Abstract
Objectives
With increasing antimicrobial resistance, rapid antimicrobial susceptibility testing (RAST) becomes important, especially in patients with bloodstream infections. EUCAST decided to develop a standardized rapid method, based on EUCAST disc diffusion, to offer susceptibility reports within 4–8 h of a positive blood culture (BC).
Methods
BC bottles were spiked with clinical isolates (n = 332) of the seven most relevant sepsis pathogens with a variety of resistance mechanisms. RAST was performed directly from the bottle and zones read after 4, 6 and 8 h. Several variables were investigated, including the effect of using different BC bottles and of a 0–18 h delay between a positive signal and the performance of RAST.
Results
For five species, most inhibition zones could be read after 4 h. The proportion of results that could be interpreted increased from 75% at 4 h to 84% after 8 h. Categorical agreement against the reference method was good, with error rates of false susceptibility of 0.2%, 0.2% and 0.2% at 4, 6 and 8 h and false resistance of 1.2%, 0.2% and 0.1% at 4, 6 and 8 h, respectively.
Conclusions
With the EUCAST RAST method, reliable AST results can be delivered within 4–8 h of positivity of BC bottles for seven important bloodstream infection pathogens. To reduce the occurrence of errors and to absorb the variability caused by using a non-standardized inoculum, material from different manufacturers and workflow-related delays, we have introduced an area in which interpretation is not permitted, the Area of Technical Uncertainty.
Introduction
With increasing antimicrobial resistance, rapid antimicrobial susceptibility testing (RAST) becomes increasingly important, especially in patients with bloodstream infection (BSI). Several authors have shown that RAST and appropriate early therapy improve the clinical outcome in BSI and septic shock.1–4 Improved management of BSI includes widening of indications for blood culture (BC), 24 h staffing of laboratories or at least 24 h access for hospital staff to BC instruments.
Disc diffusion is one of the oldest and most frequently used methods for susceptibility testing. It is applicable to a broad range of bacteria and agents, needs no special equipment and is more versatile than any other method. The EUCAST standardized method5 was developed for 16–20 h incubation. A ‘rapid’ AST method should produce results in less than 8 h to have significant impact. Laboratories have developed in-house disc diffusion RAST methods, some based on EUCAST standard methodology from cultured colonies.6–8 These studies have shown that the size of inhibition zones changes over incubation time and that WT isolates (lacking phenotypically detectable acquired resistance mechanisms to the agent) and non-WT isolates (having phenotypically detectable resistance) behave differently. In our studies, shorter incubation leads to smaller inhibition zones for WT isolates and larger zones for non-WT isolates.9 This results in poorer separation between WT and non-WT isolates. It is crucial to realize that: (i) zone diameter breakpoints developed for standard disc diffusion cannot be used with RAST; and (ii) the robustness of the test is decreased due to the poorer separation between the two populations.
EUCAST’s aims for RAST were: (i) the method should be valid for relevant BSI pathogens and agents; (ii) the time to results should be shorter than a standard work day; (iii) materials should be available in a standard microbiology laboratory; (iv) the method should be easy to perform and control; (v) it should be independent of the BC system; and (vi) it should be freely available on the EUCAST website.
This paper describes the development of a RAST method based on EUCAST standard disc diffusion methodology but with inoculation with untreated/unprocessed broth directly from positive BC bottles and a shorter incubation time (reading of results after 4, 6 and 8 h). It was validated against EUCAST breakpoints and standard methodology. Pathogens commonly isolated from patients with BSI and targeted in both the EARS-Net10 and the CAESAR11 surveillance programmes were included (Table 1).
Table 1.
Bacterial isolates and specific resistance mechanisms used in the evaluation of the RAST method
| Species (number of isolates) and antimicrobial agents tested (BMD as referencea) | Number of R isolates | Resistance mechanism identifiedb |
|---|---|---|
| E. coli (n = 60) | ||
| piperacillin/tazobactam | 14 |
|
| cefotaxime | 25 | |
| ceftazidime | 20 | |
| meropenem | 3 | |
| ciprofloxacin | 21 | |
| amikacin | 1 | |
| gentamicin | 17 | |
| tobramycin | 20 | |
| K. pneumoniae (n = 52) | ||
| piperacillin/tazobactam | 18 |
|
| cefotaxime | 20 | |
| ceftazidime | 16 | |
| meropenem | 6 | |
| ciprofloxacin | 21 | |
| amikacin | 3 | |
| gentamicin | 16 | |
| tobramycin | 17 | |
| P. aeruginosa (n = 53) | ||
| piperacillin/tazobactam | 17 | multiple and mixed resistance mechanisms |
| ceftazidime | 15 | |
| imipenem | 15 | |
| meropenem | 11 | |
| ciprofloxacin | 24 | |
| gentamicin | 14 | |
| tobramycin | 11 | |
| S. aureus (n = 54) | ||
| cefoxitin (screen) | 21 | MRSA |
| norfloxacin (screen) | 17 | |
| gentamicin | 8 | |
| erythromycin | 17 | |
| clindamycin | 7 | |
| E. faecalis (n = 23) | ||
| ampicillin | 0 |
|
| imipenem | 0 | |
| gentamicin (screen) | 17 | |
| linezolid | 2 | |
| vancomycin | 9 | |
| E. faecium (n = 34) | ||
| ampicillin | 31 |
|
| imipenem | 33 | |
| gentamicin (screen) | 19 | |
| linezolid | 6 | |
| vancomycin | 22 | |
| S. pneumoniae (n = 56) | ||
| oxacillin (screen) | 24 | benzylpenicillin non-WT (screen positive with oxacillin 1 μg disc and with benzylpenicillin MICs of 0.125–4 mg/L) |
| norfloxacin (screen) | 8 | |
| erythromycin | 26 | |
| clindamycin | 10 | |
| trimethoprim/ sulfamethoxazole | 11 | |
| Control strains (n = 5) | ||
|
– | |
BMD was used as reference, with the exceptions listed as ‘screen’, where EUCAST standard disc diffusion screen tests were used. For S. aureus, PCR was used as reference for methicillin resistance.
In some cases, resistance genes/mechanisms were identified through WGS.
Materials and methods
Bacterial isolates and reference AST data
The isolates were from international collections of clinical isolates (see Acknowledgements) and from clinical samples at Clinical Microbiology, Region Kronoberg, Sweden, between 2000 and 2017. Isolates represented WT and non-WT isolates with various resistance mechanisms (Table 1). The levels of resistance are shown in the MIC distributions in Tables S1 to S7 (available as Supplementary data at JAC Online). Species were identified using the Microflex system with MALDI Biotyper (MBT) v. 3.1 software (Bruker Daltonics) and the MBT database DB-5627. AST was performed for all isolates using broth microdilution (BMD) according to ISO 20776–112 (using EUCAST MH-F broth for Streptococcus pneumoniae) and EUCAST standard disc diffusion.13 BMD results were used as reference with the exception of discs intended for screening for specific resistance mechanisms, where the EUCAST reference method for screening was used (Table 1, Tables S1 to S7). Results were interpreted according to EUCAST Breakpoint Tables v. 8.0, 2018.14 For Staphylococcus aureus and cefoxitin, mecA status by in-house PCR was used as reference and all cefoxitin screen-positive isolates were mecA positive.
Spiked BC bottles
BC bottles, BACTEC™ Plus Aerobic/F (Becton, Dickinson and Company, Sparks MD, USA), were inoculated with a bacterial suspension of 100–200 cfu from an overnight culture on a blood agar plate. To simulate routine conditions, 5 mL sterile defibrinated horse blood (Håtunalab AB, Bro, Sweden) was added to each bottle prior to incubation in the BC instrument, BACTEC FX (Becton, Dickinson and Company). Bottles were positive after 3.5–17.5 h in the instrument (mean and median value 11.5 h). To imitate real-life conditions, bottles were removed 0–14 h after positive signal (mean value 5 h). Disc diffusion according to the EUCAST RAST method, as described below, was performed immediately.
EUCAST RAST methodology
Media
Standard EUCAST media, Mueller–Hinton (MH) and Mueller–Hinton Fastidious (MH-F) agar were used. AST was performed on 90 mm circular plates produced in-house using agar from two manufacturers in parallel: Oxoid (Thermo Fisher Scientific, Basingstoke, UK) and BBL (BD, Sparks, MD, USA), resulting in two readings per isolate and agent.
Discs
Antibiotic discs (Oxoid/Thermo Fisher Scientific), were chosen to represent relevant agents or agent groups used in the treatment of BSI (Table 1).
Procedure
The EUCAST standard disc diffusion methodology13 was modified as follows: (i) the inoculum was defined as 100–150 μL broth directly from a positive BC bottle; (ii) zones were read after 4, 6 and 8 h (with a variation of ±5 min); and (iii) all zones were read from the front of the plate with the lid removed. The inoculum was evenly distributed over the agar with a cotton swab using an automatic plate rotator (Retro C80, bioMérieux, Marcy l’Étoile, France). Inhibition zones were read manually using a calliper. The plates were reincubated within 10 min of removal from the incubator to allow readings at 6 and 8 h (Table 1). Only tests with confluent growth and clearly delineated inhibition zones were read.
Quality control (QC)
QC according to EUCAST standard methodology was performed daily to control materials and equipment used.15
QC of the RAST method was performed by inoculating BC bottles with five different QC strains (Table 1) using the same RAST methodology as described above. For each QC strain, the RAST procedure was repeated 16–20 times throughout the experiments.
Data analysis and establishment of RAST breakpoints
RAST breakpoints, specific for each species and agent and for 4, 6 and 8 h of incubation, were established to optimize susceptibility categorization. The EUCAST standard procedure to establish zone diameter breakpoints based on MIC–zone diameter correlates was used.5 To deal with the greater variation and poorer separation with the RAST method, we introduced an area between confirmed susceptible (S) and resistant (R) results where interpretation was not permitted, the Area of Technical Uncertainty (ATU). Thus, for each reading time the possible results were S, R and ATU (interpretation not permitted). The proportion of results for which an interpretation could (S or R) and could not (ATU) be offered at 4, 6 and 8 h was calculated. For results where an interpretation of S or R was possible, the proportion of very major errors (VMEs: RAST = S and reference method = R), major errors (MEs: RAST = R and reference method = S) and minor errors (mEs: RAST = S or R and reference method = intermediate, I) were calculated versus EUCAST Breakpoint Tables v. 8.0.14 The proportion of errors was calculated on the total number of tests with a readable inhibition zone. With the introduction of the ATU, there are no I (definition from 2019: ‘susceptible, increased exposure’) results with the RAST method.
The influence of variation in the RAST system
To develop a robust RAST method that would tolerate the variations inherent to a clinical laboratory, we investigated the effect on inhibition zone diameters and on bacterial growth of: (i) a delay in removing a positive bottle from the instrument; (ii) a delay in performing RAST after the removal of a positive bottle from the instrument; and (iii) the effect of using BC bottles from different manufacturers. These studies are presented in the Supplementary data (see ‘The influence of variation in the RAST system’; Table S8).
Results
Inhibition zone diameters after 4, 6 and 8 h of incubation
It was possible to measure inhibition zones after 4 h for the majority of isolates of Escherichia coli (92%), Klebsiella pneumoniae (99%), S. aureus (58%), Enterococcus faecalis (93%) and S. pneumoniae (82%) but not for Pseudomonas aeruginosa (0%) and Enterococcus faecium (44%). After 6 h, 89%–100% of all zones could be read and after 8 h it was possible to read 98%–100% of all zones (Table 2). Inhibition zones could not be read when there was: (i) insufficient growth (i.e. no growth or non-confluent growth); or (ii) a poorly delineated zone edge. For E. coli, K. pneumoniae and P. aeruginosa, thin hazy growth within the inhibition zones was sometimes observed at early readings but disappeared at 6 or 8 h. The hazy growth was always ignored if an outer zone edge was clearly visible. In general, with longer incubation time, zone diameters for WT isolates increased whereas zone diameters for non-WT isolates decreased. Thus, with longer incubation time, the gap between the S and R populations increased and the risk of overlap decreased (Figures 1 to 4; Figures S1 to S7).
Table 2.
Theoretical and actual number of tests performed, the proportion of tests that could be read and interpreted after 4, 6 and 8 h and the categorical errors with RAST at each reading time for the seven species (E. coli, K. pneumoniae, P. aeruginosa, S. aureus, E. faecalis, E. faecium and S. pneumoniae)
| E. coli (n = 60 isolates) | ||||
| Incubation time (h) | 4 | 6 | 8 | |
| Number of tests (n) | ||||
| Theoretical number of testsa | 960 | 960 | 960 | |
| Completed testsb | 958 | 958 | 958 | |
| Readable zones (% of completed tests)c | 886 (92) | 958 (100) | 958 (100) | |
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 19 | 20 | 20 | |
| Interpreted as S | 57 | 58 | 58 | |
| Interpreted as R | 24 | 22 | 22 | |
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 1.0 | 1.4 | 1.6 |
| ME | 2.2 | 0.0 | 0.0 | |
| VME | 0.1 | 0.0 | 0.0 | |
| Total errors | 3.3 | 1.4 | 1.6 | |
| K. pneumoniae (n = 52 isolates) | ||||
| Incubation time (h) | 4 | 6 | 8 | |
| Number of tests (n) | ||||
| Theoretical number of testsa | 832 | 832 | 832 | |
| Completed testsb | 831 | 831 | 831 | |
| Readable zones (% of completed tests)c | 820 (99) | 831 (100) | 831 (100) | |
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 28 | 23 | 19 | |
| Interpreted as S | 45 | 52 | 55 | |
| Interpreted as R | 27 | 25 | 25 | |
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 1.7 | 0.8 | 0.6 |
| ME | 0.7 | 0.0 | 0.0 | |
| VME | 0.0 | 0.0 | 0.1 | |
| Total errors | 2.4 | 0.8 | 0.6 | |
| P. aeruginosa (n = 53 isolates) | ||||
| Incubation time (h) | 6 | 8 | ||
| Number of tests (n) | ||||
| Theoretical number of testsa | 742 | 742 | ||
| Completed testsb | 741 | 741 | ||
| Readable zones (% of completed tests)c | 676 (91) | 727 (98) | ||
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 18 | 17 | ||
| Interpreted as S | 57 | 58 | ||
| Interpreted as R | 24 | 22 | ||
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 2.2 | 1.7 | |
| ME | 0.4 | 0.0 | ||
| VME | 0.2 | 0.0 | ||
| Total errors | 2.7 | 1.7 | ||
| S. aureus (n = 54 isolates) | ||||
| Incubation time (h) | 4 | 6 | 8 | |
| Number of tests (n) | ||||
| Theoretical number of testsa | 324 | 432 | 432 | |
| Completed testsb | 324 | 432 | 432 | |
| Readable zones (% of completed tests)c | 188 (58) | 385 (89) | 392 (91) | |
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 11 | 8 | 6 | |
| Interpreted as S | 73 | 66 | 67 | |
| Interpreted as R | 16 | 26 | 27 | |
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 0.0 | 0.0 | 0.5 |
| ME | 0.0 | 0.3 | 0.0 | |
| VME | 0.0 | 0.3 | 0.0 | |
| Total errors | 0.0 | 0.6 | 0.5 | |
| E. faecalis (n = 23 isolates)d | ||||
| Incubation time (h) | 4 | 6 | 8 | |
| Number of tests (n) | ||||
| Theoretical number of testsa | 260 | 260 | 260 | |
| Completed testsb | 260 | 260 | 260 | |
| Readable zones (% of completed tests)c | 242 (93) | 259 (100) | 260 (100) | |
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 31 | 30 | 25 | |
| Interpreted as S | 54 | 56 | 58 | |
| Interpreted as R | 15 | 14 | 17 | |
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 0.0 | 0.0 | 0.0 |
| ME | 0.0 | 0.0 | 0.0 | |
| VME | 0.0 | 0.0 | 0.0 | |
| Total errors | 0.0 | 0.0 | 0.0 | |
| E. faecium (n = 34 isolates)e | ||||
| Incubation time (h) | 6 | 8 | ||
| Number of tests (n) | ||||
| Theoretical number of testsa | 380 | 380 | ||
| Completed testsb | 380 | 380 | ||
| Readable zones (% of completed tests)c | 352 (93) | 375 (99) | ||
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 19 | 14 | ||
| Interpreted as S | 17 | 23 | ||
| Interpreted as R | 64 | 63 | ||
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 0.7 | 0.6 | |
| ME | 0.7 | 0.3 | ||
| VME | 0.0 | 0.0 | ||
| Total errors | 1.4 | 0.9 | ||
| S. pneumoniae (n = 66 isolates) | ||||
| Incubation time (h) | 4 | 6 | 8 | |
| Number of tests (n) | ||||
| Theoretical number of testsa | 560 | 560 | 560 | |
| Completed testsb | 560 | 560 | 560 | |
| Readable zones (% of completed tests)c | 461 (82) | 550 (98) | 558 (100) | |
| Results calculated on readable zones (%) | ||||
| Not interpreted as S or R (ATU) | 26 | 22 | 8 | |
| Interpreted as S | 48 | 52 | 66 | |
| Interpreted as R | 26 | 26 | 27 | |
| Errors calculated on the total number of zones interpreted as S or R (%) | ||||
| Errors | mE | 1.2 | 0.9 | 0.8 |
| ME | 1.2 | 0.5 | 0.4 | |
| VME | 0.6 | 0.9 | 1.2 | |
| Total errors | 2.9 | 2.3 | 2.3 | |
Theoretical number of tests = total number of possible isolate/agent combinations in duplicate (due to testing on media from two manufacturers).
Number of completed tests = number of completed tests after excluding missing data (e.g. disc dropped).
Readable zones = number of tests with readable inhibition zones.
For E. faecalis some isolates have been tested several times resulting in a total of 52 readings.
For E. faecium some isolates have been tested several times resulting in a total of 76 readings.
Figure 1.
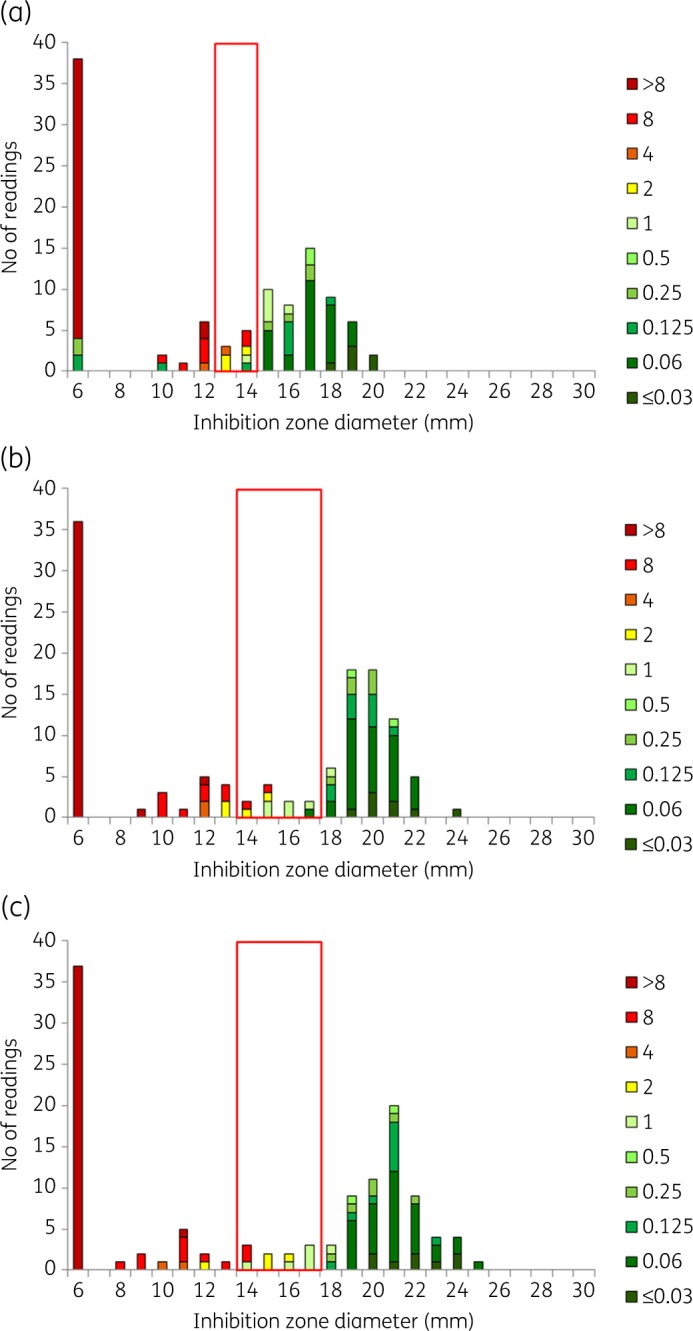
Cefotaxime BMD MIC and inhibition zone diameter distributions for RAST after (a) 4 h, (b) 6 h and (c) 8 h incubation for E. coli (n = 60) and cefotaxime 5 μg. All isolates were tested on MH agar from two manufacturers in parallel, resulting in a theoretical maximum number of 120 results. The colour coding shows MIC values (mg/L) of isolates. The red box shows the ATU where interpretation is not permitted. Zone diameters greater than the ATU are interpreted as S and zones smaller than the ATU are interpreted as R. Data for all other agent/organism combinations are available as Supplementary data (Figures S1 to S7).
Figure 4.
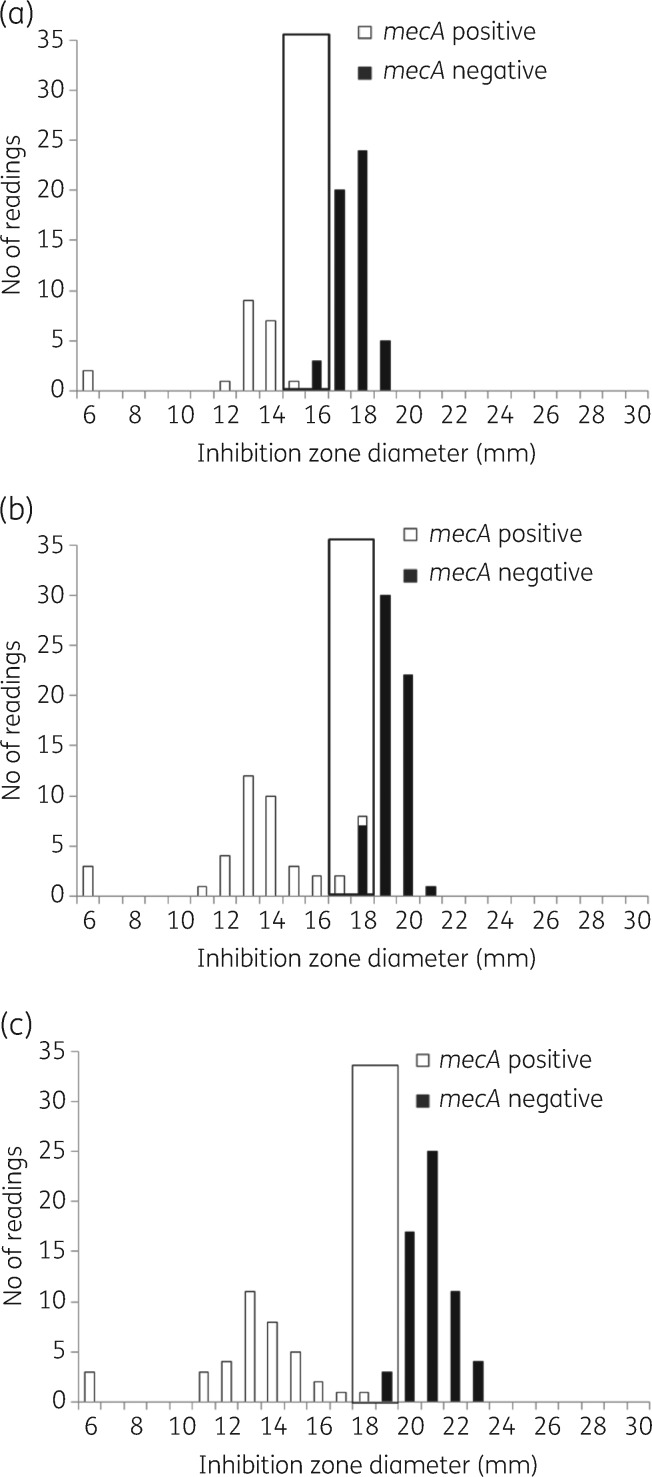
mecA status and inhibition zone diameter distributions for RAST after (a) 4 h, (b) 6 h and (c) 8 h incubation for S. aureus (n = 54) and cefoxitin 30 μg. All isolates were tested on MH agar from two manufacturers in parallel, resulting in a theoretical maximum number of 108 results. The colours of the bars correspond to presence or absence of the mecA gene. The black box shows the ATU where interpretation is not permitted. Zone diameters greater than the ATU are interpreted as S and zones smaller than the ATU are interpreted as R. Data for all other agent/organism combinations are available in Figures S1 to S7.
Figure 2.
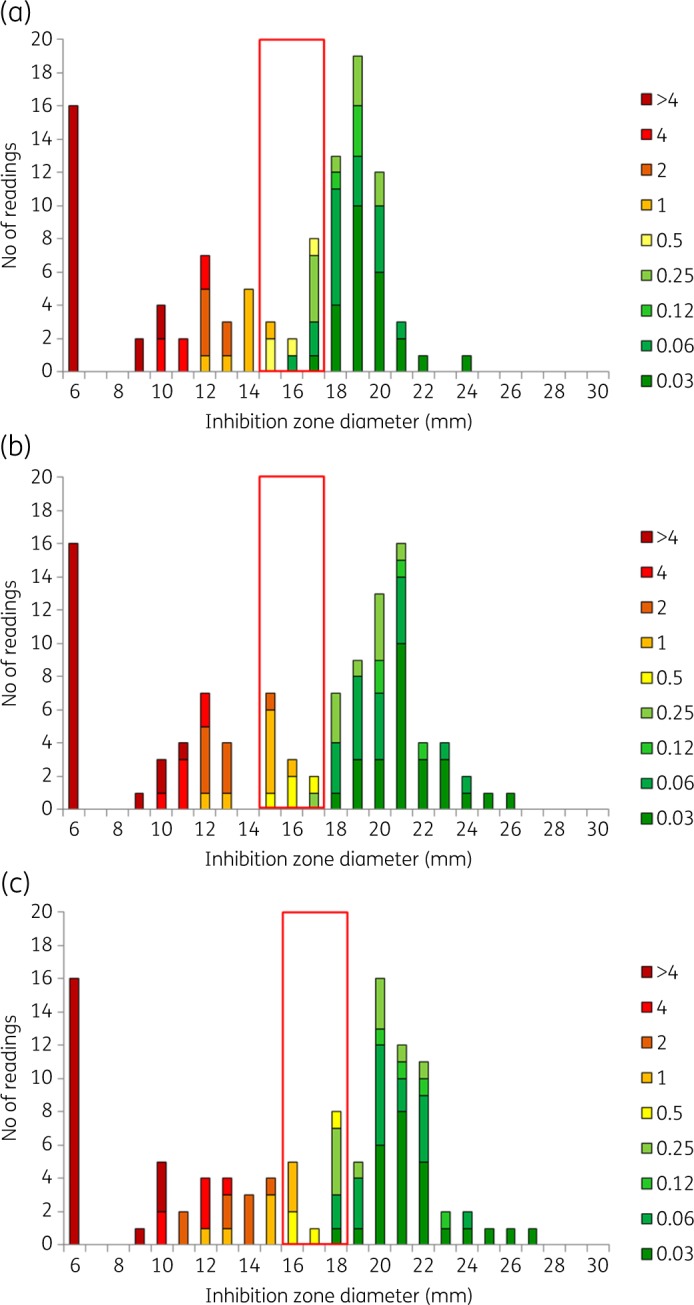
Ciprofloxacin BMD MIC and inhibition zone diameter distributions for RAST after (a) 4 h, (b) 6 h and (c) 8 h incubation for K. pneumoniae (n = 52) and ciprofloxacin 5 μg. All isolates were tested on MH agar from two manufacturers in parallel, resulting in a theoretical maximum number of 104 results. The colour coding shows MIC values (mg/L) of isolates. The red box shows the ATU where interpretation is not permitted. Zone diameters greater than the ATU are interpreted as S and zones smaller than the ATU are interpreted as R. Data for all other agent/organism combinations are available in Figures S1 to S7.
Figure 3.
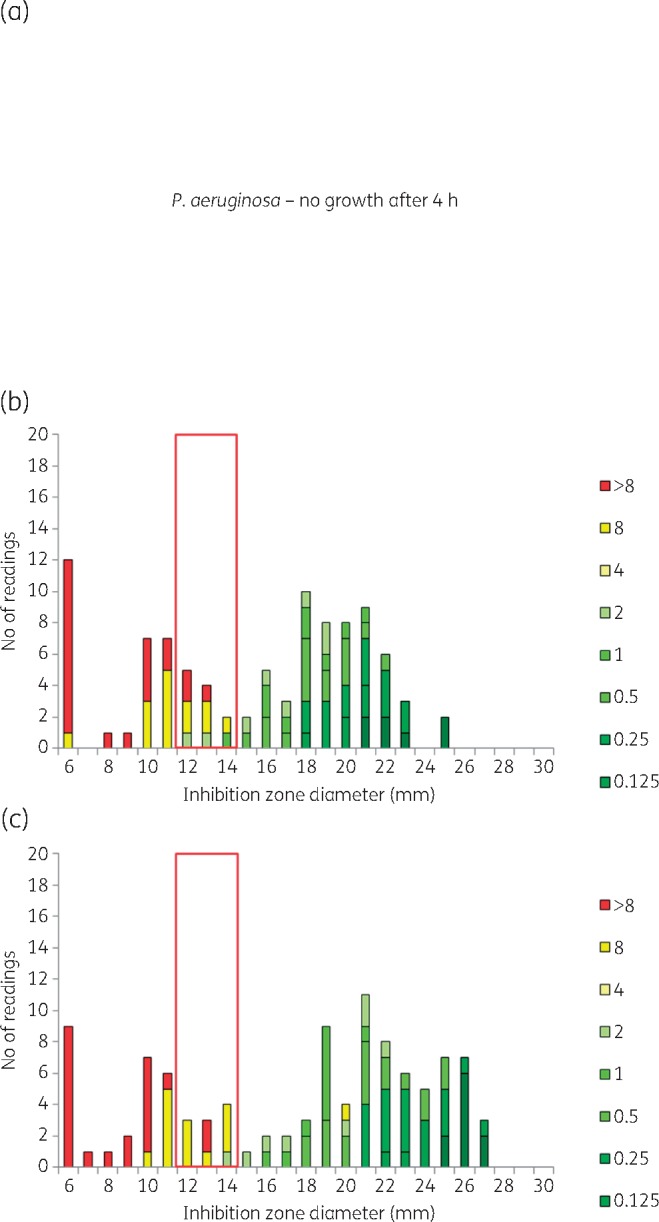
Meropenem BMD MIC and inhibition zone diameter distributions for RAST after (a) 4 h, (b) 6 h and (c) 8 h incubation for P. aeruginosa (n = 53) and meropenem 10 μg. All isolates were tested on MH agar from two manufacturers in parallel, resulting in a theoretical maximum number of 106 results. The colour coding shows MIC values (mg/L) of isolates. The red box shows the ATU where interpretation is not permitted. Zone diameters greater than the ATU are interpreted as S and zones smaller than the ATU are interpreted as R. Data for all other agent/organism combinations are available in Figures S1 to S7.
Establishment of the preliminary RAST breakpoints
Individual breakpoints for each agent, species and reading time were determined according to the outlined principles. We were able to determine preliminary zone diameter breakpoints for all investigated species/agent combinations except for S. aureus versus clindamycin due to poor separation (Table 1) and for P. aeruginosa and E. faecalis at 4 h due to poor growth.
For many species/agent combinations, a reliable distinction between S and R isolates could be achieved (Figures 1 to 4) and both S and R breakpoints could be established (Figures S1 to S7). The placement and width of the ATU depended primarily on the degree of separation between S and R isolates and, as shown, this will differ between species, agent and reading time (Figures S1 to S7).
For a few combinations, the overlap between S and R isolates was problematic: E. coli and K. pneumoniae versus piperacillin/tazobactam, S. aureus versus clindamycin, S. pneumoniae versus clindamycin and enterococci versus vancomycin. For these, it was not always possible to define both S and R breakpoints and no RAST breakpoints were defined for S. aureus versus clindamycin. For enterococci versus ampicillin and imipenem, only an S breakpoint was set for E. faecalis and only an R breakpoint for E. faecium.
The proportion of results in the ATU decreased over time from an average of 25% (range 11%–31%, depending on agent and species) after 4 h to 20% (8%–30%) after 6 h and to 16% (5%–25%) after 8 h (Table 2). With the tentative breakpoints and after excluding those tests where no interpretation was obtained (insufficient growth/no measurable zone or a result in the ATU), the error rates at 4, 6 and 8 h were as follows: VME 0.2%, 0.2%, 0.2% and ME 1.2%, 0.2% and 0.1%, respectively (Table 2). VMEs and MEs were mostly related to the shortest incubation time and to results obtained with the tentative breakpoints used for screening for fluoroquinolone resistance with norfloxacin in S. aureus and S. pneumoniae. Errors were not related to the brand of MH medium used (Table 3).
Table 3.
List of all VMEs and MEs with RAST for each species/agent combination and incubation time and the corresponding reference categorizationa
| Species | Isolate | Antimicrobial agent | Error | Incubation time (h) | RAST zone/ interpretationb |
Reference |
Standard disc diffusion discrepant from MIC | Agar manufacturer | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | 6 h | 8 h | disc diffusion | MIC | |||||||
| E. coli | A | TZP | ME | 4 | 6/R | 16/ATU | 19/ATU | — | S | BD BBL | |
| TZP | ME | 4 | 6/R | 16/ATU | 17/ATU | — | S | Oxoid | |||
| CTX | ME | 4 | 6/R | 19/S | 21/S | — | S | BD BBL | |||
| CTX | ME | 4 | 6/R | 20/S | 19/S | — | S | Oxoid | |||
| CAZ | ME | 4 | 6/R | 18/S | 18/S | — | S | BD BBL | |||
| CAZ | ME | 4 | 6/R | 18/S | 19/S | — | S | Oxoid | |||
| B | TZP | ME | 4 | 6/R | 19/ATU | 19/ATU | — | S | BD BBL | ||
| TZP | ME | 4 | 6/R | 18/ATU | 19/ATU | — | S | Oxoid | |||
| CTX | ME | 4 | 6/R | 20/S | 19/S | — | S | BD BBL | |||
| CTX | ME | 4 | 6/R | 19/S | 20/S | — | S | Oxoid | |||
| CAZ | ME | 4 | 6/R | 21/S | 19/S | — | S | BD BBL | |||
| CAZ | ME | 4 | 6/R | 19/S | 19/S | — | S | Oxoid | |||
| C | TZP | ME | 4 | 12/R | 15/ATU | 15/ATU | — | S | BD BBL | ||
| D | TZP | ME | 4 | 6/R | 16/ATU | 17/ATU | — | S | Oxoid | ||
| E | CTX | ME | 4 | 10/R | 19/S | 21/S | — | S | BD BBL | ||
| F | TOB | VME | 4 | 14/S | 14/ATU | 14/ATU | — | R | I | BD BBL | |
| K. pneumoniae | G | AMK | ME | 4 | 12/R | 13/ATU | 13/S | — | S | I | Oxoid |
| H | GEN | VME | 8 | 13/ATU | 13/ATU | 14/S | — | R | I | BD BBL | |
| P. aeruginosa | I | IPM | ME | 6 | — | 6/R | 19/S | — | S | BD BBL | |
| IPM | ME | 6 | — | 6/R | 18/S | — | S | Oxoid | |||
| J | TZP | VME | 6 | — | 17/S | 15/ATU | — | R | BD BBL | ||
| S. aureus | K | NOR | ME | 6 | — | 6/R | 15/S | S | — | Oxoid | |
| L | NOR | VME | 6 | — | 14/S | 14/ATU | R | — | BD BBL | ||
| E. faecium | M | AMP | ME | 6 | — | 6/R | 9/S | — | S | R | BD BBL |
| AMP | ME | 6, 8 | — | 6/R | 6/R | — | S | R | Oxoid | ||
| S. pneumoniae | N | SXT | ME | 4 | 6/R | 15/S | 15/S | — | S | BD BBL | |
| SXT | ME | 4 | 6/R | 14/S | 15/S | — | S | Oxoid | |||
| O | SXT | ME | 4, 6, 8 | 6/R | 6/R | 6/R | — | S | R | BD BBL | |
| SXT | ME | 4, 6, 8 | 6/R | 6/R | 6/R | — | S | I | Oxoid | ||
| P | NOR | VME | 4, 6, 8 | 12/S | 14/S | 13/S | R | — | BD BBL | ||
| NOR | VME | 4, 6, 8 | 11/S | 12/S | 12/S | R | — | Oxoid | |||
| Q | NOR | VME | 6, 8 | NG | 14/S | 13/S | R | — | BD BBL | ||
| NOR | VME | 6, 8 | NG | 13/S | 13/S | R | — | Oxoid | |||
| R | NOR | VME | 8 | NG | NG | 13/S | R | — | BD BBL | ||
| NOR | VME | 8 | NG | 11/ATU | 12/S | R | — | Oxoid | |||
AMK, amikacin; AMP, ampicillin; CAZ, ceftazidime; CTX, cefotaxime; IPM, imipenem; GEN, gentamicin; NOR, norfloxacin; SXT, trimethoprim/sulfamethoxazole; TOB, tobramycin; TZP, piperacillin/tazobactam; NG, no growth; Oxoid, from Thermo Fisher Scientific; BD BBL, from BD. A dash in either of the ‘Reference’ columns indicates that method is not used as a reference.
A single isolate may show several errors and some errors are with both MH manufacturers.
Erroneous interpretations are shown in bold.
Detection of resistance mechanisms
With the suggested breakpoints, all E. coli and K. pneumoniae isolates resistant to cefotaxime, ceftazidime or meropenem with BMD were either in the ATU or correctly categorized as R. All MRSA isolates were in the ATU or correctly categorized as R. Enterococci with high-level aminoglycoside resistance (HLAR) were either in the ATU or correctly categorized by RAST with gentamicin. Enterococci with vanA were reported as R with the suggested RAST breakpoints. Isolates with vanB were either reported as R or were not interpreted as they ended up within the ATU. All oxacillin screen-positive S. pneumoniae with standard disc diffusion were correctly categorized by RAST.
QC strains
The QC values for the reference AST methods (BMD and standard disc diffusion) were within published ranges.15
The QC procedure developed for RAST demonstrated that inhibition zones were systematically different compared with standard disc diffusion (Tables S9 to S13). These data and data from two clinical trials initiated by EUCAST to validate the RAST method (to be published separately) were used to define specific QC targets and ranges for the RAST procedure. Separate targets and ranges were needed for 4, 6 and 8 h readings. The RAST QC recommendations are used to facilitate the introduction of the methodology in the laboratory and are embedded in the RAST breakpoint table.16
The influence of variation in the RAST method
The influence of variation due to delays in the workflow at the laboratory or the use of BC bottles from different manufacturers is described in Tables S8 and S14 and Figures S8 and S9). In summary, the variations caused by either of these were absorbed by the ATU.
Discussion
It is important to avoid empirical therapy in patients with severe illnesses. With increasing antimicrobial resistance, empirical therapy will fail more often and it is especially important to shorten the period until effective therapy is administered in patients with severe illnesses.3,17 In these cases, safer options for empirical therapy are preferred, which in turn accelerate the development of resistance to last-option agents such as carbapenems, polymyxins and, more recently, β-lactam/β-lactamase inhibitor agents.4
The EUCAST standardized method for RAST directly from positive BC bottles offers results within a standard work day. The variation caused by a non-standardized inoculum and by laboratories using several different BC systems, work schedules and media from different manufacturers was absorbed by the introduction of the ATU. The method delivered results for many species and agents as soon as 4 h, and for others after 6 h, of incubation. Since presenting criteria for the original seven relevant BSI pathogens and agents on the EUCAST website, Acinetobacter baumannii has been added and we are currently developing criteria for more agents. We also developed a specific QC procedure to be used for implementation of RAST in routine laboratories.16,18
To develop a robust and reliable method, we investigated the growth characteristics of relevant pathogens, both WT isolates and non-WT isolates. It became clear that zone diameter breakpoints needed to be recalibrated for shortened incubation and a non-standardized inoculum. Interpretative robustness was achieved by developing specific breakpoints for each of the seven species and the three reading times and by the introduction of an area between S and R where susceptibility categorization was not permitted (the ATU).
As with other standard methods, one must allow for random variation in materials and procedures. When following the recommended RAST methodology, this variation (e.g. BC bottles and media from different manufacturers and reasonable variation in laboratory procedures) was sufficiently small to be absorbed by the introduction of the ATU.
The main part of the work in this study was performed using a single BC system (BD BACTEC™), but altogether four BC bottle types (from three manufacturers) were evaluated. We used discs from a manufacturer with proven good quality19 and testing was performed on MH agar from two manufacturers in parallel to include some media variation (unpublished data, J. Åhman, E. Matuschek and G. Kahlmeter).
There are limitations to the EUCAST RAST system. Not all AST results were available within 4 h; only half of the S. aureus and E. faecium grew and none of the P. aeruginosa, but all species grew after 6 h. This is well inside the span of a working day. Also, although errors were few, those that did occur were predominantly related to the shortest incubation time. The ATU was crucial to minimize VMEs and MEs. The proportion of readings in the ATU was different for different species and agents but always decreased over time from an average of 25% at 4 h to 16% at 8 h. However, since our isolates were selected to be challenging, the proportion of results in the ATU will be smaller among consecutive clinical BCs, especially in areas where resistance is less common. Also, when the result of one agent could not be interpreted, often that of others could. Abandoning the notion that a susceptibility report has to be complete before it is released, valid and useful results could be made available after 4, 6 and 8 h, gradually completing the report.
Other authors have shown that RAST methods based on disc diffusion are possible both from cultured colonies and directly from positive BCs.6–8,20–22 However, these studies all had one or several of the following limitations: longer incubation times; lower categorical agreement; lack of a defined ATU; and mostly the number of species and/or isolates was limited. Authors agree on the time dependence of the formation of inhibition zone diameters, the need for time-related and species-specific breakpoints and the need for an ATU. Some have identified the need to recalibrate zone diameter breakpoints.6–8,20 This is also supported by our results. Only a few authors have published methods where reading after 4 h was possible.21,23 The need for a lag phase cannot be eliminated or significantly shortened.24 In our study, several techniques were tried before we decided to use untreated/unprocessed broth directly from positive BC bottles, thereby achieving the shortest possible lag phase (unpublished data, E. Jonasson, E. Matuschek and G. Kahlmeter).
Published phenotypic, non-commercial initiatives are not standardized and are not broadly validated.20–22,25,26 They are often based on material (BC bottles, discs, MH medium, gradient tests, a semi-automated device etc.) from a single manufacturer and interpretation is often performed with breakpoints for standard AST. All initiatives require incubation for at least 4 h and usually longer.27
Commercial phenotypic methods for RAST from positive BC bottles are being developed and some are available.23,28–30 It is outside the scope of this article to review these and the reader is referred to two recent publications.24,27 With few exceptions, these are still under development, not yet commercially available and/or take more than 4 h. To our knowledge, the Accelerate Pheno™ system is the only commercially available system. However, it is expensive to run and has limited capacity.28,30 It is a long and arduous road to deliver a full-scale AST system.24,27
With the breakpoints and ATUs that were defined in this study, VMEs and MEs were generally low. For E. coli, K. pneumoniae, P. aeruginosa and S. aureus, 70%–90% of all readable results could be interpreted as S or R. VMEs and MEs were few (<0.2%) for these four species. For enterococci there were no VMEs and only a few MEs. The large proportion of results in the ATU for enterococci was primarily explained by the failure of vancomycin to correctly predict resistance in vanB-positive isolates with low MICs. This problem exists when using standard phenotypic AST techniques, but was accelerated by the conscious inclusion of many problematic isolates. Most errors for S. pneumoniae were related to the norfloxacin screen and mostly when zones were close to the screening breakpoint with the standard method. These isolates were S to levofloxacin and moxifloxacin with BMD and the problem was resolved when EUCAST decided to revise the screening breakpoint for the standard method.31
The EUCAST RAST method is not complicated to perform and requires only equipment and competence already available in clinical laboratories. However, it may require that laboratories review and tackle logistics related to BC, including workflow, opening hours and staffing. All parts of the chain are equally important; transportation of BC bottles to the laboratory, around-the-clock availability of BC instruments, time to species identification (which today can be reduced to 60 min)32 and laboratory staff availability. It is evident that time to AST results can be significantly reduced with RAST and that it is no longer acceptable to wait until the next day for an AST report when results can be available within 4–8 h. However, laboratories must develop systems by which the RAST result is promptly conveyed to and understood by those responsible for therapy. If achieved, this will help improve clinical outcome, especially where resistance is common.
On the basis of the results of this study, a preliminary set of breakpoints and ATUs were defined and then tested in two major clinical trials involving 55 laboratories in Europe, using different BC systems and discs and media from many different manufacturers.33,34 After having aggregated all results (spiked bottles and the results from the clinical trials) a method for RAST directly from BC bottles and a set of breakpoint tables were proposed and accepted by the EUCAST Steering Committee. The methodology, breakpoint tables, implementation guide and QC procedure are available on the EUCAST website (www.eucast.org).
Conclusions
The EUCAST RAST disc diffusion method was developed to offer a standardized method for direct AST from positive BC bottles, with specific breakpoints for each species and precise reading times (4, 6 and 8 h) as specified by EUCAST. The method absorbed variation from the use of different BC systems, MH media, workflows and opening hours. Laboratories can rapidly report reliable S and R results and, rather than an uncertain result, report a blank. Categorical agreement was acceptable and error rates low when tested on difficult isolates with an array of resistance mechanisms.
The method is not complicated to introduce into standard clinical microbiology laboratories but will require adaptation of workflow. It is cheap to run, quicker than other current methods, based on known and accepted material, more flexible than any other system and will potentially lead to a considerably shortened time for susceptibility test results to reach the bedside of the patient. With the incorporation of the ATU, unavoidable variation is prevented from causing VMEs and MEs. Guidance for the most important species and agents is now available on the EUCAST website.
Supplementary Material
Acknowledgements
The authors thank Paul Rhomberg and Ronald Jones (JMI Laboratories, Iowa, USA) for isolates from the worldwide SENTRY Antimicrobial Resistance Surveillance Program and Christian G. Giske (Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden) for contributing isolates. Part of the results from this study have been presented at the 27th ECCMID, Vienna, Austria, 2017 (Poster 165) and the 28th ECCMID in Madrid, Spain, 2018 (Oral presentation 0746).
Funding
This work was supported by the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) through its regular support of the development of EUCAST methodology, and by Region Kronoberg, Sweden, Research and Development funds (grant numbers 1075, 782331).
Transparency declarations
None to declare.
Author contributions
E.J. performed the antimicrobial susceptibility testing. E.J., E.M. and G.K. planned the study and analysed and evaluated the results. All authors contributed to writing the manuscript.
References
- 1. Buehler SS, Madison B, Snyder SR. et al. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 2016; 29: 59–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrer R, Martin-Loeches I, Phillips G. et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014; 42: 1749–55. [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Roberts D, Wood KE. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–96. [DOI] [PubMed] [Google Scholar]
- 4. Rhodes A, Evans LE, Alhazzani W. et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 5. Matuschek E, Brown DFJ, Kahlmeter G.. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect 2014; 20: O255–66. [DOI] [PubMed] [Google Scholar]
- 6. Fröding I, Vondracek M, Giske CG.. Rapid EUCAST disc diffusion testing of MDR Escherichia coli and Klebsiella pneumoniae: inhibition zones for extended-spectrum cephalosporins can be reliably read after 6 h of incubation. J Antimicrob Chemother 2017; 72: 1094–102. [DOI] [PubMed] [Google Scholar]
- 7. Hombach M, Jetter M, Blöchliger N. et al. Fully automated disc diffusion for rapid antibiotic susceptibility test results: a proof-of-principle study. J Antimicrob Chemother 2017; 72: 1659–68. [DOI] [PubMed] [Google Scholar]
- 8. van den Bijllaardt W, Buiting A, Mouton JW. et al. Shortening the incubation time for antimicrobial susceptibility testing by disk diffusion for Enterobacteriaceae: how short can it be and are the results accurate? Int J Antimicrob Agents 2017; 49: 631–7. [DOI] [PubMed] [Google Scholar]
- 9. Jonasson E, Matuschek E, Kahlmeter G, Validation of EUCAST rapid antimicrobial susceptibility testing (RAST) with disk diffusion tests directly from positive blood cultures for Pseudomonas aeruginosa, Haemophilus influenzae, Enterococcus faecalis and Enterococcus faecium. Twenty-Eighth European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain,2018. Abstract O747.
- 10.ECDC. Surveillance of Antimicrobial Resistance in Europe2017. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net).https://ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017.
- 11.Central Asian and Eastern European Surveillance of Antimicrobial Resistance. Annual report 2018. http://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/publications/2018/central-asian-and-eastern-european-surveillance-of-antimicrobial-resistance-annual-report-2018-2018.
- 12.International Organization for Standardization. Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases: 20776–1. 1996. [Google Scholar]
- 13.EUCAST. Antimicrobial susceptibility testing EUCAST disk diffusion method. Version 6.0, 2017. http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/.
- 14.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0, 2018. http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/.
- 15.EUCAST. Routine and extended internal quality control for MIC determination and disk diffusion as recommended by EUCAST. Version 8.0, 2018. http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/.
- 16.EUCAST. Zone diameter breakpoints for rapid antimicrobial susceptibility testing (RAST) directly from blood culture bottles. Version 1.0, 2018. http://www.eucast.org/rapid_ast_in_blood_cultures/breakpoints_for_short_incubation/.
- 17. Cassini A, Diaz Högberg L, Plachouras D. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2018; 18: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EUCAST. EUCAST rapid antimicrobial susceptibility testing (RAST). Version 1.0, 2018. http://www.eucast.org/rapid_ast_in_blood_cultures/methods/.
- 19. Åhman J, Matuschek E, Kahlmeter G.. The quality of antimicrobial discs from nine manufacturers – EUCAST evaluations in 2014 and 2017. Clin Microbiol Infect 2019; 25: 346–52. [DOI] [PubMed] [Google Scholar]
- 20. Chandrasekaran S, Abbott A, Campeau S. et al. Direct-from-blood-culture disk diffusion to determine antimicrobial susceptibility of Gram-negative bacteria: preliminary report from the Clinical and Laboratory Standards Institute methods development and standardization working group. J Clin Microbiol 2018; 56: 1678–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coyle MB, McGonagale LA, Plorde JJ. et al. Rapid antimicrobial susceptibility testing of isolates from blood cultures by direct inoculation and early reading of disk diffusion tests. J Clin Microbiol 1984; 20: 473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Périllaud C, Pilmis B, Diep J. et al. Prospective evaluation of rapid antimicrobial susceptibility testing by disk diffusion on Mueller-Hinton rapid-SIR directly on blood cultures. Diagn Microbiol Infect Dis 2019; 93: 41–21. [DOI] [PubMed] [Google Scholar]
- 23. Barnini S, Brucculeri V, Morici P. et al. A new rapid method for direct antimicrobial susceptibility testing of bacteria from positive blood cultures. BMC Microbiol 2016; 16: 185.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Idelevich EA, Becker K.. How to accelerate antimicrobial susceptibility testing. Clin Microbiol Infect 2019; 25: 1347–55. [DOI] [PubMed] [Google Scholar]
- 25. Heather CS, Maley M.. Automated direct screening for resistance of Gram-negative blood cultures using the BD Kiestra WorkCell. Eur J Clin Microbiol Infect Dis 2018; 37: 117–25. [DOI] [PubMed] [Google Scholar]
- 26. Hogan CA, Watz N, Budvytiene I. et al. Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn Microbiol Infect Dis 2019; 94: 116–21. [DOI] [PubMed] [Google Scholar]
- 27. van Belkum A, Bachmann T, Lüdke G. et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol 2019; 17: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charnot-Katsikas A, Tesic V, Love N. et al. Use of the Accelerate Pheno System for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J Clin Microbiol 2018; 56: 1166–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi J, Yong Jeong H, Yoon Lee G. et al. Direct, rapid antimicrobial susceptibility test from positive blood cultures based on microscopic imaging analysis. Sci Rep 2017; 7: 1148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pancholi P, Carroll KC, Buchan B. et al. Multicenter evaluation of the Accelerate PhenoTest BC Kit for rapid identification and phenotypic antimicrobial susceptibility testing using morphokinetic cellular analysis. J Clin Microbiol 2018; 56: 1329–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, 2019. http://www.eucast.org.
- 32. Martiny D, Dediste A, Vandenberg O.. Comparison of an in-house method and the commercial Sepsityper™ kit for bacterial identification directly from positive blood culture broths by matrix-assisted laser desorption-ionisation time-of-flight mass spectrometry. Eur J Clin Microbiol Infect Dis 2012; 31: 2269–81. [DOI] [PubMed] [Google Scholar]
- 33. Jonasson E, Åkerlund A, Matuschek E. et al. Multi-laboratory validation of the rapid method for antimicrobial susceptibility testing directly from positive blood cultures proposed by EUCAST. Twenty-Eighth European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain,2018. Oral presentation O0747.
- 34. Jonasson E, Matuschek E, Kahlmeter G, Multi-laboratory evaluation in southern Europe of the EUCAST method for rapid antimicrobial susceptibility testing directly from positive blood cultures. Twenty-Ninth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands,2019. Oral presentation O0254.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.