Abstract
Objective:
The objective of the study was to analyze the effect of intrathecal transplantation of autologous bone marrow-derived mononuclear cells (BMMNCs) in functional recovery of spinal cord injury (SCI) patients along with neurorehabilitation and to evaluate various factors influencing the outcome of cellular therapy.
Methods:
We conducted an open-label study including 180 sub-acute and chronic SCI patients. All patients received intrathecal autologous BMMNCs along with neurorehabilitation. 80–100 mL of bone marrow was aspirated and BMMNCs were obtained using density gradient separation. An average of 1.06 × 108 cells with 97% viability was administered through lumbar puncture immediately. After transplantation, all patients underwent neurorehabilitation. Patients were followed up after an average of 9 ± 7 months. They were assessed for functional symptomatic changes and the outcome measures used were functional independence measure (FIM) and walking index for SCI (WISCI).
Results:
Patients showed symptomatic improvement in sitting/standing balance, bed mobility, trunk stability, upper limb function, mobility, sensation, bowel/bladder functions, and activities of daily living with no serious adverse events. Scores on FIM and WISCI showed statistically significant improvement. On subgroup analysis, it was found that early intervention and more than one dose of BMMNCs demonstrate a better functional outcome. Younger patients demonstrated better improvements in functional independence. Both cervical and dorsolumbar levels of injury show significant improvements in motor and sensory deficits.
Conclusions:
Autologous BMMNC transplantation with neurorehabilitation is safe, effective, enhances functional recovery, and improves the quality of life of SCI patients in sub-acute and chronic stage.
Keywords: Autologous bone marrow-derived mononuclear cells, chronic, quality of life, spinal cord injury
Introduction
Spinal cord injury (SCI) is any damage caused to the spinal cord and nerve roots resulting in incomplete or partial loss of function below the level of injury. This may lead to temporary or permanent loss of motor, sensory, or autonomic functions. Depending on the severity and level of injury, the patient may suffer residual muscular and sensory defects. SCI can be categorized as traumatic and nontraumatic. Traumatic SCIs are most commonly caused due to road traffic accidents, falls, sports injuries, etc., while, non-traumatic SCIs are mostly caused due to infections, tumors, or vascular, and degenerative conditions.[1,2]
Decades ago, a person with severe SCI would die within a couple of years after injury due to diseases of the urinary system, cardiovascular diseases, neurological deficits, pneumonia and influenza, septicemia, other infections, suicide, etc.[3] However, in recent years, the outcome has improved considerably due to better surgical and early management techniques at the acute stage of injury.[4] The majority of patients live with residual neurological deficits in the chronic phase of an injury, making them functionally dependent for their activities of daily living (ADLs). Rehabilitation, pain relief, spasticity treatment, and prevention of secondary complications are the only treatment options currently available for SCI patients in the chronic stage.[5] However, these conventional treatments alone do not result in satisfactory functional recovery.[6] The recovery of the patients suffering from SCI is difficult due to the limited ability of the central nervous system to replace the damaged/lost cells, restore disrupted myelin and re-establish functional neural connections.[7] Hence, newer treatments are being investigated with an aim to regenerate and repair the lost neuronal functions. Cellular therapy has emerged as a promising therapeutic strategy due to its ability to address the underlying pathophysiology of chronic SCI.[8-11] It reduces neuronal damage and inflammation, promotes regeneration, tissue repair at the site of injury, remyelination of the axons and helps in neuroprotection and angiogenesis.[12-14] In experimental models of SCI, cellular therapy using different cell types has shown to overcome physical disability and promote neurological improvements.[15-18] Among clinical studies, autologous bone marrow-derived cells have shown to be the most safe and effective cell types, with the ability to promote functional recovery along with sensory and motor improvements without any severe complications.[19-23]
In our previous preliminary study, we demonstrated the safety and efficacy of intrathecal transplantation of autologous bone marrow-derived mononuclear cells (BMMNCs) in dorsolumbar and cervical chronic SCI.[24,25] In the current study, we performed a detailed analysis of factors influencing the outcome of cellular therapy in sub-acute and chronic SCI patients.
Experimental Section
Study design and objective
An open-label study was conducted including 180 patients of SCI (sub-acute and chronic) at NeuroGen Brain and Spine Institute, Navi Mumbai, India, starting from August 2012 to October 2018. The objective of the study was to analyze the effect of intrathecal transplantation of autologous BMMNCs in functional recovery of SCI patients along with neurorehabilitation and to evaluate various factors influencing the outcome of cellular therapy.
Ethics statement
The study protocol was approved by the Institutional Ethics Committee. The selection of the patients was based on the World Medical Association Declaration of Helsinki: Ethical principles for medical research involving the human subjects.[26] A written informed consent was obtained from all the patients and their caregivers after explaining the intervention in detail with all possible adverse events.
Inclusion and exclusion criteria
Inclusion criteria of the study were as follows: Patients with documented sub-acute (post-injury time [PIT] <12 months) and chronic SCI (PIT >12 months)[27] on magnetic resonance imaging (MRI) or computed tomography scan irrespective of the cause, extent, or completeness of their injury, age for more than 2 years and <80 years. Both males and females with intact higher mental functions were included in the study.
Exclusion criteria of subjects were as follows: Presence of respiratory distress, severe anemia (<9 g/dL), bone marrow disorders, abnormal liver or kidney function, and acute infections such as human immunodeficiency virus, hepatitis B virus, or hepatitis C virus, and malignancy. Other acute medical conditions, include respiratory tract infection or fever or fracture or renal failure pregnancy, and breastfeeding.
Pre-intervention protocol
All patients underwent extensive evaluation by medical and rehabilitation experts according to the protocol. Patients were evaluated for fitness pretherapy using routine blood tests and X-rays. Specific tests, such as MRI spine, electromyography, somatosensory evoked potentials, and nerve conduction velocity, were also performed. Pre-evaluation also included a complete neurological examination, psychological examination, manual muscle testing, and evaluation of motor functions according to the American Spinal Injury Association (ASIA) scale, functional independence according to functional independence measure (FIM), and walking according to walking index for SCI (WISCI) and spasticity was assessed using the modified Ashworth Scale. For the patients who were on anticoagulants, it was stopped 48 h before the procedure and restarted 96 h after the procedure.
Cellular transplantation procedure
Patients were administered granulocyte colony-stimulating factor (CSF), 72 h and 24 h before BMMNC transplantation as it helps in survival and multiplication of the cells.[28] 80–100 mL of bone marrow (based on age and body weight) was aspirated from the anterior iliac crest under local anesthesia using a bone marrow aspiration needle and placed in sterile tubes containing heparin. Bone marrow was diluted in the ratio of 1:1 with normal saline. The diluted bone marrow was subjected to density gradient separation using Ficoll-Paque media by centrifuging at 440 × g rpm for 35 min in a swinging bucket rotor without a brake at 20°C. MNCs were obtained as a buffy coat and were washed 3 times with normal saline by centrifuging at 300 × g for 15 min in a swinging bucket rotor without a brake at 20°C and finally resuspended in 1 ml of normal saline. The number of the cells was assessed manually using a hemocytometer and the viability of the cells was checked using a trypan blue exclusion assay and also confirmed by TALI machine using propidium iodide. The percentage of CD34+cells was assessed using fluorescence-activated cell sorting using BD™ Stem Cell Enumeration Kit (Catalog No. 344563). An average of 1.06 × 108 cells with 97% viability was administered through lumbar puncture immediately after separation using an 25G spinal needle at the level between the fourth and fifth lumbar vertebrae. 1 g of methyl prednisolone in 500 ml of ringer lactate was given intravenously to reduce inflammation and enhance the survival of the injected cells.[24,25]
Neurorehabilitation
After BMMNC transplantation, all the patients underwent an individualized multidisciplinary neurorehabilitation, which included physiotherapy, occupational therapy, psychological interventions, and aquatic therapy. The rehabilitation program was designed for each patient as per the detailed analysis done before the therapy. All the patients were recommended a home program under the supervision of a professional.
Adverse event monitoring and follow-up
The patients were monitored during their hospital stay of 4 days for any procedure or cell transplantation related immediate adverse events such as fever, headache, nausea, diarrhea, vomiting, pain, allergic reaction, shock, pain, or bleeding at the site of aspiration/injection, and infection.
Patients were followed up after an average of 9 months ± 7 months. At the time of each follow-up a detailed assessment was done which included neurological examination, psychological examination, manual muscle testing, and evaluation of motor functions according to the ASIA scale, functional independence according to FIM, and walking according to WISCI. They were also monitored for any long-term minor or major adverse events during this period.
Outcome measures and statistical analysis
At follow up, changes observed in symptoms were recorded. A percentage analysis was done for each symptom. The objective outcome measures used to record the effects of cellular therapy were FIM and WISCI.
Statistical analyses were performed using SPSS Statistics v20 software. Non-parametric Wilcoxon signed-rank test was used to test the statistical significance of the difference in the scales WISCI, FIM, and before and after transplantation. A significance level of 0.05 was chosen for all tests.
Results
Demographics
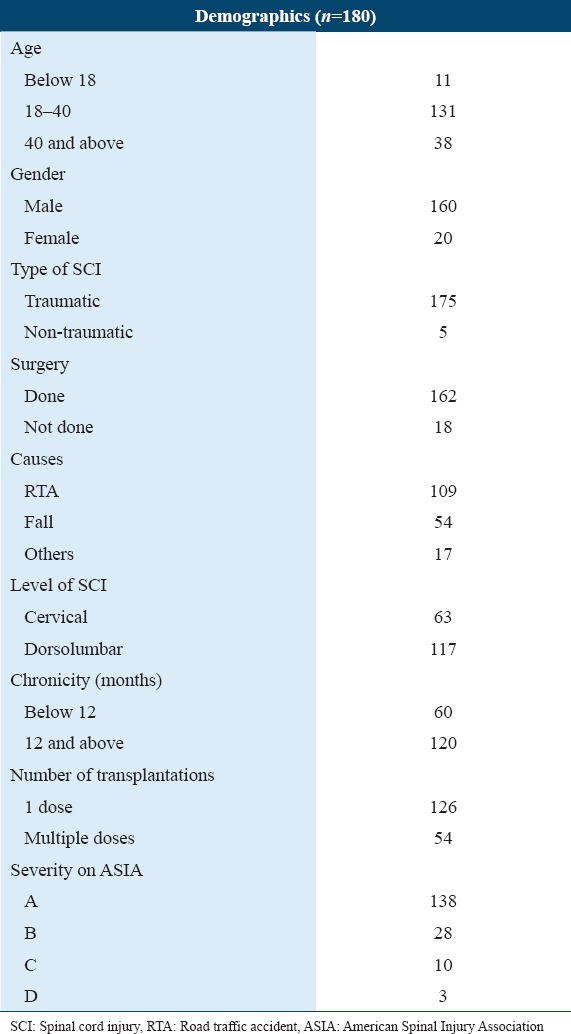
Cellular therapy was performed in 180 patients, among which 160 were males (88.88%) and 20 were females (11.11%). The age range was between 2 and 76 years, with an average age of 32.01 years. For detailed subgroup analysis, patients were categorized based on age, level of injury, and duration since SCI number of transplantations and severity on ASIA [Table 1].
Table 1.
Patient demographics and clinical data
Symptomatic analysis
Percentage analysis of the improvements in various symptoms of SCI was performed [Table 2 and Figure 1].
Table 2.
Percentage improvement in various symptoms of spinal cord injury
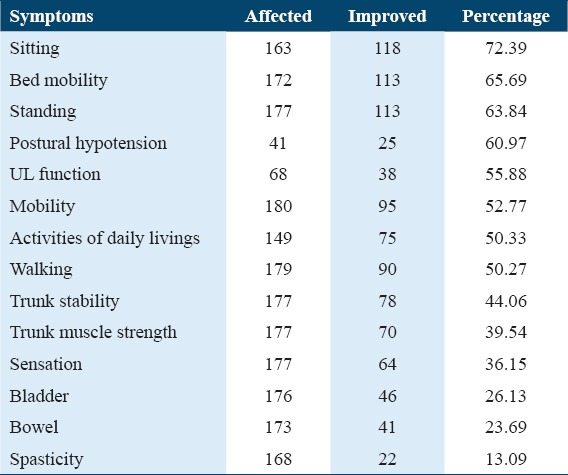
Figure 1.
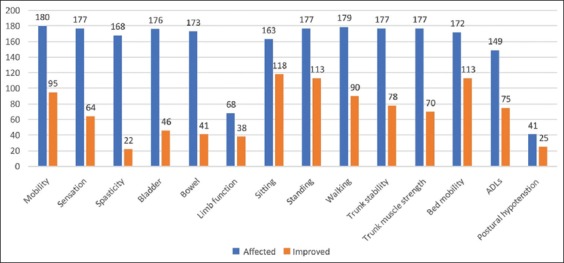
Symptomatic improvements after cell therapy
Along with percentage analysis, changes in the scores of FIM and WISCI were also analyzed. It was found that 69.44% of patients showed improved scores on the FIM scale while 37.22% showed improved scores on WISCI. The improvement in scores was also analyzed statistically using Wilcoxon signed-rank test and it was found that these improvements were statistically significant (P < 0.05) [Table 3].
Table 3.
Statistical analysis using the Wilcoxon signed-rank test

Factors affecting the outcome of intervention
Subgroup analysis was performed for various factors to study their influence on the outcome of intervention with respect to functional recovery.
Level of SCI
All patients were categorized into two groups, namely, cervical and dorsolumbar to understand the effect of level of injury on the functional outcome after cellular therapy. It was found that 70.08% of patients with dorsolumbar injury showed improved scores on the FIM scale while 68.25% of patients with cervical injury showed improved scores [Table 4].
Table 4.
Percentage analysis based on the level of injury

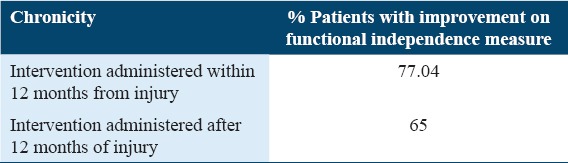
Chronicity of injury
Based on the chronicity of injury, patients were divided into two groups: Intervention administered within 12 months from injury and after 12 months from injury. It was observed that FIM scores improved in 77.04% of patients who underwent cellular therapy within 12 months of injury and in 65% of patients who underwent cellular therapy after 12 months. This indicates a better outcome in patients who were administered cellular therapy within 1 year of injury [Table 5].
Table 5.
Percentage analysis based on chronicity
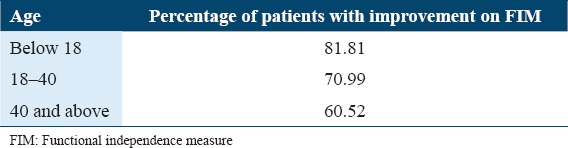
Age
All the patients were divided into three groups based on their age: Below 18 years, 18–40 years, and 40 years and above. It was found that maximum patients in the group of below 18 years of age showed improved FIM scores indicating a better outcome in the younger population of SCI [Table 6].
Table 6.
Percentage analysis of FIM score based on age
Number of transplantations
The effect of the number of cellular transplantations was analyzed by comparing the outcome on FIM in patients who underwent single and two doses. Fifty-four patients underwent a second dose. It was observed that higher percentage of patients (79.62%) who were administered the second dose showed improvements as compared to those who underwent a single dose of cellular transplantation (65.07%) [Table 7].
Table 7.
Improvements in FIM score based on the number of transplantations

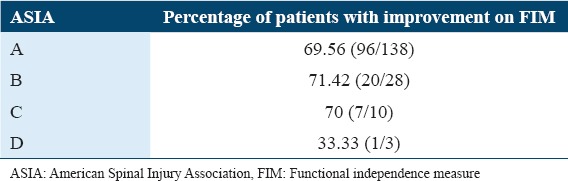
Severity based on ASIA
The functional outcome of cellular transplantation was studied with respect to the severity of injury based on ASIA. All grades on the ASIA scale showed significant improvements on percentage analysis. However, only 33% of patients with Grade D showed improvements. However, this could be inconclusive as there were only three patients in this group [Table 8].
Table 8.
Improvements on FIM based on the AISA score
Discussion
SCI is an injury to the spinal cord that results in varying degrees of paralysis and/or sensory involvement.[7] It can result in long-lasting dysfunction thus, significantly affecting the quality of life of patients. Moreover, the prevalence rate has also been increasing every year, mostly in young adults causing serious clinical, social and economic burden across the globe.[1] Despite major advances in the medical and surgical care of SCI patients, no effective treatment exists for the neurological recovery of patients with chronic SCI, and they experience substantial neurological dysfunction and lifelong disability. Various neuroprotective and neuroregenerative therapies are being extensively investigated and cell-based therapy has emerged as a promising treatment option as it addresses the underlying core pathophysiology of SCI.
Pathophysiology of SCI
The pathophysiology of SCI involves two phases; primary injury phase, which is followed by a cascade of secondary injury. Primary injury is caused due to the initial events such as shearing, laceration, compression, and distraction. Within a few minutes after the damage, the spinal cord suffers severe hemorrhages mainly in the grey matter area and subsequently leads to necrosis at the lesion site. This event initiates the occurrence of vasospasm, thrombosis, and spinal cord edema resulting in ischemia.[29,30] After the primary injury, a complex array of secondary pathophysiological events is initiated which expands the area of neural tissue injury. Various inflammatory cells such as macrophages, microglia, T-cells, and neutrophils infiltrate at the injury site and triggers the release of inflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1α, IL-1β, and IL-6.[31,32] Excitatory amino acids, such as glutamate and aspartate, are also released causing excitotoxicity at the injury site.[33] Ionic homeostasis is also lost resulting in intracellular hypercalcemia which activates calcium-dependent proteases and causes mitochondrial dysfunction of the cells.[34] All these biochemical changes in neuronal and glial cells lead to increased dysfunction and apoptotic cell death over hours to weeks after the initial insult. Glial scar is formed in the chronic phase due to the release of reactive astrocytes, fibroblasts, and activated microglial cells.[35-37] The apoptotic loss of cells causes demyelination and damage to the axon, and scar formation contributes to the inhibitory environment for axonal regeneration, thus interrupting sensory and motor neuronal transmission to and from the brain.[38,39]
Rationale for autologous BMMNCs transplantation in sub-acute and chronic SCI
Cellular transplantation has shown to promote physiological recovery in SCI either by direct cell replacement or through various paracrine mechanisms.[40] To study their clinical, functional benefits, we administered autologous BMMNCs in 180 sub-acute and chronic SCI patients, intrathecally. These cells are safe, free from ethical issues and do not undergo malignant changes or genetic abnormalities.[24-25,41] They are a mixture of mesenchymal stem cells, hematopoietic stem cells, macrophages, endothelial progenitor cells, etc., and thus exhibit a combined effect of all these cells.[42]
Bone marrow-derived cells either differentiate into neurons to restore the neuronal transmission or into oligodendrocytes or astrocytes for remyelination. Animal studies have revealed extensive remyelination after transplantation of BM cells into the demyelinated rat spinal cord.[43]
They can also carry out the repair process through various paracrine mechanisms. BMMNCs secrete various neurotropic and neuromodulatory molecules such as connective tissue growth factor, IL, vascular endothelial growth factor (VEGF), glial cell-derived neurotrophic factor, insulin-like growth factor 1, and basic fibroblast growth factor.[44] Neurotrophic factors promote neuronal sprouting, synaptogenesis, and increase neurotransmission by increasing the release of neurotransmitters. These factors can ameliorate the toxic environment thus preventing apoptosis and stimulating survival and growth of the affected neural cells thereby improving their synaptic connectivity.[45] BMMNCs secrete cytokines which promote hematopoietic cells to adhere to the endothelium promoting angiogenesis.[46] A preclinical study reported that bone marrow stem cells help in motor function recovery in rats with SCI, by upregulating VEGF mRNA expression and increasing angiogenesis in the spinal cord. The anti-inflammatory molecules secreted by these cells reduce inflammation and microglial activation thereby promoting neuroprotection.[47] These factors also induce endogenous cells to increase neuronal plasticity. The growth and neurotrophic factors stimulate the cells to form bridges at the injury site, which act as support for regrowing nerve fibers to rejoin.[48] The same has been demonstrated in another study where marrow stromal cells formed physical, nerve fiber-permissive tissue bridges which lead to long-term functional improvement in rats.[49] These cells also secrete exosomes which are involved in the transportation of biochemical molecules that play a role in cell communication. Exosomes also suppress the inflammatory response and the activation of neurotoxic reactive astrocytes, thus reducing the neural cell death.[50]
Intrathecal route of administration
To gain optimal outcome of cellular transplantation, it is vital to identify the best possible route of administration. Cells can either be transplanted directly into the site of injury or through intravenous, intraarterial, or intrathecal routes. However, direct transplantation is an invasive procedure and involves a high risk of secondary damage. Studies have revealed that through intravenous route, very few cells reach the damaged site as most of the cells get trapped in the lungs, liver, and spleen. Moreover, intraarterial infusion is accompanied by a high incidence of micro-occlusion whereas[51,52] intrathecal transplantation is relatively less invasive[53] and the cells can mobilize directly to the site of injury through CSF. This has been validated in a study where green fluorescent protein labeled MSCs could migrate from a distant site into the injured spinal cord through the CSF.[54]
Neurorehabilitation
As a part of the study protocol, after transplantation, all patients underwent a multidisciplinary personalized neurorehabilitation program, which included physiotherapy, occupational therapy, psychological therapy, and aquatic therapy. Rehabilitation plays an important role, as it further enhances the effect of stem cells.[55] A study found that on combining bone marrow stem cell therapy and exercise, the functional improvements were significantly greater as compared to single or no therapy.[56] Task-oriented training and physical therapy have shown to increase neurotrophic factors, endogenous proliferation and help in spinal reorganization.[57]
Clinical results
In the current study, patients showed improvements in various symptoms such as sitting and standing balance, bed mobility, trunk stability, upper limb function, mobility, sensation, and ADLs. It was observed that the improvements achieved were faster and better as compared to standard treatments. These observations were made by experts, patients, and their families. This may be explained by synergistic and augmentative positive effects of cell therapy with neurorehabilitation. Eleven patients out of 53 showed improved healing of pressure sore. FIM and WISCI were used as outcome measures to quantify the functional recovery after cellular transplantation. FIM is a widely accepted assessment tool to evaluate the functional independence of the patient. About 69.44% of patients improved on the FIM scale which reflects on significantly improved quality of life[58] WISCI scale was used to measure the change in ambulation of these patients.[59] Though with support, patients were able to improve their mobility and thereby elevate their mood, confidence, and self-esteem. Improvement in scores of FIM and WISCI after cellular therapy was also statistically significant, suggesting a significant relationship between the effect of cell therapy and functional improvements.
Further, subgroup analysis was performed to analyze the influence of the following factors on the outcome of intervention: (i) Level of injury, (ii) chronicity, (iii) age, (iv) number of transplantation, and (v) severity based on ASIA. After cellular therapy and rehabilitation, the majority of the patients showed improvements in motor and sensory functions based on the level of injury. Patients with injury at C3 and above need ventilator support for breathing therefore they were not included in this study. Patients with injury at the C4-C5 level showed better motor and sensory improvements in the upper limbs and trunk than lower limbs. Sometimes, changes in these patients may be apparent after a longer time and with more than one transplantation. Patients with injury at C6-D1 cervical level showed improvements in postural hypotension. Then, they initially improved in the upper limb and trunk muscle strength, and with time standing balance and ambulation were improved. Standing independently with walker and ambulation differed from person to person. If there was no other musculoskeletal complication, ambulation with calipers and walker was possible with time and regular rehabilitation. Patients with dorsolumbar level of injury showed better improvements in bed mobilities, bowel bladder sensation, trunk muscle strength, balance, and mobility (with assistive devices such as push-knee splints and walker). This suggests that lower the level of injury better is the result after cell therapy and rehabilitation.
Based on the chronicity of injury, the patients who underwent early intervention, i.e. within 12-months post-injury showed better improvements as an increase in the chronicity leads to scar formation which makes repair difficult. As compared to the acute phase, cellular therapy in the chronic phase or sub-acute phase is more likely to be successful as the environment is more permissive after the inflammatory response subsides.
When patients were analyzed based on age, better improvements were observed in the age group of below 18 years, followed by young adults (18–40 years) and patients above 40 years. This indicates that younger patients respond better to cellular transplantation, possibly for multiple reasons, including a greater number of stem cells being available, greater neuroplasticity, and better response to neurorehabilitation.
Our study also suggests that two doses of transplantation are more beneficial as compared to a single dose of BMMNC transplantation. This was also demonstrated by Park et al. in their studies. In one of their studies, they observed 60% of patients improved after multiple transplantations.[60,61]
The severity of SCI depends on the type of injury whether it is complete (ASIA A) or incomplete (ASIA- B, C, and D). Although the percentage analysis in this study showed around 70% of patients improving in Grades A, B, and C, it was observed by medical experts that incomplete injuries showed better motor and sensory response as compared to that of complete injury. Similar results were observed in a study conducted on 297 SCI patients using BMMNCs.[21] In a study done by Park et al., it was observed that after autologous bone marrow cell transplantation in conjunction with the administration of granulocyte macrophage-CSF, four patients showed neurologic improvements in their American Spinal Injury Association Impairment Scale (AIS) grades (from A to C). One patient improved to AIS Grade B from A and the last patient remained in AIS Grade A.[62]
In this study, we also monitored the adverse events to establish the safety of intrathecal transplantation of autologous BMMNCs. Throughout the follow-up duration, no patient experienced any serious adverse events. However, immediately after intervention, four patients reported minor procedure-related side effects such as fever and spinal headache which were managed with medication during their hospital stay.
Limitations
One of the limitations of this study was the lack of a control group. However, patients with duration of SCI longer than 12 months who had shown plateau in the recovery phase may be considered as self-controls. Furthermore, a neuroimaging study or a biomarker would help validate the results at a cellular level.
Conclusions
Autologous BMMNC transplantation is a safe and effective therapeutic strategy for sub-acute and chronic SCI patients. It significantly reduces functional deficits and makes these patients independent in performing their ADL. In this study, patients who underwent early intervention, i.e., within 12-months post-injury showed better improvement. Patients who were administered more than one dose of BMMNCs demonstrated enhanced functional outcomes. Increased functional independence was noted in patients below 18 years followed by young adults (18–40 years) and patients above 40 years. Both cervical and dorsolumbar levels of injury showed significant improvements in motor and sensory deficits. Cellular therapy also benefitted bladder and bowel functions. Overall, in addition to standard treatment, cellular therapy can achieve higher functional goals and help patients of SCI lead to a better quality of life.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Tator CH. Epidemiology and general characteristics of the spinal cord injury patient. In: Benzel EC, editor. Contemporary Management of Spinal Cord Injury. Park Ridge, Illinois, USA: American Association of Neurological Surgeons; 1995. pp. 9–13. [Google Scholar]
- 2.Chen Y, Tang Y, Vogel LC, Devivo MJ. Causes of spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19:1. doi: 10.1310/sci1901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord. 2000;38:604–10. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- 4.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic spinal cord injury:Current concepts and treatment update. Arq Neuropsiquiatr. 2017;75:387–93. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 5.Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000 Res. 2016;5:1017. doi: 10.12688/f1000research.7586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristante AF, Barros Filho TE, Marcon RM, Letaif OB, Rocha ID. Therapeutic approaches for spinal cord injury. Clinics. 2012;67:1219–24. doi: 10.6061/clinics/2012(10)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraus KH. The pathophysiology of spinal cord injury and its clinical implications. Semin Vet Med Surg (Small Anim) 1996;11:201–7. doi: 10.1016/s1096-2867(96)80013-2. [DOI] [PubMed] [Google Scholar]
- 8.Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010;6:363–72. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel A. Stem cell therapy in spinal cord injury:Hollow promise or promising science?J Craniovertebr Junction Spine. 2016;7:121–6. doi: 10.4103/0974-8237.181880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ul Hassan A, Hassan G, Rasool Z. Role of stem cells in treatment of neurological disorder. Int J Health Sci (Qassim) 2009;3:227–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Nandoe Tewarie RS, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. J Spinal Cord Med. 2009;32:105–14. doi: 10.1080/10790268.2009.11760761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WE. Concise review:Bone marrow for the treatment of spinal cord injury:Mechanisms and clinical applications. Stem Cells. 2011;29:169–78. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutts M, Keirstead HS. Stem cells for the treatment of spinal cord injury. Exp Neurol. 2008;209:368–77. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–51. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald JW, Liu XZ, Qu Y, Mickey SK, Turetsky D, Gottlieb DI, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nature Med. 1999;5:1410–2. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 16.Bottai D, Cigognini D, Madaschi L, Adami R, Nicora E, Menarini M, et al. Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Exp Neurol. 2010;223:452–63. doi: 10.1016/j.expneurol.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Nicolas G, Helen B, Robin JM, Nick DJ. Autologous olfactory mucosal cell transplants in clinical spinal cord injury:A randomized double-blinded trial in a canine translational model. Brain. 2012;135:3227–37. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–73. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- 19.Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, et al. Phase 1 trial of autologous bone marrow stem cell transplantation in patients with spinal cord injury. Stem Cells Int. 2016;2016:6768274. doi: 10.1155/2016/6768274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–45. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 21.Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury:A phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7:241–8. [PubMed] [Google Scholar]
- 22.Mendonça MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in sub-acute spinal cord injured patients. Clinical Neurol Neurosurg. 2012;114:935–9. doi: 10.1016/j.clineuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Gokulchandran N, Sane H, Badhe P, Kulkarni P, Lohia M, et al. Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury:An original study. J Neurorestoratol. 2013;1:13–22. [Google Scholar]
- 25.Sharma A, Sane H, Gokulchandran N, Kulkarni P, Thomas N, Bhovad P. Role of autologous bone marrow mononuclear cells in chronic cervical spinal cord injury-a longterm follow up study. J Neurol Disord. 2013;1:2. [Google Scholar]
- 26.World Medical Association. World medical association declaration of Helsinki:Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Wannapakhe J, Arayawichanon P, Saengsuwan J, Amatachaya S. Changes of functional ability in patients with spinal cord injury with and without falls during 6 months after discharge. Phys Ther. 2014;94:675–81. doi: 10.2522/ptj.20130260. [DOI] [PubMed] [Google Scholar]
- 28.Haas R, Murea S. The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells. Cytokines Mol Ther. 1995;1:249–70. [PubMed] [Google Scholar]
- 29.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–13. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 30.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–25. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 31.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol. 2003;184:313–25. doi: 10.1016/s0014-4886(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 33.Ulndreaj A, Chio JC, Ahuja CS, Fehlings MG. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 2016;16:1127–9. doi: 10.1080/14737175.2016.1207532. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–8. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma. 2007;24:991–9. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- 36.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 37.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–25. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 38.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–6. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 39.Waxman SG. Demyelination in spinal cord injury. J Neuro Sci. 1989;91:1–14. doi: 10.1016/0022-510x(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 40.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 41.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–13. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuende N, Rico L, Herrera C. Concise review:Bone marrow mononuclear cells for the treatment of ischemic syndromes:Medicinal product or cell transplantation? Stem cells Transl Med. 2012;1:403–8. doi: 10.5966/sctm.2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–63. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain:Therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu D, Lü G, Cao Y, Li G, Zhi X, Fan Z. [Effects of bone marrow mesenchymal stem cells transplantation on expression of vascular endothelial growth factor gene and angiogenesis after spinal cord injury in rats] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:837–41. [PubMed] [Google Scholar]
- 48.Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 49.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–82. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury via suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36:469–84. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 52.Sudulaguntla A, Nanjwade B, Chandy V. Stem cells:Cultivation and routes of administration. Curr Trends Biomed Eng Biosci. 2017;2:555579. [Google Scholar]
- 53.Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life:Comprehensive case studies. Cell Transplant. 2008;17:1277–93. doi: 10.3727/096368908787648074. [DOI] [PubMed] [Google Scholar]
- 54.Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P, et al. Administration of autologous bone marrow-derived mononu-clear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant. 2012;21:S79–90. doi: 10.3727/096368912X633798. [DOI] [PubMed] [Google Scholar]
- 55.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976) 2004;29:1971–9. doi: 10.1097/01.brs.0000138273.02820.0a. [DOI] [PubMed] [Google Scholar]
- 56.Emmons R, Niemiro GM, De Lisio M. Exercise as an adjuvant therapy for hematopoietic stem cell mobilization. Stem Cells Int. 2016;2016:7131359. doi: 10.1155/2016/7131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carvalho KA, Cunha RC, Vialle EN, Osiecki R, Moreira GH, Simeoni RB, et al. Functional outcome of bone marrow stem cells (CD45(+)/CD34(-)) after cell therapy in acute spinal cord injury:In exercise training and in sedentary rats. Transplant Proc. 2008;40:847–9. doi: 10.1016/j.transproceed.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 58.Sumalatha KB, Handa G, Singh U. Rehabilitation medicine implications of stem cell therapy in spinal cord injury-a review. Indian J Phys Med Rehabil. 2013;24:9–15. [Google Scholar]
- 59.Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure:A quantitative review. Arch Phys Med Rehabil. 1996;77:1226–32. doi: 10.1016/s0003-9993(96)90184-7. [DOI] [PubMed] [Google Scholar]
- 60.Jackson AB, Carnel CT, Ditunno JF, Read MS, Boninger ML, Schmeler MR, et al. Outcome measures for gait and ambulation in the spinal cord injury population. J Spinal Cord Med. 2008;31:487–99. doi: 10.1080/10790268.2008.11753644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh SK, Jeon SR. Current concept of stem cell therapy for spinal cord injury:A review. Korean J Neurotrauma. 2016;12:40–6. doi: 10.13004/kjnt.2016.12.2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913–22. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]


