Abstract
Objectives
Eumycetoma is currently treated with a combination of itraconazole therapy and surgery, with limited success. Recently, olorofim, the lead candidate of the orotomides, a novel class of antifungal agents, entered a Phase II trial for the treatment of invasive fungal infections. Here we determined the activity of olorofim against Madurella mycetomatis, the main causative agent of eumycetoma.
Methods
Activity of olorofim against M. mycetomatis was determined by in silico comparison of the target gene, dihydroorotate dehydrogenase (DHODH), and in vitro susceptibility testing. We also investigated the in vitro interaction between olorofim and itraconazole against M. mycetomatis.
Results
M. mycetomatis and Aspergillus fumigatus share six out of seven predicted binding residues in their DHODH DNA sequence, predicting susceptibility to olorofim. Olorofim demonstrated excellent potency against M. mycetomatis in vivo with MICs ranging from 0.004 to 0.125 mg/L and an MIC90 of 0.063 mg/L. Olorofim MICs were mostly one dilution step lower than the itraconazole MICs. In vitro interaction studies demonstrated that olorofim and itraconazole work indifferently when combined.
Conclusions
We demonstrated olorofim has potent in vitro activity against M. mycetomatis and should be further evaluated in vivo as a treatment option for this disease.
Introduction
The poverty-associated disease mycetoma, which was added to the Neglected Tropical Disease List in 2016 by WHO, remains a major health problem in endemic areas.1,2 Most cases occur in the mycetoma belt between latitudes 15° South and 30° North.3,4 Mycetoma presents itself as a subcutaneous chronic granulomatous infectious and inflammatory disease characterized by the formation of grains in affected tissues.3,5 In more than 80% of the cases, the foot and leg are affected.4 This disease is divided into two groups: actinomycetoma (mycetoma caused by bacteria) and eumycetoma (mycetoma caused by fungi). Although many different fungal species are found to cause eumycetoma, Madurella mycetomatis dominates other fungal species and is present in more than 70% of all patients.4,6
Eumycetoma is recalcitrant in nature, which necessitates prolonged antifungal therapy combined with massive and repeated surgical debridement. In severe cases, amputation of the affected part may be the only remaining treatment option.3,7,8 Previous reports determined that M. mycetomatis was most susceptible to the azole class of antifungal agents9–11 and is currently treated with itraconazole.12 Treatment with itraconazole may take years and, with an average monthly income of only $60/month, itraconazole at $330/month is considered to be too expensive for patients. Thus there is a dire need for another antifungal agent that is active against M. mycetomatis.13
Olorofim, formerly known as F901318 (F2G Ltd, Eccles, Manchester, UK), is the leading representative from a novel class of antifungal agents called the orotomides.14 Olorofim inhibits the fungal enzyme dihydroorotate dehydrogenase (DHODH) leading to obstruction of the pyrimidine biosynthesis pathway.14,15 Studies have demonstrated that olorofim is active against pathogenic and azole-resistant Aspergillus species,16–19Scedosporium species,20Lomentospora prolificans,20Coccidioides immitis,21Fusarium proliferatum22 and other dimorphic fungi.22 Oliver et al.14 also demonstrated that olorofim exhibited much greater potency against Aspergillus spp. compared with other leading antifungal classes. Given the potency and activity of olorofim, here we aim to evaluate its in vitro activity against M. mycetomatis and the in vitro interaction between olorofim and itraconazole as a first effort to determine whether olorofim shows potential as a new treatment for eumycetoma.
Materials and methods
In silico modelling
The M. mycetomatis DHODH sequence was obtained by BLAST analysis using the Aspergillus fumigatus DHODH protein sequence as a guide (EC 1.3.5.2). M. mycetomatis, A. fumigatus and Homo sapiens DHODH sequences were aligned using Clustal Omega (EMBL-EBI, UK) and formatted using BOXSHADE (EMBnet node, Switzerland). Mitochondrial targeting sequences of M. mycetomatis and A. fumigatus DHODH were predicted by MitoFates23 (Japan), while the transmembrane domains were predicted by Phobius24 (Stockholm Bioinformatics Centre, Sweden).
Isolates
A total of 21 M. mycetomatis isolates with different genetic25,26 and geographical backgrounds were used in this study. Among the isolates used, 14 isolates originated from Sudan, there was 1 isolate each from Algeria, Mali, India, Chad and the Netherlands and there were 2 isolates with unknown origin. Isolates were obtained from the Mycetoma Research Centre in Sudan, the Swiss Tropical Institute in Switzerland and the Westerdijk Fungal Biodiversity Institute and Erasmus Medical Centre mycetoma collection in the Netherlands. All isolates are maintained and preserved in the Erasmus Medical Centre’s mycetoma collection. Isolates were identified to species level on the basis of morphology, PCR-based RFLP and sequencing of the internal transcribed spacer (ITS) regions.27
Fungal preparation
Fungal colonies were maintained on Sabouraud dextrose agar (BD Biosciences). After 3 weeks of growth at 37°C, colonies were scraped off, sonicated at maximum power for 5 s (Soniprep 150, Beun de Ronde, The Netherlands) and then inoculated into 50 mL Greiner tubes (Sigma–Aldrich) containing RPMI-1640 culture medium supplemented with 0.35 g/L l-glutamine and 1.94 mM MOPS. The isolates were then further incubated for 7 days at 37°C. After incubation, the mycelia within were washed once with RPMI-1640 culture medium. A fungal suspension of 69%–71% transmission was then prepared (Novaspec II spectrophotometer) for in vitro susceptibility testing.
In vitro susceptibility testing
Susceptibility testing was carried out according to the previously described and validated method developed for susceptibility testing using a standardized hyphal inoculum.9,28 Antifungal activity of olorofim against M. mycetomatis was determined using the XTT assay. Efficacy of olorofim was compared with that of itraconazole. Olorofim was dissolved in DMSO and tested at a range of 0.004–2 mg/L at a 2-fold dilution rate. Itraconazole was also dissolved in DMSO and tested at a range of 0.008–16 mg/L at a 2-fold dilution rate. The assay was carried out in round-bottom microtitre plates (Greiner Bio-one, The Netherlands). Wells in the microtitre plates were filled with different concentrations of olorofim or itraconazole and 100 μL of fungal suspension. For each fungal isolate, a drug-free and a negative control were included. The microtitre plates were then sealed and placed at 37°C for 7 days. Endpoints were determined at Day 7 and supernatant was measured at 450 nm (Epoch 2, Biotek, USA). MICs of olorofim and itraconazole were determined. MIC was defined as the lowest concentration with a minimum of 80% growth reduction. With the XTT assay, 100% reduction in viable fungal mass could not be used as an endpoint, since a number of strains had pigments that influenced the colour intensity.9,28 MIC50 and MIC90 were defined as the MICs that inhibited growth of 50% and 90% of all isolates tested, respectively. All experiments were performed in triplicate.
Olorofim and itraconazole interaction
A chequerboard microdilution assay was used to evaluate the in vitro activity between olorofim and itraconazole. Olorofim was evaluated using a concentration ranging from 0.002 to 2 mg/L and itraconazole from 0.004 to 0.25 mg/L. The interaction between olorofim and itraconazole was analysed based on the FIC index and the interaction ratio (IR).29,30 FIC index values were calculated as follows:
and represent the concentration of drugs A and B, respectively, when tested in combination and and represent the concentration of drugs A and B, respectively, when acting individually. An FIC index value of ≤0.5 is considered synergistic, a value of >0.5 to 4 is considered indifferent and a value of >4 is considered antagonistic.29,30 The IRs were calculated using the formula:
Io and Ie represent the observed and expected percentage of inhibition for a given interaction, respectively. Ie is calculated as follows:
A and B represent the percentage of inhibition observed for each compound when acting alone. The interaction was considered synergistic when IR was >1.5, indifferent when IR was between 0.5 and 1.5, and antagonistic when IR was <0.5.30,31 The chequerboard assay was performed twice using M. mycetomatis genome isolate MM55. Using the XTT endpoint read, MIC was defined as the lowest concentration with a minimum of 80% growth reduction.
Statistical analysis
MICs of olorofim and itraconazole were statistically compared using a Mann–Whitney test. A P value of <0.05 was deemed statically significant.
Results
In silico modelling predicts that M. mycetomatis is susceptible to olorofim
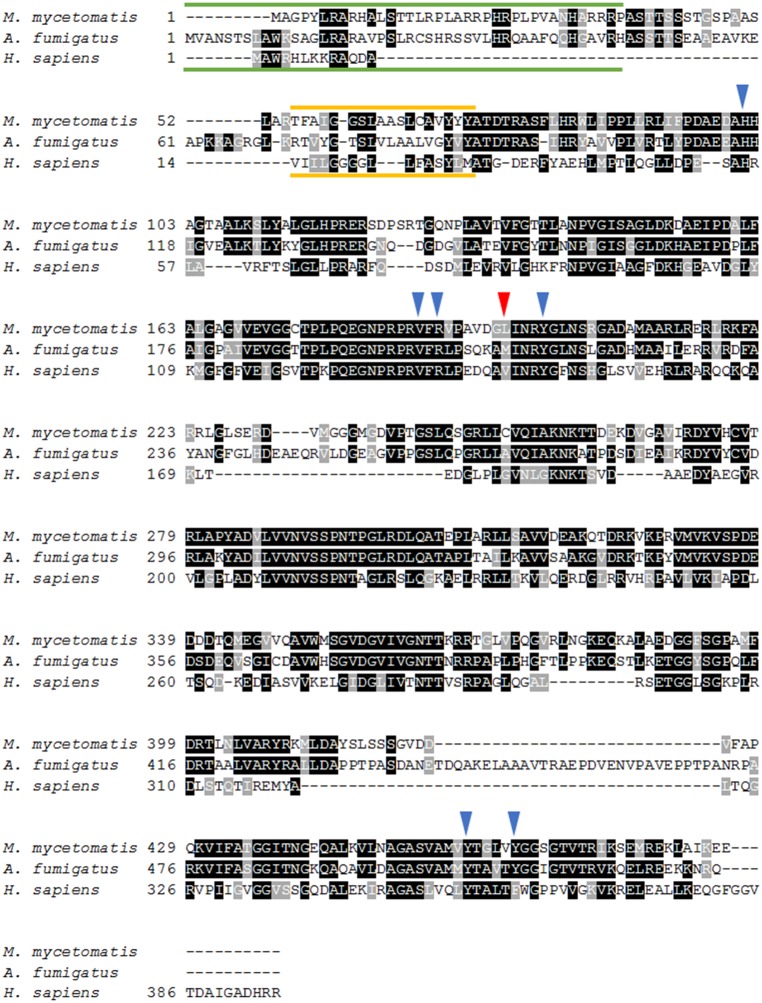
The analysis of DHODH sequences showed that the M. mycetomatis DHODH homologue (accession number: KXX79707) shares 58.7% homology with that of A. fumigatus and 40.1% homology with that of H. sapiens. When comparing the amino acid residues that are predicted to be important in olorofim binding14 in both A. fumigatus and M. mycetomatis DHODH, we observed a similarity of 86% between the two species. M. mycetomatis DHODH shares six out of seven predicted binding residues with A. fumigatus DHODH. The amino acid that differed was Leu195, which corresponded to the Met209 position in A. fumigatus (Figure 1). This is a conservative replacement, with both amino acids having hydrophobic side chains, indicating that M. mycetomatis might be susceptible to olorofim.
Figure 1.
Alignment of M. mycetomatis, A. fumigatus and human (H. sapiens) DHODH amino acid sequences. Conserved residues are highlighted in black and similar residues are highlighted in grey. The predicted mitochondrial targeting sequences are indicated by the green lines and the predicted transmembrane domains are indicated by the yellow lines. The blue arrows depict the amino acid residues predicted to be important for olorofim binding in A. fumigatus DHODH15 that are identical in M. mycetomatis. The red arrow depicts the amino acid residue predicted to be important for olorofim binding in A. fumigatus DHODH that deviates in M. mycetomatis. In A. fumigatus this amino acid is Met209, while in M. mycetomatis the amino acid is Leu195. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
M. mycetomatis is highly susceptible to olorofim in vitro
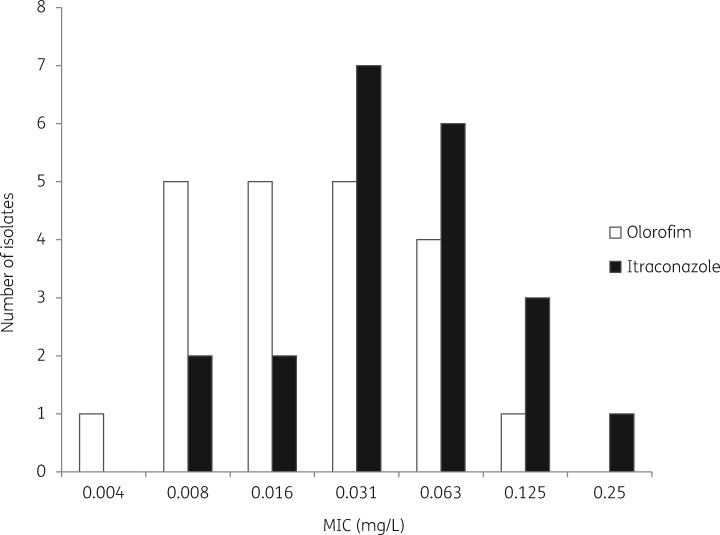
As shown in Figure 2, MICs of olorofim ranged from 0.004 to 0.125 mg/L and MICs of itraconazole ranged from 0.008 to 0.25 mg/L. M. mycetomatis is more susceptible to olorofim compared with itraconazole. Significantly lower MICs were obtained for olorofim (median = 0.016 mg/L) than for itraconazole (median = 0.031 mg/L) (P = 0.047) (Table 1). For olorofim, a concentration of 0.016 mg/L was needed to inhibit 50% of isolates and a concentration of 0.063 mg/L was needed to inhibit 90% of M. mycetomatis isolates. For itraconazole, 0.031 and 0.125 mg/L was needed to inhibit 50% and 90% of isolates, respectively.
Figure 2.
In vitro activities of olorofim and itraconazole against 21 M. mycetomatis isolates, represented by MICs.
Table 1.
In vitro susceptibility to olorofim and itraconazole, and the interaction of the combined drugs
| Olorofim | Itraconazole | Combined | |
|---|---|---|---|
| MIC, median (mg/L) | 0.016 | 0.031 | — |
| MIC, range (mg/L) | 0.004–0.125 | 0.008–0.25 | — |
| MIC50 (mg/L) | 0.016 | 0.031 | — |
| MIC90 (mg/L) | 0.063 | 0.125 | — |
| MIC for M. mycetomatis isolate MM55 (mg/L) | 0.063 | 0.063 | — |
| FIC index for M. mycetomatis isolate MM55 | — | — | 3.2 (indifferent) |
| IR for M. mycetomatis isolate MM55 | — | — | 0.91 (indifferent) |
—, not applicable.
Indifferent interaction between olorofim and itraconazole
To determine whether itraconazole and olorofim could potentially be combined in a therapy, a chequerboard assay for olorofim and itraconazole was performed on M. mycetomatis genome isolate MM55. As shown in Table 1, an FIC index of 3.2 and an IR of 0.92 were obtained. Both these values indicate that olorofim and itraconazole are indifferent when combined.
Discussion
Despite treatment with itraconazole at 200–400 mg daily, only 25.9% of eumycetoma patients are cured and 2.8% end up with amputation. This in turn leads to a high morbidity and dependency on family members. Therefore, there is an urgent need to identify novel drugs with activity against the causative agents of eumycetoma.
Since the discovery of olorofim, several studies have been carried out on the antifungal properties of olorofim, demonstrating its effectiveness against several fungal species, notably Aspergillus spp.19 Olorofim showed a lower MIC compared with other drugs tested.16,18–20 To evaluate whether olorofim would be active against M. mycetomatis, we first analysed and compared the amino acid sequence of M. mycetomatis DHODH with that of A. fumigatus. A homology of 58.7% was determined between the two DHODH sequences. Furthermore, six out of seven amino acids predicted by Oliver et al.14 to be important in olorofim binding were shared. The single remaining amino acid, Met209 in the A. fumigatus DHODH amino acid sequence, was replaced by Leu195 in M. mycetomatis. Apparently this substitution did not affect the susceptibility to olorofim as we demonstrated that M. mycetomatis is indeed susceptible to olorofim with MICs ranging from 0.004 to 0.125 mg/L. Oliver et al.14 successfully created a mutant Candida albicans DHODH that became susceptible to olorofim by replacing Phe162 and Val171 (equivalent to Val200 and Met209 in A. fumigatus) with Val162 and Met171. This indicated that these two residues at their respective positions in each species were important for olorofim binding and subsequent inhibition of DHODH. However, as for the two residues in M. mycetomatis, the presence of Leu195 (Met209 in A. fumigatus) at the latter position apparently did not impair the binding of olorofim to DHODH. Since the difference in the latter amino acid residue between M. mycetomatis and A. fumigatus did not affect susceptibility to olorofim, taking these data together, the resistance of C. albicans to olorofim is most likely due to the difference in the former of the two residues (position 162 in C. albicans, 200 in A. fumigatus and 186 in M. mycetomatis). As indicated by Oliver et al.,14 there must also be other important differences in DHODH between these species and more studies are needed to understand the importance of the amino acids involved in olorofim binding.
Olorofim is currently in a Phase II study (ClinicalTrials.gov identifier NCT03583164) for treatment of invasive fungal infections caused by Scedosporium spp., Aspergillus spp. and other resistant fungi in patients lacking suitable alternative treatment options. This study will provide a good understanding of the dosage and the efficacy of olorofim in patients, which might also be applicable for mycetoma patients. However, for actinomycetoma, the bacterial form of mycetoma, it was discovered that patients were more responsive to combination therapy than to a single drug alone. The combination therapy differs between countries; in Mexico and Sudan the Welsh regimen is used and in India the Raman regimen is used.3,32–35 Until now, combination therapy for eumycetoma has not extensively been explored in animal models and clinical trials. This is because the results of in vitro combination studies may differ according to the methodologies used and thus cannot be relied upon to predict the clinical effect that may be obtained. For M. mycetomatis, hyphal fragments are exposed to the antifungal agents in vitro, while in vivo it structures itself as grains. Therefore, the efficacy of combination therapy should always be determined both in vitro and in vivo.36 In the past, combination therapy for eumycetoma did not seem feasible since all antifungal agents with activity against the causative agents had the same mode of action, which could lead to antagonism instead of synergy.37 When azoles were combined with terbinafine in vitro by Ahmed et al.30 (2015), indifference and antagonism were noted. The in vivo study by Eadie et al.37 (2017) demonstrated that combining the drugs resulted in antagonism and treatment significantly decreased larvae survival. Eadie et al.37 confirmed the discovery of Scheven and Schwegler38 that antagonism occurs when ergosterol is inhibited via two different pathways. In this study, the combination of olorofim and itraconazole, two drugs with different modes of action, was studied. The components of the in vitro combination of olorofim and itraconazole against M. mycetomatis acted indifferently to each other, as no antagonism or synergy was noted. Since olorofim and itraconazole inhibit fungal growth via different mechanisms, this highlights the room for further evaluation in vivo and in a clinical setting where they could be potentially combined to treat eumycetoma.
In conclusion, we showed that olorofim inhibits growth of M. mycetomatis and, although olorofim and itraconazole inhibit fungal growth by different mechanisms, when combined they show no antagonism or synergism. The next step will be to study the efficacy of olorofim against M. mycetomatis in an in vivo model.39
Funding
This study was supported by internal funding.
Transparency declarations
Olorofim was obtained from F2G Ltd. Jason D. Oliver and Mike Birch are employees and shareholders of F2G Ltd. Bart Rijnders is an investigator of a Phase II study on olorofim. His employer receives patient fees for the inclusion of patients in this study. All other authors: none to declare.
References
- 1. Fahal AH, Elkhawad AO.. Managing mycetoma: guidelines for best practice. Exp Rev Dermatol 2013; 8: 301–7. [Google Scholar]
- 2.WHO. Sixty-Ninth World Health Assembly WHA69.21 - Addressing the Burden of Mycetoma. http://www.who.int/neglected_diseases/mediacentre/WHA_69.21_Eng.pdf? ua=1.
- 3. Zijlstra EE, van de Sande WWJ, Welsh O. et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis 2016; 16: 100–12. [DOI] [PubMed] [Google Scholar]
- 4. van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis 2013; 7: e2550.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fahal AH, Hassan MA.. Mycetoma. Br J Surg 1992; 79: 1138–41. [DOI] [PubMed] [Google Scholar]
- 6. Ameen M, Arenas R.. Developments in the management of mycetomas. Clin Exp Dermatol 2009; 34: 1–7. [DOI] [PubMed] [Google Scholar]
- 7. Fahal AH. Mycetoma review. Khartoum Med J 2011; 4: 514–23. [Google Scholar]
- 8. van de Sande W, Fahal A, Ahmed SA. et al. Closing the mycetoma knowledge gap. Med Mycol 2018; 56: 153–64. [DOI] [PubMed] [Google Scholar]
- 9. Kloezen W, Meis JF, Curfs-Breuker I. et al. In vitro antifungal activity of isavuconazole against Madurella mycetomatis. Antimicrob Agents Chemother 2012; 56: 6054–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Belkum A, Fahal AH, van de Sande W.. In vitro susceptibility of Madurella mycetomatis to posaconazole and terbinafine. Antimicrob Agents Chemother 2011; 55: 1771–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van de Sande WWJ, Luijendijk A, Ahmed AOA. et al. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the Sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob Agents Chemother 2005; 49: 1364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welsh O, Al-Abdely HM, Salinas-Carmona MC. et al. Mycetoma medical therapy. PLoS Negl Trop Dis 2014; 8: e3218.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van de Sande WW, Maghoub el S, Fahal AH. et al. The mycetoma knowledge gap: identification of research priorities. PLoS Negl Trop Dis 2014; 8: e2667.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oliver JD, Sibley GEM, Beckmann N. et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci USA 2016; 113: 12809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones ME. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem 1980; 49: 253–79. [DOI] [PubMed] [Google Scholar]
- 16. du Pré S, Beckmann N, Almeida MC. et al. Effect of the novel antifungal drug F901318 (olorofim) on growth and viability of Aspergillus fumigatus. Antimicrob Agents Chemother 2018; 62: e00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buil JB, Rijs A, Meis JF. et al. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J Antimicrob Chemother 2017; 72: 2548–52. [DOI] [PubMed] [Google Scholar]
- 18. Lackner M, Birch M, Naschberger V. et al. Dihydroorotate dehydrogenase inhibitor olorofim exhibits promising activity against all clinically relevant species within Aspergillus section Terrei. J Antimicrob Chemother 2018; 73: 3068–73. [DOI] [PubMed] [Google Scholar]
- 19. Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A.. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J Antimicrob Chemother 2019; 74: 1586–90. [DOI] [PubMed] [Google Scholar]
- 20. Wiederhold NP, Law D, Birch M.. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 2017; 72: 1977–80. [DOI] [PubMed] [Google Scholar]
- 21. Wiederhold NP, Najvar LK, Jaramillo R. et al. The orotomide olorofim is efficacious in an experimental model of central nervous system coccidioidomycosis. Antimicrob Agents Chemother 2018; 62: e0099-18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jorgensen KM, Astvad KMT, Hare RK. et al. EUCAST determination of olorofim (F901318) susceptibility of mold species, method validation, and MICs. Antimicrob Agents Chemother 2018; 62: e0047-18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukasawa Y, Tsuji J, Fu SC. et al. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol Cell Proteomics 2015; 14: 1113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kall L, Krogh A, Sonnhammer EL.. Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res 2007; 35: W429–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van de Sande WW, Gorkink R, Simons G. et al. Genotyping of Madurella mycetomatis by selective amplification of restriction fragments (amplified fragment length polymorphism) and subtype correlation with geographical origin and lesion size. J Clin Microbiol 2005; 43: 4349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim W, Eadie K, Horst-Kreft D. et al. VNTR confirms the heterogeneity of Madurella mycetomatis and is a promising typing tool for this mycetoma causing agent. Med Mycol 2019; 57: 434–40. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed AO, Mukhtar MM, Kools-Sijmons M. et al. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J Clin Microbiol 1999; 37: 3175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmed AOA, van de Sande WWJ, van Vianen W. et al. In vitro susceptibilities of Madurella mycetomatis to itraconazole and amphotericin B assessed by a modified NCCLS method and a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay. Antimicrob Agents Chemother 2004; 48: 2742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meletiadis J, Meis J, Mouton JW. et al. Methodological issues related to antifungal drug interaction modelling for filamentous fungi. Rev Med Microbiol 2002; 13: 101–17. [Google Scholar]
- 30. Ahmed SA, Kloezen W, Fahal AH. et al. In vitro interaction of currently used azoles with terbinafine against Madurella mycetomatis. Antimicrob Agents Chemother 2015; 59: 1373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gisi U. Synergistic interaction of fungicides in mixtures. Phytopathology 1996; 86: 1273–9. [Google Scholar]
- 32. Welsh O, Vera-Cabrera L, Welsh E. et al. Actinomycetoma and advances in its treatment. Clin Dermatol 2012; 30: 372–81. [DOI] [PubMed] [Google Scholar]
- 33. Ramam M, Bhat R, Garg T. et al. A modified two-step treatment for actinomycetoma. Indian J Dermatol Venereol Leprol 2007; 73: 235–9. [DOI] [PubMed] [Google Scholar]
- 34. Ramam M, Garg T, D’Souza P. et al. A two-step schedule for the treatment of actinomycotic mycetomas. Acta Derm Venereol 2000; 80: 378–80. [DOI] [PubMed] [Google Scholar]
- 35. Welsh O, Sauceda E, Gonzalez J. et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol 1987; 17: 443–8. [DOI] [PubMed] [Google Scholar]
- 36.EMA. Guideline on the Clinical Evaluation of Antifungal Agents for the Treatment and Prophylaxis of Invasive Fungal Diseases. CHMP/EWP/1343/01 Rev. 1, 2010.
- 37. Eadie K, Parel F, Helvert-van Poppel M. et al. Combining two antifungal agents does not enhance survival of Galleria mellonella larvae infected with Madurella mycetomatis. Trop Med Int Health 2017; 22: 696–702. [DOI] [PubMed] [Google Scholar]
- 38. Scheven M, Schwegler F.. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob Agents Chemother 1995; 39: 1779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kloezen W, van Helvert-van Poppel M, Fahal AH. et al. A Madurella mycetomatis grain model in Galleria mellonella larvae. PLoS Negl Trop Dis 2015; 9: e0003926.. [DOI] [PMC free article] [PubMed] [Google Scholar]