmayer T, Rombach S, Ramshorn F, Biever P, Bode C, Duerschmied D, Staudacher DL. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21:157. doi: 10.1186/s13054-017-1744-8. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura K, Sera A, Inoue I, et al. Should we emergently revascularize occluded coronaries for cardiac arrest?: rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–1613. doi: 10.1161/CIRCULATIONAHA.111.067538. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- 28.Bougouin W, Dumas F, Lamhaut L, Marijon E, Carli P, Combes A, Pirracchio R, Aissaoui N, Karam N, Deye N, et al. Sudden Death Expertise Center Investigators. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. 2019;0:1–11. doi: 10.1093/eurheartj/ehz753. doi: 10.1093/eurheartj/ehz753. [DOI] [PubMed] [Google Scholar]
- 29.Nagao K, Nonogi H, Yonemoto N, Gaieski DF, Ito N, Takayama M, Shirai S, Furuya S, Tani S, Kimura T, et al. Japanese Circulation Society With Resuscitation Science Study (JCS-ReSS) Group. Duration of prehospital resuscitation efforts after out-of-hospital cardiac arrest. Circulation. 2016;133:1386–1396. doi: 10.1161/CIRCULATIONAHA.115.018788. doi: 10.1161/CIRCULATIONAHA.115.018788. [DOI] [PubMed] [Google Scholar]
- 30.Duggal C, Weil MH, Gazmuri RJ, Tang W, Sun S, O’Connell F, Ali M. Regional blood flow during closed-chest cardiac resuscitation in rats. J Appl Physiol (1985) 1993;74:147–152. doi: 10.1152/jappl.1993.74.1.147. doi: 10.1152/jappl.1993.74.1.147. [DOI] [PubMed] [Google Scholar]
- 31.Lurie K, Voelckel W, Plaisance P, Zielinski T, McKnite S, Kor D, Sugiyama A, Sukhum P. Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: a progress report. Resuscitation. 2000;44:219–230. doi: 10.1016/s0300-9572(00)00160-x. doi: 10.1016/s0300-9572(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 32.Lurie KG, Mulligan KA, McKnite S, Detloff B, Lindstrom P, Lindner KH. Optimizing standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Chest. 1998;113:1084–1090. doi: 10.1378/chest.113.4.1084. doi: 10.1378/chest.113.4.1084. [DOI] [PubMed] [Google Scholar]
- 33.Toller W, Wölkart G, Stranz C, Metzler H, Brunner F. Contractile action of levosimendan and epinephrine during acidosis. Eur J Pharmacol. 2005;507:199–209. doi: 10.1016/j.ejphar.2004.11.049. doi: 10.1016/j.ejphar.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 34.Marsh JD, Margolis TI, Kim D. Mechanism of diminished contractile response to catecholamines during acidosis. Am J Physiol. 1988;254(pt 2):H20–H27. doi: 10.1152/ajpheart.1988.254.1.H20. doi: 10.1152/ajpheart.1988.254.1.H20. [DOI] [PubMed] [Google Scholar]
- 35.Schotola H, Toischer K, Popov AF, Renner A, Schmitto JD, Gummert J, Quintel M, Bauer M, Maier LS, Sossalla S. Mild metabolic acidosis impairs the beta-adrenergic response in isolated human failing myocardium. Crit Care. 2012;16:R153. doi: 10.1186/cc11468. doi: 10.1186/cc11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, Perez P, Meziani F. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 2010;36:2019–2029. doi: 10.1007/s00134-010-2045-8. doi: 10.1007/s00134-010-2045-8. [DOI] [PubMed] [Google Scholar]
- 37.Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, Symons JD, Richardson RS. α1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Exp Physiol. 2013;98:256–267. doi: 10.1113/expphysiol.2012.066613. doi: 10.1113/expphysiol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumar RW, Brown CG, Robitaille PM, Altschuld RA. Myocardial high energy phosphate metabolism during ventricular fibrillation with total circulatory arrest. Resuscitation. 1990;19:199–226. doi: 10.1016/0300-9572(90)90103-l. doi: 10.1016/0300-9572(90)90103-l. [DOI] [PubMed] [Google Scholar]
- 39.Neumar RW, Brown CG, Van Ligten P, Hoekstra J, Altschuld RA, Baker P. Estimation of myocardial ischemic injury during ventricular fibrillation with total circulatory arrest using high-energy phosphates and lactate as metabolic markers. Ann Emerg Med. 1991;20:222–229. doi: 10.1016/s0196-0644(05)80927-8. doi: 10.1016/s0196-0644(05)80927-8. [DOI] [PubMed] [Google Scholar]
- 40.Raina KD, Callaway C, Rittenberger JC, Holm MB. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation. 2008;79:249–256. doi: 10.1016/j.resuscitation.2008.06.005. doi: 10.1016/j.resuscitation.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82:1036–1040. doi: 10.1016/j.resuscitation.2011.03.034. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


