Abstract
Background:
Despite increasing emphasis on integration of palliative care with disease-directed care for advanced cancer, the nature of this integration and its effects on patient and caregiver outcomes are not well-understood.
Aim:
We evaluated the effects of integrated outpatient palliative and oncology care for advanced cancer on patient and caregiver outcomes.
Design:
Following a standard protocol (PROSPERO: CRD42017057541), investigators independently screened reports to identify randomized controlled trials or quasi-experimental studies that evaluated the effect of integrated outpatient palliative and oncology care interventions on quality of life, survival, and healthcare utilization among adults with advanced cancer. Data were synthesized using random-effects meta-analyses, supplemented with qualitative methods when necessary.
Data sources:
English-language peer-reviewed publications in PubMed, CINAHL, and Cochrane Central through November 2016. We subsequently updated our PubMed search through July 2018.
Results:
Eight randomized-controlled and two cluster-randomized trials were included. Most patients had multiple advanced cancers, with median time from diagnosis or recurrence to enrollment ranging from 8 to 12 weeks. All interventions included a multidisciplinary team, were classified as “moderately integrated,” and addressed physical and psychological symptoms. In a meta-analysis, short-term quality of life improved, symptom burden improved, and all-cause mortality decreased. Qualitative analyses revealed no association between integration elements, palliative care intervention elements, and intervention impact. Utilization and caregiver outcomes were often not reported.
Conclusions:
Moderately integrated palliative and oncology outpatient interventions had positive effects on short-term quality of life, symptom burden, and survival. Evidence for effects on healthcare utilization and caregiver outcomes remains sparse.
Keywords: Meta-analysis, palliative care, palliative medicine, quality of life, survival
Introduction
Globally, cancer is the second leading cause of death, accounting for nearly one of every six deaths.1 Cancer is associated with physical symptoms and affects quality of life, physical and psychological functioning, and family systems.2 Often concurrent with oncology care, palliative care aims to improve quality of life by managing physical symptoms and psychosocial and spiritual distress. Palliative care occurs across a continuum, beginning at the time of diagnosis of a serious illness and continuing until the end of life; it is appropriate at any stage of illness and can be provided along with curative treatment.3 Integration of palliative and oncology care is now considered standard of care for patients with advanced cancer.4–10 Furthermore, two recent meta-analyses and systematic reviews11,12 and several clinical trials13–15 support this integrated approach to improve symptoms and quality of life across care settings and disease type, including advanced cancer. Insights about the mechanism of action by which palliative care improves outcomes, a, are lacking, as is a more detailed study of particular intervention elements associated with these outcome improvements.
Although primary care practitioners and oncologists have usually incorporated primary palliative care into treatment of patients (i.e. palliative care that they themselves provide), palliative care has recently become a specialty recognized by National Accreditation Council.16 Growing recognition of the value of palliative care has led to a 150% growth in the field over the past decade17 and has increased access to services.18 Delivered in both inpatient and outpatient settings, palliative care varies in team composition, integration level, and eligibility for care. Healthcare organizations have described various integration methods, including co-rounding models for hospitalized patients, co-located outpatient services, and stand-alone services.19,20 However, the extant literature has not been synthesized to identify the most effective aspects and degrees of integration within health systems or among providers.
Previous reviews examined the effectiveness of palliative care across inpatient, outpatient, nursing home, and home-based settings and included trials patients with cardiovascular, neurologic, and other diseases.11,21 The current review complements these broader, more inclusive reviews to isolate the effects of palliative care on outcomes among patients with cancer. In addition, outpatient settings, considered the “next frontier” of community-based palliative care, are where most cancer care occurs.22,23 The current review is the first to focus exclusively on integrated palliative care and oncology care in outpatient settings. Furthermore, it is the first to utilize a theory-based approach to classifying integrated palliative and oncology care in outpatient settings and to examine the relation between integration and cancer-related patient and caregiver outcomes, hence adding to and building upon existing literature.
Methods
We followed a standard protocol for all steps of this review (PROSPERO: CRD42017057541). This work was part of a larger report for the Veterans Health Administration Evidence-based Synthesis Program. A technical report fully detailing our methods is available at https://www.hsrd.research.va.gov/publications/esp.
Search strategy and selection criteria
We conducted searches of MEDLINE® (via PubMed®), the Cochrane Central Registry of Controlled Trials, and CINAHL through 21 November 2016; we subsequently updated our PubMed search through July 2018 (Appendix Table 1). We examined the bibliographies of published reviews and contacted experts to identify additional relevant studies.
Two reviewers used prespecified eligibility criteria (Appendix Table 2) to assess all titles and abstracts. Major eligibility criteria were as follows: trial or quasi-experimental design, adults with advanced cancer, interventions delivered in outpatient settings, evidence of integration between palliative care and oncology services, primary outcomes (quality of life, survival, and healthcare utilization), and comparator of usual oncology care. We fielded a web-based survey, using standardized questions derived from the Integrated Practice Assessment Tool24 (IPAT; Appendix Table 3), to collect detailed information about integration elements, and used responses in combination with published data to determine eligibility. Authors provided requested data for 6 of 10 studies. Disagreements about study eligibility were resolved by discussion or a third investigator. Excluded studies are described in Appendix Table 4.
Data abstraction, categorization of interventions, and quality assessment
Patient characteristics, intervention/comparator details, and outcomes at two time points—at least >28days postintervention (primary) and at least 6 months postintervention (secondary)—were abstracted into a custom database. Review and reconciliation were conducted for full-text screening. Consistent with the methods of Kavalieratos et al.,11 we characterized palliative care intervention elements based on the eight domains of palliative care (e.g. physical, psychological) recommended by the National Consensus Project’s 2013 Practice Guidelines for Quality Palliative Care.25 We categorized integrated care levels using the IPAT24, which classifies practices into six groups as coordinated care (Levels 1 and 2), co-located care (Levels 3 and 4), and integrated care (Levels 5 and 6).
We used the Cochrane risk of bias tool26 for randomized controlled trials (RCTs) and the revised Newcastle—Ottawa Scale27 for cohort studies. Individual studies were assigned a summary risk of bias score (low, moderate, or high).
Data synthesis and analysis
When studies were conceptually homogeneous and there were at least three studies with the same outcome, we conducted meta-analyses with the “metaphor” library (version 1.9–7) in the R statistical package (version 3.1.2).28 End of treatment or first postintervention assessments were considered short-term outcomes with all outcomes being at least >28days postintervention; outcomes assessed at least 6 months postintervention were considered long-term outcomes. When quantitative synthesis was possible, we combined dichotomous outcomes using random-effects models and computed summary risk ratios (RRs) or hazard ratios (HRs). Continuous outcomes were summarized using the standardized mean difference (SMD). We used the Knapp—Hartung approach to adjust the standard errors of the estimated coefficients.29,30 We evaluated statistical heterogeneity using visual inspection and Cochran’s Q and I2 statistics. I2 of 25%, 50%, and 75% correspond to low, medium, and high heterogeneity.31 When quantitative synthesis was not feasible, we synthesized interventions qualitatively. Evidence from higher quality studies with more precise effect estimates was given more weight. Publication bias could not be assessed statistically because there were fewer than 10 studies in all analyses.32
To identify intervention and integration elements associated with greater effects, we used subgroup analyses and qualitative cross-case impact analysis. We analyzed the pattern of associations between prespecified intervention elements (e.g. physical, psychological) and the six integrated care levels with effects on quality of life and overall impact. To determine overall impact, we randomly ordered the studies on a spreadsheet and listed the study’s outcomes in each domain (e.g. survival, quality of life) without any identifiers. Two investigators reviewed the effects in each outcome domain and independently rated the overall intervention impact using a 4-point scale (i.e. high, moderate, low, or no impact). Disagreements were resolved by consensus.
The strength of evidence for each question was assessed using the GRADE approach, which considers study design, risk of bias, consistency, directness, and precision.33 These domains were evaluated using the GRADEpro software (gradepro.org).
Role of the funding source
This research was funded by the Veterans Health Administration, Office of Research and Development, and Quality Enhancement Research Initiative. Staff from the Quality Enhancement Research Initiative did not participate in developing the scope of work, conducting the study, or reviewing the draft report.
Results
Electronic and manual searches identified 1987 unique citations (Appendix Figure 1). Of the 182 potentially eligible studies, most were excluded because they did not include an eligible intervention (n = 45) or an eligible study design (n = 39). We included 10 unique studies13,15,34–41 and 11 companion papers42–52 (2385 patients) that focused on integrated palliative and oncology care (Table 1; Appendix Table 5). Palliative care was compared to standard oncology care in seven trials.13,15,37–41 Single trials compared palliative care to oncology care plus a symptom-management toolkit,35 oncology care plus “on-demand” palliative care,34 and delayed palliative care that began 3 months postrandomization.36 All but three studies34,37,41 were conducted in the United States, and two used cluster randomization.35,37 Most studies enrolled patients with several types of advanced cancer; the median time from diagnosis or recurrence to enrollment was 8–12weeks. Median age of participants was 64.3years (range, 59–67 years) based on eight studies; 48% of the participants were female. Median percentage of White participants was 94.4% reported in six studies.
Table 1.
Characteristics of integrated palliative care studies.
| Study designs | 8 RCTs |
| 2 cluster RCTs | |
| Study years | 2006–2017 |
| Median number of patients enrolled (range) | 151 (115–461) |
| Total number of patients enrolled | 2385 |
| Mean patient age (range) | 64.3 (59–67) reported in eight studies |
| Median percentage female (range) | 48% (31.5%–71.2%) |
| Median percentage White (range) | 94.4% (84.9%–96.5%) reported in six studies |
| Number of studies with Veterans | 2 |
| Median time since diagnosis or recurrence (range) | 8–12 weeks reported in nine studies (30–60 days to >24 months) |
| Intervention setting | 8 ambulatory |
| 2 home | |
| Cancer diagnoses | 8 multiple cancers |
| 1 lung cancer | |
| 1 pancreatic cancer | |
| Patients’ functional status | 4 studies: ECOG 0–2 |
| 6 studies: not reported | |
| Countries | 7 USA |
| 1 Canada | |
| 2 Europe | |
| Risk of bias, objective outcomes | 3 low |
| 4 unclear | |
| 3 not applicable | |
| Risk of bias, patient-reported outcomes | 2 low |
| 4 unclear | |
| 3 high | |
| 1 not applicable |
ECOG: Eastern Cooperative Oncology Group; RCT: randomized controlled trial.
The risk of bias for objective outcomes was judged low for three studies,13,15,37 unclear for four studies,34–36,41 and not applicable for the three studies not reporting these outcomes (Appendix Figure 2).38–40 Risk of bias for patient-reported outcomes was judged low for two studies,13,40 unclear for four studies,15,36,37,39 and high for the others.
Characteristics of the interventions
Palliative care was delivered by a multidisciplinary team of two to five clinicians; all included nurses, five included a palliative care physician, three included a mental health professional, and two included chaplains. All studies provided services during outpatient visits; five also included telephone-based care, and three described delivery of written materials (Appendix Table 6). In one study, telephone was the primary intervention delivery mode.13 All palliative care study interventions addressed physical and psychological symptoms. Most interventions also addressed social, spiritual, ethical and legal, and end-of-life aspects of care. Structural issues (e.g. interdisciplinary team engagement with patients or families) were addressed explicitly in half the interventions, but cultural issues were not. The intensity of services varied greatly, ranging from four sessions weekly13 to contacts every 2–4weeks until death.34
Level of integration
Additional data on integration were supplied by authors from 6 of the 10 trials (Table 2).13,15,35–37,40 Of the 10 trials, 2 were classified as having basic collaboration on site and 4 as having close collaboration on site with some systems integration. Four studies could not be classified due to no response from authors. In all trials for which the authors responded, palliative services were physically or virtually co-located in the same facility as oncology services, although not necessarily the same space. Standard communication about treatment issues, interactive communication, and routine communication exchanges between palliative and oncology clinicians occurred in at least half the studies.
Table 2.
Levels of integration and impact ratings for integrated palliative and oncology care.
| Studya | Integration elements | Level of integration Impact ratingb |
||||||
|---|---|---|---|---|---|---|---|---|
| Care teams co-located? |
Written or electronic information exchanged routinely? |
Care teams communication bidirectional? |
Information exchanged as standard and routine practice? |
Care providers have equal roles in decision- making? |
Care standardized across ALL patients? |
One joint treatment plan for cancer patients? |
||
| Bakitas et al.13 | Yes | Yes | Yes | Yes | No | No | No | Level 3 Moderate |
| Bakitas et al.36 | Yes | Yes | Unsure | Yes | No | Yes | Yes | Level 4 Low |
| Clark et al.38 | No author response | Unclear Low |
||||||
| Groenvold et al.41 | No author response | Unclear Low |
||||||
| Maltoni et al.34 | No author response | Unclear Low |
||||||
| McCorkle et al.35 | Yes | Yes | Yes | Yes | No | Yes | No | Level 4 None |
| Rummans et al.39 | No author response | Unclear Low |
||||||
| Temel et al.15 | Yes | No | Yes | Yes | Yes | No | No | Level 4 Moderate |
| Temel et al.40 | Yes | Yes | Yes | Yes | Unsure | Yes | No | Level 4 Low |
| Zimmermann et al.37 |
Yes | Yes | Yes | Yes | No | No | No | Level 3 Low |
Answers were solicited from the first author of each article. Report investigators also answered questions based on reading the published article; however, with only one exception, the report investigators’ answer was always unsure.
Impact ratings range from none to high and are independently derived based on a judgment about intervention effects (e.g. effect size or statistical significance) across six outcome domains.
Quality-of-life outcomes
Integrated palliative care improved short-term quality of life (n = 9; SMD 0.24; 95% confidence interval (CI), 0.13 to 0.35; I2 = 0.0%; Figure 1(a)). Positive effects were consistent, ranging from small to moderate in all but one study.35 At 6–12 months, quality of life was not improved (n = 6; SMD 0.15; 95% CI, –0.12 to 0.43; I2 = 28%; Appendix Figure 3A). One study found an interaction effect by cancer type; patients with lung cancer benefited greater than those with gastrointestinal cancer.40 Notably, longer term quality of life was not a primary study endpoint in any of these studies, and study dropout due to disease progression and death likely limited the ability to detect longer term differences in outcomes.
Figure 1. Integrated palliative care outcomes: (a) short-term (1–3 months) effects on quality of life, (b) effects on overall symptom burden, and (c) effects on all-cause mortality.

Values of I2 equal the percentage of total variance across studies due to heterogeneity rather than chance. Cochran’s Q test assesses the significance of I2 values. A p value associated with the Q statistic greater than 0.05 suggests presence of heterogeneity. CI: confidence interval; ESAS: Edmonton Symptom Assessment Scale; FACIT-PAL: Functional Assessment of Chronic Illness Therapy-Palliative Care; FACIT-Sp: Functional Assessment of Chronic Illness Therapy-Spiritual Well-being; FACT-G: Functional Assessment of Cancer Therapy-General; FACT-Hep: Functional Assessment of Cancer Therapy-Hepatobiliary; HR: hazard ratio; FACT-L: Functional Assessment of Cancer Therapy-Lung; HCS: Hepatobiliary Cancer Subscale; LCS: Lung cancer subscale; N: study sample size; QUAL-E: Quality of life at end of life symptom impact subscale; ROB: risk of bias; SD: standard deviation; SDS: Symptom Distress Scale; SMD: standardized mean difference; TOI: Trial Outcome Index.
Overall symptom burden outcomes
At 1–3 months postrandomization, patients assigned to integrated palliative care showed small but statistically nonsignificant improvements in symptom burden (n = 6; SMD –0.17; 95% CI, –0.45 to 0.11; I2 = 62%; Figure 1(b)). One study39 only reported effects on symptom burden as statistically nonsignificant and could not be included in the meta-analysis. All but one study36 showed small to moderate improvement in symptom burden. This outlier study delivered structured coaching sessions by telephone after consultation with specialist palliative care clinicians. A sensitivity analysis that excluded this study showed a consistent pattern of decreased symptom burden with integrated palliative care (n = 5; SMD –0.25; 95% CI, –0.39 to –0.11; I2 = 0%).
Psychological symptom outcomes
Effects of integrated palliative care on one or more psychological symptoms were reported in all but one study.37 Six studies reported effects on depression symptoms.13,15,34–36,40 There was no short-term effect on depressive symptoms reporting severity as a continuous outcome (n = 4; SMD –0.09; 95% CI, –0.32 to 0.13; I2 = 0%; Appendix Figure 3B). One15 of two studies15,34 reporting the proportion meeting the threshold for depressed mood showed an intervention effect (n = 104; 4% vs 17% meeting criteria for major depression; p = 0.04). Two studies15,34 that reported the proportion of patients with significant anxiety symptoms showed no difference between groups. Two studies38,39 found no statistically significant effect on transient mood states.
Survival outcomes
Overall survival was reported at 12 months,34,36 a mean of 14.6 months,13 and at 4.5–36 months.15,41 All studies compared integrated palliative care to usual oncology care except one36 in which the control patients began delayed palliative care 3 months postrandomization. Integrated palliative care was not associated with lower all-cause mortality (n = 5; HR, 0.84; 95% CI, 0.61 to 1.18; Figure 1(c)), but intervention effects varied substantially (I2 = 57%; Q = 9.3, p< 0.055). One study41 that tested a low-intensity intervention and included patients with longer times since diagnosis (i.e. most were between 12 and 24 months and some were >24 months) showed no effect on mortality. A sensitivity analysis that omits this study, limiting the analysis to a more homogeneous group of trials, showed lower all-cause mortality (n = 4; HR, 0.77; 95% CI, 0.61 to 0.98; I2 = 0%).
End-of-life care outcomes
In studies following patients from 6 to 35 months, patients receiving palliative care were more likely to die at home (n = 3; RR, 1.19; 95% CI, 1.05 to 1.36; I2 = 0%; Appendix Figure 3C) than those receiving usual oncology care. Effects on site of death were consistent across studies. Aggressiveness of care near the end of life was reported in three trials.15,36,37 Measures varied greatly; thus, results were qualitatively synthesized. One study15 conducted in patients with lung cancer reported a composite measure (chemotherapy 14days before death, no use of hospice care, or admission to hospice <3 days before death), finding that patients in palliative care were less likely than those in usual oncology care to receive aggressive end-of-life care (33% vs 54%; p = 0.05). Three studies reported chemotherapy use at end of life,15,36,37 one of which showed an intervention effect (chemotherapy in the last 60days of life, 32/61 vs 47/67; p = 0.05).15 One study reported on the proportion of patients receiving chemotherapy at all,37 while the other reported receipt of chemotherapy in the last 60days of life;36 neither showed an intervention effect.
Effects on healthcare utilization
Measures of utilization were reported inconsistently across studies. Thus, results were qualitatively synthesized. Emergency department and hospitalization use was reported in four trials.13,15,34,36 None of the studies found intervention effects, but in the three studies reporting the proportion of patients with an emergency department visit, visits were modestly lower (RR range, 0.73–0.93). Hospitalization rates were also modestly lower (RR range, 0.73–0.96) in the three studies reporting this rate. For emergency department visits and hospitalizations, effect estimates were imprecise and do not exclude a clinically important effect. Two studies that examined intensive care unit utilization13,36 found no intervention effect. Costs of care were reported in one study of patients with lung cancer.15 The intervention was associated with a lower mean total cost per day throughout the study period; this difference was not statistically significant (US$117; p = 0.13). Chemotherapy costs in the last 30days of life were significantly different, with the intervention yielding a US$757 mean reduction compared to standard care (p = 0.03).
Caregiver and patient experience outcomes
Caregiver experience was reported in three trials.36–38,43,44 The best data come from a cluster-randomized trial that involved consultation and follow-up monthly in the oncology-palliative care clinic by a palliative care physician and nurse. Caregiver experience assessed satisfaction with information-giving, availability of care, psychological care, and physical care (range, 16–80).37 For patients assigned to palliative care, caregiver experience was better at 3 months (mean change, 1.4 vs –3.1; p = 0.007; SMD 0.39; 95% CI, 0.02 to 0.77) and 4 months (mean change, 0.6 vs 2.4; p = 0.02; SMD 0.27; 95% CI, –0.10 to 0.65). Caregiver quality of life did not differ between groups.
Two other trials36,38,43 examined caregiver quality of life using a measure assessing physical, emotional, spiritual, and family dimensions of well-being. Palliative care was delivered by a multidisciplinary team and addressed multiple domains of quality of life. One study incorporated a specific telephone-based intervention addressing topics such as caregiver role, problem-solving, and self-care. There were no intervention effects in either study at 3 months36,43 or 27 weeks.38
Palliative care effects on caregiver depressive symptoms and burden were reported in one trial.36,44 At 3 months, caregiver depressive symptoms were lower for palliative care (Cohen’s d, –0.32; p = 0.02) but the effects did not persist in the subset of caregivers assessed after the patient died (Cohen’s d, 0.07; p = 0.88). Caregiver burden was measured with a scale that included objective, demand, and stress burden subscales. At 3 months, no effects on any of the subscales were found (Cohen’s d, 0.01–0.09; p⩾0.29). In a subanalysis among caregivers whose care recipient had died, stress burden (Cohen’s d, –0.44; p = 0.01) was lower in caregivers assigned to early palliative care.
Patient experience was reported in the cluster-randomized trial, using the same multidimensional measure completed by caregivers.37 In the palliative care arm, patient experience was better at 3 months (Cohen’s d, 0.47; p = 0.003) and 4 months (Cohen’s d, 0.73; p < 0.001).
Adverse effects
Adverse effects of integrated palliative care were not specified as an outcome and were not reported in any trials.
Association between integration levels and overall impact
Impact ratings were based on overall intervention effects on outcomes from six categories. Seven trials34,36–41 were classified as “low impact,” two trials13,15 as “moderate impact,” and one trial as “no impact”35 (Appendix Table 7). The limited number of studies and limited variability in integration levels and impact ratings precluded quantitative analyses. Qualitative analysis identified no consistent pattern of results (Table 2). Trials with Level 4 integration ratings had impact ratings of low,36,40 moderate,15 and none.35 The two trials with Level 3 integration ratings13,37 included all of the same elements of integration but had different impact ratings (moderate and low, respectively). All trials included three of the integration elements: colocation, standard/routine information exchange, and routine exchange of written or electronic information. These integration elements did not appear to be associated with a greater benefit for patients given that impact ratings ranged from none to moderate across the six trials.
Association between integration levels and quality of life
We used subgroup analyses to examine the relation between integration level and intervention effects on short-term quality of life (Figure 2). Overall, there was no association between integration level and intervention effects on short-term quality of life. Two of the six studies15,37 had a significant positive effect on short-term quality of life. Both included the following three integration elements: (1) interactive communication, (2) standard/routine information exchange, and (3) co-location.
Figure 2.
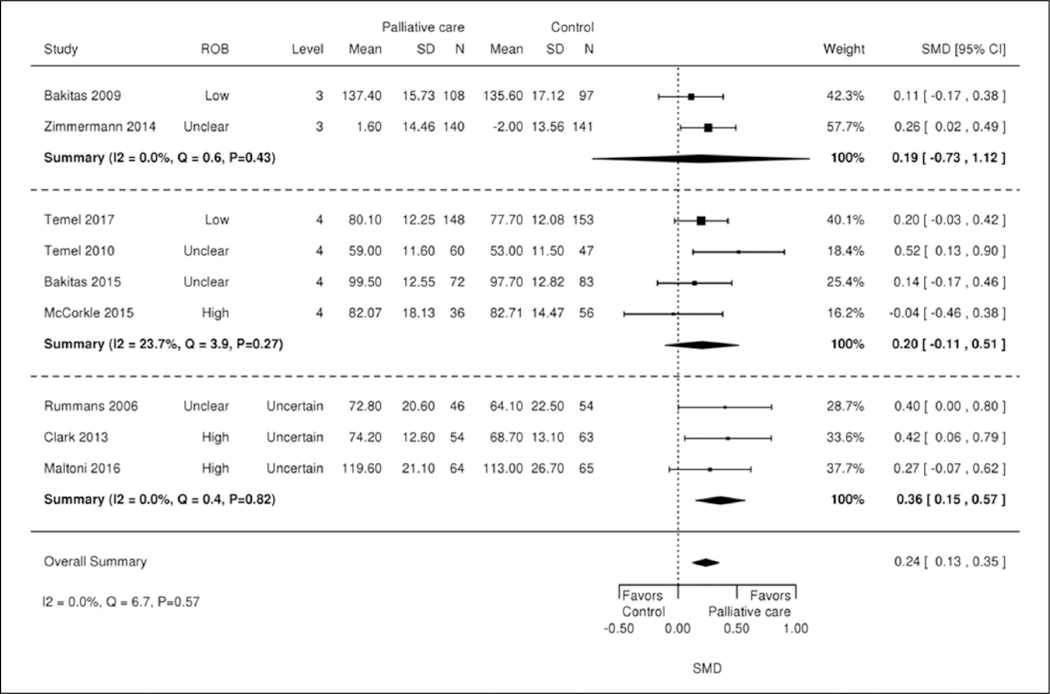
Integration category and effects on short-term (1–3 months) quality of life. CI: confidence interval; N: study sample size; ROB: risk of bias; SD: standard deviation; SMD: standard mean difference.
Association between intervention elements and overall impact
Using cross-case analyses, we did not identify an association between the eight palliative care intervention elements (e.g. structure, physical),25 and overall impact. All trial interventions involved physical and psychological aspects of palliative care. All trials except two34,41 involved social aspects of palliative care; none described cultural aspects of palliative care. Studies that had the highest impact ratings13,15 included end-of-life and ethical/legal aspects of care. Overall, trials that included more aspects of palliative care than others did not appear to have higher impact ratings.
Discussion
We evaluated palliative care integrated with oncology care for patients with advanced cancer, examining effects on outcomes of importance to patients, clinicians, and policymakers. We identified eight RCTs and two cluster-randomized trials; all were comparative effectiveness trials examining palliative care services that were moderately integrated with oncology care. All interventions were colocated in the same facility. All interventions included physical and psychological aspects of care; none included cultural aspects of care. We found a pattern of positive effects, including improved survival and short-term quality of life. Strength of evidence was rated for quality of life, symptom burden, and mortality. Ratings were as follows: high strength of evidence for mortality, moderate strength of evidence for shorter term quality of life, low strength of evidence for longer term quality of life, and very low strength of evidence for symptom burden. Concerns that contributed to the lower strength of evidence were high risk of bias and imprecision that was attributed to the 95% CI not excluding a small and small-to-moderate effect.
We aimed to describe intervention elements associated with greater benefit to patients with cancer. Most of the trials did not clearly report integration elements. Therefore, we relied on author report to classify integration level. We qualitatively examined associations between integration elements, palliative care intervention domains, and intervention impact. No association between integration level and intervention effects emerged; the key limitations of these analyses were the limited number of studies and limited variability in integration elements and impact ratings.
Current guidelines from medical professional societies7–9,53 recommend integration of palliative care and oncology care. Our results and recent systematic review and meta-analytic findings11,54,55 support these recommendations in demonstrating quality of life improvements with integrated care. Our review is unique in its focus on integrated approaches in only outpatient oncology settings, and in its application of a novel theory-driven approach to standardized classification and evaluation of key integrated palliative care elements. It is among the first to find an aggregate improvement in survival across multiple studies in oncology settings. For healthcare systems desiring to integrate palliative and oncology services, barriers and facilitators to implementation should be considered. Common barriers include personnel costs, limited staffing and space,56 patients’ limited participation in shared medical appointments,51 and perceptions that palliative care is limited to end-of-life care.57 Facilitators may include palliative care performance measures, communication and collaboration between healthcare leaders, and patient–clinician education about the complementary roles of palliative and oncology teams.56
This protocol-driven review has numerous strengths, including new data gathered from study authors, rigorous methods, and classification of integrated palliative care elements. However, the review has limitations. The classification of integrated palliative care relied on authors’ retrospective reports about integration characteristics that were used to classify each study. We were also limited by the existing literature. Palliative care intervention and integration elements were poorly defined in most studies. Interventions varied substantially in goals, delivery, intensity, target recipient, and outcome measures may not have precisely measured the same constructs. Accordingly, trials should more clearly report intervention and integration elements. Outcome measures should be standardized and include outcomes most valued by patients and input on barriers and facilitators to implementation. Identified studies included predominantly White samples in economically developed countries and included very few patients with hematologic malignancies; future research is needed with ethnically, racially, socioeconomically, and diagnostically diverse groups. Finally, studies are needed in community settings and across inpatient and outpatient settings.
Conclusion
A small but growing literature supports integrated outpatient palliative and oncology care for advanced cancer. A diverse set of moderately integrated interventions were identified. A pattern of small-to-moderate, positive, short-term effects on quality of life, symptom burden, and survival was found. Effects on other outcomes such as healthcare utilization and caregiver outcomes are less studied and warrant further attention. In addition, gaps in evidence for some policy-relevant outcomes and critical intervention elements remain. More clearly defined palliative care intervention and integration characteristics would improve understanding of the impact of integrated palliative and oncology care on outcomes. Future studies should report intervention and integration elements more carefully, adopt a standard set of outcomes, and recruit more culturally and diagnostically diverse samples.
Supplementary Material
What is already known about the topic?
Integrated palliative and oncology for advanced cancer in outpatient settings has the potential to improve outcomes.
Prior reviews on palliative care have not specifically focused on integrated approaches.
What this paper adds?
This review is the first to focus only on outpatient settings and to classify and evaluate integrated elements of palliative care.
Palliative care that is moderately integrated with oncology care lowers mortality and improves short-term quality of life.
This review is the first to find a survival benefit of integrated palliative and oncology care across multiple high-quality randomized controlled trials.
Implications for practice, theory, or policy
Results from this review support guidelines that recommend integrating palliative with oncology care in economically developed countries.
Acknowledgements
This report is based on research conducted by the Evidence-based Synthesis Program (ESP) Center located at the Durham VA Medical Center, Durham, NC, funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative. The authors would also like to acknowledge Liz Wing, MA, for providing editorial assistance. J.W.W. obtained funding for this research project. J.J.F., T.W.L., A.K., K.S., K.R., J.R.M., and J.W.W. contributed to data analysis and interpretation and drafted the initial manuscript. All authors contributed to study conceptualization and design, and data extraction all authors critically reviewed and revised the manuscript and approved the final manuscript for submission. Trial registration: PROSPERO CRD42017057541.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report is based on research conducted by the Evidence-based Synthesis Program (ESP) Center located at the Durham VA Medical Center, Durham, NC, funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative (VA ESP Project #09–009; 2017). This work was also supported by the Center of Innovation for Health Services Research in Primary Care (CIN 13–410) at the Durham VA Medical Center. T.W.L.’s work is supported by a Sojourns Scholars Award from the Cambia Health Foundation and by a Mentored Research Scholar Grant from the American Cancer Society (MRSG-15–185-01-PCSM). K.N.P.S.’s work is supported by the Rehabilitation Research and Development Service of the Veterans Affairs Office of Research & Development (1IK2 RX002348–01). A.K.’s work is supported by a grant from the Agency for Healthcare Research and Quality (5K08HS023681–02). The findings and conclusions in this document are those of the author(s) who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the US government. Therefore, no statement in this article should be construed as an official position of the Department of Veterans Affairs.
Research ethics and patient consent
As a systematic review and meta-analysis, the study did not directly involve human participants and required no approval from an Ethics Committee or Institutional Review Board.
Footnotes
Data management and sharing
Data will be made available upon request.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3): 177–193. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke K, Zhong X, Theobald D, et al. Somatic symptoms in patients with cancer experiencing pain or depression: prevalence, disability, and health care use. Arch Intern Med 2010; 170(18): 1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center to Advance Palliative Care. Definition of palliative care, https://www.capc.org/about/palliative-care/ (accessed 28 July 2017).
- 4.Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington, DC: National Academies Press, 2013. [PubMed] [Google Scholar]
- 5.American Board of Internal Medicine. Hospice & Palliative Medicine Policies, http://www.abim.org/certification/policies/internal-medicine-subspecialty-policies/hospice-palliative-medicine.aspx (accessed 14 July 2017).
- 6.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012; 30(8): 880–887. [DOI] [PubMed] [Google Scholar]
- 7.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017; 35(1): 96–112. [DOI] [PubMed] [Google Scholar]
- 8.Oncology Nursing Society. Position statements, https://www.ons.org/advocacy-policy/positions (accessed 26 July 2017).
- 9.American College of Surgeons. Cancer program standards, https://www.facs.org/quality-programs/cancer/coc/standards (accessed 26 July 2017).
- 10.National Comprehensive Cancer Network. NCCN Guidelines and compendium updated, https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=130 (accessed 14 July 2017).
- 11.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016; 316(20): 2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; (6): CD007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009; 302(7): 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakitas M, Stevens M, Ahles T, et al. Project ENABLE: a palliative care demonstration project for advanced cancer patients in three settings. J Palliat Med 2004; 7(2): 363–372. [DOI] [PubMed] [Google Scholar]
- 15.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363(8): 733–742. [DOI] [PubMed] [Google Scholar]
- 16.Von Gunten CF and Lupu D. Development of a medical subspecialty in palliative medicine: progress report. J Palliat Med 2004; 7(2): 209–219. [DOI] [PubMed] [Google Scholar]
- 17.Center to Advance Palliative Care. Growth of palliative care in U.S. hospitals: 2015 snapshot (2000–2013), https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwistfCnhYnVAhVCSyYKHbs9D6AQFggkMAA&url=https%3A%2F%2Fmedia.capc.org%2Ffiler_public%2F34%2F77%2F34770c03-a584-4079-a9ae-edb98dab6b20%2Fgrowth_snapshot_2016_final.pdf&usg=AFQjCNHBb8USyMnPXqAZDUzAG2FqoSjixg&cad=rja (accessed 14 July 2017).
- 18.Hui D, Elsayem A, DelaCruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA 2010; 303(11): 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui D and Bruera E. Models of integration of oncology and palliative care. Ann Palliat Med 2015; 4(3): 89–98. [DOI] [PubMed] [Google Scholar]
- 20.Hui D, Kim YJ, Park JC, et al. Integration of oncology and palliative care: a systematic review. Oncologist 2015; 20(1): 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaertner J, Siemens W, Meerpohl JJ, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017; 357: j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamal AH, Currow DC, Ritchie CS, et al. Community-based palliative care: the natural evolution for palliative care delivery in the U.S. J Pain Symptom Manage 2013; 46(2): 254–264. [DOI] [PubMed] [Google Scholar]
- 23.Meier DE and Beresford L. Outpatient clinics are a new frontier for palliative care. J Palliat Med 2008; 11(6): 823–828. [DOI] [PubMed] [Google Scholar]
- 24.Waxmonsky J, Auxier A, Wise Romero P, et al. Integrated Practice Assessment Tool (IPAT). 2013, http://www.integration.samhsa.gov/operations-administration/IPAT_v_2.0_FINAL.pdf (accessed 14 July 2017).
- 25.National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care—3rd edition, PA, https://www.hpna.org/HPNA_Item_Details.aspx?ItemNo=978-1-934654-35-4 (accessed 19 July 2017).
- 26.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B and O’Connel D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, https://www.google.com/urlPsa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0ahUKEwjTldKmqInVAhXH6CYKHbC8A2EQFggrMAE&url=https%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fbooks%2FNBK99082%2Fbin%2Fappb-fm4.pdf&usg=AFQjCNFFkGagLWH-3qPWOF-cPI9C2qee_Xw&cad=rja (accessed 14 July 2017).
- 28.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36(3): 1–48. [Google Scholar]
- 29.DerSimonian R and Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 30.Knapp G and Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22(17): 2693–2710. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Research and Quality (AHRQ). Methods guide for effectiveness and comparative effectiveness reviews, https://www.effectivehealthcare.ahrq.gov/topics/cer-methods-guide/overview (accessed 2 January 2018). [PubMed]
- 34.Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 2016; 65: 61–68. [DOI] [PubMed] [Google Scholar]
- 35.McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med 2015; 18(11): 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015; 33(13): 1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014; 383(9930): 1721–1730. [DOI] [PubMed] [Google Scholar]
- 38.Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer 2013; 119(4): 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol 2006; 24(4): 635–642. [DOI] [PubMed] [Google Scholar]
- 40.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017; 35(8): 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groenvold M, Petersen MA, Damkier A, et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: the Danish Palliative Care Trial. Palliat Med 2017; 31(9): 814–824. [DOI] [PubMed] [Google Scholar]
- 42.Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer 2016; 69: 110–118. [DOI] [PubMed] [Google Scholar]
- 43.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol 2015; 33(13): 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakitas M, Dionne-Odom JN, Jackson L, et al. “There were more decisions and more options than just yes or no”: evaluating a decision aid for advanced cancer patients and their family caregivers. Palliat Support Care 2017; 15(1): 44–56. [DOI] [PubMed] [Google Scholar]
- 45.Dionne-Odom JN, Hull JG, Martin MY, et al. Associations between advanced cancer patients’ survival and family caregiver presence and burden. Cancer Med 2016; 5(5): 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Follwell M, Burman D, Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 2009; 27(2): 206–213. [DOI] [PubMed] [Google Scholar]
- 47.Pirl WF, Greer JA, Traeger L, et al. Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care. J Clin Oncol 2012; 30(12): 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greer JA, Tramontano AC, McMahon PM, et al. Cost analysis of a randomized trial of early palliative care in patients with metastatic nonsmall-cell lung cancer. J Palliat Med 2016; 19(8): 842–848. [DOI] [PubMed] [Google Scholar]
- 49.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 2012; 30(4): 394–400. [DOI] [PubMed] [Google Scholar]
- 50.Nipp RD, Greer JA, El-Jawahri A, et al. Age and gender moderate the impact of early palliative care in metastatic non-small cell lung cancer. Oncologist 2016; 21(1): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maloney C, Lyons KD, Li Z, et al. Patient perspectives on participation in the ENABLE II randomized controlled trial of a concurrent oncology palliative care intervention: benefits and burdens. Palliat Med 2013; 27(4): 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Support Care 2009; 7(1): 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy M, Smith T, Alvarez-Perez A, et al. Palliative care version 1.2016. J Natl Compr Canc Netw 2016; 14(1): 82–113. [DOI] [PubMed] [Google Scholar]
- 54.Haun MW, Estel S, Rucker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017; 6: CD011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015; 4(3): 99–121. [DOI] [PubMed] [Google Scholar]
- 56.Bekelman DB, Rabin BA, Nowels CT, et al. Barriers and facilitators to scaling up outpatient palliative care. J Palliat Med 2016; 19(4): 456–459. [DOI] [PubMed] [Google Scholar]
- 57.Sharma N, Sharma AM, Wojtowycz MA, et al. Utilization of palliative care and acute care services in older adults with advanced cancer. J Geriatr Oncol 2016; 7(1): 39–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


