Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, catheter ablation, electroporation, fistula, swine
Background:
Pulsed field ablation (PFA) can be myocardium selective, potentially sparing the esophagus during left atrial ablation. In an in vivo porcine esophageal injury model, we compared the effects of newer biphasic PFA with radiofrequency ablation (RFA).
Methods:
In 10 animals, under general anesthesia, the lower esophagus was deflected toward the inferior vena cava using an esophageal deviation balloon, and ablation was performed from within the inferior vena cava at areas of esophageal contact. Four discrete esophageal sites were targeted in each animal: 6 animals received 8 PFA applications/site (2 kV, multispline catheter), and 4 animals received 6 clusters of irrigated RFA applications (30 W×30 seconds, 3.5 mm catheter). All animals were survived to 25 days, sacrificed, and the esophagus submitted for pathological examination, including 10 discrete histological sections/esophagus.
Results:
The animals weight increased by 13.7±6.2% and 6.8±6.3% (P=0.343) in the PFA and RFA cohorts, respectively. No PFA animals (0 of 6, 0%) developed abnormal in-life observations, but 1 of 4 RFA animals (25%) developed fever and dyspnea. On necropsy, no PFA animals (0 of 6, 0%) demonstrated esophageal lesions. In contrast, esophageal injury occurred in all RFA animals (4 of 4, 100%; P=0.005): a mean of 1.5 mucosal lesions/animal (length, −21.8±8.9 mm; width, −4.9±1.4 mm) were observed, including one esophago-pulmonary fistula and deep esophageal ulcers in the other animals. Histological examination demonstrated tissue necrosis surrounded by acute and chronic inflammation and fibrosis. The necrotic RFA lesions involved multiple esophageal tissue layers with evidence of arteriolar medial thickening and fibrosis of periesophageal nerves. Abscess formation and full-thickness esophageal wall disruptions were seen in areas of perforation/fistula.
Conclusions:
In this novel porcine model of esophageal injury, biphasic PFA induced no chronic histopathologic esophageal changes, while RFA demonstrated a spectrum of esophageal lesions including fistula and deep esophageal ulcers and abscesses.
What Is Known?
Unintentional thermal injury of the esophagus during radiofrequency catheter ablation of the posterior left atrium is associated with esophageal ulcers and, rarely, atrioesophageal fistulas.
Pulsed field ablation is a novel, myocardium-specific energy source effective for creating transmural atrial lesions. Older monophasic waveforms have been shown to result in minimal esophageal injury in a surgical preclinical model.
What the Study Adds?
A novel preclinical model was created to nonsurgically assess the response to esophageal injury. This was accomplished by delivering the energy source from within the inferior vena cava, against the esophagus (which was purposefully mechanically deviated toward the inferior vena cava).
Biphasic pulsed field ablation induced no chronic histopathologic esophageal changes, whereas radiofrequency catheter ablation demonstrated a spectrum of esophageal lesions including esophageal ulcers, abscess, and fistula.
During catheter ablation for atrial fibrillation, the most feared complication is atrioesophageal fistula resulting from collateral esophageal injury from lesions placed on the posterior left atrium (LA). This risk is present with all thermal energy sources that have been evaluated, including radiofrequency ablation (RFA), cryoablation, laser ablation, and ultrasound ablation.1–6 Pulsed field ablation (PFA) is a newer energy source characterized by a nonthermal ablative mechanism (ie, electroporation) that has a unique relative specificity for myocardial tissue.7–10 Therefore, PFA presents a unique opportunity to reduce or even eliminate the risk of unintended collateral injury and safety concerns such as pulmonary venous stenosis, phrenic nerve injury, and most critically, injury to the esophagus, which can range from reversible complications such as gastrointestinal dysmotility of the esophagus to the potentially fatal atrioesophageal fistula.10–14
A seminal preclinical study has indicated that electroporation mostly spares the esophageal wall, but this required an open surgical technique with direct application of a basic monopolar, monophasic pulse delivered directly atop the esophagus.13 Although this study attests to the relative esophageal sparing effects of PFA, the approach used in this study cannot be used to test catheters designed for endocardial delivery of the more modern biphasic PFA waveforms being studied preclinically and clinically.15–17 Accordingly, we developed a nonsurgical, transvenous porcine model that approximates clinical catheter ablation on the posterior LA opposite the esophagus. In this preclinical study, we used this novel model to characterize and contrast the histopathologic effects of catheter-based PFA versus RFA on the esophagus.
Methods
The data, analytic methods, and study materials will not be made available for purposes of reproducing the results or replicating the procedure. All preclinical experiments were approved by the Institutional Animal Care and Use Committee at PreClinical Med Device, Covance Laboratories, San Carlos, CA. A total of 10 female Yorkshire swine were included in the study: 6 and 4 swine underwent PFA and RFA, respectively. The ablation procedures required separate catheters and generators; thus, ablations were performed consecutively and not in a randomized fashion, with all PFA experiments being completed before the RFA experiments. As this was also the first time the model was being evaluated, sample size was not calculated.
Procedural Details
All swine were premedicated, intubated, and mechanically ventilated with isoflurane (1%–3%) after an overnight fast. Percutaneous femoral venous access was obtained and followed by systemic heparin administration. Ablation catheters were introduced into the inferior vena cava (IVC) through either 12F or 8.5F deflectable sheaths for PFA (Faradrive; Farapulse, Inc, Menlo Park, CA) or RFA (Agilis; Abbott, Inc, Minneapolis, MN). The esophagus was then carefully intubated using an esophageal balloon retractor device (DV8; Manual Surgical Sciences, Inc, Minneapolis, MN) under fluoroscopy. The distal esophageal lumen was then defined by iodinated contrast administered through an open port in the DV8 device. Following this, the DV8 device was inflated with a mixture of saline and contrast (for fluoroscopic visualization) and rotated to deviate the esophagus rightward and anteriorly toward the ablation catheter in the IVC. The deflectable sheaths were used to ensure forceful contact between the ablation catheters and the esophagus in both groups. Orthogonal fluoroscopic views were used to confirm the immediate proximity of the deviated esophagus to the ablative portion of the catheters in the IVC. When possible, the ablation catheter was used to mechanically displace luminal contrast within the esophagus to further confirm proximity.
PFA Procedure
In this cohort, ablation was performed using a clinical PFA system: a cardiac-specific waveform generator, and a 12F over-the-wire PFA catheter (Farawave, Farapulse, Inc) as described previously.15,16 The catheter itself has 5 splines, each containing 4 electrodes, and is advanced over a guidewire. The catheter tip was deployed to a basket pose, and the deflectable sheath was used to position the tip close to the esophageal margin (Figures 1 and 2). All PFA applications were delivered in a biphasic-bipolar fashion using the same waveform being studied clinically. The PFA waveforms consisted of a sequence of microsecond scale pulses at 2000 V. Four discrete locations on the distal esophagus were targeted based on the proximity of the deviated distal esophagus to the IVC. Eight PFA applications were placed at each location in the 6 swine of the PFA cohort. Each PFA application consisted of 5 pulse packets over <3 seconds. A minimum of one partial rotation of the device was performed between applications to simulate clinical workflow.
Figure 1.
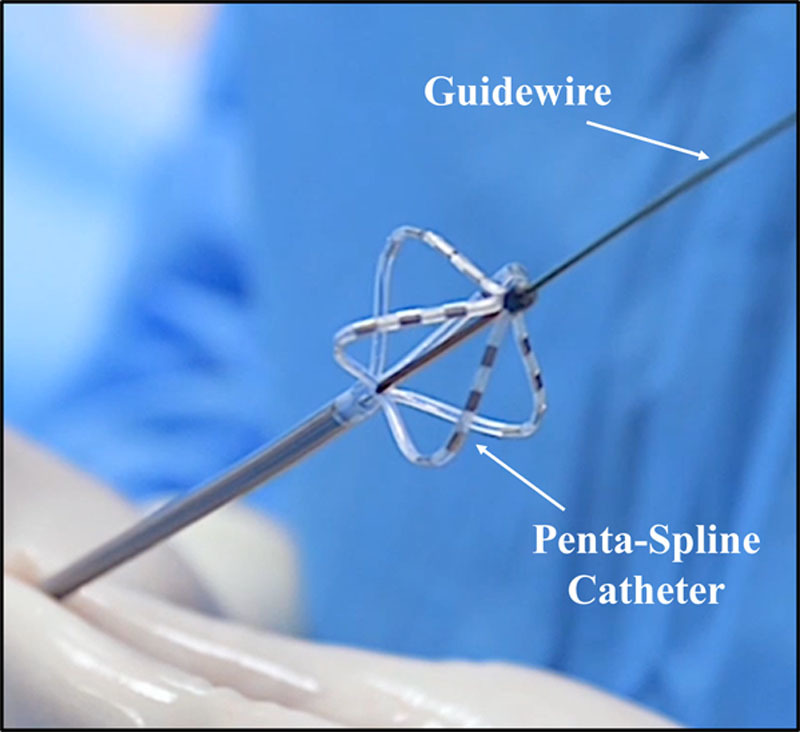
Pulsed field ablation (PFA) catheter: pentaspline over-the-wire PFA catheter in basket pose.
Figure 2.
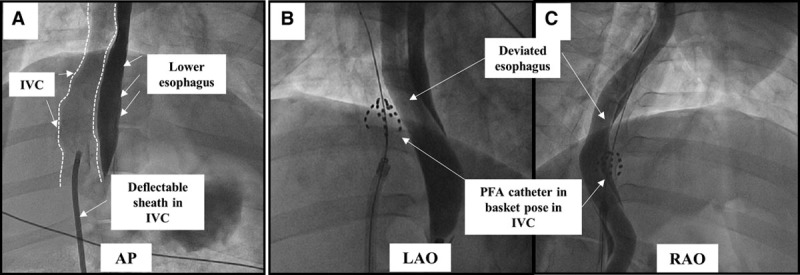
Fluoroscopic view of the esophageal injury mode: pulsed field ablation (PFA) cohort. A, Contrast angiography was performed using a long deflectable sheath placed in the inferior vena cava (IVC; outlined). In the anteroposterior (AP) view, the IVC is seen rightward of the contrast filled esophagus. B and C, Left and right anterior oblique (LAO and RAO) projections demonstrate the PFA catheter in basket pose forcefully pushed against the deviated esophagus. The PFA catheter is shown here ablating 2 different esophageal locations.
Radiofrequency Procedure
In this cohort, a 3.5-mm irrigated ablation catheter (Blazer Open-Irrigated; Boston Scientific, Inc, Minneapolis, MN) was used to deliver power-controlled radiofrequency energy with the corresponding generator (Maestro 4000; Boston Scientific, Inc) set to 30 W with saline flow rate of 17 mL/min (Figure 3). The maximal duration and electrode temperature of each radiofrequency application was set to 30 seconds and 42°C, respectively. Similar to the PFA group, 4 vertically discrete locations were ablated with clusters of 6 radiofrequency applications per location, abutting the deviated esophagus. No waiting period was used between ablations beyond what was needed to reposition the catheter. Intraluminal esophageal temperature monitoring was not performed.
Figure 3.
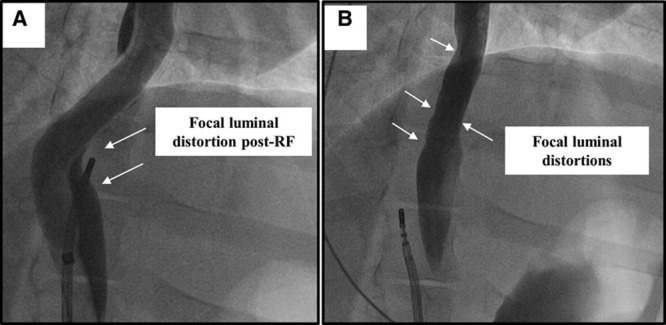
Fluoroscopic view of the esophageal injury model: radiofrequency (RF) ablation cohort. A, Focal distortion of the esophageal lumen is seen after delivery of RF at that site (deviation balloon in place). B, Luminal irregularities are seen at the site of RF injury after removal of the deviation balloon.
All swine were recovered and survived for 25 days with careful daily follow-up, at which point, all swine were humanely euthanized.
Simulation
Simulation of electric field intensity created by the applied PFA dose was performed using a 3-dimensional computational model (COMSOL, Burlington, MA) to assess the dose delivered to the esophagus specifically in this preclinical assessment. The local geometry used in this model included the ablation catheter placed within the IVC (assumed to be <2 mm thick) in the basket pose pushed up against the deviated esophagus.
Histological Investigation
On necropsy, the esophagus, IVC, and the adjoining lungs were carefully examined and photographed. The entire distal esophagus (below the level of the heart) and adjoining IVC (including adherent tissue in cases of lesion formation) were carefully removed, examined, opened longitudinally, and photographed. Following formalin fixation, 10 evenly spaced transverse sections were made spanning the esophagus and submitted for histology. Two of these 10 sections, one each from the superior most and inferior most portion of the esophagus, served as nonablated controls. All tissues were stained with hematoxylin and eosin and Masson trichrome. The examining pathologist was blinded to the energy source used during the assessment of sections.
Statistical Analyses
Study results are presented using descriptive statistics. For continuous variables, the results include number, mean, and SD where pertinent. They were compared using a Wilcoxon signed-rank test or Mann-Whitney U test as appropriate. Presented data for categorical variable includes the number and percent in each category and were compared using Fisher test. A P of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS 26.0 software (SPSS, Inc, Chicago, IL).
Results
All 10 of 10 swine completed the follow-up period of 25 days. Pre- and postprocedural weights were available for 8 of 10 swine. The mean weights of the 4 of 6 PFA swine at the time of the procedure and follow-up were 73.9±4.4 and 83.9±4.0 kg, respectively – an increase in weight by 13.7±6.2 kg (P=0.068). In the RFA cohort, the mean weights of the 4 of 4 swine were 71.9±1.3 and 76.9±5.8, respectively—an increase in weight by 6.8±6.3 kg (P=0.144). The relative amount of weight gain between groups, although reduced in the RFA cohort, did not reach statistical significance (P=0.343).
Acute Procedural Data
PFA Cohort
PFA was successfully delivered in all swine with total PFA delivery times of <1 minute/animal. In a small number of attempted deliveries (0.6%), ablation was automatically interrupted by the generator due to the catheter being overly constricted by the constrained anatomy of the porcine IVC relative to clinical pulmonary vein ostia, that is, the splines of the basket were not deployed. In these instances, the device was repositioned, and all subsequent deliveries were completed successfully. No arrhythmias or EKG changes were noted. Diaphragmatic capture was seen during all PFA applications but did not result in any noticeable dislocation of PFA catheter, which was continuously monitored with fluoroscopy. All swine had an uneventful recovery from the acute procedure.
RFA Cohort
Except for 4 instances of early termination of radiofrequency delivery due to impedance rises and 1 instance of catheter dislocation, all RFA applications were completed in all swine. There were no steam pops noted. In locations where esophageal luminal contrast was immediately adjacent to the radiofrequency catheter tip, one could easily appreciate local acute effects of radiofrequency deliver such as scalloping of the contrast within the esophageal lumen. This focal luminal distortion did not completely resolve by the end of the study—a finding that was not seen in the PFA cohort (Figure 3B).
Survival Period
One of the 4 swine in the RFA cohort demonstrated behavioral changes on day 21: it stopped eating and became less active. Subsequently, it displayed breathing difficulty, fever, and tachycardia. It received supportive medical management and was able to complete the survival process without further distress. The other 3 swine in the RFA cohort, and all 6 swine in the PFA cohort, completed the survival period without any change in behavior or appetite and displayed no signs of distress.
Pathological Examination
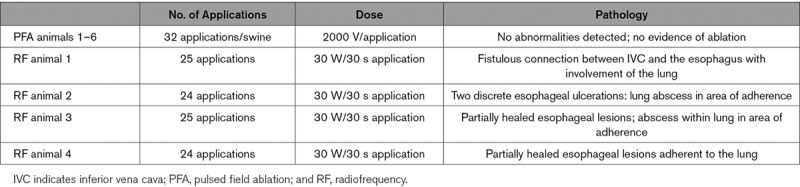
All swine (0 of 6, 0%) in the PFA cohort demonstrated no evidence of lesions on either the adventitial or the mucosal aspects of the esophagus (Figure 4A). In the RFA cohort, distinct forms of esophageal injury were evident in all 4 of 4 swine as compared with the PFA cohort (100% versus 0%, P=0.005). A mean of 1.5 full-thickness lesions/RFA swine were seen with a mean length and width of 21.8±9.0 and 4.9±1.5 mm (range, 2.96–38.17 mm) in this group. Esophageal RFA lesions manifested in one swine as a necrotic inflammatory process extending from the mucosal lesion with a fistulous connection to the IVC, lung, and the esophagus (Figure 4B; Table). The other swine demonstrated mucosal ulcerations with adhesions to surrounding tissue on the adventitial aspects of these ulcers (Figure 5A and 5B; Table). Lesions encompassed the neighboring pulmonary parenchyma with gross evidence of abscess in one swine. Other sites also demonstrated mucosal deformities but without perforation indicating the presence of healed lesions (Figure 5C). The IVC demonstrated no obvious lesions in the PFA group on gross examination. More importantly, there were no areas of scarring visible, and the luminal surface was smooth and even. On the other hand, besides the one animal in the radiofrequency group that developed the fistula, all 3 swine 00demonstrated prominent areas of thickening and discoloration, and more importantly, area of scarring with deformation of the walls was evident in 2 swine (Figure I in the Data Supplement).
Figure 4.
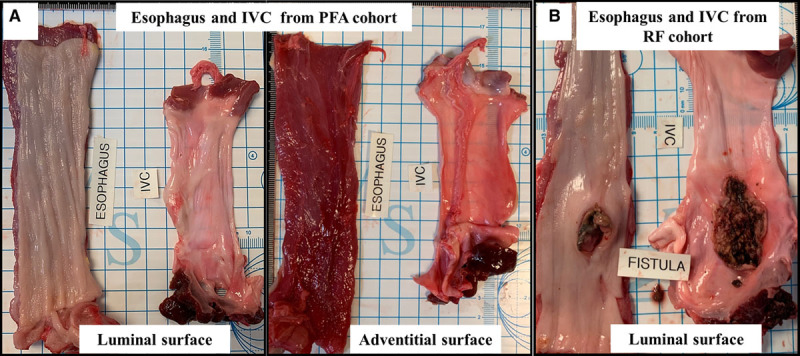
Gross necropsy views of esophageal changes at sacrifice. A, Representative images demonstrating the normal luminal and adventitial surface of the esophagus after pulsed field ablation (PFA). B, Conversely, from the radiofrequency (RF) ablation cohort, a perforating ulcer is seen on the luminal aspect of the esophagus that was separated from a larger necrotic area opening into the inferior vena cava (IVC; esophagus-IVC fistula).
Figure 5.
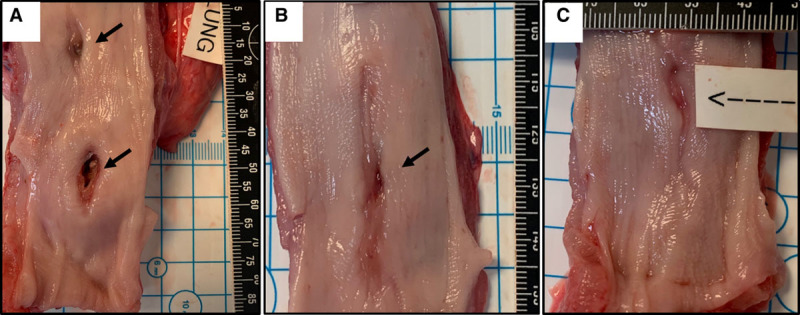
Spectrum of esophageal mucosal abnormalities in the radiofrequency ablation cohort other than fistula formation. Shown are 2 discrete ulcerations with areas of mucosal perforation in the center (A), as well as examples of partially healed mucosal lesions in the other 2 swine (B and C).
Table.
Description of the Number of Pulsed Field and Radiofrequency Applications, Dosages, and Pathological Findings
Histological Investigation
PFA Cohort
A total of 60 sections (48 lesions from the ablation zone and 12 from the control zone) were examined in the PFA cohort. There was no evidence of erosions, inflammation, or fibrosis along the entire extent of these sections (other than rare foci of inflammation around mucinous glands that was present in both control and ablation sections). All sections demonstrated the expected normal histological findings, and there were no differences noted between sections from the control and PFA sites (Figure 6A; Table). Importantly, the outer layers of the tunica muscularis and the tunica adventitia, the esophageal layer closest to the IVC, did not show any changes and in fact demonstrated normal vessels and nerve fascicles (Figure 6B).
Figure 6.
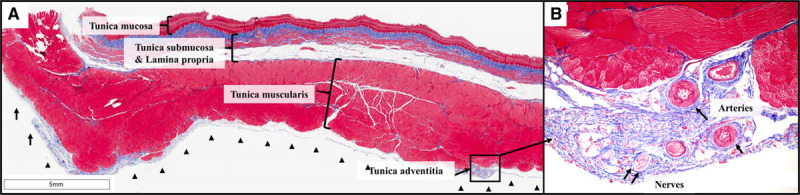
Esophageal histology: pulsed field ablation (PFA) cohort. A, Shown are all 4 layers of the esophageal wall after PFA: tunica mucosa, tunica submucosa, tunica muscularis, and the outer most layer, tunica adventitia. These are all normal along the entire circumference of the esophagus. In particular, there is no evidence of ablation lesions in the outer layers of the tunica muscularis. B, In this zoomed view (10×) of the tunica adventitia, there are normal vessels and nerve fascicles with no evidence of fibrosis or inflammation. Masson trichrome stain.
Radiofrequency Cohort
A total of 32 sections were examined; lesions were present in the majority of the submitted sections from this cohort (excluding the control sections; Figure 7A). The majority of sections demonstrated areas of acute and chronic inflammation and necrosis with areas of surrounding fibrosis that extended frequently across all layers of the esophagus consistent with the gross appearance of esophageal injury that was seen. Lesions were noted to contain granulation tissue that extended deeper into the surrounding tissues with features of sinus/fistula formation (Figure 7B through 7G). In 5 of 32 lesions, pulmonary parenchyma was specifically seen to be attached to necrotic tissue and demonstrated inflammation and abscess formation (Figure 7C and 7D). In some sections, partially healed ablation lesions were seen extending up to the mucosal layer. The tunica adventitia in many sections demonstrated areas of fibrosis that involved the nerves; there was also medial thickening of arterial walls (Figure 7E).
Figure 7.
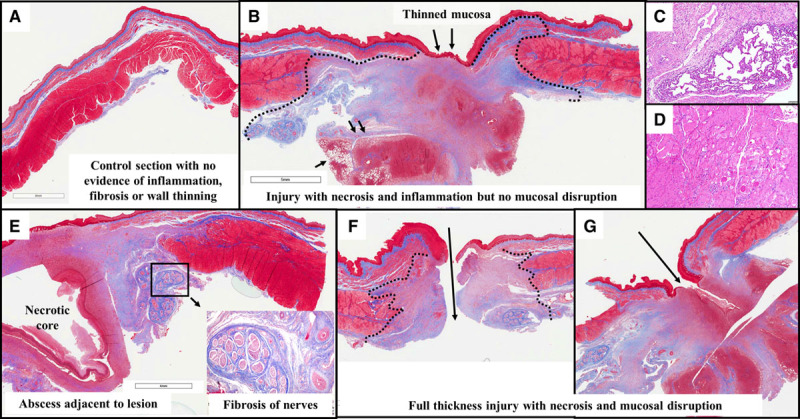
Esophageal histology: radiofrequency ablation (RFA) cohort. A, In this control section from the RFA cohort (ie, from a segment of the esophagus above the level of ablation), there is normal esophageal architecture with no evidence of lesion. B, This section demonstrates near-full-thickness injury with complete necrosis of the muscular layers below the area of mucosal thinning. Granulation tissue is seen extending into the adjacent lung (small black arrows). C, This zoomed (10×) view reveals adherent lung tissue with thickening of alveoli walls and inflammation. D, The tunica muscularis in the vicinity of the lesions shows interstitial inflammation, degenerative vacuolated myocytes, necrotic myocytes (loss of striation, nuclei, eosinophilic cytoplasm), and fibrosis. E, This section reveals a necrotic abscess core within the lesion extending up to the mucosa. The inset focuses on the adventitial lesion resulting in inflammation and fibrosis, involving the periesophageal nerve trunks and thickened arterial walls. F, This section depicts a perforating ulcer with disruption of the wall. G, Here, a fistula tract extends into granulation tissue well beyond the mucosal layer.
Simulation
Because catheter/electrode geometry can have significant effects on the generated pulsed electrical field, simulations were performed to evaluate the somewhat IVC-constrained basket pose geometry used in the above clinical studies (Figure 8A), in comparison to the more open/expanded basket pose used clinically when at the pulmonary vein ostia (Figure 8B). The electrical fields corresponding to these 2 basket poses are shown in Figure 8C and 8D, respectively. The electric field is displayed as an intensity color plot projected on the surface of the esophagus in various views. As shown, the peak electric field intensity at the esophageal surface in the constrained basket pose is ≈900 V/cm, whereas the maximum field intensity in the more open clinical basket pose configuration is substantially less (≈700 V/cm). Thus, the field distribution that the esophagus was exposed to in this preclinical assessment was a supraclinical dose.
Figure 8.
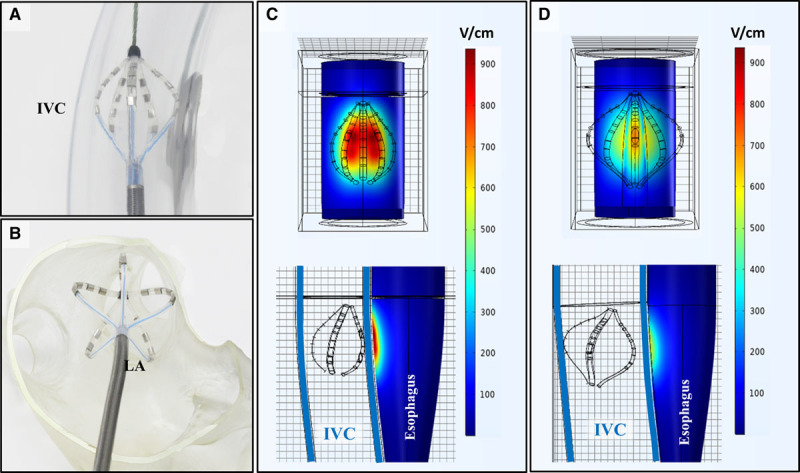
Pulsed field ablation (PFA) simulations. A, Basket pose catheter deployment within an inferior vena cava (IVC) model in the preclinical setting, compared with (B) the basket pose deployment in the left atrium (LA) during clinical cases. C, Simulation results demonstrating the field distribution of PFA on the esophagus in the basket pose used in the preclinical model (A). D, For comparison, simulation demonstrating the lower field strength exposure of the esophagus during PFA with the clinical basket pose (note larger overall diameter of the deployed spines).
Discussion
In this study, we describe a novel in vivo survival animal model for assessing the esophageal tissue response to catheter ablation. We used this model to evaluate the pathological effects of PFA and RFA on the esophagus at a 25-day time point and reached several conclusions. First, modern biphasic PFA, as delivered by the study catheter/generator in a dose that has demonstrated durable LA lesions in the clinical setting, completely spared the esophagus in all swine. The esophageal injury rate was 0 of 6 (0%), even by detailed histological examination. Second, irrigated RFA, at a clinically relevant dose of 30 W for 30 seconds, reproduced a spectrum of esophageal injury patterns in all swine including mucosal ulcerations in various stages of healing and fistulous connections between IVC, lung, and esophagus. The esophageal injury rate was significantly greater in this group at 4 of 4 (100%; P=0.005).
Esophageal Injury Models
Preclinical evaluation of esophageal injury in response to catheter ablation has been difficult to study because of the naturally distant anatomic relationship of the esophagus and the LA in large animal models. This led to the use of models such as acute nonsurvival studies where the esophagus is directly ablated, survival models where the ablation is performed directly on the surgically exposed cervical esophagus, or within the inferior pulmonary veins with the esophagus deviated anteriorly by an esophageal balloon.13,18–20 Surgical approaches are limited by the inability to create physiological conditions such as ablation from within the blood pool, whereas the use of the inferior pulmonary veins to access the esophagus is restricted to catheters that can be easily maneuvered into these often smaller caliber distal pulmonary veins.
The model used in the present study required deviating the esophagus against an ablation catheter placed in the IVC, to approximate the necessary anatomic proximity required to predispose esophageal injury during posterior LA ablation. This model has several advantages in that the ablation catheters were able to deliver the ablative energy: (1) within the blood pool similar to endocardial LA ablation; (2) in an in vivo, nonsurgical, transvenous model allowing for clinical workflows and chronic assessments; (3) against a thin-walled IVC, which, although thinner than the posterior LA wall (a worst-case assessment), is more relevant than direct surgical application; and (4) over a large area of esophageal tissue (the lower half of the esophagus) to allow multiple lesions to be placed for assessment. Also, the electrical conductivity of the vascular tissue of the IVC is similar to the conductivity of the cardiac muscle of the posterior LA wall.21 Thus, ablation from within the IVC should recapitulate well the exposure of the esophagus during PFA of the posterior LA.
PFA and the Esophagus
PFA developed for cardiac use differs significantly from contemporary thermal energy sources such as RFA in that its ablative effects are limited largely to the myocardium; radiofrequency and other thermal energies create lesions by tissue destruction of all structures that achieve lethal temperatures, irrespective of tissue type. Several preclinical studies now support this unique attribute of PFA.11–14 Similarities between the swine and human esophagus form the basis for using swine as a clinically relevant model for catheter ablation. Specifically, the swine esophageal layers are similar to that of humans: both have similar sizes and share histological features such as a mucosal layer, submucosal glands, muscularis propria, and an adventitial layer (Figure 6).
Assessments of esophageal susceptibility to PFA have thus far been limited to one surgical study where the investigators used a catheter specifically designed for the experiment to deliver a monophasic/monopolar waveform and another nonsurvival study where a clamp mechanism was applied directly to the esophagus.13,22 In the former study, the direct PFA applications led to the early development (≈48 hours) of intraepithelial vesicles in the squamous epithelial lining of the esophageal mucosa that resolved over a week, suggesting a relative sensitivity of the mucosal layers to PFA. On pathological exam, the swine (at days 2 and 60) demonstrated no scarring macroscopically, but histology revealed small areas of inflammation/scaring within the tunica muscularis. These histological changes spared the mucosal and submucosal layers. Similar changes limited to the muscular layer were also seen in the nonsurvival study that delivered monophasic PFA directly to the esophagus.22
The tunica muscularis lesions seen in these studies are in contrast with the complete lack of any esophageal changes seen in our study. The complete sparing of the esophagus (assessed at the chronic time point) seen in our study could be attributed to the significantly different waveform used (biphasic/bipolar waveform versus monophasic/monopolar) and the device design. It is possible that relative motion between the IVC and the esophagus during pulse delivery from muscular activation could affect the exposure of the esophagus to PFA. To compensate for this potential limitation and to realize the maximum possible exposure to which the esophagus could be exposed to clinical LA ablation, 8 applications at 4 discrete sites (all within the lower esophagus) were delivered with the catheter in basket pose and deflected directly atop the esophagus. The lack of damage despite this supraclinical exposure is encouraging.
Importantly, only the basket pose was used for PFA delivery in this study as opposed to the usual clinical configurations of flower and basket combinations. The basket pose was purposefully chosen as the swine IVC cannot accommodate the flower configuration given its anatomic size constraints. Further, the basket poses observed in this study were more compressed than those observed during clinical use. The simulation performed (Figure 8) suggests that the basket pose used in this experiment resulted in a more concentrated delivery of PFA in its immediate vicinity, potentially increasing the exposure of the deviated esophagus to stronger ablative fields. This supports not only the validity of the model, in that the esophagus was indeed exposed to PFA, but the nature and intensity of the field is such that esophageal tissue was not electroporated as confirmed by histology.
The 10 sections examined along the entire length of the esophagus in all swine indicate that the likelihood of missing a healed lesion is unlikely. Finally, it is also important to appreciate that PFA itself can be delivered in a variety of different pulse designs, waveforms, and catheter designs, all of which would require their own individual safety assessments. Indeed, the safety of PFA described here is specific to the device and waveform used; other PFA waveforms and catheter delivery systems must also undergo similar safety evaluations to determine their effects on the esophagus.
PFA Clinical Experience
The esophageal sparing nature of PFA using the system is consistent with the clinical experience to date. In a recent clinical report, 81 paroxysmal AF patients underwent pulmonary vein isolation with this PFA catheter, without esophageal temperature monitoring or mechanical deviation. In all cases, there were no esophagus-related safety events. Given the low incidence of atrioesophageal fistula, evaluations were also performed to characterize subclinical PFA-related damage. Postprocedure esophagogastroduodenoscopy in 29 patients did not reveal any lesions or adverse effects. Furthermore, late gadolinium-enhanced magnetic resonance imaging, performed in 8 patients, identified no esophageal hyperintensities consistent with damage, despite clear hyperenhancement of the immediately adjacent atrial tissue.
RFA and the Esophagus
All 4 swine in the RFA cohort demonstrated esophageal injury with lesions varying from partially healed lesions to transmural necrotic lesions with fistulizing components. These findings are consistent with prior preclinical and clinical esophageal injury patterns that have been described.3–5,22 Indeed, the esophageal findings of this study replicate those of a prior in vivo esophageal injury model that assessed a thermal ablation energy source (focused ultrasound22). The pathological findings seen in our study are also similar to the above study suggesting that thermal-based transmural coagulation necrosis of the esophageal wall is likely the common initiating mechanism. Based on the greater prevalence of ulcerations compared with fistulous connections in both studies, it seems likely that with greater degrees of esophageal injury, some ulcers fail to heal and progress to significant necrosis and inflammation of surrounding tissue, ultimately leading to fistula formation into adjacent organs. The anatomic separation that results after the esophagus is returned to its normal location, upon removal of the deviating balloon in our model, likely explains why fistulation to the IVC with serious/fatal consequences did not occur in any swine.
A single power-duration combination (30 W for 30 seconds) was studied in this experiment with nonforce sensing irrigated catheters. This parameter was chosen to represent a clinically relevant setting for the posterior wall. While some have recently advocated a high power–short duration radiofrequency delivery strategy for the posterior LA ablation, this was not studied in the present article; our goal here was rather to support the feasibility of the model itself. The occurrence of esophageal injury in all 4 swine indicates that the esophagus was indeed in close proximity to the IVC and that the lower half of the swine esophagus is capable of demonstrating familiar injury patterns.
Limitations
The model used in this experiment requires sustained deviation of the esophagus toward the thin-walled and predominantly fibrous IVC and is, therefore, by design, only an approximation of clinical conditions. In addition, the lack of contact force in the radiofrequency arm and the differences in relation to blood flow, heat sink, histological features, and mean pressures between the IVC and the LA are significantly different; thus, our study’s conclusions may not exactly reflect actual clinical situations. Also, a lack of endoscopic assessments and pathological examinations at earlier survival time points limits our assessment of esophageal sparing by PFA to only the 25-day time point; transient intermediate changes to the esophagus cannot be ruled out. Although we chose not to monitor luminal esophageal temperature in either arm due to concerns of inaccurate temperature assessment in the setting of esophageal deviation, the lack of this data is a limitation. The IVC was not submitted for histological assessment, so we are unable to describe the effects of ablation on the IVC wall itself. However, it has previously been demonstrated that PFA of the superior vena cava does not have any appreciable ablative effect on vein wall tissue.12 Finally, these preclinical findings need to be confirmed by larger clinical studies.
Conclusions
Biphasic, bipolar PFA delivered using the multispline catheter used clinically in a novel esophageal injury model demonstrates no histopathologic changes to the esophagus at 25 days. Replication of a clinical radiofrequency dose yielded uniform and severe esophageal injury.
Sources of Funding
This study was supported by Farapulse, Inc.
Disclosures
Dr Reddy has stock and consults for Farapulse and also is a consultant for Boston Scientific. He has equity in Manual Surgical Sciences. Dr Koruth received grant support and consults for Farapulse. Dr Dukkipati has stock in Farapulse. Dr Neuzil received grant support and consults for Farapulse. R. Brose, Dr Viswanathan, and E.D. Buck are employees of Farapulse. The other authors report no conflicts. A complete list of conflicts with other medical companies not related to this manuscript is provided in the Data Supplement.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- IVC
- inferior vena cava
- LA
- left atrium
- PFA
- pulsed field ablation
- RFA
- radiofrequency ablation
For Sources of Funding and Disclosures, see page 207.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.119.008303.
References
- 1.Nair KK, Danon A, Valaparambil A, Koruth JS, Singh SM. Atrioesophageal fistula: a review. J Atr Fibrillation. 2015;8:1331. doi: 10.4022/jafib.1331. doi: 10.4022/jafib.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koruth JS, Chu EW, Bhardwaj R, Dukkipati S, Reddy VY. Esophageal damage during epicardial ventricular tachycardia ablation. JACC Clin Electrophysiol. 2017;3:1470–1471. doi: 10.1016/j.jacep.2017.03.016. doi: 10.1016/j.jacep.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation. 2017;136:1247–1255. doi: 10.1161/CIRCULATIONAHA.117.025827. doi: 10.1161/CIRCULATIONAHA.117.025827. [DOI] [PubMed] [Google Scholar]
- 4.Lim HW, Cogert GA, Cameron CS, Cheng VY, Sandler DA. Atrioesophageal fistula during cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:208–213. doi: 10.1111/jce.12313. doi: 10.1111/jce.12313. [DOI] [PubMed] [Google Scholar]
- 5.Black-Maier E, Pokorney SD, Barnett AS, Zeitler EP, Sun AY, Jackson KP, Bahnson TD, Daubert JP, Piccini JP. Risk of atrioesophageal fistula formation with contact force-sensing catheters. Heart Rhythm. 2017;14:1328–1333. doi: 10.1016/j.hrthm.2017.04.024. doi: 10.1016/j.hrthm.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Neven K, Schmidt B, Metzner A, Otomo K, Nuyens D, De Potter T, Chun KR, Ouyang F, Kuck KH. Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circ Arrhythm Electrophysiol. 2010;3:260–265. doi: 10.1161/CIRCEP.109.922930. doi: 10.1161/CIRCEP.109.922930. [DOI] [PubMed] [Google Scholar]
- 7.Kaminska I, Kotulska M, Stecka A, Saczko J, Drag-Zalesinska M, Wysocka T, Choromanska A, Skolucka N, Nowicki R, Marczak J, et al. Electroporation-induced changes in normal immature rat myoblasts (H9C2). Gen Physiol Biophys. 2012;31:19–25. doi: 10.4149/gpb_2012_003. doi: 10.4149/gpb_2012_003. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Fan Q, Ji Z, Qiu X, Li Z. The effects of irreversible electroporation on nerves. PLoS One. 2011;6:e18331. doi: 10.1371/journal.pone.0018831. doi: 10.1371/journal.pone.0018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409–1415. doi: 10.1109/TBME.2006.873745. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 10.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 11.van Driel VJ, Neven KG, van Wessel H, du Pré BC, Vink A, Doevendans PA, Wittkampf FH. Pulmonary vein stenosis after catheter ablation: electroporation versus radiofrequency. Circ Arrhythm Electrophysiol. 2014;7:734–738. doi: 10.1161/CIRCEP.113.001111. doi: 10.1161/CIRCEP.113.001111. [DOI] [PubMed] [Google Scholar]
- 12.van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12:1838–1844. doi: 10.1016/j.hrthm.2015.05.012. doi: 10.1016/j.hrthm.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Neven K, van Es R, van Driel V, van Wessel H, Fidder H, Vink A, Doevendans P, Wittkampf F. Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ Arrhythm Electrophysiol. 2017;10:e004672. doi: 10.1161/CIRCEP.116.004672. doi: 10.1161/CIRCEP.116.004672. [DOI] [PubMed] [Google Scholar]
- 14.du Pré BC, van Driel VJ, van Wessel H, Loh P, Doevendans PA, Goldschmeding R, Wittkampf FH, Vink A. Minimal coronary artery damage by myocardial electroporation ablation. Europace. 2013;15:144–149. doi: 10.1093/europace/eus171. doi: 10.1093/europace/eus171. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, Sediva L, Chovanec M, Dukkipati SR, Jais P. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–326. doi: 10.1016/j.jacc.2019.04.021. doi: 10.1016/j.jacc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. doi: 10.1016/j.jacep.2018.04.005. doi: 10.1016/j.jacep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, Buck ED, Speltz M, Dukkipati SR, Reddy VY. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol. 2019;12:e007781. doi: 10.1161/CIRCEP.119.007781. doi: 10.1161/CIRCEP.119.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong J, Stewart MT, Cheek DS, Francischelli DE, Kirchhof N. Cardiac ablation via electroporation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3381–3384. doi: 10.1109/IEMBS.2009.5332816. doi: 10.1109/IEMBS.2009.5332816. [DOI] [PubMed] [Google Scholar]
- 19.Ripley KL, Gage AA, Olsen DB, Van Vleet JF, Lau CP, Tse HF. Time course of esophageal lesions after catheter ablation with cryothermal and radiofrequency ablation: implication for atrio-esophageal fistula formation after catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:642–646. doi: 10.1111/j.1540-8167.2007.00790.x. doi: 10.1111/j.1540-8167.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, Howard B. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–764. doi: 10.1016/j.hrthm.2018.10.030. doi: 10.1016/j.hrthm.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel C, Peyman A, Grant EH. Electrical conductivity of tissue at frequencies below 1 MHz. Phys Med Biol. 2009;54:4863–4878. doi: 10.1088/0031-9155/54/16/002. doi: 10.1088/0031-9155/54/16/002. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama K, Nakagawa H, Seres KA, Jung E, Merino J, Zou Y, Ikeda A, Pitha JV, Lazzara R, Jackman WM. Canine model of esophageal injury and atrial-esophageal fistula after applications of forward-firing high-intensity focused ultrasound and side-firing unfocused ultrasound in the left atrium and inside the pulmonary vein. Circ Arrhythm Electrophysiol. 2009;2:41–49. doi: 10.1161/CIRCEP.108.807925. doi: 10.1161/CIRCEP.108.807925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


