Abstract
Background
Diagnosis of endometrial (womb) cancer is normally made at an early stage, as most women with the disease experience abnormal vaginal bleeding, which prompts them to seek medical advice. However, delays in presentation and referral can result in delay in diagnosis and management, which can lead to unfavourable treatment outcomes. This is particularly a problem for pre‐ and peri‐menopausal women. Providing educational information to women and healthcare providers regarding symptoms relating to endometrial cancer may raise awareness of the disease and reduce delayed treatment.
Objectives
To assess the effectiveness of health education interventions targeting healthcare providers, or individuals, or both, to promote early presentation and referral for women with endometrial cancer symptoms.
Search methods
We searched CENTRAL, MEDLINE and Embase. We also searched registers of clinical trials, abstracts of scientific meetings and reference lists of review articles.
Selection criteria
We planned to include randomised controlled trials (RCTs), both individually randomised and cluster‐RCTs. In the absence of RCTs we planned to include well‐designed non‐randomised studies (NRS) with a parallel comparison assessing the benefits of any type of health education interventions.
Data collection and analysis
Two review authors independently evaluated whether potentially relevant studies met the inclusion criteria for the review, but none were found.
Main results
A comprehensive search of the literature yielded the following results: CENTRAL (1022 references), MEDLINE (2874 references), and Embase (2820 references). After de‐duplication, we screened titles and abstracts of 4880 references and excluded 4864 that did not meet the review inclusion criteria. Of the 16 references that potentially met the review inclusion, we excluded all 16 reports after reviewing the full texts. We did not identify any ongoing trials.
Authors' conclusions
There is currently an absence of evidence to indicate the effectiveness of health education interventions involving healthcare providers or individuals or both to promote early presentation and referral for women with endometrial cancer symptoms. High‐quality RCTs are needed to assess whether health education interventions enhance early presentation and referral. If health education interventions can be shown to reduce treatment delays in endometrial cancer, further studies would be required to determine which interventions are most effective.
Plain language summary
Do health education interventions lead to early treatment for women with symptoms of endometrial (womb) cancer
Background Endometrial (womb) cancer arises from the uncontrolled growth of abnormal cells in the lining of the womb. Diagnosis when the disease is still at an early stage (cancer is still within the womb, without spread into nearby tissues) is common, as most women with the disease experience abnormal vaginal bleeding and go to their doctors. However, delayed management of endometrial cancer still occurs. This is particularly a problem for women who are about to go through or are in the menopause. Providing educational information to women and healthcare providers regarding symptoms relating to endometrial cancer may raise awareness of the disease and reduce delayed treatment. We undertook this review to assess whether endometrial cancer education led to women with endometrial cancer symptoms visiting their doctors and being referred for treatment earlier than when there was no education.
The aim of the review We undertook this review to assess whether endometrial cancer education led to women with endometrial cancer symptoms visiting their doctors and being referred for treatment earlier than when there was no educational information available.
Main findings We planned to include randomised controlled trials (studies in which people or groups of people are allocated by chance to two or more groups, treating them differently). In the absence of randomised controlled trials, we planned to include studies where participants were not randomised but that included an assessment of the benefits of health education compared to no health education. We searched scientific databases and checked the titles and abstracts of 4880 possibly relevant articles and assessed the full text of 16 of these references. However, we found no studies that met our inclusion criteria.
Conclusions There is currently an absence of evidence to indicate whether providing health education to healthcare providers, or individuals or both, promotes early presentation and referral for women with symptoms of endometrial cancer.
Summary of findings
Summary of findings for the main comparison. Health education interventions to promote early presentation and referral for women with symptoms of endometrial cancer.
| Health education interventions to promote early presentation and referral for women with symptoms of endometrial cancer | ||||||
|
Patient or population: adult women with symptoms of endometrial cancer Settings: individual‐ or community‐level settings Intervention: any types of health education intervention Comparison: head‐to‐head intervention or a control (presumably usual/standard practice) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intevention | |||||
| Overall survivala | No included study | |||||
| Disease‐free survivalb | No included study | |||||
| Delayed referralc | No included study | |||||
| Delayed presentationd | No included study | |||||
| Referral time (days)e | No included study | |||||
| Presentation time (days)f | No included study | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
|
aSurvival until death from all causes.
bSurvival until the appearance of a new lesion of disease. cTime from primary care first appointment to time of primary care referral to secondary care of longer than 14 days. dTime from symptom of postmenopausal bleeding to the first appointment with a responsible specialist of longer than 14 days and longer than three months for irregular bleeding if premenopausal. eTime from primary care first appointment to time of primary care referral to secondary care. fTime from symptom onset to arrival at primary care hospital. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
Description of the condition
Uterine cancer is the fifth most common cancer affecting women worldwide, with an estimated 382,000 new cases and 90,000 uterine cancer deaths, occurring globally in 2018 (Bray 2018). Endometrial (womb) cancer, which arises from the endometrium (inner lining of the womb), is the most common type of uterine cancer. The highest incidence of endometrial cancer is in North America and Europe (Beesley 2010). Endometrial cancer incidence, particularly aggressive subtypes, is increasing across different populations (Beesley 2010; Cote 2015), and in low‐ and middle‐income countries it is now the most common gynaecological cancer. Predisposing factors for endometrial cancer include high body mass index, diabetes mellitus, nulliparity, infertility, unopposed oestrogen therapy, oestrogen‐producing tumours (rare tumours that secrete oestrogen), early menarche or late menopause, and Lynch syndrome (Colombo 2016).
After a diagnosis of endometrial cancer has been made, staging is performed to determine the extent of the disease. Staging of endometrial cancer (investigations to determine whether the cancer is confined to the womb or has spread to other parts of the body) is mainly achieved as a result of histological examination of the womb and other tissues following surgery. The International Federation of Gynecology and Obstetrics (FIGO) staging system for endometrial cancer is provided in Appendix 1 (Amant 2018). Staging enables physicians to plan the best treatments after surgery and can help predict long‐term survival. The majority of cases of endometrial cancer (70% to 75%) are diagnosed at FIGO stages I or II (Colombo 2016). The five‐year overall survival of women with endometrial cancer stages I or II ranges from 75% to 90%. In contrast, the five‐year overall survival for stages III and IV are only 55% to 65% and 20% to 25%, respectively (Colombo 2016).
Description of the intervention
Timely diagnosis of symptomatic cancer is likely to have benefits for women in terms of improved survival, earlier‐stage diagnosis and improved quality of life, although these benefits may vary between cancers (Neal 2015). Early diagnosis of cancer requires patients and healthcare providers to be aware of the early symptoms and signs of cancer, which should lead to prompt access to healthcare services and referral to a specialised health centre for further diagnostic tests and management (WHO 2007). Low cancer awareness (which may include lack of knowledge or false beliefs about cancer symptoms and the risk of developing cancer) among individuals can contribute to a delay in their presentation (Allgar 2005). Raising public awareness and education about identifying the early symptoms of cancer should reduce delayed diagnosis for patients (Car 2016).
Health educational interventions aim to improve the knowledge, awareness, attitudes, and skills of a target population (Mansell 2011). In the context of this review, we have defined health education interventions as interventions that facilitate knowledge and awareness to promote early presentation in the general population and interventions that aim to promote early referral among healthcare providers by increasing their knowledge or influencing their attitudes, using a variety of formats or programmes.
Health education intervention for promoting cancer awareness among individuals can be delivered by either individual‐level interventions or community‐level interventions (Austoker 2009). The intervention provided at an individual level may include a face‐to‐face session with a health professional or an educational leaflet given to an identified individual. Community‐level educational interventions may include media campaigns, health education websites, or leaflets or posters distributed indiscriminately in a public space (Austoker 2009).
Healthcare providers in the primary care setting play a major role in identifying people with symptoms suspicious of cancer since this is the first point of healthcare access for most people (NICE 2017; Swann 2018). People who have so‐called 'red flag' symptoms are then typically referred to a specialised healthcare centre for further diagnosis and treatment. A previous systematic review reported a trend of poor treatment outcomes among people with symptomatic cancers of a range of types who had long waiting times for definitive treatment (Neal 2015), although a small study in women diagnosed with endometrial cancer suggested that primary care doctors referred women who had worse prognosis disease more promptly (Morrison 2003). Based on these findings, reducing the delay in referral may improve outcomes. Several Cochrane Reviews observed an improvement in professional practice after implementing various educational interventions (Forsetlund 2009; Giguère 2012; O'Brien 2007). These may include lectures, printed educational materials, continuing education meetings, workshops, videos, and Internet triage packages to raise the awareness of 'red flag' symptoms of cancer (Mansell 2011).
How the intervention might work
Early diagnosis of endometrial cancer, ideally before the disease spreads, is clinically achievable and relatively uncomplicated, as most women with the disease experience abnormal vaginal bleeding, either postmenopausal bleeding or abnormal pre‐menstrual bleeding (Jamison 2013; Saso 2011). Women with endometrial cancer typically present with postmenopausal bleeding, which is defined as unexplained vaginal bleeding more than 12 months after menstruation has stopped due to menopause and in those who are not taking hormone replacement therapy (NICE 2017). The estimates of the probability of endometrial cancer in women presenting with postmenopausal bleeding varies from 4% to 11% (Bani‐Irshaid 2011; Clarke 2020; Escoffery 2002; Gredmark 1995; Lee 1995). The risk of endometrial cancer among women with postmenopausal bleeding increases with age (Gredmark 1995). The UK National Institute for Health and Care Excellence (NICE) recommends the urgent referral of women with postmenopausal bleeding, ensuring an appointment within two weeks for further evaluation if they have postmenopausal bleeding and are aged 55 years or over (NICE 2017). The guidelines also recommend consideration of a referral for an appointment within two weeks for endometrial cancer evaluation in women aged under 55 years with postmenopausal bleeding (NICE 2017). Other suspicious symptoms of endometrial cancer include an abnormal vaginal discharge or heavy or prolonged periods in premenopausal women. Presentation with a pelvic or abdominal mass or pelvic pain is relatively rare and may be associated with advanced cancer (Jamison 2013; Saso 2011).
Promoting recognition of possible warning symptoms and signs of endometrial cancer among individuals and healthcare providers remains a critical goal. However, primary healthcare providers encounter endometrial cancer comparatively rarely, which could lead to low levels of knowledge and awareness. Educational intervention may, therefore, enhance the appreciation of the need for early referral by improving knowledge and awareness to providers about 'red flag' symptoms of cancer (Rose 2001). A previous systematic review indicated that educational interventions delivered to individuals or communities may increase cancer awareness in other types of cancer (Austoker 2009).
Why it is important to do this review
Delays in the management of women with endometrial cancer are not uncommon (Dolly 2016; Elit 2014; O'Leary 2013; Strohl 2016; Zhou 2018). Zhou 2018 noted the delayed general practitioner referrals for women with endometrial cancer, which were more likely for pre‐menopausal women. Dolly 2016 observed that the mean interval time from the diagnosis of endometrial cancer to treatment was 47.5 days. Recently, Strohl 2016 reported that approximately 25% of women with endometrial cancer experienced a surgical delay, which was defined as a surgical wait time greater than six weeks. Delay in surgical treatment is likely associated with poor access to speciality care (Shalowitz 2017).
Delay in the management of women with endometrial cancer has a negative impact on survival (Dolly 2016; Elit 2014; Strohl 2016). Survival for women with surgical wait times of more than six weeks was worse than for those treated within six weeks of diagnosis, when controlling for women's age, ethnicity, insurance status, level of educational attainment, and comorbidity (Strohl 2016).
Delayed presentation and referral may contribute to the overall delay in management for women with gynaecologic cancers, leading to unfavourable treatment outcomes (Rose 2015; Shalowitz 2015; Shalowitz 2017). To ensure the best possible outcomes for women with endometrial cancer, timely presentation, diagnosis, and referral to an experienced healthcare setting is required. A previous systematic review noted that educational interventions increased women’s participation in cervical cancer screening programmes (Musa 2017). Our aim was to conduct this review with the goal of evaluating the effectiveness of health education interventions for promoting early presentation and referral for women with suspected symptoms of endometrial cancer.
Objectives
To assess the effectiveness of health education interventions targeting healthcare providers, or individuals, or both, to promote early presentation and referral for women with endometrial cancer symptoms.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs), both individually randomised (studies in which individuals were randomised to either the intervention or the control arm of the experiment, or randomised to receive different interventions) and cluster‐RCTs (studies that have as the unit of randomisation a group or community level, or where clusters of professionals or groups of professionals are implementing interventions). We planned to exclude cross‐over RCTs due to the nature of the question of the review. If we identified no RCTs, we planned to include non‐randomised studies (NRS) with a parallel comparison. We intended to include NRS that analysed results for intervention effects adjusted for baseline characteristics, that is, participants' age and menopausal status. We planned to exclude NRS without a concurrent comparison group.
Types of participants
We planned to include woman aged 18 years or older in any setting who experienced symptoms suspicious for endometrial cancer. We planned to include any healthcare providers of any age, gender, or profession (e.g. nurse, doctor, allied staff), in any public or private healthcare facility. In addition, as we planned to recruit cluster‐RCTs to this review, participants could thus be communities or healthcare institutions or other units. We planned to perform a separate analysis for different types of participants (individuals who experienced suspicious symptoms of endometrial cancer and healthcare providers).
Types of interventions
Interventions of interest were any health education interventions performed with the aim of promoting the early presentation and referral of women with symptoms suspicious of endometrial cancer compared with the control (presumably usual or standard practice), or directed, head‐to‐head educational interventions. Interventions could target individuals, healthcare providers, or both. We planned to include studies regardless of their level of delivery of the intervention (individual or public or community). Interventions aimed at the individual level could be health education outreach visits, meetings, or printed educational materials. Community‐based health education interventions could be mass media campaigns, health education website, or posters distributed indiscriminately in public areas.
Types of outcome measures
Primary outcomes
Overall survival: defined as survival of women with endometrial cancer from diagnosis from all causes
Disease‐free survival: defined as survival of women with endometrial cancer until the appearance of a new lesion of disease
Secondary outcomes
Delayed referral: defined as the time from primary care first appointment to time of primary care referral to secondary care of longer than 14 days (NICE 2017)
Delayed presentation: defined as the time from the symptom of postmenopausal bleeding to the first appointment with a responsible specialist of longer than 14 days and longer than three months for irregular bleeding if premenopausal
Referral time: defined as the time from primary care first appointment to the time of primary care referral to secondary care (days)
Presentation time or time of help‐seeking: defined as the time from symptom onset to arrival at primary care hospital (days)
Conversion rate: defined as the proportion of referrals for suspected cancer who were then shown to have endometrial cancer
Detection rate: defined as the proportion of endometrial cancers that were detected
Time from presentation to receiving definite treatment (days)
Women's satisfaction with the referral process: using visual analogue scale or as defined by the study authors
Physicians' satisfaction with the referral process: using visual analogue scale or as defined by the study authors
Quality of life: evaluated among women with endometrial cancer after treatment using a scale that has been validated through reporting of norms in a peer‐reviewed publication, for example, European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐EN24 endometrial‐specific quality of life questionnaire (Greimel 2011)
Cost‐effectiveness of the intervention: using a validated scale, for example, European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO‐MCBS; Cherny 2015). If data permit, we will analyse either cost per case of endometrial cancer detected or incremental cost‐effectiveness ratio (ICER)
We planned to present a 'Summary of findings' table to report the following outcomes listed in order of priority
Overall survival
Disease‐free survival
Delayed referral
Delayed presentation
Referral time
Presentation time
Search methods for identification of studies
We searched the following sources, irrespective of the language of publication, publication status, or sample size.
Electronic searches
We searched the following electronic databases on 17 February 2020:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 2), in the Cochrane Library;
MEDLINE via Ovid (1946 to February week 1, 2020);
Embase via Ovid (1980 to 2020 week 7).
We identified all relevant articles on PubMed, and we planned to conduct a further search for newly published articles using the ’related articles’ feature.
Appendix 2; Appendix 3; Appendix 4 display the search strategies for CENTRAL, MEDLINE, and Embase.
Searching other resources
Ongoing studies
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/), and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), to identify any ongoing trials. If we identified any ongoing unpublished trials, we planned to approach the principal investigators and major co‐operative groups active in this area to ask for relevant data.
Grey literature
We searched the OpenGrey (www.opengrey.eu/), and Index to ProQuest Dissertations & Theses: UK & Ireland databases for grey literature.
Handsearch
We searched within reference lists of all included studies and within previous systematic reviews on the same topic. We also searched the reports of conferences in the following sources: Annual Meeting of the American Society of Gynecologic Oncologists; Annual Meeting of the International Gynecologic Cancer Society; Annual Meeting of the European Society of Medical Oncology (ESMO); Annual Meeting of the American Society of Clinical Oncology (ASCO); Annual Meeting of the British Gynaecological Cancer Society (BGCS); Biennial Meeting of the Asian Society of Gynecologic Oncology (ASGO); Biennial Meeting of the Asia and Oceania Federation of Obstetrics and Gynaecology (AOFOG); Biennial Meeting of the European Society of Gynaecologic Cancer (ESGO); and Biennial Meeting of the International Gynecologic Cancer Society (IGCS).
Data collection and analysis
Selection of studies
We transferred all titles and abstracts retrieved by electronic searching to Covidence 2019. After the removal of duplicated results, two review authors (CC and CK) independently examined the remaining references. We excluded those studies that clearly did not meet the inclusion criteria and obtained full‐text copies of potentially relevant references. Two review authors (CC and CK) independently assessed the eligibility of the retrieved reports/publications. Any disagreements were resolved through discussion or by consulting a third review author (KC, PL, or AA) if necessary. We planned to identify and exclude duplicates and collate multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We applied the details obtained from the selection process in Covidence 2019 to create a PRISMA flow diagram (Liberati 2009), and 'Characteristics of excluded studies’ table.
Data extraction and management
Not applicable ‐ see Differences between protocol and review
Assessment of risk of bias in included studies
Not applicable ‐ see Differences between protocol and review
Measures of treatment effect
Not applicable ‐ see Differences between protocol and review
Unit of analysis issues
Not applicable ‐ see Differences between protocol and review
Dealing with missing data
Not applicable ‐ see Differences between protocol and review
Assessment of heterogeneity
Not applicable ‐ see Differences between protocol and review
Assessment of reporting biases
Not applicable ‐ see Differences between protocol and review
Data synthesis
Not applicable ‐ see Differences between protocol and review
Subgroup analysis and investigation of heterogeneity
Not applicable ‐ see Differences between protocol and review
Sensitivity analysis
Not applicable ‐ see Differences between protocol and review
Main outcomes of 'Summary of findings' table for assessing the quality of the evidence
We have presented a 'Summary of findings' table (Table 1), based on the methods described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019). However, there were no studies that met the review inclusion criteria (see Differences between protocol and review).
Results
Description of studies
Results of the search
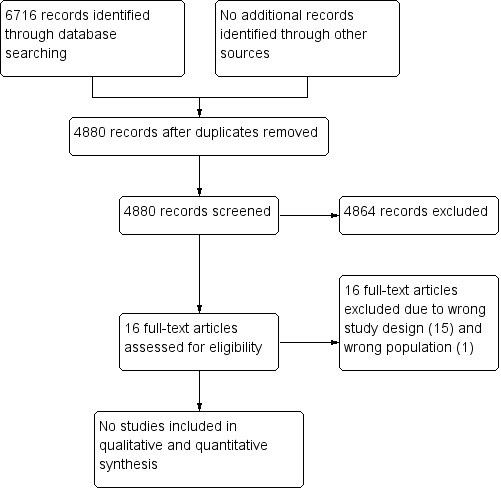
A broad search of the databases in February 2020 yielded the following results: CENTRAL (1022 references), MEDLINE (2874 references), and Embase (2820 references). We found no additional references on searching other sources. We did not identify any ongoing trials. After de‐duplication, we screened titles and abstracts of 4880 references and excluded 4864 references that did not meet the review inclusion criteria. Of the 16 references that potentially met our criteria for inclusion, we excluded all 16 reports after reviewing the full texts (see Characteristics of excluded studies). Figure 1 displays the PRISMA flow chart for study selection in this review.
1.
Study flow diagram.
Included studies
There were no studies that met the review inclusion criteria.
Excluded studies
After excluding non‐relevant and duplicate records, we retrieved 16 possibly eligible studies for a more detailed assessment. We excluded Mbah 2015 because this record was a protocol of an ongoing RCT, which was undertaken to assess the effectiveness of coach training compared to printed educational materials for enhancing cancer screening (see Characteristics of excluded studies). We excluded the remaining 15 studies because they were not comparative studies conducted to assess the effect of health education interventions for promoting early presentation and referral for women with symptoms of endometrial cancer (Abdelraheim 2017; Anwar 2002; Bellhouse 2018; Burbos 2011; Cooper 2014; Doll 2016; Johnson 2011; Kang 2013; Kwon 2007; Mohamed 2003; Ramanathan 2011; Redman 2000; Robinson 2009; Smailyte 2015; Vandborg 2011; see Characteristics of excluded studies).
Risk of bias in included studies
Not applicable
Allocation
Not applicable
Blinding
Not applicable
Incomplete outcome data
Not applicable
Selective reporting
Not applicable
Other potential sources of bias
Not applicable
Effects of interventions
See: Table 1
We were unable to conduct meta‐analyses as no studies are included in this review.
Discussion
Summary of main results
In this review, we planned to determine the benefits of any health education interventions performed with the aim of promoting the early presentation and referral of women with symptoms suspicious of endometrial cancer compared with standard or usual care, or as specified in the included studies. Interventions could have targeted individuals, healthcare providers, or both. There were no eligible RCTs in this review. We, therefore, tried to find NRS with a parallel comparison that had analysed results for the effects of health education interventions. However, there was also no existing NRS that met the review inclusion criteria.
Overall completeness and applicability of evidence
Not relevant as there are no included studies.
Quality of the evidence
No definite judgment can be made as there were no studies that fulfilled the review inclusion criteria.
Potential biases in the review process
With assistance from the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers' Information Specialist, we were able to conduct a comprehensive literature search, including a search of the grey literature, conference proceedings and abstracts, and registered databases of ongoing trials. The possibility of publication bias, therefore, is unlikely even though there were no studies that met the review inclusion criteria. There were no issues associated with bias secondary to conflicts of interests of the authors of this review.
Agreements and disagreements with other studies or reviews
Health education is commonly integrated into the cancer prevention programme. A previous systematic review, conducted to evaluate the impact of health education on participation in cervical cancer screening programmes, confirmed the significantly increased rate of cervical cancer screening among women receiving cervical cancer education compared to those who underwent usual practice (Musa 2017). However, there are currently no studies evaluating the benefits of health education interventions to enhance early presentation and referral for women with symptoms of endometrial cancer, despite it being much more common than cervical cancer in high‐income countries.
Theoretically, delayed presentation of women with endometrial cancer would certainly not be problematic as more than 90% of endometrial cancers present with abnormal vaginal bleeding (Redman 2000). However, a British prospective survey noted that low awareness of symptoms that can signal endometrial cancer is one of the causes leading to delays in treating endometrial cancer (Johnson 2011). Although the majority of women with endometrial cancer (86.6%) presented with abnormal vaginal bleeding, almost half admitted that they were unaware that abnormal bleeding was a symptom of endometrial cancer. Consequently, approximately half of women with endometrial cancer waited more than a month to see their general practitioners (GP) after their first vaginal bleed and 12% waited more than six months before they went to see their GP (Johnson 2011).
Physician's delay in the recognition of the symptoms relating to endometrial cancer has been acknowledged to be the main reason in failing to refer and diagnose early. Approximately 18% of women with endometrial cancer who presented with abnormal bleeding needed to approach their GP more than once before they initiated investigation or referral (Johnson 2011). In addition, some women reported that they were not urgently referred to access gynaecological services because their GP had told them that their vaginal bleeding was normal (Johnson 2011). This is especially a problem for pre‐ and peri‐menopausal women.
Health education may play an important role in increasing the recognition of healthcare providers regarding the symptoms of endometrial cancer. Cooper 2014 reported higher recognition of symptoms related to gynaecological cancers among the providers who reported using education materials promoting awareness of gynaecologic cancer symptoms compared to those who did not.
Associations between poor health literacy of women with endometrial cancer, low recognition of healthcare providers and delays in the management of endometrial cancer noted in Johnson 2011 are intriguing and may suggest the role of health education interventions to increase public awareness of potential symptoms of endometrial cancer and to heighten recognition in providers to minimise delays in treating endometrial cancer.
Authors' conclusions
Implications for practice.
At present, there is an absence of evidence to indicate the effectiveness of health education interventions involving healthcare providers or individuals, or both, to promote the early presentation and early referral for women with symptoms suspicious of endometrial cancer. This again highlights that endometrial cancer is under‐resourced, in terms of funding and research, given its relatively high incidence in high‐income countries, compared to other cancer types (Bray 2018; Crosbie 2014).
Implications for research.
High‐quality studies are needed to assess whether health education interventions can enhance early presentation and referral for women with symptoms of endometrial cancer. If health education interventions can be shown to reduce delays in treating endometrial cancer, further studies are required to determine which type of health education intervention yields the best results. As the results of randomised controlled trials may be available only after a considerable amount of time, evidence drawn from well‐designed non‐randomised studies are also needed, given the dearth of information in this area. Any studies should include an assessment of the referral pathway times to adequate provision of information for clinical‐decision making.
Acknowledgements
We thank Gail Quinn, Clare Jess, and Tracey Harrison for their contribution to the editorial process; Jo Platt for designing the search strategy; and Jo Morrison for clinical and editorial advice.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
We would like to thank the referees for their many helpful suggestions and comments, these including Andrew Bryant, Emma Crosbie, Stuart Rundle and Katharine Tylko‐Hill.
Appendices
Appendix 1. The International Federation of Gynecology and Obstetrics (FIGO) staging for carcinoma of the endometrium
| 2018 FIGO staging | Descriptions | ||
| Ia | Tumour contained to the corpus uteri | ||
| IAa | No or less than half myometrial invasion | ||
| IBa | Invasion equal to or more than half of the myometrium | ||
| IIa | Tumour invades the cervical stroma but does not extend beyond the uterusb | ||
| IIIa | Local and/or regional spread of tumour | ||
| IIIAa | Tumour invades the serosa of the corpus uteri and/or adnexaec | ||
| IIIBa | Vaginal involvement and/or parametrial involvementc | ||
| IIICa | Metastases to the pelvis and/or para‐aortic lymph nodesc | ||
| IIIC1a | Positive pelvic nodes | ||
| IIIC2a | Positive para‐aortic lymph nodes with or without positive pelvic lymph nodes | ||
| IVa | Tumour invades bladder and/or bowel mucosa and/or distant metastases | ||
| IVAa | Tumour invasion of bladder and/or bowel mucosa | ||
| IVBa | Distant metastases, including intra‐abdominal metastases and/or inguinal lymph nodes | ||
|
aEither grade 1, grade 2, or grade 3 bEndocervical glandular involvement only should be considered as Stage I and no longer as Stage II cPositive cytology has to be reported separately without changing the stage | |||
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Uterine Neoplasms] explode all trees #2 uter* near/5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*) #3 endometri* near/5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*) #4 womb near/5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*) #5 corpus uteri near/5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*) #6 vag* bleed* near/3 (menopaus* or pre‐menopaus* or between period* or unusual* or heav* or abnormal* or unexplain*) #7 discharge* near/3 (menopaus* or pre‐menopaus* or between period* or unusual* or heav* or abnormal* or unexplain*) #8 menstruat* near/3 (menopaus* or pre‐menopaus* or between period* or unusual* or heav* or abnormal* or unexplain*) #9 post menopaus* bleed* or PMB #10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 #11 MeSH descriptor: [Referral and Consultation] explode all trees #12 refer or referral* or referred #13 consult* or red flag symptom* #14 earl* near/3 (refer* or treat* or manag* or alert* or eval* or suspic*) #15 urgent* near/3 (refer* or treat* or manag* or alert* or eval* or suspic*) #16 #11 or #12 or #13 or #14 or #15 #17 MeSH descriptor: [Health Promotion] explode all trees #18 MeSH descriptor: [Health Knowledge, Attitudes, Practice] this term only #19 MeSH descriptor: [Health Education] in all MeSH products #20 health* near/3 (promot* or knowledge* or practice* or educat*) #21 earl* near/3 (warning* or indicat* or sign* or symptom* or interven* or identif* or investigat*) #22 urgent* near/3 (warning* or indicat* or sign* or symptom* or interven* or identif* or investigat*) #23 #17 or #18 or #19 or #20 or #21 or #22 #24 seek help* or access* or engage* or attend* or identif* or eval* or present* or explor* or investigat* or pursue* or inquir* or search* #25 #23 and #24 #26 #16 or #25 #27 #10 and #26
Appendix 3. MEDLINE Ovid search strategy
1. exp Uterine Neoplasms/ 2. ((uterus or uterine or endometri* or womb or corpus uteri) adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)).ti,ab. 3. ((vag* bleed* or discharge* or menstruat*) adj3 (menopaus* or pre‐menopaus* or between period* or unusual* or heav* or abnormal* or unexplain*)).ti,ab. 4. (post menopaus* bleed* or PMB).ti,ab. 5. 1 or 2 or 3 or 4 6. exp "Referral and Consultation"/ 7. (refer or referral* or referred).ti,ab. 8. consult* or red flag symptom*.ti,ab. 9. ((earl* or urgent*) adj3 (refer* or treat* or manag* or alert* or eval* or suspic*)).ti,ab. 10. 6 or 7 or 8 or 9 11. Health Promotion/ 12. Health Knowledge, Attitudes, Practice/ 13. Health Education/ 14. (health* adj3 (promot* or knowledge* or practice* or educat*)).ti,ab. 15. ((earl* or urgent*) adj3 (warning* or indicat* or sign* or symptom* or interven* or identif* or investigat*)).ti,ab. 16. 11 or 12 or 13 or 14 or 15 17. (seek help* or access* or engage* or attend* or identif* or eval* or present* or explor* or investigat* or pursue* or inquir* or search*).ti,ab. 18. 16 and 17 19. 10 or 18 20. 5 and 19 21. randomized controlled trial.pt. 22. controlled clinical trial.pt. 23. randomized.ab. 24. placebo.ab. 25. clinical trials as topic.sh. 26. randomly.ab. 27. trial.ti. 28. exp case‐control studies/ 29. exp Cohort Studies/ 30. (cohort* or prospective* or retrospective*).mp. 31. ((case adj control*) or (case adj series)).mp. 32. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. (animals not (humans and animals)).sh. 34. 32 not 33 35. 20 and 34
Appendix 4. Embase Ovid search strategy
1. exp uterus cancer/ 2. ((uterus or uterine or endometri* or womb or corpus uteri) adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)).ti,ab. 3. ((vag* bleed* or discharge* or menstruat*) adj3 (menopaus* or pre‐menopaus* or between period* or unusual* or heav* or abnormal* or unexplain*)).ti,ab. 4. (post menopaus* bleed* or PMB).ti,ab. 5. 1 or 2 or 3 or 4 6. exp patient referral/ 7. (refer or referral* or referred).ti,ab. 8. consult*.mp. or red flag symptom*.ti,ab. 9. ((earl* or urgent*) adj3 (refer* or treat* or manag* or alert* or eval* or suspic*)).ti,ab. 10. 6 or 7 or 8 or 9 11. health promotion/ 12. attitude to health/ 13. health education/ 14. (health* adj3 (promot* or knowledge* or practice* or educat*)).ti,ab. 15. ((earl* or urgent*) adj3 (warning* or indicat* or sign* or symptom* or interven* or identif* or investigat*)).ti,ab. 16. 11 or 12 or 13 or 14 or 15 17. (seek help* or access* or engage* or attend* or identif* or eval* or present* or explor* or investigat* or pursue* or inquir* or search*).ti,ab. 18. 16 and 17 19. 10 or 18 20. 5 and 19 21. controlled clinical trial/ 22. crossover procedure/ 23. double‐blind procedure/ 24. randomized controlled trial/ 25. single‐blind procedure/ 26. random*.mp. 27. factorial*.mp. 28. (crossover* or cross over* or cross‐over*).mp. 29. placebo*.mp. 30. (double* adj blind*).mp. 31. (singl* adj blind*).mp. 32. assign*.mp. 33. allocat*.mp. 34. volunteer*.mp. 35. exp case control study/ 36. cohort analysis/ 37. case study/ 38. prospective study/ 39. retrospective study/ 40. (cohort* or prospective* or retrospective*).mp. 41. ((case adj control*) or (case adj series)).mp. 42. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 43. 20 and 42
Appendix 5. 'Risk of bias' assessment in randomised controlled trials
We will base the 'Risk of bias' assessment on Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), as follows.
-
Random sequence generation
Low risk of bias, e.g. participants assigned to treatments on the basis of a computer‐generated random sequence or a table of random numbers
High risk of bias, e.g. participants assigned to treatments on the basis of date of birth, clinic ID number, or surname, or no attempt to randomise participants
Unclear risk of bias, e.g. not reported, information not available
-
Allocation concealment
Low risk of bias, e.g. where the allocation sequence could not be foretold.
High risk of bias, e.g. allocation sequence could be foretold by participants, investigators, or treatment providers.
Unclear risk of bias, e.g. not reported
-
Blinding of participants and personnel
Low risk of bias if participants and personnel were adequately blinded.
High risk of bias if participants and personnel were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
-
Blinding of outcome assessors
Low risk of bias if outcome assessors were adequately blinded.
High risk of bias if outcome assessors were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
-
Incomplete outcome data: we will record the proportion of participants whose outcomes were not reported at the end of the study. We will determine this domain for each outcome as follows.
Low risk of bias, e.g. if less than 20% of participants were lost to follow‐up, and reasons for loss to follow‐up were similar in both treatment arms.
High risk of bias, e.g. if more than 20% of participants were lost to follow‐up, or reasons for loss to follow‐up differed between treatment arms.
Unclear risk of bias, e.g. if loss to follow‐up was not reported.
-
Selective outcome reporting
Low risk of bias, e.g. the study reports all outcomes specified in the protocol.
High risk of bias, e.g. it is suspected that the study has selectively reported outcomes.
Unclear risk of bias, e.g. it is unclear whether outcomes have been selectively reported.
-
Other bias
Low risk of bias, e.g. the review authors do not suspect any other source of bias, and the trial appears to be methodologically sound.
High risk of bias, e.g. the review authors suspect that the trial is prone to an additional bias.
Unclear risk of bias, e.g. the review authors are uncertain whether an additional bias may be present.
Appendix 6. Risk Of Bias In Non‐randomized Studies – of Interventions (ROBINS‐I) tool
| 'Risk of bias' judgements in ROBINS‐I: pre‐intervention and at‐intervention domains | |||||||
| Risk judgement | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | ||||
| Low | No confounding expected | All participants who would have been eligible for the target trial were included in the study and start of follow‐up and start of intervention coincide for all participants. |
Intervention status is well‐defined and based solely on information collected at the time of intervention. | ||||
| Moderate | Confounding expected, all known important confounding domains appropriately measured and controlled for; and reliability and validity of measurement of important domains were sufficient, such that we do not expect serious residual confounding |
Selection into the study may have been related to intervention and outcome, but the authors used appropriate methods to adjust for the selection bias; or start of follow‐up and start of intervention do not coincide for all participants, but (a) the proportion of participants for which this was the case was too low to induce important bias; (b) the authors used appropriate methods to adjust for the selection bias; or (c) the review authors are confident that the rate (hazard) ratio for the effect of intervention remains constant over time. |
Intervention status is well‐defined, but some aspects of the assignments of intervention status were determined retrospectively. | ||||
| Serious | Switches in treatment, co‐interventions, or problems with implementation fidelity are apparent and are not adjusted for in the analyses. | Proportions of missing participants differ substantially across interventions; or reasons for missingness differ substantially across interventions; and missing data were addressed inappropriately in the analysis; or the nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. |
The methods of outcome assessment were not comparable across intervention groups; or the outcome measure was subjective (i.e. likely to be influenced by knowledge of the intervention received by study participants) and was assessed by outcome assessors aware of the intervention received by study participants; or error in measuring the outcome was related to intervention status. |
||||
| Critical | Substantial deviations from the intended intervention are present and are not adjusted for in the analysis. | There were critical differences between interventions in participants with missing data that were not, or could not, be addressed through appropriate analysis. | The methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. | ||||
| No information | No information is reported on whether there is deviation from the intended intervention. | No information is reported about missing data or the potential for data to be missing. |
No information is reported about the methods of outcome assessment. |
||||
| Risk of bias judgements in ROBINS‐I: postintervention domains | |||||||
| Judgement | Bias due to deviations from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | |||
| Low | No bias due to deviation from the intended intervention is expected, e.g. if both the intervention and comparator are implemented over a short time period, and subsequent interventions are part of routine medical care, or if the specified comparison relates to initiation of intervention regardless of whether it is continued | Data were reasonably complete; or proportions of and reasons for missing participants were similar across intervention groups; or analyses that addressed missing data are likely to have removed any risk of bias. |
The methods of outcome assessment were comparable across intervention groups; and the outcome measure was unlikely to be influenced by knowledge of the intervention received by study participants or the outcome assessors were unaware of the intervention received by participants; and any error in measuring the outcome is unrelated to intervention status. |
There is clear evidence (usually through examination of a pre‐registered protocol or statistical analysis plan) that all reported results correspond to all intended outcomes, analyses, and subcohorts. | |||
| Moderate | Bias due to deviation from the intended intervention is expected, and switches, co‐interventions, and some problems with intervention fidelity are appropriately measured and adjusted for in the analyses. Alternatively, most (but not all) deviations from intended intervention reflect the natural course of events after initiation of intervention. | Proportions of missing participants differ across interventions; or reasons for missingness differ minimally across interventions; and missing data were not addressed in the analysis. |
The methods of outcome assessment were comparable across intervention groups; and the outcome measure is only minimally influenced by knowledge of the intervention received by study participants; and any error in measuring the outcome is only minimally related to intervention status. |
The outcome measurements and analyses are consistent with an a priori plan; or are clearly defined and both internally and externally consistent; and there is no indication of selection of the reported analysis from among multiple analyses; and there is no indication of selection of the cohort or subgroups for analysis and reporting on the basis of the results. |
|||
| Serious | Switches in treatment, co‐interventions, or problems with implementation fidelity are apparent and are not adjusted for in the analyses. | Proportions of missing participants differ substantially across interventions; or reasons for missing participants differ substantially across interventions; and missing data were addressed inappropriately in the analysis; or the nature of the missing data means that the risk of bias cannot be removed through appropriate analysis. |
The methods of outcome assessment were not comparable across intervention groups; or the outcome measure was subjective (i.e. likely to be influenced by knowledge of the intervention received by study participants) and was assessed by outcome assessors aware of the intervention received by study participants; or error in measuring the outcome was related to intervention status. |
Outcome measurements or analyses are internally or externally inconsistent; or there is a high risk of selective reporting from among multiple analyses; or the cohort or subgroup is selected from a larger study for analysis and appears to be reported on the basis of the results. |
|||
| Critical | Substantial deviations from the intended intervention are present and are not adjusted for in the analysis. | There were critical differences between interventions in participants with missing data that were not, or could not, be addressed through appropriate analysis. |
The methods of outcome assessment were so different that they cannot reasonably be compared across intervention groups. | There is evidence or strong suspicion of selective reporting of results, and the unreported results are likely to be substantially different from the reported results. | |||
| No information | No information is reported on whether there is deviation from the intended intervention. | No information is reported about missing data or the potential for data to be missing. | No information is reported about the methods of outcome assessment. | There is too little information to make a judgement (e.g. if only an abstract is available for the study). | |||
| Source: Sterne 2016 | |||||||
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelraheim 2017 | Wrong study design |
| Anwar 2002 | Wrong study design |
| Bellhouse 2018 | Wrong study design. |
| Burbos 2011 | Wrong study design |
| Cooper 2014 | Wrong study design |
| Doll 2016 | Wrong study design |
| Johnson 2011 | Wrong study design |
| Kang 2013 | Wrong study design |
| Kwon 2007 | Wrong study design |
| Mbah 2015 | Wrong population. |
| Mohamed 2003 | Wrong study design |
| Ramanathan 2011 | Wrong study design |
| Redman 2000 | Wrong study design |
| Robinson 2009 | Wrong study design |
| Smailyte 2015 | Wrong study design |
| Vandborg 2011 | Wrong study design |
GP: general practitioner; RCT: randomised controlled trial
Differences between protocol and review
Types of outcome measures
We deleted some secondary outcomes listed in the review protocol (Cheewakriangkrai 2019), as they seem to be less important: delayed treatment, cancer‐related mortality, and proportion of women diagnosed with stage III to IV endometrial cancer.
Considerations for future updates of the review
We did not identify any studies suitable for inclusion in the review. Therefore it was not relevant to carry out the following procedures: extracting data, assessing the risk of bias in included studies, measuring of treatment effect, assessing heterogeneity between the results of studies, assessing reporting biases using funnel plots, or conducting any subgroup analyses or sensitivity analyses. In future updates of the review, we will employ the following methods if we identify included studies.
Data extraction and management
Two review authors (CC and CK) will independently extract the study characteristics and outcome data from the included studies using Covidence 2019. We plan to note in the 'Characteristics of included studies’ table if outcome data were not reported in a usable way. We plan to resolve any disagreements by consensus or by involving a third review author (KC, PL, or AA). A second review author (PP) will check the study characteristics for accuracy against the study report.
We plan to extract the following data from the included studies.
Author, year of publication, and journal citation (including language)
Country
Setting
Study designs and study methodology: individual randomised controlled trial (RCT)/cluster‐RCT/non‐randomised study (NRS)
Inclusion and exclusion criteria
Operation definitions of delay in referral and delay in treatment
Study population, characteristics, and outcomes: sample size, detailed characteristics including levels of healthcare settings, and types of professionals
Intervention details: any health education interventions performed with the aim of promoting early referral to a specialised centre, single or multifaceted intervention, level of intervention given
Comparison: standard/usual care/as specified in the included studies
Risk of bias (see Appendix 5)
Outcomes: for each outcome, we will extract the outcome definition and unit of measurement (if relevant). For adjusted estimates, we will record variables adjusted for in analyses. The unit of analysis will depend on the type of RCT (see Unit of analysis issues below).
Results: we will extract the number of participants allocated to each intervention group, the total number analysed for each outcome and missing participants. For NRS, we will extract the number of participants categorised in the group to which the intervention was received.
Notes: funding for the study, and notable conflicts of interest of study authors.
If we find more than one publication of the same study, we plan to use the most recent publication for data extraction and plan to collate multiple reports of the same study.
We plan to extract the results as follows.
For time‐to‐event data (survival outcomes), we plan to extract the log of the hazard ratio (log(HR)) and its standard error from the trial reports. If these are not reported, we plan to estimate the log(HR) and its standard error using the methods cited in Parmar 1998.
For dichotomous outcomes (e.g. delayed referral, delayed presentation, and delayed treatment), we plan to extract the number of people in each treatment arm who experienced the outcome of interest and the number of people assessed at the endpoint, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. referral time and presentation time), we plan to extract the final value and standard deviation (SD) of the outcome of interest and the number of people assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (MD) between treatment arms.
Where possible, we plan to extract all data extracted on the basis of intention‐to‐treat analysis, in which participants were analysed in the groups to which they were assigned.
Assessment of risk of bias in included studies
We plan to assess and report on the methodological quality and risk of bias of the included RCTs in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), which recommends the explicit reporting of the following individual elements for RCTs (Appendix 5).
Selection bias: random sequence generation and allocation concealment
Performance bias: blinding of participants and personnel (participants and treatment providers)
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data (i.e. incomplete follow‐up outcomes and treatment‐related complications)
Reporting bias: selective reporting of outcomes
Other potential sources of bias
Two review authors (CC and CK) will independently apply the 'Risk of bias’ tool, resolving any differences by discussion or by appeal to a third review author (KC, PL, or AA). We will judge each item as being at high, low, or unclear risk of bias as set out in the criteria presented in Appendix 5 (Higgins 2017).
We will assess the following biases in cluster‐RCTs:
recruitment bias;
baseline imbalance;
loss of clusters;
incorrect analysis; and
comparability with individually randomised trials (Higgins 2017).
If we identify no RCTs, we will include NRS. We will assess the risk of bias in NRS according to the Cochrane Risk Of Bias In Non‐randomized Studies ‐ of Interventions (ROBINS‐I) tool, and we plan to record results in the template (Sterne 2016). We plan to classify NRS at high risk of bias when they are at 'serious’ risk according to the Cochrane ROBINS‐I tool.
We plan to assess the included studies for their risk of bias based on the following seven domains in the ROBINS‐I tool (Appendix 6).
Bias due to confounding
Bias in the selection of participants into the study
Bias in the classification of interventions
Bias due to deviations from the intended intervention
Bias due to missing data
Bias in measurement of outcomes
Bias in the selection of the reported result
We plan to provide a quote from the study report or a statement, or both, as justification for the judgment for each item in the ’Risk of bias’ table. When interpreting treatment effects and meta‐analyses, we plan to take into account the risk of bias for the studies that contribute to that outcome. Where information on the risk of bias relates to unpublished data or correspondence with a study author, we will note this in the table.
Measures of treatment effect
We will apply the following measures for the effect of treatment.
For time‐to‐event outcomes (e.g. overall and disease‐free survival), we plan to apply the hazard ratio (HR) with 95% confidence interval (CI).
For dichotomous outcomes (e.g. delayed referral, delayed presentation, delayed treatment, and death (if not possible to treat as a time‐to‐event outcome and obtain an HR)), we plan to analyse data based on the number of events and the number of people assessed in the intervention and comparison groups. We plan to use these to calculate the RR and 95% CI.
For continuous outcomes (e.g. quality of life measures, cost‐effectiveness, and satisfaction score), we plan to analyse data based on the mean, SD, and number of people assessed for both the intervention and comparison groups to calculate the MD between treatment arms with a 95% CI. If the MD is reported without individual group data, we plan to use this to report the study results. If more than one study measures the same outcome using different tools, we plan to calculate the standardised mean difference (SMD) and 95% CI using the inverse‐variance method.
Unit of analysis issues
We will include studies where individual people were randomised and cluster‐randomised studies. For individual RCTs, the unit of analysis will be per woman randomised. As we plan to recruit cluster‐randomised trials to this review, we will avoid unit of analysis errors by performing meta‐analysis (if appropriate) using effect estimates and their standard errors (SEs) where the trial has been correctly analysed.
On the other hand, for a trial without appropriate adjustment of clustering, we will approximate the correct analyses based on the 'inflating standard error' approach cited in (Higgins 2019a), as follows.
Calculating the design effect, which is 1 + (M − 1) ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient (note: for unknown ICC, the estimated ICC will be (a) yielded from either a similar study, or (b) assume an ICC of 0.10 (Campbell 2001))
Multiplying SE of the effect estimate by the square root of the design effect (note: we will apply the natural log form for dichotomous and time‐to‐event outcomes)
Performing meta‐analysis using the generic inverse‐variance method in Review Manager 5 (Review Manager 2014)
In NRS, the unit of analysis is the participant(s) receiving the intervention. We will follow the methods stated in the Cochrane Handbook for Systematic Reviews of Interventions for carrying out the calculations or determining the statistical outcomes (Reeves 2019). In a study with multiple intervention groups, where possible, we will combine all relevant experimental intervention groups into a single group to create a single pair wise comparison (Higgins 2019b).
Dealing with missing data
We will report the percentage of observations with missing data in each included study. We will contact the original investigators to request missing data. If we cannot contact the investigators or are unable to obtain the missing data, we will analyse only the available data and not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We will clinically assess heterogeneity by visual inspection of the forest plots. We also plan to assess statistical heterogeneity in each meta‐analysis using the I² statistic (Higgins 2003), and Chi² test. We will regard heterogeneity as substantial if the I² statistic is greater than 50%, or there is a low P value (< 0.10) in the Chi² test for heterogeneity (Deeks 2001; Deeks 2019). If there is substantial statistical heterogeneity, we will carry out subgroup analyses to assess the differences between the included studies. However, if there is clinical, methodological, or considerable statistical heterogeneity (I² statistic greater than 75%) across included studies, we plan to apply a narrative approach to data synthesis (Deeks 2019).
Assessment of reporting biases
We plan to examine funnel plots corresponding to the meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias if we identify more than 10 studies. We will assess funnel plot asymmetry visually; if we identify the asymmetry of funnel plots, we plan to perform exploratory analyses to investigate the possible impact (Sterne 2011).
Data synthesis
We will use the random‐effects model with an inverse variance weighting for all meta‐analyses (DerSimonian 1986). We plan to perform statistical analysis using Review Manager 5 (Review Manager 2014).
For time‐to‐event outcome (e.g. overall and disease‐free survival), we will pool HRs using the generic inverse‐variance method.
For any dichotomous outcome (e.g. delay in referral or delay in treatment), we will calculate the RRs for each study, which we will then pool.
For continuous outcome (e.g. satisfaction score), we will pool the MDs between the treatment arms if all studies measure the outcome on the same scale; otherwise, we will pool SMDs.
Subgroup analysis and investigation of heterogeneity
We will carry out subgroup analysis for the following factors to assess the impact of the following variables on the effect size.
Single or multifaceted/integrated intervention
Income status of the country (e.g. low‐ and middle‐income countries versus high‐income countries)
Characteristics of the population (e.g. disadvantaged or advantaged population or general population versus minority groups)
We plan to assess subgroup differences by interaction tests available within Review Manager 5 (Review Manager 2014). We plan to report the results of subgroup analyses quoting the Chi² statistic and P value, the interaction test, and I² statistic value.
Sensitivity analysis
We plan to perform a sensitivity analysis in order to assess the effect of the following factors on the primary outcomes.
Repeating the analysis excluding unpublished studies (if any)
Repeating the analysis excluding RCTs judged to be at high or unclear risk of bias for allocation concealment (in case of RCT available)
Repeating the analysis excluding studies that were not originally adjusted for clustering (in case of cluster‐RCT available)
Repeating the analysis excluding NRS judged to be a high risk of bias according to the Cochrane ROBINS‐I tool (in case of no RCT available)
Main outcomes of 'Summary of findings' table for assessing the quality of the evidence
In future updates of the review, we will employ the following methods if we identify included studies
We will present the results of the meta‐analysis for the outcomes as outlined in the Types of outcome measures section and we planned to use GRADEpro GDT to develop the 'Summary of findings' table. We will present the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, and publication bias) but also to external validity such as directness of results (Langendam 2013). We will apply the GRADE checklist and GRADE Working Group quality of evidence definitions (Meader 2014).
We plan to downgrade the evidence from 'high' quality by one level for each serious limitation (or by two levels for each very serious limitation), as follows
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
Contributions of authors
Chalong Cheewakriangkrai: conceived the review question; developed, co‐ordinated, and completed the review Chumnan Kietpeerakool: conceived the review question; developed and completed the review Apiwat Aue‐aungkul: developed and completed the review Kittipat Charoenkwan: developed and completed the review Porjai Pattanittum: editing and advisory role Denny John: editing and advisory role Pisake Lumbiganon: co‐ordinated the development of the review, editing and advisory role
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, Faculty of Medicine, Chiang Mai University, Thailand.
Department of Obstetrics and Gynaecology, Faculty of Medicine, Khon Kaen University, Thailand.
Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Thailand.
Campbell South Asia, New Delhi, India.
Cochrane Thailand, Thailand.
External sources
Thailand Research Fund (Distinguished Professor Award), Thailand.
Long‐term Institutional Development HUBs (LID‐HUBs), the Human Reproduction Programme (HRP) Alliance for Research Capacity Strengthening, Department of Reproductive Health and Research, World Health Organization, Geneva, Switzerland.
Declarations of interest
Chalong Cheewakriangkrai: none known Chumnan Kietpeerakool: none known Apiwat Aue‐aungkul: none known Kittipat Charoenkwan: none known Porjai Pattanittum: none known Denny John: none known Pisake Lumbiganon: none known
New
References
References to studies excluded from this review
Abdelraheim 2017 {published data only}
- Abdelraheim AR, Khairy M, Mohammed M, Lawrence A. Two week waits: what are we waiting for?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;215:112‐7. [DOI] [PubMed] [Google Scholar]
Anwar 2002 {published data only}
- Anwar T, Kiwanuka AI, Watson A. Referral to treatment time interval for patients with endometrial cancer in a district general hospital. Journal of Obstetrics and Gynaecology 2002;22(5):551‐2. [DOI] [PubMed] [Google Scholar]
Bellhouse 2018 {published data only}
- Bellhouse S, McWilliams L, Firth J, Yorke J, French DP. Are community‐based health worker interventions an effective approach for early diagnosis of cancer? A systematic review and meta‐analysis. Psycho‐oncology 2018;27(4):1089‐99. [DOI] [PubMed] [Google Scholar]
Burbos 2011 {published data only}
- Burbos N, Musonda P, Rufford B. Diagnostic performance of urgent referrals for suspected gynaecological malignancies. Archives of Gynecology and Obstetrics 2011;284(6):1495‐500. [DOI] [PubMed] [Google Scholar]
Cooper 2014 {published data only}
- Cooper CP, Gelb CA, Rodriguez J, Hawkins NA. Promoting gynecologic cancer awareness at a critical juncture‐‐where women and providers meet. Journal of Cancer Education 2014;29(2):247‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doll 2016 {published data only}
- Doll KM, Meng K, Gehrig PA, Brewster WR, Meyer AM. Referral patterns between high‐ and low volume centers and associations with uterine cancer treatment and survival: a population‐based study of Medicare, Medicaid, and privately insured women. American Journal of Obstetrics and Gynecology 2016;215(4):447.e1‐447.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2011 {published data only}
- Johnson N, Miles T, Bailey D, Tylko‐Hill K, Das N, Ahson G, et al. Delays in treating endometrial cancer in the South West of England. British Journal of Cancer 2011;104(12):1836‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2013 {published data only}
- Kang MY, Sykes P, Herbison PY, Petrich S. Retrospective analysis on time frames of referral, diagnosis and treatment of patients with endometrial carcinomas in Dunedin Hospital, 2008‐2011. New Zealand Medical Journal 2013;126(1384):84‐95. [PubMed] [Google Scholar]
Kwon 2007 {published data only}
- Kwon JS, Carey MS, Cook EF, Qiu F, Paszat LF. Addressing wait times for endometrial cancer surgery in Ontario. Journal of Obstetrics and Gynaecology Canada 2007;29(12):982‐7. [DOI] [PubMed] [Google Scholar]
Mbah 2015 {published data only}
- Mbah O, Ford JG, Qiu M, Wenzel J, Bone L, Bowie J, et al. Mobilizing social support networks to improve cancer screening: the COACH randomized controlled trial study design. BMC Cancer 2015;15:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2003 {published data only}
- Mohamed H, Nair P. One‐stop clinic for postmenopausal bleeding at district general hospital: does it have a role?. Journal of Obstetrics and Gynaecology 2003;23(2):182‐4. [DOI] [PubMed] [Google Scholar]
Ramanathan 2011 {published data only}
- Ramanathan SA, Baratiny G, Stocks NP, Searles AM, Redford RJ. General practitioner referral patterns for women with gynaecological symptoms: a randomised incomplete block study design. Medical Journal of Australia 2011;195(10):602‐6. [DOI] [PubMed] [Google Scholar]
Redman 2000 {published data only}
- Redman CW. An audit of the management of uterine malignancy within the West Midlands. West Midlands Gynaecological Oncology Group. BJOG 2000;107(4):552‐5. [DOI] [PubMed] [Google Scholar]
Robinson 2009 {published data only}
- Robinson KM, Ottesen B, Christensen KB, Krasnik A. Diagnostic delay experienced among gynecological cancer patients: a nationwide survey in Denmark. Acta Obstetricia et Gynecologica Scandinavica 2009;88(6):685‐92. [DOI] [PubMed] [Google Scholar]
Smailyte 2015 {published data only}
- Smailyte G, Jasilionis D, Vincerzevskiene I, Krilaviciute A, Ambrozaitiene D, Stankuniene V, et al. Educational differences in incidence of cancer in Lithuania, 2001‐2009: evidence from census‐linked cancer registry data. European Journal of Cancer Prevention 2015;24(3):261‐6. [DOI] [PubMed] [Google Scholar]
Vandborg 2011 {published data only}
- Vandborg MP, Christensen RD, Kragstrup J, Edwards K, Vedsted P, Hansen DG, et al. Reasons for diagnostic delay in gynecological malignancies. International Journal of Gynecological Cancer 2011;21(6):967‐74. [DOI] [PubMed] [Google Scholar]
Additional references
Allgar 2005
- Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. British Journal of Cancer 2005;92(11):1959‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amant 2018
- Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. International Journal of Gynaecology and Obstetrics 2018;143 Suppl 2:37‐50. [DOI] [PubMed] [Google Scholar]
Austoker 2009
- Austoker J, Bankhead C, Forbes LJ, Atkins L, Martin F, Robb K, et al. Interventions to promote cancer awareness and early presentation: systematic review. British Journal of Cancer 2009;101(Suppl 2):S31‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bani‐Irshaid 2011
- Bani‐Irshaid I, Al‐Sumadi A. Histological findings in women with postmenopausal bleeding: Jordanian figures. Eastern Mediterranean Health Journal 2011;17(7):582‐6. [PubMed] [Google Scholar]
Beesley 2010
- Beesley VL, Janda M, Eakin EG, Auster JF, Chambers SK, Aitken JF, et al. Gynecological cancer survivors and community support services: referral, awareness, utilization and satisfaction. Psycho‐oncology 2010;19:54‐61. [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
Campbell 2001
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20(3):391‐9. [PUBMED: 11180309] [DOI] [PubMed] [Google Scholar]
Car 2016
- Car LT, Papachristou N, Urch C, Majeed A, El‐Khatib M, Aylin P, et al. Preventing delayed diagnosis of cancer: clinicians' views on main problems and solutions. Journal of Global Health 2016;6(2):020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherny 2015
- Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti‐cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO‐MCBS). Annals of Oncology 2015;26(8):1547‐73. [DOI] [PubMed] [Google Scholar]
Clarke 2020
- Clarke MA, Long BJ, Sherman ME, Lemens MA, Podratz KC, Hopkins MR, et al. A prospective clinical cohort study of women at increased risk for endometrial cancer. Gynecologic Oncology 2020;156(1):169‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2016
- Colombo N, Creutzberg C, Amant F, Bosse T, González‐Martín A, Ledermann J, et al. ESMO‐ESGO‐ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow‐up. International Journal of Gynecological Cancer 2016;26(1):2‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cote 2015
- Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali‐Fehmi R. The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer Epidemiology, Biomarkers & Prevention 2015;24(9):1407‐15. [DOI] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 21 January 2019. Melbourne, Australia: Veritas Health Innovation, 2019.
Crosbie 2014
- y5g0Crosbie E, Morrison J. The emerging epidemic of endometrial cancer: time to take action. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.ED000095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks J, Altman D, Bradburn M. Statistical Methods for Examining Heterogeneity and Combining Results From Several Studies in Meta‐Analysis. 2nd Edition. London: BMJ, 2001. [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dolly 2016
- Dolly D, Mihai A, Rimel BJ, Fogg L, Rotmensch J, Guirguis A, et al. A delay from diagnosis to treatment Is associated with a decreased overall survival for patients with endometrial cancer. Frontiers in Oncology 2016;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elit 2014
- Elit LM, O'Leary EM, Pond GR, Seow HY. Impact of wait times on survival for women with uterine cancer. Journal of Clinical Oncology 2014;32(1):27‐33. [DOI] [PubMed] [Google Scholar]
Escoffery 2002
- Escoffery CT, Blake GO, Sargeant LA. Histopathological findings in women with postmenopausal bleeding in Jamaica. West Indian Medical Journal 2002;51(4):232‐5. [PubMed] [Google Scholar]
Forsetlund 2009
- Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O'Brien MA, Wolf F, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giguère 2012
- Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD004398.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gredmark 1995
- Gredmark T, Kvint S, Havel G, Mattsson LA. Histopathological findings in women with postmenopausal bleeding. British Journal of Obstetrics and Gynaecology 1995;102(2):133‐6. [DOI] [PubMed] [Google Scholar]
Greimel 2011
- Greimel E, Nordin A, Lanceley A, Creutzberg CL, Poll‐Franse LV, Radisic VB, et al. Psychometric validation of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Endometrial Cancer Module (EORTC QLQ‐EN24). European Journal of Cancer 2011;47(2):183‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019a
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Jamison 2013
- Jamison PM, Noone AM, Ries LA, Lee NC, Edwards BK. Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiology, Biomarkers & Prevention 2013;22:233‐41. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 1995
- Lee WH, Tan KH, Lee YW. The aetiology of postmenopausal bleeding ‐ a study of 163 consecutive cases in Singapore. Singapore Medical Journal 1995;36(2):164‐8. [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gotzsche P, Ioannidis J. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339:2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansell 2011
- Mansell G, Shapley M, Jordan JL, Jordan K. Interventions to reduce primary care delay in cancer referral: a systematic review. British Journal of General Practice 2011;61(593):e821‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2003
- Morrison J, Gillespie S, MacKenzie IZ. 'Two week wait' standards for suspected gynaecological malignancy. On target, but missing the point?. Journal of the British Menopause Society 2003;9:170‐2. [DOI] [PubMed] [Google Scholar]
Musa 2017
- Musa J, Achenbach CJ, O'Dwyer LC, Evans CT, McHugh M, Hou L, et al. Effect of cervical cancer education and provider recommendation for screening on screening rates: a systematic review and meta‐analysis. PLoS One 2017;12(9):e0183924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neal 2015
- Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon‐Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. British Journal of Cancer 2015;112 Suppl 1:S92‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence (NICE). Suspected cancer: recognition and referral (NICE guideline [NG12]). nice.org.uk/guidance/ng12 (accessed prior to 21 January 2019).
O'Brien 2007
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard‐Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Leary 2013
- O'Leary E, Elit L, Pond G, Seow H. The wait time creep: changes in the surgical wait time for women with uterine cancer in Ontario, Canada, during 2000‐2009. Gynecologic Oncology 2013;131(1):151‐7. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Reeves 2019
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non‐randomized studies on intervention effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2001
- Rose PW, Watson E, Yudkin P, Emery J, Murphy M, Fuller A, et al. Referral of patients with a family history of breast/ovarian cancer ‐ GPs' knowledge and expectations. Family Practice 2001;18(5):487‐90. [DOI] [PubMed] [Google Scholar]
Rose 2015
- Rose PW, Rubin G, Perera‐Salazar R, Almberg SS, Barisic A, Dawes M, et al. Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: a primary care vignette survey. BMJ Open 2015;5(5):e007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saso 2011
- Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem‐Maghami S. Endometrial cancer. BMJ 2011;343:d3954. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Shalowitz 2015
- Shalowitz DI, Vinograd AM, Giuntoli RL. Geographic access to gynecologic cancer care in the United States. Gynecologic Oncology 2015;138(1):115‐20. [DOI] [PubMed] [Google Scholar]
Shalowitz 2017
- Shalowitz DI, Epstein AJ, Buckingham L, Ko EM, Giuntoli RL 2nd. Survival implications of time to surgical treatment of endometrial cancers. American Journal of Obstetrics and Gynecology 2017;216(3):268.e1‐18. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strohl 2016
- Strohl AE, Feinglass JM, Shahabi S, Simon MA. Surgical wait time: a new health indicator in women with endometrial cancer. Gynecologic Oncology 2016;141(3):511‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swann 2018
- Swann R, McPhail S, Witt J, Shand B, Abel GA, Hiom S, et al. Diagnosing cancer in primary care: results from the National Cancer Diagnosis Audit. British Journal of General Practice 2018;68(666):e63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Cancer Control: Prevention. Geneva: WHO Press, 2007. [Google Scholar]
Zhou 2018
- Zhou Y, Mendonca SC, Abel GA, Hamilton W, Walter FM, Johnson S, et al. Variation in 'fast‐track' referrals for suspected cancer by patient characteristic and cancer diagnosis: evidence from 670 000 patients with cancers of 35 different sites. British Journal of Cancer 2018;118(1):24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cheewakriangkrai 2019
- Cheewakriangkrai C, Kietpeerakool C, Aue‐aungkul A, Charoenkwan K, Pattanittum P, John D, et al. Health education interventions to promote early presentation and referral for women with symptoms of endometrial cancer. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD013253] [DOI] [PMC free article] [PubMed] [Google Scholar]


