Abstract
A versatile approach to fabricate PEG-peptide copolymer gels was utilized to design niches to promote chondrogenic differentiation of human mesenchymal stem cells (hMSCs). The sequences RGD and KLER were chosen as motifs to modify PEG gels through a thiol-acrylate polymerization. The KLER sequence, a binding site from decorin protein, is known to bind strongly to collagen type II and is responsible for matrix organization, while RGD promotes general survival of encapsulated cells. hMSCs were encapsulated at 2 × 106 cells/mL into 10 wt % PEG gels with 1 mM CRGDSG in the presence or absence of 5 mM CKLERG. A scrambled sequence served as a control. The gels were cultured in control and chondrogenic media, containing 5 ng/mL TGFβ1 over a 6-week period. Cell/gel constructs were analyzed at various time points for glycosaminoglycan (GAG) content, type II collagen deposition, immunostaining, and gene analysis. After 14 days in chondrogenic cultures, cells in RGDS and KLER functionalized gels produced 2.5 times as much GAG/cell as those in gels containing only RGD. By day 28, hMSCs within the chondrogenic KLER gels produced 27-fold higher hydroxyproline than that of day 0, whereas cells in chondrogenic culture with RGDS alone produced twofold of initial. Immunostained images indicated that col II was more predominant in the KLER-derivatized gels than others, and enhanced chondrogenic differentiation in KLER containing gels was further supported by RT-PCR analysis of type II collagen and aggrecan expression. Collectively, these results demonstrate how incorporation of matrix-binding peptide interacts with hMSCs inducing chondrogenic differentiation and cartilage-specific ECM deposition.
Keywords: human mesenchymal stem cells, biomaterials, decorin, chondrogenesis
INTRODUCTION
Design of gels for 3D cell culture and the promotion of tissue regeneration often require the incorporation of cellular cues to direct cell response, extracellular matrix (ECM) deposition, and tissue evolution. Cells respond directly to their surrounding environment through multiple cell–cell and cell-ECM interactions.1 As a result, synthetic gel niches are evolving to incorporate selected functionalities to direct cell interactions and cell responses such as proliferation, migration, differentiation, and eventually matrix deposition and tissue repair.2 Directions in the development of bioactive gel carriers have included the use of large ECM components such as collagen3,4 or alginate scaffolds5,6; tethered or encapsulated growth factors, namely TGFβ7,8 and BMPs9,10; adhesive proteins: fibronectin, laminin, and vitronectin11,12; or adhesive peptide sequences: RGD, YIGSR, REDV, PHSRN.12–17 Along with approaches to integrate functionality in gels, new directions have emerged to encapsulate, entrap, or conjugate these biomacromolecules, and poly(ethylene glycol) (PEG) is often used as a base platform.18–20
PEG is a highly hydrated bioinert polymer that is used in clinical medicine, especially as a conjugate to drugs to increase their stability, solubility, and availability. PEG gels also serve as a major platform for cell delivery in tissue engineering, as the high water content and tissue-like elasticity imparts desired properties. From a fundamental perspective, PEG’s resistance to protein adsorption allows one to study the influence of specific functionalities (e.g. cell adhesion) on cell function, since PEG avoids problems with classic materials and nonspecific interactions of proteins.11,21
A variety of methods to direct the function of encapsulated cells has been studied via modifications of PEG or other similar hydrophilic scaffolds. Numerous reports have demonstrated the effectiveness of immobilized RGD, the most widely investigated adhesive peptide motif, on promoting cell attachment, survival, migration, and differentiation.22–25 Furthermore, the entrapment or immobilization of growth factors within PEG gels has shown directed response from cells within. For instance, the covalent attachment of TGFβ to a PEG gel improved smooth muscle cell ECM production.8 Epidermal growth factor when conjugated to PEG retained biological activity and directed proper morphology and function when presented to seeded hepatocytes.26 The incorporation of enzymatically degradable peptide sequences into the crosslinks of PEG gels enables cell-directed remodeling of the gel structure and promotion of migration. Specifically, studies show the cleavage of covalently attached oligopeptides to PEG hydrogels by means of cell-secreted enzymes, collagenase, plasmin, or matrix metalloproteases, directing cellular migration through the gel.27,28
Cells also respond to extracellular signals through mechanisms other than direct integrin–receptor interactions with the ECM.29,30 The ECM of a cell is known to bind many different components, such as growth factors, as a means to present the signal at the proper time, place, and context.31–33 Other interactions are directed by electrostatic forces, as in the ECM of a chondrocyte, where the charges found on chondroitin sulfate or keratin sulfate play a role in directing matrix deposition.34–36 Furthermore, there is evidence that small ECM components, such as decorin, can interact with growth factor receptors, lessening the risk of tumorgenesis in tissues.37 This provides evidence that indirect interactions of a cell with an ECM component are just as important as direct adhesive interactions between a cell and a ligand, and therefore should be investigated to determine their ability to direct cell function. An example of an ECM molecule that is important in dictating the composition and organization of cartilage ECM is decorin.38
Decorin is a small leucine-rich protoeglycan (SLRP) found in the extracellular and pericellular matrices of chondrocytes39–41. This SLRP interacts with collagens I, II, IV, VI, etc., as well as other ECM components and growth factors, such as fibronectin, thombrospondin, TGFβ, and epidermal growth factor receptors39,41,42. Decorin plays a key role in matrix deposition by aiding in the fibril growth, extension, and ultimately organization of type II collagen in the cartilage matrix.43,44 A collagen triple-helical structure has been found to be stabilized once bound to decorin at two major sites, those of RELH and KLER. RELH and KLER are found within the concave regime of decorin and are complementary to the preferential binding site near the N-terminus of collagen type II45,46. In this manner, the decorin binding sites of RELH and KLER bind to and organize collagen type II in the matrix, inhibit collagenase degradation, and regulate the influence of TGFβ on chondrocytes.47,48
This work focuses on understanding how integration of peptide sequences, specifically those that allow for cell adhesion and those that bind type II collagen, into a PEG gel will influence chondrogenesis of human mesenchymal stem cells (hMSCs). The gels were synthesized using a thiol-acrylate phototopolyermization chemistry.49 Specifically, poly(ethylene glycol) diacrylate (PEGDA) was copolymerized with cysteine-containing peptides, namely CRGDSG, to promote cell survival,50 and CKLERG, to promote cartilage-specific matrix deposition and organization. We hypothesize that incorporation of both CRGDSG and CKLERG, into hMSC-containing PEG gels are vital in first initiating hMSC differentiation to chondrocytes and induction of cartilage ECM production.
MATERIALS AND METHODS
Cell harvest and culture
Human mesenchymal stem cells (hMSCs) were purchased from Cambrex Bio Science (Walkersville, MD) and cultured in low glucose DMEM supplemented with 10% fetal bovine, serum 1 μg/mL amphotericin B, 50 U/mL penicillin, 50 μg/mL streptomycin, and 20 μg/mL gentamicin. hMSCs used in this study were collected from one donor and encapsulated at passage 3 to reduce the variability from the primary cell isolate.
Synthesis of PEG and peptide motifs
Poly (ethylene glycol) diacrylate (PEGDA) was synthesized by dissolving PEG (Mn = 4600 Da) in anhydrous dichloromethane and purging with argon for 15 min. Triethylamine (TEA) in a 2:1 molar excess was added to the solution, and allowed to mix under argon for 15 min. Acryloyl chloride at a 2.5:1 molar excess was mixed with anhydrous dichloromethane and added dropwise to the PEG/TEA solution that reacted overnight under argon purge. The product was rotovapped and filtered to remove excess salts, precipitated in cold ethyl ether, filtered, and dried in a dessicator. After complete drying, the PEGDA was redissolved in dIH2O and dialyzed (Spectrum, 1000 MWCO) over a 24-h period with three dIH2O exchanges. A 1H NMR analysis of the dialyzed PEGDA product revealed an average of 88% acrylation by comparing acrylate hydrogen to ethylene oxide backbone hydrogen integrations.
The peptide sequences CKLERG (cysteine–lysine–leucine–glutamic acid–arginine–glycine, N-terminus to C-terminus), CLKREG (scramble sequence) (cysteine–leucine–lysine–arginine–glutamic acid–glycine), and CRGDSG (cysteine–arginine–glycine–aspartic acid–serine–glycine, N-terminus to C-terminus) were synthesized using a solid phase peptide synthesizer (Applied Biosystems, model 433A). The fabricated peptide sequences were cleaved from the resin and deprotected using trifluoro acetic acid (Sigma), phenol, and triisopropylsilane (Sigma). Both sequences were then precipitated in diethyl ether and desiccated for 2 days. The dried product was redissolved in dIH2O and lyophilized. Matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) spectroscopy was run on the lyophilized product to verify peptide molecular weight and structure. The final product was incorporated into the PEG hydrogel network through a photointiated thiol-acrylate reaction with the thiol groups located on the cysteines.49 Figure 1 displays the chemical structure of the PEGDA, CRGDGS, and CKLERG used in these studies.
Figure 1.
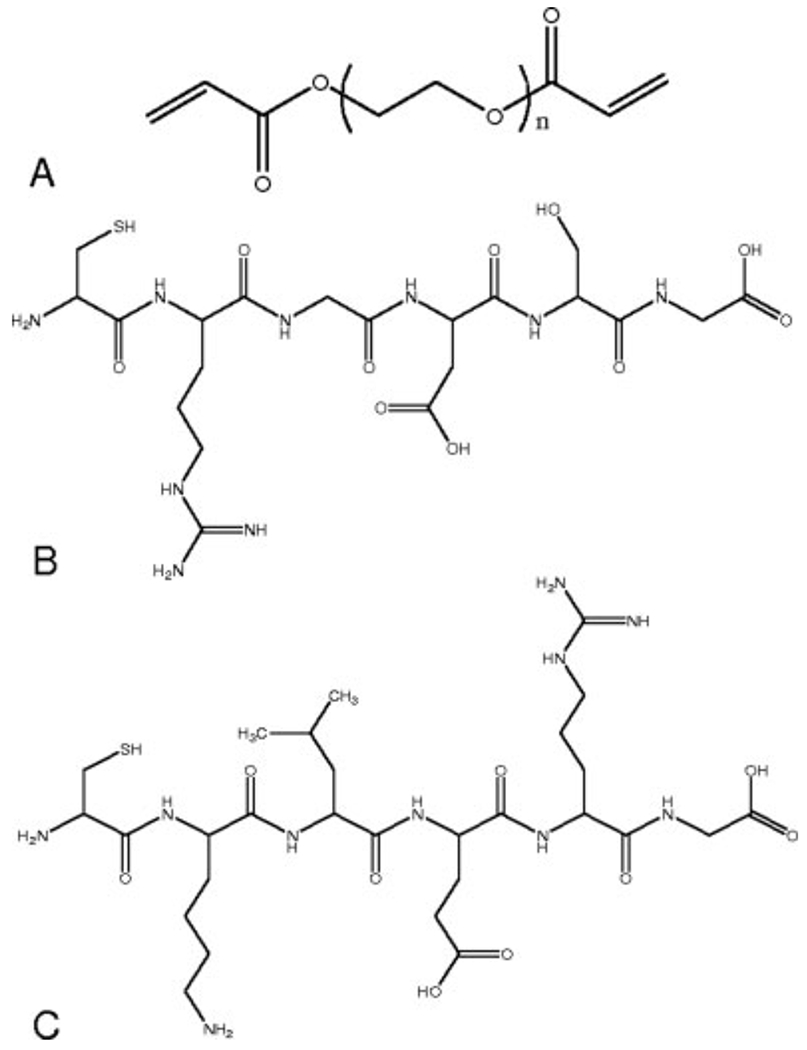
Chemical structures of (A) diacrylated macromere PEG4600DA (n = 105), (B) CRGDSG, and (C) CKLERG peptide. PEG4600DA, CRGDSG, and CKLERG were used in hydrogel fabrication.
hMSC encapsulation in peptide-tailored PEG hydrogels
hMSCs were encapsulated in a PEG-peptide copolymer system via a photoinitiated thiol-acrylate polymerization mechanism similar to a previously described thiol-methacrylate reaction.51 This technique was used to efficiently and easily tether in the peptide sequence of interest through a robust, cytocompatible polymerization process. In both cases, a solution of 10 wt % PEGDA was dissolved in PBS and the photoinitiator, 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-propyl)ketone (I2959), was added at 0.05 wt %. All PEG gels containing 1 mM CRGDSG and the presence or absence of CKLERG (5 mM) were examined. A scrambled sequence of CLKREG (5 mM) was used as a control. The exact concentrations were determined experimentally based upon amine measurements using the fluoraldehyde assay.52 The well-mixed macromer solution was filtered and added to an hMSC pellet that had been trypsinized from culture, centrifuged, and counted. The final cell pellet, containing 2 million cells/mL, was triturated with the macromer solution and added in 40-μL volume aliquots to 1-mL syringes with the tips removed. Each macromer/cell solution was photopolymerized at 365-nm light with an intensity of ~5 mW/cm2 for 10 min at room temperature. After photopolymerization, the cell-laden gel disks (5 mm × 1 mm) were removed and placed in either control stem cell media or chondrogenic media [Dulbecco’s high glucose modified eagle medium, ITS+ premix (6.25 μg/mL bovine insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenous acid, 5.33 μg/mL linoleic acid, 1.25 μg/mL bovine serum albumin), 100 nM dexamethasone, 50 μg/mL ascorbic acid 2-phosphate, 100 μg/mL sodium pyruvate, 100 μg/mL penicillin–streptomycin, amphotericin B] and supplemented with 5 ng/mL TGFβ1. The constructs were cultured for up to 6 weeks at 37°C and 5% CO2 with media being changed twice weekly.
Cell proliferation and matrix production
Gel were collected at 0, 1, 2, 3, 4, 5, and 6 weeks (N = 6). The constructs were digested for 16 h at 60°C in a papain solution (125 μg/mL papain, 10 mM cysteine) and further processed after digestion with a tissue homogenizer. As a measure of cell number, double-stranded DNA content was measured via the PicoGreen assay (Pierce). The same digests were then used to measure the amount of sulfated glycosaminoglycan (GAG) and hydroxyproline (OHP)53 in each construct. Sulfated GAGs were quantified using an assay based on dimethylmethylene blue (DMMB)54; chondroitin sulfate A was used as a standard. Production of total collagen was quantitatively measured via the OHP assay, where OHP was detected through base-catalyzed hydrolysis of the collagen digests. The samples were then neutralized with acid and reacted with a choloramine T solution followed by a final reaction with p-dimethylbenzaldehyde. Colorimetric differences in the samples were quantified using a standard curve of OHP. GAG and OHP values were normalized against the amount of DNA per sample to achieve an average production per cell.
Histological analysis of collagen type
Collagen deposition and type were visualized using immunohistological staining. Two constructs were removed from each culture platform at 1, 2, 3, 4, 5, and 6 week time points and fixed in 4% paraformaldehyde buffer overnight. The fixed samples were then cryosectioned at 40 μm and rinsed in PBS to remove Histoprep freezing medium. Staining of the slides was performed following the VectaStain Universal ABC kit (Vector Labs). The sections were fixed onto the glass slides with acetone and treated with 3% bovine serum albumin (BSA) block to avoid nonspecific binding of the antibodies. Slides were treated with either collagen type I or type II primary antibodies. Primary antibodies raised against collagen I (rabbit) and collagen II (rabbit) were diluted in 1% BSA solution, and staining with the antibodies was performed on each of the sections for 1 h. A biotinylated secondary antibody (anti-rabbit IgG) was diluted as recommended by the manufacturer and incubated on the slides for 30 min. Following this incubation treatment, an avidin and biotinylated horseradish peroxidase complex was incubated on the slides for 30 min, after which a peroxidase substrate (Vector Nova Red) was allowed to bind to the HRP component, creating a red stain in areas of antibody binding. Finally, the slides were treated with a DAPI dye for 10 min to locate the cell nuclei. Images were taken using a fluorescent microscope, Nikon Eclipse TE300 camera and related software.
Expression of chondrogenic markers
Constructs were removed at 0, 1, 2, 3, 4, 5, and 6 week time points (N = 3), and total RNA was extracted using a guanidinium thiocyanate/phenol reagent (TRI Reagent, Sigma) according to the manufacturer’s protocols. RNA samples were treated with DNase removing genomic contaminants and then quantified with the RiboGreen assay (Molecular Probes) as described by the manufacturer. The samples were diluted to contain equivalent amounts of RNA, which was then converted to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Real time PCR, using an iCycler Real-Time PCR machine (Bio-Rad), was used to analyze gene expression levels of aggrecan, collagen I and II, and the hMSC marker nucelostemin. All values were normalized to GAPDH. Forward and reverse primers are referenced in Table I.55,56 PCR was performed following the program: 95°C for 3 min followed by 45 cycles for 15 s at 95°C and 60°C for 60 s.
TABLE I.
| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| GAPDH | ATGGGGAAGGTGAAGGTCG | TAAAAGCAGCCCTGGTGACC |
| Collagen I | CAGCCGCTTCACCTACAGC | TTTTCTATTCAATCACTGTCTTGCC |
| Collagen II | GGCAATAGCAGGTTCACGTACA | CGATAACAGTCTTGCCCCACTT |
| Aggrecan | TCGACCACAGCGAGGCC | TCGAGGACAGCGAGGCC |
| Nucleostemin | CAAGCATTGAGGAACTAAGAC | GCAATAGTAACCTAATGAGCC |
Statistics
Data is presented as mean ± standard deviation of the replicates. A Student’s t-test was utilized to compare data sets, with p values less than 0.05 signifying significant differences.
RESULTS
Differentiation of encapsulated hMSCs through monitoring matrix deposition
The role of the gel composition and media composition on the extent of chondrogenic differentiation of encapsulated hMSCs was first monitored through biological assays measuring cartilage-specific matrix components. Early stages of chondrogenesis are characterized by the deposition of GAGs, which was monitored via the DMMB assay. In Figure 2, chondroitin sulfate secretion was measured over a 6-week time course for hMSCs cultured in gels containing CRGDSG alone or in combination with CKLERG or CLKREG (scramble). The amount of GAG produced by the encapsulated hMSCs was normalized to the cell amount of DNA in the same exact hydrogel to remove effects from differences in proliferation. Those hMSCs cultured in control media [Fig. 2(A)] did not show a significant amount of GAG production over the entire course of the study (<0.08 μg ChSA/ng DNA). The same low basal level of GAG/cell was witnessed for cells in the chondrogenic media and cultured in gels with the scramble CLKREG sequence [Fig. 2(B)]. Interestingly, cells in RGDS functionalized gels in chondrogenic culture did not show a significant increase in GAG production until day 28, reaching ~0.1 μg ChSA/ng DNA. The production then decreased to basal levels by day 42. Levels of GAG/DNA secreted by cells in CKLERG-containing gels in chondrogenic culture increased steadily, reaching a high value of 0.22 μg ChSA/ng DNA on day 21. Afterwards, the GAG/DNA values decreased to 0.05 μg ChSA/ng DNA by day 42.
Figure 2.
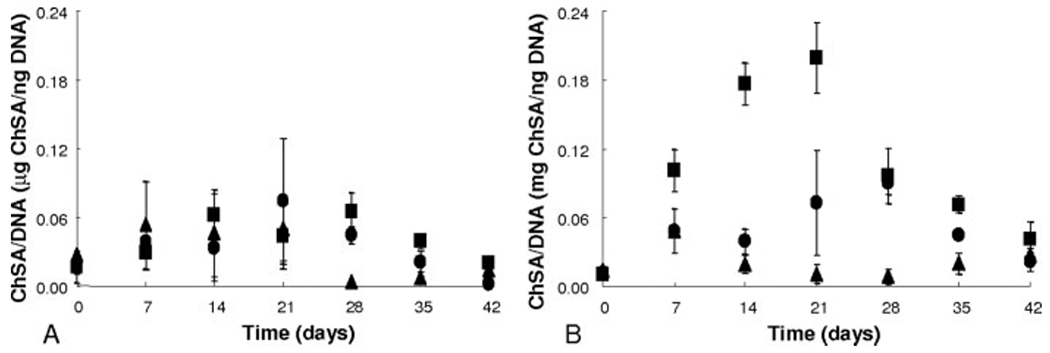
Chondroitin sulfate production as a measure of glycosaminoglycan deposition by hMSCs in cultures as normalized to DNA. Gel compositions are 1 mM CRGDSG (●), 1 mM CRGDSG and 5 mM CLKREG (i.e. scramble) (▲), or 1 mM CRGDSG and 5 mM CKLERG (■) cultured in control media (panel A) and chondrogenic media (panel B).
The total amount of collagen produced by encapsulated hMSCs was also measured as a function of the culture conditions. Total collagen production, along with immunostained images selective for collagen type II deposition, provides further insight into the degree of chondrogenic differentiation. Figure 3(A,B) depicts the amount of OHP produced by hMSCs in various gel formulations normalized to OHP levels on day 0. Collagen levels for all control culture platforms continually decreased to ~50% of the initial levels by day 42 [Fig. 3(A)]. In chondrogenic media, the hMSCs in gels with the scrambled sequence (CLKREG) showed no significant increase or decrease in OHP production over the course of the study [Fig. 3(B)]. Those cells cultured in chondrogenic media in the CRGDSG hydrogels reached a high of ~200% OHP production on days 21 through 35, then decreasing to ~150% of the initial value by day 42. hMSCs encapsulated in CKLERG gels and chondrogenic culture began depositing collagen by day 7 (~500% of the initial levels) only to reach a high level of production ~2700% of initial values by days 28 and 35. The extremely high levels of total collagen production, as seen with cells in CKLERG gels cultured in chondrogenic media, decreased slightly to ~1200% by day 42. The statistically significant and dramatic elevations in collagen production, as seen in the OHP assay, was confirmed to be that of collagen type II, especially in the chondrogenic CKLERG culture platform, though immunostaining techniques [Fig. 3(C,D)]. Immunostained images specific for collagen type I showed slight col I deposition at early time points, but did not coincide with the large amounts of col II staining as seen surrounding cells in the CKLERG gels cultured in chondrogenic media (data not shown). Collagen deposited within the pericellular and ECM of encapsulated hMSCs was stained red, and the nuclei of the cells were counterstained with DAPI. As seen in Figure 3(C), those hMSCs cultured in control CKLERG hydrogels do not show a significant amount of red staining by day 28, whereas Figure 3(D) reveals that those hMSCs cultured in chondrogenic CKLERG platforms have deposited a large amount of collagen type II, as depicted by the areas of heavy red staining.
Figure 3.
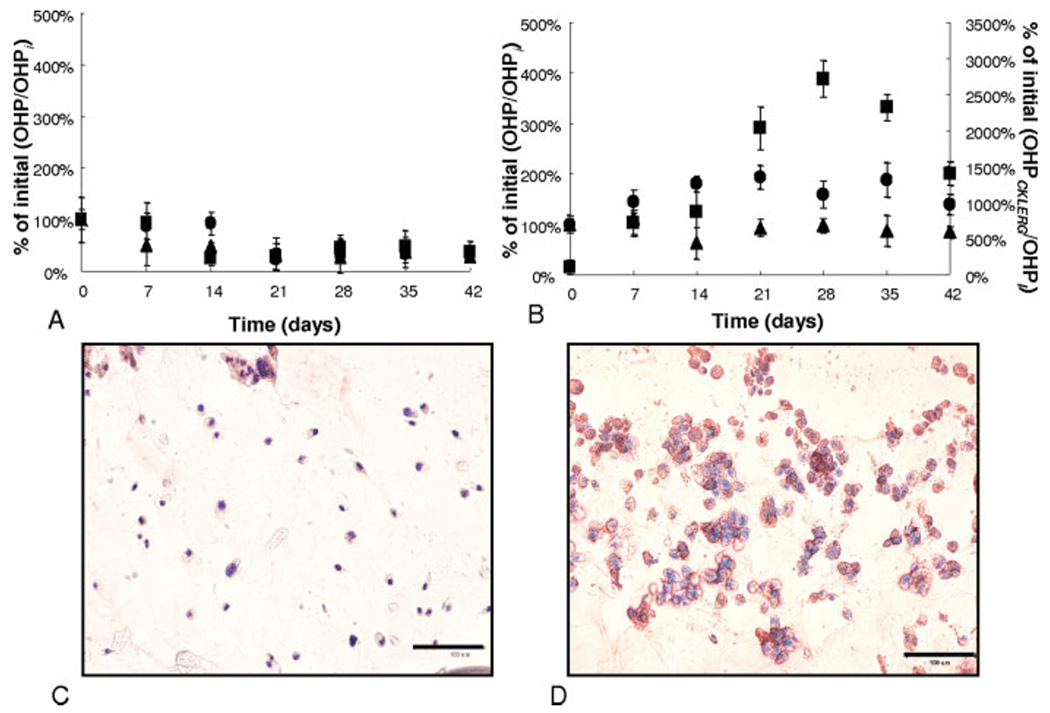
Hydroxyproline (OHP) production as normalized to initial OHP production on day 0 (OHPi). 1 mM CRGDSG (●), 1 mM CRGDSG and 5 mM CLKREG (i.e. scramble) (▲), and control 1 mM CRGDSG and 5 mM CKLERG (■) whereby control media (A) and chondrogenic media (B) were plotted on the primary y-axis while the chondrogenic 1 mM CRGDSG and 5 mM CKLERG (■) was plotted on the secondary y-axis. hMSCs encapsulated in 1 mM CRGDSG and 5 mM CKLERG platforms were immunostained for collagen type II deposition (red staining) and counterstained for cell nuclei (blue) on day 28 of control media culture (C) or chondrogenic culture (D). Scale bar = 100 μm.
Expression of chondrogenic markers
Further characterization of the chondrogenic differentiation of the hMSCs in the gel cultures was achieved through monitoring mRNA levels of nucleostemin, a marker for undifferentiated hMSCs,56 aggrecan, collagen type I and collagen type II. The results displayed in Figure 4 suggest that hMSCs cultured in control media, regardless of gel composition, [Fig. 4(A)], are experiencing no significant change in their phenotype (i.e., differentiation), as the levels of nucleostemin remained relatively constant and high. However, as seen in Figure 4(B), those hMSCs cultured in chondrogenic media, with the exception of the CLKREG (scramble) gels, showed a statistically significant decrease in nucelostemin gene expression levels by ~day 14. Further, the levels of collagen type I production, as seen in Figure 4(C), show no clear trends in time or with gel composition. The chondrogenic cultures [Fig. 4(D)], all show a significant rise in col I expression at day 14, which then decreases steadily to a final level of ~0.5 by day 42.
Figure 4.
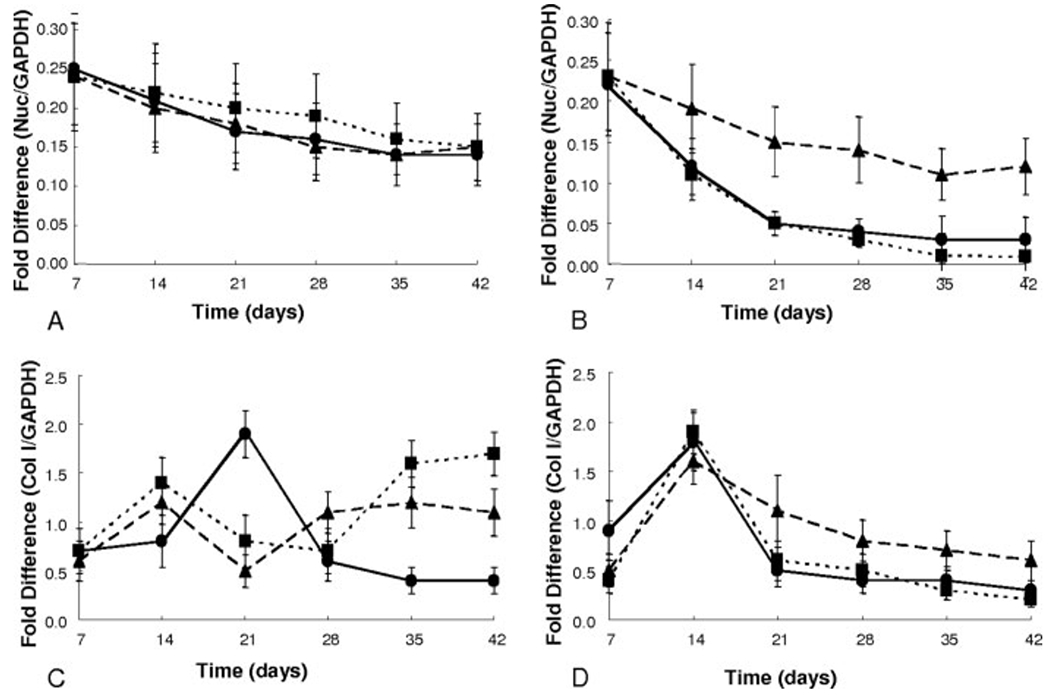
mRNA gene expression levels as represented by fold difference over housekeeping gene GAPDH for nucleostemin (A,B) and collagen type I (C,D) data are depicted: 1 mM CRGDSG (●), 1 mM CRGDSG and 5 mM CLKREG (i.e. scramble) (▲), and 1 mM CRGDSG and 5 mM CKLERG (■) whereby control media (A,C) and chondrogenic media (B,D).
When examining the chondrogenic markers of aggrecan and type II collagen, cells in control media show basal levels of expression of both genes throughout the culture period [Fig. 5(A,C)]. hMSCs encapsulated in CLKREG (scramble) gels and cultured in chondrogenic media showed a slight increase in aggrecan expression by day 7 and col II by day 35; however, these levels were immediately reduced to basal control culture expression levels for the remainder of the study [Fig. 5(B,D)]. Aggrecan expression levels for cells in CRGDSG gels in chondrogenic culture remained high at ~0.017-fold difference for days 7 and 14 decreasing to basal levels by day 35. Cells encapsulated in CKLERG gels in chondrogenic culture followed the same trend with expression levels ~1.5 times higher as cells in CRGDSG gels. Collagen type II expression for cells in CRGDSG gels in chondrogenic media increased to ~1.0-fold expression levels by day 21 and remained stable at that level for the entire culture period. Cells in CKLERG cultures with chondrogenic media reached a level of ~1.0-fold expression levels by day 21 as well, further increasing to a high of ~2.3-fold expression levels by day 35. Col II expression levels for the cells in CKLERG gels decreased to ~1.7 by day 42 of chondrogenic culture. The increase and decrease in gene expression levels specifically relating to chondrogenic markers, such as aggrecan and col II, followed consistently with biological matrix assay performed as mentioned earlier.
Figure 5.
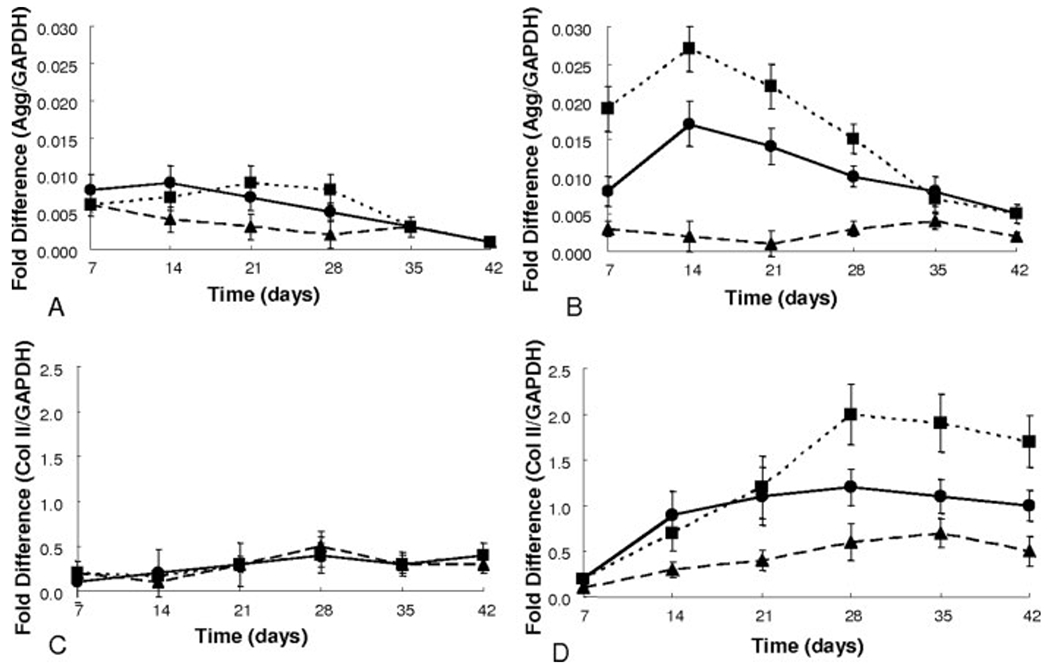
mRNA gene expression levels as represented by fold difference over housekeeping gene GAPDH for aggrecan (A,B) and collagen type II (C,D) data are depicted: 1 mM CRGDSG (●), 1 mM CRGDSG and 5 mM CLKREG (i.e. scramble) (▲), and 1 mM CRGDSG and 5 mM CKLERG (■) whereby control media (A,C) and chondrogenic media (B,D).
DISCUSSION
Stem cells receive a complex array of signals from their niches that regulates their properties in both time and space. Here, PEG gels were used as a platform to introduce peptide fragments as a means to mimic important ECM interactions. Much of the focus on peptide-modified gels has been the incorporation of adhesive ligands, mainly in the form of the peptide sequence RGDS. Previous research suggests that adhesion is an important part of newly developing tissue and has also been linked to the initial stages of hMSC differentiation into chondrocytes.39 In the research presented here, we investigated the additional influence of a nonadhesive, nonintegrin binding peptide sequence, CKLERG on the chondrogenesis of encapsulated hMSCs. As mentioned previously, the sequence KLER is a major binding site within the decorin SLRP found extensively in cartilage ECM. Decorin is known to bind collagen type II and aids in cartilage ECM organization and development.46
Incorporation of both a cell adhesive sequence, RGDS, and a cartilage ECM binding peptide, KLER, was shown to further promote chondrogenic differentiation of hMSCs and cartilage-specific ECM production. Early stage cartilage ECM production, as measured via the DMMB assay, relates the amount of GAG produced as normalized to DNA. As seen from Figure 2(A), those cells cultured in control media in all gel compositions maintained basal levels of GAG deposition, relaying that a significant portion of these cells were not undergoing chondrogenic differentiation. Figure 2(B) shows that cells in CRGDSG gels in chondrogenic media increased GAG production within the first 3 weeks of culture. However, the cells in CKLERG gels cultured in chondrogenic media produced 2.5 times as much GAG/cell as did those cells only exposed to CRGDSG. These results coincide with the initial increase in aggrecan expression as seen with the RT-PCR data relating that hMSCs encapsulated within CKLERG gels are allowed to interact with the RGD sequence, expending their much needed ATP source to begin the differentiation process, which is further influenced by the presence of KLER.
Furthermore, total collagen was measured and through the results, those cells in CRGDSG gels exposed to chondrogenic media produced a large amount of total collagen after 2 weeks into the study. This rise in collagen production after the onset of GAG production, as seen with the previously mentioned results, follows nicely with the studied timeline for hMSC chondrogenesis. Through the time course of hMSC differentiation, cells will initially adhere to fibronectin in their surrounding environment, begin depositing GAGs within their pericellular matrix, and eventually produce collagen type II to aid in completing the ECM highly rich in GAGs and collagen type II.41 The levels of OHP produced by hMSCs entrapped in the CKLERG gels in chondrogenic media showed extremely elevated levels reaching an ultimate high on day 28 of ~27-fold increase over the initial time point. These data, along with the positive collagen type II staining depicted in the immunostained sections of the gels in Figure 3(C,D), provide evidence that the rise in OHP is related to a strong deposition of collagen type II around these differentiated hMSCs in this particular culture platform. Collagen type I staining (data not shown) did not depict heavy staining corresponding to the days of elevated OHP expression. In addition, the decrease in collagen type I gene expression levels for cells in CKLERG gels by day 21 of culture and an increase in collagen type II gene expression further supports the premise that the hMSCs are differentiating and producing a cartilage ECM. Finally, the low levels of GAG, OHP, aggrecan and col II expression, as well as the high levels of nucelostemin expression as seen with hMSCs in the chondrogenic scramble platform, demonstrate that the increase in chondrogenic response of these cells was not directed by any type of peptide sequence. The mere fact that the scramble peptide did not positively affect the hMSCs in culture provides evidence that the CKLERG sequence generates a specific ECM interaction causing the needed cell-signaling cascades to direct chondrogenesis.
This evidence supports the claim that in order to induce a specific cellular behavior a more complex cell carrier is useful to design and employ. In this system, hMSCs were encapsulated in a material containing the much needed adhesive component, CRGDSG, and a second peptide that caused a different interaction with the cell. Research suggests that natively decorin will interact with the cell through electrostatic interactions but most importantly through binding with the ECM and affecting organization in such a manner.44 This PEG-peptide system creates a niche that accentuates the role of the native environment. hMSCs require binding to fibronectin to initiate chondrogenesis and the production of GAGs,57 whereby the decorin moiety provides the draw for collagen production and ECM organization to better elaborate the cartilage matrix. Therefore, in designing a hydrogel for chondrogenic differentiation of hMSCs and subsequent evolution of cartilaginous tissue that recapitulate aspects of native matrix components aids and extends the influence and direction of cellular growth and tissue development.
Acknowledgments
Contract grant sponsor: National Institute of Health; contract grant number: DE12998
Contract grant sponsor: Graduate Assistantship in Areas of National Need Fellowship
References
- 1.Albelda SB, Buck CA. Integrins and other cell adhesion molecules. Faseb J 1990;4:2868–2880. [PubMed] [Google Scholar]
- 2.Yang S, Leong K-F, Du A, Chua C-K. The design of scaffolds for use in tissue engineering. I. Traditional factors. Tissue Eng 2001;7:679–689. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm 2001;221:1–22. [DOI] [PubMed] [Google Scholar]
- 4.Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials 2001;22:3145–3154. [DOI] [PubMed] [Google Scholar]
- 5.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol 1990;8:71–78. [DOI] [PubMed] [Google Scholar]
- 6.Johnson FC, Craig DQM, Mercer AD. Characterization of the clock structure and molecular weight of sodium alginates. J Pharm Pharmacol 1997;49:639–643. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Yaszemski MJ, Mikos AG. TGF-β1 release from biodegradable polymer microparticles: Its effects on marrow stromal osteoblast function. J Bone Joint Surg Am 2001;83 (Suppl 1, Pt 2):S82–S91. [PubMed] [Google Scholar]
- 8.Mann BK, Schmedlen RH, West JL. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 2001;22:439–444. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res 2000;50:405–409. [DOI] [PubMed] [Google Scholar]
- 10.Saito NO, Okada T, Horiuchi H, Murakami N, Takahashi J, Hawata M, Ota H, Nozaki K, Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol 2001;19:332–335. [DOI] [PubMed] [Google Scholar]
- 11.Hubbell JA. Bioactive biomaterials. Curr Opin Biotechnol 1999;10:123–129. [DOI] [PubMed] [Google Scholar]
- 12.West JLH, Hubbell JA. Bioactive Polymers. Boston: Birkhauser; 1997. p 83–95. [Google Scholar]
- 13.Drumheller PD, Hubbell JA. Polymer networks with grafted cell adhesion peptides for highly biospecific cell adhesive substrates. Anal Biochem 1994;222:380–388. [DOI] [PubMed] [Google Scholar]
- 14.Seeger JM, Klingman N. Improved endothelial cell seeding with cultured cells and fibronectin-coated grafts. J Surg Res 1985;38:641–647. [DOI] [PubMed] [Google Scholar]
- 15.Bearinger JP, Castner DG, Healy KE. Biomolecular modification of p(AAm-co-EG/AA) IPNs supports osteoblast adhesion and phenotypic expression. J Biomater Sci Polym Ed 1998;9:629–652. [DOI] [PubMed] [Google Scholar]
- 16.Massia SP, Rao SS, Hubbell JA. Covalently immobilized laminin peptide Tyr-Ile-Gly-Ser-Arg (YIGSR) supports cell spreading and co-localization of the 67-kilodalton laminin receptor with α-actinin and vinculin. J Biol Chem 1993;268:8053–8059. [PubMed] [Google Scholar]
- 17.Hirano Y, Okuno M, Hayashi T, Goto K, Nakajima A. Cell-attachment activities of surface immobilized oligopeptides RGD, RGDS, RGDV, RGDT, and YIGSR toward 5 cell-lines, J Biomater Sci Polym Ed 1993;4:235–243. [DOI] [PubMed] [Google Scholar]
- 18.West JLH, Hubbell JA. Photopolymerized hydrogel materials for drug delivery applications. React Polym 1995;25:139–147. [Google Scholar]
- 19.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials 2001;22:3045–3051. [DOI] [PubMed] [Google Scholar]
- 20.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 1998;19:1287–1294. [DOI] [PubMed] [Google Scholar]
- 21.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 2003;9:679–688. [DOI] [PubMed] [Google Scholar]
- 22.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984;309:30–33. [DOI] [PubMed] [Google Scholar]
- 23.Shin H, Jo S, Mikos AG. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethyleneglycol) spacer. J Biomed Mater Res 2002;61:169–179. [DOI] [PubMed] [Google Scholar]
- 24.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002;23:4315–4323. [DOI] [PubMed] [Google Scholar]
- 25.Park KH, Na K, Chung HM. Enhancement of the adhesion of fibroblasts by peptide containing an Arg-Gly-Asp sequence with poly(ethylene glycol) into a thermo-reversible hydrogel as a synthetic extracellular matrix. Biotechnol Lett 2005;27: 227–231. [DOI] [PubMed] [Google Scholar]
- 26.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med 1996;2:1022–1027. [DOI] [PubMed] [Google Scholar]
- 27.West JLH, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999;32:241–244. [Google Scholar]
- 28.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun Y, Jacobs HA, Kim SW. Mechanism of thrombin inactivation by immobilized heparin. J Biomed Mater Res 1996;30:423–427. [DOI] [PubMed] [Google Scholar]
- 30.Mauray S, De Raucourt E, Chaubet F, Maiga-Revel O, Sternberg C, Fischer AM. Comparative anticoagulant activity and influence on thrombin generation of dextran derivatives and of a fucoidan fraction. J Biomater Sci Polym Ed 1998;9:373–387. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Zheng J, Imanishi Y, Yonezawa K, Kasuga M. Proteinfree cell culture on an artificial substrate with covalently immobilized insulin. Proc Natl Acad Sci USA 1996;93:3598–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. Faseb J 2001;15:1300–1302. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder-Tefft JB, Benta H, Estridge TD. Collagen and heparin matrices for growth factor delivery. J Controlled Release 1997;49:291–298. [Google Scholar]
- 34.Lee V, Cao L, Zhang Y, Kiani C, Adams ME, Yang BB. The roles of matrix molecules in mediating chondrocyte aggregation, attachment, and spreading. J Cell Biochem 2000;79:322–333. [DOI] [PubMed] [Google Scholar]
- 35.Sandy JD, O’Neill JR, Ratzlaff LC. Acquisition of hyaluronate-binding affinity in vivo by newly synthesized cartilage proteoglycans. Biochem J 1989;258:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole CA, Flint MH, Beaumont BW. Chondrons extracted from canine tibial cartilage: Preliminary report on their isolation and structure. J Orthop Res 1988;6:408–419. [DOI] [PubMed] [Google Scholar]
- 37.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem 2000;275:35153–35161. [DOI] [PubMed] [Google Scholar]
- 38.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 1999;274:18843–18846. [DOI] [PubMed] [Google Scholar]
- 39.Tenni R, Viola M, Welser F, Sini P, Giudici C, Rossi A, Tira ME. Interaction of decorin with CNBr peptides from collagens I and II: Evidence for multiple binding sites and essential lysyl residues in collagen. Eur J Biochem 2002;269:1428–1437. [DOI] [PubMed] [Google Scholar]
- 40.Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci USA 1986;83:7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole CA, Honda T, Skinner SJ, Schofield JR, Hyde KF, Shinkai H. Chondrons from articular cartilage (II): Analysis of the glycosaminoglycans in the cellular microenvironment of isolated canine chondrons. Connect Tissue Res 1990;24:319–330. [DOI] [PubMed] [Google Scholar]
- 42.Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem 2000;275:32879–32887. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 2006;98:1436–1449. [DOI] [PubMed] [Google Scholar]
- 44.Dugan TA, Yang VW, McQuillan DJ, Hook M. Decorin modulates fibrin assembly and structure. J Biol Chem 2006; 281:38208–38216. [DOI] [PubMed] [Google Scholar]
- 45.Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry 1996;35:8795–8799. [DOI] [PubMed] [Google Scholar]
- 46.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem 1996;271:31767–31770. [DOI] [PubMed] [Google Scholar]
- 47.Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol 2006;25:332–341. [DOI] [PubMed] [Google Scholar]
- 48.Geng Y, McQuillan D, Roughley PJ. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol 2006;25:484–491. [DOI] [PubMed] [Google Scholar]
- 49.Salinas CN, Anseth KS. Characterization of thiol-acrylate mixed-mode photopolymerization for the incorporation of peptides into a PEG gel. Macromolecules 2008;41:6019–6026. [Google Scholar]
- 50.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol 2005;24:208–218. [DOI] [PubMed] [Google Scholar]
- 51.Cramer NBB, Christopher N. Kinetics of thiol-ene and thiol-acrylate photopolymerizations with real-time Fourier transform infrared. J Polym Sci Part A 2001;39:3311–3319. [Google Scholar]
- 52.Roth M Fluorescence reaction for amino acids. Anal Chem 1971;43:880–882. [DOI] [PubMed] [Google Scholar]
- 53.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–447. [DOI] [PubMed] [Google Scholar]
- 54.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883:173–177. [DOI] [PubMed] [Google Scholar]
- 55.Martin I, Jakob M, Schafer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 2001;9:112–118. [DOI] [PubMed] [Google Scholar]
- 56.Kafienah W M S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells 2006;24:1113–1120. [DOI] [PubMed] [Google Scholar]
- 57.Tavella S, Bellese G, Castagnola P, Martin I, Piccini D, Doliana R, Colombatti A, Cancedda R, Tacchetti C. Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation. J Cell Sci 1997;110 (Pt 18):2261–2270. [DOI] [PubMed] [Google Scholar]


