Abstract
Background:
Individuals who are resilient are more likely to engage in functional tasks and exercise post hip fracture. There may be a genetic predisposition to being resilient.
Objectives:
This study tested the direct and indirect association of 10 candidate genes, age, cognition, gender, comorbidities, pain and social activity on resilience, function and exercise post hip fracture.
Method:
This was a descriptive study including 172 community dwelling older adults. Measures included: age, gender, cognition (Modified Mini Mental Status Exam), comorbidities, social activities (self-report), DNA (GRM1, NTRK1, NTRK2, GNB3, NPY, SLC6A15. SLC6A4, BDNF, CR1TR1, FKBP5), pain (areas of pain and Numeric Rating Scale), function (Physical and Instrumental Activities of Daily Living; Lower Extremity Gains Score; Short Physical Performance Battery; Grip Strength) and exercise (Yale Physical Activity Scale).
Results:
The majority of participants were Caucasian (93%), 50% were women and the average age was 81.09 (SD = 7.42). There were significant associations between resilience and single nucleotide polymorphisms from GRM1, NTRK1, NTRK2, GNB3, NPY and SLC6A15. Resilience, age, cognition, social activity, pain and genetic variability were directly and/or indirectly associated with exercise and/or function.
Discussion:
This study highlights the importance of resilience for engagement in exercise and function after hip fracture and provides preliminary evidence for a genetic role for resilience.
Keywords: Genetics, Resilience, Function, Exercise
Background
Hip fractures commonly occur among older adults, with over 1.5 million occurring each year worldwide (Cheng et al., 2011). Unfortunately, approximately 50% of individuals who sustain a hip fracture do not return to their pre-fracture level of function (Brauer et al., 2009; United States Deparmtent of Health and Human, 2004). At 12 months post hip fracture these individuals remain unable to rise from an armless chair independently or step symmetrically, and approximately 38%–50% need assistance to walk a short distance or are unable to walk (Binder et al., 2004; Cecchi et al., 2018; Gruber-Baldini et al., 2003; Kammerlander et al., 2011; Mangione et al., 2005; Morri et al., 2018; Munter et al., 2018). In addition, they engage in limited exercise (Ingemarsson et al., 2003; Morri et al., 2018; Munter et al., 2018; Resnick et al., 2011), defined as planned, structured activity geared toward improving physical fitness (Caspersen et al., 1985).
Engaging in functional tasks and exercise post hip fracture has been shown to improve recovery and prevent or reduce the functional decline that can otherwise occur (Chudyk et al., 2009; Moayyeri, 2008; Pahikanti and Von Ah, 2012; Pedersen et al., 2013). It has been repeatedly shown that strength training and walking programs improve function, enhance strength and aerobic capacity, and prevent disability among those who sustained hip fractures (Binder et al., 2004; Latham et al., 2014; Tsauo et al., 2005).
Although known to be beneficial, there are many challenges to engaging older adults in functional tasks or exercise (Benedetti et al., 2009; Browning et al., 2009). This is particularly true for those who have sustained a hip fracture or other types of acute events (Hill et al., 2011; Resnick et al., 2007; Taraldsen et al., 2014). The social ecological model provides one way to understand the challenges associated with function and physical activity post hip fracture (Gregson et al., 2003). This model suggests that an individual’s behavior is affected by intrapersonal, interpersonal, environment and policy related factors and the ongoing interactions that occur among these factors. The purpose of this study was to focus on the intrapersonal and interpersonal factors that influence function and physical activity and test the direct and indirect impact of 10 candidate genes, age, cognition, gender, comorbidities, pain and social interactions on resilience, function and exercise at two months post hip fracture.
Intrapersonal factors include such things as: genotype, age, gender, comorbidities, acute medical problems, pain and psychological factors such as resilience (Flierl et al., 2010; Ingemarsson et al., 2003). Resilience refers to the capacity to spring back from a physical, emotional, financial, or social challenge. Being resilient indicates that the individual has the human ability to adapt in the face of tragedy, trauma, adversity, hardship, and ongoing significant life stressors (Newman, 2005). Resilient individuals tend to demonstrate adaptive behavior, especially with regard to social functioning, morale, and somatic health, and are less likely to succumb to illness (Resnick et al., 2015; van Kessel, 2013; Wild et al., 2013). Increasingly, there is evidence that resilience is related to motivation and recovery following physical or psychological trauma (Charney, 2004; Chow et al., 2007; Sanders et al., 2008). Thus, individuals who are resilient are more likely to engage in functional tasks and exercise post hip fracture (Robinson et al., 2014; Ziaian et al., 2012).
Resilience is an intrapersonal aspect of behavior and is a component of the individual’s personality. Resilience, however, develops and changes over time through ongoing experiences with the physical and social environment and interpersonal interactions. Physiologically, resilience has been associated with the individual’s flexibility in his or her neurochemical stress response systems and the neural circuitry involved in stress responses. Prior research has shown that being exposed to acute or chronic stress can result in depression, anxiety or other types of negative psychological and physical outcomes (Farhang et al., 2014; Southwick and Charney, 2012; Taliaz et al., 2011). Stress leads to alterations in brain structures associated with cognition, mood and behavior within the hypothalamic pituitary-adrenocortical (HPA) axis (Bowes and Jaffee, 2013). Numerous neurotransmitters, neuropeptides and hormones have also been associated with response to stress and thereby influence the individual’s resilience in response to the stressor (Charney, 2004).
Serotonin has been the most commonly studied neurotransmitter and the serotonin gene, solute carrier family 6 neurotransmitter transporter (SLC6A4) is the only gene noted to be associated with resilience among older adults (Feder et al., 2009; O’Hara et al., 2012; Resnick et al., 2015). Some additional genes have inconsistently been associated with resilience. These genes include brain-derived neurotrophic factor (BDNF), corticotropin-releasing hormone receptor 1 (CRHR1), peptidyl-prolyl cis-trans isomerase (FKBP5), glutamate receptor metabotropic 1 (GRM1), solute carrier family 6 member 15 (SLC6A15) and solute carrier family 6 member 4 (Feder et al., 2009; Shaoa et al., 2013). Other genes have been associated with response to stress and depression in animal models and/or humans and, therefore, are anticipated to possibly be associated with resilience. These genes include neuropeptide Y (NYP), neurotrophic tyrosine receptor kinase-1 and 2 (NTRK-1; NTRK-2) and guanine nucleotide binding protein beta polypeptide 3 (GNB3) (Duclot and Kabbaj, 2013; Farhang et al., 2014; Lin et al., 2009; López-León et al., 2008; Pizzorusso et al., 1999; Taliaz et al., 2011). We, therefore, considered the direct influence of these genes on resilience among older adults post hip fracture and their indirect effect on function and exercise through resilience.
In addition to intrapersonal factors, interpersonal factors can influence performance of functional tasks and time spent in exercise post hip fracture. Interpersonal factors include interactions individuals encounter through the recovery process (Hill et al., 2011); verbal encouragement from a therapist, spouse or significant other to exercise, for example, or seeing others exercise post hip fracture can impact behavior (Resnick et al., 2011; Resnick et al., 2007; Resnick & D’Adamo, 2011). Gaining a better understanding of the intra- and interpersonal factors that impact recovery post hip fracture, particularly with regard to genetics and resilience, will help identify individuals who may be at risk for having low resilience. These identified individuals may be less likely to successfully recover post hip fracture. Once identified, targeted approaches can be implemented to help these individuals strengthen their resilience and optimize recovery.
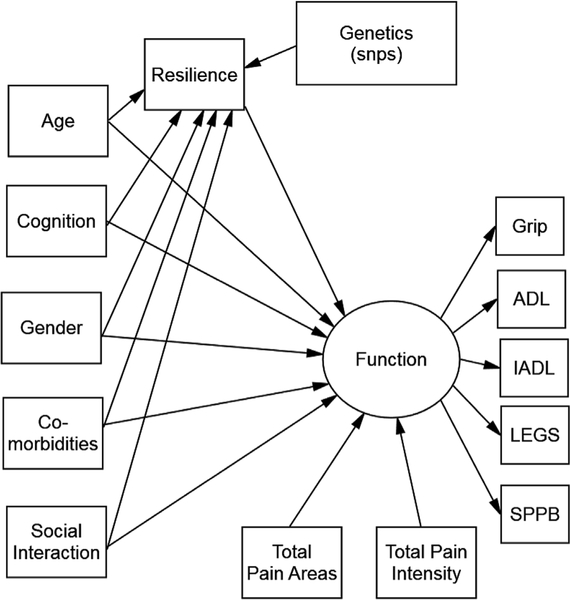
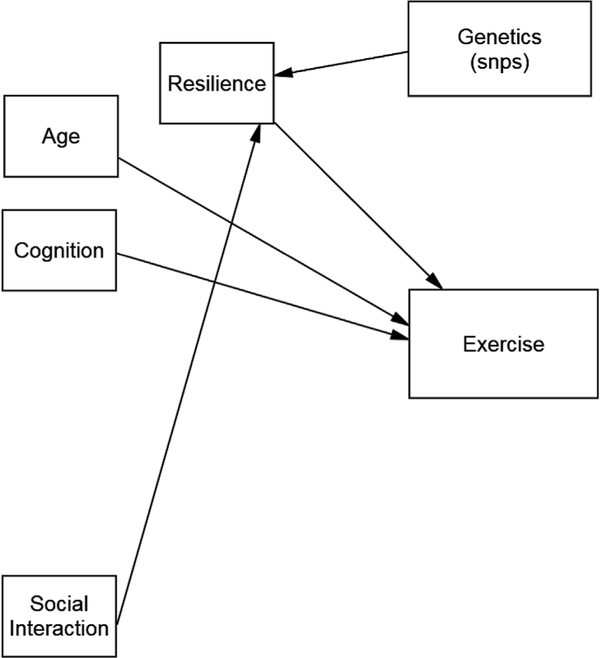
The hypothesized models tested in this study are shown in Figs. 1 and 2. Fig. 1 explores the factors associated with time spent in functional activities and Fig. 2 explores the factors associated with time spent in exercise. Specifically, we hypothesized that variation in the identified candidate genes, age, cognition, gender, comorbidities and social support were directly associated with resilience and all of these variables were directly or indirectly associated with function, as noted in Fig. 1 and with exercise, as noted in Fig. 2.
Fig. 1.
Hypothesized model forfunction.
Fig. 2.
Hypothesized model for exercise.
Methods
Design and sample
Data for this analysis came from the seventh cohort of the Baltimore Hip Studies (BHS-7). BHS-7 was a prospective cohort study comparing men and women, frequency matched (1:1) based on calendar time of fracture and hospital to assure equal numbers of men and women. Eligible individuals were community-dwelling, aged 65 years or older and admitted for surgical repair of a hip fracture to one of eight study hospitals located in the Baltimore metropolitan area. Individuals were excluded if they had a pathological fracture, were bedbound during the 6 months prior to the fracture, non-English-speaking, resided more than 70 miles from the hospital, weighed more than 300 pounds, or had some type of hardware in the contralateral hip (either due to hip fracture or hip replacement). The first 200 study participants were included in this analysis. To recruit these participants, 911 hip fracture patients were screened, 517 (57%) were eligible (222 males; 295 females), and 105 men and 107 women consented to participate. A total of 23 participants were withdrawn (8 participants failed to provide data at the baseline and 2-month follow up visit, 6 were found to be ineligible and another 9 participants were removed from the analysis sample as a result of an IRB-requested post procedure audit) leaving 189 participants. Of these 189 participants, 172 (91%) had a DNA sample and were included in this analysis. The parent study was approved by the Institutional Review Boards of the University of Maryland Baltimore, and review boards within the participating hospitals.
Measures
Following consent, study personnel (nurses and trained evaluators) performed a baseline face-to-face structured interview (within 22 days of hospital admission) including a questionnaire for demographic information and comorbidities and a blood sample was obtained. At two, six and twelve months post hip fracture, participants were interviewed again face-to-face and asked about function, physical activity, psychosocial factors, and pain and were observed completing basic functional tasks. Another blood sample was also obtained at the follow up time points. A proxy was asked to respond to survey questions for any participants that scored < 36 on the Modified Mini-Mental State Examination (3MS) (Bassett et al., 1990; Magaziner et al., 1996). With the exception of baseline demographic information (age, gender, and education), the 2 month follow up data was used for all analyses. Blood samples for DNA were obtained from the first blood sample provided. All measures have established evidence of reliability and validity when used with older adults.
Cognitive status was evaluated using the Modified Mini-Mental State Examination (3MS) (MacKnight et al., 1999). The 3MS ranges from 0 to 100. As previously established, a score of less than 79 was indicative of cognitive impairment (The Modified Mini-Mental State (3MS) The Modified Mini-Mental State (3MS) Test, 2014). To describe comorbidities, the modified Charlson Comorbidity Index (Charlson et al., 1987) was completed using data from medical records.
Function
Function was conceptualized using a measurement model that included five measures of function: Physical Activities of Daily Living (PADL), Instrumental Activities of Daily Living (IADL), lower extremity performance based on the Lower Extremity Gain Scale (LEGS) (Hawkes et al., 2004; Zimmerman et al., 2006), physical performance based on the Short Physical Performance Battery (SPPB) (Guralnik et al., 1994), and grip strength. PADL focused on lower extremity functioning using a modified form of the Functional Status Index (Jette, 1980) and patients were asked about their ability to perform 12 lower extremity functional tasks with no assistance, equipment or human assistance, or if they did not perform the task for health or other reasons during the past week. Scores ranged from 0 to 12 with higher scores representing greater impairment (Jette et al., 1987). IADLs were obtained using the modified version of the Older Americans Resources and Services Instrument (OARS) (Plotnikoff et al., 2000). This measure includes the evaluation of performance on seven tasks of daily living during the preceding two weeks. The scale ranges from zero to seven with higher scores representing greater impairment.
LEGS is a performance-based measure that focuses on clinically relevant aspects of functioning post hip fracture (Zimmerman et al., 2006). Higher scores were indicative of better performance. The SPPB (Guralnik et al., 1994) was completed by having patients perform tasks that mimic daily activities. Grip strength was evaluated using the JAMAR Hydraulic Hand Dynamometer (Ashford et al., 1996; Bohannon and Schaubert, 2005; Roberts et al., 2011).
Exercise
Exercise was measured with the exercise subscale of the Yale Physical Activity Survey (YPAS) (Dipietro et al., 1993; Pescatello et al., 1994). The YPAS is an interviewer-administered questionnaire which includes five subscales of common groups of work including: house work, yard work, exercise, recreational activities and caretaking performed during a typical week (Dipietro et al., 1993; Pescatello et al., 1994). The exercise subscale defines exercise as moderate level physical activity including such things as walking at a moderate or brisk pace of 3–4.5 miles per hour or 100 steps per minute, biking, swimming, or jogging. Social interaction was based on self-report of the number of social activities the individual participated in during the two weeks prior to interview (House et al., 1982).
Resilience
Resilience was evaluated using a modified version of the 25-Item Resilience Scale (Wagnild, 2003; Wagnild and Young, 1993). The Resilience Scale is a general measure of resilience for adults across the lifespan. The measure was modified based on prior work noting that 10 of the original 25 items did not fit the measurement model based on Rasch Analysis and confirmatory factor analysis (Resnick and Inguito, 2011). These 10 items were redundant or unclear for older participants and thus were removed, resulting in a 15-item scale. Responses ranged from 1 (disagree) to 7 (agree). The final scores ranged from 15 to 105, with higher scores indicating more resilience.
Pain
Pain was evaluated based on a sum of the total areas of pain and a sum of overall pain intensity across all those areas. Specifically participants were asked if, in the past month, they experienced upper extremity, hip, knee, back or ankle pain. In addition, to reflect intensity, they were asked to rate their pain in each area on a scale of 0–10. Scores ranged from 0 to 50 with higher scores indicative of more intense pain.
Genotyping
DNA was extracted from 3.5 to 7 ml of whole blood drawn into a BD vacutainer® ACD blood collection tube (BD, Ref# 364606). Subjects were genotyped using the Affymetrix 1M SNP chip (v6.0, Santa Clara, CA USA) by the Genomics Core Laboratory at the University of Maryland according to the manufacturer’s recommendations. Genotype calling was done using Birdseed algorithm (v2.0) which is part of the Birdsuite tools (Korn et al., 2008). Ten candidate genes and associated single nucleotide polymorphisms (SNPs) were included in the analysis. As noted above the 10 genes that were included in model testing were GRM1, NTRK1, NTRK2, GNB3, NPY, BDNF, SLC6A4, CRHR1 COMT, and SLC6A15.
Statistical analysis
Descriptive analyses were used to describe the sample and structural equation modeling was used to test our hypothesized models using SPSS and AMOS (Arbuckle, 1997). Data were checked for missing values, normality and outliers. The measurement model for function was tested prior to full model testing. Model fit was assessed using chi-square statistic (χ2)/df, and the root mean square error of approximation (RMSEA) (Bollen, 1989). Path significance was based on the Critical Ratio (CR) (Arbuckle, 1997). Significance for parameter estimates was set at p ≤ .05. In all of the models tested, all paths in the measurement model for function were significant and there was a good fit of the data to the measurement model (χ2/df of 1.98, p = .08; RMSEA = 0.05).
Results
As per study design, 50% of the participants were women. The majority of the participants were Caucasian (93%), and the average age was 81.09 (SD = 7.42). As shown in Table 1, cognitive testing indicated that participants were without significant cognitive impairment with a mean score of 82.55 (SD = 19.28) on the 3MS. At 2 months post hip fracture, the participants had fair resilience (mean 84.01, SD 13.11), engaged in limited time in exercise (less than 3 min per week) and had functional impairments with a mean of 15.27 (SD = 10.09) on LEGS, 3.03 (SD = 2.45) on the SPPB, a 3.37 (SD = 1.10) on IADLs, a 7.57(SD = 3.21) on PADL and a mean grip strength of 24.07 (SD = 8.82). Overall the participants had pain in two locations (mean 2.33, SD = 1.39) and a mean total pain intensity across all areas of 9.43 (SD = 9.94). Pain intensity was reported to be lowest at the hip.
Table 1.
Description of study participants on all model variables.
| Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|
| Model Variables | ||||
| Age | 65 | 96 | 81.09 | 7.42 |
| Comorbidities | 0 | 8 | 2.22 | 1.89 |
| Years of Schooling | 5 | 20 | 13.26 | 3.43 |
| Resilience | 43 | 105 | 84.01 | 13.11 |
| Exercise time hours/week | 0 | 17.50 | 2.79 | 3.28 |
| Modified Mini Mental Score | 4 | 100 | 82.55 | 19.28 |
| Social Interaction | 0 | 26 | 5.92 | 6.96 |
| Total areas of Pain | 0 | 5 | 2.33 | 1.39 |
| Total pain intensity | 0 | 39 | 9.43 | 9.94 |
| Hip pain | 0 | 10 | 2.88 | 3.41 |
| Knee pain | 1 | 10 | 5.34 | 2.71 |
| Back pain | 1 | 10 | 5.47 | 2.64 |
| Foot/ankle pain | 1 | 10 | 5.63 | 2.39 |
| Upper extremity pain | 0 | 10 | 5.30 | 2.12 |
| Model of Function | ||||
| Lower Extremity Gains Scale | .00 | 0–35 | 15.27 | 10.09 |
| Short Physical Performance Battery | .00 | 11.00 | 3.03 | 2.45 |
| Grip Strength in kilograms | 2.00 | 47.00 | 24.07 | 8.82 |
| Instrumental Activities of Daily Living | 0.00 | 4.00 | 3.37 | 1.10 |
| Physical Activities of Daily Living | 0.00 | 12.00 | 7.57 | 3.21 |
Model results
Full model testing using function as the primary outcome resulted in 12 of the hypothesized paths being significant although there was a poor fit of the data to the full hypothesized model (χ2/df of 7.5, a NFI of 0.32 and a RMSEA of 0.20). Removing non-significant paths resulted in no improvement in model fit (revised models had a χ2/df of 6.2, the NFI was 0.48 and the RMSEA was 0.12; χ2 difference of 1.3 and df difference of 29, p > .05). As shown in Fig. 3, for the sake of parsimony, the smaller revised model was used for all model testing. Genetic variability and social activities were associated with resilience and accounted for 4–8% of the variance in resilience. Resilience, age, cognition, social activity, and pain intensity were directly associated with function and genetic variability was indirectly associated with function through resilience. Together these variables accounted for 35% of the variance in function.
Fig. 3.
Significant paths for function model.
With regard to exercise, full model testing showed that only five of the hypothesized paths were significant and there was a poor fit of the data to the model (χ2/df of 8.1, an NFI of 0.14 and a RMSEA of 0.20). Repeat testing of the revised model with non-significant paths removed (Fig. 4) was done and there was a significant improvement in model fit (revised model had a χ2/df of 3.6, the NFI was 0.49 and the RMSEA was 0.12; χ2 difference of 4.5 and df difference of 21, p < .05). Six of the 10 genes hypothesized to be included in the models were noted to be significantly associated with resilience and function or exercise. Specifically, variability in GRM1, NTRK1, NTRK2, GNB3, NPY and SLC6A15 was associated with resilience as was participation in social activities. Together, these factors accounted for 4–7% of the variance in resilience in all models testing the factors that influenced exercise. In addition, resilience, age and cognition were directly associated with exercise. Genetic variability was indirectly associated with exercise through resilience. Together, these variables accounted for 20% of the variance in exercise. Path results of model testing for each of the genes and their associated SNPs are provided in Appendices 1 and 2 online.
Fig. 4.
Significant paths for exercise model.
Discussion
The findings from this study provide support for the association between genetics and resilience and the mediating effect of resilience on function and exercise post hip fracture. Specifically, GRM1, NTRK1, NTRK2, GNB3, NPY and SLC6A15 were the six genes associated with resilience. Prior research has also noted the effects of these genes on psychological factors including depression, stress and resilience (Fox et al., 2009; Graham et al., 2013; Wald et al., 2013). Unlike prior research (Dunn et al., 2014; Graham et al., 2013; O’Hara et al., 2012; Rana et al., 2014; Resnick et al., 2015), we did not find an association between FKBP5, BDNF, CRHR1 or SLC6A4 and resilience. We anticipate that some of the differences in our findings and that of others may be due to the way in which resilience was measured. Individuals may be resilient across a variety of challenges: economically, psychologically, socially, physically or cognitively, or they may generally be resilient with regard to any type of challenge they experience. Most studies considering genetic associations with resilience considered psychological resilience (Graham et al., 2013; O’Hara et al., 2012) or physical resilience (Resnick et al., 2015). In the current study, resilience was measured using a general measure of resilience.
The findings from this study provide additional support for the relationship between resilience and function and time spent in exercise (Pérez-López et al., 2014; Resnick & D’Adamo, 2011; Sun et al., 2014). Particularly for older adults in the post hip fracture period, performing functional tasks and engaging in exercise is challenging and stressful. Testing of the full hypothesized model demonstrated that age, gender, comorbidities and cognition did not influence resilience. In addition to genetic variability, only social activities were associated with resilience. Given the cross-sectional nature of this study it was not possible to determine causation. It is possible that those who were more resilient engaged in more social activities. Conversely, it is also possible that social activities strengthened resilience by building self-efficacy and confidence through participation in activities (Resnick, 2014; Resnick et al., 2011).
Demonstrating the importance of resilience in relation to functional performance and exercise post hip fracture suggests a need to implement interventions to strengthen resilience among older adults post hip fracture. Strengthening self-efficacy and outcomes expectations has consistently been shown to strengthen resilience with regard to recovery from a stressful event. Self-efficacy expectations are defined as an individuals’ judgment of their confidence to carry out specific behaviors (Bandura, 1995, 1997). Outcome expectations are the belief that if a behavior is performed there will be a certain outcome. The theory of self-efficacy, which is based on Social Cognitive Theory (Bandura, 1995, 1997), proposes that the stronger the individuals’ perceived self-efficacy and outcome expectations, the more vigorous and persistent their efforts to initiate and adhere to a behavior (Bandura, 1995, 1997). Four sources of information have been noted to influence an individuals’ cognitive appraisal of efficacy expectations: (1) enactive mastery experience or actually performing the behavior; (2) verbal persuasion or encouraging someone to perform the behavior; (3) vicarious experience or seeing like others perform the behavior; and 4) the impact of physiological and affective cues experienced during an activity such as pain, anxiety or enjoyment. Implementing interventions using these four sources of information can strengthen self-efficacy and outcome expectations and thereby strengthen resilience.
Knowing that there is a genetic association with resilience is important clinically as being able to identify individuals who are more or less likely to be resilient based on genetic testing allows clinicians to allocate resources and implement interventions with those most likely to need them. The genes found to be associated with resilience in this study affect things like neuroplasticity (the ability of the brain to reorganize itself), serotonin (a neurotransmitter that influences mood) or glutamate (a neurotransmitter important for memory, cognition and function). All of these genes are important for recovery in the post hip fracture period. The negative effects of these genes can be reversed post hip fracture through interventions such as exercise, stress reduction techniques (e.g. meditation, massage) and building self-efficacy around recovery by using techniques such as: engaging individuals in physical activity, providing verbal encouragement and positive reinforcement, exposing patients to positive role models and eliminating unpleasant sensations such as fear of falling or getting hurt.
Study participants spent very little time participating in exercise and this decreased over time. It is likely that the majority of the exercise that was occurring at 2 months post hip fracture was associated with ongoing physical therapy. In addition to resilience, the only factors that were directly associated with exercise were age and cognitive status. As would be expected, those who were younger and more cognitively intact spent more time exercising. Although males are generally believed to engage in more exercise than women (United States Bureau of Labor Statistics, 2015), this was not noted in this study. It is possible that with increased age and/or following an acute event such as a hip fracture gender differences with regard to exercise are not maintained. Pain was also not associated with time spent in exercise. This was consistent whether or not pain was based on the number of areas in which the individual experienced pain or intensity of pain across all the areas that pain was experienced.
The factors associated with function were similar to those reported related to exercise, with the exception of the impact of pain intensity and social activities on functional performance. Specifically, those who were younger, more intact cognitively, had less intense pain and participated in more social activities had better function. These findings suggest that there is a need to decrease pain to facilitate participation in functional activities. Moreover, it was of note that the intensity of pain experienced by participants was lowest in their hip when compared to other areas (i.e., knees, shoulders). This supports the need to consider multiple areas of pain typical in older adults when attempting to understand the impact of pain on function and physical activity post hip fracture. Following a hip fracture and surgical repair, altered gait patterns and use of assistive devices can exacerbate pain in other weight bearing joints. Care providers should anticipate the risk for exacerbation of pain in other joints post hip fracture and implement appropriate interventions to address joint pain, particularly in the non-affected joints.
The hypothesized model only explained a small percentage of the variance in resilience. With the exception of social activities, only intrapersonal factors were included in this model. Environmental factors, psychological status (e.g., depression) and other possible factors may also influence resilience and should be considered in future work.
Study limitations and conclusion
This study was limited by the small sample size, select nature of participants and because it was cross-sectional. Replication of the models is needed with a larger, more representative sample of hip fracture patients. In addition, the measure of exercise was based on verbal report or proxy reporting and may not be reflective of actual performance of exercise. Further we recognize that we tested multiple models and thus it is possible that some of the paths may have been significant based on chance. Despite these limitations, this study highlights the importance of resilience for engagement in exercise and function post hip fracture. It also provides preliminary evidence for a genetic role for resilience. Future research should continue to consider and expand on these as well as other factors within the social ecological model than can influence behavior. In so doing recovery in the post hip fracture period can be optimized.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institute on Aging, R37 AG009901, and the Claude D. Pepper Older Americans Independence Center, P30 AG028747.
Funding source and involvement
This research was supported by grants from the National Institute on Aging, R37 AG009901, and the Claude D. Pepper Older Americans Independence Center, P30 AG028747. The funders had no involvement in any aspect of the study or manuscript development.
Footnotes
Ethical Statement
All of the authors of this manuscript participated in the planning and carrying out of the research and in all aspects of manuscript development and review.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijotn.2019.03.005.
Conflicts of interest
All authors declare that there are no conflicts of interest related to the manuscript.
References
- Arbuckle J, 1997. Amos Users’ Guide Version 3.6. Small Waters Corporation, Chicago. [Google Scholar]
- Ashford R, Nagelburg S, Adkins R, 1996. Sensitivity of the Jamar Dynamometer in detecting submaximal grip effort. J. Hand Surg 21, 4–2–5. [DOI] [PubMed] [Google Scholar]
- Bandura A, 1995. Self-efficacy in Changing Societies. Cambridge University Press, New York. [Google Scholar]
- Bandura A, 1997. Self-efficacy: the Exercise of Control. W.H. Freeman and Company, New York, New York. [Google Scholar]
- Bassett SS, Magaziner J, Hebel JR, 1990. Reliability of proxy response on mental health indices for aged, community-dwelling women. Psychol. Aging 5, 127–132. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Di Gioia A, Conti L, Berti L, Esposti L, Tarrini G, 2009. Physical activity monitoring in obese people in the real life environment. J. NeuroEng. Rehabil 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB, 2004. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. Journal of the American Medical Association 292 (7), 837–846. [DOI] [PubMed] [Google Scholar]
- Bohannon R, Schaubert KL, 2005. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J. Hand Ther 18 (4), 426–427. [DOI] [PubMed] [Google Scholar]
- Bollen KA, 1989. Structural Equations with Latent Variables. Wiley-Interscience. [Google Scholar]
- Bowes L, Jaffee S, 2013. Bilogy, genes and resilience: toward a multidisciplinary approach. Trauma Violence Abuse 14 (3), 195–208. [DOI] [PubMed] [Google Scholar]
- Brauer C, Coca-Perraillon M, Cutler D, Rosen A, 2009. Incidence and mortality of hip fractures in the United States. Journal of the American Medical Association 302 (14), 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning C, Sims J, Kendig H, Teshuva K, 2009. Predictors of physical activity behavior in older community-dwelling adults. J. Allied Health 38 (1), 8–17. [PubMed] [Google Scholar]
- Caspersen C, Powell K, Christenson G, 1985. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Report 100 (2), 126–131. [PMC free article] [PubMed] [Google Scholar]
- Cecchi F, Pancani S, Antonioli D, Avila L, Barilli M, Gambini M, Landucci P, Romano E, Sarti C, Zingoni M, Gabrielli MA, Vannetti F, Pasquini G, Macchi C, 2018. Fatigue and pain limit independent mobility and physiotherapy after hip fracture surgery. BMC Geriatr. 18 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR, 1987. A new method of classifying prognostic comorbidity in longitudinal studies: Prognostic development and validation. Journal of Chronic Disease 40 (5), 373–383. [DOI] [PubMed] [Google Scholar]
- Charney D, 2004. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatry 161, 195–216. [DOI] [PubMed] [Google Scholar]
- Cheng S, Levy A, Lefalvre K, Guy P, Kuramoto L, Sobolev B, 2011. Geographic trends in incidence of hip fracture: a comprehensive literature review. Osteoporos. Int 22, 2575–2586. [DOI] [PubMed] [Google Scholar]
- Chow S, Hamagani F, Nesselroade J, 2007. Age differences in dynamical emotion-cognition linkages. Psychol. Aging 22 (4), 765–780. [DOI] [PubMed] [Google Scholar]
- Chudyk A, Jutai JW, Petrella RJ, Speechley M, 2009. Systematic review of hip fracture rehabilitation practices in the elderly. Arch. Phys. Med. Rehabil 90, 249–253. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER, 1993. A survey for assessing physical activity among older adults. Med. Sci. Sports Exerc 25 (5), 628–642. [PubMed] [Google Scholar]
- Duclot F, Kabbaj M, 2013. Individual differences in novelty seeking predict subsequent vulnerability to social defeat through a differential epigentic regulation of brain derived neurotrophic factor expression. J. Neurosci 33 (27), 11048–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn E, Solovieff N, Lowe S, Gallagher P, Chaponis J, Rosand J, ... Smoller J, 2014. Interaction between genetic variants and exposure to Hurricane Katrina on post-traumatic stress and post-traumatic growth: a prospective analysis of low income adults. J. Affect. Disord 152–154, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang S, Barar J, Fakhari A, Mesgariabbasi M, Khani S, Omidi Y, Farnam A, 2014. Asymmetrical expression of BDNF and NTRK3 genes in frontoparietal cortex of stress resilient rats in an animal model of depression. Synapse 68 (9), 387–393. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS, 2009. Psychobiology and molecular genetics of resilience. Neuroscience 10, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl M, Gerhardt D, Hak D, Morgan S, Stahel P, 2010. Key issues and controversies in the acute management of hip fractures. Orthopedics 33, 102–110. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C, 2009. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proc. Biol. Sci 276 (1663), 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D, Helmer D, Harding M, Kosten T, Petersen N, Nielsen D, 2013. Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in veterans. J. Psychiatr. Res 47 (6), 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson J, Foerster S, Orr R, Jones L, Benedict J, Clarek B, Zotz K, 2003. System, environment and policy changes: using the Social Ecological Model as a framework for evaluating nutrition eductaion and social marketing programs with low income audiences. J. Nutr. Educ 33, S4–S15. [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini A, Zimmerman S, Morrison R, Grattan L, Dolan M, Hebel J, ... Magaziner J, 2003. Cognitive impairment in hip fracture patients: Timing of detection and longitudinal follow-up. J. Am. Geriatr. Soc 51, 1227–1236. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, ... Wallace RB, 1994. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journals of Gerontology A: Biological Sciences and Medical Sciences 49 (2), M85–M94. [DOI] [PubMed] [Google Scholar]
- Hawkes W, Williams G, Zimmerman S, Lapuerta P, Li T, Orwig D, ... Magaziner J, 2004. Determining a clinically meaningful difference in a performance-based measure to assess recovery from hip fracture: the Lower Extremity Gain Scale (LEGS). J. Clin. Epidemiol 57 (10), 1019–1024. [DOI] [PubMed] [Google Scholar]
- Hill AM, Hoffman T, McPhail S, Beer C, Hill KD, Brauer SG, Haines TP, 2011. Factors associated with older patients’ engagement in exercise after hospital discharge. Arch. PM&R (Phys. Med. Rehabil.) 92 (9), 1395–1403. [DOI] [PubMed] [Google Scholar]
- House JS, Robbins C, Metzner HL, 1982. The association of social relationships and activities with mortality: prospective evidence from the Tecumseh Community Health Study. Am. J. Epidemiol 116, 123–140. [DOI] [PubMed] [Google Scholar]
- Ingemarsson A, Frandin K, Mellstrom D, Moller M, 2003. Walking ability and activity level after hip fracture in the elderly – a follow-up. J. Rehabil. Med 35, 76–83. [DOI] [PubMed] [Google Scholar]
- Jette AM, 1980. Functional Status Index: reliability of a chronic disease evaluation instrument. Arch. Phys. Med. Rehabil 61 (September), 395–401. [PubMed] [Google Scholar]
- Jette A, Harris B, Cleary P, Campion E, 1987. Functional recovery after hip fracture. Arch. Phys. Med. Rehabil 68, 735–740. [PubMed] [Google Scholar]
- Kammerlander C, Gosch M, Kammerlander KU, Luger TJ, Blauth M, Roth T, 2011. Longterm functional outcome in geriatric hip fracture patients. Archives of Orthopedic Trauma Surgery 131 (10), 1435–1444. [DOI] [PubMed] [Google Scholar]
- Korn J, Kuruvilla F, McCarroll S, Wysoker A, Nemesh J, Cawley S, Altshuler D, 2008. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat. Genet 40 (10), 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham N, Harris B, Bean J, Heeren T, Goodyear C, Zawacki S, Jette A, 2014. Effect of a home based exercise program on functional recovery following rehabilitation after hip fracture. Journal of the American Medical Association 311 (7), 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Chen P, Chang H, Gean P, Tsai H, Yang Y, Lu R, 2009. Interaction of serotonin-related genes affects short-term antidepressant response in major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry 33 (7), 1167–1172. [DOI] [PubMed] [Google Scholar]
- López-León S, Janssens A, González-Zuloeta Ladd A, Del-Favero J, Claes S, Oostra B, van Duijn C, 2008. Meta-analyses of genetic studies on major depressive disorder. Mol. Psychiatr 13 (8), 772–785. [DOI] [PubMed] [Google Scholar]
- MacKnight C, Graham J, Rockwood K, 1999. Factors associated with inconsistent diagnosis of dementia between physicians and neuropsychologists. J. Am. Geriatr. Soc 47 (11), 1294–1299. [DOI] [PubMed] [Google Scholar]
- Magaziner J, Bassett SS, Hebel JR, Gruber-Baldini A, 1996. Use of proxies to measure health and functional status in epidemiologic studies of community-dwelling women aged 65 years and older. Am. J. Epidemiol 143 (3), 283–292. [DOI] [PubMed] [Google Scholar]
- Mangione KK, Craik RL, Tomlinson SS, Palombaro KM, 2005. Can elderly patients who have had a hip fracture perform moderate- to high-intensity exercise at home? Phys. Ther 85 (8), 727–739. [PubMed] [Google Scholar]
- Moayyeri A, 2008. The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Ann. Epidemiol 18 (11), 827–835. [DOI] [PubMed] [Google Scholar]
- Morri M, Chiari P, Forni C, Magli O, Gazineo D, Franchini N, Marconato L, Giamboi R, Cotti A, 2018. What factors are associated with the recovery of autonomy after a hip fracture? A propsective, multicentric cohort study. Arch. PM&R (Phys. Med. Rehabil.) 99 (5), 893–899. [DOI] [PubMed] [Google Scholar]
- Munter KH, Clemmesen CG, Foss NB, Palm H, Kristensen MT, 2018. Disability & rehabilitation 40 pp. 1808–1816 15. [DOI] [PubMed] [Google Scholar]
- Newman R, 2005. APA’s resilience initiative. Prof. Psychol. Res. Pract 36 (2), 227–229. [Google Scholar]
- O’Hara R, Marcus P, Thompson WK, Flournoy J, Vahia I, Lin X, et al. , 2012. 5HTTLPR short allele, resilience and successful aging in older adults. American Journal of Geriatric Psyciatry 20 (5), 452–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahikanti L, Von Ah D, 2012. Impact of early mobilization protocol on the medical surgical inpatient population: an integrated review of the literature. Clin. Nurse Spec 26, 87–94. [DOI] [PubMed] [Google Scholar]
- Pedersen MM, Bodilsen AC, Petersen J, Beyer N, Andersen O, Lawson-Smith L, et al. , 2013. Twenty four hour mobility during acute hospitalization in older medical patients. Journal of Gerontology A Biological Sciences Medicine and Science 68, 331–337. [DOI] [PubMed] [Google Scholar]
- Pérez-López F, Pérez-Roncero G, Fernández-Iñarrea J, Fernández-Alonso A, Chedraui P, Llaneza P, 2014. Resilience, depressed mood, and menopausal symptoms in postmenopausal women. Menopause 21 (2), 159–164. [DOI] [PubMed] [Google Scholar]
- Pescatello L, DiPietro L, Fargo A, Ostfeld A, Nadel E, 1994. The impact of physical activity and physical fitness on health indicators among older adults. J. Aging Phys. Act 2, 2–13. [Google Scholar]
- Pizzorusso T, Berardi N, Rossi F, Viegi A, Venstrom K, Reichardt L, Maffei L, 1999. TrkA activiation in the rat visual cortex by antirat trkA IgG prevents the effect of monocular deprivation. Eur. J. Neurosci 11 (1), 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff R, Brez S, Hotz S, 2000. Exercise behavior in a community sample with diabetes: understanding the determinants of exercise behavioral change. Diabetes Educat. 26 (3), 450–459. [DOI] [PubMed] [Google Scholar]
- Rana BK, Darst BF, Bloss C, Shih PB, Depp C, Nievergelt CM, ... Jeste DV, 2014. Candidate SNP associations of optimism and resilience in older adults: Exploratory study of 935 communty dwelling adults. Am. J. Geriatr. Psychiatry 22 (10), 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, 2014. Resilience in older adults. Top. Geriatr. Rehabil 30 (2), 155–163. [Google Scholar]
- Resnick B, D’Adamo C, 2011. Factors associated with exercise among older adults in a continuing care retirement community. Rehabil. Nurs 36 (2), 47–53. [DOI] [PubMed] [Google Scholar]
- Resnick B, Inguito P, 2011. Testing the reliability and validity of the resilience measure. Arch. Psychiatr. Nurs 25 (1), 11–20. [DOI] [PubMed] [Google Scholar]
- Resnick B, Orwig D, Yu-Yahiro J, Hawkes W, Shardell M, Hebel J, Magaziner J, 2007. Testing the effectiveness of the Exercise Plus Program in older women post-hip fracture. Ann. Behav. Med 34 (1), 67–76. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E, Boltz M, Hawkes W, Shardell M, Orwig, Magaziner J, et al. , 2011a. Physical activity in the post hip fracture period. J. Aging Phys. Act 19 (4), 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, Klinedinst J, Yerges-Armstrong L, Choi E, Dorsey S, 2015. The impact of genetics on physical resilience and successful aging. J. Aging Health 3, 1–21. [DOI] [PubMed] [Google Scholar]
- Roberts H, Denison H, Martin H, Patel H, Syddall H, Cooper, et al. , 2011. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardized approach. Age Ageing 40, 423–429. [DOI] [PubMed] [Google Scholar]
- Robinson J, Larson C, Cahill SP, 2014. Relations between resilience, positive and negative emotionality, and symptoms of anxiety and depression. Psychological Trauma: Theory, Research, Practice, and Policy 6 (Suppl. 1), S92–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A, Lim S, Sohn W, 2008. Resilience to urban poverty: Theoretical and empirical considerations for population health. American Journal of Public Health 98 (6), 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaoa W, Fan S, Lei Y, Yao G, Chen JJ, Zhou J, et al. , 2013. Metabolomic identification of molecular changes associated with stress resilience in the chrnoic mild stress rat model of depression. Metabolomics 9, 433–443. [Google Scholar]
- Southwick S, Charney D, 2012. The science of resilience: implications for the prevention and treatment of depression. Science 338 (5), 79–81. [DOI] [PubMed] [Google Scholar]
- Sun J, Buys N, Jayasinghe R, 2014. Effects of community-based meditative Tai Chi programme on improving quality of life, physical and mental health in chronic heartfailure participants. Aging Ment. Health 18 (3), 289–295. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A, 2011. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci 31 (12), 4475–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraldsen K, Sletvold O, Thingstad P, Saltvedt I, Granat M, Lydersen, Helbostad J, et al. , 2014. Physical behavior and function early after hip fracture surgery in patients receiving comprehensive geriatric care or orthopedic care-A randomized controlled trial. Journals of Gerontology A Biological Sciences and Medical Sciences 69 (3), 338–345. [DOI] [PubMed] [Google Scholar]
- The Modified Mini-Mental State (3MS) Test, 2014. Scoring manual. Available at: http://www.dementia-assessment.com.au/cognitive/3MS_improved.pdf Last accessed December, 2015.
- Tsauo J, Leu W, Chen Y, Yang R, 2005. Effects on function and quality of life of postoperative home-based physical therapy for patients with hip fracture. Arch. Phys. Med. Rehabil 86 (10), 1953–1957. [DOI] [PubMed] [Google Scholar]
- United States Bureau of Labor Statistics, 2015. Sports and exercise. Available at: http://www.bls.gov/spotlight/2008/sports/ Last accessed December, 2015.
- United States Department of Health and Human Services, 2004. Bone and Health and Osteoporosis: A Report to the Surgeon General. United States Department of Health and Human Servies, Rockville, MD. [Google Scholar]
- van Kessel G, 2013. The ability of older people to overcome adversity: a review of the resilience concept. Geriatr. Nurs 34 (2), 122–127. [DOI] [PubMed] [Google Scholar]
- Wagnild G, 2003. Resilience and successful aging: Comparison among low and high income older adults. J. Gerontol. Nurs 29 (12), 42–49. [DOI] [PubMed] [Google Scholar]
- Wagnild G, Young H, 1993. Development and psychometric evaluation of the resilience scale. J. Nurs. Meas 1 (2), 165–177. [PubMed] [Google Scholar]
- Wald I, Degnan K, Gorodetsky E, Charney D, Fox N, Fruchter E, ... Bar-Haim Y, 2013. Attention to threats and combat-related posttraumatic stress symptoms: prospective associations and moderation by the serotonin transporter gene. JAMA Psychiatry 70 (4), 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild K, Wiles J, Allen RES, 2013. Resilience: Thoughts on the value of the concept for critical gerontology. Ageing Soc. 33 (1). [Google Scholar]
- Ziaian T, de Anstiss H, Antoniou G, Baghurst P, Sawyer M, 2012. Resilience and its association with depression, emotional and behavioural problems, and mental health service utilization among refugee adolescents living in South Australia. International Journal of Population Research, 485956 Available at: http://www.hindawi.com/journals/ijpr/2012/485956/ Last accessed December, 2015. [Google Scholar]
- Zimmerman S, Hawkes W, Hebel J, Fox K, Lydick E, Magaziner J, 2006. Lower Extremity Gain Scale (LEGS) to assess hip fracture recovery performance. Archives of Physiscal Medicine and Rehabilitation 87 (3), 430–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.