Abstract
The review demonstrates that control of posture and locomotion is provided by systems across the caudal-to-rostral extent of the neuraxis. A common feature of the neuroanatomic organization of the postural and locomotor control systems is the presence of key nodes for convergent input of multisensory feedback in conjunction with efferent copies of the motor command. These nodes include the vestibular and reticular nuclei and interneurons in the intermediate zone of the spinal cord (Rexed’s laminae VI–VIII). This organization provides both spatial and temporal coordination of the various goals of the system and ensures that the large repertoire of voluntary movements is appropriately coupled to either anticipatory or reactive postural adjustments that ensure stability and provide the framework to support the intended action. Redundancies in the system allow adaptation and compensation when sensory modalities are impaired. These alterations in behavior are learned through reward- and error-based learning processes implemented through basal ganglia and cerebellar pathways respectively. However, neurodegenerative processes or lesions of these systems can greatly compromise the capacity to sufficiently adapt and sometimes leads to maladaptive changes that impair movement control. When these impairments occur, the risk of falls can be significantly increased and interventions are required to reduce morbidity.
INTRODUCTION
Control of balance, posture, and locomotion is provided by systems across the caudal-to-rostral extent of the neuraxis. These systems are organized to meet five primary goals: (1) maintain vertical support against gravity; (2) maintain balance by keeping the center of mass within the base of support; (3) provide postural stability that is appropriate for the task; (4) control foot trajectory to ensure safe ground clearance; and (5) attenuate the transmission of accelerations to the head in order to stabilize the visual and vestibular apparatus (Winter, 1989, 1993; Rothwell, 1994). These goals must be achieved in response to unexpected displacements of body segments, termed reactive postural control, or in anticipation of changes in posture and balance related to volitional actions or expected disturbances to balance, termed anticipatory postural control.
Figure 1.1 presents a generalized model of the components required for anticipatory and reactive control of balance, support, and postural stability. This model emphasizes that motor commands that act upon the musculoskeletal system must be coupled with commands to locomotor and postural control centers. Sensory feedback from self-generated or imposed movement can modulate central motor, locomotor, and postural commands based on the difference between the sensed and desired outcome of movement. The core components of the nervous system that accomplish these goals include input from sensory systems (visual, vestibular, and somatosensory systems) that provide feedback about the location and movement of objects in the external environment, orientation and motion of the head (body) in space, and the relative position and motion of the body segments, higher-order supraspinal centers that plan, initiate, and execute movement based on goals, reward, and multisensory input, and lower-order subcortical centers (brainstem nuclei and spinal cord) that integrate motor commands with multisensory feedback to ensure the volitional and automatic actions are coupled to appropriate postural adjustments. The components and interconnections of the nervous system that contribute to vertical support, postural stability, and locomotion are summarized in Figure 1.2.
Fig. 1.1.
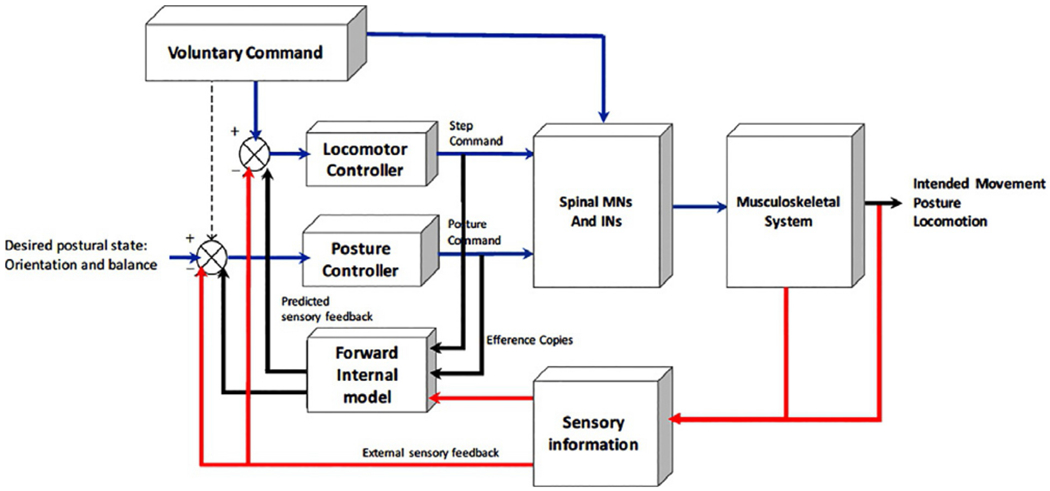
General model of the organization of the control of posture, locomotion, and voluntary movement. This model emphasizes that the voluntary command for movement (e.g., step initiation) must be coupled with parallel commands to a locomotor and postural control network. Efference copies of the locomotor and postural commands are relayed to a forward internal model (internal body schema), which, in conjunction with sensory information, is used to compare the desired postural/movement state with the perceived postural/movement state. Differences are used to update the locomotor and postural controller commands. INs, interneurons; MNs, motoneurons. (Adapted from Mille et al. (2012).)
Fig. 1.2.
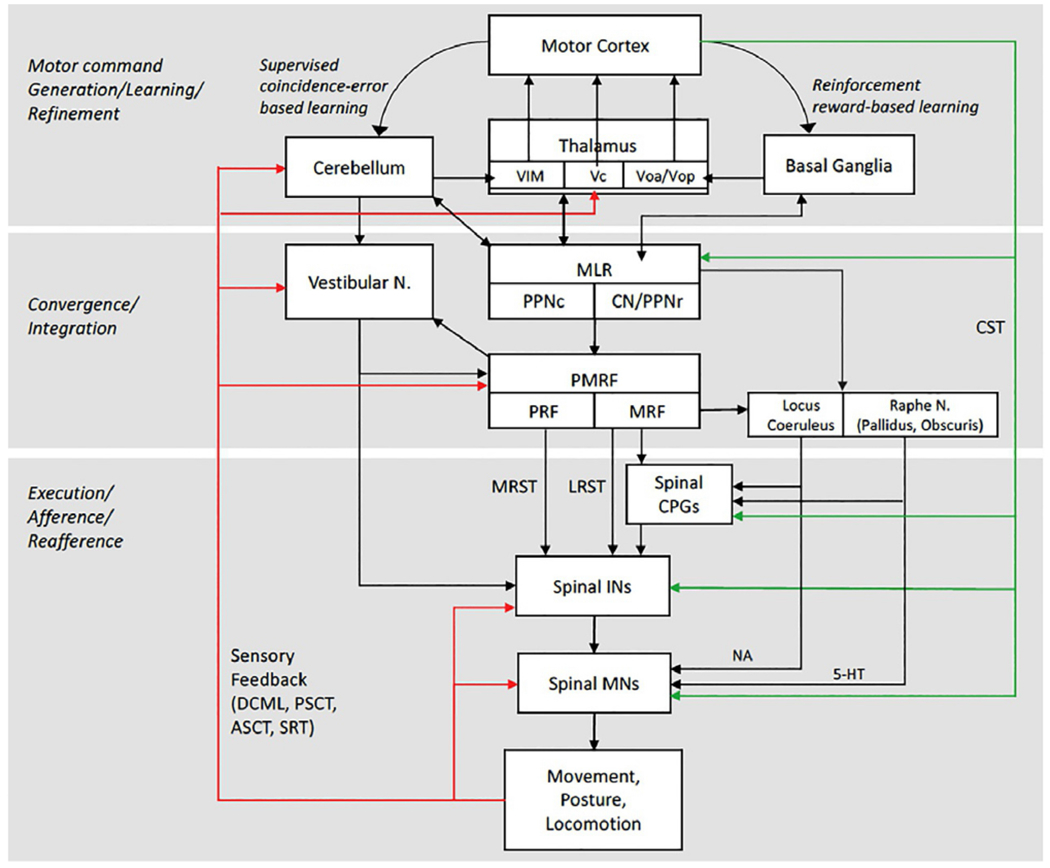
Model of the core components of the nervous system, and their connectivity, that contribute to the control of balance, vertical support, posture, and locomotion and their integration with voluntary motor commands. 5-HT, serotonin; ASCT, anterior spinocerebellar tract; CPGs, central pattern generators; CST, corticospinal tract; DCML, dorsal column medial lemniscal tract; INs, interneurons; LRST, lateral (medullary) reticulospinal tract; MLR, mesencephalic locomotor region; MNs, motoneurons, MRF, medullary reticular formation; MRST, medial (pontine) reticulospinal tract; NA, noradrenaline (norepinephrine); PMRF, pontomedullary reticular formation; PPNc, caudal region of pedunculopontine nucleus; PPNr, rostral region of pedunculopontine nucleus; PRF, pontine reticular formation; PSCT, posterior spinocerebellar tract; SRT, spinoreticular tract; Vc, ventrocaudal nucleus of the thalamus; VIM, ventral intermediate nucleus of the thalamus; Voa/Vop, ventralis oralis anterior and ventralis oralis posterior of the thalamus.
This review will describe and discuss the functional neuroanatomy and roles of the principal sensory systems, cortical and subcortical structures, ascending and descending pathways and reflexes that provide reactive or anticipatory control of support, balance, and postural stability during bipedal human standing and locomotion.
SENSORY SYSTEMS AND REFLEX PATHWAYS FOR REACTIVE POSTURAL CONTROL
Sensory feedback from the visual, vestibular, and somatosensory systems provides information to the nervous system that is used to establish an internal schema of the orientation and motion of the body and its relationship to the external environment. Each sensory modality provides functionally specific information that contributes to the internal schema. Yet, a seminal feature of the sensory control of posture and locomotion is that each sensory modality does not function independently. Instead, there is extensive convergence and integration of multisensory input, at multiple levels of the neuraxis, on to regions that receive motor (or locomotor) commands and project to motor and premotor neurons in the spinal cord (Bronstein, 2016). This convergence allows the nervous system to modulate output depending upon the reliability and salience of each input and the goals of the intended movement. It also provides the capacity to adapt and compensate for corruption or loss of input from one modality. The sensory receptors, movement signals encoded, and pathways mediating feedback are reviewed below.
PROPRIOCEPTIVE AND EXTEROCEPTIVE RECEPTORS
Knowledge of the relative locations, orientations, and rate of movement of the body segments and their relationship to the base of support is conveyed to the nervous system through proprioceptive and cutaneous receptors. The term proprioception refers to the unconscious processing of sensory information pertaining to body or limb position and motion in space that is derived from receptors in the muscles, tendons, and joint capsules (Konczak et al., 2009). Kinesthesia is the term used to describe the conscious awareness of the body or limb position in space. Dysfunction or loss of proprioception can significantly compromise the control of movement and regulation of balance and posture and requires compensatory upregulation of other sensory systems (vision, vestibular sense).
Muscle spindles
Signals encoding muscle length and rate of muscle stretch are provided by muscle spindles embedded in skeletal muscles. This feedback modulates muscle activity and informs the nervous system of the relative position and motion of the joint. Fast-conducting afferent feedback from muscle spindles plays a critical role in reactive postural control (e.g., stretch reflexes), regulating the stiffness of the joint, providing kinesthetic sense, and has a strong influence on the phase and pattern of locomotion.
The muscle spindle complex consists of intrafusal muscle fibers that run in parallel and are attached to either end of an adjacent extrafusal muscle fiber (Fig. 1.3A). Each intrafusal fiber has contractile regions at each end that are innervated by specialized fusimotor efferents from the ventral horn of the spinal cord (gamma static, gamma dynamic, and beta dynamic) and a noncontractile central region that is innervated by primary and secondary sensory endings that wind around the fiber. Stretch of the central region provides the deformation stimulus that depolarizes and fires the sensory endings. Three types of intrafusal fibers are contained within the spindle complex: long nuclear bag1 and bag2 fibers and shorter nuclear chain fibers. Long nuclear bag1 fibers are innervated by axons from dynamic fusimotor efferents. Dynamic fusimotor discharge contracts the polar ends of the bag1 fibers and markedly increases the sensitivity to changes in muscle length (stretch velocity) (Fig. 1.3B) (for review, see Mileusnic et al., 2006). Bag2 and chain fibers are innervated by static fusimotor efferents that provide control of length sensitivity.
Fig. 1.3.
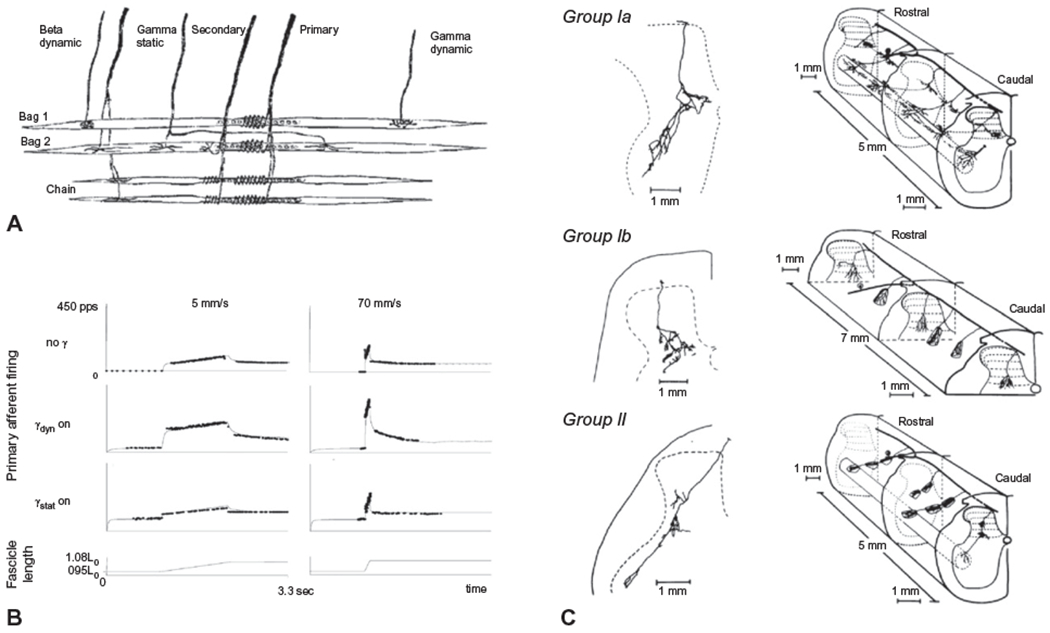
(A) Diagram of a biologic muscle spindle and a model of spindle structure. A muscle spindle consists of three types of intrafusal fibers that receive fusimotor inputs (gamma static and dynamic) while giving rise to primary (Ia) and secondary (II) afferents. (B) Model of spindle output during 6-mm whole-muscle ramp stretches. Primary afferent responses at two different velocities (5 and 70 mm/s) are presented either in the absence of fusimotor stimulation (no gamma), with dynamic fusimotor stimulation at 70 pps, or during constant static fusimotor stimulation at 70 pps. Solid thin lines represent model output; experimental data are shown as dots. (C) Examples of the pattern of termination of single group Ia, group Ib, and group II muscle afferents from the triceps surae muscle in the cat. ((A) and (B) adapted from Mileusnic MP, Brown IE, Lan N, et al. (2006) Mathematical models of proprioceptors. I. Control and transduction in the muscle spindle. J Neurophysiol 96: 1772–1788, with permission from the American Physiological Society; (C) reproduced from Brown (1982) The dorsal horn of the spinal cord. Q J Exp Physiol 67: 193–212, with permission from John Wiley.)
Primary muscle afferents (group Ia) innervate all three types of intrafusal fibers and thus the discharge rate of Ia afferents encodes both muscle stretch velocity and length. The response properties of primary endings are nonlinear, being most sensitive to small displacements of the muscle fascicle but less sensitive to larger displacements. In contrast, secondary muscle afferents (group II) innervate the static intrafusal fibers and demonstrate a relatively linear increase in discharge in relation to muscle length. Muscle spindle afferents project to the spinal cord, bifurcate before entering the dorsal horn, and send ascending and descending branches via the dorsal columns. Branches from these axons enter the dorsal horn and terminate predominantly in Rexed’s laminae VI, VII, and IX (Brown, 1982) (Fig. 1.3C). Group Ia afferents have relatively large-diameter myelinated axons with conduction velocities in the range of 72–120 m/s. The terminals of Ia afferents make monosynaptic excitatory connections with alpha motoneurons of the homonymous muscle group and Ia inhibitory interneurons with connections to motoneurons of the antagonist muscle. The excitatory connections between the Ia afferent and alpha motoneurons form the basis of the short-latency stretch reflex (tendon jerk) and provide rapid reactive adjustments to disturbances in posture or intended movement. Group II afferents are slower conducting (42–72 m/s) than la axons and terminate on interneurons that provide either excitatory or inhibitory input to motoneurons in the ventral horn (Bannatyne et al., 2006). The inhibitory interneurons project both ipsilaterally and contralaterally. Muscle spindle afferents also project rostrally to supraspinal centers (see section on ascending pathways, below). These ascending projections provide the input required for the perception of muscle/joint position and error feedback for long-latency modulation or correction of posture and movement.
Fusimotor activity, and corresponding spindle sensitivity and gain, is controlled by descending supraspinal input from corticospinal or reticulospinal tracts or premotor interneuron networks within the spinal cord. Coactivation of fusimotor and alpha motoneurons ensures that the sensitivity of the spindle is maintained during muscle contractions that shorten fiber length. Furthermore, the fusimotor system increases the dynamic range of the spindle output and thus provides the capacity to modulate the gain of the stretch reflexes in response to intended or imposed displacements of the body (Fig. 1.3B) (Mileusnic et al., 2006). Muscles that provide vertical support and balance control (e.g., tibialis anterior, soleus, and quadriceps) have Ia sensory endings that can be tuned to be exquisitely sensitive to small changes in muscle length or slow rates of stretch (e.g., 0.1–1.5 Hz) that occur during quasistationary tasks such as quiet standing (Day et al., 2017). This allows the proprioceptive system to rapidly respond to subtle changes in anterior or posterior sway. During locomotion, sensitivity of the spindles in the same muscles is dramatically modulated in a phase-dependent manner and plays a prominent role in regulating the rhythm, phase, and muscle activation patterns of the locomotor cycle. For example, group I and II afferent feedback evoked by stretch of the hip flexors (e.g., sartorius muscle) is important for signaling the transition between the end of stance and initiation of swing (Lam and Pearson, 2001).
Golgi tendon organs
Golgi tendon organs (GTOs) are mechanoreceptors that provide output encoding the level of tensile load applied to the tendon (for review, see Mileusnic and Loeb, 2006). For this reason, GTOs, particularly those in the lower-limb extensors, are critical for sensing the forces exerted to resist imposed loads or the force of gravity acting on the body and regulating extensor activity required for maintaining vertical support and postural stability. These receptors are located in series between the muscle fibers and the collagen strands that compose the tendon. Each GTO is innervated by a single myelinated Ib afferent. Muscle contraction straightens the collagen fibers surrounding the GTO and compresses and depolarizes the sensory ending. Ib axons are fast conducting (72–120 m/s), bifurcate when entering the spinal cord, and send branches rostrally and caudally via the dorsal columns. Branches that enter the gray matter principally terminate in Rexed’s laminae V–VII (Fig. 1.3C) and innervate premotor interneurons.
The reflex actions evoked by activation of Ib afferents can be quite complex. The classic GTO Ib reflex (termed autogenic inhibition) is characterized disynaptic inhibition of synergistic motoneurons, via Ib inhibitory interneurons, and di- or trisynaptic excitation of antagonist motoneurons. The ascending and descending branches of the Ib afferent axon allow these effects to be elicited at multiple joints. Unlike intrafusal muscle fibers, GTOs do not receive efferent projections that can modulate the sensitivity of the receptor. Nonetheless, the function of the Ib inhibitory reflex pathway can be dramatically modulated in a context- and task-dependent manner. For example, Ib reflex inhibition is reduced during standing compared with sitting (Faist et al., 2006) and further attenuated during situations of postural threat (e.g., unstable or small support surface) (Horslen et al., 2017).
GTO Ib reflexes are modulated in both strength and sign (switch from motoneuron suppression to excitation) depending upon the phase of locomotion (Duysens et al., 2000). During the stance of gait, stimulation of Ib afferents innervating the ankle extensors facilitates activity in extensor motoneurons whereas stimulation during swing evokes inhibition in the same muscles. This “reflex reversal” of the Ib reflex response demonstrates that interneuron networks, other than the Ib inhibitory interneurons, contribute to GTO reflex. Load-related feedback from the lower-limb extensors also plays an important role in regulating the locomotor rhythm and can evoke a rapid transition from flexion to extension or prolong the extension phase of the cycle (Frigon and Gossard, 2010).
Joint receptors
Sense of joint position is also derived from receptors lining the synovial joints. These receptors include Golgi endings (type III) in the joint ligaments (similar to tendon organs), Ruffini endings (type I), and paciniform-like corpuscles (type II) located in the joint capsule and free nerve endings (type IV) innervating the connective tissue (Rothwell, 1994). Golgi, Ruffini endings, and free nerve endings are innervated by group I, II, and III afferent fibers respectively. The concentration of receptors is highest near the extremes of the joint range of motion; thus, these receptors are considered to contribute little to proprioceptive feedback within the normal range of motion (Burke et al., 1988). However, distension of the capsule, such as occurs following effusion of the joint, can markedly increase the firing of joint afferents (Lundberg et al., 1978; Grigg and Hoffman, 1982). Group II knee joint afferents excite premotor interneurons (group II and Ib interneurons) in the spinal cord that function to suppress activity in the extensor muscles. This syndrome of arthrogenic muscle inhibition can lead to extensor muscle weakness, vertical collapse, and falls.
Cutaneous receptors
Cutaneous receptors of the feet provide feedback about the distribution of pressure beneath the foot (base of support), the direction, level, and rate of load bearing (pressure), and the compliance and geometry of the support surface. Four receptor structures of the glabrous skin provide this information: Merkel discs, Meissner corpuscles, Pacinian corpuscles, and Ruffini endings. Merkel discs and Meissner corpuscles are located near the surface of the skin at the dermal–epidermal junction, are highly sensitive to localized vertical pressure, and contribute to the sense of light touch. Afferents innervating Merkel discs show an initial high rate of discharge followed by slower, irregular and sustained firing (slow-adapting afferents) (Rothwell, 1994). In contrast, Meissner’s corpuscles respond to both vertical and shear forces applied to the skin and quickly adapt to maintained pressure (fast-adapting afferents).
The receptive fields of afferents innervating Merkel discs and Meissner corpuscles are relatively small, thus these receptors provide localized, rapid, and sustained information about the compliance and contours of the support surface. In contrast, Ruffini endings and Pacinian corpuscles are in the deep layers of the skin and their afferents have large receptive fields. Pacinian corpuscles are highly sensitive to rapid indentation of the skin (thresholds as low as 10 μm), are innervated by rapidly adapting afferents (fast-adapting afferents) and can respond to high-frequency vibrations in the 300–400-Hz range.
Ruffini endings are less sensitive than Pacinian corpuscles, are innervated by slowly adapting afferents (slow-adapting type II), and provide information about the direction of translational forces applied to the skin. Axons of cutaneous mechanoreceptors bifurcate rostrally and caudally when entering the spinal cord and send collaterals that innervate the dorsal horn across several spinal segments of the spinal cord. The majority of slow- and fast-adapting afferent terminals are concentrated in Rexed’s laminae II–V (Woolf, 1987). Ascending projections travel to supraspinal structures and provide signals that encode features related to the static and dynamic state of contact with the supporting surface.
The actions of cutaneous reflexes are dependent upon the modality and location of the stimulus. When the leg is not load bearing, activation of cutaneous receptors on the sole of the foot evokes a rapid withdrawal reflex characterized by excitation of the leg flexors and suppression of the extensors. However, the sensitivity, perceptual thresholds, and sign of reflex responses (reflex reversal) evoked by activation of cutaneous receptors on the sole of the foot are critically dependent upon the state of vertical support (e.g., standing vs. sitting) and phase of locomotion (Duysens et al., 1990; De Serres et al., 1995). Thresholds are higher during quiet standing than during sitting (Mildren et al., 2016). Perceptual thresholds also differ across the sole of the foot, with lower thresholds in the ball and arch of the foot and higher thresholds in the regions of the heel and toe (Inglis et al., 2002). Cutaneous afferents from the feet have powerful synaptic effects on the coordination and phase of locomotion via input to spinal central pattern generators (CPGs) (Frigon, 2017) and the pontomedullary reticular formation (Drew et al., 1996).
ASCENDING PATHWAYS MEDIATING PROPRIOCEPTIVE AND EXTEROCEPTIVE FEEDBACK
Ascending sensory information contributes to the conscious awareness of the body or limb position in space (kinesthesia), but also allows the nervous system to compare the expected with the resultant sensory consequences of movement (Fig. 1.1). This allows motor commands and the gain of reflex pathways to be modulated with respect to postural demands and response goals. The main pathway of transmission of proprioceptive and exteroceptive information is via the dorsal column medial lemniscal pathway, posterior and anterior spinocerebellar tracts, and spinoreticular tracts (Figs 1.1 and 1.4).
Fig. 1.4.
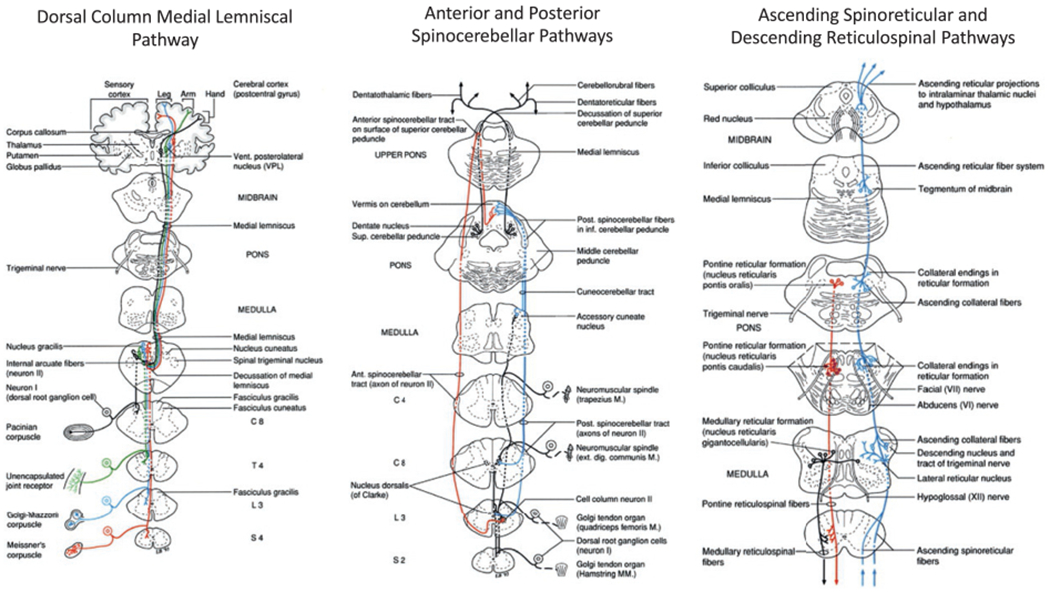
Primary ascending sensory pathways that contribute to the control of posture, balance, and locomotion. (Reproduced from Parent A (1996) Carpenter’s human neuroanatomy, 9th edn. Baltimore, MD: Williams and Wilkins, with permission from Wolters Kluwer.)
Dorsal column medial lemniscal pathway
The fastest-conducting pathway conveying proprioceptive and cutaneous signals is via the dorsal column medial lemniscal pathway. Branches of the muscles, spindle Ia and II afferents, and cutaneous and joint receptor axons travel via the ipsilateral posterior white columns (fasciculus gracilis, fasciculus cuneatus), synapse on to internal arcuate fibers in the nucleus gracilis or cuneatus at the level of the medulla, decussate, then travel via the medial lemniscus to the somatosensory region of the contralateral thalamus (Hassler’s ventro-caudal, Vc, thalamus) (Carpenter, 1991). Thalamocortical projections from muscle spindle afferents mainly target Brodmann’s area 3a of the primary somatosensory cortex, near the base of the central sulcus, while cutaneous afferents project to area 3b of the primary sensorimotor cortex (posterior bank of the central sulcus). Substantial sensory conduction delays or lesions of the posterior columns can significantly attenuate or abolish kinesthesia and are often associated with deficits in postural stability and gait.
Posterior spinocerebellar tract
Both the posterior and anterior spinocerebellar tracts are specialized for the transmission of proprioceptive and exteroceptive signals from the lower limb to the cerebellum. The posterior spinocerebellar tract is derived from large cells of the dorsal nucleus of Clarke. These cells receive powerful monosynaptic input from axon collaterals of muscle spindles (group Ia and II), GTOs (Ib), or pressure receptors (Carpenter, 1991). Proprioceptive and cutaneous inputs are segregated and somatotopically organized and this structure is maintained to the cerebellar terminations. Ascending fibers from the dorsal nucleus have conduction velocities ranging from 30 to 110 m/s and ascend ipsilaterally via the posterolateral region of the lateral funiculus, through the inferior cerebellar peduncle, and terminate as mossy fibers in rostral and caudal portions of the vermis (lobule I–IV, pyramis and paramedian lobule; the paleocerebellum). The dorsal nucleus of Clarke is not present above the level of C8. For this reason, some uncrossed Ia and Ib afferent fibers terminate on to cells of the accessory cuneate nucleus in the medulla and ascend via the cuneocerebellar tract to lobule V of the cerebellar cortex. Cells of the posterior spinocerebellar and cuneocerebellar tract are unaffected by spinal processing or descending motor commands (Cohen et al., 2017).
Anterior spinocerebellar tract
The anterior spinocerebellar tract is composed of axons originating from Rexed’s laminae V–VII (Carpenter, 1991). Unlike the posterior spinocerebellar tract, cells of the anterior tract receive convergent input from proprioceptive and cutaneous afferents. In addition, these cells receive collateral projections from descending pathways including the corticospinal and vestibulospinal systems, thus providing supraspinal modulation of ascending activity according to task and postural demands. Axons of the anterior tract immediately cross at the level of the spinal cord through anterior commissural fibers and ascend contralaterally along the anterolateral funiculus, through the superior cerebellar peduncle, and terminate in the contralaleral or ipsilateral anterior cerebellar vermis (lobules I–IV). Collaterals of these fibers also terminate on regions of the dorsal and medial accessory olivary nuclei. Conduction velocities of the anterior spinocerebellar tract range from 70 to 120 m/s.
Spinoreticular tract
Axons from proprioceptive (Ia, II, and Ib) afferents synapse on to cells in the posterior horn that give rise to the spinoreticular tract (Carpenter, 1991). These fibers ascend predominantly via the anterolateral portion of the ipsilateral spinal cord then send extensive collaterals that terminate across the extent of the pontomedullary reticular formation, including the nucleus reticularis pontis caudalis and oralis and nucleus reticularis gigantocellularis; regions with reticulospinal neurons that contribute to the control of anticipatory and reactive postural adjustments, locomotion, and postural tone.
VESTIBULAR APPARATUS
The vestibular system rapidly senses linear and angular changes in the motion and orientation of the head. The primary functions of this system are to: (1) stabilize gaze as the head moves relative to the body; (2) stabilize head movement (contributes to stabilizing the visual image on the retina); (3) provide a sense of self-motion; and (4) activate and modulate postural reflexes that contribute to the maintenance of vertical support and control of balance (Fig. 1.5A). These functions are accomplished by rapid reflex pathways that control eye and neck movements (vestibulo-ocular and vestibulocollic reflexes) and longer latency responses via output to nuclei (vestibular nuclei and reticular formation) that control postural reflexes and vertical support.
Fig. 1.5.
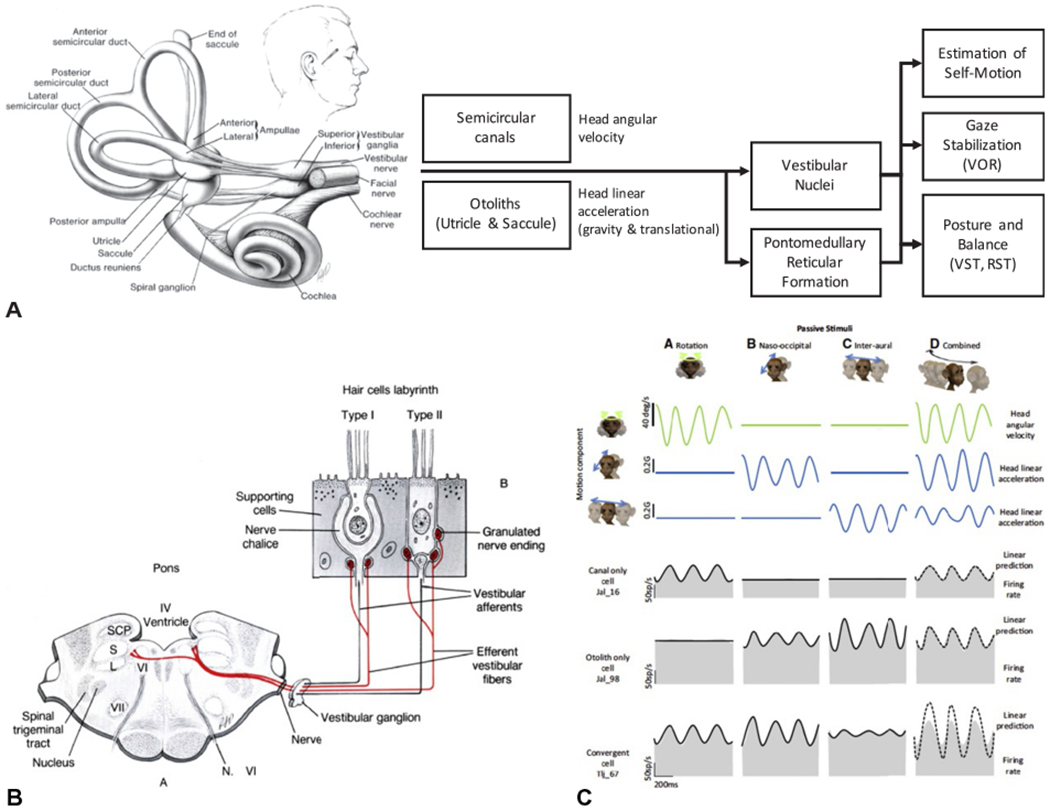
(A) The vestibular apparatus and a simplified summary of its outputs and functions with respect to the control of posture and balance. (B) Summary of the structure, afferents, and efferents of the hair cells. (C) Model of the response of vestibular nucleus neurons to passive pure linear, pure rotational, and combined motion of the head. The bottom three rows show the changes in firing rates of a vestibular neuron that receives input from the semicircular canals, otolith, or combined input from both. RST, reticulospinal tract; VOR, vestibulo-ocular reflex; VST, vestibulospinal tract. ((A) Adapted with permission from Cullen KE (2012) The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35: 185–196. (B) Reproduced with permission from Parent A (1996) Carpenter’s human neuroanatomy, 9th edn. Baltimore, MD: Williams and Wilkins. (C) Reproduced from Carriot et al. (2015).)
The sensing organs of the vestibular system are contained bilaterally within the vestibular portion of the inner ear. Each vestibular apparatus consists of three mutually orthogonal semicircular canals (anterior vertical, posterior vertical, and horizontal or lateral ducts) and two otolith organs, the utricle and saccule, that are oriented perpendicular to one another (Fig. 1.5A). The semicircular canals sense angular motion of the head while the otoliths sense linear acceleration (gravity and translational motion).
Motion sense in semicircular canals is encoded by hair cells located within a thickened zone of epithelium near the ends of the canal (Fig. 1.5B). The hair cells extend into a gelatinous diaphragm, called the cupula. Angular acceleration of the head (rotation, flexion, extension) generates motion of the endolymphatic fluid contained within the canals, displaces the cupula, deforms the hair cells, and causes the sensory endings to discharge.
The firing rate of semicircular canal afferents is approximately proportional to the angular velocity of the head and in phase with motion at frequencies above 0.1 Hz and up to 4.0 Hz (Fig. 1.5C) (Haggerty et al., 2017). The otolith organs have a similar system for sensing motion but have a structure specialized for encoding linear motion, in particular, sensing dynamic responses that are in phase with linear accelerations, including gravity. When the head is erect, the utricle is oriented nearly perpendicular to the line of gravity, therefore the hair cells preferentially respond when the head is tilted with respect to gravity or during linear accelerations. In contrast, the saccule is oriented vertically so that the hair cells are preferentially activated during vertical accelerations of the head.
The organization of the hair cell (a line of stereocilia; Fig. 1.5B) ensures that discharge of the afferent fiber is directionally specific; motion in one direction depolarizes the hair cell while motion in the opposite direction hyperpolarizes the ending. Since the mechanical orientation of each hair cell is different, motion of the head in one direction will depolarize some cells more than others, resulting in a net population output that is unique to the direction of head acceleration. Unlike the ampulla of the canals, the mass of the gelatinous otoliths prevents the hair cells from returning from their resting position when stimulated by gravity. Thus, afferents from the otoliths can fire tonically (persistent) or phasically in response to changes in motion or orientation. Sensitivity of vestibular hair cells and their afferents is modulated by efferent input from a cluster of cells located dorsal and/or ventral to the facial nerve. Efferent input increases baseline afferent discharge and decreases afferent sensitivity (Mathews et al., 2017).
Vestibular afferents from the otoliths and semicircular canals project ipsilaterally, via cranial nerve VIII, to the vestibular nuclei (lateral, medial, superior, and inferior (descending) vestibular nuclei) (Carpenter, 1991) and also project, via disynaptic and polysynaptic pathways, to the pontomedullary reticular formation (Abzug and Peterson, 1973; Peterson and Abzug, 1975). Two classes of afferent fibers projecting from neuroepithelial hairs cells have been described based on the variability (regularity) of these discharge patterns: large-diameter irregular and smaller-diameter regular afferent fibers. Regular afferents transmit twice as much information and are twice as sensitive for detecting head motion with the normal physiologic frequency range as irregular afferents (4 vs. 8 °/s) (Cullen, 2012). In contrast, irregular afferent fibers are more sensitive to higher-frequency head movements (e.g., 15 Hz). Both classes of afferent fibers project to the vestibular nuclei and this information is processed, in conjunction with convergent multisensory input, to provide the sense of self versus external motion and to activate and modulate postural reflexes and balance.
VISION
The visual apparatus and pathways convey information about object contrast, luminance, size, distance to the eye (depth perception), and spatial frequency (level of detail present in a stimulus per degree of visual angle) (Bronstein and Guerraz, 1999) (Fig. 1.6). The visual system uses this information to inform the nervous system of the static and dynamic features of the surrounding environment and the orientation and motion of the body relative to these features. This information is used to plan and execute anticipatory actions and their associated postural adjustments or initiate reactions to abrupt changes in the visual field.
Fig. 1.6.
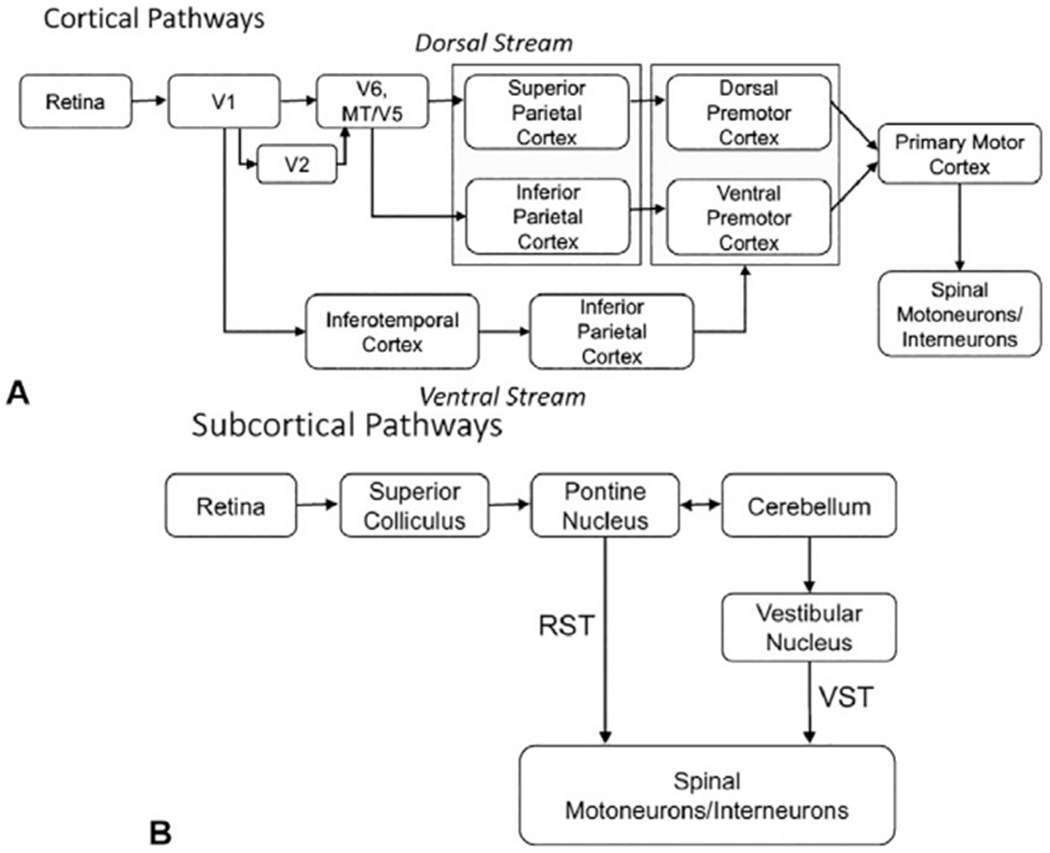
Summary of the cortical (A) and subcortical (B) pathways linking the visual system input structures and connections of the visual system to structures that control movement and posture. RST, reticulospinal tract; VST, vestibulospinal tract.
Postural responses to elements in the visual field are predominantly mediated by inputs from the retina to the primary visual cortex (V1, calcarine sulcus, area 17) via the optic nerve, optic chiasm, optic tracts, and lateral geniculate nucleus. Output from the primary visual cortex projects to two functionally specialized visual pathways devoted to extracting visual attributes from the visual field: the dorsal (V1 to posterior parietal cortex) and ventral (V1 to inferotemporal cortex) streams (Ungerleider and Mishkin, 1982; Goodale and Milner, 1992). The ventral stream (“vision-to-perception” or “what” pathway) subserves the recognition and discrimination of shapes and objects. This information is used to generate a visual “knowledge-base” (Goodale, 1996) that can be used to preplan future actions. Accordingly, the ventral stream contributes to anticipatory actions through connections from the inferotemporal cortex, to prefrontal regions of the frontal cortex, to premotor and primary motor cortex.
The dorsal stream (“vision-for-action” pathway) contributes to real-time control of action by transforming information about the spatial location, shape, orientation, and motion of objects into the coordinate frames of the effectors (limbs) being used to perform the action (Goodale, 1996). Visual information flows across the dorsal stream from area V1 to extrastriate areas V6, MT/V5, and then to superior and inferior regions of the posterior parietal cortex. Visual processing through the superior parietal cortex (areas V6A, PEc, MIP, LIP) is specialized for the recognition of object motion and self-motion and is considered to play an important role in error detection and the rapid correction of limb trajectories such as during obstacle avoidance (Marigold and Drew, 2011; Drew and Marigold, 2015; Potocanac and Duysens, 2017). Motor actions mediated by the superior parietal pathway are mediated by a pathway to the dorsal premotor cortex, then primary motor cortex, whereby descending commands are sent to spinal motoneurons via the corticospinal tract. In contrast, visual input to the inferior parietal cortex (areas AIP, PFG, PF) encodes the spatial location of stationary and moving objects and projects to the ventral premotor cortex and may play a preferential role in target acquisition (e.g., grasp of an object to regain balance).
The polysynaptic organization of the dorsal visual stream results in relatively slow reaction times to visual stimuli, in the order of 170 ms, or more and responses to optokinetic stimuli can take up to 10 seconds to reach steady state (Haggerty et al., 2017). Yet, if an object in the visual field unexpectedly moves, adjustments can be made from the planned trajectory of the upper or lower limb in the order of 120 ms (Patla et al., 1991; Day and Brown, 2001; Weerdesteyn et al., 2004; Reynolds and Day, 2005). It has been proposed that these rapid reactions to visual perturbations are mediated by a subcortical pathway from V1, to the superior colliculus, to the pontine reticular nucleus and then to spinal motor networks via fast-conducting reticulospinal projections to spinal motor networks. Rapid corrections could also be mediated by connections to the cerebellum and output to reticulospinal or vestibulospinal systems.
SUPRASPINAL CONTROL OF POSTURE AND LOCOMOTION
Motor and premotor cortex and corticospinal tract
Frontal regions of the cortex, including the prefrontal, premotor, and primary motor cortex, are critical for the selection, planning, initiation, and execution of intended movements, including transitions in behavior (standing to walking, walking to running), alterations in whole-body or limb trajectory, and modifying the spatial and temporal aspects of gait. These functions can be planned and executed proactively according to endogenous desires (self-initiated or internally generated movements) or in reaction to sensory stimuli (exogenous or externally generated movements). Internally generated movements are selected in prefrontal regions of the cortex, including the dorsolateral prefrontal and presupplementary motor areas, then prepared in mesial premotor regions of Brodmann’s area 6, including the supplementary and cingulate motor areas (Deecke, 1987; Jahanshahi et al., 1995). Externally generated (sensory cued) movements are initiated by sensory input that is polysynaptically relayed to frontal cortex via posterior parietal or cerebellothalamocortical connections, then prepared in more lateral motor regions of area 6, including the dorsal and ventral premotor cortex. Execution of movement is through the primary motor cortex (Brodmann’s area 4).
Each of the motor and premotor regions (premotor cortex, supplementary motor cortex, cingulate motor cortex) sends extensive projections to the spinal cord via the corticospinal tract. The primary motor cortex is specialized for the precise control of muscle activation patterns due to a relatively high proportion of fibers with direct monosynaptic input to spinal motoneurons (cortico-motoneuronal connections) (Lemon, 1993). This type of specialized connection is well suited for the precise control of limb trajectories in the presence of obstacles (Drew and Marigold, 2015). Projections from the premotor regions (area 6) comprise more than 30% of the corticospinal tract and the extent of limb representations equals or exceeds that in primary motor cortex (Dum and Strick, 1991, 1996, 2002).
While around 10% of projections from the primary motor cortex make monosynaptic connections to spinal motoneurons, the vast majority of terminations from motor and premotor regions are on to interneurons in Rexed’s laminae V–VIII and IX (predominantly VII) (Dum and Strick, 1996). These inputs converge and integrate with input from other descending systems and sensory feedback to the spinal cord. While the function of premotor connections to the intermediate zone of the spinal cord is poorly understood, spinal interneurons in these regions can show preparatory movement-related changes in activity with a time course similar to that seen in premotor cortical regions (Prut and Fetz, 1999; Fetz et al., 2002). These findings suggest that premotor activity may contribute to the anticipatory modulation of reflex pathways and the excitability of spinal motor networks.
As described in the introduction (Fig. 1.1), movements initiated by motor cortical commands must be coupled with postural and locomotor controllers to ensure that postural disturbances generated by movement are anticipated and corrected for immediately prior to, or during, the intended action (Gahery and Massion, 1981; Hugon et al., 1982) (Fig. 1.7). Accordingly, motor and premotor cortical areas have direct projections to the vestibular nuclei, mesencephalic locomotor region (MLR), pontomedullary reticular formation, and intermediate zones of the spinal cord. The vestibular nuclei receive extensive projections from primary motor (area 4), premotor and supplementary motor areas (area 6), and primary somatosensory sensory (areas 3b, 3a, and 2)(Fukushima, 1997). Similarly, the pontomedullary reticular formation receives direct proj ections from primary motor, premotor, and supplementary motor areas (Keizer and Kuypers, 1984, 1989; Kably and Drew, 1998). This likely explains the presence of both movement preparation- and movement execution-related activity in vestibulospinal and reticulospinal neurons and spinal interneurons (Prut and Fetz, 1999; Buford and Davidson, 2004).
Fig. 1.7.
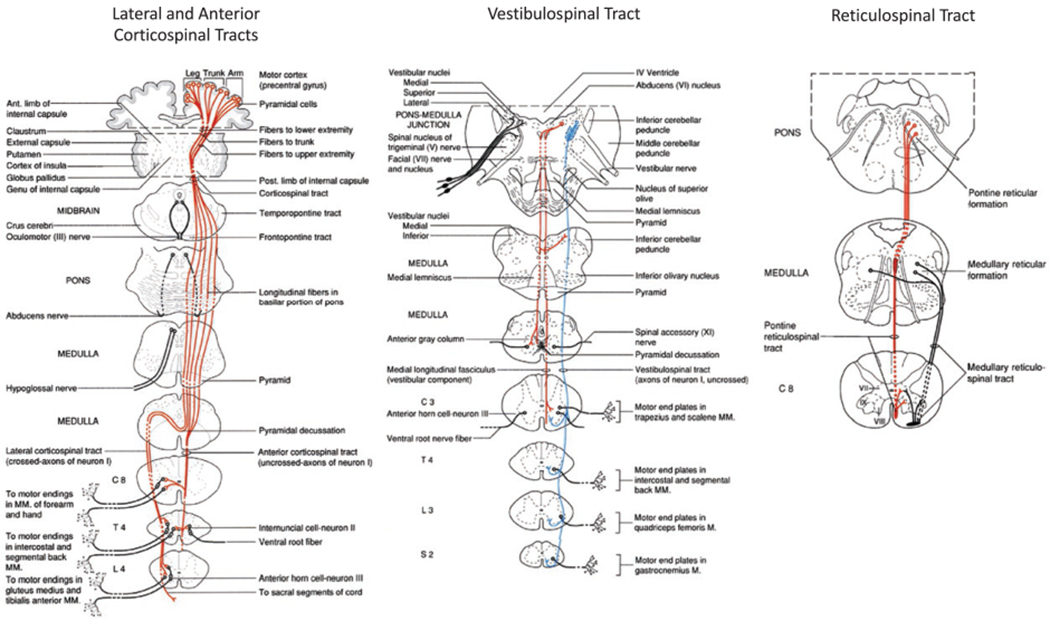
Primary descending motor pathways that contribute to the control of posture, balance, and locomotion. (Reproduced from Parent A (1996) Carpenter’s human neuroanatomy, 9th edn. Baltimore, MD: Williams and Wilkins, with permission from Wolters Kluwer.)
Basal ganglia
The motor circuit of the basal ganglia is considered to be important for the selection and suppression of movement (Mink, 1996), the execution of automatic actions (Wu and Hallett, 2005), and scaling of motor output (Pfann et al., 2001). These motor functions are honed through reinforcement (reward-based) learning and are dependent upon dopaminergic input to the striatum. The basal ganglia do not project directly to the spinal cord. For this reason, any influence the basal ganglia has on posture, balance, and locomotion must be mediated indirectly by modulating the activity of thalamocortical pathways or locomotor and muscle tone circuits in the pedunculopontine nucleus (PPN) of the pontine tegmentum.
The actions of the basal ganglia are exerted by modulating activity in the two primary output structures: the internal segment of the globus pallidus and substantia nigra pars reticulata. The output neurons are tonically active at rest, with firing rates of 60–80 Hz, and send inhibitory (GABAergic) projections to either the ventro-oralis nuclei of the thalamus (Voa, Vop) or the PPN (part of the MLR; see below). Thus, movement is facilitated by the reduced firing rates in basal ganglia output nuclei and suppressed by increased firing rates. Movement facilitation through the basal ganglia is mediated by corticostriatal projections to medium spiny neurons that express D1 dopamine receptors and send direct inhibitory (GABAergic) projections on to the output neurons of the basal ganglia (Albin et al., 1989). This circuit has been termed the direct pathway.
In contrast, movement suppression is driven by corticostriatal projections to medium spiny neurons that express D2 dopamine receptors and exert their inhibitory actions through the indirect pathway. This circuit is composed of an inhibitory projection to the globus pallidus externus and a subsequent disinhibition of glutamatergic (excitatory) input from the subthalamic nucleus to the output nuclei. Decisions to rapidly suppress movement, such as in response to a “stop” signal, can be also driven by direct excitatory corticosubthalamic nucleus connections that can induce short-latency increases in basal ganglia output.
In keeping with this model, selective activation of medium spiny neurons in the putamen that express D1 receptors (indirect pathway) has been shown to facilitate movement and locomotion in rodents whereas activation of neurons that express D2 receptors suppresses movement and locomotion (Kravitz et al., 2010; Roseberry et al., 2016). Nigrostriatal dopaminergic projections act to strengthen corticostriatal synaptic connectivity in D1-expressing medium spiny neurons (facilitates activity in the direct pathway) and suppress connectivity in D2-expressing medium spiny neurons (suppresses activity in the indirect pathway) (Surmeier et al., 2007). Diseases causing a reduction in dopaminergic innervation of the striatum are associated with increased activity in the indirect pathway and excessive inhibition of thalamocortical pathways and the PPN, resulting in the hypokinetic, bradykinetic, postural instability and gait dysfunction syndromes that characterize parkinsonism.
Cerebellum
The cerebellum is considered to be critical for the detection and correction of motor errors and the subsequent refinement of motor output through the process of supervised (coincidence error-based) motor learning (Houk et al., 2007). Accordingly, the cerebellum is the recipient of multisensory, cortical, and subcortical input. Similar to the basal ganglia, cerebellar output does not project directly to the spinal cord, thus the effects of cerebellar processing on motor control are mediated indirectly through proj ections to both cortical and subcortical targets.
The cerebellar cortex can be functionally subdivided into three longitudinal zones, organized from medial to lateral, that differ with respect to the topography of olivocerebellar inputs and the targets of efferent projections (Rothwell, 1994): (1) the medial or vermal zone, including the flocculonodular lobe, that projects to the fastigial nucleus and vestibular nuclei; (2) the intermediate or paravermal or intermediate zone that projects mainly to the interposed nucleus; and (3) the large hemisphere or lateral zone projects primarily to the dentate nucleus. The medial (vermal) zone predominantly receives inputs from the vestibular nerve (cranial nerve VIII) and vestibular nuclei as well as projections from the superior colliculus and visual cortex.
Output projections from the fastigial nucleus are excitatory and travel via the superior and inferior peduncles to lateral vestibulospinal neurons in the vestibular nucleus, reticulospinal neurons in the reticular formation, and the PPN in the pontine tegmentum. Some fibers project to the contralateral ventral intermediate nucleus of the thalamus and are relayed to the motor cortex. A subset of Purkinje cells in the medial zone bypass the fastigial nucleus and send GABAergic (inhibitory) projections that terminate directly in the vestibular nucleus. This circuitry suggests that the medial zone predominantly contributes to reactive postural control by modulating output from the vestibular and reticular nuclei in response to vestibular and visual stimuli.
The vermal region of the cerebellum also has a presumed “locomotor” region that contributes to the coordination of brainstem CPGs (Takakusaki, 2017). The intermediate (paravermal) zone receives input from ascending spinocerebellar and cuneocerebellar tracts that transmit activity from proprioceptive and cutaneous afferents. The Purkinje cells of the intermediate zone synapse on to neurons in the interpositus nucleus, which in turn projects, via the superior cerebellar peduncle, to the contralateral red nucleus and ventral intermediate nucleus of the thalamus, then to the motor cortex (in particular the premotor cortex). Purkinje cells and neurons of the interpositus nucleus in the intermediate zone typically show activity that follows the onset of movements, suggesting that this pathway serves to coordinate and shape reactive postural control in response to limb movements and touch through predominantly motor cortical pathways.
The lateral zone of the cerebellum (part of the neocerebellum) is the newest and largest part of the human cerebellum. Inputs are from the primary motor, premotor, and supplementary motor areas and somatic sensory areas of the cortex by way of corticopontine fibers, pontine nuclei, and pontocerebellar fibers. Thus, the corticopontocerebellar pathway carries information about the preparation, initiation, and execution of voluntary movements and the sensory consequences.
It has been proposed that input from the sensorimotor cortex provides an efferent copy of the motor command that the cerebellum uses to encode an error signal based on the difference between expected (efferent) and sensed (reafferent) sensory feedback. Consistent with this idea, Purkinje cells and neurons in the dentate nucleus show activity changes that can precede movement onset by hundreds of milliseconds (Thach, 1975). Projections from the neocerebellar cortex terminate in the dentate nucleus. Fibers from the dentate nucleus make up most of the superior cerebellar peduncle and synapse in the contralateral parvocellular red nucleus and ventral intermediate nucleus thalamus. Thus, the corticopontocerebellar-thalamocortical loop integrates feedforward commands with sensory information to shape and modulate subsequent motor commands.
The input–output organization of the cerebellum is maintained across all regions. The cerebellar cortex receives two types of input: mossy fibers which are the terminal axons from the spinal cord (e.g., spinocerebellar tracts), vestibular system, pontomedullary reticular formation, and climbing fibers which originate from the inferior olive. Mossy fibers terminate on granule cells in the granular layer of cortex and also send a collateral branch that terminates on Purkinje cells. Climbing fibers synapse on to the dendritic tree of Purkinje cells but also branch to the deep nuclei. The details of the circuitry and synaptic processing of inputs to the cerebellar cortex are beyond the scope of this review, but the result of this processing culminates in changes in the activity of Purkinje cells.
The firing patterns of Purkinje cells are characterized by “simple” spikes (approximately 1 ms in duration), which are generated by synaptic inputs from the parallel fibers of granule cells, or “complex” spikes generated by climbing fiber input. Simple spikes encode movement kinematics and position errors. Complex spikes also encode position errors and kinematic parameters and serve to modulate the information encoded in Purkinje cell simple spikes in advance of a change in behavior (Streng et al., 2017a, b).
The inferior olive is a hub for convergent inputs from the spinal cord (spino-olivary tract), motor cortex, superior colliculus, vestibular nuclei, trigeminal nuclei, and pretectum. In this manner, the olivocerebellar system provides multimodal input that contributes to error-based motor learning and shapes subsequent motor output by modulating the activity of both cerebellar nuclei and Purkinje cells (predominantly via the induction of plasticity at the parallel fiber–Purkinje cell synapse) (Lang et al., 2017). Output from the Purkinje cell is inhibitory (GABAergic) and terminates on the deep cerebellar nuclei. At rest, Purkinje cells discharge at rates between 40 and 80 Hz, thus exerting tonic inhibition on the deep nuclei and suppressing output from the cerebellum. The deep cerebellar nuclei (fastigial, interposed, or dentate nucleus) send glutamatergic projections that facilitate the activity of target structures and pathways. Accordingly, increased activity in Purkinje cells reduces cerebellar output while lesions of cerebellar cortex can disinhibit the deep nuclei and facilitate downstream pathways.
Effects of cerebellar lesions on postural stability and muscle tone are critically dependent upon the zone (medial, intermediate, lateral) and level of cortical and deep cerebellar nuclei involvement (Rothwell, 1994). In general, a syndrome of hypotonia, ataxia (dyscoordination: hypermetria, dysdiadochokinesia), and postural instability is the motor hallmark of cerebellar lesions. These pathologic features are predominantly caused by the loss of excitatory output from the deep cerebellar nuclei. Hypotonia is characterized by deficits in the capacity to generate force and reduced sensitivity of muscle stretch. These changes reflect reduced excitatory drive to both alpha and gamma (fusimotor) spinal motoneurons from descending corticospinal, reticulospinal, and vestibulospinal systems. In cats, lesions of the anterior lobe that affect both the deep nuclei (fastigial, interpositus) and cortex can result in a marked in increase in extensor muscle tone and profound gait ataxia. This is thought to be due to the loss of direct inhibition of the lateral vestibular (Deiter’s) nucleus from Purkinje cell output and a net increase in vestibulospinal output to the extensor muscles. Inactivation or dysfunction of cerebellar cortical output, with sparing of the deep cerebellar nuclei, is associated with a syndrome of dyscoordination (hypermetria, dydiadochokinesia) and kinetic tremor. These changes reflect the loss of cerebellar cortical processing that provides error-based corrections of movement and supervised (coincidence error-based) learning.
BRAINSTEM CONTROL OF POSTURAL REFLEXES, MUSCLE TONE, AND LOCOMOTION
Vestibular nuclei and the vestibulospinal tract
The vestibular nuclei are one of the principal targets of output from the vestibular apparatus but also serve as a hub for the convergence of inputs from multisensory systems and supraspinal centers (for review, see McCall et al., 2017). The vestibular nuclei are organized in two longitudinal columns immediately caudal to the floor of the fourth ventricle. The lateral cell column consists of the superior, lateral, and inferior (descending) nuclei while the medial vestibular nucleus composes the medial column (Carpenter, 1991). Vestibular afferents from the semicircular canals and otoliths terminate ipsilaterally in all the vestibular nuclei. Afferents from semicircular canals project primarily to the superior vestibular nucleus and rostral part of the medial vestibular nucleus. Afferents from the utricle predominantly project to the ventral part of the lateral vestibular nucleus whereas cells that innervate the saccule terminate in dorsolateral regions of the inferior vestibular nucleus.
Cerebellar projections to the vestibular nuclei predominately originate from the medial zone (vestibulocerebellum) and project bilaterally, via the fastigial nucleus, to portions of the inferior and lateral vestibular nuclei. These regions also receive direct inhibitory projections from Purkinje cells in the cerebellar cortex that bypass the deep nuclei. The vestibular nuclei also receive input from the cortex, including premotor (area 6) and primary somatosensory (areas 2 and 3a) regions, and from the cervical spinal cord (McCall et al., 2017). The convergence of input to the vestibular nuclei provides the capacity to execute and modulate vestibular-elicited reflexes in anticipation of planned movements or in reaction to sensory stimuli in the environment. The vestibular nuclei also have extensive commissure connections between sides and reciprocal connections to supraspinal, brainstem, and spinal regions controlling posture and balance to ensure that vestibulospinal influences are coordinated with the actions of the other pathways.
The vestibular nuclei influence postural control via two descending pathways to the spinal cord, the lateral and medial vestibulospinal tracts (Fig. 1.8). The medial vestibulospinal tract is primarily composed of axons from the medial vestibular nucleus, but fibers from the lateral and descending vestibular nucleus also contribute to the tract (Carpenter, 1991). Axons of the medial tract travel in the medial longitudinal fasciculus and principally terminate in upper cervical regions of the spinal cord that innervate upper-body musculature, particularly neck musculature (the extent to which the medial tract innervates lower segments of the spinal in humans is unknown). Thus, the medial vestibular nucleus and medial vestibulospinal tract provide the circuitry necessary to rapidly control vestibulocollic reflexes and neck postural changes associated with alterations in head orientation.
Fig. 1.8.
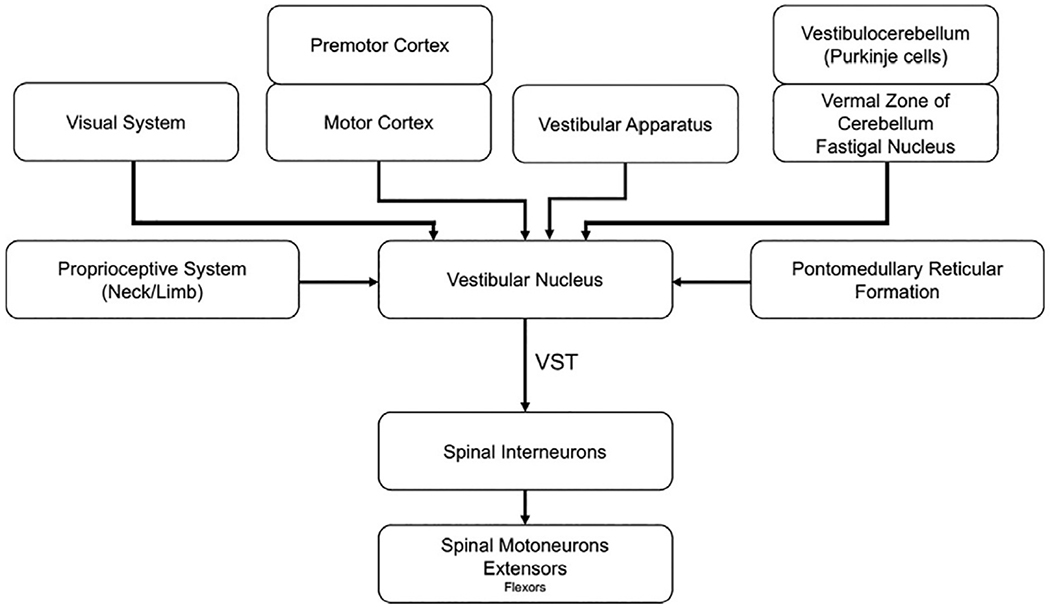
Summary of the input and output pathways of the vestibular nucleus. VST, vestibulospinal tract. (Adapted from McCall AA, Miller DM, Yates BJ (2017) Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front Neurol 8: 112.)
The lateral vestibulospinal tract is the largest of the two tracts and is primarily composed of axons from neurons in the lateral vestibular nucleus (Dieter’s nucleus) with some contribution from the descending (inferior) nucleus. The fibers descend ipsilaterally in the ventrolateral columns and have branches that innervate multiple levels of the spinal cord, thus providing the capacity to modulate spinal motoneuron activity across segments (Abzug et al., 1974). The primary sites of termination of vestibulospinal projections are on to interneurons in Rexed’s laminae VII and VIII, thus this pathway indirectly influences spinal motoneuron activity via di- or polysynaptic connections. Rexed’s lamina VII is also a major site of termination proprioceptive afferents and descending projections from reticulospinal and corticospinal pathways. Thus, vestibulospinal pathways are well positioned to modulate the excitability of reflex responses to anticipated or imposed postural displacements.
Experiments in animals have shown that electric stimulation of the lateral vestibular nucleus evokes a net increase in the excitability of extensor motoneurons and inhibitory effects on flexor motoneurons (Wilson and Yoshida, 1969; Grillner et al., 1970; McCall et al., 2017). The facilitatory effects of vestibulospinal inputs on extensor motoneuron excitability suggest that the predominant function of this pathway is to provide appropriate levels of extensor tone to achieve vertical support against gravity.
Mesencephalic locomotor region
The MLR is a region located in the midbrain reticular formation that contributes to the generation of locomotor rhythm generation (intensity and duration) and control of postural tone. The MLR is traditionally described as being composed of two nuclei, the cuneiform nucleus (CN) and the PPN. The CN is located ventral to the tegmentum and dorsal to the PPN and contains both glutamatergic and GABAergic neurons. Descending projections from this nucleus extensively target reticulospinal neurons in the pontomedullary reticular formation.
The PPN is located in the lateral tegmentum ventral to the inferior colliculus and is composed of two distinct regions that differ with respect to the distribution of neuron types and afferent–efferent connectivity (Martinez-Gonzalez et al., 2011): (1) a caudal region (PPN pars compacta) that contains a heterogeneous population of cholinergic, glutamatergic, and GABAergic neurons, receives inputs from the cortex (frontal motor cortical regions), basal ganglia and dorsal raphe nucleus, and projects to the thalamus, subthalamic nucleus, ventromedial regions of the pontomedullarly reticular formation, spinal cord and brainstem regions that control muscle tone during rapid eye movement sleep (sublaterodorsal nucleus, lateral pontine tegmentum, ventrolateral periaqueductal gray); and (2) a rostral region that also contains a heterogeneous population of neurons but is distinct from the caudal region due to a substantially higher density of GABAergic neurons and extensive reciprocal connectivity with the basal ganglia. These differences between subdivisions of the PPN suggest that the caudal zone preferentially contributes to the modulation of brain state (e.g., arousal) and muscle tone whereas the rostral zone has a close functional relationship with the CN and basal ganglia that contributes to the control of postural reflexes and locomotion.
In keeping with this idea, high-frequency electric stimulation (> 100 Hz) in more caudal regions of the MLR (near the caudal PPN) is associated with suppression of muscle tone (Garcia-Rill and Skinner, 1987; Garcia-Rill et al., 2004; Takakusaki et al., 2011; Takakusaki, 2017). Stimulation in more rostral regions (rostral PPN and CN) evokes a locomotor-like pattern of alternating flexor–extensor muscle activity in the legs (Jordan et al., 2008; Takakusaki, 2017).
Recent studies using optogenetics have shown that glutamatergic neurons in the region of the rostral PPN and CN contribute to the generation of locomotor intensity and state (Roseberry et al., 2016). This function is mediated by direct excitatory projections to glutamatergic reticulospinal neurons in the medial medullary reticular formation (nucleus reticularis gigantocellularis) (Capelli et al., 2017), whereas a parallel projection to muscarinoceptive neurons that terminate on reticulospinal neurons acts to amplify and extend the duration of locomotor output (locomotor “boost”) (Smetana et al., 2010).
The MLR receives input from motor regions of the cortex, cerebellum, and basal ganglia. Motor regions of the frontal cortex have both indirect (via basal ganglia and cerebellum) and direct input to locomotor-generating regions of the MLR (Takakusaki, 2017). This provides supraspinal control of locomotor mode and intensity. The basal ganglia input to glutamatergic neurons of the MLR can either facilitate or suppress movement and locomotion through changes in the direct or indirect pathways respectively (Roseberry et al., 2016). The cerebellum also projects to the MLR, thus providing a conduit for movement error-based changes in locomotion or postural tone. Experiments in cats have shown that electric stimulation of a restricted zone of white matter (hook bundle of Russel), through which fastigial nucleus efferents decussate and project to the MLR, reticular formation, or vestibular nucleus, can produce bilateral changes in posture tone, or elicits quadripedal locomotion (Mori et al., 1999). This finding has been interpreted to suggest that a cerebellar circuit with output through the fastigial nucleus (presumed cerebellar locomotor region) to the MLR contributes to the supraspinal generation or control of locomotion. While neuroimaging studies have shown activity in this region during locomotor-like tasks in humans, little is known about the role this area plays in posture or locomotion (Jahn et al., 2008).
Pontomedullary reticular formation and reticulospinal tract
The pontomedullary reticular formation and reticulospinal tract is a major descending system for the control of movement and a critical hub for sensorimotor integration that allows the nervous system to appropriately couple voluntary actions with posture and locomotion (Fig. 1.9). This system has been shown to play a prominent role in: (1) anticipatory and reactive postural adjustments; (2) control of locomotor intensity and mode (walking vs. running); and (3) regulation of muscle tone (Drew et al., 1986; Prentice and Drew, 2001; Schepens and Drew, 2004; Takakusaki, 2017). These complex motor functions are coordinated by the convergence of cortical and subcortical motor commands with sensory feedback and topographically organized descending projections to axial muscles and flexor and extensor muscles of the limbs (Peterson et al., 1979).
Fig. 1.9.
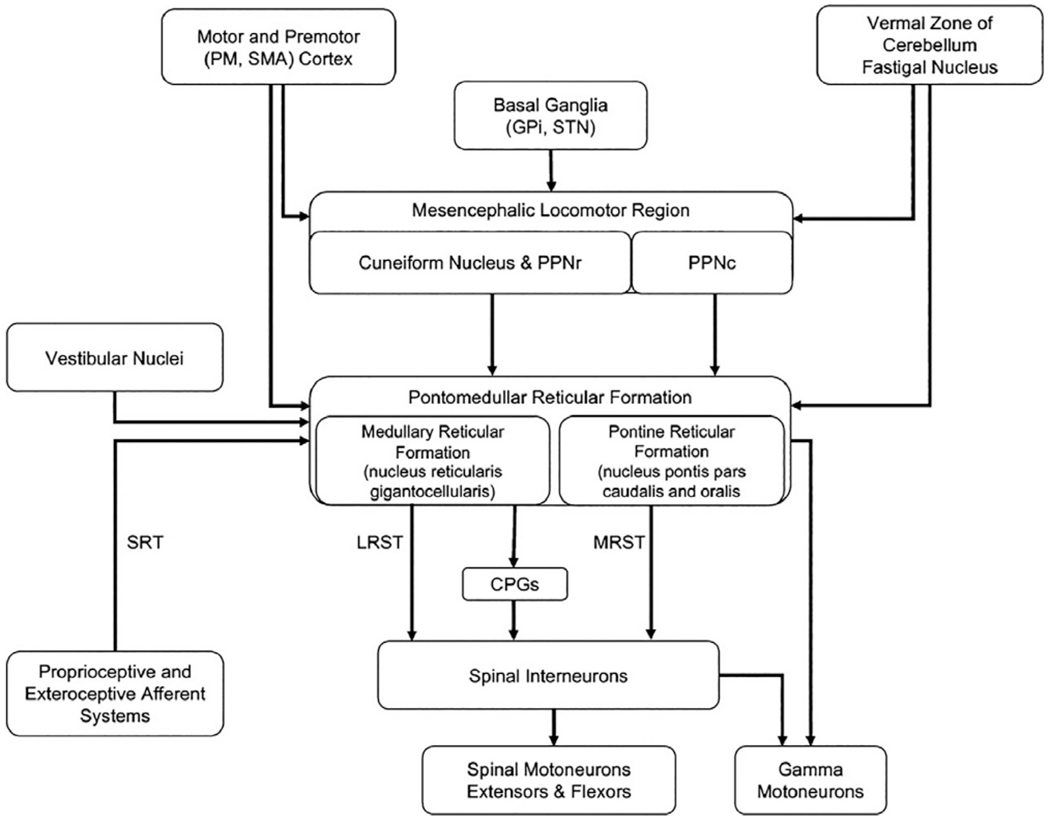
Summary of the input and output pathways to the mesencephalic locomotor region and pontomedullary reticular formation. CPGs, central pattern generators; GPi, globus pallidus internus; LRST, lateral (medullary) reticulospinal tract; MRST, medial (pontine) reticulospinal tract; PM, premotor cortex; PPNc, caudal region of pedunculopontine nucleus; PPNr, rostral region of pedunculopontine nucleus; SMA, supplementary motor area; SRT, spinoreticular tract; STN, subthalamic nucleus.
The medullary reticular formation consists of three main regions: a paramedian reticular nuclear group, a central group (nucleus reticularis ventralis and gigantocellularis), and a lateral nuclear group (magnocellular, parvicellular nuclei). The pontine reticular formation includes the nucleus reticularis pontis, pars oralis, and pars caudalis. Neurons with activity related to locomotion, anticipatory and reactive postural control, and reaching are predominantly located in medial regions of the pontomedullary reticular formation, including the nucleus reticularis gigantocellularis, pontis caudalis, pontis oralis, and magnocellularis (Drew et al., 1986; Matsuyama and Drew, 2000; Buford and Davidson, 2004; Schepens and Drew, 2004). These regions contain neurons whose descending axonal projections form the lateral (medullary) and medial (pontine) reticulospinal tracts (see below).
The medial pontomedullary reticular formation receives both crossed and uncrossed input from the cortex, particularly from motor regions of the frontal cortex, including the primary motor cortex, premotor cortex, and supplementary motor area (Peterson et al., 1974; Jinnai, 1984; He and Wu, 1985; Canedo and Lamas, 1993; Kably and Drew, 1998). Cortical input comes from both collaterals of descending corticospinal axons and direct corticoreticular projections. This organization provides higher-order control of reticulospinal activity and the capacity to couple voluntary motor commands (corticospinal-mediated) with appropriate postural adjustments (Fig. 1.1). Regions that receive input from motor regions of the frontal cortex also have convergent input from the MLR, cerebellum, vestibular nuclei, and spinoreticular pathways.
Output from the otoliths and semicircular canals is relayed to reticulospinal neurons in dorsorostral zones of the nucleus reticularis gigantocellularis and caudal pontine reticular formation via di- and polysynaptic connections through the vestibular nuclei (Peterson, 1972; Peterson and Abzug, 1975; Peterson et al., 1980). This input serves to modulate reticulospinal output in concert with gravity-dependent postural reflexes. Cerebellar inputs predominantly originate from medial and intermediate zones of the cerebellum (fastigial and interpositus nuclei), thus providing error-related kinematic feedback (Eccles et al., 1975; Matsuyama and Jankowska, 2004; Takahashi et al., 2014). Spinoreticular inputs arise from proprioceptive and cutaneous receptors and predominantly terminate in caudal regions of nucleus reticularis gigantocellularis (Parent, 1996). Cutaneous afferent feedback to medullary reticulospinal neurons is particularly prominent during locomotion (Drew et al., 1996; Baker, 2011). Convergence of these inputs on to regions of the medial pontomedullary reticular formation that output to the reticulospinal tract provides the organization to modulate and adapt postural commands in accordance with changes in sensory feedback and voluntary motor commands.
The reticulospinal tract, in conjunction with the vestibulospinal tract, comprises the medial descending motor system (Lawrence and Kuypers, 1968). The importance of these systems is exemplified by experiments in nonhuman primates showing that bilateral lesions of the lateral descending motor pathways (corticospinal tract) resulted in an immediate period of flaccid paralysis, but this was subsequently followed by a marked recovery of postural control, locomotion, and reaching while chronic impairment in the control of hand movements remained (Lawrence and Kuypers, 1968).
The lateral (medullary) reticulospinal tract is predominantly derived from the axons of neurons in the nucleus reticularis gigantocellularis, whereas the medial (pontine) reticulospinal tract arises from nucleus reticularis pontis, pars oralis, and pars caudalis (Carpenter, 1991). Axons of reticulospinal neurons in the pontine reticular formation initially descend bilaterally through the medullary tegmental field, then continue ipsilaterally via the ventral funiculus of the spinal cord and comprise the medial reticulospinal tract. Projections from the medullary reticulospinal formation descend bilaterally (although the ipsilateral tract predominates) just lateral to the medial longitudinal fasciculus, then enter the spinal cord and either join with the pontine fibers that compose the medial reticulospinal tract or travel via ventrolateral funiculus in the lateral reticulospinal tract (Carpenter, 1991). A subset of reticulospinal neurons make monosynaptic connections on to alpha and gamma motoneurons in the ventral horn (lamina IX), while the majority terminate on premotor interneurons in the intermediate zone of the spinal cord (laminae VII and VIII). Parallel inputs to alpha and gamma motoneurons provide the reticulospinal system with the means to modulate muscle stretch sensitivity in accordance with the task requirements.
Our understanding of the topographic and functional organization of the medial pontomedullarly reticular formation has largely been derived from chemical or electric stimulation studies in cats (Grillner et al., 1968). Two zones have been described that differ with respect to effects of stimulation on spinal motoneuron activity. Stimulation in the dorsorostral region of the nucleus reticularis gigantocellularis and caudal pontine reticular formation predominantly evokes monosynaptic excitatory postsynaptic potentials in motoneurons of axial and flexor and extensor muscles of the limbs (responses were most prevalent in proximal muscles) (Grillner et al., 1968; Jankowska et al., 1968; Riddle et al., 2009; Frigon, 2017). In contrast, stimulation in more ventrocaudal regions of the nucleus reticularis gigantocellularis was most commonly associated with di- and multisynaptic inhibition of spinal motoneurons. These two zones have been hypothesized to play a role in the maintenance of posture and regulation of muscle tone during both awake behaviors and sleep (Takakusaki, 2017).
Control of muscle tone can be exerted by direct and indirect inhibitory or excitatory synaptic connections to spinal motoneurons, input to gamma motoneurons that alter the sensitivity of muscle spindles to stretch (Carpenter, 1991), or presynaptic inhibition of primary sensory afferents (Takakusaki, 2017). Trains of electric stimulation applied to either zone of the medial pontomedullary reticular formation can also evoke stereotypical patterns of muscle activation characterized by the coupling of upper-limb flexion with shoulder abduction on the ipsilateral side in conjunction with contralateral-limb extension and shoulder adduction (Davidson and Buford, 2004, 2006; Herbert et al., 2010). Similarly, the medial pontomedullary reticular formation is considered to play a role in the ipsilateral coupling of hip extension and adduction, knee extension and ankle plantarflexion, and contralateral coupling of hip flexion and abduction, knee flexion, and ankle dorsi-flexion (Thelen et al., 2003; Sanchez et al., 2017). Overexpression of these patterns is thought to contribute to the abnormal upper- and lower-limb synergies that emerge following lesions of the corticofugal pathways (e.g., stroke) (Brunnstrom, 1970).
The pontomedullary reticular formation is also an important structure for the generation of locomotion. Reticulospinal neurons in both dorsal and ventral regions of the medial medullary reticular formation receive extensive input from the MLR (cuneiform and rostral regions of the PPN). Glutamatergic input from the MLR drives the locomotor intensity and state (Roseberry et al., 2016). Electric stimulation of the medullary reticular formation during treadmill locomotion in the cat evokes responses characterized by activation of flexors and extensors that are modulated with the phase of the gait cycle, coupling of the ipsilateral flexors and contralateral extensors, or can reset the locomotor rhythm (Frigon, 2017). These findings demonstrate that the medullary reticular formation and lateral reticulospinal tract strongly influence activity in spinal CPG circuits.
The descending projections of the reticulospinal tract share two features with the lateral vestibulospinal tract: (1) a large proportion of axons have collaterals that innervate at multiple levels of the spinal cord; and (2) predominantly terminate on to premotor interneurons in Rexed’s laminae VII and VIII. Unlike the vestibulospinal inputs which primarily facilitate extensor and suppress flexor muscle activity, reticulospinal tract inputs facilitate or suppress both extensor and flexor activity in a task- or posture-dependent manner and often have reciprocal effects on the ipsilateral and contralateral side. This difference further supports the idea that the vestibulospinal system is specialized for the extensor muscle activity to control vertical support of support against gravity, whereas the reticulospinal input contributes to execution of complex muscle patterns (synergies) that provide task-appropriate postural support in conjunction with the intended movement (Schepens and Drew, 2004).
Locus coeruleus and caudal raphe (monoamine control of spinal motoneurons)
Descending neuromodulatory inputs from the brainstem act to increase the response of spinal motoneurons to inputs and prolong the firing in the absence of synaptic input. For this purpose, motoneurons are densely innervated by serotonergic and noradrenergic, and to a lesser extent dopaminergic, inputs (Montague et al., 2013) (Fig. 1.10A). The primary sources of the serotonergic and noradrenergic inputs are from the caudal raphe nucleus (nucleus raphe pallidus and obscurus) and locus coeruleus respectively (Maratta et al., 2015). These inputs facilitate motoneuron activity by: (1) amplifying and prolonging synaptic input (can increase the gain by up to fivefold) and (2) facilitating self-sustained firing through the induction of persistent inward currents (Fig. 1.10B) (for review, see Heckmann et al., 2005, 2009).
Fig. 1.10.
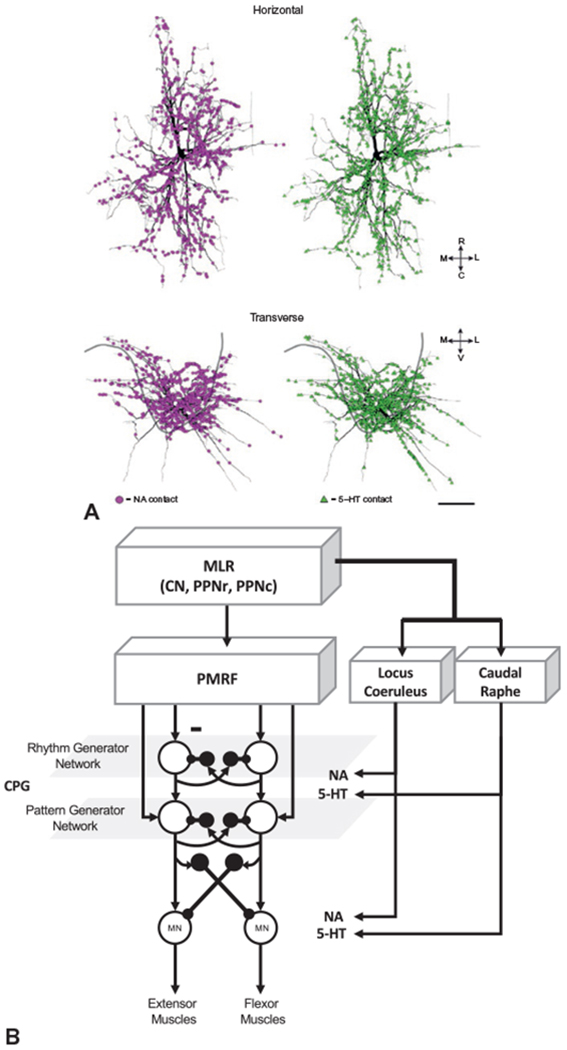
(A) Reconstruction of a spinal motoneuron showing the location and density of serotonergic (5-HT, green) and noradrenergic (NA, purple) inputs to the dendrites. (B) Summary of pathways mediating neuromodulatory (NA and 5-HT) inputs to the central pattern generator (CPG) network and spinal motoneurons from the brainstem. CN, cranial nerve; MLR, mesencephalic locomotor region; MN, motoneuron; PMRF, pontomedullary reticular formation; PPNc, caudal region of pedunculopontine nucleus; PPNr, rostral region of pedunculopontine nucleus. ((A) Reproduced from Montague SJ, Fenrich KK, Mayer-Macaulay C, et al. (2013) Nonuniform distribution of contacts from noradrenergic and serotonergic boutons on the dendrites of cat splenius motoneurons. J Comp Neurol 521: 638–656, with permission from John Wiley.)
Persistent inward currents allow the motoneuron to continue firing in the absence of synaptic input. These mechanisms are considered to play an important role in postural control by reducing the need for sustained synaptic input. Consistent with this hypothesis, estimates of the strength of persistent inward currents are higher in extensor compared with flexor muscles (Wilson et al., 2015). Loss of the descending monoaminergic pathways, such as occurs in spinal cord injury, has been proposed to contribute to the induction of spasticity and muscle spasms (ElBasiouny et al., 2010; Murray et al., 2010). The descending monoaminergic inputs from the locus coeruleus and caudal raphe nucleus are also proposed to play a role in changing the excitability of CPG networks in the spinal cord. Descending monoaminergic pathways from the locus coeruleus and caudal raphe nucleus also innervate inhibitory interneurons that are part of the CPG network. These inputs are required for inter- and intralimb coordination during locomotion (Jordan and Slawinska, 2011).
SPINAL CONTROL OF LOCOMOTION, CENTRAL PATTERN GENERATORS
As described above, the MLR and pontomedullary reticular formation are important for controlling the intensity and mode of locomotion. This control is exerted by descending reticulospinal projections to the spinal cord. However, the locomotor rhythm and the right–left and flexor–extensor patterns of muscle activity that govern bipedal gait in humans are principally controlled by a network of interneurons in the spinal cord that are collectively termed CPGs. Details of the interneuron types and their interconnectivity are beyond the scope of this chapter (McCrea and Rybak, 2008; Jordan and Slawinska, 2011).
In general, the CPG network is composed of two layers (Fig. 1.11). The first is comprised of bilateral, reciprocally connected flexor and extensor interneurons that generate the locomotor rhythm. The rhythm-generating layer projects to a second network of reciprocally connected interneurons that coordinate the muscle activation pattern. The key features of these organization are: each limb is controlled by a separate CPG, each CPG contains two groups of excitatory interneurons (the half-centers) that control the activity of flexors and extensors, reciprocal inhibitory interconnections between the half-centers ensure that only one center is active at a time, phase switching occurs when the excitability of one half-center falls below a critical value and the opposing center is released from inhibition (McCrea and Rybak, 2008). Note that each layer of the CPG network, including the spinal motoneurons, receives extensive somatosensory feedback from the limbs and descending projections from supraspinal centers. Somatosensory inputs have strong effects on locomotor phase, particularly during phase transitions (e.g., hip flexion during the transition from stance to swing), modulating limb trajectory in response to imposed displacements and loading, and reinforcing flexor–extensor activity (Frigon, 2017). Similarly, all layers of the CPG network receive descending supraspinal input (corticospinal, reticulospinal, vestibulospinal) that provides control of the locomotor pattern, intensity, and mode (transition from walking to running), alterations in limb trajectory, and the excitability of postural reflexes that accompany locomotion.
Fig. 1.11.
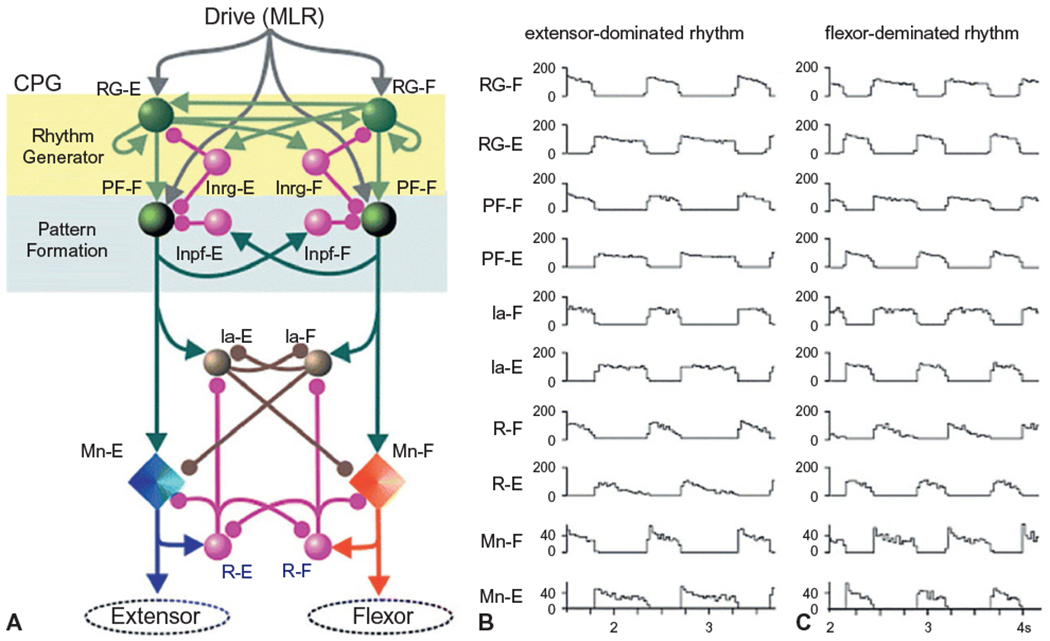
(A) Model of a two-level central pattern generator (CPG) network. The CPG structure is organized into a layer of interneurons that control rhythm generation and a layer that controls pattern generation. Note the reciprocal connections between the flexors and extensors. Sensory feedback is transmitted to all layers of the network (not shown). (B and C) Locomotion patterns generated by the two-level model. Note the alternating activities of the flexor and extensor rhythm- (RG) and pattern- (PF) generating networks, flexor and extensor inhibitory interneurons (Ia) and Renshaw cells (R), and extensor spinal motoneuron activity (Mn). In panel B, the mesencephalic locomotor region drive to RG-E is larger than to RG-F and results in a rhythm with a longer duration extensor phase. Panel C demonstrates the rhythm generated when RG-F drive is larger than RG-E. (Reproduced from Rybak et al. (2006).)
ACKNOWLEDGMENTS
Thank you to Dr. Sommer Amundsen Huffmaster for review of the manuscript and my mentor, Dr. William Tatton, who taught me the importance of “anatomy first.” Support for this work was provided, in part, by National Institutes of Health grants RO1 NS088697 and RO1 NS070264.
References
- Abzug C, Peterson BW (1973). Antidromic stimulation in the ponto-medullary reticular formation of local axon branches of contralateral vestibular neurons. Brain Res 64: 407–413. [DOI] [PubMed] [Google Scholar]
- Abzug C, Maeda M, Peterson BW et al. (1974). Cervical branching of lumbar vestibulospinal axons. J Physiol 243: 499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375. [DOI] [PubMed] [Google Scholar]
- Baker SN (2011). The primate reticulospinal tract, hand function and functional recovery. J Physiol 589: 5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I et al. (2006). Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci 26: 2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM (2016). Multisensory integration in balance control. Handb Clin Neurol 137: 57–66. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Guerraz M(1999). Visual-vestibular control of posture and gait: physiological mechanisms and disorders. Curr Opin Neurol 12: 5–11. [DOI] [PubMed] [Google Scholar]
- Brown AG (1982). The dorsal horn of the spinal cord. Q J Exp Physiol 67: 193–212. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S (1970). Movement therapy in hemiplegia: a neurophysiological approach, Harper & Row, New York, NY. [Google Scholar]
- Buford JA, Davidson AG (2004). Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G(1988). Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol 402: 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canedo A, Lamas JA (1993). Pyramidal and corticospinal synaptic effects over reticulospinal neurones in the cat. J Physiol 463: 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli P, Pivetta C, Soledad Esposito M et al. (2017). Locomotor speed control circuits in the caudal brainstem. Nature. 10.1038/nature24064. [DOI] [PubMed] [Google Scholar]
- Carpenter MB (1991). Core text of neuroanatomy, fourth edition, Williams & Wilkins, Baltimore. [Google Scholar]
- Carriot J, Jamali M, Brooks JX et al. (2015). Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: implication for perception. J Neurosci 35: 3555–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O, Harel R, Aumann TD et al. (2017). Parallel processing of internal and external feedback in the spinocerebellar system of primates. J Neurophysiol 118: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE (2012). The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA (2004). Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA (2006). Bilateral actions of the reticulospinal tract on armand shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39. [DOI] [PubMed] [Google Scholar]
- Day BL, Brown P (2001). Evidence for subcortical involvement in the visual control of human reaching. Brain 124: 1832–1840. [DOI] [PubMed] [Google Scholar]
- Day J, Bent LR, Birznieks I et al. (2017). Muscle spindles in human tibialis anterior encode muscle fascicle length changes. J Neurophysiol 117: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deecke L (1987). Bereitschaftspotential as an indicator of movement preparation in supplementary motor area and motor cortex. Ciba Found Symp 132: 231–250. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Yang JF, Patrick SK (1995). Mechanism for reflex reversal during walking in human tibialis anterior muscle revealed by single motor unit recording. J Physiol 488: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Marigold DS (2015). Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol 33: 25–33. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S (1986). Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol 55: 375–401. [DOI] [PubMed] [Google Scholar]
- Drew T, Cabana T, Rossignol S (1996). Responses of medullary reticulospinal neurones to stimulation of cutaneous limb nerves during locomotion in intact cats. Exp Brain Res 111: 153–168. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL (1996). Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 16: 6513–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2002). Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682. [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA et al. (1990). Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82: 351–358. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H (2000). Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Nicoll RA, Schwarz WF et al. (1975). Reticulospinal neurons with and without monosynaptic inputs from cerebellar nuclei. J Neurophysiol 38: 513–530. [DOI] [PubMed] [Google Scholar]
- ElBasiouny SM, Schuster JE, Heckman CJ (2010). Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121: 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M et al. (2006). In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res 1076: 87–92. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y et al. (2002). Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev 40: 53–65. [DOI] [PubMed] [Google Scholar]
- Frigon A (2017). The neural control of interlimb coordination during mammalian locomotion. J Neurophysiol 117: 2224–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Gossard JP (2010). Evidence for specialized rhythm-generating mechanisms in the adult mammalian spinal cord. J Neurosci 30: 7061–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K (1997). Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res 117: 1–16. [DOI] [PubMed] [Google Scholar]
- Gahery Y, Massion J (1981). Coordination between posture and movement. Trends in Neurosciences 4: 199–202. [Google Scholar]
- Garcia-Rill E, Skinner RD (1987). The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res 411: 1–12. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Homma Y, Skinner RD (2004). Arousal mechanisms related to posture and locomotion: 1. Descending modulation. Prog Brain Res 143: 283–290. [PubMed] [Google Scholar]
- Goodale MA (1996). Visuomotor modules in the vertebrate brain. Can J Physiol Pharmacol 74: 390–400. [PubMed] [Google Scholar]
- Goodale MA, Milner AD (1992). Separate visual pathways for perception and action. Trends Neurosci 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Grigg P, Hoffman AH (1982). Properties of Ruffini afferents revealed by stress analysis of isolated sections of cat knee capsule. J Neurophysiol 47: 41–54. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S (1968). Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinalmotoneurones. Brain Res 10: 477–480. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S (1970). The vestibulospinal tract. Effects on alpha-motoneurones in the lumbosacral spinal cord in the cat. Exp Brain Res 10: 94–120. [DOI] [PubMed] [Google Scholar]
- Haggerty SE, Wu AR, Sienko KH et al. (2017). A shared neural integrator for human posture control. J Neurophysiol: jn00428 02016. 10.1152/jn.00428.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XW,Wu CP (1985). Connections between pericruciate cortex and the medullary reticulospinal neurons in cat: an electrophysiological study. Exp Brain Res 61: 109–116. [DOI] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ (2005). Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156. [DOI] [PubMed] [Google Scholar]
- Heckmann CJ, Mottram C, Quinlan K et al. (2009). Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120: 2040–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert WJ, Davidson AG, Buford JA (2010). Measuring the motor output of the pontomedullary reticular formation in the monkey: do stimulus-triggered averaging and stimulus trains produce comparable results in the upper limbs? Exp Brain Res 203: 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Inglis JT, Blouin JS et al. (2017). Both standing and postural threat decrease Achilles’ tendon reflex inhibition from tendon electrical stimulation. Journal of Physiology-London 595: 4493–4506; 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Bastianen C, Fansler D et al. (2007). Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philos Trans R Soc Lond B Biol Sci 362: 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon M, Massion J, Wiesendanger M (1982). Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflugers Arch 393: 292–296. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Kennedy PM, Wells C et al. (2002). The role of cutaneous receptors in the foot.Adv Exp Med Biol 508: 111–117. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG et al. (1995). Self-initiated versus externally triggered movements I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118: 913–933. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T et al. (2008). Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage 39: 786–792. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lundberg A et al. (1968). Inhibitory effects evoked through ventral reticulospinal pathways. Arch Ital Biol 106: 124–140. [PubMed] [Google Scholar]
- Jinnai K (1984). Electrophysiological study on the corticoreticular projection neurons of the cat. Brain Res 291: 145–149. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Slawinska U (2011). Chapter 12—modulation of rhythmic movement: control of coordination. Prog Brain Res 188: 181–195. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB et al. (2008). Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191. [DOI] [PubMed] [Google Scholar]
- Kably B, Drew T (1998). Corticoreticular pathways in the cat I. Projection patterns and collaterization. Journal of Neurophysiology 80: 389–405. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG (1984). Distribution of corticospinal neurons with collaterals to lower brain stem reticular formation in cat. Exp Brain Res 54: 107–120. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HG (1989). Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 74: 311–318. [DOI] [PubMed] [Google Scholar]
- Konczak J, Corcos DM, Horak F et al. (2009). Proprioception and motor control in Parkinson’s disease. J Mot Behav 41: 543–552. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR et al. (2010). Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Pearson KG (2001). Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J Neurophysiol 86: 1321–1332. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM (1968). The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Apps R, Bengtsson F et al. (2017). The roles of the olivocerebellar pathway in motor learning and motor control. A consensus paper. Cerebellum 16: 230–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN (1993). The G. L. Brown Prize Lecture. Cortical control of the primate hand. Exp Physiol 78: 263–301. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED (1978). Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. J Physiol 284: 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratta R, Fenrich KK, Zhao E et al. (2015). Distribution and density of contacts from noradrenergic and serotonergic boutons on the dendrites of neck flexor motoneurons in the adult cat. J Comp Neurol 523: 1701–1716. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Drew T (2011). Contribution of cells in the posterior parietal cortex to the planning of visually guided locomotion in the cat: effects of temporary visual interruption. J Neurophysiol 105: 2457–2470. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J (2011). Topographical organization of the pedunculopontine nucleus. Front Neuroanat 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MA, Camp AJ, Murray AJ (2017). Reviewing the role of the efferent vestibular system in motor and vestibular circuits. Front Physiol 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Drew T (2000). Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat I. Walking on a level surface. J Neurophysiol 84: 2237–2256. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Jankowska E (2004). Coupling between feline cerebellum (fastigial neurons) and motoneurons innervating hindlimb muscles. J Neurophysiol 91: 1183–1192. [DOI] [PubMed] [Google Scholar]
- McCall AA, Miller DM, Yates BJ (2017). Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front Neurol 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildren RL, Strzalkowski ND, Bent LR (2016). Foot sole skin vibration perceptual thresholds are elevated in a standing posture compared to sitting. Gait Posture 43: 87–92. [DOI] [PubMed] [Google Scholar]
- Mileusnic MP, Loeb GE (2006). Mathematical models of proprioceptors II. Structure and function of the Golgi tendon organ. J Neurophysiol 96: 1789–1802. [DOI] [PubMed] [Google Scholar]
- Mileusnic MP, Brown IE, Lan N et al. (2006). Mathematical models of proprioceptors I. Control and transduction in the muscle spindle. J Neurophysiol 96: 1772–1788. [DOI] [PubMed] [Google Scholar]
- Mille ML, Creath RA, Prettyman MG et al. (2012). Posture and locomotion coupling: a target for rehabilitation interventions in persons with Parkinson’s disease. Parkinsons Dis 2012: 754186 10.1155/2012/754186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW (1996). The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Montague SJ, Fenrich KK, Mayer-Macaulay C et al. (2013). Nonuniform distribution of contacts from noradrenergic and serotonergic boutons on the dendrites of cat splenius motoneurons. J Comp Neurol 521: 638–656. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B et al. (1999). Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol 82: 290–300. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ et al. (2010). Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A (1996). Carpenter’s human neuroanatomy, 9th edition Williams and Wilkins, Baltimore, MD. [Google Scholar]
- Patla AE, Prentice SD, Robinson C et al. (1991). Visual control of locomotion: strategies for changing direction and for going over obstacles. J Exp Psychol Hum Percept Perform 17: 603–634. [DOI] [PubMed] [Google Scholar]
- Peterson BW (1972). Responses of vestibular nuclear neurons to macular input. Prog Brain Res 37: 109–120. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Abzug C (1975). Properties of projections from vestibular nuclei to medial reticular formation in the cat. J Neurophysiol 38: 1421–1435. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Anderson ME, Filion M (1974). Responses of ponto-medullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res 21: 19–44. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K (1979). Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36: 1–20. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Fukushima K, Hirai N et al. (1980). Responses of vestibulospinal and reticulospinal neurons to sinusoidal vestibular stimulation. J Neurophysiol 43: 1236–1250. [DOI] [PubMed] [Google Scholar]
- Pfann KD, Buchman AS, Comella CL et al. (2001). Control of movement distance in Parkinson’s disease. Mov Disord 16: 1048–1065. [DOI] [PubMed] [Google Scholar]
- Potocanac Z, Duysens J (2017). Online adjustments of leg movements in healthy young and old. Exp Brain Res 235: 2329–2348. [DOI] [PubMed] [Google Scholar]
- Prentice SD, Drew T (2001). Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol 85: 679–698. [DOI] [PubMed] [Google Scholar]
- Prut Y, Fetz EE (1999). Primate spinal interneurons show pre-movement instructed delay activity. Nature 401: 590–594. [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Day BL (2005). Visual guidance of the human foot during a step. J Physiol 569: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN (2009). Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry TK, Lee AM, Lalive AL et al. (2016). Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164: 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC (1994). Control of human voluntary movement, 2nd edition Chapman & Hall, London. [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M et al. (2006). Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577: 617–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez N, Acosta AM, Lopez-Rosado R et al. (2017). Lower extremity motor impairments in ambulatory chronic hemiparetic stroke: evidence for lower extremity weakness and abnormal muscle and joint torque coupling patterns. Neurorehabil Neural Repair 31: 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T (2004). Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238. [DOI] [PubMed] [Google Scholar]
- Smetana R, Juvin L, Dubuc R et al. (2010). A parallel cholinergic brainstem pathway for enhancing locomotor drive. Nat Neurosci 13: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng ML, Popa LS, Ebner TJ (2017a). Climbing fibers control Purkinje cell representations of behavior. J Neurosci 37: 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng ML, Popa LS, Ebner TJ (2017b). Climbing fibers predict movement kinematics and performance errors. J Neurophysiol jn: 00266 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M et al. (2007). D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30: 228–235. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Shinoda Y (2014). Convergent synaptic inputs from the caudal fastigial nucleus and the superior colliculus onto pontine and pontomedullary reticulospinal neurons. J Neurophysiol 111: 849–867. [DOI] [PubMed] [Google Scholar]
- Takakusaki K (2017). Functional neuroanatomy for posture and gait control. J Mov Disord 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Obara K, Nozu T et al. (2011). Modulatory effects of the GABAergic basal ganglia neurons on the PPN and the muscle tone inhibitory system in cats. Arch Ital Biol 149: 385–405. [DOI] [PubMed] [Google Scholar]
- Thach WT (1975). Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res 88: 233–241. [DOI] [PubMed] [Google Scholar]
- Thelen DD, Riewald SA, Asakawa DS et al. (2003). Abnormal coupling of knee and hip moments during maximal exertions in persons with cerebral palsy. Muscle Nerve 27: 486–493. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982). Two cortical visual systems In: Ingle DJ, Goodale MA, RJW Mansfield (Eds.), Analysis of visual behavior. MIT Press, Cambridge, MA, pp. 549–586. [Google Scholar]
- Weerdesteyn V, Nienhuis B, Hampsink B et al. (2004). Gait adjustments in response to an obstacle are faster than voluntary reactions. Hum Mov Sci 23: 351–363. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M (1969). Comparison of effects of stimulation of Deiters’ nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol 32: 743–758. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Thompson CK, Miller LC et al. (2015). Intrinsic excitability of human motoneurons in biceps brachii versus triceps brachii. J Neurophysiol 113: 3692–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA (1989). Biomechanics of normal and pathological gait: implications for understanding human locomotor control. J Mot Behav 21: 337–355. [DOI] [PubMed] [Google Scholar]
- Winter DA (1993). Knowledge base for diagnostic gait assessments. Med Prog Technol 19: 61–81. [PubMed] [Google Scholar]
- Woolf CJ (1987). Central terminations of cutaneous mechanoreceptive afferents in the rat lumbar spinal cord. J Comp Neurol 261: 105–119. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M(2005). A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 128: 2250–2259. [DOI] [PubMed] [Google Scholar]


