Presenting an optimised synthesis of the fungus-derived antibiotic CJ-15,801 which shows selective activity against Staphylococcus aureus and Plasmodium falciparum.
Presenting an optimised synthesis of the fungus-derived antibiotic CJ-15,801 which shows selective activity against Staphylococcus aureus and Plasmodium falciparum.
Abstract
The biosynthesis of the essential metabolic cofactor coenzyme A (CoA) has been receiving increasing attention as a new target that shows potential to counter the rising resistance to established antimicrobials. In particular, phosphopantothenoylcysteine synthetase (PPCS)—the second CoA biosynthesis enzyme that is found as part of the bifunctional CoaBC protein in bacteria, but is monofunctional in eukaryotes—has been validated as a target through extensive genetic knockdown studies in Mycobacterium tuberculosis. Moreover, it has been identified as the molecular target of the fungal natural product CJ-15,801 that shows selective activity against Staphylococcus aureus and the malaria parasite Plasmodium falciparum. As such, CJ-15,801 and 4′-phospho-CJ-15,801 (its metabolically active form) are excellent tool compounds for use in the development of new antimicrobial PPCS inhibitors. Unfortunately, further study and analysis of CJ-15,801 is currently being hampered by several unique challenges posed by its synthesis. In this study we describe how these challenges were overcome by using a robust palladium-catalyzed coupling to form the key N-acyl vinylogous carbamate moiety with retention of stereochemistry, and by extensive investigation of protecting groups suited to the labile functional group combinations contained in this molecule. We also demonstrate that using TBAF for deprotection causes undesired off-target effects related to the presence of residual tertiary ammonium salts. Finally, we provide a new method for the chemoenzymatic preparation of 4′-phospho-CJ-15,801 on multi-milligram scale, after showing that chemical synthesis of the molecule is not practical. Taken together, the results of this study advances our pursuit to discover new antimicrobials that specifically target CoA biosynthesis and/or utilization.
Introduction
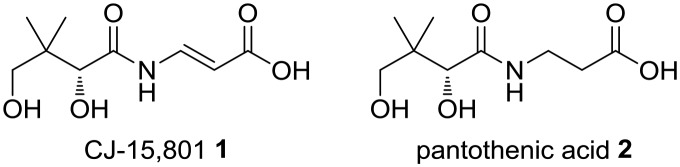
The search for new antimicrobials with targets that are distinct from those currently being used clinically—and for which resistance is steadily increasing—has led to the identification of the biosynthesis and utilisation of the essential cofactor coenzyme A (CoA) as a promising new target.1,2 From the perspective of specific molecular targets, phosphopantothenoylcysteine synthetase (PPCS), which catalyses the second reaction of CoA biosynthesis, has drawn particular interest. This follows recent findings indicating that silencing of the Mycobacterium tuberculosis (Mtb) coaBC gene, which encodes the bifunctional CoaBC protein that carries PPCS activity in this organism and most other bacteria, is bactericidal in vitro as well as in an in vivo mouse model.3 In addition, the fungal natural product CJ-15,801 (1) from Seimatosporium spp.4—a close structural analogue of pantothenic acid (2), the vitamin precursor of CoA (Fig. 1)—was found to show selective antibiotic action against Staphylococcus aureus (including drug-resistant strains) due to inhibition of its PPCS activity following metabolic activation by the first CoA biosynthetic enzyme, pantothenate kinase (PanK).5 CJ-15,801 also shows antimicrobial activity against the malaria parasite Plasmodium falciparum,6 with recent findings suggesting that a similar mechanism of action (metabolic activation by PanK followed by inhibition of PPCS) is in operation in its case.7 While these studies make a convincing case for pursuing PPCS as a target for the development of new bactericidal and anti-parasitic compounds, CJ-15,801 remains the only reported small molecule inhibitor of the enzyme. This raises its importance as a lead and key tool compound in PPCS inhibitor development campaigns. Unfortunately, significant challenges in its synthesis have resulted in limited access to the material required for follow-up studies, especially in the case of in vivo experiments.
Fig. 1. The natural product antibiotic CJ-15,801 1 is a structural analogue of pantothenic acid 2 (vitamin B5).
The efficiency of the synthesis of CJ-15,801 and its analogues is largely determined by two factors: first, the method used to introduce the N-acyl vinylogous carbamate moiety with the required E-stereochemistry, and second, the protection/deprotection protocol used on the 1,3-diol and carboxylic acid functional groups. Previous syntheses of CJ-15,801 have highlighted the latter to be especially problematic,8–10 as the partially deprotected intermediates are prone to decomposition driven by the lactonization of the pantoic acid moiety to form pantolactone and 4-aminoacrylate, which decomposes further. A new synthesis of CJ-15,801 that aims to increase the scale of its production would therefore need to address both of these challenges, and ideally also provide an entry for the synthesis of its metabolically active form, 4′-phospho-CJ-15,801 (8).
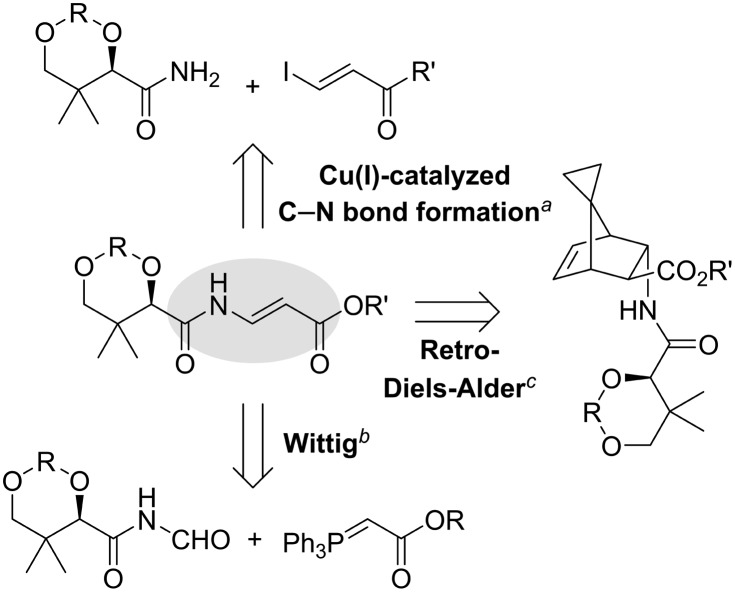
In previous studies, the N-acyl vinylogous carbamate moiety of CJ-15,801 was introduced by one of three strategies (Scheme 1), all of which suffer from some drawbacks in the context of establishing a practical and atom-efficient synthesis. The first uses an oxygen- and water-sensitive Cu(i) catalyst for formation of the key C–N bond that complicates any attempts at a practical large scale synthesis.8 The second, which introduces the enamide in two steps from pantoamide (3a) by means of a Wittig olefination of an imide precursor, suffers from low atom efficiency (due to the use of the triphenylphosphonium ylide) and relatively poor stereoselectivity.9,10 Finally, a report in which the enamide is formed by retro Diels–Alder cleavage of a bicyclic precursor was presented as a scalable synthesis,11 even though the preparation of the precursors require an undesirable multistep synthesis.12 Two additional reports on the synthesis of the cis-isomer of 1 have also been published,13,14 but these are not relevant as the natural product requires the E-configuration for bioactivity.5
Scheme 1. Synthetic strategies previously used to introduce the N-acyl vinylogous carbamate functionality of CJ-15,801 (1). aPorco and co-workers.8bMarquez and co-workers.9,10 cReddy and co-workers.11.
Considering these shortcomings, we re-examined the synthesis of CJ-15,801 with a focus on improving the efficiency and scalability of its preparation, and opening avenues for the preparation of its phosphorylated active form. In this process, we uncovered significant off-target effects in bioactivity tests when the inhibitor was combined with certain counterions. Taken together, the findings of this study create an important foundation for future research focused on the discovery of antimicrobials targeting CoA biosynthesis, and more specifically those directed at PPCS enzymes.
Results and discussion
A search of the synthetic literature suggested an alternative method to introduce CJ-15,801's N-acyl vinylogous carbamate moiety based on a Pd-catalyzed coupling used by Tanoury et al. to synthesize the key intermediate of the interleukin-1β converting enzyme (ICE) inhibitor VX-765 on a one kilogram scale.15 In this reaction, β-bromoacrylates and β-bromoacrylamides were selectively converted into the corresponding vinylogous carbamates or ureas in excellent yields using Pd(OAc)2 as catalyst in the presence of water, an inorganic base (K2CO3) and a phase transfer catalyst (cetyltrimethylammonium bromide, CTAB). Importantly, the stereochemistry of the starting material was retained in the product, although isomerization was found to occur in some cases upon prolonged heating after reaction completion.
Next, the scope of the Pd-catalyzed coupling was considered in the context of needing access to variety of potential protecting groups, as these would have to be evaluated for their ability to yield the target molecule without causing significant degradation. Therefore pantoamides 3,16 of which the 1,3-diol was protected with an acetonide, p-methoxybenzylidene (CHPMP), carbonate or di-tert-butylsilylene (DTBS) group, were coupled to the allyl, methyl, tert-butyl, 2-(trimethylsilyl)ethyl (TMSE), p-methoxybenzyl (PMB), cyanoethyl (CE) and benzyl β-bromoacrylates 4 (Scheme 2). Performing the coupling reaction with these precursors in various combinations on a 1–2 mmol (0.1–0.4 g) scale using the conditions outlined by Tanoury et al.15 gave the protected intermediates 5 in good to excellent yields, except in the case of 5f (Table 1). Moreover, all the major products had the required E-configuration; however, we found that in some cases small quantities (4–12%) of the undesired Z-isomer was also obtained after chromatography. Performing the Pd-catalyzed coupling of amide 3a and bromoacrylate 4a at a temperature of 80 °C (instead of the usual 55 °C) led to the exclusive formation of the Z-isomer of 5a, albeit in a slightly lower overall yield (43% vs. 55%). This result lends support to the analysis of Tanoury et al. that the Z-isomers are formed upon heat-induced isomerization and demonstrates that these isomers can be synthesized by conducting the coupling reactions at elevated temperatures.
Scheme 2. Pd-Catalyzed coupling of protected pantoamides 3 with protected E-β-bromoacrylates 4 to yield protected CJ-15,801 intermediates 5. Reaction conditions: pantoamide 3 (1 equiv.), β-bromo-acrylate 4 (1.1 equiv.), Pd(OAc)2 (0.1 equiv.), Xantphos (0.15 equiv.), K2CO3 (2 equiv.), CTAB (0.2 equiv.), toluene, 55 °C for 5 h; H2O (3 equiv.) added after the first hour.
Table 1. Isolated yields from the Pd-catalyzed coupling of protected pantoamides 3 to E-β-bromoacrylates 4.
| Entry | Pantoamide 3 (R1) | E-β-Bromo-acrylate 4 (R2) | Product | Yield (%) |
| 1 | 3a (CMe2) | 4a (allyl) | 5a | 55 a |
| 2 | 3a (CMe2) | 4b (Me) | 5b | 71 b |
| 3 | 3a (CMe2) | 4c (tBu) | 5c | 59 |
| 4 | 3a (CMe2) | 4d (TMSE) | 5d | 67–80 c , d |
| 5 | 3b (CHPMP) | 4e (PMB) | 5e | 82 |
| 6 | 3c (C O) | 4f (CE) | 5f | 35 |
| 7 | 3d (SitBu2) | 4d (TMSE) | 5g | 82–86 c |
| 8 | 3a (CMe2) | 4f (CE) | 5h | 70–71 c |
| 9 | 3a (CMe2) | 4g (Bn) | 5i | 83 |
a Z-Product also obtained in 11% yield.
b Z-Product also obtained in 4% yield.
cRange of yields depending on scale of synthesis.
d Z-Product also obtained in 29% yield (large scale) or 12% yield (small scale).
To date, CJ-15,801 has only successfully been obtained from protected intermediates 5a, 5c and 5d, i.e. when the 1,3-diol was protected by using an acetonide and the carboxylic acid with an allyl, tert-butyl or TMSE ester (Table 2, entries 1–6).8,10,17,18 In all cases, deprotection of these intermediates proceeded in a step-wise manner, with the acetonide being removed first from 5a and 5c, while in the case of 5d it was removed only after deprotection of the TMSE ester. This approach gave yields of between 40% and 61% for the deprotection steps. Importantly, Marquez and co-workers also attempted a one-pot deprotection strategy in the case of 5c, but reported that this gave a lower overall yield of 20% (Table 2, entry 5).10 We set out to investigate whether these yields could be improved by an alternative protecting group strategy.
Table 2. Strategies for deprotection of intermediates 5 to yield CJ-15,801 (1).
| Entry | Precursor | R1 | R2 | Reaction conditions | Yield a (%) | Ref. |
| 1 | 5a | CMe2 | Allyl | 1. BiCl3, H2O, MeCN, rt, 8 h; 2. Pd(PPh3)4, N-hydroxyphthalimide (supported), THF, 35 °C, 12 h | 60 | 8 |
| 2 | 5a | CMe2 | Allyl | 1. BiCl3, H2O, MeCN, rt, 8 h; 2. Pd(PPh3)4, THF, rt, 8 h | 61 | 5 |
| 3 | 5c | CMe2 | t Bu | 1. BiCl3, H2O, MeCN, rt, O/N; 2. HCO2H | 40 | 10 |
| 4 | 5c | CMe2 | t Bu | 1. PTSA, MeOH/H2O, 22 h; 2. HCO2H, 2 h | 47 | 17 |
| 5 | 5c | CMe2 | t Bu | HCO2H, rt, 1.5 h then PTSA, MeOH, 1.25 h | 20 | 10 |
| 6 | 5d | CMe2 | TMSE | 1. TBAF, THF, rt, 19 h; 2. BiCl3, H2O, MeCN, rt, 19 h | 44 | 10 |
| 7 | 5e | CHPMP | PMB | BiCl3, H2O, MeCN, rt | IM b | This work |
| 8 | 5f | CO | CE | LiOH, THF, rt | IM b | This work |
| 9 | 5g | SitBu2 | TMSE | TBAF, THF, rt, 4 h then DOWEX, CaCO3, MeOH, rt, 1 h | 70–93 c , d | This work |
| 10 | 5h | CMe2 | CE | 1. BiCl3, H2O, MeCN, rt, 18 h; 2. K2CO3, H2O, MeOH, rt, 1 h | 53–66 d | This work |
aOverall isolated yields.
bIM, inseparable mixture.
cPartial TBA salt.
dRange of yields dependent on scale of synthesis.
The combination of protecting groups used in the case of intermediates 5e, 5f and 5g should allow for a single step deprotection by treatment with a mild or Lewis acid (5e), a base (5f) or with tetrabutylammonium fluoride (TBAF) (5g) respectively. We hypothesized that using a single global deprotection would lead to improved yields and would facilitate purification of the final product. Unfortunately, attempts at deprotecting 5e and 5f in this manner were not as successful as anticipated. Subjecting 5e to the BiCl3-based deprotection conditions used previously only gave an inseparable mixture of products (Table 2, entry 7), as did 5f upon treatment with LiOH in THF (Table 2, entry 8). In contrast, treatment of 5g with an excess of TBAF in THF on a ∼0.2 mmol (∼0.1 g) scale, followed by a previously reported work-up with DOWEX (sulfonic acid) resin and CaCO3 to remove excess TBAF and TBAF-derived products19 gave CJ-15,801 in an apparently greater than quantitative yield. NMR spectroscopic analysis revealed that this was due to the presence of residual tetrabutylammonium (TBA) ion in the sample (∼0.42 equiv. relative to 1). This finding was unexpected, as the DOWEX/CaCO3 work-up was reported to remove >99% of TBAF-derived materials in the deprotection of a variety of tert-butyldimethylsilyl-protected alcohols.19 However, close scrutiny of the NMR spectra provided in the original report revealed that residual TBA was also present in amounts ranging from 0.06–0.2 equivalents in most of the provided examples. In the case of 1 the ratio is likely higher due to its carboxylate acting as counterion to the TBA cation. Nonetheless, correcting for the presence of the TBA counterion, the yield of 1 by deprotection of 5g was found to be an excellent 93% (Table 2, entry 9), or ∼76% overall from the pantoamide/bromoacrylate precursors.
In light of these successes, we set out to investigate the scalability of the synthesis of 1via the intermediate 5g. DTBS-protected pantoamide 3d and 3-bromoacrylate 4d were first prepared on >2 gram scale as coupling reactants, followed by application of the Pd-catalyzed coupling on a ∼8 mmol (∼2.6 g) scale to give 3.1 g (86%) of 5g—a similar yield to that obtained in the small scale preparation (Table 1, entry 7). For the deprotection step, we investigated several strategies whereby the amount of residual TBA present in the final product could be minimized. These included varying the amount of TBAF used in the deprotection, performing multiple rounds of the fluoride precipitation/cation-exchange during work-up, and using an alternative method that relies on catalytic fluoride (either TBAF or CsF) to effect cleavage of the silyl groups.20 However, even in the best case (obtained using 2.5 equiv. of TBAF and 6 sequential rounds of the resin-based work-up) the product was obtained with 0.23 equiv. of residual TBA still present, and a concomitant reduction in the yield of the deprotection step to 70% (Table 2, entry 9).
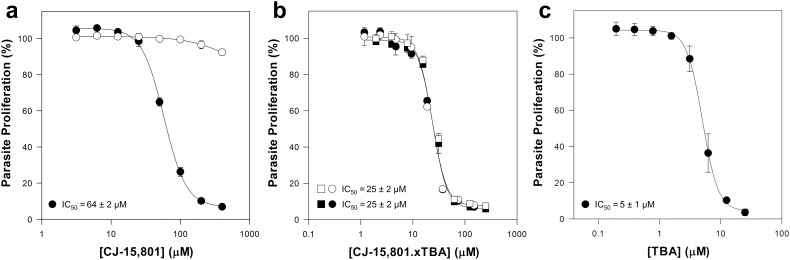
With CJ-15,801 prepared in this manner in hand, we set out to determine whether it shows the same biological activity as observed for previous preparations. As the antiplasmodial activity of CJ-15,801 has been characterized in several published studies,6,7,18 we chose this system to perform the comparative analysis. Since pantothenic acid (2) antagonises CJ-15,801's parasite growth inhibition, the test was performed in both normal medium (that contains ∼1 μM 2) and medium to which 100 μM 2 was added to confirm on-target activity. The results show that CJ-15,801 prepared as previously described10 gave the usual dose response profile that was shifted in the presence of additional pantothenic acid (2) (Fig. 2a). However, the CJ-15,801 prepared from 5g as described above showed increased potency (IC50 value of 25 μM compared to 64 μM), and its dose response curve was not shifted by additional pantothenic acid (Fig. 2b), indicating that the antiplasmodial activity of the preparation was not due to inhibition of a CoA-related target. To test if the increased potency could be ascribed to the presence of the TBA counterion, we also tested its antiplasmodial activity separately. Interestingly, TBA alone had an IC50 value of ∼5 μM (Fig. 2c). Previous studies have demonstrated that TBA acts as a potent potassium ion channel blocker,21 and this may explain its antiplasmodial activity. Unfortunately, this finding indicates that the presence of even low amounts of TBA renders the CJ-15,801 salt mixture unsuitable for use in in vitro and in vivo experiments.
Fig. 2. Comparative analysis of the antiplasmodial activity of CJ-15,801 prepared either from 5d (ref. 10) using a previously published method (panel a) or by means of the TBAF-mediated global deprotection of 5g (panel b) that gave CJ-15,801 containing residual TBA (the value of x is estimated at 0.23). Experiments in panels a and b were carried out in the presence of pantothenic acid (2), either 1 μM (black symbols) or 100 μM (white symbols). The antiplasmodial dose response curve of TBA is shown in panel c. The data are averaged from 3–4 independent experiments (panel a and c). Error bars in panels a and c represent SEM. The data in panel b were generated in four independent experiments, two averaged from experiments carried out with one preparation of CJ-15,801 (squares) and another two averaged from experiments carried out with an independent preparation (circles). Error bars in panel b represent range/2. IC50 values represent mean ± SEM from 3 (panel c) or 4 (panels a and b) independent experiments (either TBAF or CsF) to effect cleavage of the silyl groups.20 However, even in the best case (obtained using 2.5 equiv. of TBAF and 6 sequential rounds of the resin-based during work-up) the product was obtained with 0.23 equiv. of residual TBA still present, and a concomitant reduction in the yield of the deprotection step to 70% (Table 2, entry 9).
We therefore considered other protecting group combinations that would circumvent the use of TBAF. After several such combinations were tested, we found that the acetonide/CE-protected intermediate 5h showed the most promise for an efficient and scalable synthesis of CJ-15,801. Pd-Catalyzed coupling of protected pantoamide 3a and 3-bromoacrylate 4f on a ∼15 mmol scale gave 3.3 g of 5h in a 70% yield (Table 1, entry 8). Deprotection was found to proceed optimally when performed sequentially, removing the acetonide first with BiCl3, followed by removal of the ester by treatment with K2CO3 (Table 2, entry 10). This protocol gave overall isolated yields of between 53–66% depending on the scale of the reaction, and gave 1 of high purity that is suitable for use in both ex vivo and in vivo studies.
For in vitro inhibition studies of PPCS enzymes and CoaBC proteins, the metabolically active form of the inhibitor, i.e. 4′-phospho-CJ-15,801 (8), is required. In the only synthesis of 8 published to date, 1.4 mg of the target molecule was obtained by enzymatic phosphorylation of 1 followed by purification by preparative HPLC.5 We therefore investigated if the new synthetic routes developed in this study could also be used in an optimized synthesis of 8 (Scheme 3). Towards this end, the acetonide was removed from 5h and 5i respectively to give the corresponding esters 6a and 6b. These were phosphorylated using either amidite chemistry or with dibenzyl chlorophosphate to give the all-CE (7a) and all-Bn (7b) protected versions of 8 respectively.
Scheme 3. Attempts at the chemical and chemoenzymatic synthesis of 4′-phospho-CJ-15,801 (8).
Unfortunately, deprotection of 7a using several base-catalyzed methods (K2CO3/MeOH; DBU, TMSCl; NH4OH/MeOH, 50 °C), as well as deprotection of 7b using either Birch reduction or BCl3, failed to give the target molecule as the major product (hydrogenolysis of 7b causes reduction of the key double bond6). Instead, mixtures of products were obtained from which we were unable to purify the desired molecule. This suggests that 8 shows similar acid and base-sensitive degradation profiles as its parent compound 1.
We therefore revisited the previous S. aureus PanK-mediated preparation of 8 with the goal of increasing the scale of the reaction and simplifying the purification procedure. From previous experience, we found that using 2 equiv. ATP as phosphate donor significantly complicates the purification of the product. We therefore investigated the use of sub-stoichiometric amounts of ATP coupled with an ATP-regeneration system. We were pleased to find that the reaction proceeded to completion when using only 2.5 mol% ATP (relative to the amount of 1) and phosphoenolpyruvate (PEP) and pyruvate kinase for its regeneration in situ. However, upon using C18 SPE for purification—our preferred method, as the ease of access to SPE and the ability to apply it to multi-milligram scale reactions would greatly simplify the procedure—large amounts of the Tris–HCl (trisaminomethane hydrochloride) buffer used in the reaction were found to co-elute with the product. To determine if this problem could be circumvented, the reaction was performed in a range of buffers, comparing the impact on conversion and evaluating the extent of co-elution of the buffer and the product. We found a solution in using volatile buffers such as ammonium acetate or ammonium bicarbonate, both of which gave full conversion after overnight incubation and allowed for the purification of ∼60 mg material by C18 SPE as desired.
Unfortunately, 1H and 13C NMR spectroscopic analysis of the product mixture showed the presence of peaks that are not attributable to the desired product 8. However, HPLC (with UV detection) and MS analysis indicated a purity of >95%. This suggests that the contaminants were low molecular weight components, likely pyruvate derivatives that co-elute with 8 during purification by SPE. As such, these contaminants are not expected to influence the outcome of in vitro assays, which already contain pyruvate.
The purity of the desired product was subsequently determined to be ∼60% by using UV-vis absorbance and comparison to a standard curve prepared using pure material. This value agreed with integration of the signals in the 1H NMR spectrum. These findings indicate that the chemoenzymatic method is amenable to the production of multi-milligram quantities of 4′-phospho-CJ-15,801 (8). This is a significant improvement on the scale of the previously reported synthesis, even when considering the presence of the low molecular weight contaminants. If required, the material prepared in this manner may be purified further by preparative HPLC using the established methods.5
Conclusions
We have presented a method for the scalable synthesis of the natural product antibiotic CJ-15,801 (1) based on the efficient formation of its N-acyl vinylogous carbamate moiety in the desired E-configuration by means of a Pd-mediated coupling reaction, and an optimized deprotection protocol. We demonstrated that the material prepared using a TBAF-mediated deprotection is not suitable for biological testing due to off-target effects that can be attributed to the residual TBA counterion present in the final preparation. This finding highlights the general potential for false positive results from material prepared using protocols involving TBAF. Finally, we report a chemoenzymatic method for the preparation of multi-milligram quantities of the metabolically active phosphorylated form of the inhibitor.
Our report reinforces the necessity of improved synthetic protocols to facilitate the preparation of promising inhibitors in sufficient quantities and in high purity to allow for in vitro and in vivo follow-up studies to be performed, and to support future lead development efforts. We hope that this work will facilitate such studies of CJ-15,801 and related molecules to allow for the full potential of PPCS enzymes as promising antimicrobial targets to be gauged.
Experimental
Materials and methods
All chemicals were purchased from Merck KGaA and were used without further purification unless otherwise noted. S. aureus PanK was obtained as previously described.22,23 Pyruvate kinase was from Merck KGaA. Tetrahydrofuran (THF) was distilled under nitrogen from sodium wire using benzophenone as an indicator. Dichloromethane (CH2Cl2) was distilled under nitrogen from calcium hydride. N,N-Dimethylformamide (DMF) was dried and purified by shaking up over potassium hydroxide, followed by distillation under reduced pressure and a nitrogen atmosphere, and was stored over 3 Å molecular sieves. All column chromatography was performed using Merck silica gel 60 (particle size 0.040–0.063 mm) using combinations of hexane, ethyl acetate (EtOAc), CH2Cl2 and methanol (MeOH) as eluents. Thin layer chromatography (TLC) was carried out on aluminium-backed Merck silica gel 60 F254 plates. Visualization was performed with a UV lamp followed by spraying with a cerium ammonium molybdate or ninhydrin solution, followed by heating. All 1H and 13C NMR spectra were obtained using 300 MHz Varian VNMRS (75 MHz for 13C), 400 MHz Varian Unity Inova (101 MHz for 13C) or 600 MHz Varian Unity Inova (125 MHz for 13C) instruments at the Central Analytical Facility (CAF) of Stellenbosch University. Chemical shifts (δ) were recorded using the residual solvent peak or an external reference. All chemical shifts are reported in ppm and all spectra were obtained at 25 °C. Proton spectral data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, br = broad), coupling constant in Hz, and integration. All high resolution mass spectra (HRMS) were performed on a Waters API Q-TOF Ultima spectrometer at the mass spectrometry unit of CAF using either positive or negative ion mode ESI as appropriate.
Optimized synthesis of CJ-15,801 (1)
(R)-2,2,5,5-Tetramethyl-[1,3]dioxane-4-carboxylic acid amide (3a)
The synthesis of 3a was performed according to the literature procedure of Aquino et al.16 To an oven-dried two-neck round bottom flask containing 2,4-dihydroxy-3,3-dimethylbutyramide (1.58 g, 10.7 mmol, 1.0 equiv.) was added anhydrous acetone (27 mL) and CH2Cl2 (27 mL). To this solution was added isopropylmethyl ether (2.10 mL, 21.4 mmol, 2.0 equiv.) and toluenesulfonic acid (p-TsOH) (204 mg, 1.07 mmol, 0.1 equiv.). The resulting mixture was then stirred for 1 h at ambient temperature followed by filtration and neutralization by the addition of triethylamine (300 μL). The resulting mixture was then dried over Na2SO4. After filtration the solution was concentrated in vacuo and the amide purified using flash chromatography (hexane/EtOAc; 2 : 1) to afford 3a as a yellow oil (1.24 g, 62%) which solidified on standing. The NMR data are in agreement with the literature spectra.161H NMR (400 MHz, DMSO-d6): δ 7.10 (br s, 2H), 5.21 (d, J = 6.0 Hz, 1H), 4.47 (t, J = 5.5 Hz, 1H), 3.66 (d, J = 6.0 Hz, 1H), 3.30 (dd, J = 10.3, 5.4 Hz, 1H), 3.18 (dd, J = 10.3, 5.4 Hz, 1H), 0.82 (s, 3H), 0.81 (s, 3H).
2-Cyanoethyl (E)-3-bromoacrylate (4f)
(E)-β-Bromoacrylic acid24,25 (300 mg, 1.99 mmol, 1.0 equiv.) and DMAP (25 mg, 2.0 mmol, 1.0 equiv.) in an oven-dried two-neck flask were dissolved by addition of CH2Cl2 (4 mL) and 3-hydroxyproprionitrile (150 mg, 1.99 mmol, 1.0 equiv.) using oven-dried syringes. The solution was cooled to 0 °C and DIC (339 μL, 2.19 mmol) was added drop-wise using an oven-dried syringe. The reaction was allowed to warm to ambient temperature and stirred for 1 h. The reaction mixture was then filtered through celite and concentrated in vacuo. The resulting syrup was taken up in EtOAc (50 mL) and washed with saturated NaHCO3 (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo. The desired product was purified using flash chromatography (hexane/EtOAc; 9 : 1) to give the product as a clear liquid (388 mg, 96%), which was used immediately in the subsequent step. 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 13.9 Hz, 1H), 6.50 (d, J = 13.9 Hz, 1H), 4.30 (t, J = 6.2 Hz, 2H), 2.70 (t, J = 6.2 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 163.2, 128.4, 127.5, 116.7, 77.2, 59.1, 17.8.
2-Cyanoethyl (R,E)-3-(2,2,5,5-tetramethyl-1,3-dioxane-4-carboxamido)acrylate (5h)
The reactions were conducted according to an adapted procedure of Tanoury et al.15 To an oven-dried Schlenk tube under a nitrogen atmosphere were added Pd(OAc)2 (6 mg, 0.027 mmol, 0.1 equiv.), Xantphos (23 mg, 0.04 mmol, 0.15 equiv.), K2CO3 (74 mg, 0.534 mmol, 2.0 equiv.), CTAB (19 mg, 0.053 mmol, 0.2 equiv.), pantoamide 3a (50 mg, 0.27 mmol, 1.0 equiv.), bromoacrylate 4f (60 mg, 0.29 mmol, 1.1 equiv.) and toluene (0.4 M with respect to amide). The suspension was then degassed under high vacuum until no further gas evolution was observed, after which it was warmed to 55 °C. After stirring for 1 h, 3 equiv. of water was added and the reaction stirred for a further 4 h at 55 °C. After cooling to ambient temperature the reaction was diluted with EtOAc, washed with water and the organic layer dried over Na2SO4. After filtration and concentration in vacuo the required E-isomer (60 mg, 71%) was obtained exclusively after purification by flash chromatography (hexane/EtOAc 2 : 1) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 12.1 Hz, 1H), 8.01 (dd, J = 14.1, 12.1 Hz, 1H), 5.64 (d, J = 14.1 Hz, 1H), 4.34 (t, J = 6.3 Hz, 2H), 4.20 (s, 1H), 3.71 (d, J = 11.8 Hz, 1H), 3.31 (d, J = 11.8 Hz, 1H), 2.73 (t, J = 6.3 Hz, 2H), 1.51 (s, 3H), 1.45 (s, 3H), 1.04 (s, 3H), 1.00 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 168.0, 166.4, 137.3, 116.8, 101.4, 99.5, 77.2, 76.7, 71.2, 58.4, 33.4, 29.4, 21.8, 18.8, 18.6. HRMS (ESI/QTOF) m/z: [M – H]– calcd for C15H21N2O5: 309.1450; found: 309.1448.
Small scale synthesis of CJ-15,801 (1)
BiCl3 (51 mg, 161 μmol, 0.2 equiv.) was added to 5h (250 mg, 806 μmol, 1.0 equiv.) in CH3CN (7 mL) and H2O (290 μL) and the resulting mixture was stirred at ambient temperature overnight. The filtrate was evaporated under vacuum to give a crude product that was subsequently purified by flash column chromatography (DCM, then 95 : 5 DCM : MeOH) to give 6a as a yellow oil (179 mg, 82%). 1H NMR (600 MHz, CDCl3) δ 9.24 (d, J = 11.9 Hz, 1H), 8.03 (dd, J = 14.1, 11.9 Hz, 1H), 5.66 (d, J = 14.1 Hz, 1H), 4.78 (d, J = 4.5 Hz, 1H), 4.35 (t, J = 6.2 Hz, 2H), 4.16 (d, J = 4.5 Hz, 1H), 3.52 (s, 1H), 2.74 (t, J = 6.2 Hz, 2H), 0.98 (s, 3H), 0.95 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 172.1, 167.1, 138.1, 117.3, 101.5, 77.7, 71.1, 58.6, 39.6, 21.0, 20.4, 18.3. HRMS (ESI/QTOF) m/z: [M + H]+ calcd for C12H19N2O5: 271.1294; found: 271.1286.
K2CO3 in MeOH (10% w/v; 200 mg in 2 mL) was added to 6a (17 mg, 63 μmol, 1.0 equiv.) and the mixture was stirred at ambient temperature for 1 h. DOWEX 50 W × 8-40 was added to the reaction mixture until acidified to pH 5.5. The resin was washed with MeOH (20 mL) and the filtrate concentrated in vacuo. CJ-15,801 1 was obtained after flash column chromatography (EtOAc/MeOH/CH3CN/H2O 5 : 1 : 1 : 1) as a light yellow oil (11 mg, 81%). The NMR data were in agreement with the literature spectra.8
Large scale synthesis of CJ-15,801 (1)
For the large scale synthesis the precursors 3d and 4f were prepared on a gram scale according to the methods outlined above for the respective small scale syntheses in comparable or improved yields (∼50% for 3d and 80% for 4f respectively). The gram-scale Pd-catalyzed coupling was performed according to the general method using amide 3a (2.88 g, 15.4 mmol, 1.0 equiv.) and bromoacrylate 4f (3.73 g, 16.9 mmol, 1.1 equiv.). (E)-5h (3.35 g, 70%) was obtained as a yellow oil after purification by flash chromatography (hexane/EtOAc 2 : 1). Deprotection was accomplished in two-steps by first combining (E)-5h (1.46 g, 4.70 mmol, 1.0 equiv.) in CH3CN (30 mL) with BiCl3 (148 mg, 0.47 mmol, 0.1 equiv.) and H2O (1 mL) and stirring the resulting mixture at ambient temperature overnight. The filtrate was evaporated under vacuum to give the crude product that was subsequently purified by flash column chromatography (hexane/EtOAc 1 : 4) to give 6a as a yellow oil (966 mg, 76%). In the second deprotection step, K2CO3 in MeOH (10% w/v; 9 g in 90 mL) was added to 6a (966 mg, 3.58 mmol) and the mixture was stirred at ambient temperature for 1 h. DOWEX 50 W × 8-40 (previously washed with deionised water) was added to the reaction mixture until the pH reached 5.5. The resin was washed with MeOH (100 mL) and the filtrate concentrated in vacuo. CJ-15,801 (1) was obtained after flash column chromatography (EtOAc/MeOH/CH3CN/H2O 5 : 1 : 1 : 1) as a light yellow oil (544 mg, 70%). The NMR data were in agreement with the literature spectra.8
4′-Phospho-CJ-15,801 (8)
A reaction mixture with a total volume of 12.3 mL contained 30 mM NH4OAc (or NH4HCO3), 10 mM MgCl2, 20 mM KCl, 0.38 mM ATP (2.5 mol%) and 15 mM CJ-15,801 (1) (40 mg, 0.184 mmol), 16 mM disodium phosphoenolpyruvate (Na2PEP) at a final pH adjusted to ∼7.0. The mixture was split into 1 mL reactions across 12 × 1.5 mL microfuge tubes, and the reaction initiated by the addition of purified S. aureus PanK (0.033 mg) and pyruvate kinase (0.04 units) to each tube. After incubation at 37 °C overnight, the reaction was terminated and the proteins precipitated by heat-shock treatment at 95 °C for 6 minutes. Following centrifugation at 17000 × g for 10 minutes and syringe filtration (Acrodisc PSF with GXF/0.45 μm GHP membrane, 13 mm; PALL Life Sciences) of the supernatant, the resulting filtrates were combined, concentrated in vacuo and purified using C18 SPE (Phenomenex C18-E 10 g/60 mL, equilibrated with 50 mL MeOH followed by 100 mL H2O) with H2O as eluent. Collected fractions were analysed for the presence of the desired product by HPLC (see ESI† for details). The product-containing fractions were combined and repeatedly lyophilized to constant weight to yield a white solid (64.5 mg). Purity determination by comparison to a standard curve indicated a 70% yield of 4′-phospho-CJ-15,801 (8) (38.4 mg, 60% purity). 1H NMR (300 MHz, D2O): δ 7.69 (d, J = 14.2 Hz, 1H), 5.75 (d, J = 14.2 Hz, 1H), 4.20 (s, 1H), 3.86–3.76 (m, 1H), 3.70–3.62 (m, 1H), 1.01 (s, 3H), 0.95 (s, 3H). Contaminant peaks at δ 5.52 (t, J = 1.7 Hz), 5.26 (t, J = 1.6 Hz), 3.70–3.44 (m), 1.56 (s), 1.40 (s). 13C NMR (75 MHz, D2O): δ 175.8, 174.6, 134.5, 103.4, 75.2, 71.4 (d, 2JC,P = 5.4 Hz), 71.4 (d, 2JC,P = 5.4 Hz), 63.1, 39.4 (d, 2JC,P = 7.6 Hz), 39.4 (d, 2JC,P = 7.6 Hz), 21.3, 19.2. Contaminant peaks at δ 109.4, 72.7, 69.5, 68.4. HRMS (ESI+): m/z [M + H]+ calcd for C9H17NO8P: 298.0686; found: 298.0688; [M + Na]+ calcd for C9H16NO8PNa: 320.0506; found: 320.0510.
Antiplasmodial activity tests
The biological activity of CJ-15,801 and TBA (as the hydroxide) on the growth of P. falciparum were determined through a SYBR safe-based assay as described previously,7 with RPMI medium (supplemented with 11 mM glucose, 200 μM hypoxanthine, 24 μg mL–1 gentamicin and 6 g L–1 Albumax II) containing either 1 μM or 100 μM pantothenic acid (2).
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The CJ-15,801 used to obtain the data shown in Fig. 2a was a kind gift from Rudi Marquez and Alan Sewell, which is gratefully acknowledged. We are also grateful to the Canberra branch of the Australian Red Cross Blood Service for providing red blood cells. This work was supported by a CPRR grant (#78988) from the National Research Foundation (NRF) of South Africa and a National Institutes of Health (NIH) award (R01AI136836) to ES. RD received grant-holder and free-standing postdoctoral fellowships from the NRF and postdoctoral study support from the Oppenheimer Memorial Trust, RvdW and LB received NRF Scare Skills doctoral bursaries and KJM an NRF Innovation doctoral bursary. ETT was supported by a Research Training Program scholarship from the Australian Government.
Footnotes
†Electronic supplementary information (ESI) available: Additional experimental information and electronic copies of selected spectra. See DOI: 10.1039/c9md00312f
References
- Moolman W. J., de Villiers M., Strauss E. Biochem. Soc. Trans. 2014;42:1080–1086. doi: 10.1042/BST20140131. [DOI] [PubMed] [Google Scholar]
- Spry C., Kirk K., Saliba K. J. FEMS Microbiol. Rev. 2008;32:56–106. doi: 10.1111/j.1574-6976.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- Evans J. C., Trujillo C., Wang Z., Eoh H., Ehrt S., Schnappinger D., Boshoff H. I. M., Rhee K. Y., Barry C. E., Mizrahi V. ACS Infect. Dis. 2016;2:958–968. doi: 10.1021/acsinfecdis.6b00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie Y., Dekker K. A., Hirai H., Ichiba T., Ishiguro M., Shiomi Y., Sugiura A., Brennan L., Duignan J., Huang L. H., Sutcliffe J., Kojima Y. J. Antibiot. 2001;54:1060–1065. doi: 10.7164/antibiotics.54.1060. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen R., Hammons J. C., Meier J. L., Dahesh S., Moolman W. J. A., Pelly S. C., Nizet V., Burkart M. D., Strauss E. Chem. Biol. 2012;19:559–571. doi: 10.1016/j.chembiol.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba K. J., Kirk K. Mol. Biochem. Parasitol. 2005;141:129–131. doi: 10.1016/j.molbiopara.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Tjhin E. T., Spry C., Sewell A. L., Hoegl A., Barnard L., Sexton A. E., Siddiqui G., Howieson V. M., Maier A. G., Creek D. J., Strauss E., Marquez R., Auclair K., Saliba K. J. PLoS Pathog. 2018;14:e1006918. doi: 10.1371/journal.ppat.1006918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Shen R., Su S., Porco, Jr. J. A. Org. Lett. 2004;6:27–30. doi: 10.1021/ol0360041. [DOI] [PubMed] [Google Scholar]
- Villa M. V. J., Targett S. M., Barnes J. C., Whittingham W. G., Marquez R. Org. Lett. 2007;9:1631–1633. doi: 10.1021/ol070336e. [DOI] [PubMed] [Google Scholar]
- Sewell A. L., Villa M. V. J., Matheson M., Whittingham W. G., Marquez R. Org. Lett. 2011;13:800–803. doi: 10.1021/ol103114w. [DOI] [PubMed] [Google Scholar]
- Kashinath K., Swaroop P. S., Reddy D. S. RSC Adv. 2012;2:3596–3598. [Google Scholar]
- Mukherjee S., Corey E. J. Org. Lett. 2010;12:1024–1027. doi: 10.1021/ol100032u. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C., Mathison C. J. N. Angew. Chem., Int. Ed. 2005;44:5992–5997. doi: 10.1002/anie.200501853. [DOI] [PubMed] [Google Scholar]
- Lee J. M., Ahn D.-S., Jung D. Y., Lee J., Do Y., Kim S. K., Chang S. J. Am. Chem. Soc. 2006;128:12954–12962. doi: 10.1021/ja0639315. [DOI] [PubMed] [Google Scholar]
- Tanoury G. J., Chen M., Dong Y., Forslund R. E., Magdziak D. Org. Lett. 2008;10:185–188. doi: 10.1021/ol702532h. [DOI] [PubMed] [Google Scholar]
- Aquino F., Pauling H., Walther W., Plattner D. A., Bonrath W. Synthesis. 2000:731–737. doi: 10.1055/s-2000-6403. [DOI] [Google Scholar]
- Pett H., Jansen P., Hermkens P., Botman P., Beuckens-Schortinghuis C., Blaauw R., Graumans W., van de Vegte-Bolmer M., Koolen K., Rutjes F., Dechering K., Sauerwein R., Schalkwijk J. Malar. J. 2015;14:169. doi: 10.1186/s12936-015-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry C., Sewell A. L., Hering Y., Villa M. V. J., Weber J., Hobson S. J., Harnor S.S. J.J., Gul S., Marquez R., Saliba K. J. Eur. J. Med. Chem. 2018;143:1139–1147. doi: 10.1016/j.ejmech.2017.08.050. [DOI] [PubMed] [Google Scholar]
- Kaburagi Y., Kishi Y. Org. Lett. 2007;9:723–726. doi: 10.1021/ol063113h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLauro A. M., Seo W., Phillips S. T. J. Org. Chem. 2011;76:7352–7358. doi: 10.1021/jo200848j. [DOI] [PubMed] [Google Scholar]
- Faraldo-Gómez J. D., Kutluay E., Jogini V., Zhao Y., Heginbotham L., Roux B. J. Mol. Biol. 2007;365:649–662. doi: 10.1016/j.jmb.2006.09.069. [DOI] [PubMed] [Google Scholar]
- Barnard L., Mostert K. J., van Otterlo W. A. L., Strauss E. ACS Infect. Dis. 2018;4:736–743. doi: 10.1021/acsinfecdis.7b00240. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Barnard L., Mottaghi K., Tempel W., Antoshchenko T., Hong B. S., Allali-Hassani A., Smil D., Vedadi M., Strauss E., Park H.-W. ACS Infect. Dis. 2016;2:627–641. doi: 10.1021/acsinfecdis.6b00090. [DOI] [PubMed] [Google Scholar]
- Weir J. R., Patel B. A., Heck R. F. J. Org. Chem. 1980;45:4926–4931. [Google Scholar]
- Luo Y., Roy I. D., Madec A. G. E., Lam H. W. Angew. Chem., Int. Ed. 2014;53:4186–4190. doi: 10.1002/anie.201310380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.