Abstract
Human skin demonstrates a striking variation in tone and color that is evident among multiple demographic populations. Such characteristics are determined predominantly by the expression of the genes controlling the quantity and quality of melanin, which can alter significantly due to the presence of small nucleotide polymorphism affecting various steps of the melanogenesis process and generally linked to the lighter skin phenotypes. Genetically determined, constitutive skin color is additionally complemented by the facultative melanogenesis and tanning responses; with high levels of melanin and melanogenic factors broadly recognized to have a protective effect against the UVR-induced molecular damage in darker skin. Long-term sun exposure, together with a genetic makeup responsible for the ability to tan or the activity of constitutive melanogenic factors, triggers defects in pigmentation across all ethnic skin types. However, sun exposure also has well documented beneficial effects that manifest at both skin homeostasis and the systemic level, such as synthesis of vitamin D, which is thought to be less efficient in the presence of high levels of melanin or potentially linked to the polymorphism in the genes responsible for skin darkening triggered by UVR. In this review, we discuss melanogenesis in a context of constitutive pigmentation, defined by gene polymorphism in ethnic skin types, and facultative pigmentation that is not only associated with the capacity to protect the skin against photo-damage but could also have an impact on vitamin D synthesis through gene polymorphism. Modulating the activities of melanogenic genes, with the focus on the markers specifically altered by polymorphism combined with differential requirements of sun exposure in ethnic skin types, could enhance the applications of already existing skin brightening factors and provide a novel approach toward improved skin tone and health in personalized skincare.
Keywords: ethnic skin types, melanogenesis, hyper-pigmentation, vitamin D
Introduction
Fine skin complexion is defined as clear and unblemished; ideal skin tone is associated with even distribution of the skin pigment – melanin; hyper-pigmentation is characterized by spots or patches that are darker than the skin surrounding them. These specific changes in pigmentation are predominantly linked to the excessive exposure to ultraviolet radiation (UVR) from sunlight, which is, in a broader sense, a well described factor also associated with photo-damage and premature ageing of the skin.1
Skin problems linked to photo-damage are generally considered to affect lighter skin; this is because melanin provides protection against UVR and lighter skin types are more susceptible to its damaging effects. However, the skin types predominantly affected by hyper-pigmentation problems are the darker phenotypes, including Oriental, Indian and occasionally African origin, which are also characterized by higher melanin content.2–5 It is therefore plausible to predict a fine balance in the melanin content required for sufficient protection against photo-damage, which is usually associated with darker skin tone, whilst keeping undesirable hyper-pigmentation responses low. Such balance could be affected if the melanogenesis process was insufficient and its protective effect against UVR exceeded, resulting in the enhanced sensitivity of the skin to sun-induced photo-damage.
Presently, sun protection products are broadly recommended as essential application to counteract the harmful effects of excessive UVR exposure. Such approach has resulted in significant reduction of skin problems caused by photo-damage; leading to further preference toward the sunscreens with high SPF (sun protection factor) which therefore have stronger ability to absorb or reflect the UVR reaching the skin.
However, the parallel evidence that is now emerging also demonstrates that sun exposure has direct and significant benefits in promoting health of the skin, predominantly through stimulation of vitamin D synthesis. Exposure to UVR causes photo-activation of 7-dehydrocholesterol in the skin and synthesis of vitamin D3, which is subsequently circulated and metabolized further to the active form of vitamin D. This mechanism is responsible for more than 90% of the vitamin D production in the body.6,7 Vitamin D is a pro-hormone essential for calcium metabolism, immune function, and skin health.8 In skin, deficient levels of vitamin D are associated with decrease in the optimal differentiation of keratinocytes.9–11
Lighter skin types have the capacity for maximum photo-activation and conversion of 7-dehydrocholesterol into vitamin D that can be stimulated at low intensities of UVR. However, increasing evidence suggests that excessive usage of sunscreens also prevents the synthesis of vitamin D, which can be reduced by 95%.12,13 The prevalence of vitamin D deficiency is also increased in people with darker skin types, such as Indian and African, which need longer time in the sun to produce a similar quantity of vitamin D compared to the Caucasian type. Vitamin D levels are additionally linked to the distribution of the populations across northern and southern latitudes.14
Based on this, the question emerges regarding the available approaches to skincare that would enable sufficient protection against photo-damage and amelioration of hyper-pigmentation whilst simultaneously preserving the beneficial effects of sun exposure. In this review, we discuss this topic in a context of personalized skincare and the possible different approaches required for each skin type. Melanogenesis is a complex process based on a cascade of biochemical reactions regulated by a range of genes.15 Many of these genes are subject to genetic modifications that alter their biological activities and determine the specific melanogenic traits in corresponding skin types.16 The biomarkers fall into several different categories, which are associated with different stages of melanogenesis, including the facultative, UVR-induced tanning responses in addition to the genetically determined traits contributing to specific skin type. Several of these genes are also linked to vitamin D metabolism, particularly in the light Caucasian skin where they could play important roles in the melanogenic responses of the skin to UVR.17 Differences in the constitutive pigmentation traits are also associated with specific hyper-pigmentation problems that are differently manifested in different skin types and could be linked to altered sensitivities to UVR and photo-damage. Finally, we summarize the main applications for skin lightening and improvement of skin tone and propose additional avenues for future considerations regarding the skin-type based approaches toward skin sensitivity to photo-damage and harnessing the benefits of sun exposure.
Melanogenesis: Complex Reactions Behind Skin Color and Tone
Skin color and tone are determined by the presence of melanin, which is a pigment synthesized in the epidermis by neural crest-derived cells, melanocytes, forming an epidermal melanin unit with approximately 40 keratinocytes at the dermal-epidermal junction.18,19 After maturation the melanin is transferred in specialized membrane organelles, melanosomes, into the surrounding keratinocytes and distributed in the supra-basal layers of the epidermis, where they determine the color of the skin and protect against the effects of UVR.20–22 Keratinocytes in the basal layer contain the majority (60–80%) of the total melanosomes, which are localized predominantly over cell nuclei providing photo-protection against UVR-induced DNA damage.23
Melanin is a macromolecular biopolymer derived from tyrosine through series of biochemical reactions.15,24 Melanogenesis is a complex process controlled at different physiological stages, involving a range of enzymes, structural proteins, and intermediate molecules that regulate development of melanocytes, biogenesis and survival of melanosomes as well as synthesis and maturation of melanin and transfer of melanosomes to keratinocytes. Constitutive pigmentation of the skin is also influenced by paracrine regulation of melanogenesis that originates as a result of cross-talk between melanocytes and keratinocytes as well as dermal fibroblasts25–67 (Table 1).
Table 1.
Genes and Biomarkers of Melanogenesis Relevant to Personalized Skincare
| Key Steps of Melanogenesis | |||
|---|---|---|---|
| UV-induced | |||
| 1 | UV-induced DNA synthesis of POMC (proopiomelanocortin). Processing of POMC to α-MSH (α-melanocyte-stimulating-hormone) and ACTH (adrenocorticotropic hormone)25,26 | ||
| 2 | Binding of α-MSH or ACTH to MC1R (melanocortin 1 receptor) and its activation25–27 | ||
| 3 | Activation of ADCY (adenylate cyclase) and increased formation of cAMP25,26,28 | ||
| 4 | Activation of PKA (protein kinase A) and phosphorylation of CREB (cAMP responsive-element binding) family of transcription factors25,26,28 | ||
| 5 | CREB-mediated expression of MITF (microphthalmia transcription factor), master regulator of melanocyte development and survival25,26,28 | ||
| 6 | MITF-induced expression of TYR (tyrosinase), TYRP1 (tyrosinase-related protein 1) and TYRP2/DCT (tyrosinase related protein-2/dopachrome tautomerase) through interactions with M- and E-boxes present in the promoter regions. TYR and TYRP1 are delivered to stage II melanosomes25,26,29 | ||
| Genetic | |||
| 7 | Enzymatic oxidation of tyrosine by TYR to DOPA (l-3,4-dihydroxyphenylalanine) and DOPAquinone30,31 | ||
| Eumelanin pathway | Pheomelanin pathway | ||
| 8a | Spontaneous conversion of DOPAquinone, via DOPAchrome, to DHI (5,6-dihydroxyindole) and DHICA (5,6-dihydroxyindole-2-carboxylic acid) accelerated by TYRP2/DCT32–34 | 8b | Reaction of DOPAquinone with cysteine to produce 5SCD (5-S-cysteinyldopa) and 2SCD (2-S-cysteinyldopa)32,35 |
| 9a | Oxidization of DHI and DHICA by TYR and TYRP1 to eumelanin polymer32–34 | 9b | Oxidization to intermediates which polymerize to pheomelanin32,35 |
| Additional key players in melanogenesis | |||
| Melanin synthesis | |||
| 10 | ASIP (agouti signaling protein); an antagonist of MC1R. Binding to MC1R leads to decreased TYR activity resulting in pheomelanin production27,36 | ||
| 11 | IRF4 (interferon regulatory factor 4); involved in transcription of TYR, TYRP1 and TYRP2. MITF directly or indirectly regulates IRF4 expression37 | ||
| 12 | ATRN (attractin) Group XI C-type lectin, trans-membrane protein, functions as accessory receptor for Agouti protein38 | ||
| 13 | Wnt/β-catenin; activation of nuclear β-catenin by Wnt leads to increased expression of MITF and melanogenesis39 | ||
| 14 | GSS (glutathione synthetase); involved in GSH (glutathione) biosynthesis. Role in the switch between eumelanogenesis and pheomelanogenesis through interactions with TYR and DOPAquinone40 | ||
| 15 | GGT7 (gamma-glutamyltransferase 7); membrane-associated protein involved in metabolism of glutathione and the trans-peptidation of amino acids40 | ||
| 16 | RALY (RALY heterogeneous nuclear ribonucleoprotein); RNA binding protein40 | ||
| 17 | EIF2S2 (eukaryotic translation initiation factor 2); functions in the early steps of protein synthesis40,41 | ||
| 18 | EIF6 (eukaryotic translation initiation factor 6); role in protein synthesis42 | ||
| 19 | DRD2 (dopamine receptor D2); signaling shows increase with increasing UV exposure43 | ||
| Melanocyte biogenesis and survival | |||
| 20 | KITLG (hyper-pigmentation c-KIT receptor). Development and migration of melanocyte lineages, activates MAPK (mitogen activated protein kinase) leading to up-regulated expression of MITF, which activates keratinocytes to produce factors promoting melanosome phagocytosis44–46 | ||
| 21 | EDA (ectodysplasin A). Trans-membrane protein of the TNF (tumor necrosis factor) family, cytokine involved in the epithelial-mesenchymal signaling47 | ||
| 22 | ITCH (itchy homolog); E3 ubiquitin-protein ligase, induces proteasomal degradation40 | ||
| 23 | HERC2 (HECT and RLD domain containing E3 ubiquitin protein ligase 2); regulates ubiquitin-dependent retention of repair proteins on damaged chromosomes48 | ||
| 24 | BNC2 (basonuclin 2); zinc finger protein, cell survival after oxidative stress49 | ||
| 25 | SMARCA2 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 2). Member of the large ATP-dependent chromatin remodeling complex SNF/SWI required for transcriptional activation of repressed genes50 | ||
| 26 | DDB1 (DNA damage- binding protein 1); involved in DNA nucleotide excision repair, functions as a core component of the E3 ubiquitin ligase complexes51 | ||
| 27 | EGFR (epidermal growth factor receptor); induces cell proliferation and differentiation52 | ||
| 28 | FGF7 (fibroblasts growth factor); enhances melanin synthesis and melanocyte proliferation, stimulates melanosome transfer after UVB irradiation53 | ||
| 29 | SCF (stem cell factor); fibroblast factor, enhances melanin synthesis and melanocyte proliferation54 | ||
| 30 | UQCC (ubiquinol-cytochrome c reductase complex); trans-membrane protein involved in FGF regulated growth control40 | ||
| 31 | VLDLR (very-low-density-lipoprotein receptor); trans-membrane receptor involved in endocytosis55 | ||
| 32 | PROCR (protein C receptor); cell survival and proliferation56 | ||
| 33 | ADAM17 (ADAM metallopeptidase domain 17); involved in cell adhesion and migration57 | ||
| 34 | ADAMTS 20 (ADAM metallopeptidase with thrombospondin type 1 motif 20); involved in cell adhesion and migration58 | ||
| Melanosome biogenesis, maturation and trafficking | |||
| 35 | Pme17 (Premelanosome protein); early melanosome development and maturation, fibrils optimize condensation of melanin59 | ||
| 36 | OCA2 (oculocutaneous albinism type 2); chloride anion channel protein, effector of melanosomal pH, glutathione metabolism, processing and trafficking of tyrosinase to melanosomes60–62 | ||
| 37 | SLC45A2/MATP (solute carrier family 45 member 2). Membrane transporter; ion transport; increases pH and TYR activity62,63 | ||
| 38 | SLC24A4 (solute carrier family 24 member 4). Membrane transporter; impact on TYR activity62 | ||
| 39 | SLC24A5/NCKX5 (solute carrier family 24 member 5). Membrane transporter; a putative NA+/Ca2+ ion exchanger pump, impact on TYR activity62 | ||
| 40 | MFSD12 (major facilitator superfamily domain containing 12); trans-membrane solute transporter in endosomes and lysosomes in melanocytes. Depletion of MFSD12 increases eumelanin content51 | ||
| 41 | TMEM38 (trans-membrane protein 38); lysosomal protein, monovalent cation channel, functions in maintenance of intracellular calcium47 | ||
| 42 | SNX13 (sorting nexin 13); involved in the intracellular trafficking and lysosomal degradation47,55 | ||
| 43 | EDEM2 (ER degradation enhancing alpha-mannosidase like protein 2), protein folding and trafficking40 | ||
| 44 | DTNBP1 (dystrobrevin binding protein 1); melanosome biogenesis64 | ||
| 45 | MAP1LC3A (microtubule associated protein 1 light chain 3 alpha); mediates the physical interactions between microtubules and elements of the cytoskeleton40 | ||
| 46 | MYO5A (myosin VA); transport of melanosomes in melanocytes, target of MITF65 | ||
| 47 | LYST (lysosomal trafficking regulator,CHS1); vesicular transport protein, regulated by MITF66 | ||
| 48 | EXOC2 (exocyst complex component 2); exocytosis, melanosome trafficking, actin remodeling67 | ||
Ethnic Skin Phenotypes are Defined by the Genes Controlling Melanogenesis
The color and tone of the skin are determined by the quantities and qualities of the synthesized melanin, which is one of the most variable phenotypes in humans. The geographic patterns of skin pigmentation demonstrate a strong correlation with latitude and UVR intensity; skin tends to be darker in tropical and equatorial regions with higher levels of UVR compared to the regions more distant from the equator.68,69
Constitutive pigmentation depends on the amount of melanin and relative ratio of eumelanin (brown/black pigment) and pheomelanin (yellow-red pigment) as well as the size, quantity, and distribution of melanosomes within the epidermis.16,70 Skin color is however not affected by the differences in melanocyte densities, which remain constant in every skin type.23,71
Skin types show variations in melanosome size and distribution, for example in African skin melanosomes are larger and more dispersed in basal keratinocytes whilst in European skin melanosomes are smaller and clustered together.72–74 In addition, melanosomes derived from dark skin have a neutral pH and higher activity of melanogenic enzymes whilst melanosomes derived from light skin are more acidic and have lower melanogenic activity.75,76
Constitutive skin pigmentation is above all a polygenic trait, with the quantities and type of melanin controlled by the genes with allelic variants through single nucleotide polymorphism (SNP), which is associated with changes in gene activity usually leading to lighter skin phenotype.16 In addition, a number of other genes involved in melanogenesis also demonstrate changes in the levels of expression linked to skin lightening and sun sensitivity, whilst constitutive levels of gene expression are typically higher in dark skin.
Comparison of the major melanogenic genes that are subject to allelic variation or changes in the transcriptional activity reveals the possible functional patterns in four major ethnic skin types. The proportion of the affected genes is predominant in Caucasian skin. This number is significantly decreased in Oriental skin, with evident further reduction in darker skin types of Indian and African origin (Table 2 and Figure 1).
Table 2.
Single Nucleotide Polymorphism (SNP) and Changes in Gene Expression Affecting Melanogenic Traits in Different Skin Types
| Gene | Skin Type | Gene Alterations and Phenotypes |
|---|---|---|
| Melanin synthesis | ||
| MC1R | Caucasian | Allelic diversity; sun sensitivity and freckles26,45,77,78 |
| Oriental | Allelic diversity; lighter skin reflectance and freckles81,82 | |
| MITF | Caucasian | Allelic diversity, polymorphism correlates with the levels of serum 25[OH]D, impact on vitamin D status and deficiency17,64,67 |
| IRF4 | Caucasian | Allelic diversity, reduced skin tanning response, freckling and sun sensitivity79,80 |
| GSS | Caucasian | Allelic diversity40 |
| GGT7 | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| RALY | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| EIF2S2 | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| EIF6 | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| ASIP | Caucasian | Polymorphism associated with sensitivity to sun and freckling45,66,84,86 |
| Oriental | Allelic diversity66 | |
| ATRN | Oriental | Allelic diversity58 |
| DRD2 | Oriental | Allelic diversity83 |
| TYR | Caucasian | Involved in normal variation of pigmentation. Polymorphism correlated with the levels of serum 25[OH]D, impact on vitamin D status and deficiency17,84,88,89 |
| Indian | Accounts for the differences between darkest and lightest skin reflectance90 | |
| TYRP1 | Caucasian | Polymorphism correlated with the levels of serum 25[OH]D, impact on vitamin D status and deficiency17,83,84 |
| Oriental | Allelic diversity68,158 | |
| Indian | Allelic diversity. Frequently associated with red-bronze skin159 | |
| African | Allelic diversity. Frequently associated with red-bronze skin159 | |
| TYRP2/DCT | Caucasian | Allelic diversity68,158 |
| Oriental | Allelic diversity68,83,158 | |
| Indian | Allelic diversity58,158 | |
| African | Allelic diversity158 | |
| Melanocyte biogenesis and survival | ||
| ITCH | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| UQCC | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| PROCR | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| KITLG | Caucasian | Allelic diversity45,83,84 |
| Oriental | Allelic diversity83,84 | |
| EDA | Caucasian | Allelic diversity47 |
| EDA | Oriental | Allelic diversity47 |
| BNC2 | Caucasian | Allelic diversity40,66,84,85 |
| Oriental | Allelic diversity66,84 | |
| EGFR | Caucasian | Allelic diversity83 |
| Oriental | Allelic diversity83 | |
| ADAM17 | Oriental | Allelic diversity58 |
| ADAMTS20 | Oriental | Allelic diversity58 |
| DDB1 | Caucasian | Allelic diversity51 |
| Oriental | Allelic diversity51 | |
| Indian | Allelic diversity51 | |
| African | Allelic diversity51 | |
| HERC2 | Caucasian | Allelic diversity40,51 |
| Indian | Allelic diversity51 | |
| African | Allelic diversity51 | |
| SMARCA2 | African | Allelic diversity55 |
| VLDLR | African | Allelic diversity55 |
| Melanosome biogenesis and trafficking | ||
| EDEM2 | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| DTNBP1 | Caucasian | Allelic diversity. Polymorphism correlated with the levels of serum 25[OH]D64 |
| MAP1LC3 | Caucasian | Lower expression levels in the lightly-pigmented melanocytes40 |
| EXOC2 | Caucasian | Polymorphism correlated with the levels of serum 25[OH]D, impact on vitamin D status and deficiency17,67 |
| MYO5A | Caucasian | Allelic diversity. Polymorphism correlated with the levels of serum 25[OH]D64 |
| LYST | Oriental | Allelic diversity66 |
| SLC24A4 | Caucasian | Allelic diversity45,80,84 |
| Indian | Allelic diversity84 | |
| SLC45A2/MATP | Caucasian | Polymorphism associated with olive skin and immature melanosomes63,78,160 |
| Oriental | Allelic diversity78 | |
| Indian | Allelic diversity78 | |
| African | Allelic diversity78 | |
| OCA2 | Caucasian | Allelic diversity45,51,84 |
| Oriental | Allelic diversity; major gene contributing to skin lightening58,84,161,162 | |
| Indian | Allelic diversity51,84 | |
| African | Allelic diversity51,84 | |
| SLC24A5 | Caucasian | Allelic diversity. Mutations disrupt melanosomal maturation and melanin biosynthesis78,84,87 |
| Oriental | Allelic diversity78,87 | |
| Indian | Allelic diversity at very high frequencies78,84,87 | |
| African | Allelic diversity at very high frequencies51,78,87 | |
| MFSD12 | African | Allelic diversity51 |
| SNX13 | African | Allelic diversity55 |
| TMEM38 | African | Allelic diversity51 |
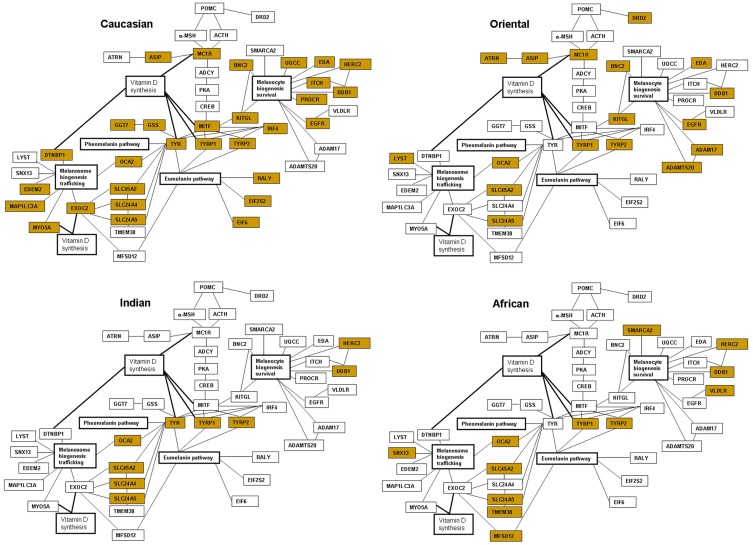
Figure 1.
Interactive networks of the major melanogenic genes and gene polymorphism or altered gene expression affecting pigmentation in four ethnic skin types. The biomarkers have been assembled based on the literature;25–90,158–162 the genes affected in each skin type are marked yellow.
The individual genes altered by polymorphism can be further identified as predominant within each skin type and organized in the functional categories based on the role within the melanogenic pathway. In Caucasian skin, such specific group of the polymorphic genes is comprised of the enzymes and transcription factors responsible for the early steps of the melanin synthesis pathway. The melanogenic genes that are specifically affected by polymorphism in Caucasian skin, MC1R (melanocortin 1 receptor),26,45,77,78 MITF (microphthalmia transcription factor),17,64,67 IRF4 (interferon regulatory factor 4)79,80 and GSS (glutathione synthetase)40 play a role in melanin synthesis and in a switch between eumelanin and pheomelanin production. The genes are also involved in the pathways of melanogenic responses to UVR; therefore decreased tanning and increased sun sensitivity would prevail as a result of decreased activity of these factors. Additional genes demonstrate lower expression levels in the lightly-pigmented melanocytes in Caucasian skin, including GGT7 (gamma-glutamyl transferase 7), RALY (heterogenous nuclear ribonucleoprotein), EIF2S2 (eukaryotic translation initiation factor 2) and EIF6 (eukaryotic translation initiation factor 6).40 Caucasian skin also bears a polymorphism in the genes involved in melanosome biogenesis and trafficking such as DTNBL1 (dystrobrevin binding protein 1),64 EXOC2 (exocyst complex component 2),17,67 and MYO5A (myosin VA).64 A number of genes with likely roles in melanocyte biogenesis and survival and melanosome trafficking also demonstrate lower expression in the lightly-pigmented melanocytes, including ITCH (itchy homolog), UQCC (ubiquinol-cytochrome c reductase complex), PROCR (protein C receptor), EDEM2 (ER degradation enhancing alpha-mannosidase like protein 2) and MAP1LC3 (microtubule associated protein 1 light chain 3 alpha).40 In addition to direct association with lighter skin, the polymorphism affecting the genes such as MITF, TYR (tyrosinase), TYRP1 (tyrosinase-related protein 1), EXOC2, MYO5A, and DTNBP1 is also correlated with the levels of serum 25[OH]D, indicating a direct impact on vitamin D status and deficiency in Caucasian individuals.17,64,67
Polymorphic genes specific to Oriental skin fall within the markers of melanin synthesis and responses to UVR exposure such as ATRN (attractin),58 MC1R,81,82 and DRD2 (dopamine receptor 2)83 as well as melanocyte biogenesis and survival, including ADAM17 (ADAM metallopeptidase domain 17), ADAMTS20 (ADAM metallopeptidase with thrombospondin type 1 motif 20),58 and melanosome trafficking, LYST (lysosomal trafficking regulator,CHS1).66 The majority of the polymorphic genes in Oriental skin fall within the group of biomarkers involved in melanocyte biogenesis and survival, with additional genes including KITGL (hyper-pigmentation c-KIT receptor),45,83,84 EDA (ectodysplasin A),47 BNC2 (basonuclin 2),40,66,84,85 and EGFR (epidermal growth factor receptor)83 also harboring SNP modifications in Caucasian skin. Both Oriental and Caucasian skin demonstrate polymorphism in ASIP (agouti signaling protein),45,66,84,86 indicative of potentially enhanced sun sensitivity in these genetic backgrounds.
Majority of the gene polymorphism in Indian skin types falls within a group of melanosome biogenesis and maturation, ion channels and transport membrane proteins SLC45A2/MATP (solute carrier family 45 member 2),78 SLC24A4 (solute carrier family 24 member 4),84 OCA2 (oculocutaneous albinism type 2),51,84 SLC24A5 (solute carrier family 24 member 5)78,84,87 but the SNPs in SLC45A2/MATP, OCA2 and SLC24A5 are also present across all other skin types (Table 2). Both Indian and Caucasian skin share polymorphism in TYR83,84,88-90 and SLC24A4,45,80,84 suggesting that the likely main skin-lightening traits are determined by decrease in the synthesis of melanin and/or the presence of immature melanosomes with altered activity of tyrosinase.
Polymorphic genes in African skin are the biomarkers of melanocyte biogenesis and survival SMARCA2 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 2)55 and VLDLR (very-low-density-lipoprotein receptor)55 as well as melanosome biogenesis and trafficking SNX13 (sorting nexin 13),55 TMEM38 (trans-membrane protein 38)51 and MFSD12 (major facilitator superfamily domain containing 12).51 Genes altered by polymorphism in African skin also represent biomarkers of melanosome biogenesis and maturation including SLC45A2/MATP,78 OCA251,84 and SLC24A551,78,87 but the SNPs are also present across all other skin types (Table 2).
Finally, the strongly positive melanogenic traits are present in both Indian and African skin for ASIP and KITGL, resulting in alleles that would favor higher tyrosinase activity and higher melanin index as a result of significantly enhanced melanogenic responses to UVR characterizing the darker skin. The melanogenic markers can be assembled in the interactive networks representing major genes and polymorphic clusters present in each skin type, together with the emerging links to vitamin D synthesis (Figure 1).
Effect of UVR on Melanogenic Responses and Their Variation in Skin Types
In addition to constitutive pigmentation, which is defined as genetically determined basal melanin production, facultative pigmentation can be described as enhanced production of melanin due to exposure to UVR, the most important environmental factor regulating the melanogenesis process. UVR is also the major environmental stress leading to the development of hyper-pigmentation disorders.
Based on the wavelength, UVR reaching the skin is classified into UVA and UVB. UVA (320–400 nm) is less energetic but can penetrate deep into the skin, reaching the basal layer of the epidermis and dermis. UVA exposure is a major factor in the photo-aging process, leading to the production of reactive oxygen species (ROS), an increase in inflammatory mediators such as IL-1 or IL-6, expression of matrix metalloproteinases (MMPs) and lipid peroxidation.1 UVB (290–320 nm) is more energetic and capable of inducing direct DNA damage through the induction of cyclobutane pyrimidine dimers and 6–4 photoproducts in the epidermis.91 UVB exposure is associated with sunburn and expression of melanogenic enzymes resulting in increased pigmentation.92 Whole UV spectrum is responsible for the cellular responses that are involved in the stimulation of pigmentation and development of pigmentary lesions.
Melanin has protective effect against DNA damage induced by UVA/UVB and there is a clear relationship between these factors in the skin types of different ethnic origins and constitutive pigmentation.23,93,94 For example, predominant type of UVR-induced DNA damage, cyclobutane pyrimidine dimers (CPDs), demonstrate a uniform distribution throughout the epidermis, including melanocytes and basal layer, and the upper dermis in Caucasian skin. In contrast, CPDs are significantly reduced in the epidermis of Indian and African skin and can be mostly detected in the suprabasal layers, with a gradual reduction in the basal layer, indicative of better photo-protection against mutagenesis and faster repair of DNA damage in darkly pigmented skin.95–97 In addition, activation of DNA repair can also be regulated by MC1R, which is frequently affected by SNP in lightly pigmented skin of Caucasian origin.98,99
Exposure to UVR triggers several reactions that ultimately lead to darkening of the skin, including oxidation and polymerization of melanin, redistribution of melanosomes, increase in expression of α-MSH (α-melanocyte-stimulating-hormone) and MITF and transfer of melanin from the lower to upper epidermis.92,100,101 Melanocytes synthesize two types of melanin that are chemically and functionally different.70 Eumelanin is photo-protective through the ability to dissipate >99.9% of UVR and visible light, limiting the extent of UVR penetration within epidermis and scavenging ROS. In contrast, pheomelanin is highly photo-reactive, enhancing the UVR-induced production of ROS and further damage of the cells.102–104
The ratio of eumelanin to pheomelanin is dependent on the catalytic activity of rate-limiting enzyme tyrosinase (TYR) and the availability of cysteine. High TYR activity/low concentrations of cysteine lead to the synthesis of eumelanin, whilst low TYR activity/high concentrations of cysteine lead to the synthesis of pheomelanin. The differences in skin color are not determined by the quantity of melanocytes, which remains constant, but by the activity of melanocytes including the relative levels of eumelanin and pheomelanin.105–107
Decreased ratio of eumelanin to pheomelanin is associated with increased photo-sensitivity, predisposition to freckles, and decreased tanning responses. Direct genetic link to the MC1R gene expression points at its likely role as a major contributing factor affecting the eumelanin/pheomelanin ratio. Consistently, a number of gene polymorphisms and loss-of-function mutations in the MC1R gene result in a decrease in eumelanin production, frequently linked with red hair phenotypes and fair skin that is susceptible to damage.108
The melanogenic response of human skin to UVR occurs in three phases: immediate pigment darkening (IPD), persistent pigmentation (PPD), and delayed tanning (DT). IPD occurs immediately during or after UVR exposure and is transient, whilst PPD lasts longer upon more intense UVR.109,110 Both IPD and PPD rely on oxidation and polymerization of existing melanin or its precursors rather then de novo synthesis, together with redistribution of melanosomes within melanocytes and keratinocytes.111–113 Such responses are not protective against erythema or DNA damage.114–116 In contrast, DT response is detected several days later after UVR exposure and is associated with activation of melanin synthesis pathway.117
Lighter skin phenotypes might have reduced IPD tanning response to UVR, with the threshold of the irradiation dose required to produce IPD/PPD higher than the dose inducing sunburn.118 IPD is associated with reversible oxidation of DHI (5,6-dihydroxyindole) and DHICA (5,6-dihydroxyindole-2-carboxylic acid), whilst PPD develops by irreversible oxidative cleavage of indolequinone to PTCA (pyrrole-2,3,5-tricarboxylic acid) and cross-linking of dihydroxyindole to PTeCA (pyrrole-2,3,4,5-tetracarboxylic acid).119–121 PPD is also associated with photo-degradation of pheomelanin, however its contribution to PPD could be masked by higher content of eumelanin.122
DT involves de novo melanogenesis driven by increased activity of TYR in melanocytes as well as enhanced multiplication and transfer of melanosomes to keratinocytes. Melanin synthesis in DT is initiated by DNA damage in keratinocytes, which leads to up-regulation of POMC (proopiomelanocortin) and its processing into α-MSH. Subsequent binding of α-MSH to MC1R in melanocyte results in activation of melanogenesis, which has a photo-protective effect against further DNA damage. Factors synthesized and secreted by keratinocytes, such as ET-1 (endothelin 1) and IL-1 (interleukin 1) also play a role in a cross-talk between melanogenesis and inflammation.100,123 DT response and tanning abilities are directly related to skin types, showing proportional association with Fitzpatrick classification and constitutive skin color.124 Decreased ability to tan and solar elastosis in Caucasian skin type have been associated with altered activity of IRF4. However, visible changes in pigmentation in tanning typically do not result from significant increase in the melanin content, but rather its re-distribution in the epidermal layers in all skin types.125–127
The entire UVR spectrum is also involved in photo-aging; directly related to the penetration properties in UVA and UVB depending on skin color and melanin content. Enhanced and impaired melanogenic response, particularly linked to TYR activity and inflammation can also lead to the defects in skin pigmentation, which are the primary sign of photo-aging in Indian and Oriental skin.2,4
Defects of Skin Pigmentation and Their Associations with Skin Types
Defects in the pigmentation can be triggered or exacerbated by long-term sun exposure and the type, onset, and frequency of the hyper-pigmented lesions are dictated by the skin complexion and genetic background. For example, hyper-pigmented spots, frequently considered as a sign of photo-aging, develop earlier and are more pronounced in Oriental and Indian skin types compared to the Caucasian skin type.2–4
Hyper-pigmentation can be classified into three main types:
actinic lentigines (AL, lentigo senilis) are light to dark brown spots ranging in size from millimeters to centimeters located mainly on photo-exposed areas such as face, hands, forearms and upper back.128 Actinic lentigines are the clinical signs of photo-aging and considered an indicator of the amount of sun exposure over the course of a life-time.129,130 Changes are characterized by elastosis, hyper-pigmented basal layer due to an increased total content of melanin in the keratinocytes (hypermelaninosis), increased expression of TYR and mitochondria quantities in melanocytes, with unaltered size of melanosomes and melanocytes densities along dermal-epidermal junctions.128,131 Actinic lentigines demonstrate broadened and elongated rete ridges of the dermal-epidermal junction resulting in protrusions of the epidermis into the dermis, together with altered expression of KGF (keratinocyte growth factor), FGF7 (fibroblast growth factor 7), SCF (stem cell factor) and the components of the dermal extra-cellular matrix.132,133 This type of photo-damage affects mainly Caucasian and Indian skin, where it has been associated with variations in MC1R and SLC45A2 genes. Genetic variations in four other genes, namely IRF4, MC1R, ASIP and BNC2 have been associated with lentigines acquired during aging in Caucasian skin.49,134
Additional changes to pigmentation, freckles or ephelides are small, 1–2 mm in diameter, red to light brown spots that are induced by sunlight and most frequently found on the face, arms, neck and chest. Melanocytes in ephelides contain multiple large melanosomes and the genes involved in formation of hyper-pigmented spots include MC1R, IRF4, ASIP, TYR, BNC2 and OCA2. Ephelides are characteristic predominantly in individuals with fair skin and often partially disappear with age.131,135
Post-inflammatory hyper-pigmentation (PIH) occurs as a result of an inflammatory reaction induced by allergic contact such as drug sensitization and endogenous causes such as atopic dermatitis. This type of damage appears as brown patches on photo-exposed areas including face, shoulders and trunk.136 Inflammation in the epidermis results in the release of reactive oxygen species, cytokines, eicosanoids, prostaglandins and leukotrienes that stimulate the melanocytes leading to increased melanin production. The inflammation also causes melanocyte hyperplasia, damage to the basement membrane through collagen IV degradation, leakage of melanins from basal keratinocytes, accumulation of melanophages at the proximity of blood vessels and in the dermis and dermal hyper-pigmentation.137 This type of damage affects all skin types but is more prevalent in Oriental and Indian skin types. Moreover, in both skin types it is more common in darker constitutive pigmentation background due to increased reactivity of melanocytes.136,138,139 Post-inflammatory hyper-pigmentation has a common association with acne in African, Indian and Oriental skin types and can persist after the original acne lesions have been resolved. Similar problems are frequently linked to cosmetic therapies such as laser treatment or chemical peels performed on skin with higher pigmentation levels.140–142
Melasma (M) is hypermelanosis of hormonal origin, which may be stimulated by high levels of estrogen and progesterone and is characterized by large dark brown patches with irregular borders in sun-exposed areas, especially the face.143 On the histological level there is increased elastosis, disruption of basement membrane, flattening of rete ridges, increased micro-vasculature and infiltration of mast cells.144
Enhanced synthesis of melanin is associated with up-regulated expression of TRP1 (tyrosinase-related protein 1), TRP2 (tyrosinase-related protein 2), MITF, melanocyte hypertrophy and activation of a-MSH, corticotrophin and IL-1 in response to UVR.145,146 In addition to hormonal link and inflammation, the most important environmental factor triggering melasma is acute sun exposure. Higher prevalence of melasma onset is observed in darker skin types and the populations living in areas with greater exposure to UVR are more likely to develop this type of pigmentation defect.147 It is also correlated with specific features that depend on skin type, for example accumulation of melanophages and melanin in dermis is more prevalent in darker skin.146 Facial melasma is particularly common in Indian skin types and in the middle- to older age groups.148
Changes in skin pigmentation, particularly those related to UVR exposure, chronic inflammation and immunosuppression, together with constitutive pigmentation background are linked to several types of skin cancer. Compared to Caucasian populations, skin cancers such as basal cell carcinoma (BCC) and melanoma are in general significantly less frequent in darker skin types, as a result of increased photo-protective UVR-filtering effects of epidermal melanin. Major risk factors for melanoma are increased exposure to UVR, fair complexion and freckling. In contrast, squamous cell carcinoma (SCC), is most frequently diagnosed in African skin. Predisposing factors for SCC are burns, chronic inflammation, and scarring.149
Current Approaches and Ingredients for Skin Brightening and Improvement of Skin Tone
Current applications toward management of skin pigmentation defects and improvement of skin tone are important aspects in the field of cosmetics, beauty therapy and dermatology. They can include inhibition of the enzymes at different stages of melanogenesis such as TYR, MITF and MC1R, number and size of melanosomes, interference with melanosomal maturation and transfer of melanin, destruction of melanocytes, exfoliation, dermabrasion, ultrasound and laser therapies. Successful treatments usually combine two or more methods with synergistic effect. Active ingredients are selected from both synthetic and natural sources for the capacity to control pigmentation whilst remaining minimally toxic.150 Identification of new or improved combination of ingredients with a defined mechanism and therapeutic profiles would also be based on the detailed structure–activity relationship studies. Although many of these applications have inhibitory activity against melanogenesis, very few became a commercial product based on cytotoxicity, cutaneous absorption and clinical trials151–157 (Table 3).
Table 3.
Current Applications and Active Ingredients for Improvement of Skin Tone
| Synthetic Inhibitors of TYR |
| Molecules with broad chemical nature and mechanisms of action. The most popular include hydroquinone (HQ) and its derivatives monobenzyl ether of hydroquinone (MBEH), monomethyl ether of hydroquinone (MMEH), benzaldehyde analogs; chalcone analogs; phenolic amines and derivatives of 4-phenylimidazole-2-thione, mequinol, N-acetyl glucosamine, benzimidazole-2-thiol, phenylthiol, phenylthiourea (PTU), p-aminobenzoic acid (PABA), quinazoline, biphenyl derivatives, indole derivatives and thiosemicarbazone.32,151,152 |
| Botanical extracts |
| Usually contain a combination of active natural ingredients that work in synergy. Some skin-lightening compounds in such extracts include aloesin, anisic acid, arbutin, trans-cinnamaldehyde, p-coumaric acid, cumic acid, epicatechin gallate, ellagic acid, glabridin, hesperidin, kaempferol, 2-oxyresveratrol, resveratrol, azelaic acid, aurone, hydroxystilbenes, hydroxycinnamic acid derivatives, chalcones and trihydroxyflavones, caffeic acid and ginsenoside Rb1.151,152 |
| Derivatives of resorcinol (un-substituted 4-alkylresorcinols) |
| De-pigmenting derivatives of resorcinol (1,3-benzendiol) include 4-cycloalkylresorcinol, 4-cycloalkylmethylresorcinols, 4-haloresorcinol, 4-(1,3) dithian-2-ylresorcinols and 4-n-butyl resorcinol (rucinol).32 |
| Antioxidants |
| Reduce the synthesis of melanin by quenching ROS generated through exposure to UVR and oxidization of TYR and DOPA.153,154 Antioxidants with the capacities to interfere with melanogenesis are phytic acid, glutathione, ubiquinone, resveratrol, kojic acid and ferulic acid.151 |
| Vitamins |
| The capacities to increase turnover rate of melanin (vitamin A), inhibit the transfer of melanosomes from melanocytes to keratinocytes (vitamin B3), interfere with the glycosylation of TYR (vitamin B5), de-activation of UVR-induced ROS and inhibition of TYR (vitamin C) and protection against UVR-induced inflammation (vitamin E).151 |
| Inhibitors of melanosome transfer |
| Ingredients interfering with melanosome transfer include niacinamide and lectins.47 |
| Molecules cyto-toxic to melanocytes |
| Molecules with inhibitory effect on melanocyte activity or survival include fomiferin and its derivative fomiferin-3,4-dimethyl ether, fraxidin methyl ether, hernlarin, imperatorin, kuhlmannin, obliquin, osajin and its derivative osajin-4-methyl ether, pachyrrhizin, prenyletin, robustic acid, sphondin, warangalone and xanthyletin.32 |
| Peptides and oligopeptides |
| Reduce pigmentation through interaction with the protease-activated receptor 2 (PAR-2) in keratinocytes affecting melanin and melanosome uptake by keratinocytes. Custom designed oligopeptides between 6 and 12 amino acids, dipeptides or cyclic peptides have the capacity to translocate to melanosomes and inhibit TYR.32,152 |
| Inhibitors of MC1R/αMSH |
| The molecules that indirectly inhibit TYR expression through down-regulation of cAMP include glyceollin, methyl and ethyl linoenates, platycodin, bisabolangelone, chrysin, paeonol.152 |
| Alpha hydroxy acids (AHA) and their derivatives |
| Applications as superficial chemical peels, target stratum corneum and accelerate desquamation of the outer epidermal layers, increasing melanin turnover. Increase the enzymatic activities leading to epidermolysis and promoting the synthesis of elastin fibers. The most commonly used are glycolic, lactic, citric, malic, pyruvic and salicylic acids and their derivatives.151 |
| Inhibitors of wnt/β-catenin signaling pathway |
| Wnt/β-catenin signaling pathway enhances MITF gene expression and melanogenesis; ingredients inhibiting the pathway through enhanced degradation of β-catenin include cardamonin, fingolimod, pyridinyl imidazoles and andrographolide.155,156 |
| Small interfering RNA (siRNA) |
| Based on double-stranded, ~21 base pairs RNA with the sequence complementary to the target mRNA. Mediate gene silencing and inhibition of TYR by binding the mRNA and degrading it at the site of application.32 |
| Inhibitors of adaptor protein (AP)-complexes |
| AP-1 and its interacting partner kinesin family member 13A (KIF13A) are required for the transfer of melanogenic enzymes to melanosomes and their maturation. Inhibition of KIF13A or AP-1 decreases the expression of melanogenic enzymes and synthesis of melanin.32 |
| Anti-inflammatory factors |
| Dexamethasone, fluocinolone acetonide and tranexamic acid have applications in treatment of hyper-pigmentation defects.47 |
| Physical therapies in association with UV-blocking agents |
| Based on laser treatment, cryotherapy and dermoabrasion.157 |
| Active ingredients stimulating melanogenesis |
| The potential capacities to protect the skin from photodamage by increasing the melanin content. The ingredients include pyrazoles, indole alkaloids, cannabinoid derivatives and 2-bromopolymitate derivatives.32 |
Proposed Treatments and Solutions toward Personalized Skin Tone Applications
Based on the genetic biomarkers that determine skin types discovered so far, it can be proposed that hyper-pigmentation is linked to the variation in specific traits regulating melanogenic pathways, which are also reflected in skin sensitivity to UVR. Such traits could in turn form a basis for design of skin tone applications compatible with each skin type. Given the variety of the biomarkers, the melanogenic traits would include the quantity and quality of melanin, melanosome biogenesis and maturation, trafficking of melanosomes in melanocyte, transfer of melanosomes to keratinocytes and distribution of melanin in the suprabasal and basal layers. These factors would also be expressed differentially in evenly pigmented and hyper-pigmented skin.
The novel approaches toward hyper-pigmentation in a context of personalized skincare can be based on the following points:
eumelanin is a factor protecting against UVR, the most damaging factor in lighter skin with relatively low levels of melanin.23,93,94
Presence of SNP in the melanogenic gene usually results in down-regulation of the activity, therefore generally associated with skin lightening leading to skin tone variations.
Hyper-pigmentation is linked to increased skin sensitivity and UVR damage, therefore could manifest as deficiency in the protective mechanisms of melanin irrespective of skin type.
Exposure to the sun has beneficial effects on the synthesis of vitamin D; with ~90% synthesized through short 15–20 min daily exposure for lighter skin, which is below the threshold level that induces delayed tanning. Vitamin D3 is produced in the skin through irradiation of 7-dehydrocholesterol, which is then metabolized to blood serum 25-hydroxyvitamin D3 [25(OH)D] and 1α 25-dihydroxyvitamin [D3 (1,25(OH 2D))].6,7 This mechanism is less efficient in darker skin due to the effects of higher melanin content, which is proposed to have an inhibitory effect on vitamin D synthesis. However, lower activity of the pigmentation genes due to the polymorphism is linked to vitamin D deficiency (with serum level of 25[OH]D as a biomarker) in Caucasian type, indicative of the likely link between vitamin D synthesis and melanogenic capacities related to sun exposure.17,64,67
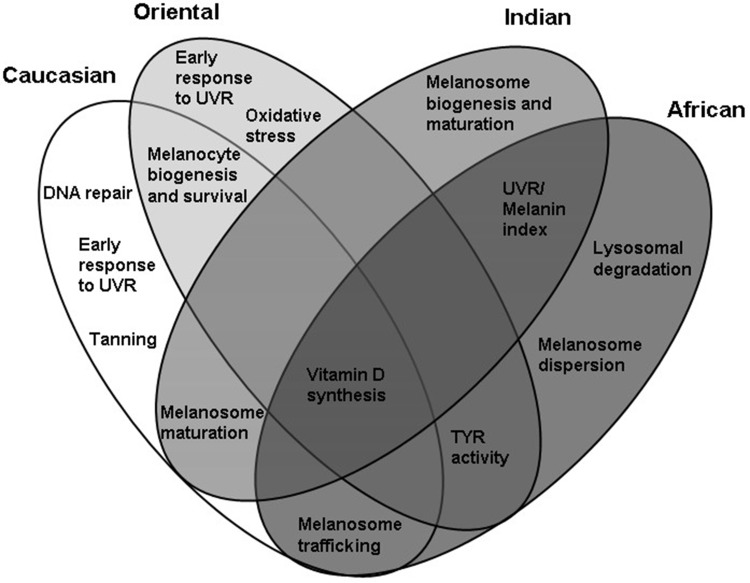
Based on this, the applications toward hyper-pigmentation can be developed based on the genetic makeup linked to the observed and predicted altered activities of the melanogenic factors specific for each skin type. Such applications, modulating the gene activity would target two mainstreams of melanogenesis, namely facultative and constitutive pigmentation with the specific genetic components considered as unique for each skin type or overlapping between them (Figure 2).
Figure 2.
Proposed functional cluster targets for enhancement of skin tone and prevention of pigmentation problems in four skin types whilst retaining the capacity for vitamin D synthesis and defense against molecular damage caused by UVR.
Caucasian Skin: Enhancement of DNA Repair, Delayed Tanning and Melanosome Trafficking in Facultative Pigmentation
This skin type demonstrates a significant accumulation of the traits associated with decreased melanogenic responses to UVR and facultative pigmentation. Enhancing UVR-induced melanogenesis as a protective mechanism against molecular damage could be an efficient way of achieving even skin tone in Caucasian skin. Applications would focus on modulating the activity of early melanogenic genes that are activated by UVR and linked to sun sensitivity and DNA repair such as MC1R and its antagonist ASIP. Such applications could affect the activity of MITF, TYR and TYRP1 genes associated with tanning and increased production of melanin through synthesis of melanogenic precursors for oxidation and polymerization. Additional biomarkers of interest could be IRF4 and GSS; involved in regulation of a switch between eumelanin and pheomelanin ratio and sun sensitivity through TYR expression. The potential skin brightening targets could also include the genes involved in melanosome biogenesis and trafficking such as DTNBL1, EXOC2 and MYO5A. The activity of MITF, TYR, TYRP1, DTNBL1, MYO5A and EXOC2 could also correlate with serum 25[OH]D and vitamin D status, therefore enhancement of tanning responses in formulations applied during sun exposure could be beneficial for even skin tone whilst retaining the ability to synthesize vitamin D.
Oriental Skin: Early Melanogenic Traits Associated with Sun Sensitivity and Oxidative Stress, Melanocyte Biogenesis and Survival
Oriental skin demonstrates polymorphism in the traits linked to early melanogenic responses to UVR exposure, sun sensitivity and oxidative stress whilst retaining high activity of TYR. Modulating the activity of genes such as ASIP and ATRN (antagonists of MC1R) in combination with activity of the genes involved in oxidative stress such as BNC2 or DRD2 could be beneficial for sun protection through control of early and facultative melanogenesis. Such applications could be combined with the inhibitors of constitutive TYR to control hyper-pigmentation whilst promoting synthesis of vitamin D in Oriental skin. Other potential targets of skin-brightening applications could also involve the genes associated with melanocyte biogenesis and survival such as KITGL, EDA and EGFR, which are also relevant to Caucasian skin.
Indian Skin: Melanosome Biogenesis and Maturation in Constitutive Pigmentation
Indian skin type does not demonstrate a significant polymorphism in the genes associated with tanning, indicative of the robust responses to UVR. The potential skin tone targets in Indian skin could instead involve the genes responsible for melanosome maturation, ion channels and effectors of melanosomal pH such as SLC45A2/MATP, SLC24A4, OCA2 and SLC24A5. The skin brightening applications could be based on the active ingredients modulating pH, TYR activity as well as processing and trafficking of tyrosinase to melanosomes. The ingredients targeting these genes could be combined with the inhibitors of constitutive TYR expression, to control hyper-pigmentation whilst promoting synthesis of vitamin D. Some aspects of this control could be additionally applicable to Caucasian skin, which also demonstrates the polymorphism in SLC45A2/MATP, SLC24A4, OCA2, SLC24A5 and TYR genes.
African Skin: Melanosomal Trafficking and Dispersion, Lysosomal Targets in Melanocytes
Similar to Indian skin, African skin type would demonstrate significant tanning response to UVR based on the gene polymorphism. The potential skin tone targets could involve the genes involved in melanosome trafficking and dispersion such as MFSD12 as well as lysosomal degradation and maintenance of intracellular calcium such as SNX13 and TMEM38. The ingredients targeting these genes could be combined with the inhibitors of constitutive TYR, to control hyper-pigmentation whilst promoting synthesis of vitamin D (Figure 2).
Summary
This review summarized the main components of the melanogenesis pathways, with a focus on the genes controlling UVR-induced, facultative melanogenesis and genetically determined, constitutive melanogenesis. Skin color is determined by the quantity and quality of melanin, which is regulated differently in ethnic skin types due to small nucleotide polymorphism (SNP) in the specific genes controlling different steps of the melanogenesis process. Gene polymorphism usually results in decreased activity of the gene, generally leading to lighter skin variants among each population. High melanin levels are well recognized as a protective factor against UVR-induced cellular and molecular damage, therefore a significant level of such damage is normally detected in lightly-pigmented skin. Skin responses to UVR are also associated with tanning, which occurs in three phases: immediate pigment darkening (IPD), persistent pigmentation (PPD), and delayed tanning (DT). Decreased ability to tan has been linked to hyper-pigmentation problems such as solar elastosis in lighter skin types. Defects in pigmentation can be triggered or exacerbated by long-term sun exposure and are evident across all skin types. Sun exposure has however a beneficial effect, such as the synthesis of vitamin D, which is thought to be less efficient in darkly pigmented skin due to the inhibitory effect of melanin on vitamin D production. However, gene polymorphism in several major melanogenic enzymes has also been linked to vitamin D deficiency in Caucasian type, indicative of the likely association between vitamin D synthesis and melanogenic capacities related to sun exposure. Given this, novel applications for a treatment or prevention of hyper-pigmentation could be developed based on the differences in the activities of melanogenic genes specific for each skin type. Such treatments could rely on targeting two aspects of melanogenesis separately, with more focus on facultative pigmentation in lighter skin types that are generally more prone to photo-damage whilst retaining high capacity for vitamin D synthesis. Darker skin types would benefit more from targeting expanded network of the genes at the base of constitutive pigmentation, perhaps combined with the already existing inhibitors of main melanogenic enzymes for enhancement of skin capacity toward synthesis of vitamin D. In such context, the skin lightening applications could be extended into personalized skincare and beneficial sun exposure.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.D’Orazio JD, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi: 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimes PE. Management of hyper-pigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77–85. doi: 10.1016/j.sder.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 3.Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48:S143–S148. doi: 10.1067/mjd.2003.274 [DOI] [PubMed] [Google Scholar]
- 4.Eun HC. Cutaneous photodamage in Asians. J Dermatol. 2001;28:614–616. doi: 10.1111/j.1346-8138.2001.tb00045.x [DOI] [PubMed] [Google Scholar]
- 5.Ho SG, Chan HH. The Asian dermatologic patient: review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009;10:153–168. doi: 10.2165/00128071-200910030-00002 [DOI] [PubMed] [Google Scholar]
- 6.Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732–736. doi: 10.1111/bjd.2009.161.issue-4 [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl. 2):V28–V33. doi: 10.1359/jbmr.07s211 [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, Vitamin D. A D-lightful health perspective. Nutr Rev. 2008;66:S182–S194. doi: 10.1111/j.1753-4887.2008.00104.x [DOI] [PubMed] [Google Scholar]
- 9.Christakos S, Hewison M, Gardner DG, et al. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287:45–58. doi: 10.1111/nyas.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto K, Azuma Y, Kiyoki M, et al. Involvement of endogenously produced 1,25 dihydroxyvitamin D-3 in the growth and differentiation of human keratinocytes. Biochim Biophys Acta. 1991;1092(3):311–318. doi: 10.1016/S0167-4889(97)90006-9 [DOI] [PubMed] [Google Scholar]
- 12.Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841–846. [PubMed] [Google Scholar]
- 13.Lehmann U, Hirche F, Stangl GI, et al. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled Trial. J Clin Endocrinol Metab. 2013;98(11):4339–4345. doi: 10.1210/jc.2012-4287 [DOI] [PubMed] [Google Scholar]
- 14.Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man: variation in solar ultraviolet at different latitudes may have caused racial differentiation in man. Science. 1967;157(3788):501–506. doi: 10.1126/science.157.3788.501 [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282(38):27557–27561. doi: 10.1074/jbc.R700026200 [DOI] [PubMed] [Google Scholar]
- 16.Barsh GS. What controls variation in human skin color? PLoS Biol. 2003;1:e27. doi: 10.1371/journal.pbio.0000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156(1):39–47. doi: 10.1210/en.2014-1238 [DOI] [PubMed] [Google Scholar]
- 18.Francisco S, Stefania B, Mauro P, et al. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick TB, Breathnach AS. [The epidermal melanin unit system]. Dermatol Wochenschr. 1963;147:481–489. [PubMed] [Google Scholar]
- 20.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev [DOI] [PubMed] [Google Scholar]
- 21.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. [DOI] [PubMed] [Google Scholar]
- 22.Park HY, Kosmadaki M, Yaar M, et al. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009;66:1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todokora T, Kobayashi N, Zmudzka BZ, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje [DOI] [PubMed] [Google Scholar]
- 24.d’Ischia M, Wakamatsu K, Cicoira F, et al. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015;28:520–544. doi: 10.1111/pcmr.12393 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Borron JC, Abdel-Malek Z, Jimenez-Cervantes C. MC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014;27:699–720. doi: 10.1111/pcmr.2014.27.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaumont KA, Wong SS, Ainger SA, et al. Melanocortin MC 1 receptor in human genetics and model systems. Eur J Pharmacol. 2011;660:103–110. doi: 10.1016/j.ejphar.2010.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x [DOI] [PubMed] [Google Scholar]
- 28.Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22:3035–3041. doi: 10.1038/sj.onc.1206443 [DOI] [PubMed] [Google Scholar]
- 29.Goding CR. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14:1712–1728. [PubMed] [Google Scholar]
- 30.Hearing VJ, Jimenez M. Mammalian tyrosinase. The critical regulatory control point in melanocyte pigmentation. Int J Biochem. 1987;19:1141–1147. doi: 10.1016/0020-711X(87)90095-4 [DOI] [PubMed] [Google Scholar]
- 31.Halaban R, Patton RS, Cheng E, et al. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem. 2002;277:14821–14828. doi: 10.1074/jbc.M111497200 [DOI] [PubMed] [Google Scholar]
- 32.Pillaiyar T, Manickam M, Jung SH. Inhibitors of melanogenesis: a patent review (2009 – 2014). Expert Opin Ther Pat. 2015;25(7):775–788. doi: 10.1517/13543776.2015.1039985 [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Urabe K, Winder A, et al. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994;13:5818–5825. doi: 10.1002/embj.1994.13.issue-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito S, Suzuki N, Takebayashi S, Commo S, Wakamatsu K. Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res. 2013;26:817–825. doi: 10.1111/pcmr.2013.26.issue-6 [DOI] [PubMed] [Google Scholar]
- 35.Wakamatsu K, Ohtara K, Ito S. Chemical analysis of late stages of pheomelanogenesis: conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res. 2009;22:474–486. doi: 10.1111/j.1755-148X.2009.00580.x [DOI] [PubMed] [Google Scholar]
- 36.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. [DOI] [PubMed] [Google Scholar]
- 37.Praetorius C, Grill C, Stacey S, et al. A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell. 2013;155:1022–1033. doi: 10.1016/j.cell.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hida T, Wakamatsu K, Sviderskaya EV, et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: a cAMP-independent pathway. Pigment Cell Melanoma Res. 2009;22(5):623–634. doi: 10.1111/j.1755-148X.2009.00582.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widlund HR, Horstmann MA, Price ER, et al. Beta-catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Visser M, Duffy DL, et al. Genetics of skin color variation in Europeans: genome-wide association studies with functional follow-up. Hum Genet. 2015;134:823–835. doi: 10.1007/s00439-015-1559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez-Pacheco N, Flores C, Alonso S, et al. Identification of a novel locus associated with skin colour in African-admixed populations. Sci Rep. 2017;7:44548. doi: 10.1038/srep44548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouillard AD, Gundersen GW, Fernandez NF, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford). 2016;2016:pii:baw100. doi: 10.1093/database/baw100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tammaro A, Cavallotti C, Gaspari AA, et al. Dopaminergic receptors in the human skin. J Biol Regul Homeost Agents. 2012;26:789–795. [PubMed] [Google Scholar]
- 44.Picardo M, Cardinali G. The genetic determination of skin pigmentation: KITLG and the KITLG/c-Kit pathway as key players in the onset of human familial pigmentary diseases. J Invest Dermatol. 2011;131:1182–1185. doi: 10.1038/jid.2011.67 [DOI] [PubMed] [Google Scholar]
- 45.Sulem P, Gudbjartsson DF, Stacey SN, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13 [DOI] [PubMed] [Google Scholar]
- 46.Hemesath TJ, Price ER, Takemoto C, et al. MAP kinase links the transcription factor microphthalmia to c-Kit signaling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681 [DOI] [PubMed] [Google Scholar]
- 47.Del Bino S, Duval C, Bernerd F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int J Mol Sci. 2018;19(9):2668. doi: 10.3390/ijms19092668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and theOCA2 promoter. Genome Res. 2012;22(3):446–455. doi: 10.1101/gr.128652.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs LC, Hamer MA, Gunn DA, et al. A genome-wide association study Identifies the skin color genes IRF4, MC1R, ASIP, and BNC2 influencing facial pigmented spots. J Investig Dermatol. 2015;135:1735–1742. doi: 10.1038/jid.2015.62 [DOI] [PubMed] [Google Scholar]
- 50.Mehrotra A, Mehta G, Aras S, Trivedi A, de la Serna IL. SWI/SNF chromatin remodeling enzymes in melanocyte differentiation and melanoma. Crit Rev Eukaryot Gene Expr. 2014;24(2):151–161. doi: 10.1615/CritRevEukaryotGeneExpr.v24.i2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crawford NG, Kelly DE, Hansen MEB, et al. Loci associated with skin pigmentation identified in African populations. Science. 2017;358(6365):eaan8433. doi: 10.1126/science.aan8433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon-Thomson C, Mason RS, Moore GP. Regulation of epidermal growth factor receptor expression in human melanocytes. Exp Dermatol. 2001;10(5):321–328. doi: 10.1034/j.1600-0625.2001.100504.x [DOI] [PubMed] [Google Scholar]
- 53.Cardinali G, Bolasco G, Aspite N, et al. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J Investig Dermatol. 2008;128:558–567. doi: 10.1038/sj.jid.5701063 [DOI] [PubMed] [Google Scholar]
- 54.Yoshida H, Kunisada T, Grimm T, et al. Review: melanocyte migration and survival controlled by SCF/c-kit expression. J Investig Dermatol Symp Proc. 2001;6(1):1–5. doi: 10.1046/j.0022-202x.2001.00006.x [DOI] [PubMed] [Google Scholar]
- 55.Martin AR, Lin M, Granka JM, et al. An unexpectedly complex architecture for skin pigmentation in Africans. Cell. 2017;171:1340–1353. doi: 10.1016/j.cell.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirley SH, Grimm EA, Kusewitt DF. Ultraviolet radiation and the slug transcription factor induce proinflammatory and immunomodulatory mediator expression in melanocytes. J Skin Cancer. 2012;410925:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawaguchi M, Hozumi Y, Suzuki T. ADAM protease inhibitors reduce melanogenesis by regulating PMEL17 processing in human melanocytes. J Dermatol Sci. 2015;78(2):133–142. doi: 10.1016/j.jdermsci.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 58.Edwards M, Bigham A, Tan J, et al. Association of the OCA2 polymorphism His615Arg with melanin content in East Asian populations: further evidence of convergent evolution of skin pigmentation. PLoS Genet. 2010;6:e1000867. doi: 10.1371/journal.pgen.1000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Morya VK, Nguyen DH, et al. Unrevealing the role of P-protein on melanosome biology and structure, using siRNA-mediated down regulation of OCA2. Mol Cell Biochem. 2015;403:61–71. doi: 10.1007/s11010-015-2337-y [DOI] [PubMed] [Google Scholar]
- 61.Bellono NW, Escobar IE, Lefkovith AJ, et al. An intracellular anion channel critical for pigmentation. Elife. 2014;3:e04543. doi: 10.7554/eLife.04543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellono NW, Escobar IE, Oancea E. A melanosomal two-pore sodium channel regulates pigmentation. Sci Rep. 2016;6:26570. doi: 10.1038/srep26570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sturm RA. A golden age of human pigmentation genetics. Trends Genet. 2006;22:464–468. doi: 10.1016/j.tig.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 64.Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429–1438. [PubMed] [Google Scholar]
- 65.Alves CP, Yokoyama S, Goedert L, et al. MYO5A gene is a target of MITF in melanocytes. J Invest Dermatol. 2017;137(4):985–989. doi: 10.1016/j.jid.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 66.McEvoy B, Beleza S, Shriver MD. The genetic architecture of normal variation in human pigmentation: an evolutionary perspective and model. Hum Mol Genet. 2006;15(2):R176–R181. doi: 10.1093/hmg/ddl217 [DOI] [PubMed] [Google Scholar]
- 67.Slominski A, Postlethwaite AE. Skin under the sun: when melanin pigment meets vitamin D. Endocrinology. 2015;156(1):1–4. doi: 10.1210/en.2014-1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sturm RA, Duffy DL. Human pigmentation genes under environmental selection. Genome Biol. 2012;13:248. doi: 10.1186/gb-2012-13-9-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16:523–531. doi: 10.1034/j.1600-0749.2003.00072.x [DOI] [PubMed] [Google Scholar]
- 71.Whiteman DC, Parsons PG, Green AC. Determinants of melanocyte density in adult human skin. Arch Dermatol Res. 1999;291:511–516. doi: 10.1007/s004030050446 [DOI] [PubMed] [Google Scholar]
- 72.Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Racial differences in the fate of melanosomes in human epidermis. Nature. 1969;222:1081–1082. doi: 10.1038/2221081a0 [DOI] [PubMed] [Google Scholar]
- 73.Konrad K, Wolff K. Hyper-pigmentation, melanosome size, and distribution patterns of melanosomes. Arch Dermatol. 1973;107:853–860. doi: 10.1001/archderm.1973.01620210021005 [DOI] [PubMed] [Google Scholar]
- 74.Alaluf S, Atkins D, Barrett K, et al. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002;15:112–118. doi: 10.1034/j.1600-0749.2002.1o071.x [DOI] [PubMed] [Google Scholar]
- 75.Smith DR, Spaulding DT, Glenn HM, Fuller BB. The relationship between Na(+)/H(+) exchanger expression and tyrosinase activity in human melanocytes. Exp Cell Res. 2004;298:521–534. doi: 10.1016/j.yexcr.2004.04.033 [DOI] [PubMed] [Google Scholar]
- 76.Fuller BB, Spaulding DT, Smith DR. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Exp Cell Res. 2001;262:197–208. doi: 10.1006/excr.2000.5092 [DOI] [PubMed] [Google Scholar]
- 77.Beaumont KA, Shekar SL, Newton RA, et al. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177 [DOI] [PubMed] [Google Scholar]
- 78.Norton HL, Kittles RA, Parra E, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203 [DOI] [PubMed] [Google Scholar]
- 79.Nan H, Kraft P, Qureshi AA, et al. Genome-wide association study of tanning phenotype in a population of European ancestry. J Invest Dermatol. 2009;129:2250–2257. doi: 10.1038/jid.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han J, Kraft P, Nan H, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:e1000074. doi: 10.1371/journal.pgen.1000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng S, Lu XM, Luo HR, Xiang-Yu JG, Zhang Y. Melanocortin-1 receptor gene variants in four Chinese ethnic populations. Cell Res. 2001;11:81–84. doi: 10.1038/sj.cr.7290070 [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi K, Watanabe C, Kawaguchi A, et al. Association of melanocortin 1 receptor gene (MC1R) polymorphisms with skin reflectance and freckles in Japanese. J Hum Genet. 2012;57:700–708. doi: 10.1038/jhg.2012.96 [DOI] [PubMed] [Google Scholar]
- 83.Lao O, de Gruijter JM, van Duijn K, et al. Signatures of positive selection in genes associated with human skin pigmentation as revealed from analyses of single nucleotide polymorphisms. Ann Hum Genet. 2007;71(3):354–369. doi: 10.1111/j.1469-1809.2006.00341.x [DOI] [PubMed] [Google Scholar]
- 84.Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:R9–R17. doi: 10.1093/hmg/ddp003 [DOI] [PubMed] [Google Scholar]
- 85.Jacobs LC, Wollstein A, Lao O, et al. Comprehensive candidate gene study highlights UGT1A and BNC2 as new genes determining continuous skin color variation in Europeans. Hum Genet. 2012;132:147–158. doi: 10.1007/s00439-012-1232-9 [DOI] [PubMed] [Google Scholar]
- 86.Duffy DL, Zhao ZZ, Sturm RA, et al. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol. 2010;130:520–528. doi: 10.1038/jid.2009.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basu Mallick C, Iliescu FM, Mols M, et al. The light skin allele of SLC24A5 in South Asians and Europeans shares identity by descent. PLoS Genet. 2013;9:e1003912. doi: 10.1371/journal.pgen.1003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rooryck C, Morice-Picard F, Elçioglu NH, et al. Molecular diagnosis of oculocutaneous albinism: new mutations in the OCA1-4 genes and practical aspects. Pigment Cell Melanoma Res. 2008;21:583–587. doi: 10.1111/j.1755-148X.2008.00496.x [DOI] [PubMed] [Google Scholar]
- 89.Jin Y, Ferrara T, Gowan K, et al. Next-generation DNA re-sequencing identifies common variants of TYR and HLA-A that modulate the risk of generalized vitiligo via antigen presentation. J Invest Dermatol. 2012;132:1730–1733. doi: 10.1038/jid.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stokowski RP, Pant PV, Dadd T, et al. A genomewide association study of skin pigmentation in a South Asian population. Am J Hum Genet. 2007;81:1119–1132. doi: 10.1086/522235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Premi S, Wallisch S, Mano CM, et al. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842–847. doi: 10.1126/science.1256022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi N, Nakagawa A, Muramatsu T, et al. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J Investig Dermatol. 1998;110:806–810. doi: 10.1046/j.1523-1747.1998.00178.x [DOI] [PubMed] [Google Scholar]
- 94.Rijken F, Bruijnzeel PL, Van Weelden H, Kiekens RC. Responses of black and white skin to solar-simulating radiation: differences in DNA photodamage, infiltrating neutrophils, proteolytic enzymes induced, keratinocyte activation, and IL-10 expression. J Investig Dermatol. 2004;122:1448–1455. doi: 10.1111/j.0022-202X.2004.22609.x [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi Y, Takahashi K, Zmudzka BZ, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje [DOI] [PubMed] [Google Scholar]
- 96.Coelho SG, Zmudzka BZ, Yin L, et al. Non-invasive diffuse reflectance measurements of cutaneous melanin content can predict human sensitivity to ultraviolet radiation. Exp Dermatol. 2013;22:266–271. doi: 10.1111/exd.2013.22.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Del Bino S, Sok J, Bessac E, Bernerd F. Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res. 2006;19:606–614. doi: 10.1111/j.1600-0749.2006.00338.x [DOI] [PubMed] [Google Scholar]
- 98.Hauser JE, Kadekaro AL, Kavanagh RJ, et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19:303–314. doi: 10.1111/pcr.2006.19.issue-4 [DOI] [PubMed] [Google Scholar]
- 99.Kadekaro AL, Wakamatsu K, Ito S, Abdel-Malek ZA. Cutaneous photoprotection and melanoma susceptibility: reaching beyond melanin content to the frontiers of DNA repair. Front Biosci. 2006;11:2157–2173. doi: 10.2741/1958 [DOI] [PubMed] [Google Scholar]
- 100.Eller MS, Gilchrest BA. Tanning as part of the eukaryotic SOS response. Pigment Cell Res. 2000;13(8):94–97. doi: 10.1034/j.1600-0749.13.s8.17.x [DOI] [PubMed] [Google Scholar]
- 101.Coelho SG, Zhou Y, Bushar HF, et al. Long-lasting pigmentation of human skin, a new look at an overlooked response to UV. Pigment Cell Melanoma Res. 2009;22:238–241. doi: 10.1111/j.1755-148X.2009.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hill HZ, Li W, Xin P, Mitchell DL. Melanin: a two edged sword? Pigment Cell Res. 1997;10:158–161. doi: 10.1111/pcr.1997.10.issue-3 [DOI] [PubMed] [Google Scholar]
- 103.Kadekaro AL, Kavanagh RJ, Wakamatsu K, et al. Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res. 2003;16:434–447. doi: 10.1034/j.1600-0749.2003.00088.x [DOI] [PubMed] [Google Scholar]
- 104.Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000;13(8):140–144. doi: 10.1034/j.1600-0749.13.s8.25.x [DOI] [PubMed] [Google Scholar]
- 105.Del Marmol V, Ito S, Bouchard B, et al. Cysteine deprivation promotes eumelanogenesis in human melanoma cells. J Invest Dermatol. 1996;107:698–702. doi: 10.1111/1523-1747.ep12365591 [DOI] [PubMed] [Google Scholar]
- 106.Morgan AM, Lo J, Fisher DE. How does pheomelanin synthesis contribute to melanomagenesis?: two distinct mechanisms could explain the carcinogenicity of pheomelanin synthesis. Bioessays. 2013;35:672–676. doi: 10.1002/bies.201300020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ito S, Wakamatsu K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem Photobiol. 2008;84:582–592. doi: 10.1111/j.1751-1097.2007.00238.x [DOI] [PubMed] [Google Scholar]
- 108.Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA. Red hair is the null phenotype of MC1R. Hum Mutat. 2008;29(8):E88–E94. doi: 10.1002/humu.v29:8 [DOI] [PubMed] [Google Scholar]
- 109.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. 2013;12:54–64. doi: 10.1039/C2PP25152C [DOI] [PubMed] [Google Scholar]
- 110.Rosen CF, Jacques SL, Stuart ME, Gange RW. Immediate pigment darkening: visual and reflectance spectrophotometric analysis of action spectrum. Photochem Photobiol. 1990;51:583–588. doi: 10.1111/php.1990.51.issue-5 [DOI] [PubMed] [Google Scholar]
- 111.Routaboul C, Denis A, Vinche A. Immediate pigment darkening: description, kinetic and biological function. Eur J Dermatol. 1999;9:95–99. [PubMed] [Google Scholar]
- 112.Maeda K, Hatao M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J Investig Dermatol. 2004;122:503–509. doi: 10.1046/j.0022-202X.2004.22223.x [DOI] [PubMed] [Google Scholar]
- 113.Lavker RM, Kaidbey KH. Redistribution of melanosomal complexes within keratinocytes following UV-A irradiation: a possible mechanism for cutaneous darkening in man. Arch Dermatol Res. 1982;272:215–228. doi: 10.1007/BF00509049 [DOI] [PubMed] [Google Scholar]
- 114.Black G, Matzinger E, Gange RW. Lack of photoprotection against UVB-induced erythema by immediate pigmentation induced by 382 nm radiation. J Investig Dermatol. 1985;85:448–449. doi: 10.1111/1523-1747.ep12277170 [DOI] [PubMed] [Google Scholar]
- 115.Beitner H. Immediate pigment-darkening reaction. Photodermatol. 1988;5:96–100. [PubMed] [Google Scholar]
- 116.Gange RW. Comparison of pigment responses in human skin to UVB and UVA radiation. Prog Clin Biol Res. 1988;256:475–485. [PubMed] [Google Scholar]
- 117.Ortonne JP. The effects of ultraviolet exposure on skin melanin pigmentation. J Int Med Res. 1990;18(3):8C–17C. [PubMed] [Google Scholar]
- 118.Agin PP, Desrochers DL, Sayre RM. The relationship of immediate pigment darkening to minimal erythemal dose, skin type, and eye color. Photodermatol. 1985;2:288–294. [PubMed] [Google Scholar]
- 119.Ito S, Wakamatsu K, Sarna T. Photodegradation of eumelanin and pheomelanin and its pathophysiological implications. Photochem Photobiol. 2018;94:409–420. doi: 10.1111/php.2018.94.issue-3 [DOI] [PubMed] [Google Scholar]
- 120.Ito S, Kikuta M, Koike S, et al. Roles of reactive oxygen species in UVA-induced oxidation of 5,6-dihydroxyindole-2-carboxylic acid-melanin as studied by differential spectrophotometric method. Pigment Cell Melanoma Res. 2016;29:340–351. doi: 10.1111/pcmr.2016.29.issue-3 [DOI] [PubMed] [Google Scholar]
- 121.Ito S, Pilat A, Gerwat W, et al. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013;26:357–366. doi: 10.1111/pcmr.12078 [DOI] [PubMed] [Google Scholar]
- 122.Del Bino S, Ito S, Sok J, et al. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell Melanoma Res. 2015;28:707–717. doi: 10.1111/pcmr.2015.28.issue-6 [DOI] [PubMed] [Google Scholar]
- 123.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.1988.01670060015008 [DOI] [PubMed] [Google Scholar]
- 125.Tadokoro T, Yamaguchi Y, Batzer J, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Investig Dermatol. 2005;124:1326–1332. doi: 10.1038/nature05660 [DOI] [PubMed] [Google Scholar]
- 126.Coelho SG, Miller SA, Zmudzka BZ, Beer JZ. Quantification of UV-induced erythema and pigmentation using computer-assisted digital image evaluation. Photochem Photobiol. 2006;82:651–655. doi: 10.1562/2005-08-02-TSN-635 [DOI] [PubMed] [Google Scholar]
- 127.Hennessy A, Oh C, Diffey B, Wakamatsu K, Ito S, Rees J. Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res. 2005;18:220–223. doi: 10.1111/(ISSN)1600-0749 [DOI] [PubMed] [Google Scholar]
- 128.Choi W, Yin L, Smuda C, et al. Molecular and histological characterization of age spots. Exp Dermatol. 2017;26:242–248. doi: 10.1111/exd.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bastiaens M, Hoefnagel J, Westendorp R, et al. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17:225–229. doi: 10.1111/j.1600-0749.2004.00131.x [DOI] [PubMed] [Google Scholar]
- 130.Monestier S, Gaudy C, Gouvernet J, Richard MA, Grob JJ. Multiple senile lentigos of the face, a skin ageing pattern resulting from a life excess of intermittent sun exposure in dark-skinned caucasians: a case-control study. Br J Dermatol. 2006;154:438–444. doi: 10.1111/bjd.2006.154.issue-3 [DOI] [PubMed] [Google Scholar]
- 131.Praetorius C, Sturm RA, Steingrimsson E. Sun-induced freckling: ephelides and solar lentigines. Pigment Cell Melanoma Res. 2014;27(3):339–350. doi: 10.1111/pcmr.2014.27.issue-3 [DOI] [PubMed] [Google Scholar]
- 132.Lin CB, Hu Y, Rossetti D, et al. Immuno-histochemical evaluation of solar lentigines: the association of KGF/KGFR and other factors with lesion development. J Dermatol Sci. 2010;59(2):91–97. doi: 10.1016/j.jdermsci.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 133.Chen N, Hu Y, Li WH, Eisinger M, Seiberg M, Lin CB. The role of keratinocyte growth factor in melanogenesis: a possible mechanism for the initiation of solar lentigines. Exp Dermatol. 2010;19:865–872. doi: 10.1111/j.1600-0625.2009.00957.x [DOI] [PubMed] [Google Scholar]
- 134.Tschachler E, Morizot F. Ethnic differences in skin aging In: Gilchrest BA, Krutmann J, editors. Skin Aging. Berlin/ Heidelberg, Germany: Springer; 2006:23. [Google Scholar]
- 135.Baron AE, Asdigian NL, Gonzalez V, et al. Interactions between ultraviolet light and MC1R and OCA2 variants are determinants of childhood nevus and freckle phenotypes. Cancer Epidemiol Biomarkers Prev. 2014;23:2829–2839. doi: 10.1158/1055-9965.EPI-14-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lacz NL, Vafaie J, Kihiczak NI, Schwartz RA. Postinflammatory hyper-pigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362–365. doi: 10.1111/ijd.2004.43.issue-5 [DOI] [PubMed] [Google Scholar]
- 137.Park JY, Park JH, Kim SJ, et al. Two histopathological patterns of postinflammatory hyper-pigmentation: epidermal and dermal. J Cutan Pathol. 2017;44:118–124. doi: 10.1111/cup.12849 [DOI] [PubMed] [Google Scholar]
- 138.Dunwell P, Rose A. Study of the skin disease spectrum occurring in an Afro-Caribbean population. Int J Dermatol. 2003;42:287–289. doi: 10.1046/j.1365-4362.2003.01358.x [DOI] [PubMed] [Google Scholar]
- 139.Davis EC, Callender VD. Postinflammatory hyper-pigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20–31. [PMC free article] [PubMed] [Google Scholar]
- 140.Negishi K, Akita H, Tanaka S, et al. Comparative study of treatment efficacy and the incidence of post-inflammatory hyper-pigmentation with different degrees of irradiation using two different quality-switched lasers for removing solar lentigines on Asian skin. J Eur Acad Dermatol Venereol. 2013;27:307–312. doi: 10.1111/j.1468-3083.2011.04385.x [DOI] [PubMed] [Google Scholar]
- 141.Chan HH, Manstein D, Yu CS, et al. The prevalence and risk factors of post-inflammatory hyper-pigmentation after fractional resurfacing in Asians. Lasers Surg Med. 2007;39:381–385. doi: 10.1002/lsm.20512 [DOI] [PubMed] [Google Scholar]
- 142.Kang HJ, Na JI, Lee JH, Roh MR, Ko JY, Chang SE. Postinflammatory hyper-pigmentation associated with treatment of solar lentigines using a Q-Switched 532-nm Nd: YAG laser: a multicenter survey. J Dermatolog Treat. 2017;28:447–451. doi: 10.1080/09546634.2016.1254330 [DOI] [PubMed] [Google Scholar]
- 143.Ortonne JP, Arellano I, Berneburg M, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009;23:1254–1262. doi: 10.1111/j.1468-3083.2009.03295.x [DOI] [PubMed] [Google Scholar]
- 144.Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci. 2016;17:824. doi: 10.3390/ijms17060824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kang HY, Suzuki I, Lee DJ, et al. Transcriptional profiling shows altered expression of wnt pathway-and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Investig Dermatol. 2011;131:1692–1700. doi: 10.1038/jid.2011.109 [DOI] [PubMed] [Google Scholar]
- 146.Kang HY, Bahadoran P, Suzuki I, et al. In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp Dermatol. 2010;19:e228–e233. doi: 10.1111/j.1600-0625.2009.01057.x [DOI] [PubMed] [Google Scholar]
- 147.Cestari TF, Dantas LP, Boza JC. Acquired hyper-pigmentations. An Bras Dermatol. 2014;89:11–25. doi: 10.1590/abd1806-4841.20142353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hourblin V, Nouveau S, Roy N, de Lacharriere O. Skin complexion and pigmentary disorders in facial skin of 1204 women in 4 Indian cities. Indian J Dermatol Venereol Leprol. 2014;80:395–401. doi: 10.4103/0378-6323.140290 [DOI] [PubMed] [Google Scholar]
- 149.Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21(4):170–178. [PMC free article] [PubMed] [Google Scholar]
- 150.Petit L, Pierard GE. Skin lightening products revisited. Int J Cosmet Sci. 2003;25:169–181. doi: 10.1046/j.1467-2494.2003.00182.x [DOI] [PubMed] [Google Scholar]
- 151.Kamakshi J. Fairness via formulations: a review of cosmetic skin-lightening ingredients. Cosmet Sci. 2012;63:43–54. [PubMed] [Google Scholar]
- 152.Pillaiyar T, Namasivayam V, Manickam M, Jung SH. Inhibitors of melanogenesis: an updated review. J Med Chem. 2018;61(17):7395–7418. doi: 10.1021/acs.jmedchem.7b00967 [DOI] [PubMed] [Google Scholar]
- 153.Roshchupkin D, Pistov MY, Patapenlo AY. Inhibition of UV induced erythema by antioxidants. Arch Dermatol Res. 1979;266:91–94. doi: 10.1007/BF00412867 [DOI] [PubMed] [Google Scholar]
- 154.Muizzuddin N, Shakoori AR, Marenus KD. Effect of antioxidants and free radical scavengers on protection of human skin against UVB, UVA and IR irradiation. Skin Res Technol. 1999;5:260–265. doi: 10.1111/srt.1999.5.issue-4 [DOI] [PubMed] [Google Scholar]
- 155.Bellei B, Pitisci A, Izzo E, Picardo M. Inhibition of melanogenesis by the pyridinyl imidazole class of compounds: possible involvement of the Wnt/beta-catenin signaling pathway. PLoS One. 2012;7:e33021. doi: 10.1371/journal.pone.0033021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3beta/beta-catenin signal pathway. J Dermatol Sci. 2015;79:74–83. doi: 10.1016/j.jdermsci.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 157.Kono T, Groff WF, Sakurai H, et al. Comparison study of intense pulsed light versus a long-pulse pulsed dye laser in the treatment of facial skin rejuvenation. Ann Plast Surg. 2007;59:479–483. doi: 10.1097/SAP.0b013e3180327943 [DOI] [PubMed] [Google Scholar]
- 158.Alonso S, Izagirre N, Smith-Zubiaga I, et al. Complex signatures of selection for the melanogenic loci TYR, TYRP1 and DCT in humans. BMC Evol Biol. 2008;8:74. doi: 10.1186/1471-2148-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rooryck C, Morice F, Didier L, et al. Genetic basis of oculocutaneous albinism. Expert Rev Dermatol. 2009;4:611–622. doi: 10.1586/edm.09.53 [DOI] [Google Scholar]
- 160.Cook AL, Chen W, Thurber AE, et al. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J Invest Dermatol. 2009;129:392–405. doi: 10.1038/jid.2008.211 [DOI] [PubMed] [Google Scholar]
- 161.Yang Z, Zhong H, Chen J, et al. A genetic mechanism for convergent skin lightening during recent human evolution. Mol Biol Evol. 2016;33:1177–1187. doi: 10.1093/molbev/msw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Abe Y, Tamiya G, Nakamura T, et al. Association of melanogenesis genes with skin color variation among Japanese females. J Dermatol Sci. 2013;69:167–172. doi: 10.1016/j.jdermsci.2012.10.016 [DOI] [PubMed] [Google Scholar]