Supplemental Digital Content is available in the text
Keywords: Biomarker, meta-analysis, sepsis, soluble urokinase-type plasminogen activator receptor (suPAR), systematic review
Abbreviations: APACHE II, the Acute Physiology and Chronic Health Evaluation II, AUC, the area under the receiver-operating characteristic curve, CIs, confidence intervals, CRP, C-reactive protein, DOR, diagnostic odds ratio, EWS, early warning score, IL, interleukin, LBP, lipopolysaccharide-binding protein, NLR, negative likelihood ratio, PCT, procalcitonin, PLR, positive likelihood ratio, PRISMA, the preferred reporting items for systematic reviews and meta-analyses, SIRS, systemic inflammatory response syndrome, SROC, summary receiver-operating characteristic, suPAR, soluble urokinase-type plasminogen activator receptor, WOS, web of science
ABSTRACT
Background:
Soluble urokinase-type plasminogen activator receptor (suPAR) has the potential to diagnose infectious diseases. Due to the lack of reliable biomarkers and the importance of timely diagnosis for sepsis treatment, we conducted this systematic review and meta-analysis to evaluate the value of suPAR diagnosis and prognosis for sepsis.
Methods:
PubMed, Embase, Web of Science, and Cochrane Library databases were searched for studies, which reported the value of suPAR diagnosis and/or prognosis in patients with sepsis.
Results:
A total of 30 studies involving 6,906 patients were included. Sensitivity and specificity of suPAR for diagnosing sepsis were 0.76 [95% confidence interval (CI), 0.63–0.86] and 0.78 (95% CI, 0.72–0.83), respectively. The area under the summary receiver-operating characteristic curve (AUC) was 0.83 (95% CI, 0.80–0.86). Pooled sensitivity and specificity for predicting mortality were 0.74 (95% CI, 0.67–0.80) and 0.70 (95% CI, 0.63–0.76), respectively, with AUC of 0.78 (95% CI, 0.74–0.82). In addition, AUC for differentiating sepsis from systemic inflammatory response syndrome (SIRS) was 0.81 (95% CI, 0.77–0.84), and the sensitivity and specificity were 0.67 (95% CI, 0.58–0.76) and 0.82 (95% CI, 0.73–0.88), respectively.
Conclusion:
suPAR is a feasible biomarker for timely diagnosis and prognosis of sepsis. Compared with effective value of procalcitonin (PCT) identified by previous meta-analysis, suPAR has similar clinical guiding value, whereas suPAR exhibits higher specificity, which can facilitate the deficiencies of PCT. suPAR also shows a diagnostic value in differentiating sepsis from SIRS. Considering the lack of biomarkers for sepsis and the similar clinical value of suPAR and PCT, suPAR should be considered as a biomarker in clinical practice for sepsis.
INTRODUCTION
Soluble urokinase-type plasminogen activator receptor (suPAR) is a soluble form of uPAR which is a membrane-bound receptor for uPA. SuPAR is widely found in body fluids, including plasma, urine, blood, serum, and cerebrospinal fluid. The concentration of suPAR is positively correlated with immune system activity and is a biomarker to indicate the severity and deterioration of the disease, such as arthritis, liver fibrosis, malaria, and bacterial infection (1–5). The concentration is relatively stable under physiological conditions (6, 7). However, when the body is in the state of inflammation or other diseases, the immune system is activated and suPAR level is upregulated, which will greatly increase the content of suPAR in serum (1). Therefore, the level of suPAR has the potential to guide the diagnosis and prognosis of sepsis.
According to the guidelines for sepsis 3.0, sepsis is a disorder of the host's response to infection, resulting in life-threatening organ dysfunction (8). It needs to be differentiated from other infections or systemic inflammation such as bacteremia and systemic inflammatory response syndrome (SIRS). Timely diagnosis and accurate treatment are essential to reduce the mortality of sepsis (9). Unfortunately, the current clinical diagnosis of sepsis remains a challenge. Biomarkers are a commonly used diagnostic tool for diseases, with the advantages of fully automatic identification, short turnaround time, and low production costs (1). However, for patients with sepsis, biomarkers that contribute to decision-making are still insufficient. The guidelines of the Surviving Sepsis Campaign (SSC) updated in 2018 only recommend procalcitonin (PCT) as a biomarker for sepsis (10). There is an urgent need for accurate and effective early biomarkers.
Systematic reviews published to date indicate that suPAR has a moderate diagnostic value for bacterial infection or systemic inflammation (11, 12). Backes et al. suggested that suPAR is not specific enough to diagnosing infections, whereas their review included only a limited number of studies (11). However, a large number of subsequent original studies have shown that suPAR has a reliable diagnostic and prognostic value for patients with sepsis. It is still unclear whether suPAR can be an effective biomarker for the diagnosis and prognosis of sepsis. This study was designed to evaluate the timely diagnosis and prognosis efficacy of suPAR in patients with sepsis, to assess the potential of assisting clinical decision-making, and to provide a reference for the clinical application of new biomarkers for sepsis.
MATERIALS AND METHODS
All methods followed the PRISMA guidelines for conducting this systematic review and meta-analysis (13, 14).
Data sources and searches
The review authors searched for medical literature before March 2019. The research has been conducted in electronic databases such as the Cochrane Library, PubMed, Excerpt Medica Database (Embase), Web of Science (WOS), and in the reference lists from review articles, irrespective of publication date, status, or language. The search was conducted with the following keywords: suPAR and sepsis or septicemia or septicemia or septic shock. Search strategies in Cochrane Library, PubMed, Embase, and WOS can be found in the Supplement, Supplemental Digital Content.
The present meta-analysis includes studies that meet the following criteria:
-
1.
Adult patients with confirmed or suspected sepsis (over 18 years of age). Hospitalization in intensive care unit or emergency department.
-
2.
The studies included the results of suPAR diagnosis and/or prognosis in patients with sepsis. And the selected studies were able to extract information from the 2 × 2 contingency table. Sepsis or infection was diagnosed by the latest reference standard in the original study, such as sepsis3.0, sepsis2.0, and microbiological testing.
-
3.
No publication date, status, or language restrictions were applied. Clinical original articles were included, whereas the secondary study, conference abstracts, editorials, and animal experiments were excluded.
Study selection
Two review authors (QH, HX) independently assessed the studies to be included based on the titles, abstracts, and keywords. If a study was found relevant to our topic, at least two reviewers will further evaluate the full text to determine whether it meets the inclusion criteria or not. In case of inconsistencies between the reviewers, the third reviewer (JL) will be consulted. To further ensure the eligibility of a study, the authors have been consulted when additional information is needed in the study, for example, the details of results and methods or allocation concealment. A study diagram was prepared for this purpose to demonstrate the entire process of literature research and the selection of studies.
Data extraction and quality assessment
The data were extracted by two review authors independently (TS, PY) and the resulting differences were resolved by the third reviewer (KY). The extracted data included lead author, publication year, country of origin, and participant characteristics (age, sex, and hospitalization, prevalence, mortality rate, and the measured time of suPAR levels). The optimal cutoff threshold, values for sensitivity, specificity, true-positive, true-negative, false-positive, and false-negative, and the area under the receiver-operating characteristic (ROC) curve (AUC). Where data were missing, a letter will be written to the authors to request for the data. If the letter brings about no response after 4 weeks, an e-mail will be sent. Still no response, we will use estimates based on available data. The outcomes which cannot be pooled or analyzed are described in the literature.
Two review authors (J Liu, J Lu) evaluated each involved study independently; they applied the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (15). The quality and bias of the included studies were assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) (16) by two independent authors (J Liu, J Lu). In case of any inconsistencies, agreement has been reached through discussion among all the authors. The quality has been assessed from two perspectives, including risk of bias and applicability concerns. The summary charts have been made to show the assessment of the risk of bias (Fig. S1, Supplemental Digital Content).
Data synthesis and analysis
This study used StataSE14.0 (StataCorp; College Station, Tex) to analyze the extracted data. The Spearman correlation coefficient was used to evaluate the threshold effect of suPAR diagnosis and prognostic accuracy. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated. The accuracy of the diagnostic and prognostic effects was evaluated by constructing a summary receiver-operating characteristic (SROC) curve. AUC reflects the accuracy of diagnostic experiments, 0.5 to 0.7 indicates low accuracy, 0.7 to 0.9 indicates moderate accuracy, and >0.9 indicates high accuracy. Heterogeneity was assessed by Q test (significant heterogeneity if P < 0.05) and I2 test (significant heterogeneity if I2 > 50%). If substantive heterogeneity (I2 > 50%) exists, univariate meta-regression and subgroup analysis are performed to analyze the sources of heterogeneity. The tested items of meta-regression analysis were from the baseline characteristics table. The median cutoff values of the included studies were calculated, and the studies were divided into high cutoff point subgroup (cutoff values ≥ the median) and low cutoff point subgroup (cutoff values < the median). Based on the results, forest plots were made to demonstrate the cumulative effect of suPAR. Deek's funnel plot was used to assess the publication bias. The α value was set at 0.05.
RESULTS
A flow chart of the study selection process (Fig. 1) was prepared according to the PRISMA guidelines (17). After reviewing the title and abstract, 111 articles were screened for full-text review. Of these, 81 articles failed to meet the inclusion criteria. Only 30 studies fulfilled all criteria. Among them, Georgescu et al. (18, 19) and Kofoed et al. (20, 21) produced two articles from two different studies, respectively. In summary, 30 studies involving 6,906 patients were included in our systematic review and meta-analysis.
Fig. 1.
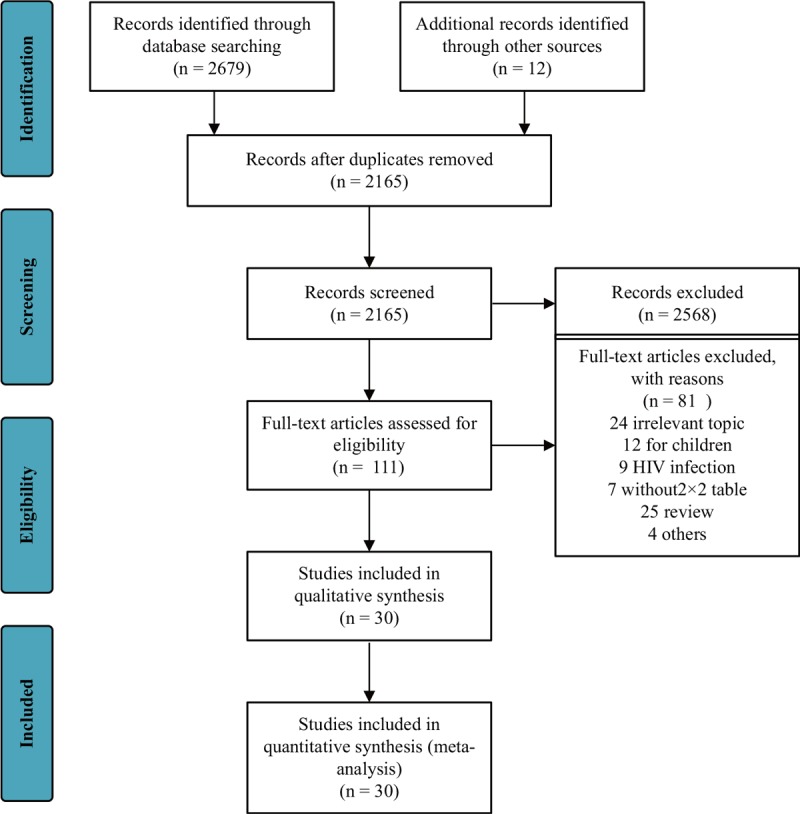
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram and exclusion criteria.
The baseline characteristics of the 30 identified studies (18–47) are summarized, as shown in Table 1. Of the included studies (18, 21–23, 26, 27, 30, 31, 35, 37–40, 42, 44, 45, 47), 17 studies including 2,722 patients reported the results of suPAR in the diagnosis of sepsis, and 19 studies including 5,622 patients reported the usefulness of suPAR for predicting poor outcomes in sepsis (19, 20, 22, 24, 25, 27–30, 32–34, 36, 38, 39, 41, 43, 44, 46). Of these, there were eight studies reported the diagnostic value of suPAR in bacterial sepsis (18, 21, 23, 27, 31, 35, 40, 42), and eight reported the results about the value of suPAR predicting mortality from bacterial sepsis (19, 20, 25, 27, 32–34, 41). In addition, five studies investigated the effect of suPAR in differentiating sepsis from SIRS (22, 31, 42, 45, 47). Most of the included studies tested suPAR levels at admission or within 24 h after admission, thus the test results can reflect the timely diagnostic and prognosis value of suPAR. All included studies used an enzyme-linked immunosorbent assay kit (most of them used ViroGates, Birkerød, Denmark) to test the level of suPAR. The optimal cutoff threshold of each study was retrospectively determined based on the ROC curve. The median cutoff value for suPAR in the included studies where it was used for the diagnosis of sepsis was 7.5 ng/mL (range: 2.7–12.0 ng/mL), and the median cutoff value where it was used for mortality prediction was 9.6 ng/mL (range: 6.3–14.3 ng/mL).
Table 1.
Characteristics of included studies
| Study type | Author | Year | Country | Study design | Clinical setting | Reference standard | Sample size | Age, y | Morbidity, % | Cutoff1, ng/mL | Mortality, % | Cutoff2, ng/mL | Tested sample | Measured time | Type of assay kit |
| P | Agustín Julián-Jiménez | 2019 | Spain | MPR | ED | Sepsis-3 | 136 | 84.5 | NA | NA | 9.5 | 7.1 | Plasma | D0 | ViroGates |
| D | Jing-jing Zhao | 2018 | China | PR | ICU | Sepsis: International Guidelines for Management of Severe Sepsis and Septic Shock; SIRS: the 1991 ACCP/SCCM | 88 | 62.5 | 63.0* | 5.5 | NA | NA | Serum | D0 | ViroGates |
| 50.0† | 8.4 | ||||||||||||||
| D | L Lazaridis | 2018 | Greece | PR | ICU | Sepsis-3 | 100 | 61.3 | 60.0 | 12.0 | NA | NA | Serum | D1 | ViroGates |
| P | Xiaoling Wu | 2017 | China | PR | ICU | Berlin definition | 162 | 57.2 | NA | NA | 30.4 | 14.3 | Plasma | D0 | ViroGates |
| D&P | Mian Zeng | 2016 | China | PR | ICU | Sepsis: International Guidelines for Management of Severe Sepsis and Septic Shock; SIRS: the 1991 ACCP/SCCM | 126 | 59.0 | 73.9 | 9.5 | 37.8 | 12.0 | Plasma | D1 | USCN Life Science |
| D | Xuan Liu | 2016 | China | PR | ICU | SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions | 137 | 69.5 | NA | NA | 17.1 | 10.8 | Plasma | D1 | ViroGates |
| D&P | Walaa S. Khater | 2016 | Egypt | PR | ICU | 2001 International Sepsis Definitions Conference criteria | 80 | 68.9 | 50.0 | 4.4 | 60.0 | 6.3 | Serum | D1 | Quantikine |
| P | Panagiotis Tsirigotis | 2016 | Greece | PR | ICU | SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions | 105 | 66.6 | NA | NA | 36.2 | 7.6 | Serum | D1 | ViroGates |
| D | Mitra Barati | 2015 | Iran | PR | ICU | International guidelines Based on the American College of Chest Physicians/ Society of Critical Care Medicine (ACCP/SCCM) Sepsis Directory | 107 | 62.4 | 48.2‡ | 8.5 | NA | NA | Plasma | D1 | USCN Life Science |
| 62.5§ | 9.5 | ||||||||||||||
| P | Anca Meda Georgescu1 | 2015 | Romania | PR | ICU | The 1991 ACCP/SCCM | 67 | NA | NA | NA | 71.6 | 10.6 | Plasma | D1 | ViroGates |
| D&P | Anca Meda Georgescu2 | 2015 | Romania | PR | ICU | Blood cultures | 49 | 71.0 | 28.6 | 9.9 | 77.6 | 11.5 | Serum | D0 | ViroGates |
| D | Anne J. M. Loonen | 2014 | Netherlands | RR | ED | Blood cultures | 125 | 62.2 | 16.0 | 7.5 | NA | NA | Serum | NA | ViroGates |
| D | M. Reichsoellner | 2014 | Austria | PR | ED | Blood cultures | 159 | 65.9 | 69.2 | 7.6 | NA | NA | Plasma | D0 | ViroGates |
| D | Matti Vänsk | 2014 | Finland | PR | NA | Guidelines of the American College of Chest Physicians/Society of Critical Care Medicine | 99 | 56.0 | 21.2 | 4.0 | NA | NA | Plasma | D0 | ViroGates |
| P | R. B. Raggam | 2014 | Austria | PR | ED et al. | Blood cultures | 902 | 63.0 | NA | NA | 13.0 | 9.2 | Plasma | D0 | ViroGates |
| D&P | Katia Donadello | 2014 | Belgium | PR | ICU | the criteria proposed by the International Sepsis Forum | 258 | 62.0 | 36.4 | 5.6 | 18.1 | 10.2 | Serum | D0 | ViroGates |
| D | Martin Hoenigl | 2013 | Austria | PR | ED | Blood cultures | 132 | 67.3 | 41.7 | 7.9 | NA | NA | Serum | D0 | ViroGates |
| P | B. Suberviola | 2013 | Spain | PR | ICU | 2001 International Sepsis Definitions Conference criteria | 137 | 62.6 | NA | NA | 29.9 | 9.6 | Serum | D0 | ViroGates |
| D | Selcuk Kaya | 2013 | Turkey | PR | NA | Blood cultures | 118 | 46.8 | 45 | 5.9 | NA | NA | Serum | D1 | ViroGates |
| P | Evangelos J Giamarellos-Bourboulis | 2012 | Greece | MPR | ICU et al. | 2001 International Sepsis Definitions Conference criteria | 1914 | 66.1 | NA | NA | 21.9 | 12.0 | Serum | D1 | ViroGates |
| D&P | R. Uusitalo-Seppala | 2012 | Finland | PR | ED | Blood cultures | 539 | 61.0 | 9.1 | 6.6 | 6.1 | 6.4 | Plasma | D0 | ViroGates |
| P | R. Huttunen | 2011 | Finland | PR | NA | Blood cultures | 132 | 62.0 | NA | NA | 13.6 | 11.0 | Plasma | D1 | ViroGates |
| D&P | Athina Savva | 2011 | Greece | MPR | ICU et al. | SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions | 180 | 58.4 | 73.9 | 10.5 | 27.8 | 12.9 | Serum | D1 | ViroGates |
| D | Gürdal Yilmaz | 2011 | Turkey | PR | NA | SIRS criteria defined in 1992 | 138 | 43.6 | 61.6 | 2.8 | NA | NA | Plasma | D0 | ViroGates |
| D&P | Alexander Koch | 2011 | Germany | PR | ICU | SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions | 273 | 64.0 | 72.2 | 8.0 | 35.9 | 8.0 | Serum | D0 | ViroGates |
| P | T. Mölkänen | 2011 | Finland | PR | NA | Blood cultures | 59 | NA | NA | NA | 32.2 | 9.3 | Serum | D2–5 | ViroGates |
| P | K. Kofoed | 2008 | Denmark | PR | ED et al. | SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions | 151 | 56.0 | NA | NA | 6.0 | 6.6 | Plasma | D0 | Luminex |
| D | K. Kofoed | 2007 | Denmark | PR | ED et al. | SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions | 151 | 56.0 | 63.6 | 2.7 | NA | NA | Plasma | D0 | Luminex |
| P | Holger Jon Møller | 2006 | Denmark | PR | NA | Blood cultures | 141 | 83.5 | NA | NA | 16.3 | 8.3 | Plasma | D0 | NA |
| P | P. Wittenhagen | 2004 | Denmark | MPR | NA | Blood cultures | 141 | 64.0 | NA | NA | 17.0 | 10.0 | Plasma | D1 | NA |
Three different sets of data in the Jing-jing Zhao's study: *represents for infection rate of sepsis; †represents the rate of sepsis from SIRS.
Two different sets of data in the Mitra Barati's study: ‡represents the rate of sepsis from SIRS; §represents infection rate of sepsis.
ARDS, acute respiratory distress syndrome; D&P, diagnosis and prognosis value; D, diagnosis value; ED, emergency department; ICU, intensive care units; MPR, multicenter prospective recruitment; NA, not available; P, prognosis value; PR, prospective recruitment; RR, retrospective recruitment; SIRS, systemic inflammatory response syndrome.
Cut-off1: the cutoff value of prevalence; Cutoff2: the cutoff value of mortality.
D0: the measured time of suPAR was at the time of admission; D1: the measured time of suPAR was within 24 h of admission; D2–5: the measured time of suPAR was within 2 to 5 days of admission.
Diagnostic value of suPAR for sepsis
The pooled sensitivity of suPAR in diagnosing sepsis was 0.76 [95% confidence interval (CI), 0.63–0.86; I2 = 93.71%, Q = 254.49 (P < 0.01)] and the specificity was 0.78 [95% CI, 0.72–0.83; I2 = 78.46%, Q = 74.29 (P < 0.01); Fig. 2]. The PLR and NLR were 3.5 (95% CI, 2.6–4.7) and 0.30 (95% CI, 0.18–0.50), respectively, and the DOR was 12 (95% CI, 6–24). The AUC was 0.83 (95% CI, 0.80–0.86; Fig. 3), indicating that suPAR has a moderate diagnostic accuracy in sepsis.
Fig. 2.
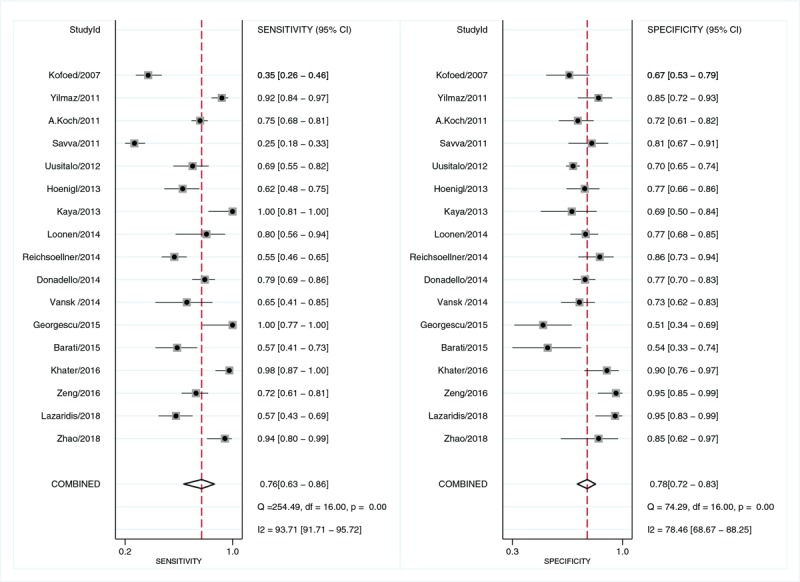
Forest plot of the sensitivity and specificity of suPAR for the diagnosis of sepsis.
Fig. 3.
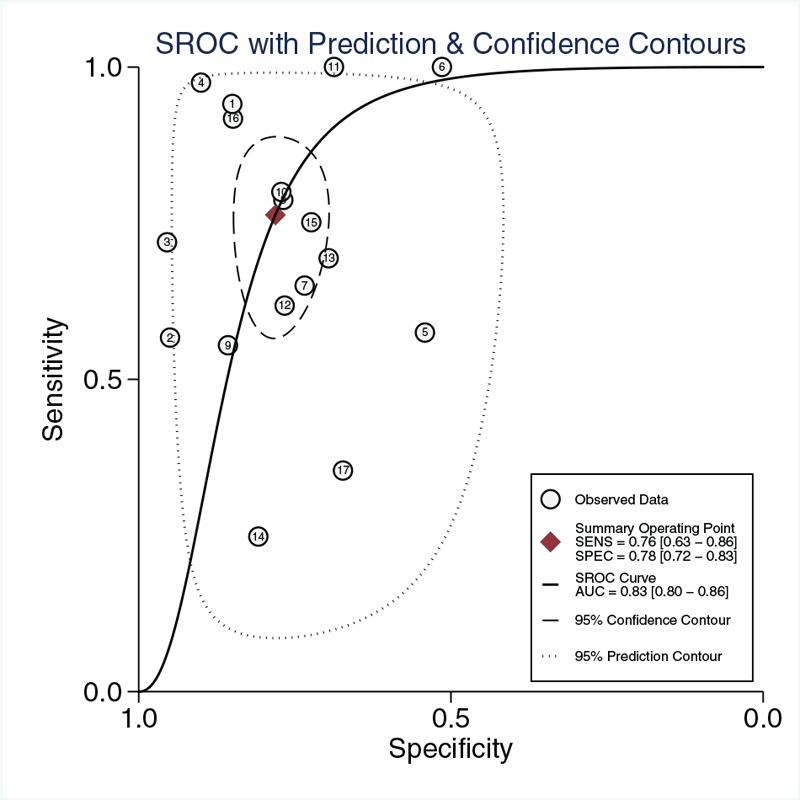
Summary receiver-operating characteristics curve for studies evaluating the diagnosis value of suPAR for sepsis.
As for publication bias, there was no significant difference detected by Deek's funnel plot (P = 0.20; Fig. S2, Supplemental Digital Content). In addition, there was no significant difference in threshold effects (Spearman correlation coefficient = 0.007; P = 0.98). Because of the non-negligible heterogeneity in the included studies, we used univariate meta-regression and subgroups to analyze sources of sensitivity and specific heterogeneity. The region, clinical setting, reference standard for diagnosis sepsis, tested sample, measured time, and type of assay kit for detecting the level of suPAR were used as covariates. Meta-regression analysis showed that the tested variables failed to explain the source of heterogeneity in sensitivity and specificity (Fig. S3 Supplemental Digital Content). Subgroup analysis is presented in Table 2.
Table 2.
Subgroup analysis of the diagnostic and prognostic value of suPAR based on different variables
| Study type | Variables | Studies, no. (patients, no.) | Sensitivity (95% CI) | Specificity (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
| Diagnostic value | Sepsis | 17 (2722) | 0.76 (0.63–0.86) | 0.78 (0.72–0.83) | 3.50 (2.60–4.70) | 0.30 (0.18–0.50) | 12 (6–24) | 0.83 (0.80–0.86) |
| Sepsis from SIRS | 5 (637) | 0.67 (0.58–0.76) | 0.82 (0.73–0.88) | 3.70 (2.40–5.80) | 0.40 (0.30–0.53) | 9 (5–18) | 0.81 (0.77–0.84) | |
| Bacterial Sepsis | 8 (1316) | 0.81 (0.57–0.94) | 0.73 (0.66–0.79) | 3.10 (2.30–4.00) | 0.25 (0.10–0.66) | 12 (4–38) | 0.79 (0.75–0.82) | |
| Cutoff <7.5 ng/mL | 8 (1471) | 0.85 (0.67–0.94) | 0.77 (0.70–0.82) | 3.60 (2.50–5.40) | 0.20 (0.08–0.50) | 18 (5–66) | 0.83 (0.80–0.86) | |
| Cutoff ≥7.5 ng/mL | 9 (1251) | 0.66 (0.50–0.78) | 0.79 (0.69–0.87) | 3.20 (2.10–4.90) | 0.43 (0.29–0.64) | 7 (4–15) | 0.80 (0.76–0.83) | |
| Prognostic value | Sepsis | 19 (5622) | 0.74 (0.67–0.80) | 0.70 (0.63–0.76) | 2.50 (2.00–3.00) | 0.38 (0.30–0.47) | 7 (5–9) | 0.78 (0.74–0.82) |
| Bacterial Sepsis | 8 (2114) | 0.68 (0.59–0.76) | 0.77 (0.66–0.85) | 2.90 (2.10–4.10) | 0.41 (0.34–0.51) | 7 (5–10) | 0.77 (0.73–0.81) | |
| Cutoff <9.6 ng/mL | 9 (2386) | 0.72 (0.66–0.77) | 0.65 (0.58–0.72) | 2.10 (1.60–2.60) | 0.43 (0.34–0.56) | 5 (3–8) | 0.74 (0.70–0.78) | |
| Cutoff ≥9.6 ng/mL | 10 (3236) | 0.74 (0.61–0.84) | 0.75 (0.64–0.83) | 3.00 (2.10–4.10) | 0.35 (0.24–0.51) | 9 (5–14) | 0.81 (0.77–0.84) |
AUC, area under the receiver-operating characteristic curve; CI, confidence interval; DOR, diagnostic odds ratio; NLR, negative likelihood ratio; PLR, positive likelihood ratio.
Prognostic value of suPAR for sepsis mortality
Twelve studies reported the results of suPAR used to predict mortality in sepsis patients. As shown in Figure 4, the pooled sensitivity of suPAR for predicting mortality of sepsis was 0.74 [95% CI, 0.67–0.80; I2 = 67.96%, Q = 56.18 (P < 0.01)] and the specificity was 0.70 [95% CI, 0.63–0.76; I2 = 88.73%, Q = 159.69 (P < 0.01)]. The PLR and NLR were 2.5 (95% CI, 2.0–3.0) and 0.38 (95% CI, 0.30–0.47), respectively, and the DOR was 7 (95% CI, 5–9). The AUC was 0.78 (95% CI, 0.74–0.82; Fig. 5), indicating that suPAR has moderate accuracy in predicting sepsis mortality.
Fig. 4.
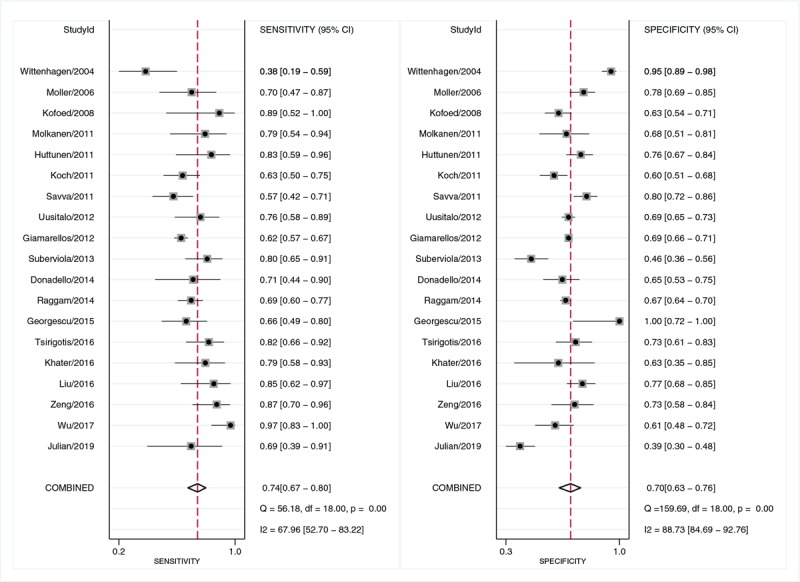
Forest plot of sensitivity and specificity of suPAR for the prediction of mortality in sepsis.
Fig. 5.
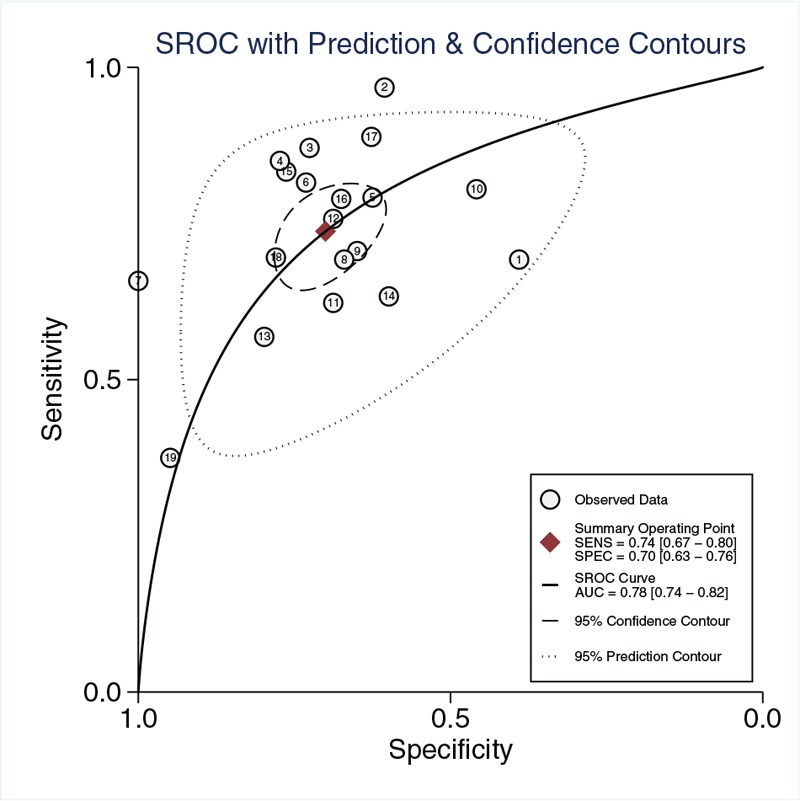
Summary receiver-operating characteristics curve for evaluating prediction value of mortality of suPAR in sepsis.
No significant threshold effects were found in these studies (Spearman correlation coefficient=0.257; P = 0.29). However, Deek's funnel plot showed a publication bias (P = 0.01; Figure S4, Supplemental Digital Content). In the meta-regression analysis, heterogeneity sources among the study were assessed by using the multicenter design, region, clinical setting, reference standard for diagnosis sepsis, tested sample, measured time, and type of assay kit. The findings showed that the multicenter design and region significantly accounted for the heterogeneity of sensitivity. As for the heterogeneity of specificity, clinical setting, reference standard for diagnosis sepsis, and the suPAR-level measured time showed significant statistical difference (Figure S5, Supplemental Digital Content). The subgroup analysis is presented in Table 2.
Subgroup analysis of the diagnostic and prognostic value of suPAR
As for the diagnostic value of suPAR in differentiating sepsis from SIRS, the pooled sensitivity and specificity were 0.67 [95% CI, 0.58–0.76; I2 = 68.45%, Q = 12.68 (P = 0.01)] and 0.82 [95% CI, 0.73–0.88; I2 = 61.65%, Q = 10.43 (P = 0.03)], respectively. The PLR and NLR were 3.7 (95% CI, 2.4–5.8) and 0.40 (95% CI, 0.30–0.53), respectively. The DOR was 9 (95% CI, 5–18), and the AUC was 0.81 (95% CI, 0.77–0.84).
The sensitivity and specificity for diagnosing bacterial sepsis were 0.81 [95% CI, 0.57–0.94; I2 = 92.44%, Q = 92.63 (P < 0.01)] and 0.73 [95% CI, 0.66–0.79; I2 = 72.00%, Q = 25.00 (P < 0.01)]. The PLR and NLR were 3.1 (95% CI, 2.3–4.0) and 0.25 (95% CI, 0.10–0.66), respectively. The DOR was 12 (95% CI, 4–38), and the AUC was 0.79 (95% CI, 0.75–0.82). The pooled sensitivity and specificity for predicting mortality of bacterial sepsis were 0.68 [95% CI, 0.59–0.76; I2 = 59.87%, Q = 17.44 (P = 0.01)] and 0.77 [95% CI, 0.66–0.85; I2 = 89.31%, Q = 65.47 (P < 0.01)], respectively. The PLR and NLR were 2.9 (95% CI, 2.1–4.1) and 0.41 (95% CI, 0.34–0.51), respectively, and the DOR was 7 (95% CI, 5–10). The AUC was 0.77 (95% CI, 0.73–0.81).
In the subgroup analysis of diagnostic studies based on cutoff value, the studies reported cutoff value <7.5 ng/mL showed the pooled sensitivity and specificity were 0.85 [95% CI, 0.67–0.94; I2 = 95.12%, Q = 143.57 (P < 0.01)] and 0.77 [95% CI, 0.70–0.82; I2 = 84.31%, Q = 51.14 (P < 0.01)], respectively. The PLR and NLR were 3.6 (95% CI, 2.5–5.4) and 0.20 (95% CI, 0.08–0.50), respectively, and the DOR was 18 (95% CI, 5–66). The AUC was 0.83 (95% CI, 0.80–0.86). As for the cutoff value ≥7.5 ng/ml, the pooled sensitivity and specificity were 0.66 [95% CI, 0.50–0.78; I2 = 92.42%, Q = 105.54 (P < 0.01)] and 0.79 [95% CI, 0.69–0.87; I2 = 79.87%, Q = 39.74 (P < 0.01)], respectively. The PLR and NLR were 3.2 (95% CI, 2.1–4.9) and 0.43 (95% CI, 0.29–0.64), respectively, and the DOR was 7 (95% CI, 4–15). The AUC was 0.80 (95% CI, 0.76–0.83).
In the subgroup analysis of prognostic studies based on cutoff value, the studies reported cutoff value<9.6 ng/mL showed the pooled sensitivity and specificity were 0.72 [95% CI, 0.66–0.77; I2 = 0.00%, Q = 7.07 (P = 0.53)] and 0.65 [95% CI, 0.58–0.72; I2 = 85.11%, Q = 53.74 (P < 0.01)], respectively. The PLR and NLR were 2.1 (95% CI, 1.6–2.6) and 0.43 (95% CI, 0.34–0.56), respectively, and the DOR was 5 (95% CI, 3–8). The AUC was 0.74 (95% CI, 0.70–0.78). As for the cutoff value ≥9.6 ng/ml, the pooled sensitivity and specificity were 0.74 [95% CI, 0.61–0.84; I2 = 82.06%, Q = 50.17 (P < 0.01)] and 0.72 [95% CI, 0.64–0.83; I2 = 91.43%, Q = 105.01 (P < 0.01)], respectively, The PLR and NLR were 3.0 (95% CI, 2.1–4.1) and 0.35 (95% CI, 0.24–0.51), respectively, and the DOR was 9 (95% CI, 5–14). The AUC was 0.81 (95% CI, 0.77–0.84).
DISCUSSION
This meta-analysis shows that suPAR has moderate diagnosis and prognosis value for sepsis. The pooled data of 17 studies, including 2,722 patients, showed that the AUC of suPAR in the diagnosis of sepsis was 0.83. A total of 19 studies, including 5,622 patients, showed the AUC of the prognosis value of suPAR was 0.78. This indicates that elevated suPAR levels are associated with a high risk of poor outcomes of sepsis. In subgroup analysis, the AUC of diagnosis and prognosis value for bacterial sepsis were 0.79 and 0.77, respectively. Furthermore, suPAR also demonstrated moderate diagnostic value to discriminate between sepsis and SIRS with an AUC of 0.81 in the pooled data.
Currently, the PCT is the only biomarker recommended in the SSC guidelines, and demonstrates that PCT can help clinical decision-making in patients with sepsis (11). Serum C-reactive protein (CRP) is also a widely studied biomarker in sepsis patients. Although CRP can be used to indicate acute inflammation, its specificity is relatively low, which limits its application in the diagnosis of sepsis (48). For PCT, a meta-analysis by Chengfen Y et al. (49) showed that PCT is a promising diagnostic biomarker for sepsis with a pooled sensitivity and specificity of 74% and 70%, respectively. Because the biomarkers mentioned above lack specificity for sepsis and the levels may be elevated in other inflammatory diseases, these biomarkers are more useful for ruling out sepsis than for ruling it in (50). Compared with the diagnosis value of PCT identified by previous meta-analysis, we found that suPAR and PCT have similar diagnostic accuracy. More importantly, suPAR exhibited higher specificity (PCT: 70% vs suPAR: 78%) than PCT and CRP, suggesting that suPAR has an advantage in identifying nonsepsis populations and is more distinguishable than other biomarkers in patients with different inflammatory diseases (41). Therefore, suPAR has the potential to complement existing biomarker defects.
Identifying of sepsis from noninfectious original SIRS is critical for clinical diagnosis and treatment. The sepsis 3.0 guidelines emphasize the existence of dysregulation of host response to infection and organ dysfunction caused by infection in the definition of sepsis (8). However, SIRS only reflects the appropriate immune response, not the immune response disorder because its pathological mechanism is infection and the concomitant inflammatory response. Regarding the accuracy of suPAR in distinguishing between sepsis and SIRS, Wentao Ni et al. (12) considered suPAR as a low accurate biomarker (AUC = 0.68). Interestingly, our study found that suPAR has a promising diagnostic value (AUC = 0.81) for differentiating sepsis from noninfectious original SIRS. Moreover, it better differentiates between infectious and noninfectious causes of critical illness than CRP, lipopolysaccharide-binding protein (LBP), and interleukin (IL)-6. This suggests that the level of suPAR has the potential to identify infectivity from noninfectivity (51), which will improve the clinical guidance value of suPAR and aid decision-making.
Existing biomarkers fail to show the ideal accuracy for predicting sepsis mortality (52, 53). A meta-analysis by Liu et al. (54) found that the PCT levels at admission were moderately accurate for the prognosis of sepsis, with sensitivity of 76% and specificity of 64%. Compared with the identified effect value of PCT we found that suPAR was slightly better in terms of specificity of prognostic value (PCT: 64% vs suPAR: 70%). Although the value of using suPAR alone to prognosis sepsis is limited, the elevated suPAR levels in the initial stage is still associated with a high risk of death. Wentao Ni et al. (12) showed that the mortality rate of bacterial infection increased by 3.37 times with the elevated suPAR level. A primary study reported that the use of combined indicators to predict sepsis mortality can be effective in improving accuracy (43). And this study suggested that combining the Acute Physiology and Chronic Health Evaluation II (APACHE II) score with the serum suPAR level could be a measurement for predicting sepsis outcomes. Due to the limitation of suPAR in prognosis sepsis, further investigation is required to evaluate the predictive efficacy of suPAR in combination with other indicators.
The suPAR measurement time of the included studies was concentrated at the time of admission or within 24 h after admission. Therefore, these results suggest that the level of suPAR could be a timely and effective criterion for the diagnosis and prognosis of sepsis. There are early clinical screening tools, such as early warning score (EWS), which predicts sepsis by analyzing available electronic data (55). EWS shows extremely opposite sensitivity and specificity of 0.16 and 0.97, respectively (56). Although EWS has good specificity, its low sensitivity significantly reduces diagnostic accuracy. In addition, the cumbersome testing items included in the score also increase the difficulty of clinical practice (57). Unlike other early detection indicators of sepsis, as a biomarker, suPAR has non-negligible advantages, including fully automated identification, short turnaround time, and low production costs.
At present, there is no gold standard for diagnosing sepsis; no tool for selecting, evaluating, and de-escalating treatment; and no reliable biomarker for allocating risk profiles or predicting outcome (58). In addition, accurate and timely diagnosis can provide a strong guarantee for screening high-risk populations and improving clinical courses and outcomes (59). Examination of a disease-specific biomarker may help in the identification, early diagnosis and prevention of disease, and monitoring during treatment (60). Clinicians are constantly seeking biomarkers to diagnosis sepsis and distinguish sepsis from SIRS. The SSC guidelines recommended only one biomarker in clinical practice, the PCT (10), which is insufficient to aid the diagnosis and clinical decision of sepsis. Therefore, effective biomarkers are needed to promote the diagnosis of sepsis and to monitor the course of the disease. Our study suggests that suPAR is a promising biomarker for sepsis. To assess the potential of suPAR assisting clinical decision-making, we compared the effect size of suPAR for sepsis with that of PCT identified in previous meta-analysis (49, 54). The level of suPAR has the similar clinical guiding value to PCT, whereas suPAR exhibits higher specificity than PCT in the diagnosis and prognosis of sepsis. Hence, suPAR can partially facilitate the deficiencies of PCT in terms of specificity. In addition, the level of suPAR has the potential to identify infectivity from noninfectiousness, which will improve the clinical guiding value of suPAR and aid decision-making. Considering the lack of biomarkers for sepsis and the similar clinical value of suPAR and PCT, suPAR has the potential value in the clinical practice for sepsis patients.
According to the sepsis 3.0 guidelines, it is a need to identify sepsis from SIRS (8). In previous studies, to the best of our knowledge, most systematic reviews focused on the relationship between suPAR and bacterial infection or SIRS (11, 12). The sepsis was treated as one of the subgroups for preliminary analysis. In addition, the number of included original studies is limited. The meta-analysis by Wentao Ni et al. (12) focused on bacterial infections and only studied bacterial-induced sepsis, whereas the pathogenic microorganisms of sepsis include bacteria, fungi, viruses, and parasites. The sepsis population they studied was not representative enough for sepsis. They found that suPAR is ineffective in distinguishing sepsis from SIRS. However, in our study, suPAR showed an effective ability to distinguish sepsis from SIRS, thus indicating that suPAR has the potential to identify infectivity from noninfectiousness. In addition, Ni et al. only studied sepsis in a subgroup analysis and did not analyze the heterogeneity of effective values of suPAR for sepsis. They cannot rule out a substantive heterogeneity or analyze the sources of heterogeneity. Considering the lack of representative sepsis patients and the inadequate information and analysis of sepsis, the conclusions of previous studies can hardly guide clinical practice and cannot be directly applied to sepsis patients. The clinical value of suPAR in the diagnosis, prognosis, and therapeutic guidance of sepsis still needs to be well defined.
Compared with meta-analyses of interventions including randomized controlled trials, those including diagnostic studies have more publication bias (61). Publication bias exists in studies which reported the prognostic value. Excluding studies without enough data could contribute to publication and reporting bias. Thus, the prognosis value of suPAR may be overestimated. As for the significant degree of heterogeneity, we performed meta-regression and subgroup analysis to explore the source of heterogeneity. Meta-regression analysis revealed that no tested variables substantially affected the sensitivity and specificity of suPAR for sepsis diagnosis. However, due to the diversity of reference standards for diagnosis sepsis among included studies, univariate meta-regression cannot fully explain the impact of the reference standard on heterogeneity, thus it should be carefully considered. Through analysis of prognosis studies, it was found that multicenter design and region have an impact on the heterogeneity of sensitivity, and the clinical setting, reference standards, and measurement time affect the heterogeneities of specificity. Given the impact of different types of infectious original in sepsis on heterogeneity, we found no significant reduction in the heterogeneity of bacterial sepsis. There were no significant threshold effects in both diagnostic and prognostic studies. As shown in Table 1, higher cutoff points (≥7.5 ng/mL for diagnostic studies; ≥9.6 ng/mL for prognostic studies) indicate a higher specificity of suPAR for sepsis diagnosis and prognosis. Minimal evidence of sensitivity heterogeneity was obtained in prognostic studies at lower cutoff points, indicating that the cutoff value of suPAR can also account for the heterogeneity.
There are several limitations in the current meta-analysis. First, the level of suPAR is associated with a variety of diseases, including sepsis and malignant tumors, kidney damage, and inflammatory bowel disease. The influencing factors of other diseases such as different complications may lead to confounding factors, which is difficult to assess in included studies. Second, it is hard to obtain raw data for each included study, which restricts us to determine the optimal cutoff point for suPAR for the sepsis diagnosis and prognosis. Third, due to the lack of original studies comparing the effective value of suPAR and PCT in patients with sepsis, it is hard to obtain accurate results. However, the trend can still be drawn by comparing the results with the existing meta-analysis of the diagnostic and prognostic value of PCT for sepsis. It is a need for further investigation is required to compare the clinical value of suPAR and that of PCT in patients with sepsis. Finally, although we have analyzed the source of heterogeneity through meta-regression and subgroup analysis, the substantial heterogeneity in the results should still be considered carefully.
CONCLUSIONS
In conclusion, the results of this systematic review and meta-analysis suggest that suPAR is a feasible biomarker for patients with sepsis. Compared with the diagnosis and prognosis value of PCT identified by previous meta-analysis, the level of suPAR has the similar clinical guiding value to PCT, whereas suPAR exhibited a higher specificity than PCT which can partially facilitate the deficiencies of PCT. In addition, the elevated level of suPAR also has the potential to identify sepsis from SIRS of noninfectious origin, which is helpful in aiding decision-making and improves the clinical guiding value of suPAR. Further investigation is required to evaluate the predictive efficacy of suPAR in combination with other indicators. Considering the lack of reliable biomarkers for sepsis, the effective clinical value of suPAR, and the importance of timely diagnosis for the treatment of sepsis, suPAR should be considered as a biomarker in clinical practice for patients with sepsis.
Supplementary Material
Acknowledgments
The authors thank the support of the First Clinical Hospital of Lanzhou University, the first clinical medical college of Lanzhou University, Evidence-based Medicine Center of Lanzhou University, and all the authors who participated in this study.
Footnotes
Authors’ contributions: JL, QH, KY, PY, HX, and TS participated in the design of the project, conducted the literature review, and participated in the analysis. QH and JL wrote this article. LZ and JL were responsible for the statistical analysis and participated in data interpretation. JL was the principal investigator for the project. All authors approved the final version of the article.
Funding: The authors remain independently of any funding influence.
Competing interests: The authors have no competing of interest nor any financial interest in any product mentioned in this paper.
The authors report no conflicts of interest.
REFERENCES
- 1.Samraj RS, Zingarelli B, Wong HR. Role of biomarkers in sepsis care. Shock 40 (5):358–365, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toldi G, Beko G, Kádár G, MácSai E, Kovács L, Vásárhelyi B, Balog A. Soluble urokinase plasminogen activator receptor (suPAR) in the assessment of inflammatory activity of rheumatoid arthritis patients in remission. Clin Chem Lab Med 51 (2):327–332, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Perch M, Kofoed P, Fischer TK, Co F, Rombo L, Aaby P, Eugen-Olsen J. Serum levels of soluble urokinase plasminogen activator receptor is associated with parasitemia in children with acute Plasmodium falciparum malaria infection. Parasite Immunol 26 (5):207–211, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Andersen O, Eugen-Olsen J, Kofoed K, Iversen J. Haugaard SB. suPAR associates to glucose metabolic aberration during glucose stimulation in HIV-infected patients on HAART. J Infect 57 (1):55–63, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Andersen ES, Ruhwald M, Moessner B, Christensen PB, Andersen O, Eugen-Olsen J, Weis N. Twelve potential fibrosis markers to differentiate mild liver fibrosis from cirrhosis in patients infected with chronic hepatitis C genotype 1. Eur J Clin Microbiol Infect Dis 30 (6):761–766, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers 27 (3):157–172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sier CF, Sidenius N, Mariani A, Aletti G, Agape V, Ferrari A, Casetta G, Stephens RW, Brunner N, Blasi F. Presence of urokinase-type plasminogen activator receptor in urine of cancer patients and its possible clinical relevance. Lab Invest 79 (6):717–722, 1999. [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. J Am Med Assoc 315 (8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39 (2):165–228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 43 (3):304–377, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med 38 (9):1418–1428, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni W, Han Y, Zhao J, Cui J, Wang K, Wang R, Liu Y. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: a systematic review and meta-analysis. Sci Rep 6:39481, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge L, Tian JH, Li YN, Pan JX, Li G, Wei D, Xing X, Pan B, Chen YL, Song FJ, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol 93:45–55, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Chen Y, Yao L, Zhou Q, Wu Q, Estill J, Wang Q, Yang K, Norris SL. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol 98:1–8, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62 (10):e1–e34, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155 (8):529–536, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Jadad AR, Tugwell P. Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 12 (2):195–208, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Georgescu A-M, Szederjesi J, Voidazan S, Dobreanu M, Copotoiu SM, Hutanu A, Azamfirei L. Soluble urokinase-type plasminogen activator receptor (suPAR)—a possible biomarker for bacteremia in sepsis. Revista Romana De Medicina De Laborator 23 (1):59–73, 2015. [Google Scholar]
- 19.Georgescu AM, Szederjesi J, Voidazan S, Dobreanu M, Azamfirei L. Serum soluble urokinase-type plasminogen activator receptor level is associated with higher mortality in patients with infectious systemic inflammatory response syndrome. Am J Infect Control 43 (6):S31–S32, 2015. [Google Scholar]
- 20.Kofoed K, Eugen-Olsen J, Petersen J, Larsen K, Andersen O. Predicting mortality in patients with systemic inflammatory response syndrome: an evaluation of two prognostic models, two soluble receptors, and a macrophage migration inhibitory factor. Eur J Clin Microbiol Infect Dis 27 (5):375–383, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, Larsen K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care 11 (2):R38, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng M, Chang M, Zheng H, Li B, Chen Y, He W, Huang C. Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. Am J Emerg Med 34 (3):375–380, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz G, Koksal I, Karahan SC, Mentese A. The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in systemic inflammatory response syndrome. Clin Biochem 44 (14–15):1227–1230, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Hu K, Yu L, Wang H, Long D. Correlation of plasma suPAR expression with disease risk and severity as well as prognosis of sepsis-induced acute respiratory distress syndrome. Int J Clin Exp Pathol 10 (12):11378–11383, 2017. [PMC free article] [PubMed] [Google Scholar]
- 25.Wittenhagen P, Kronborg G, Weis N, Nielsen H, Obel N, Pedersen SS, Eugen-Olsen J. The plasma level of soluble urokinase receptor is elevated in patients with Streptococcus pneumoniae bacteraemia and predicts mortality. Clin Microbiol Infect 10 (5):409–415, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Vanska M, Purhonen A-K, Koivula I, Jantunen E, Hamalainen S, Pulkki K, Juutilainen A. Soluble form of urokinase-type plasminogen activator receptor as a diagnostic and prognostic marker in hematological patients with neutropenic fever. Leuk Lymphoma 55 (3):718–721, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Uusitalo-Seppala R, Huttunen R, Tarkka M, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, Rintala EM. Soluble urokinase-type plasminogen activator receptor in patients with suspected infection in the emergency room: a prospective cohort study. J Intern Med 272 (3):247–256, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Tsirigotis P, Chondropoulos S, Frantzeskaki F, Stamouli M, Gkirkas K, Bartzeliotou A, Papanikolaou N, Atta M, Papassotiriou I, Dimitriadis G, et al. Thrombocytopenia in critically ill patients with severe sepsis/septic shock: prognostic value and association with a distinct serum cytokine profile. J Crit Care 32:9–15, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Suberviola B, Castellanos-Ortega A, Ruiz Ruiz A, Lopez-Hoyos M, Santibanez M. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and proADM in a single determination on ICU admission. Intensive Care Med 39 (11):1945–1952, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Savva A, Raftogiannis M, Baziaka F, Routsi C, Antonopoulou A, Koutoukas P, Tsaganos T, Kotanidou A, Apostolidou E, Giamarellos-Bourboulis EJ, et al. Soluble urokinase plasminogen activator receptor (suPAR) for assessment of disease severity in ventilator-associated pneumonia and sepsis. J Infect 63 (5):344–350, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Reichsoellner M, Raggam RB, Wagner J, Krause R, Hoenigl M. Clinical evaluation of multiple inflammation biomarkers for diagnosis and prognosis for patients with systemic inflammatory response syndrome. J Clin Microbiol 52 (11):4063–4066, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raggam RB, Wagner J, Prüller F, Grisold A, Leitner E, Zollner-Schwetz I, Valentin T, Krause R, Hoenigl M. Soluble urokinase plasminogen activator receptor predicts mortality in patients with systemic inflammatory response syndrome. J Intern Med 276 (6):651–658, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Moller HJ, Moestrup SK, Weis N, Wejse C, Nielsen H, Pedersen SS, Attermann J, Nexo E, Kronborg G. Macrophage serum markers in pneumococcal bacteremia: prediction of survival by soluble CD163. Crit Care Med 34 (10):2561–2566, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Molkanen T, Ruotsalainen E, Thorball CW, Jarvinen A. Elevated soluble urokinase plasminogen activator receptor (suPAR) predicts mortality in Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 30 (11):1417–1424, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Loonen AJM, de Jager CPC, Tosserams J, Kusters R, Hilbink M, Wever PC, van den Brule AJC. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One 9 (1):e87315, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Shen Y, Li Z, Fei A, Wang H, Ge Q, Pan S. Prognostic significance of APACHE II score and plasma suPAR in Chinese patients with sepsis: a prospective observational study. BMC Anesthesiol 16 (1):46, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazaridis L, Karatzas S, Prekates A, Toutouzas K, Mathas C, Popp J, Olsen J, Giamarellos-Bourboulis E. Integration of biomarkers and clinical signs for the early diagnosis of sepsis. Crit Care 2018; 22: [Google Scholar]
- 38.Koch A, Voigt S, Kruschinski C, Sanson E, Dueckers H, Horn A, Yagmur E, Zimmermann H, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 15 (1):R63, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khater WS, Salah-Eldeen NN, Khater MS, Saleh AN. Role of suPAR and lactic acid in diagnosing sepsis and predicting mortality in elderly patients. Eur J Microbiol Immunol 6 (3):178–185, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaya S, Koksal I, Mentese A, Sonmez M, Sumer A, Yildirim SS, Yilmaz G. The significance of serum urokinase plasminogen activation receptor (suPAR) in the diagnosis and follow-up of febrile neutropenic patients with hematologic malignancies. Int J Infect Dis 17 (11):E1056–E1059, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Huttunen R, Syrjanen J, Vuento R, Hurme M, Huhtala H, Laine J, Pessi T, Aittoniemi J. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med 270 (1):32–40, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Hoenigl M, Raggam RB, Wagner J, Valentin T, Leitner E, Seeber K, Zollner-Schwetz I, Krammer W, Prueller F, Grisold AJ, et al. Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clin Biochem 46 (3):225–229, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Giamarellos-Bourboulis EJ, Norrby-Teglund A, Mylona V, Savva A, Tsangaris I, Dimopoulou I, Mouktaroudi M, Raftogiannis M, Georgitsi M, Linner A, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care 16 (4):R149, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donadello K, Scolletta S, Taccone FS, Covajes C, Santonocito C, Cortes DO, Grazulyte D, Gottin L, Vincent JL. Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. J Crit Care 29 (1):144–149, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Barati M, Shekarabi M, Chobkar S, Talebi-Taher M, Farhadi N. Evaluation of diagnostic value of soluble urokinase-type plasminogen activator receptor in sepsis. Arch Clin Infect Dis 10 (1):e26346, 2015. [Google Scholar]
- 46.Julian-Jimenez A, Yanez MC, Gonzalez-Del Castillo J, Salido-Mota M, Mora-Ordonez B, Arranz-Nieto MJ, Chanovas-Borras MR, Llopis-Roca F, Modol-Deltell JM, Munoz G. Prognostic power of biomarkers for short-term mortality in the elderly patients seen in Emergency Departments due to infections. Enferm Infecc Microbiol Clin 37 (1):11–18, 2019. [DOI] [PubMed] [Google Scholar]
- 47.Zhao JJ, Lou XL, Chen HW, Zhu FT, Hou YQ. Diagnostic value of decoy receptor 3 combined with procalcitonin and soluble urokinase-type plasminogen activator receptor for sepsis. Cell Mol Biol Lett 23:22, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henriquez-Camacho C, Losa J. Biomarkers for sepsis. Biomed Res Int 2014:547818, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chengfen Y, Tong L, Xinjing G, Zhibo L, Lei X. Accuracy of procalcitonin for diagnosis of sepsis in adults: a meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 27 (9):743–749, 2015. [PubMed] [Google Scholar]
- 50.Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 9:107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reinhart K, Meisner M. Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin 27 (2):253–263, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Andriolo BN, Andriolo RB, Salomao R, Atallah AN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev 1:Cd010959, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivers EP, Jaehne AK, Nguyen HB, Papamatheakis DG, Singer D, Yang JJ, Brown S, Klausner H. Early biomarker activity in severe sepsis and septic shock and a contemporary review of immunotherapy trials: not a time to give up, but to give it earlier. Shock 39 (2):127–137, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 10 (6):e0129450, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umscheid CA, Betesh J, VanZandbergen C, Hanish A, Tait G, Mikkelsen ME, French B, Fuchs BD. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 10 (1):26–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roney JK, Whitley BE, Maples JC, Futrell LS, Stunkard KA, Long JD. Modified early warning scoring (MEWS): evaluating the evidence for tool inclusion of sepsis screening criteria and impact on mortality and failure to rescue. J Clin Nurs 24 (23–24):3343–3354, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Moore LJ, Jones SL, Kreiner LA, McKinley B, Sucher JF, Todd SR, Turner KL, Valdivia A, Moore FA. Validation of a screening tool for the early identification of sepsis. J Trauma 66 (6):1539–1546, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 15 (5):581–614, 2015. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. Springerplus 5 (1):2091, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spoto S, Cella E, de Cesaris M, Locorriere L, Mazzaroppi S, Nobile E, Lanotte AM, Pedicino L, Fogolari M, Costantino S, et al. Procalcitonin and MR-proadrenomedullin combination with SOFA and qSOFA scores for sepsis diagnosis and prognosis: a diagnostic algorithm. Shock 50 (1):44–52, 2018. [DOI] [PubMed] [Google Scholar]
- 61.Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 48 (1):119–130, 1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


