Abstract
Insulin-like growth factor binding protein-2 (IGFBP-2) stimulates osteoblast differentiation but only male Igfbp2 null mice have a skeletal phenotype. The trophic actions of IGFBP-2 in bone are mediated through its binding to receptor tyrosine phosphatase beta (RPTPβ). Another important ligand for RPTPβ is pleiotrophin (PTN), which also stimulates osteoblast differentiation. We determined the change in PTN and RPTPβ in Igfbp2–/– mice. Analysis of whole bone mRNA in wild-type and knockout mice revealed increased expression of Ptn. Rptpβ increased in gene-deleted animals with females having greater expression than males. Knockdown of PTN expression in osteoblasts in vitro inhibited differentiation, and addition of PTN to the incubation medium rescued the response. Estradiol stimulated PTN secretion and PTN knockdown blocked estradiol-stimulated differentiation. PTN addition to IGFBP-2 silenced osteoblast stimulated differentiation, and an anti-fibronectin-3 antibody, which inhibits PTN binding to RPTPβ, inhibited this response. Estrogen stimulated PTN secretion and downstream signaling in the IGFBP-2 silenced osteoblasts and these effects were inhibited with anti-fibronectin-3. Administration of estrogen to wild-type and Igfbp2–/– male mice stimulated an increase in both areal bone mineral density and trabecular bone volume fraction but the increase was significantly greater in the Igfbp2–/– animals. Estrogen also stimulated RPTPβ expression in the null mice. We conclude that loss of IGFBP-2 expression is accompanied by upregulation of PTN and RPTPβ expression in osteoblasts, that the degree of increase is greater in females due to estrogen secretion, and that this compensatory change may account for some component of the maintenance of normal bone mass in female mice.
Keywords: sexual dimophism, osteocalcin, receptor tyrosine phosphatase beta, insulin-like growth factor-1, bone mineral density
Insulin-like growth factor binding protein-2 (IGFBP-2) stimulates osteoblast proliferation and differentiation in vitro and bone formation in vivo (1–4). IGFBP-2 knockout mice have decreased trabecular bone mass, decreased bone mineral density, and a decreased bone formation rate (4, 5). Injection of IGFBP-2, or a peptide containing the region of IGFBP-2 that binds to its cell surface receptor, rescued the phenotype. Injection of a peptide that contained the region of the IGFBP-2 sequence that binds to and activates its receptor restored approximately 70% of the difference in bone mineral density between knockout and wild-type animals as determined by micro-computed tomography (CT) analysis (4). IGFBP-2 functions coordinately with insulin-like growth factor I (IGF-I) to stimulate osteoblast differentiation (1, 6). IGF-I deletion results in decreased bone volume and mineralization in mice, and deletion of the IGF-I receptor results in even smaller bones and deficient mineralization (7). However, coordinate interaction between IGF-I and IGFBP-2 is not mediated via IGFBP-2 binding IGF-I; rather both proteins bind to distinct cell surface receptors that initiate signaling events which converge to activate the protein kinase B (AKT) pathway, which is necessary for osteoblast differentiation (6). IGFBP-2 has been shown to bind to a cell surface protein termed receptor tyrosine phosphatase beta (RPTPβ) (8). This protein contains tyrosine phosphatase activity, and following ligand occupancy RPTPβ polymerizes, thereby negating its phosphatase activity. A specific target of this phosphatase is the AKT pathway inhibitor Phosphatase and Tensin Homolog (PTEN). Tyrosine phosphorylation of PTEN impairs its ability to inhibit AKT activation (9). Therefore, ligand occupancy of RPTPβ by IGFBP-2 results in enhanced IGF-I-stimulated AKT phosphorylation (1). Further studies have demonstrated that inhibition of IGFBP-2 binding to RPTPβ specifically inhibited not only IGF-I-stimulated AKT activation but also osteoblast differentiation (1, 6).
Although IGFBP-2 deletion resulted in significant changes in bone mass in mice, this effect was limited to male mice (5). Female mice retained a normal phenotype and had no decrease in bone mass or mineralization. To further analyze the role of estrogen, we performed ovariectomy in the Igfbp2–/– mice. Analysis of the mice 12 weeks after ovariectomy showed that the OVX Igfbp2–/– mice lost a greater percentage of areal bone mineral density (aBMD) than OVX Igfbp2+/+ mice (10). In addition, micro-CT analysis revealed a significantly greater reduction in trabecular bone mass in the distal femur of the OVX Igfbp2–/– mice (76%) than the OVX Igfbp2+/+ mice (48%) due to decreases in trabecular number. Furthermore, histomorphometry revealed that the OVX Igfbp2–/– mice had significantly increased bone resorption accompanied by decreased bone formation and mineral apposition rates compared with OVX Igfbp2+/+ animals. These findings suggest that there is a factor in female mice that could compensate for the loss of IGFBP-2 and that this factor is estrogen inducible.
RPTPβ has been shown to bind to multiple ligands other than IGFBP-2 (11). An important ligand that induces RPTPβ polymerization is pleiotrophin (PTN) (12, 13). Like IGFBP-2, PTN contains heparin binding domains that mediate its binding to RPTPβ (12). PTN has been shown in multiple studies to stimulate osteoblast proliferation and differentiation, and deletion of PTN in mice results in diminished bone volume fraction (ie, BV/TV) in some but not all studies, and decreased mineral bone mineralization (14–17). When female transgenic mice overexpressing PTN were analyzed they had significantly greater increases in bone mass and bone formation rate than male littermates (18). This suggested that osteoblast proliferation and differentiation are more PTN dependent in females than in males. These findings taken together with our prior observations strongly suggested that PTN could be the factor that compensates for loss of IGFBP-2 in female mice, thereby preserving osteoblast differentiation following Igfbp2 deletion. These studies were undertaken to test that hypothesis.
Materials and Methods
Immobilon-P membrane, estradiol and anti-β-actin antibody (RRID: AB_476692) (19) were purchased from Millipore-Sigma (St. Louis, MO). Anti-phospho-AKT (S473) (RRID: AB_329825) (20), collagen 1A (RRID: AB_2800036) (21), and anti-PTEN (AB_10694066) (22) antibodies were from Cell Signaling Technology Inc. (Danvers, MA). An anti-PTN antibody (RRID: AB_374039) (23) was purchased from GeneTex (Irvine, CA). Polyclonal antibodies for mouse osteocalcin and IGFBP-2 were generated in our laboratory. Anti-phospho-tyrosine (pY99) (RRID: AB_628123) (24) and anti-fibronectin-3 (FN3) (RRID: AB_629496) (25) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). PTN protein was purchased from R&D Systems (Minneapolis, MN). All other reagents were obtained from Millipore-Sigma unless otherwise stated.
Animals
Generation of the B6.129-Igfbp2tm1Jep strain, referred to as Igfbp2−/− mice, has been described previously (5, 26). All mice used in this study were produced and maintained in our research colony at Maine Medical Center Research Institute, where mice were housed in polycarbonate cages on sterilized paper bedding and maintained under 14-hour light, 10-hour dark cycles. Sterilized water and Teklad Global 18% Protein Rodent Diet (Harland Laboratories) were available ad libitum. Female and male Igfbp2−/− and Igfbp2+/+ control mice were all ages 24 weeks were subjected to dual-energy X-ray absorptiometry (DXA) analysis and fasted for 6 hours. In a second set of experiments, Igfbp2−/− and Igfbp2+/+ male mice were all ages 8 weeks at which time DXA analysis was preformed and then the cohort was implanted subcutaneously with 17-B estradiol pellets (0.18-mg pellet, 60-day release, #SE121) or placebo pellets (#C-111, Innovative Research of America, Sarasota, FL). At 16 weeks of age the mice were once again subjected to DXA analysis and killed as above. For each experiment, the numbers of mutant and control mice used are provided in the Results. All experimental studies and procedures involving mice were reviewed and approved by the Institutional Animal Care and Use Committee at the Maine Medical Center Research Institute.
Dual-energy X-ray absorptiometry
DXA for whole-body aBMD (g/cm2) exclusive of the head was performed using the PIXImus densitometer (GE-Lunar). A phantom standard provided by the manufacturer was assessed each day for instrument calibration (27).
Microcomputed tomography
Micro-architecture of distal trabecular bone and midshaft cortical bone were analyzed in femora from 24-week-old +/+ and −/− male and female mice as well as the 16-week-old estradiol-treated and control cohort by high-resolution micro-CT (VivaCT-40, 10-μm resolution; Scanco Medical AG). Bones were scanned at an energy level of 55 kVp and intensity of 145 μA, as described previously (28). The VivaCT-40 is calibrated weekly using a phantom provided by Scanco. All scans were analyzed using the manufacturer’s software (Scanco, version 4.05). Acquisition and analysis of micro-CT data were performed in accordance with published guidelines (29).
Quantitative real-time polymerase chain reaction
Total RNA was prepared from whole bones using a standard TRIzol (Life Technologies) method for tissues. A total of 500 ng of RNA was then converted to complementary (c)DNA in a reverse transcription reaction using the Applied Biosciences High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The cDNA was then diluted 1:10 with water. Quantification of mRNA expression was carried out using an iQ SYBR Green Supermix in a iQ5 thermal cycler and detection system (Bio-Rad). The GeNorm kit (PrimerDesign, Southampton, UK) was used to find the most stable housekeeping gene for bone, which was determined to be Hprt. Primers were designed and tested to be 95% to 100% efficient by PrimerDesign. The sequences of the primers used in this study are as follows: Hprt: sense: 5′-TCCTCCTCAGACCGCTTTT-3′, antisense: 3′-AGGTATACAAAACAAATCTAGGTCAT-5′; Ptn: sense: 5′-ACCAAGCCCAAGCCTCAA-3′ antisense: 3′-GCGTCTTTTAATCCAGCATCTT-5′; RPTPβ: sense: 5′-GAGTCTGAGAAGAAAGCGGTTAT-3′, antisense: 3′-GTGAGCAGTCTGGAAGCATTT-5′
Cell culture
Osteoclast cultures
Cultures were performed using previously described methods (5). Briefly, bone marrow cells were isolated by flushing the femurs from 8-week-old Igfbp2+/+ and Igfbp2–/– male and female mice. Osteoclast-like cells were generated by plating the bone marrow cells at 1 × 107 cells per well in 12-well plates in differentiation medium: α-minimal essential medium (MEM) supplemented with 5% Fetal Calf Serum, Macrophage Colony Stimulating Factors (30 ng/mL), and RANKL (100 ng/mL). The medium was changed at day 3, and at day 6 osteoclasts were harvested for RNA using the methods described above.
MC-3T3 E1 clone 4 (CL4) cells were obtained from ATCC (Manassas, VA). Cells were cultured in α-MEM (glucose 1000 mg/L) containing 10% fetal bovine serum (Atlanta Biologics, Flowery Branch, GA). After confluency, culture medium was changed to differentiation medium (DM), which contained 7.5% fetal bovine serum, penicillin (100 unit/mL) /streptomycin (100 ng/mL), 50 µg/mL ascorbic acid, and 2 mM phosphate buffer (NaHPO4, pH7.4). Fresh DM was applied every 72 hours.
The animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of University of North Carolina at Chapel Hill. Neonatal calvarial osteoblasts were isolated from 3- to 5-day-old C57/B6J or IGFBP-2 knockout female or both sexes of mice as indicated. Briefly, calvariae were digested 5 times with collagenase type 2 (250 unit/mL) and trypsin (0.05%) plus ethylenediamine tetra-acetate (0.02%) in the phosphate-buffered saline. The cells released from digests 2 to 5 were collected as primary calvarial osteoblasts and maintained in α-MEM (glucose 1000 mg/L) supplemented with 10% FBS and nonessential amino acid growth medium. Upon reaching confluent density the medium was changed to differentiation medium containing the same components listed previously. The cells were used without passage.
Construction of cDNAs and establishment of IGFBP-2-silenced and LacZ-silenced cells
MC-3T3 cells expressing shRNA targeting IGFBP-2 and control shRNA were established following the procedures described previously (1).
Transient transfection with siRNA targeting PTN
Small interfering (si)RNA targeting PTN (SI01392636) and a control siRNA (sc-37007) were purchased from QIAGEN (Germantown, MD) and Santa Cruz Biotechnology, Inc. (Dallas, TX), respectively. MC-3T3 CL4 cells were transfected using a concentration of 60 nM and the PepMute Plus reagent (SignaGen Laboratories, MD) following the manufacturer’s instructions. The experiments were initiated 24 hours or at the stated time after transfection.
Immunoprecipitation and immunoblotting
The cell monolayers were lysed in a modified radioimmunoprecipitation assay (RIPA) buffer as previously described (1). Immunoprecipitation was performed by incubating 0.5 mg of cell lysate protein with 1 µg of an anti-pY99 antibody at 4°C overnight. Immunoblotting was performed as previously described (1) using a dilution of 1:500 for an anti-PTN antibody, a dilution of 1:1000 for an anti-pAKT (S473) antibody, a dilution of 1:2000 for an anti-PTEN antibody, a dilution of 1:3000 for an anti-osteocalcin antibody, and a dilution of 1:5000 for an anti- β-actin antibody. The proteins were visualized using enhanced chemiluminescence (Thermo Fisher Scientific, Rockford, IL). Total cellular protein in the lysates was determined using Bicinchoninic Acid Assay (Thermo Fisher Scientific).
Statistical analysis
All in vitro experiments were replicated at least 3 times to assure reproducibility unless otherwise stated. Scanning densitometry was utilized to quantify changes in band intensity. The scanning units obtained from the band of interest/loading control from 3 separate experiments were used for data analysis for the in vitro studies. The results were analyzed for statistically significant differences using Student’s t-test when two data points were compared or ANOVA was applied when multiple points were compared. All mouse data are expressed as the mean ± SEM. Results were analyzed for statistically significant differences using a two-way ANOVA followed by Sidak’s multiple comparison post hoc test. All statistics, including regression analysis, were performed with Prism 6 statistical software (GraphPad Software, Inc). Statistical significance was set at P < .05.
Results
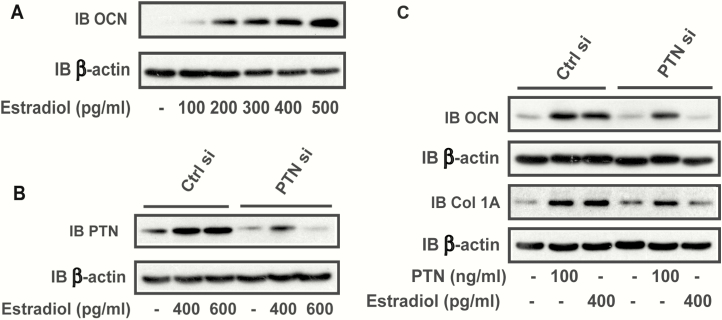
Initially we confirmed that Igfbp2–/– male mice had significantly reduced aBMD compared with wild-type males at 6 months of age. In contrast, the female Igfbp2–/– mice exhibited increased aBMD compared with controls (Fig. 1A). In addition, female Igfbp2–/– exhibited increases in trabecular BV/TV compared with the relative (wild-type control) decrease in male knockout mice (Fig. 1B). To determine if there was a difference in PTN expression that may correlate with these sex-dependent changes in bone mass, whole bone femoral mRNA expression was performed, and revealed that the knockout of IGFBP-2 in both sexes had a significant increase in PTN expression. However, the difference was substantially greater in female mice than male mice (Fig. 1C). Notably RPTPβ expression was also increased in both male and female knockout animals (Fig. 1D). Additionally, we analyzed PTN and RPTPβ expression in osteoclasts isolated from control and knockout animals. The results showed that PTN was significantly decreased in the female Igfbp2–/– mice compared with controls and RPTPβ was decreased in the Igfbp2–/– mice from both sexes (Fig. 1E and 1F). To confirm that these changes occurred at the protein level, calvarial osteoblasts were isolated from control and knockout animals and cell lysates were analyzed by immunoblotting for PTN. Lysates obtained from day-3, -6, and -9 cultures showed that there was an increased level of PTN expression in the cells derived from the female knockout mice compared with the cells obtained from control animals (Fig. 2A). To determine if this difference was cell autonomous, MC3T3 (CL-4) cells were transfected with an Igfbp-2 shRNA and PTN expression was analyzed. Following silencing of IGFBP-2 (Fig. 2B) there was an increase in PTN compared with LacZ-silenced cells and this increase was sustained on both days 3 and 6 of differentiation (Fig. 2C).
Figure 1.
Sexual dimorphisms in bone mass and expression of pleiotrophin (PTN) and receptor tyrosine phosphatase beta (RPTPβ) in male and female Igfbp2–/– mice at 6 months of age. (A) Femoral areal bone mineral density (aBMD). (B) Distal femur trabecular bone volume/total volume (BV/TV) in Igfbp2–/– and Igfbp2+/+ male and female mice (n = 10/sex/genotype). Femoral mRNA expression of (C) PTN (D) RPTPβ (n = 6/sex/genotype). In vitro osteoclast expression of Ptn (E) Rptpβ (F) (n = 6/sex/genotype). For all analysis *P < .05; **P < .01; ***P < .001; ****P < .0001.
Figure 2.
Pleiotrophin (PTN) expression is enhanced in calvarial osteoblasts isolated from insulin-like growth factor binding protein-2 (IGFBP-2) knockout mice or in IGFBP-2 knockdown MC3T3 cells. (A, B, C) Lysates were prepared from calvarial osteoblasts isolated from female IGFBP-2 knockout (–) mice or female wild type (+) mice following the instructions described in methods (A) or from IGFBP-2 knockdown (IGFBP-2 Si) or control knockdown (LacZ Si) MC3T3 cells (B, C). Cell lysates were harvested at the indicated time points and were immunoblotted with an anti-PTN (PTN) (A, C), anti-IGFBP-2 (B), or anti-β-actin antibody.
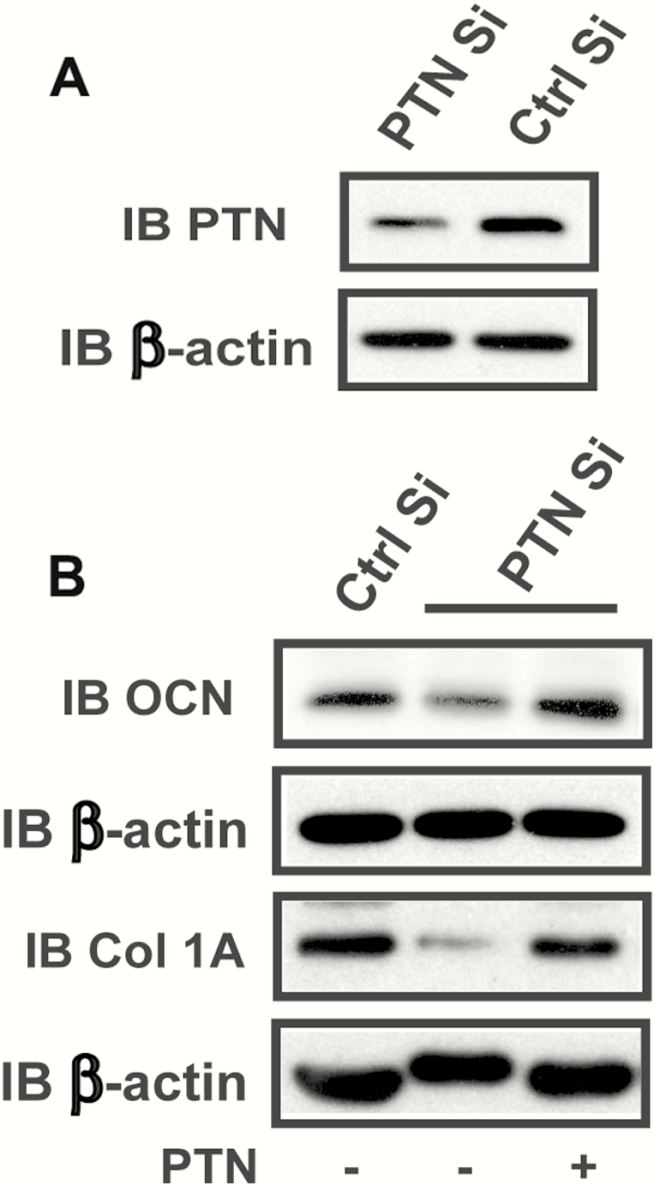
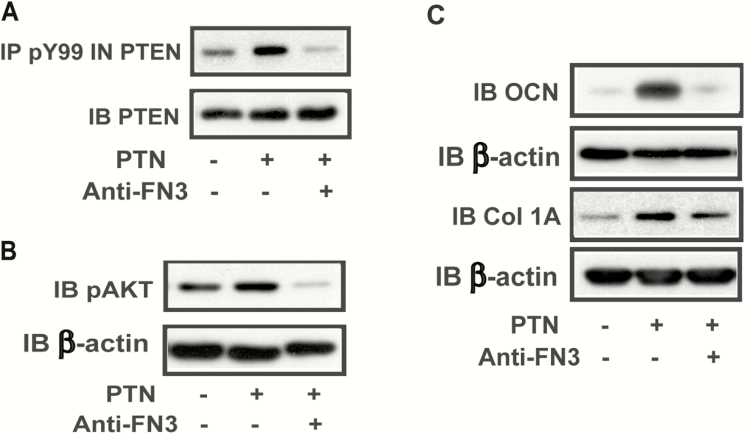
IGFBP-2 increases significantly during the first 9 days of differentiation and decreases thereafter. Therefore, we analyzed the time course of PTN expression. PTN expression increased during the first 3 days of differentiation in CL4 osteoblasts in vitro (ie, a 8.4 ± 2.6-fold increase at day 3, P < .05) and it continued to increase up to days 9 to 12 and then diminished substantially by day 15 (ie, a 60.0 ± 6% reduction compared with day 12, P < .05) (Fig. 3A). A similar result was obtained using primary calvarial osteoblasts (Fig. 3B). We have shown in prior studies that IGFBP-2 expression during the first 9 days of differentiation is required for osteoblast differentiation and that inhibition of IGFBP-2-stimulated downstream signaling elements (eg, AMPK and AKT) after day 9 does not result in any reduction in the percentage of cells that differentiate. The time course of PTN expression suggested that it may function in a similar manner at the same time point in the differentiation cycle. To determine if PTN was linked to differentiation, we used a siRNA that is specific for PTN. Exposure of MC-3T3 CL4 cells to this siRNA resulted in a significant decrease in PTN synthesis (Fig. 4A) and a significant decrease in osteocalcin production (eg, 61.3 ± 1.0 % reduction, P < .01) (Fig. 4B). Collagen 1A was also significantly decreased (eg, 82.2 ± 4.6% reduction, P < .01) In contrast, when PTN was added back to cultures that had been exposed to the siRNA, osteocalcin and collagen 1A synthesis were rescued (eg, 2.5 ± 0.5-fold and 4.0 ± 1.5-fold increases, respectively, P < .05), demonstrating that PTN was required for optimum osteoblast differentiation (Fig. 4B). To determine if PTN was functioning similarly to IGFBP-2, we added an anti-fibronectin-3 antibody (which blocks IGFBP-2 binding to RPTPβ) to cultures exposed to PTN and determined its effect on PTEN tyrosine phosphorylation and AKT activation, signaling events that are linked to RPTPβ binding and inactivation of its phosphatase activity (1). PTN stimulated PTEN tyrosine phosphorylation, leading to increased AKT phosphorylation and the addition of the antibody resulted in attenuation of this stimulation (Fig. 5A and B). PTN also stimulated osteocalcin (eg, a 3.4 ± 1.4-fold increase, P < .05), and collagen 1A expression (4.0 ± 0.6-fold increase, P < .01). These changes were significantly attenuated following antibody treatment (eg, a 54.5 ± 12.5% reduction, P < .05 for OCN; and 57.3 ± 15.7% reduction, P < .05 for collagen 1A) (Fig. 5C).
Figure 3.
Pleiotrophin (PTN) expression is upregulated at the early stage of osteoblasts differentiation but downregulated at the late stage of differentiation. (A, B) Lysates were prepared from the CL4 cells (A) or calvarial osteoblasts isolated from the wild type mice (both sexes) (B).The cultures were exposed to differentiation medium at day 0 and were analyzed at the indicated time points. The lysates were immunoblotted with an anti-PTN antibody or anti-β-actin antibody.
Figure 4.
Knockdown of pleiotrophin (PTN) inhibits osteocalcin expression which is restored by adding PTN exogenously. (A) Lysates were harvested from the CL4 cells transfected with a control siRNA (Ctrl Si) or a siRNA targeting PTN (PTN Si). The lysates were immunoblotted with an anti-PTN antibody or anti-β-actin antibody. (B) CL4 cells were treated with a control siRNA or a siRNA targeting PTN in the presence or absence of PTN. Cell lysates were immunoblotted with an anti-osteocalcin (OCN), anti-collagen 1A (Col 1A) or anti-β-actin antibody.
Figure 5.
Pleiotrophin (PTN)-stimulated PTEN tyrosine phosphorylation, AKT activation and osteocalcin expression are attenuated by an anti-fibronectin antibody. (A) Lysates were prepared from the CL4 cells treated with exogenous PTN in the presence or absence of an anti-fibronectin-3 (FN3) antibody. Cell lysates were immunoprecipitated with an anti-phospho-tyrosine (pY99) antibody and immunoblotted with an anti-PTEN antibody. The same amount of lysate was immunoblotted with an anti-PTEN antibody. (B, C) Lysates were prepared from the CL4 cells treated with exogenous PTN in the presence or absence of an anti-FN3 antibody. Cell lysates were immunoblotted with an anti-pAKT antibody (B) or anti-osteocalcin (OCN), anti-collagen 1A (Col 1A) (C) or anti-β-actin antibody.
To confirm that estrogen could induce osteoblast differentiation in our test system, we utilized CL-4 cells. The results showed that estrogen stimulated osteocalcin expression in a dose-dependent manner (Fig. 6A). To examine whether estrogen could induce PTN and if the induction of PTN was necessary for osteoblast differentiation, a siRNA targeting PTN was utilized. As predicted, estradiol stimulated PTN in the control, silenced cells but not significantly in PTN siRNA cells, (Fig. 6B). When osteocalcin synthesis was examined, estradiol stimulated osteocalcin but there was no increase in osteocalcin in response to estradiol in the PTN siRNA-treated cultures. Osteocalcin and collagen 1A induction could be rescued by PTN addition (Fig. 6C). To further test this hypothesis, CL-4 cells were exposed to IGFBP-2 shRNA. Although IGFBP-2 deletion increased PTN (Fig. 2B), PTN addition stimulated osteoblast differentiation and the anti-fibronectin-3 antibody inhibited this response (Fig. 7A). Similarly, estradiol stimulated a further increase PTN (Fig. 7B). These changes were accompanied by an estradiol-induced increase in AKT activation that could be inhibited with anti-fibronectin-3 antibody (Fig. 7C). To further prove that this resulted in a change in RPTPβ activity, we examined PTEN tyrosine phosphorylation, a direct target of the RPTPβ phosphatase (8). As shown in Fig. 7D, tyrosine phosphorylation of PTEN was stimulated in the presence of estradiol and inhibited by anti-fibronectin-3. To determine if these effects altered osteoblast differentiation, we immunoblotted cell lysates for collagen 1A. Estradiol stimulated collagen 1A production and this was inhibited following exposure to anti-fibronectin-3 (Fig. 7E).
Figure 6.
Estradiol-stimulated osteoblasts differentiation requires the presence of pleiotrophin (PTN). (A) Cell lysates were prepared from the CL4 cells treated with the different amounts of estradiol. The lysates were immunoblotted with an anti-osteocalcin (OCN) antibody or anti-β-actin antibody. (B) Lysates were prepared using CL4 cells transfected with a control siRNA (Ctrl si) or a siRNA targeting PTN (PTN si) in the presence or absence of estradiol. The lysates were immunoblotted with an anti- PTN antibody or anti-β-actin antibody. (C) CL4 cells were treated with a control siRNA or a siRNA targeting PTN in the presence or absence of PTN or estradiol. Cell lysates were immunoblotted with an anti-osteocalcin (OCN) or anti-collagen 1A (Col 1A) or anti-β-actin antibody.
Figure 7.
Pleiotrophin (PTN) or estradiol stimulate osteoblast differentiation which is attenuated by an anti-fibronectin-3 antibody in IGFBP-2 knockdown cells. (A) Cell lysates were prepared from the LacZ-silenced or IGFBP-2 knockdown CL4 cells in the absence or presence of PTN or anti-fibronectin (FN3) antibody. Cell lysates were immunoblotted with an anti-collagen 1A (Col 1A), or anti-β-actin antibody. (B) Lysates were prepared from the LacZ-silenced or IGFBP-2 knockdown CL4 cells that had been cultured in the absence or presence of estradiol. The lysates were immunoblotted with an anti- PTN or anti-β-actin antibody. (C, D, E) Lysates were prepared from the LacZ-silenced or IGFBP-2 knockdown CL4 cells cultured in the presence or absence of estradiol or anti-fibronectin (FN3) antibody. The lysates were immunoblotted with an anti-pAKT or anti-β-actin antibody (C). Lysates were immunoprecipitated with an anti-phospho-tyrosine (pY99) antibody and immunoblotted with an anti-PTEN antibody. The same amount of cell lysate was immunoblotted with an anti-PTEN antibody (D). Cell lysates were immunoblotted with an anti-collagen 1A (Col 1A) or anti-β-actin antibody (E).
To determine the significance of these events in vivo, estradiol was administered for 8 weeks to both Igfbp2+/+ and Igfbp2–/– male mice. Areal BMD and femoral trabecular bone mass were determined. Both genotypes exhibited increased aBMD with estrogen treatment; however, the increase was significantly greater in the Igfbp2–/– mice (Fig. 8A). Likewise both genotypes gained trabecular bone volume fraction with estrogen treatment; however, the knockout male mice exhibited a greater percent gain in femoral BV/TV relative to controls (Fig. 8B). To determine the mechanism accounting for the differential response to estradiol treatment, both PTN and RPTPβ femoral mRNA expression were analyzed. Igfbp2–/– control femoral PTN/ RPTPβ expression levels were increased compared with Igfbp2+/+ controls as seen in the 6-month cohort (Fig. 8C and D). However, we did not observe any further increase in PTN with estrogen treatment in either genotype (Fig. 8C). We believe this is possibly due to the pellets containing insufficient estrogen in the period prior to sacrifice. In contrast, RPTPβ was significantly increased in both groups in response to estrogen but the degree of increase was only significant in the Igfbp2–/– femurs (Fig. 8D). The findings show there is an increase in PTN in response to IGFBP-2 knockdown and a substantially greater increase in RPTPβ expression in the Igfbp2–/– mice in response to estrogen, a change that would enhance sensitivity to the anabolic effects of PTN. Both changes could function cooperatively to enhance bone mass, and this response would be consistent with the observation that females preserve their bone phenotype even in the absence of IGFBP-2 as a result of compensatory changes in both of these proteins.
Figure 8.
Estradiol treatment significantly stimulates bone mass in Igfbp2–/– and Igfbp2+/+ male mice and RPTPβ expression in null males. (A) Whole body areal bone mineral density (aBMD) changes during estrogen treatment between 8 and 16 weeks of age (n = 10/treatment/genotype). (B) Distal femur trabecular bone volume/total volume (BV/TV) in Igfbp2+/+ and Igfbp2–/– after 8 weeks of estradiol treatment. Femoral mRNA expression of PTN (C) and RPTPβ (D) (n = 6/ treatment /genotype). For all analysis *P < .05; **P < .01; ***P < .001; ****P < .0001.
Discussion
Our findings clearly demonstrate that PTN levels increase after Igfbp2 gene deletion in both male and female mice, but the degree of increase in PTN mRNA expression was substantially higher in females than males. Since we had previously reported that Igfbp2 deletion resulted in a skeletal phenotype with reduced bone mass in males, but not females, we propose that the disproportionate increase in PTN in females and the PTN receptor, RPTPβ, provides a compensatory response that accounts for this difference (5). These changes are cell autonomous, as supported by several observations. First, serum concentrations of PTN are quite low and several studies have demonstrated that locally synthesized PTN is a trophic factor for bone (30, 31). Second, the compensatory change in PTN after IGFBP-2 knockdown could be demonstrated in osteoblasts in culture using either MC-3T3 cells in which Igfbp2 expression was knocked down or primary calvarial osteoblasts derived from the Igfbp2-deficient mice. In both cases PTN production was increased in the absence of IGFBP-2. Our findings support the conclusion that PTN is utilizing the same receptor (RPTPβ) as IGFBP-2 (1). This is supported by the observation that exposure of cultures, co-incubated with PTN, and an antibody that inhibits IGFBP-2 and PTN binding to RPTPβ resulted in inhibition of PTN-stimulated AKT activation and osteoblast differentiation. Although PTN and RPTPβ are expressed in osteoclasts and their expression is reduced in the IGFBP-2–/– mice we found no difference between males and females arguing against a change in osteoclast activity that is mediated through PTN. Therefore, we propose that following Igfbp2 gene deletion the disproportionate increases in PTN and RPTPβ in the female mice compensate for the loss of IGFBP-2 and stimulate the RPTPβ/PTEN/AKT pathway to maintain osteoblast differentiation.
Several studies show that PTN is an anabolic factor for bone. Proteomic analysis of bone cell condition medium showed that bone cell chips derived from pigs released a variety of mitogens, including transforming growth factor beta, galectin-1, and PTN (32). PTN stimulates osteoblast proliferation and differentiation in vitro (13, 14). Importantly, PTN did not stimulate commitment of C2/C12 cells to the osteoblast lineage but once preosteoblasts had formed, it was a potent stimulant of osteoblast differentiation (16). Similarly, scaffolds co-cultured with osteoblast precursors showed that PTN enhanced alkaline phosphatase-positive colony formation and increased type I collagen as well as osteocalcin (33). New bone matrix formed after subcutaneous injection of these PTN- associated constructs into mice. PTN addition to a fibrous glass scaffold containing BMP-4 enhanced ectopic osteogenesis when implanted into rats subcutaneously (34). Three studies showed the mechanical loading was associated with an increase in PTN expression and 1 study showed an increase in RPTPβ in the same loading model (16, 35, 36). The PTN was induced following the loading, localized around osteocytes and periosteum.
In bone injury models there is upregulation of PTN mRNA expression and increased PTN is detected on the cell surface (37). During fracture healing, PTN expression is localized in both osteoblasts and endothelial cells during repair (38). Since angiogenesis is a critical step in bone healing, the anabolic effect PTN on endothelium is thought to function coordinately with its effects on osteoblasts and improve fracture healing. Mice overexpressing PTN show enhanced collagen 10, alkaline phosphatase, and calcification at the site of injury (39). They also have increased cortical bone area and periosteal thickening at the surface.
PTN transgenic mice showed increased expression in mesenchymal tissue and a phenotype characterized by increased bone thickness (15, 37, 40). They exhibited increased volume in both the cortical and trabecular compartments. Similarly, femoral bone mineral content was increased in transgenic mice compared with wild-type controls. Overexpression of PTN provided some protection against microgravity’s negative effects on the skeleton during spaceflight and this was attributed to stimulation of osteoblasts (41). Unlike the overexpression studies, targeted disruption of PTN was not associated with failure to increase bone size, but growth retardation was noted in weight bearing bones after 2 months of age. However, adult mice showed a more severely altered phenotype with low bone formation rates and osteopenia (15).
Studies that have analyzed bone during pathophysiologic conditions have shown significant differences in PTN expression compared with normal controls. Microarray analysis of human osteoblasts derived from femoral neck biopsies of normal and osteoporotic patients showed that PTN was one of 4 genes showing the greatest difference in expression (42). PTN mRNA expression was disproportionately reduced in cells derived from femoral neck biopsies from osteoporotic individuals. Genetic analysis of Single Nucleotide Polymorphisms in older men showed PTN Single Nucleotide Polymorphisms were associated with reduced trabecular volumetric BMD (43). Finally, a polymorphism 1277 C > T in the PTN promoter was associated with reduced lumbar spine BMD (44). Therefore, a variety of studies support the hypothesis that PTN is an important trophic factor for bone and that it functions primarily as a stimulant of osteoblast differentiation.
The results presented herein taken together with the results of our previous papers support the conclusion that IGFBP-2 and PTN are functioning by similar mechanisms. Both proteins stimulate the differentiation of preosteoblasts into osteoblasts and both are secreted by osteoblasts in mid-differentiation (36, 45). Inhibition of the secretion of either protein results in diminished osteoblast differentiation (1, 33, 45, 46). PTN and IGFBP-2 are upregulated in response to loading (34, 47). Both proteins stimulate endothelial migration and proliferation as well as angiogenesis, an important component of bone formation (48, 49). Most importantly both bind to RPTPβ and binding to this receptor leads to inactivation of its phosphatase activity thereby allowing optimum stimulation of the AKT pathway, which has been linked to osteoblast differentiation (12, 45). RPTPβ is expressed in differentiated osteoblasts and is important for osteoblast development. Genetic deletion of RPTPβ resulted in diminished numbers of osteoblasts and a reduced bone formation rate (50). Furthermore, the effect was shown to be cell autonomous. The mice were osteopenic and had decreased bone formation rates. Ex vivo analysis showed that cells lacking RPTPβ did not properly differentiate into osteoblasts. Our results show that downregulation of IGFBP-2 in osteoblasts in culture was associated with upregulation of PTN, suggesting a possible feedback mechanism. RPTPβ was also upregulated in the Igfbp2–/– mice.
One of the primary downstream targets of RPTPβ is PTEN. Interestingly, PTEN deletion has been shown to lead to upregulation of PTN secretion, again suggesting an inherent feedback mechanism that allows compensation (51). That this reciprocal regulation is important for increasing bone mass is supported by our finding that loss of IGFBP-2 in female mice is associated with a major increase in PTN and RPTPβ expression in bone and these changes are associated with an increase in BV/TV, whereas male mice have significantly less of an increase and they have a decrease in BV/TV.
The absence of a skeletal phenotype in female Igfbp2–/– mice could be explained by induction of PTN by estrogen, which has been shown to induce PTN mRNA expression in estrogen sensitive tissues (52). Most importantly, our findings show the direct addition of estrogen to osteoblast cultures results in an increase in PTN secretion and that blocking PTN association with RPTPβ following estrogen stimulation results in attenuation of estrogen-stimulated PTEN tyrosine phosphorylation and AKT activation. Furthermore, estrogen was minimally effective in stimulating osteocalcin production following PTN knockdown. These findings strongly suggest that PTN is functioning to stimulate the same signaling pathway that is stimulated by IGFBP-2 and that estrogen stimulation of PTN synthesis results in autocrine/paracrine activation of this signaling pathway. The predominant effect of estrogen is further supported by studies in PTN transgenic mice. Following induction of estrogen deficiency, PTN overexpression resulted in preservation of bone mass that was equal to wild-type mice without ovariectomy (53). The importance of estrogen was further supported by a study which compared the responses to PTN overexpression in male and female mice. Mineral apposition and bone formation rates as well as BMD were increased significantly in the transgenic females compared with controls but not in the male mice (18). The importance of estrogen is further supported by our finding that estrogen induced PTN in normal male mice and that it induced RPTPβ in both normal and Igfbp2–/– animals. These changes were accompanied by a substantial increase in aBMD and BV/TV.
PTN is expressed in a wide variety of tissues including neural and hematopoietic stem cell precursors. PTN has been shown to regulate hematopoietic stem cell maintenance and regeneration (54) and IGFBP-2 is also a potent stimulant of hematopoietic stem cell regeneration (55). PTN has been shown to play a role in endothelial cell maturation and development and blocking PTN binding to its receptor inhibits angiogenesis (56, 57). Similarly, IGFBP-2 stimulates endothelial progenitors to incorporate in tube formation, and IGFBP-2 knockout zebrafish embryos show defective angiogenesis (58). As for endothelial cells, both proteins have been shown to alter fat cell differentiation. Specifically, IGFBP-2 inhibits preadipocyte differentiation into mature adipocytes and mice overexpressing IGFBP-2 are relatively resistant to a high-fat diet (59). IGFBP-2 preferentially inhibits visceral fat. Similarly, PTN is overexpressed in visceral fat compared with subcutaneous fat and direct addition of PTN to preadipocyte cultures inhibits fat cell differentiation (60, 61). These findings suggest that IGFBP-2 and PTN may function coordinately in several cell types to regulate differentiation.
Our study has limitations. We have not proven causality in vivo by knocking out PTN and showing that estradiol fails to stimulate an increase in bone mass. We also have not carefully distinguished between those effects of estrogen that are PTN dependent and those that are PTN independent. Nevertheless, our results show a strong association between estrogen-dependent changes in PTN/ RPTPβ and bone mass acquisition in Igfbp2–/– mice. We also were able to show a causal relationship between estrogen stimulation of PTN and osteoblast differentiation in vitro.
In summary, these studies demonstrate that PTN secretion by osteoblasts is stimulated by estrogen and that PTN secretion is coordinately regulated with IGFBP-2. Knockdown of IGFBP-2 increases PTN expression in both male and female mice but the results suggest that the degree of increase in male mice is not sufficient to maintain normal bone mass following IGFBP-2 deletion. In females the increase in PTN is substantially greater and this increase is estrogen dependent. The response of females is further amplified by the fact that RPTPβ,which mediates PTN actions, is also upregulated in response to estrogen and IGFBP-2 deletion, thereby providing enhanced sensitivity to the increase in PTN that occurs in the bone microenvironment. In contrast, in Igfbp2–/– males there is less change in RPTPβ, but estrogen treatment of males resulted in a change in RPTPβ that was equivalent to females. These changes were associated with increased aBMD and BV/TV. Therefore, the findings suggest that the sexual dimorphism in response to IGFBP-2 deletion is due in part to the ability of estrogen to induce PTN and RPTPβ, two components of a signaling system that is essential for normal osteoblast differentiation.
Acknowledgments
The authors wish to thank Ms. Christine Wai for her technical assistance.
Financial Support: This work was supported by a grant from the national institutes of health NIH/ NIAMS/ORWH: 3R01 AR061164-05S1.
Glossary
Abbreviations
- BMD
bone mineral density
- BV
bone volume
- DXA
dual-energy X-ray absorptiometry
- IGF
insulin-like growth factor
- IGFBP-2
insulin-like growth factor binding protein-2
- RPTPβ
receptor tyrosine phosphatase beta
- PTN
pleiotrophin
- TV
total volume
Additional Information
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Xi G, Wai C, DeMambro V, Rosen CJ, Clemmons DR. IGFBP-2 directly stimulates osteoblast differentiation. J Bone Miner Res. 2014;29(11):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conover CA, Johnstone EW, Turner RT, et al. Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res. 2002;12(3):178–183. [DOI] [PubMed] [Google Scholar]
- 3. Palermo C, Manduca P, Gazzerro E, Foppiani L, Segat D, Barreca A. Potentiating role of IGFBP-2 on IGF-II-stimulated alkaline phosphatase activity in differentiating osteoblasts. Am J Physiol Endocrinol Metab. 2004;286(4):E648–E657. [DOI] [PubMed] [Google Scholar]
- 4. Kawai M, Breggia AC, DeMambro VE, et al. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286(16):14670–14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeMambro VE, Clemmons DR, Horton LG, et al. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149(5):2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xi G, Shen X, Rosen CJ, Clemmons DR. IRS-1 functions as a molecular scaffold to coordinate IGF-I/IGFBP-2 signaling during Osteoblast differentiation. J Bone Miner Res. 2016;31(6):1300–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohan S, Kesavan C. Role of insulin-like growth factor-1 in the regulation of skeletal growth. Curr Osteoporos Rep. 2012;10(2):178–186. [DOI] [PubMed] [Google Scholar]
- 8. Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. 2012;32(20):4116–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu DN, Pei DS, Wang Q, Zhang GY. Down-regulation of PTEN by sodium orthovanadate inhibits ASK1 activation via PI3-K/Akt during cerebral ischemia in rat hippocampus. Neurosci Lett. 2006;404(1-2):98–102. [DOI] [PubMed] [Google Scholar]
- 10. DeMambro VE, Le PT, Guntur AR, et al. Igfbp2 deletion in ovariectomized mice enhances energy expenditure but accelerates bone loss. Endocrinology. 2015;156(11):4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohebiany AN, Nikolaienko RM, Bouyain S, Harroch S. Receptor-type tyrosine phosphatase ligands: looking for the needle in the haystack. Febs J. 2013;280(2):388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meng K, Rodriguez-Peña A, Dimitrov T, et al. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000;97(6):2603–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perez-Pinera P, Chang Y, Deuel TF. Pleiotrophin, a multifunctional tumor promoter through induction of tumor angiogenesis, remodeling of the tumor microenvironment, and activation of stromal fibroblasts. Cell Cycle. 2007;6(23):2877–2883. [DOI] [PubMed] [Google Scholar]
- 14. Zhou HY, Ohnuma Y, Takita H, Fujisawa R, Mizuno M, Kuboki Y. Effects of a bone lysine-rich 18 kDa protein on osteoblast-like MC3T3-E1 cells. Biochem Biophys Res Commun. 1992;186(3):1288–1293. [DOI] [PubMed] [Google Scholar]
- 15. Fan JB, Liu W, Yuan K, et al. EGFR trans-activation mediates pleiotrophin-induced activation of Akt and Erk in cultured osteoblasts. Biochem Biophys Res Commun. 2014;447(3):425–430. [DOI] [PubMed] [Google Scholar]
- 16. Imai S, Heino TJ, Hienola A, et al. Osteocyte-derived HB-GAM (pleiotrophin) is associated with bone formation and mechanical loading. Bone. 2009;44(5):785–794. [DOI] [PubMed] [Google Scholar]
- 17. Lehmann W, Schinke T, Schilling AF, et al. Absence of mouse pleiotrophindoes not affect bone formation in vivo. Bone 2004;35:1247–55. [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto-Gotoh T, Ohnishi H, Tsujimura A, et al. Bone mass increase specific to the female in a line of transgenic mice overexpressing human osteoblast stimulating factor-1. J Bone Miner Metab. 2004;22(3):278–282. [DOI] [PubMed] [Google Scholar]
- 19.RRID: AB_476692, https://scicrunch.org/resolver/AB_476692. Accessed October 14, 2019.
- 20.RRID: AB_329825, https://scicrunch.org/resolver/AB_329825. Accessed October 14, 2019.
- 21.RRID: AB_2800036, https://scicrunch.org/resolver/AB_2800036. Accessed October 14, 2019.
- 22.RRID: AB_10694066, https://scicrunch.org/resolver/AB_10694066. Accessed October 14, 2019.
- 23.RRID: AB_374039, https://scicrunch.org/resolver/AB_374039. Accessed October 14, 2019.
- 24.RRID: AB_628123, https://scicrunch.org/resolver/AB_628123. Accessed October 14, 2019.
- 25.RRID: AB_629496, https://scicrunch.org/resolver/AB_629496. Accessed October 14, 2019.
- 26. Wood TL, Rogler LE, Czick ME, Schuller AG, Pintar JE. Selective alterations in organ sizes in mice with a targeted disruption of the insulin-like growth factor binding protein-2 gene. Mol Endocrinol. 2000;14(9):1472–1482. [DOI] [PubMed] [Google Scholar]
- 27. Delahunty KM, Shultz KL, Gronowicz GA, et al. Congenic mice provide in vivo evidence for a genetic locus that modulates serum insulin-like growth factor-I and bone acquisition. Endocrinology. 2006;147(8):3915–3923. [DOI] [PubMed] [Google Scholar]
- 28. Motyl KJ, Bishop KA, DeMambro VE, et al. Altered thermogenesis and impaired bone remodeling in Misty mice. J Bone Miner Res. 2013;28(9):1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- 30. Souttou B, Juhl H, Hackenbruck J, et al. Relationship between serum concentrations of the growth factor pleiotrophin and pleiotrophin-positive tumors. J Natl Cancer Inst. 1998;90(19):1468–1473. [DOI] [PubMed] [Google Scholar]
- 31. Tare RS, Oreffo RO, Clarke NM, Roach HI. Pleiotrophin/Osteoblast-stimulating factor 1: dissecting its diverse functions in bone formation. J Bone Miner Res. 2002;17(11):2009–2020. [DOI] [PubMed] [Google Scholar]
- 32. Caballé-Serrano J, Bosshardt DD, Buser D, Gruber R. Proteomic analysis of porcine bone-conditioned medium. Int J Oral Maxillofac Implants. 2014;29(5):1208–115d. [DOI] [PubMed] [Google Scholar]
- 33. Yang X, Tare RS, Partridge KA, et al. Induction of human osteoprogenitor chemotaxis, proliferation, differentiation, and bone formation by osteoblast stimulating factor-1/pleiotrophin: osteoconductive biomimetic scaffolds for tissue engineering. J Bone Miner Res. 2003;18(1):47–57. [DOI] [PubMed] [Google Scholar]
- 34. Sato Y, Takita H, Ohata N, Tamura M, Kuboki Y. Pleiotrophin regulates bone morphogenetic protein (BMP)-induced ectopic osteogenesis. J Biochem. 2002;131(6):877–886. [DOI] [PubMed] [Google Scholar]
- 35. Liedert A, Augat P, Ignatius A, Hausser HJ, Claes L. Mechanical regulation of HB-GAM expression in bone cells. Biochem Biophys Res Commun. 2004;319(3):951–958. [DOI] [PubMed] [Google Scholar]
- 36. Kaspiris A, Chronopoulos E, Grivas TB, et al. Effects of mechanical loading on the expression of pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta in a rat spinal deformity model. Cytokine. 2016;78:7–15. [DOI] [PubMed] [Google Scholar]
- 37. Imai S, Kaksonen M, Raulo E, et al. Osteoblast recruitment and bone formation enhanced by cell matrix-associated heparin-binding growth-associated molecule (HB-GAM). J Cell Biol. 1998;143(4):1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li G, Bunn JR, Mushipe MT, He Q, Chen X. Effects of pleiotrophin (PTN) over-expression on mouse long bone development, fracture healing and bone repair. Calcif Tissue Int. 2005;76(4): 299–306. [DOI] [PubMed] [Google Scholar]
- 39. Lamprou M, Kaspiris A, Panagiotopoulos E, Giannoudis PV, Papadimitriou E. The role of pleiotrophin in bone repair. Injury. 2014;45(12):1816–1823. [DOI] [PubMed] [Google Scholar]
- 40. Tare RS, Oreffo RO, Sato K, Rauvala H, Clarke NM, Roach HI. Effects of targeted overexpression of pleiotrophin on postnatal bone development. Biochem Biophys Res Commun. 2002;298(3):324–332. [DOI] [PubMed] [Google Scholar]
- 41. Tavella S, Ruggiu A, Giuliani A, et al. Bone turnover in wild type and pleiotrophin-transgenic mice housed for three months in the International Space Station (ISS). PLoS One. 2012;7(3):e33179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trost Z, Trebse R, Prezelj J, Komadina R, Logar DB, Marc J. A microarray based identification of osteoporosis-related genes in primary culture of human osteoblasts. Bone. 2010;46(1):72–80. [DOI] [PubMed] [Google Scholar]
- 43. Zmuda JM, Yerges-Armstrong LM, Moffett SP, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group Genetic analysis of vertebral trabecular bone density and cross-sectional area in older men. Osteoporos Int. 2011;22(4):1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mencej-Bedrač S, Preželj J, Komadina R, Vindišar F, Marc J. -1227C>T polymorphism in the pleiotrophin gene promoter influences bone mineral density in postmenopausal women. Mol Genet Metab. 2011;103(1):76–80. [DOI] [PubMed] [Google Scholar]
- 45. Xi G, Rosen CJ, Clemmons DR. IGF-I and IGFBP-2 Stimulate AMPK Activation and Autophagy, Which Are Required for Osteoblast Differentiation. Endocrinology. 2016;157(1):268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bolomsky A, Schreder M, Meißner T, et al. Immunomodulatory drugs thalidomide and lenalidomide affect osteoblast differentiation of human bone marrow stromal cells in vitro. Exp Hematol. 2014;42(7):516–525. [DOI] [PubMed] [Google Scholar]
- 47. Reijnders CM, Bravenboer N, Holzmann PJ, Bhoelan F, Blankenstein MA, Lips P. In vivo mechanical loading modulates insulin-like growth factor binding protein-2 gene expression in rat osteocytes. Calcif Tissue Int. 2007;80(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li G, Cui Y, McIlmurray L, Allen WE, Wang H. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res. 2005;23(3):680–685. [DOI] [PubMed] [Google Scholar]
- 49. Azar WJ, Azar SH, Higgins S, et al. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology. 2011;152(9):3332–3342. [DOI] [PubMed] [Google Scholar]
- 50. Schinke T, Gebauer M, Schilling AF, et al. The protein tyrosine phosphatase Rptpzeta is expressed in differentiated osteoblasts and affects bone formation in mice. Bone. 2008;42(3):524–534. [DOI] [PubMed] [Google Scholar]
- 51. Li G, Hu Y, Huo Y, et al. PTEN deletion leads to up-regulation of a secreted growth factor pleiotrophin. J Biol Chem. 2006;281(16):10663–10668. [DOI] [PubMed] [Google Scholar]
- 52. Lee JY, Jeong W, Lim W, et al. Chicken pleiotrophin: regulation of tissue specific expression by estrogen in the oviduct and distinct expression pattern in the ovarian carcinomas. PLoS One. 2012;7(4):e34215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Masuda H, Tsujimura A, Yoshioka M, et al. Bone mass loss due to estrogen deficiency is compensated in transgenic mice overexpressing human osteoblast stimulating factor-1. Biochem Biophys Res Commun. 1997;238(2):528–533. [DOI] [PubMed] [Google Scholar]
- 54. Himburg HA, Yan X, Doan PL, et al. Pleiotrophin mediates hematopoietic regeneration via activation of RAS. J Clin Invest. 2014;124(11):4753–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huynh H, Iizuka S, Kaba M, et al. Insulin-like growth factor-binding protein 2 secreted by a tumorigenic cell line supports ex vivo expansion of mouse hematopoietic stem cells. Stem Cells. 2008;26(6):1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang N, Deuel TF. Pleiotrophin and midkine, a family of mitogenic and angiogenic heparin-binding growth and differentiation factors. Curr Opin Hematol. 1999;6(1):44–50. [DOI] [PubMed] [Google Scholar]
- 57. Hamma-Kourbali Y, Bernard-Pierrot I, Heroult M, et al. Inhibition of the mitogenic, angiogenic and tumorigenic activities of pleiotrophin by a synthetic peptide corresponding to its C-thrombospondin repeat-I domain. J Cell Physiol. 2008;214(1):250–259. [DOI] [PubMed] [Google Scholar]
- 58. Wood AW, Schlueter PJ, Duan C. Targeted knockdown of insulin-like growth factor binding protein-2 disrupts cardiovascular development in zebrafish embryos. Mol Endocrinol. 2005;19(4):1024–1034. [DOI] [PubMed] [Google Scholar]
- 59. Wheatcroft SB, Kearney MT, Shah AM, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56(2):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hoggard N, Cruickshank M, Moar KM, Bashir S, Mayer CD. Using gene expression to predict differences in the secretome of human omental vs. subcutaneous adipose tissue. Obesity (Silver Spring). 2012;20(6):1158–1167. [DOI] [PubMed] [Google Scholar]
- 61. Yi C, Xie WD, Li F, et al. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett. 2011;585(20):3303–3309. [DOI] [PubMed] [Google Scholar]