Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, e-cigarette, respiratory failure, review, vape
Objectives:
Exposure to vaping is associated with a growing list of respiratory syndromes including an acute progressive form with life-threatening hypoxemic respiratory failure and pathologic changes of lung injury termed vaping-associated respiratory distress syndrome.
Data Sources:
Center from Disease Control, Departments of Public Health, MEDLINE (via PubMed), and the Cochrane Library.
Study Selection, Data Extraction and Data Synthesis:
Cases, series, and public health reports of cases that met the Centers for Disease Control and Prevention case definition of vaping-associated respiratory disease were extracted by an author with perfect verification by a second. Cases were classified on the basis of toxin exposure, symptoms, oxygen saturation, progression to respiratory failure, and pathologic features, and a clinically actionable system of classification was based on expert opinion.
Conclusions:
The reported spectrum of vaping-associated respiratory diseases allows clinical classification of cases into groups with distinct evaluation, management, and recommendations for prevention and follow-up. Clinical stratification also identifies a small proportion of vaping-exposed patients who are at risk for progression to hypoxemic respiratory failure and an acute respiratory distress syndrome–like illness.
Our understanding of the widening spectrum, large number of vaping-exposed individuals (1), increasing number of probable and confirmed cases (2), and mortality risks of vaping-related respiratory syndromes (3) has generated growing public health responses (4) including the enforcement of the Massachusetts ban on vaping shops and sales (5). Among the most concerning aspects of the growing number of reports of vaping-related respiratory disease (6–8) is that some victims experience rapid progression to hypoxemic respiratory failure and death (3, 8). A recent Centers for Disease Control and Prevention (CDC) advisory reported that among 2,016 cases, there were 42 deaths from 24 states (4).
The spectrum of the impact of vaping on patients ranges from anxiety about the health risks or costs of addiction to progressive symptoms of a life-threatening disorder. Our recommendations for management are based on the presence of vaping exposure and clinical findings that allow cases to be placed into three groups with distinct evaluation and management care plan goals and strategies. This framework helps critical care professionals quantify vaping exposure and efficiently identify patients at high risk for developing respiratory failure. We propose the term “vaping-associated respiratory distress syndrome” (VARDS) for symptomatic vaping-exposed hypoxemic patients who also have an abnormal chest imaging study. We also offer management suggestions for the sub-group of vaping-exposed patients who meet the case definition for the acute respiratory distress syndrome (ARDS) (3).
SCREENING AND RISK STRATIFICATION
Patients with a history of vaping in the last 90 days (CDC case definition) (9) should receive services based on their symptoms and preferences. Patients with recent exposure to vaping fumes and symptoms that can be caused by vaping and are not attributable to other causes have indications for a chest imaging study and screening oximetry. The presence or absence of infiltrates, vaping-associated symptoms, and normal or abnormal oxygen saturation allows triage according to the strata presented in Table 1. We offer this classification scheme as a clinically actionable expert opinion-based starting point for validation studies.
TABLE 1.
Worcester Vaping Clinical Classification System

CDC CASE DEFINITION
The CDC has proposed the following four required criterion for public health reporting of confirmed e-cigarette or vaping product use associated lung injury (EVALI) (4) cases (9): 1) Using an e-cigarette (vaping) or dabbing during the 90 days before symptom onset; 2) having a pulmonary infiltrate, such as opacities on plain film chest radiograph or ground-glass opacities on chest CT; 3) the absence of clinical evidence of a pulmonary infection on initial work-up: Minimum criteria include negative respiratory viral panel, influenza polymerase chain reaction, or rapid test if local epidemiology supports testing. All other clinically indicated respiratory infectious disease testing (e.g., urine antigen for Streptococcus pneumoniae and Legionella, sputum culture if productive cough, bronchoalveolar lavage culture when indicated, blood cultures, HIV–related opportunistic respiratory infections when appropriate) must be negative; and 4) no medical record evidence of alternative plausible diagnoses (e.g., cardiac, rheumatologic, or neoplastic process).
DEFINING VAPING EXPOSURE
A positive response to a query about personal exposure to e-cigarette or vaping fumes should be followed by ascertainment of the type of electronic nicotine delivery system (ENDS) and the method of exposure (device aerosolized or applied by dabbing or dripping). Vaping devices have evolved from first generation of cig-a-like products that are powered by rechargeable batteries and store vaping solutions in replaceable cartridges (cartomizers), to second generation mid-size electronic cigarettes, to third generation advanced personal mechanical modifiable vaporizers, to fourth generation regulated modifiable devices. Solution delivery can be from an internal single or multifill chamber or from an attached reservoir. Regulated and direct coil delivery generally causes higher levels of exposure because they generate denser aerosols than fixed delivery methods and generate droplets of larger size that can also damage segmental airways. The composition of the solution(s) that were vaporized should be determined including the medium chain triglyceride, glycol-, or glycerine-based streaming agents, lecithin, vitamin E, or terpene-based solvent, the active agent, and any adulterants that were applied to herbal ingredients. Available solutions include those that administer nicotine, nicotine salts, cannabidiol, tetrahydro-cannabinol (THC), synthetic cannabinoids, a mix of cannabinoids, and flavorants. A lexicon of terms to enable effective communication about exposure is available as supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCX/A141).
Individuals who are addicted or choose to vape should be encouraged to retain the packing materials and sales receipts they received at the time of product purchase and to bring these materials when they seek medical care. The date of first exposure, vaping frequency, and the type of device allow estimation of total exposure. The CDC recommends that the collection of vaping solutions should be coordinated with local public health departments by a qualified toxicology laboratory (4) and must be done in strict compliance with local and federal statutes that regulate the transportation of these substances.
RISK GROUP STRATIFIED EVALUATION AND MONITORING
Low risk asymptomatic patients (group 1) who are concerned about the health risks or economic burdens of vaping should be encouraged to accept help to escape addiction. The optimal approach is individualized based on whether the patient is among the 13% of the recently vaping-exposed who exclusively inhale nicotine-containing products, the 32% who exclusively inhale THC containing products, or the 55% that are exposed to both or other substances (10).
Vaping-exposed patients with symptoms of cough, chest pain, weight loss, fatigue, or dyspnea of any severity who are not explained by other conditions meet the CDC case definition for suspected EVALI (4). They require evaluation, and the case may need to be reported. When these patients have a resting oxygen saturation of 95% or more and levels of 88% or more with exercise or levels of oxygen saturation that are near their abnormal baseline levels, they are in group 2 and at an intermediate level of risk. It is acceptable to perform additional testing at an outpatient facility with the expectation that they will seek urgent evaluation should they develop significant dyspnea or experience progression of their symptoms.
The hypoxemic patients of group 3 are at the highest risk for progressing to respiratory failure and require inpatient oximetry monitoring and prevention of additional exposure for at least the first 48 hours to detect, prevent, and manage progressive hypoxemia and have urgent intervention should they progress to respiratory failure (Fig. 1). We consider patients who have the three CDC defining criteria for EVALI and 4) a chest imaging study with new and otherwise unexplained lung abnormalities and 5) have acute hypoxemia defined as a decrement from baseline to a resting oxygen saturation of less than 95% at rest or less than 88% with exercise to have VARDS.
Figure 1.
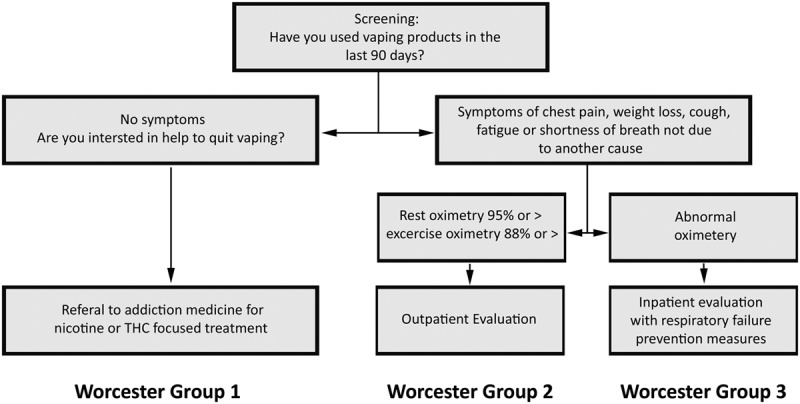
Symptom- and oximetry-based risk stratification allows identification of patients with indications for monitoring.
RISK STRATA–GUIDED DIAGNOSTIC TESTING
The low risk asymptomatic patients of group 1 do not require vaping-directed diagnostic testing until they develop chest pain, weight loss, fatigue, or dyspnea that are not explained by other conditions. The presence of respiratory symptoms and hypoxemia after exposure to a toxin is an indication for urgent evaluation that includes chest imaging and oximetry. When the chest radiograph is normal or minimally abnormal, other diseases that produce hypoxemia such as pulmonary venous thromboembolism should be carefully considered (Fig. 2). The evaluation of group 2 and 3 patients is guided by the results of oximetry and chest radiography. Risk of adverse outcomes is lower for patients with normoxia than those with acute hypoxemia and lower for those with normal compared with abnormal radiographs.
Figure 2.
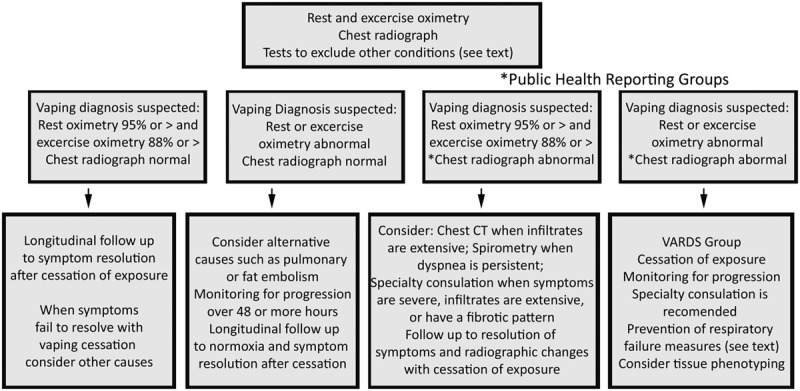
Evaluation of group 2 and 3 patients based on oximetry and chest radiography. *Reporting is strongly recommended and is required by some public health departments.
The key feature of making a diagnosis of EVALI or VARDS is the attribution of symptoms to vape fume exposure rather than to another condition. Accurate diagnosis requires clinical skill, accurate radiographic interpretation, thoughtful selection of laboratory and diagnostic testing, and both inclusionary and exclusionary reasoning. Recent exposure to other toxins or individuals with febrile respiratory illnesses, lack of vaccination when symptoms develop during a viral endemic season, the presence of fevers, chills, or localizing signs of infection, and clustering with non-fume exposed cases suggest respiratory tract infection as the cause of respiratory symptoms. Exposure to immunosuppressive medications or HIV risk factors also suggests that an opportunistic infection may be present. The presence of an inflammatory condition or features of a rheumatological disease suggests that the symptoms may not be attributable to vaping.
Because vaping-related toxins often cause coincident gastrointestinal symptoms including abdominal pain, nausea, vomiting, and diarrhea, their occurrence near the time of respiratory symptoms favors attribution to vaping. Case clustering around a common vape juice is a strong inclusionary factor. The association of symptoms with abnormal respiratory system physical findings is an indication for radiological evaluation.
Non-specific inclusionary laboratory markers include leukocytosis, elevated liver function tests, abnormal inflammatory markers such as erythrocyte sedimentation rate or C-reactive protein and oil-red-O staining of sputum pneumocytes for fat containing cells. Exclusionary factors include positive respiratory viral or influenza tests, Legionella and Pneumococcal urinary antigen tests, positive fungal markers, sputum Gram stain demonstrating neutrophils and bacteria or the presence of microbes in sputum or blood cultures.
Tissue-directed phenotyping should be considered when chest imaging demonstrates substantial bilateral abnormalities, and infectious or inflammatory causes have not been ruled out. Chest CT can provide details that can direct the acquisition of respiratory tract cultures and tissue. It can also suggest a fibrotic phenotype that has been associated with giant-cell interstitial pneumonia (11) when cobalt, lead, or another heavy metal has been released from an ENDS heating coil (12).
Series of chest CT studies of patients with rapidly progressive or subacute vaping-associated respiratory disease (12) have displayed a variety of patterns including those associated with acute eosinophilic pneumonia (13), diffuse alveolar damage, organizing pneumonia (12), endogenous lipoid pneumonia, giant-cell interstitial pneumonia (12), hypersensitivity pneumonitis (14), lipoid pneumonia (15), and diffuse alveolar hemorrhage (16). The enhanced definition of the anatomic distribution of abnormal tissue helps to define the optimal site for obtaining respiratory system cultures, cells, and tissues.
When infection has not been excluded or the patient is immunocompromised and at risk for opportunistic infections, a bronchoscopic sampling for cultures, cells studies, and laboratory testing can be helpful. Bronchoscopic examination of the airways can also identify airway mucosal injury and epithelial disruption caused by the inhalation of the heated toxins that are present in vape fumes (see below).
Acute eosinophilic pneumonia is suggested when greater than 25% of the cells recovered from bronchoalveolar lavage fluid (BAL) are eosinophils (17), and the hypersensitivity pneumonitis phenotype is suggested when the CD4/CD8 ratio of BAL lymphocytes is less than 1 (18). Analysis of BAL cells can also identify patients with exogenous lipoid pneumonia from the ingestion of lipids and endogenous lipoid pneumonia produced in response to tissue injury in the setting of airway obstruction from patients with other phenotypes.
Oil-red-O staining of lower airway histiocytes (alveolar macrophages or pneumocytes) is a well-known technique that is being explored in the context of vaping (19). Based on our original description of how this technique best distinguishes lipoid pneumonia from other forms of lung disease (20), we believe that using the quantitative lipid laden alveolar macrophage index (range 0–400) with a cut off value of 100 would better separate lipoid pneumonia cases from patients with other pathologies than the qualitative positive percentage technique (20). We found that combining a staining intensity measure with lipid positive percentage provided superior discriminatory power when 100 cells were counted.
Pathologic examination of open or bronchoscopically obtained biopsies allows identification of granulomatous (21), fibrotic, eosinophilic, hemorrhagic, and acute lung injury architectures. The focus on lipoid pneumonia as a pathologic phenotype for patients with subacute presentation of vaping-related respiratory disease has recently been expanded to include lung injury pathologies by examination of patients who presented with progressive dyspnea, cough, chest pain, fatigue, weight loss, hypoxemia with progression to respiratory failure. These patients were found to have histopathologic changes of acute lung injury, including acute fibrinous pneumonitis, diffuse alveolar damage, usually bronchiolocentric organizing pneumonia, and bronchiolitis (3).
Different toxins generated by the heating of the mix of flavorants, aldehyde or alcohol-based solvents, tocopherols, hydrocarbon-based oils, and adulterants delivered at doses that vary by alternative vaping techniques are expected to produce a spectrum of respiratory tissue responses. The increasingly broad spectrum of clinical, radiographic, and pathologic presentations strongly implies that vaping produces a variety of respiratory syndromes. It is increasingly clear that the vaping-related respiratory syndromes include alternative pathologic phenotypes including lipoid (22), acute eosinophilic pneumonia (13), hypersensitivity pneumonitis (14), and acute lung injury variants (3, 7).
The current CDC guidance documents acknowledge that specialist consultation is helpful and pathologic examination of respiratory tract tissues is indicated when recommended by a specialist (4). Phenotypic specificity is helpful for distinguishing cases that are likely to benefit from early treatment with steroids (lipoid pneumonia, acute eosinophilic pneumonia, and hypersensitivity pneumonitis) from the tissue injury variants that may be harmed by the immunosuppressive side effects of steroids and phenotypes destined for recovery with avoidance of exposure alone.
RISK GROUP–GUIDED MANAGEMENT AND PREVENTION PLANS
Group 1 (asymptomatic) patients who affirm a desire to quit vaping should be offered addiction medicine support for substance-directed treatment. There is evidence that patients who have addiction to THC or nicotine-containing products can benefit from cognitive-behavioral therapy, contingency management, motivational enhancement therapy, and multidimensional family therapy (4, 23, 24).
Normoxic, asymptomatic patients with abnormal radiographs should have a therapeutic trial of vaping cessation and evaluation for other conditions that may be responsible for their symptoms (Fig. 2). Hypoxic patients with normal pulmonary imaging studies should be evaluated for alternative explanations for their symptoms such as pulmonary or fat embolism. These patients require close follow-up for deterioration, and specialist evaluation is recommended when hypoxemia persists. Unlike patients with abnormal chest radiographs, public health reporting is not currently recommended or required for patients with normal radiographs.
Group 2 patients who are normoxic, symptomatic, and have abnormal chest imaging should be carefully examined for evidence of infection. More precise anatomic definition of the abnormalities evaluated by chest CT should be considered when risk factors for neoplastic disease, including significant tobacco exposure, are present or when radiographic abnormalities are extensive. Spirometry is indicated for those with severe, persistent, or progressive shortness of breath. Group 2 patients should be encouraged to promptly report progression of their symptoms and should be followed to resolution of their symptoms and radiographic abnormalities.
Defining when a combination of clinical, radiographic, and pathologic features strongly implicates the presence of specific diagnosis is fundamental to deciding to prescribe immunosuppressives or to focus on cessation of exposure will best promote respiratory tissue healing from the toxins. In order to expand the range of therapeutic options, we present a case of vaping-associated airway injury associated with improvement after the removal of necrotic tissue that was obstructing the airways that subtended the infiltrates.
REPORT OF AN AIRWAY TISSUE INJURY VARIANT VARDS CASE
A 29-year-old man with a history of polysubstance abuse including cocaine, marijuana, and cigarettes presented with the acute onset of pleuritic chest pain and progressive dyspnea on exertion 24–48 hours after consuming alcohol, nasally insufflating cocaine, vaping an oil-based THC containing solution followed by loss of consciousness. On arrival to the hospital, the patient was found to have sinus tachycardia and acute hypoxemic respiratory failure with a chest CT demonstrating bilateral lower zone predominant, nodular infiltrates centered around airways, and a segmental pulmonary embolism. His urinary toxicology screen was positive for cannabinoids, and a HIV test was negative. The patient was managed with oxygen, anticoagulation, and was evaluated with a bronchoscopic examination of his airways.
The airways that subtended both abnormal lower zone opacities were covered with opaque necrotic material that was removed by suction (Fig. 3). The BAL fluid demonstrated neutrophils without eosinophils or lipid laden pneumocytes. Cultures were all sterile. His procedure was associated with rapid resolution of his symptoms and resolution of his hypoxemia. The patient subsequently achieved complete functional recovery, and he has abstained from vaping.
Figure 3.
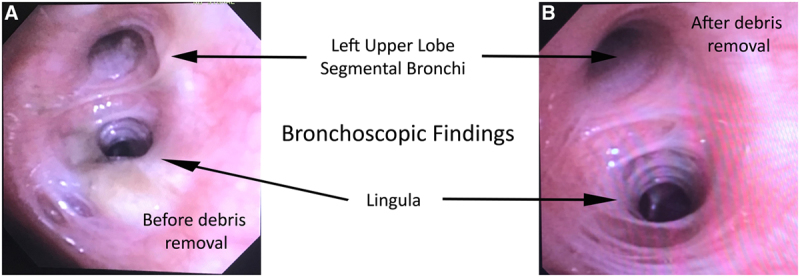
Bronchoscopic view of the airways before (A) and after (B) removal of necrotic debris.
The endoluminal findings of airway occlusion from vaping fume-related necrotic debris accumulation suggest that vaping can cause one of the defining features of endogenous lipoid pneumonia, that is, airway obstruction (25). This case of early intervention to remediate airway occlusion and rapid physiologic improvement has led to the hypothesis that expeditious opening of the airways can interrupt the progression to the deranged endogenous lipid metabolism (26) that has been observed in the mouse model of endogenous lipoid pneumonia.
In addition to the two low lipid phenotype patients of the prior report (19), this case also demonstrates that patients with the airway injury predominant phenotype can rapidly improve with airway clearance, high-flow oxygen, non-invasive ventilation, and heal to an optimal outcome without being exposed to immunosuppressive anti-inflammatory medications.
Risk group 3 patients require monitoring for progressive symptoms and serial or continuous oximetry to detect worsening hypoxemia and effective measures to prevent exposure to vaping fumes. Airway clearance techniques such as cough encouragement and prevention of atelectasis with incentive spirometry and conservative fluid management strategies should be used to prevent progression to respiratory failure. Falling oxygen saturation should be managed with escalation of oxygen therapies, with the application of a high-flow system, before moderate hypoxemia is present. Inhaled bronchodilators should be prescribed to patients with wheezing or documented obstructive deficits. Patients with elevated respiratory rates should be managed with non-invasive ventilation before hypercarbia is present, and invasive mechanical ventilation is indicated for those who present with or progress to respiratory failure. Because severe vaping cases share clinical and pathologic features with ARDS (3), we favor conservative fluid management and a lung-protective ventilation strategy. We prefer a volume-targeted mode with a tidal volume goal of 6 cc/kg and a plateau pressure of less than 30 cm water when it achieves adequate gas exchange. We recommend pressure-controlled ventilation and paralysis be considered when volume-targeted ventilation is not effective. When oxygenation is inadequate, we recommend considering inhaled epoprostenol or nitric oxide, prone position ventilation, and consideration of rescue with extracorporeal membrane oxygenation. Parenteral steroids should be considered for all patients with progressive hypoxemic respiratory failure particularly when other etiologies have been ruled out and contra-indications are not identified. In addition, measures should be taken to prevent exposure by others to any remaining vaping solution that has been associated with a case of hypoxemic respiratory failure.
Those with steroid sensitive phenotypes including acute eosinophilic pneumonia, hypersensitivity pneumonia, and lipoid pneumonia should be treated promptly with a course of steroids. We recommend that patients without signs of respiratory failure or are improving and do not have evidence of a steroid sensitive phenotype be managed without exposure to immunosuppressives.
Group 3 patients should have spirometry at a time when they are able to perform the required maneuvers. These patients require follow-up to the resolution of symptoms, clearing of their radiographic abnormalities, and normalization of their spirograms. Patients who have difficulty with cessation of vaping should be encouraged to accept help from an addiction medicine professional.
We must work with our patients to better identify the substances that they are vaping and develop culturally effective methods for encouraging abstinence. We must not attribute to vaping respiratory illnesses that are caused by infections. Our approach to the needs of patients who are exposed to vaping fumes should be guided by their symptoms, physical findings, gas exchange, radiographic abnormalities, and preferences. We should use anti-inflammatories when there is evidence of a steroid sensitive condition and avoid steroids when abstinence results in rapid improvement. Those at risk for acute respiratory failure must be identified, monitored, evaluated, and treated.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Miech R, Johnston L, O’Malley PM. Adolescent vaping and nicotine use in 2017-2018 - U.S. National estimates. N Engl J Med. 2019; 380:192–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hswen Y, Brownstein JS. Real-time digital surveillance of vaping-induced pulmonary disease. N Engl J Med. 2019; 381:1778–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019; 381:1780–1781 [DOI] [PubMed] [Google Scholar]
- 4.Siegel DA, Jatlaoui TC, Koumans EH, et al. ; Lung Injury Response Clinical Working Group; Lung Injury Response Epidemiology/Surveillance Group. Update: Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury MMWR Morb Mortal Wkly Rep. 2019; 68:919–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrison J.Massachusetts. vaping ban allowed to stand for now amid court challenge. USA Today. October 12, 2019. Available at: https://www.usatoday.com/story/news/nation/2019/10/04/massachusetts-vaping-ban-allowed-remain-amid-court-challenge/3858590002/. Accessed February 3, 2020.
- 6.Drazen JM, Morrissey S, Campion EW. The dangerous flavors of E-cigarettes. N Engl J Med. 2019; 380:679–680 [DOI] [PubMed] [Google Scholar]
- 7.Christiani DC. Vaping-induced lung injury. N Engl J Med. 2019. Sep 6. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - Preliminary report. N Engl J Med. 2019. Sep 6. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Schier JG, Meiman JG, Layden J, et al. CDC 2019 Lung Injury Response Group: Severe pulmonary disease associated with electronic-cigarette product use - interim guidance. MMWR Morb Mortal Wkly Rep. 2019; 68:787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion: Outbreak of lung injury associated with e-cigarette use, or vaping. 2020. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed February 3, 2020.
- 11.Lin C, Choi H. Granulomatosis due to electronic cigarette use CHEST. 2019; 156:A2093 [Google Scholar]
- 12.Henry TS, Kanne JP, Kligerman SJ. Imaging of vaping-associated lung disease. N Engl J Med. 2019; 381:1486–1487 [DOI] [PubMed] [Google Scholar]
- 13.Arter ZL, Wiggins A, Hudspath C, et al. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019; 27:100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommerfeld CG, Weiner DJ, Nowalk A, et al. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018; 141:e20163927. [DOI] [PubMed] [Google Scholar]
- 15.Holdorf J, Carpenter D, Kopec S. Is it the E.N.D.S.? CHEST. 2016; 150:502A [Google Scholar]
- 16.Agustin M, Yamamoto M, Cabrera F, et al. Diffuse alveolar hemorrhage induced by vaping. Case Rep Pulmonol. 2018; 2018:9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki Y, Suda T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019; 68:413–419 [DOI] [PubMed] [Google Scholar]
- 18.Caillaud DM, Vergnon JM, Madroszyk A, et al. ; French Group of Environmental Immunoallergic Bronchopulmonary Diseases. Bronchoalveolar lavage in hypersensitivity pneumonitis: A series of 139 patients. Inflamm Allergy Drug Targets. 2012; 11:15–19 [DOI] [PubMed] [Google Scholar]
- 19.Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019; 381:1488–1489 [DOI] [PubMed] [Google Scholar]
- 20.Corwin RW, Irwin RS. The lipid-laden alveolar macrophage as a marker of aspiration in parenchymal lung disease. Am Rev Respir Dis. 1985; 132:576–581 [DOI] [PubMed] [Google Scholar]
- 21.Narang R, Narang D, Narang S, et al. Good, bad, and ugly on vaping. CHEST. 2015; 148:385A [Google Scholar]
- 22.Dicpinigaitis PV, Trachuk P, Fakier F, et al. Vaping-associated acute respiratory failure due to acute lipoid pneumonia. Lung. 2019. Oct 3. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Budney AJ, Moore BA, Rocha HL, et al. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006; 74:307–316 [DOI] [PubMed] [Google Scholar]
- 24.Gravely S, Thrasher JF, Cummings KM, et al. Discussions between health professionals and smokers about nicotine vaping products: Results from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction. 2019; 114Suppl 171–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betancourt SL, Martinez-Jimenez S, Rossi SE, et al. Lipoid pneumonia: Spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010; 194:103–109 [DOI] [PubMed] [Google Scholar]
- 26.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019; 129:4290–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


