Purpose of review
The purpose of this review is to assess whether dietary fish oil supplements can be appropriate for patients with elevated triglycerides and cardiovascular risk based on a comprehensive analysis of their composition, and level of regulatory oversight.
Recent findings
Approximately 19 million people in the United States take fish oil supplements, many for the purpose of treating or preventing heart disease. Unlike prescription products, fish oil supplements are classified as food by the Food and Drug Administration (FDA) and are not required to undergo manufacturing oversight or clinical testing. Analysis of widely used dietary fish oil supplements show that they may have lower amounts of ω-3 than advertised as well as significant levels of saturated fat and oxidized oils which actually may contribute to dyslipidemia. Clinical outcome trials have failed to show a consistent cardiovascular benefit with fish oil supplements and other low-dose mixed ω-3 fatty acids.
Summary
In light of limited regulatory oversight and evidence of quality concerns, dietary fish oil supplements are not an appropriate substitute for FDA approved prescription ω-3 fatty acids for their indicated use in treatment of elevated triglycerides or the prevention of cardiovascular events.
Keywords: fish oil, oxidized lipids, supplement
INTRODUCTION
The United States Department of Health and Human Services on complementary and alternative medicine use among adults and children states that fish oil supplements are the most commonly used supplements among adults in the United States [1]. Fish consumption is recommended in the 2015–2020 Dietary Guidelines for Americans and by the American Heart Association [2]. Although widely available, fish oil supplements are not subject to approval and oversight by the Food and Drug Administration (FDA), as they are not over-the-counter (OTC) drugs, so their content and chemical integrity are not carefully regulated [3]. Additionally, their efficacy is not assessed prior to marketing as they are classified by the FDA as a food product [3]. Yet millions of consumers, including patients with dyslipidemia, take dietary fish oil supplements, many in the hopes of preventing or treating heart disease, despite the absence of supporting evidence for their clinical efficacy.
Box 1.
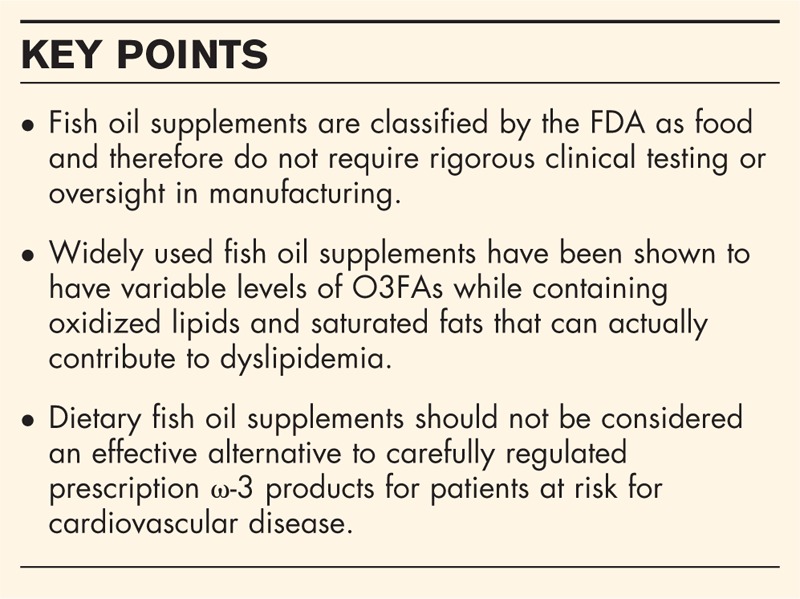
no caption available
DIETARY FISH OIL SUPPLEMENT MANUFACTURING PROCESS, QUALITY, CONTENT, AND EFFICACY
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both ω-3 polyunsaturated fatty acids (O3FAs), are the valued components in fish oil supplements. However, these and other fatty acids are particularly susceptible to oxidation because of multiple and conjugated double bonds. There are various contributors to fatty acid oxidation, including thermal-oxidation, autooxidation, photo-oxidation, metal-catalyzed oxidation, and ionically catalyzed oxidation [4]. During the manufacturing process of fish oil supplements, fatty acids may be exposed to such oxidation pathways without careful regulatory oversight. For example, to aid in the coagulation of tissue protein and expression of the oils, the harvested fish used for these supplements are heated while exposed to atmospheric oxygen. In addition, fish oil becomes oxidized through exposure to metals and enzymes found naturally in the fish [5–7]. Primary oxidation products of fatty acids include hydroperoxides, which often degrade further into secondary oxidation products such as aldehydes and ketones. These chemical changes contribute to a loss of biological integrity of fish oil supplements and produce the easily identifiable rancid odor. During oxidation, levels of EPA and DHA decline, whereas peroxide levels increase [8], and the products of the oxidation reactions may contribute to cardiovascular risk and other chronic diseases.
Independent studies from various laboratories have verified concerns about the integrity of fish oil supplements as well as the actual levels of O3FA compared with what is advertised. A study funded by the US Department of Agriculture found that only 10 out of 47 fish oil supplements had EPA levels at or above the dose indicated on their labels. Further, only twelve of these products reported accurate amounts of DHA, whereas 74% contained less than the stated label amounts of EPA or DHA [9]. In another study from New Zealand, 32 fish oil supplements were analyzed for fatty acid content, of which only 9% had O3FA levels that met or exceeded stated label amounts [10]. Of the total number of products tested, more than 80% were found to have levels of lipid peroxides, a biomarker of oxidation, which exceeded industry standards. Further, only 8% of the supplements met international standards for peroxide and total oxidation levels [10].
In another study, the quality of leading fish oil supplements (by sales) in the United States was compared with a prescription O3FA product with respect to EPA and DHA levels, the quantity and oxidation status of other fatty acids [11]. In addition to EPA and DHA, the supplements contained more than 30 different fatty acid variants. This included 10–14 saturated fat species that constituted up to 36% of the total fatty acid content. In one of the products, the amount of two saturated fats, palmitic acid and myristic acid, was nearly equivalent to the total EPA and DHA content (28 and 30%, respectively). The supplements were also analyzed and compared with a prescription ω-3 formulation for total oxidation (TOTOX), a composite of primary oxidation products measured as peroxide value and secondary oxidation products represented by anisidine value. The supplements exceeded the international threshold (as determined by the US Council for Responsible Nutrition) for peroxide value and TOTOX, and two of the three exceeded the threshold for anisidine value. By comparison, the FDA-approved O3FA prescription product did not have detectable levels of oxidized lipids or saturated fats. The fish oil supplement which contained a mixture of oxidized and nonoxidized EPA and DHA failed to inhibit lipoprotein oxidation compared with nonoxidized O3FAs. The data summarizing these findings can be found in Fig. 1a and b.
FIGURE 1.
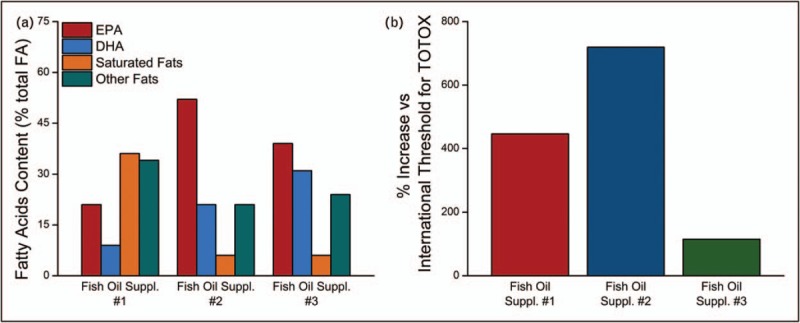
Summary of fatty acid content in three leading (by sales) fish oil supplements in the United States (a) and summary of oxidation (TOTOX) products in three leading (by sales) fish oil supplements from the United States (b). The international threshold for TOTOX levels in supplements is 26. Results show as percentage change versus the threshold for TOTOX values in three leading fish oil supplements following normalization to 1 g total ω-3 fatty acid. There was no evidence of significant oxidation in the prescription product analyzed (data not shown). Data taken from [11].
Similar findings with fish oil supplements and oxidation were reported in another North American study, which analyzed multiple lots of 16 fish oil supplements with respect to EPA and DHA content and oxidation levels [12]. Of the 16 products analyzed, only nine met their stated label claims for total EPA and DHA. Three of the products that failed to meet the stated EPA and DHA claims were manufactured by GOED (Global Organization for EPA and DHA) members. With regard to oxidation (measured as peroxide value), five of the products tested exceeded the limit established in the GOED Voluntary Monograph and the European Pharmacopeia Monograph. The two products that contained fatty acids (either partially or completely) as ethyl esters had the highest levels of oxidation with one having more than five times the limit.
Elevated levels of oxidized fatty acids in fish oil supplements may be expected to compromise any biological benefits. Although the favorable lipid and antiinflammatory effects of these products are often cited as a way to prevent cardiovascular disease, these benefits may be partly or completely negated by the lipid oxidation by-products prominently found in fish oil supplements [13,14]. In a randomized controlled trial involving healthy patients, administration of oxidized fish oils over seven weeks contributed to dyslipidemia, including an elevation in non-high density lipoprotein (HDL) cholesterol levels, compared with patients administered a primarily nonoxidized fish oil [15]. The lack of therapeutic efficacy with oxidized fish oil supplements includes no improvement in lipid or inflammatory parameter levels [16] as well as blood lipid levels [17].
Beyond lipid oxidation and saturated fats in these products, there is evidence that fish oil processing technology may contribute contaminants linked to increased risk of cancer and cardiovascular disease. An analysis of oils obtained from marine fish species indicated elevated levels of dioxin and dioxin-like polychlorinated biphenyls (dl-PCBs) higher than the maximum allowable limits for human consumption [18]. Refinement of the oils with activated carbon filters significantly reduced the presence of these compounds, but not nondioxin-like PCBs because of the lack of affinity these compounds for activated carbon. This indicates that the activated carbon alone is not sufficient for removing all harmful organic pollutants. Furthermore, results from this study showed that the refinement process increased levels of 2 and 3-monochloroproane-1,2-diols (MCPDs) in fish oils with a higher prevalence of 3-MCPDs. This is significant because 3-MCPDs are classified as a possible human carcinogen by the International Agency for Research on Cancer. The above studies indicate that the presence of oxidized oils and other organic pollutants may be present in commercial fish oil products and thus call into question their efficacy and safety for human consumption.
BIOLOGICAL CONSEQUENCES OF INGESTING OXIDIZED LIPIDS
The biological consequences of ingesting oxidized lipids and the related oxidative products have been characterized in cellular and animal studies but the application to human pathology requires further investigation. For example, 13-hydroperoxyoctadecadienoic acid (13-HPODE), a product of lipid oxidation, has been shown to significantly alter cellular functions. This includes an increase in vascular adhesion molecule expression in smooth muscle cells [19], causing human endothelial cells to express both intracellular and surface adhesion molecules [20], and activating kinase expression in aortic cells [19,21]. 13-HPODE also induces inflammation by causing elevated caspase-3 activity in enteric epithelial cells and by activating MCP1 in human vascular smooth muscle cells [22–24]. Lipid peroxidation, once initiated, increases exponentially, which alters membrane fluidity, cell signaling, and cell transport [25]. Evidence also suggests that long-term lipid peroxidation may contribute to the development of various diseases, including Alzheimer's disease and cancer [26,27]. Oxidized polyunsaturated fatty acids (PUFAs) have also been associated with hemolytic anemia, hepatomegaly, liver inflammation, and cardiomyopathy [28].
Oxidative modification of lipids has been linked to an increase in inflammation through the nuclear factor kappa-B (NF-κB) pathway [29] and insulin resistance [30,31]. The degree of oxidative stress in an organism can be quantified by measuring oxidation product levels, antioxidant levels, and the potency of antioxidant enzymes. Hydroperoxides lead to increased levels of H2O2 and subsequent catalase upregulation [32,33]. Multiple studies have shown that oxidized oils and their related oxidation products compromise cellular functions. One study found that treating cells with lipid hydroperoxides caused an eight-fold rise in cell apoptosis [34]. Additionally, rats fed oxidized oils had significantly lower α-tocopherol and glutathione levels in erythrocytes when compared with controls [35]. A similar study found that plasma α-tocopherol levels were reduced by 60% when pigs were fed a diet containing oxidized fats, thus compromising the endogenous antioxidant system and increasing risk for disease progression [36].
The period when oxidation of membranes and lipoproteins occurs is referred to as the lag phase and is an indicator of the antioxidant capacity of membranes and lipoproteins. The longer the lag phase, the greater the antioxidant capacity of the lipid particles [37]. Feeding rats oxidized oils can shorten low density lipoprotein (LDL) oxidation lag phase by as much as 25% [38]. In a clinical study, patients that consumed oxidized oil had a shortened plasma LDL-cholesterol oxidation lag phase by approximately 25% [39]. LDL that is more prone to oxidation has been linked to an increased risk for atherosclerosis [40–42].
Elevated levels of lipid hydroperoxides and reactive aldehydes predict cardiovascular events in patients with coronary artery disease (CAD) even after adjusting for traditional risk factors including cholesterol levels, obesity, and blood pressure [43,44]. These studies showed an increase in hospitalization for heart failure and revascularization for patients with CAD and elevated circulating lipid peroxides at baseline. In healthy patients fed oxidized oil, chylomicrons were found to have significantly higher levels of serum thiobarbituric acid-reactive substances (TBARS) when compared with controls [39].
Animal studies also provide supporting evidence for biological consequences of oxidized fatty acid consumption, including a 33% increase in plasma cholesterol levels with mice fed a diet containing oxidized O3FAs as compared with control [45]. Healthy rats fed oxidized oil rich in peroxides had a five-fold increase in serum lipoprotein peroxide levels, indicating that dietary peroxides are readily incorporated into lipoproteins [46]. Animals fed a diet containing oxidized oil also had aortic atheromas that were twice as large as the lesions found in control animals [47], whereas TBARS increased by 80% when rats were fed highly oxidized oil [48]. A separate study found that feeding mice a diet containing oxidized fat increased plasma LDL-cholesterol by 26% compared with control animals [49]. Finally, feeding rats oxidized oils resulted in increased levels of oxidation products in lipoproteins and caused the levels of oxidation products in the liver to double [50]. A similar study showed that liver levels of the antioxidant α-tocopherol were reduced by 50% in rats fed oxidized oils [51].
CLINICAL FINDINGS FROM FISH OIL SUPPLEMENTS AND PRESCRIPTION FORMULATIONS
Early epidemiological studies as well as outcome-based randomized clinical trials have suggested that consuming higher levels of the O3FAs may reduce the risk of heart disease [52–55]. More recent and comprehensive reviews of the clinical data with O3FAs have cast doubt on this conclusion [56▪], including a recent review that examined the effectiveness of 24 supplements and diets in cardiovascular prevention [57▪]. The authors evaluated nine systematic reviews and four randomized controlled trials, which encompassed 277 trials and 992 129 participants. Findings indicated that few nutritional supplements or dietary interventions offered any protection against cardiovascular disease or death. Supplementation with O3FA, in particular, yielded ‘low-certainty’ evidence that they were associated with reduced risk for myocardial infarction and coronary heart disease.
Large clinical outcome trials using a mixed and low-dose (1 g/day) O3FA have failed to demonstrate cardiovascular benefit. The ASCEND trial - A Study of Cardiovascular Disease in Diabetes trial; NCT00135226 reported the effect of 1 g/day of prescription O3FA therapy in 15 480 patients with diabetes but without evidence of atherosclerotic cardiovascular disease [58▪]. The results failed to demonstrate a reduction of first serious vascular events in the O3FA arm. This is consistent with most prior studies of O3FA mixtures at low doses. A similar failure was reported in the VITAL study - Vitamin D and Omega-3 Trial; NCT01169259 among more than 25 000 patients with low cardiovascular (CV) risk using again a low-dose prescription O3FA 1 g/day [59]. In this primary prevention trial, there was no statistically significant decrease in the primary composite cardiovascular endpoint or cancer-associated endpoints. Although there was a reduction in certain secondary endpoints such as MI, these are only hypothesis generating and require an appropriately powered study to test. Thus, in these two studies with over 40 000 patients, there was a consistent lack using a low-dose O3FA mixture with respect to primary prevention.
In contrast to negative studies with fish oil supplements or low-dose mixed O3FA prescription products, there was a beneficial clinical outcome with a higher dose EPA-only prescription formulation. The Reduction of Cardiovascular Events with Icosapent Ethyl - Intervention Trial (REDUCE-IT) trial was a randomized, double-blinded, placebo-controlled trial designed to examine the benefits of icosapent ethyl, a prescription, highly purified ethyl ester of EPA at 4 g/day [60▪▪]. The primary objective of this study was to determine if highly purified EPA treatment reduces ischemic events in statin-treated patients with elevated and high baseline fasting triglyceride (TG) levels (150–499 mg/dl) and cardiovascular risk for clinical events. The trial enrolled 8179 men and women with established atherosclerotic cardiovascular disease or with type 2 diabetes and one additional risk factor. The primary endpoint was a composite of time to first event for cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina and reported a highly statistically significant (P < 0.001) 25% relative risk reduction. Reductions in key secondary endpoints were also significant, including a 20% reduction in CV death, 31% reduction in MI, and a 28% reduction in stroke. Another study called STRENGTH (Statin Residual Risk Reduction with Epanova in High CV Risk Patients with Hypertriglyceridemia; NCT02104817) evaluated if a 4 g/d mix of EPA and DHA was beneficial in statin-treated patients with elevated TGs. Recently, this trial was terminated due to futility at the recommendation of the independent data monitoring committee. Thus, the only O3FA proven to be of CV benefit on top of statin therapy in outcome trials remains prescription EPA.
CONCLUSION
Fish oil supplements are classified by the FDA as food, and thus, their approval and ongoing production does not require robust FDA review. Fish oil supplements – unlike prescription and OTC medications – do not need to demonstrate effectiveness in placebo-controlled clinical trials. Patients who consume these products are often confused by statements suggesting a benefit to cardiovascular health despite affirmative clinical evidence. In fact, a comprehensive review of the basic and clinical evidence shows that widely used fish oil supplements contain lower amounts of O3FAs than specified on the label as well as saturated fats and oxidized oils that may actually contribute to dyslipidemia and increased cardiovascular risk. Consumption of oxidized oils has been linked to increases in non-HDL cholesterol and vascular changes associated with atherosclerosis, including inflammation, LDL oxidation, and endothelial dysfunction (Fig. 2). The results of a recent study showed a significant cardiovascular benefit with a highly purified and quality-controlled prescription preparation of EPA (4 g/day). Thus, dietary fish oil supplements should not be considered an effective substitute for appropriate dosage of prescription O3FA products, which are supported by strict testing and ongoing oversight by FDA (Fig. 3).
FIGURE 2.
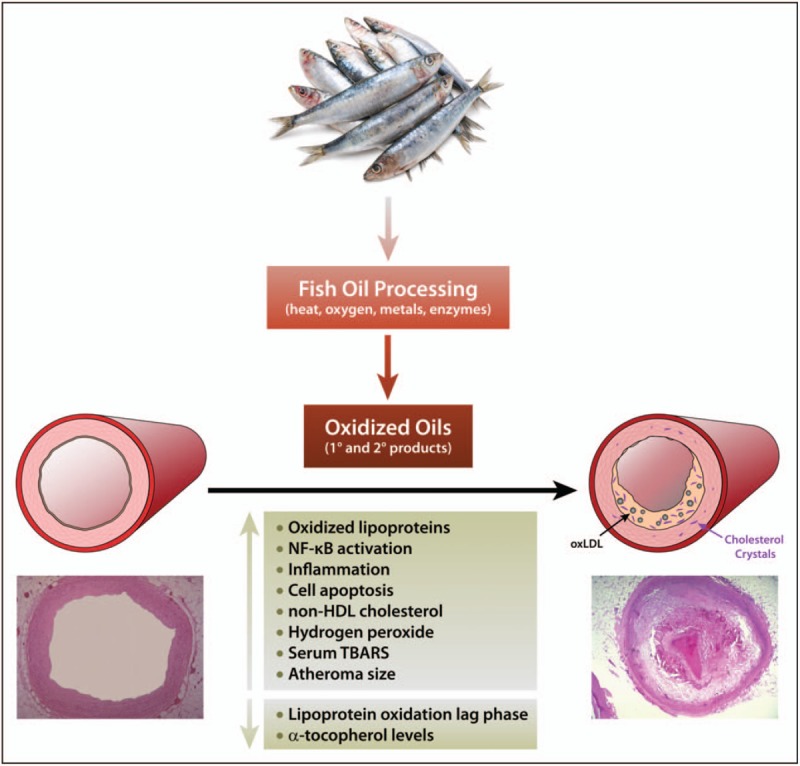
Summary of risks associated with ingestion of oxidized oils, including oxidized oils sourced from fish.
FIGURE 3.
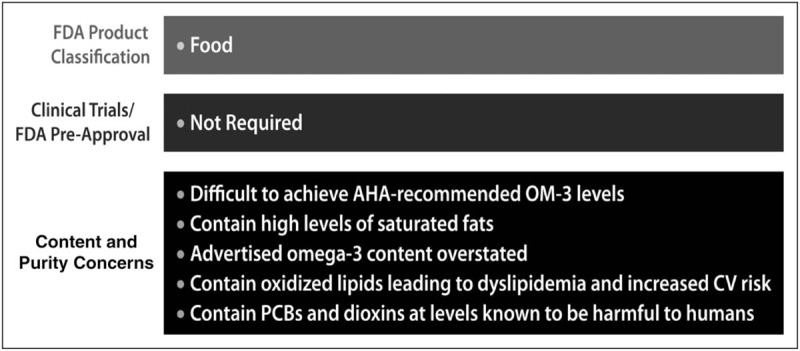
Overview of fish oil supplement classification and content.
Acknowledgements
The authors wish to thank Dr. Robert F. Jacob for expert scientific discussions and editorial assistance.
Financial support and sponsorship
None.
Conflicts of interest
R.P.M. acknowledges consulting and research funding from Amarin Pharma, Inc. (Amarin), Novartis, and Pfizer. The other authors have no disclosures.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 2008; 12:1–23. [PubMed] [Google Scholar]
- 2.Rimm EB, Appel LJ, Chiuve SE, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation 2018; 138:e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilleman D, Smer A. Prescription omega-3 fatty acid products and dietary supplements are not interchangeable. Manage Care 2016; 25:46–52. [PubMed] [Google Scholar]
- 4.Frankel EN. Lipid oxidation. Bridgwater, UK: The Oily Press; 2005. [Google Scholar]
- 5.Bimbo AP. The emerging marine oil industry. J Am Oil Chem Soc 1987; 64:706–715. [Google Scholar]
- 6.Richards MP, Li R. Effects of released iron, lipid peroxides, and ascorbate in trout hemoglobin-mediated lipid oxidation of washed cod muscle. J Agric Food Chem 2004; 52:4323–4329. [DOI] [PubMed] [Google Scholar]
- 7.Undeland I, Ekstrand B, Lingnert H. Lipid oxidation in herring (Clupea harengus) light muscle, dark muscle, and skin, stored separately or as intact fillets. J Am Oil Chem Soc 1998; 75:581–590. [Google Scholar]
- 8.Fritsche KL, Johnston PV. Rapid autoxidation of fish oil in diets without added antioxidants. J Nutr 1988; 118:425–426. [DOI] [PubMed] [Google Scholar]
- 9.Kleiner AC, Cladis DP, Santerre CR. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J Sci Food Agric 2015; 95:1260–1267. [DOI] [PubMed] [Google Scholar]
- 10.Albert BB, Derraik JG, Cameron-Smith D, et al. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep 2015; 5:7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason RP, Sherratt SC. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem Biophys Res Commun 2017; 483:425–429. [DOI] [PubMed] [Google Scholar]
- 12.Ritter JC, Budge SM, Jovica F. Quality analysis of commercial fish oil preparations. J Sci Food Agric 2013; 93:1935–1939. [DOI] [PubMed] [Google Scholar]
- 13.Khan F, Elherik K, Bolton-Smith C, et al. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc Res 2003; 59:955–962. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt EB, Arnesen H, de Caterina R, et al. Marine n-3 polyunsaturated fatty acids and coronary heart disease. Part I. Background, epidemiology, animal data, effects on risk factors and safety. Thromb Res 2005; 115:163–170. [DOI] [PubMed] [Google Scholar]
- 15.Rundblad A, Holven KB, Ottestad I, et al. High-quality fish oil has a more favourable effect than oxidised fish oil on intermediate-density lipoprotein and LDL subclasses: a randomised controlled trial. Br J Nutr 2017; 117:1291–1298. [DOI] [PubMed] [Google Scholar]
- 16.Poppitt SD, Howe CA, Lithander FE, et al. Effects of moderate-dose omega-3 fish oil on cardiovascular risk factors and mood after ischemic stroke: a randomized, controlled trial. Stroke 2009; 40:3485–3492. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Hernandez VM, Gallar M, Sanchez-Soriano J, et al. Effect of omega-3 dietary supplements with different oxidation levels in the lipidic profile of women: a randomized controlled trial. Int J Food Sci Nutr 2013; 64:993–1000. [DOI] [PubMed] [Google Scholar]
- 18.Merkle S, Giese E, Rohn S, et al. Impact of fish species and processing technology on minor fish oil components. Food Control 2017; 73:1379–1387. [Google Scholar]
- 19.Natarajan R, Reddy MA, Malik KU, et al. Signaling mechanisms of nuclear factor-kappab-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2001; 21:1408–1413. [DOI] [PubMed] [Google Scholar]
- 20.Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest 1995; 95:1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao GN, Alexander RW, Runge MS. Linoleic acid and its metabolites, hydroperoxyoctadecadienoic acids, stimulate c-Fos, c-Jun, and c-Myc mRNA expression, mitogen-activated protein kinase activation, and growth in rat aortic smooth muscle cells. J Clin Invest 1995; 96:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997; 326 (Pt 1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwarakanath RS, Sahar S, Reddy MA, et al. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-kappa B). J Mol Cell Cardiol 2004; 36:585–595. [DOI] [PubMed] [Google Scholar]
- 24.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J 2004; 384:201–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev 2010; 39:4067–4079. [DOI] [PubMed] [Google Scholar]
- 26.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006; 391:499–510. [DOI] [PubMed] [Google Scholar]
- 27.Sayre LM, Zelasko DA, Harris PL, et al. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem 1997; 68:2092–2097. [DOI] [PubMed] [Google Scholar]
- 28.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr 1993; 57:779S–785S. discussion 785S–786S. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg R, Haenen GR, van den Berg H, Bast A. Transcription factor NF-kappaB as a potential biomarker for oxidative stress. Br J Nutr 2001; 86: Suppl 1: S121–S127. [DOI] [PubMed] [Google Scholar]
- 30.Festa A, D’Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000; 102:42–47. [DOI] [PubMed] [Google Scholar]
- 31.Libby P. Inflammation in atherosclerosis. Nature 2002; 420:868–874. [DOI] [PubMed] [Google Scholar]
- 32.Meilhac O, Zhou M, Santanam N, Parthasarathy S. Lipid peroxides induce expression of catalase in cultured vascular cells. J Lipid Res 2000; 41:1205–1213. [PubMed] [Google Scholar]
- 33.Santanam N, Aug N, Zhou M, et al. Overexpression of human catalase gene decreases oxidized lipid-induced cytotoxicity in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1999; 19:1912–1917. [DOI] [PubMed] [Google Scholar]
- 34.Wang TG, Gotoh Y, Jennings MH, et al. Lipid hydroperoxide-induced apoptosis in human colonic CaCo-2 cells is associated with an early loss of cellular redox balance. FASEB J 2000; 14:1567–1576. [DOI] [PubMed] [Google Scholar]
- 35.Keller U, Brandsch C, Eder K. The effect of dietary oxidized fats on the antioxidant status of erythrocytes and their susceptibility to haemolysis in rats and guinea pigs. J Anim Physiol Anim Nutr (Berl) 2004; 88:59–72. [DOI] [PubMed] [Google Scholar]
- 36.Eder K, Stangl GI. Plasma thyroxine and cholesterol concentrations of miniature pigs are influenced by thermally oxidized dietary lipids. J Nutr 2000; 130:116–121. [DOI] [PubMed] [Google Scholar]
- 37.Cadenas E, Sies H. The lag phase. Free Radic Res 1998; 28:601–609. [DOI] [PubMed] [Google Scholar]
- 38.Eder K, Keller U, Hirche F, Brandsch C. Thermally oxidized dietary fats increase the susceptibility of rat LDL to lipid peroxidation but not their uptake by macrophages. J Nutr 2003; 133:2830–2837. [DOI] [PubMed] [Google Scholar]
- 39.Staprans I, Rapp JH, Pan XM, et al. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler Thromb 1994; 14:1900–1905. [DOI] [PubMed] [Google Scholar]
- 40.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med 2000; 28:1815–1826. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D. A critical look at the evidence for the oxidation of LDL in atherogenesis. Atherosclerosis 1997; 131: Suppl: S5–S7. [DOI] [PubMed] [Google Scholar]
- 42.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991; 88:1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walter MF, Jacob RF, Bjork RE, et al. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: the PREVENT study. J Am Coll Cardiol 2008; 51:1196–1202. [DOI] [PubMed] [Google Scholar]
- 44.Walter MF, Jacob RF, Jeffers B, et al. Serum levels of TBARS predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol 2004; 44:1996–2002. [DOI] [PubMed] [Google Scholar]
- 45.Penumetcha M, Khan-Merchant N, Parthasarathy S. Enhanced solubilization and intestinal absorption of cholesterol by oxidized linoleic acid. J Lipid Res 2002; 43:895–903. [PubMed] [Google Scholar]
- 46.Staprans I, Rapp JH, Pan XM, Feingold KR. The effect of oxidized lipids in the diet on serum lipoprotein peroxides in control and diabetic rats. J Clin Invest 1993; 92:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staprans I, Rapp JH, Pan XM, et al. Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 1996; 16:533–538. [DOI] [PubMed] [Google Scholar]
- 48.Garrido-Polonio C, Garcia-Linares MC, Garcia-Arias MT, et al. Thermally oxidised sunflower-seed oil increases liver and serum peroxidation and modifies lipoprotein composition in rats. Br J Nutr 2004; 92:257–265. [DOI] [PubMed] [Google Scholar]
- 49.Khan-Merchant N, Penumetcha M, Meilhac O, Parthasarathy S. Oxidized fatty acids promote atherosclerosis only in the presence of dietary cholesterol in low-density lipoprotein receptor knockout mice. J Nutr 2002; 132:3256–3262. [DOI] [PubMed] [Google Scholar]
- 50.Brandsch C, Eder K. Effects of peroxidation products in thermoxidised dietary oil in female rats during rearing, pregnancy and lactation on their reproductive performance and the antioxidative status of their offspring. Br J Nutr 2004; 92:267–275. [DOI] [PubMed] [Google Scholar]
- 51.Brandsch C, Nass N, Eder K. A thermally oxidized dietary oil does not lower the activities of lipogenic enzymes in mammary glands of lactating rats but reduces the milk triglyceride concentration. J Nutr 2004; 134:631–636. [DOI] [PubMed] [Google Scholar]
- 52.De Caterina R, Madonna R, Zucchi R, La Rovere MT. Antiarrhythmic effects of omega-3 fatty acids: from epidemiology to bedside. Am Heart J 2003; 146:420–430. [DOI] [PubMed] [Google Scholar]
- 53.Harrison N, Abhyankar B. The mechanism of action of omega-3 fatty acids in secondary prevention postmyocardial infarction. Curr Med Res Opin 2005; 21:95–100. [DOI] [PubMed] [Google Scholar]
- 54.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002; 288:2569–2578. [DOI] [PubMed] [Google Scholar]
- 55.Lee KW, Lip GY. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM 2003; 96:465–480. [DOI] [PubMed] [Google Scholar]
- 56▪.Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 2018; 3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review of clinical trials using ω-3 fatty acids showing a consistent lack of cardiovascular benefit for fish oil supplements and low-dose mixed O3FAs.
- 57▪.Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med 2019; 171:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive analysis of systematic reviews and trials showing that few nutritional supplements or dietary interventions offered any protection against cardiovascular disease or death.
- 58▪.Group AS, Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018; 379:1540–1550. [DOI] [PubMed] [Google Scholar]; The ASCEND trial failed to demonstrate a reduction of first serious vascular events with 1 g/day of prescription O3FA therapy in 15 480 patients with diabetes but without evidence of atherosclerotic cardiovascular disease.
- 59.Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2018; 380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60▪▪.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019; 380:11–22. [DOI] [PubMed] [Google Scholar]; Results of the REDUCE-IT trial showing that highly purified EPA treatment reduced the primary endpoint of cardiovascular events (MACE) by 25% in patients with elevated and high baseline fasting TG levels and cardiovascular risk for clinical events.


