Abstract
BACKGROUND
In 2014, the total prevalence of diabetes was estimated to be 422 million people worldwide. Due to the aging population and continued increase in obesity rates, the prevalence is expected to rise to 592 million by 2035. Diabetes can lead to several complications, including cardiovascular disease, stroke, peripheral arterial disease, nephropathy, neuropathy, retinopathy, lower extremity amputation, and musculoskeletal impairments.
CLINICAL QUESTION
Up to 80% of patients referred for outpatient physical therapy have diabetes or are at risk for diabetes, providing an opportunity for physical therapists to intervene. Therefore, we asked, “What is the role of physical therapists in fighting the diabetes epidemic?”
KEY RESULTS
Physical therapists commonly prescribe physical activity for the treatment of diabetes and other chronic diseases, such as cardiovascular disease and osteoarthritis. Physical therapists may also screen for risk factors for diabetes and diabetes-related complications and modify traditional musculoskeletal exercise prescription accordingly. Physical therapists must advocate for regular physical activity as a key component of the treatment of chronic diseases in all patient interactions.
CLINICAL APPLICATION
This commentary (1) describes the diabetes epidemic and the health impact of diabetes and diabetes-related complications, (2) highlights the physical therapist’s role as front-line provider, and (3) provides recommendations for physical therapists in screening for diabetes risk factors and diabetes-related complications and considerations for patient management. We focus on type 2 diabetes.
Keywords: complications, diabetes mellitus, disease management, epidemiology, physical therapist
Diabetes is a serious disease that has been identified by world leaders as 1 of 4 noncommunicable diseases to be targeted for action.110 Worldwide prevalence of diabetes nearly quadrupled from 108 million persons in 1980 to 422 million in 2014, while the age-adjusted prevalence nearly doubled from 4.7% to 8.5% during the same period.110 As it has in the past 2 decades, the prevalence of diabetes is expected to rise further as the population ages and adult obesity rates continue to increase.46,76 Implementation of effective interventions to delay the onset of diabetes and reduce the effects of established diabetes is desperately needed.
It is estimated that up to 80% of patients referred to outpatient physical therapy have diabetes, prediabetes, or diabetes risk factors,61 providing the perfect opportunity for physical therapists to intervene. Diabetes has the potential to negatively impact every tissue important for maintaining optimal function of the body’s systems required to produce human movement, collectively known as the human movement system (eg, musculoskeletal, nervous, endocrine, pulmonary, cardiovascular, integumentary). It is vital that physical therapists assert their role as front-line providers for patients with or at risk for diabetes.
Given the impact of diabetes on health and well-being, it is vital that physical therapists assert their role as front-line providers for patients with or at risk for diabetes. We therefore asked, “What is the role of physical therapists in fighting the diabetes epidemic?”
We propose that physical therapists intervene in 3 important ways.
Provide Guidance on Physical Activity Participation for Patients Who Have or Are at Risk for Diabetes
Physical activity is an important component in the treatment of chronic diseases, including diabetes, reducing morbidity and mortality.84 However, for patients with diabetes, physical activity programs must be carefully prescribed, such that they account for diabetes-associated pathophysiology and complications.
Regularly Screen Patients for Risk Factors for Diabetes and Diabetes-Related Complications
Once risk factors are identified, physical therapists can provide treatment that includes education in self-management strategies and prescription of safe and rewarding physical activity, an effective treatment for diabetes and associated comorbidities.
Advocate Regular Physical Activity as a Key Component of the Treatment of Chronic Diseases in All Patient Interactions
In this clinical commentary, we highlight the diabetes epidemic and provide recommendations for screening, examination, and preventive care practices that can be immediately implemented by physical therapists to reduce the impact of diabetes and its associated complications. Additionally, we highlight published, evidence-based recommendations to promote physical activity among people with diabetes.
PATHOPHYSIOLOGY OF DIABETES MELLITUS
Diabetes mellitus is a group of chronic metabolic conditions all characterized by elevated blood glucose levels resulting from the body’s inability to produce insulin, resistance to insulin action, or both (TABLE 1).9 This group of conditions consists of 4 clinically distinct types: (1) type 1 diabetes, which results from autoimmune beta-cell destruction in the pancreas and is characterized by a complete lack of insulin production; (2) type 2 diabetes, which develops when there is increased resistance to the action of insulin and the body cannot produce enough insulin to overcome the resistance; (3) gestational diabetes, which is a form of glucose intolerance that affects some women during pregnancy; and (4) a group of other types of diabetes caused by specific genetic defects of beta-cell function or insulin action, diseases of the pancreas, drugs, or chemical toxicity.5 We focus on type 2 diabetes in this commentary.
TABLE 1.
| Normal | Prediabetes | Diabetes | |
|---|---|---|---|
| Fasting plasma glucose, mg/dL | <100 | 100–125 | >126 |
| 2-h plasma glucose after 75-g OGTT, mg/dL | <140 | 140–199 | >200 |
| Random plasma glucose, mg/dL | ... | 140–199 | >200 |
| Glycated hemoglobin, % | <5.7 | 57–6.4 | ≥6.5 |
Abbreviation: OGTT, oral glucose tolerance test.
Type 2 diabetes accounts for 90% to 95% of all diagnosed diabetes cases.17 Type 2 diabetes typically begins as insulin resistance. As the body is unable to produce enough insulin to address the resistance, the pancreas may reduce or eventually stop the production of insulin.80 The term “prediabetes” is used to define individuals with a high risk of future diabetes. However, not all individuals who meet the definition of prediabetes will develop diabetes.1 Prediabetes includes people with elevated but subdiabetic fasting glucose levels (ie, “impaired fasting glucose”), impaired glucose tolerance, elevated glycated hemoglobin (HbA1c), or a history of gestational diabetes. The diabetes epidemic has been attributed to increasing sedentary behavior, a diet that provides excess energy (simple carbohydrates and saturated fats), and obesity.113 Laboratory values used to diagnose diabetes and prediabetes are provided in TABLE 1.
RISK FACTORS FOR TYPE 2 DIABETES MELLITUS
There are nonmodifiable and modifiable risk factors for development of type 2 diabetes (TABLE 2). Nonmodifiable risk factors include age, sex, socioeconomic position, race/ethnicity, genetic predisposition, history of gestational diabetes, and low birth weight.36,113 While European studies show a higher risk of diabetes in men compared with women,53 this was not consistently observed in the United States.68 In the United States, the risk of developing type 2 diabetes was higher among those in lower socioeconomic positions, including lower levels of education, occupation, and income.3 American Indians/Alaska Natives have the highest prevalence of diabetes, followed by non-Hispanic blacks and Hispanics. African Americans are more likely to develop diabetes than white and Asian individuals.36 For American Indians, the rates of diagnosed diabetes range from 5.5% to 33.5% in different tribes and population groups.22 Although genetic factors also play a role, primary risk factors appear to be those that are not genetic.93
TABLE 2.
Risk Factors for Diabetes That Are Modifiable and Nonmodifiable With Physical Therapist–Led Intervention
| Modifiable | Nonmodifiable |
|---|---|
| Overweight/obesity • Body mass index, ≥25 kg/m2 • Visceral fat • Waist circumference - Female, >35 inches - Male, >40 inches Inactivity • <30 min of moderate to vigorous activity per day, 5 d/wk Hypertension • Elevated, 120–129/<80 • Hypertension stage 1, 130–139/80–89 • Hypertension stage 2, ≥140/≥90 • Hypertension stage 3, >180/>120 (requires emergency care) Smoking Diet • Refined grains, red/processed meat, sugar-sweetened beverages, lack of moderate alcohol intake Psychosocial • Depression, increased stress, low social support, poor mental health Abnormal fasting cholesterol • Triglyceride, ≥150 mg/dL • High-density lipoprotein - Female, <50 mg/dL - Male, <40 mg/dL |
Age • >45 y Sex • Male Socioeconomic • Lower level of education, occupation, housing conditions, and income Race/ethnicity • American Indian, Alaska Native, African American, Hispanic, Asian American, Pacific Islander Family history/genetic predisposition History of gestational diabetes Low birth weight |
Modifiable risk factors include higher body mass index, physical inactivity, poor nutrition, hypertension, smoking, and alcohol use, among others.113 Increased body mass index is consistently one of the strongest risk factors for the development of diabetes.52 Additionally, distribution of body fat,88 specifically an increased waist-to-hip ratio, increases the risk of diabetes.58 Lower levels of physical activity and more television viewing time increase the risk of type 2 diabetes.45,55 Smoking increases the risk of diabetes, regardless of age.109 Psychosocial factors such as depression, increased stress, lower social support, and poor mental health are also associated with an increased risk of developing diabetes.11,33,35,42,72,100 Different aspects of the environment have also been linked to type 2 diabetes development. Increased levels of noise, poor housing conditions, and air pollution were associated with increased risk.30,92
Identifying and addressing modifiable risk factors can reduce the risk of diabetes. People who achieve recommended levels of moderate activity are about 30% less likely to develop diabetes than their inactive counterparts.55 Brisk walking for at least 2.5 hours per week was associated with reduced risk of type 2 diabetes compared to almost no walking, independent of body mass index.55 Higher levels of walkability and green space were associated with lower diabetes risk.30,92 A person’s risk of diabetic complication can be reduced up to 12% with a 10-mmHg decrease in blood pressure.22 Diets favoring higher intake of whole grains, green, leafy vegetables, and coffee; lower intake of refined grains, red and processed meat, and sugar-sweetened beverages; and moderate intake of alcohol have been linked with reduced risk of type 2 diabetes.68 A healthier diet can help to reduce HbA1c levels. For each percentage-point reduction in HbA1c level, there can be a 40% reduction in risk of microvascular complications.22 A Mediterranean diet with extra-virgin olive oil supplementation reduced diabetes risk by 40% compared to a low-fat control diet.91
HEALTH COMPLICATIONS
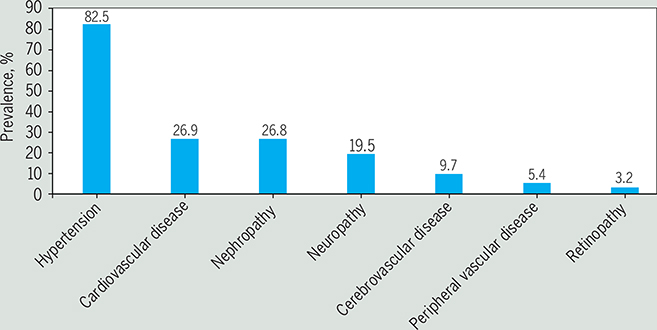
Diabetes may affect many different organ systems and can lead to serious microvascular and macrovascular complications, such as (1) cardiovascular disease, (2) stroke, (3) peripheral artery disease (PAD), also referred to as peripheral vascular disease, (4) nephropathy or chronic kidney disease, (5) diabetic peripheral neuropathy (DPN), (6) nontraumatic lower extremity amputations (NLEAs), and (7) retinopathy.31 The prevalence of the most common diabetes complications among people with type 2 diabetes is shown in the FIGURE.82 Complications can be either episodic (eg, foot ulcers or infections), those that can be treated and may recur, or progressive (eg, nephropathy), those that result in further damage to the organ and greater loss of functionality that are generally irreversible.
FIGURE.
Prevalence of complications and comorbidities among persons with diabetes in 2013. Data source: Pantalone et al.82
Heart Disease and Stroke
Cardiovascular diseases account for up to 65% of all deaths in people with diabetes.41 According to the Centers for Disease Control and Prevention, people with diabetes are about twice as likely to die from heart disease or stroke compared to those without diabetes.20 Over 70% of people with diabetes, which is significantly higher than the rate in those without diabetes, have high blood pressure.82 While risk factors for cardiovascular disease development among people with diabetes are similar to those for people without diabetes (ie, hypertension, hypercholesterolemia, and smoking), the presence of just one of these risk factors leads to poorer outcomes among people with diabetes compared to those without diabetes.99 Over the past 20 years, preventive care (self-care management strategies, diet, and participation in physical activity) targeting diabetes and the risk factors that cause these complications has improved significantly in the United States. The rates of complications from heart disease and stroke declined in adults with diagnosed diabetes from 1990 to 2010, with myocardial infarction accounting for the greatest reduction (68%).43
Peripheral Artery Disease
Peripheral artery disease results from narrowing of peripheral arterial vasculature, affecting primarily the limbs, stomach, and kidneys. Early in the process, PAD may be asymptomatic and is often underdiagnosed.81 Once symptoms are present, they are of 2 types: (1) intermittent claudication, which presents as pain, ache, or discomfort occurring during physical activity, such as walking, but resolving with rest; and (2) pain at rest caused by limb ischemia, which indicates poor blood flow to the affected limb.60 During 1999 to 2004, prevalence of PAD was about 11%.16 The risk of PAD increases with older age, smoking, and longer duration of diabetes.29,81 People with PAD are at an increased risk of lower extremity amputation and mortality.75,81 Patients with intermittent claudication, considered to benefit from exercise, improved their walking time and distance after participation in exercise compared to placebo or usual care.67
Retinopathy (Vision Loss)
Diabetic retinopathy is 1 of the 5 most common causes of severe visual impairment (visual acuity of 20/200 or worse) in the United States—an estimated 286 000 people aged 40 years or older had diabetic retinopathy during 2005 to 2008.62 As one of the longest-duration prospective studies of the epidemiology of diabetic retinopathy, the Wisconsin Epidemiologic Study of Diabetic Retinopathy reported a 10-year incidence of retinopathy of 74%.63 The 25-year follow-up of this cohort showed that 97% developed diabetic retinopathy, with about half progressing to sight-threatening disease.64,65 Risk factors for diabetic retinopathy include hyperglycemia, hypertension, dyslipidemia, longer duration of diabetes, pregnancy, puberty, and cataract surgery.23 If detected early, diabetic retinopathy’s progression can be slowed dramatically, and eyesight can be retained in many patients.57 The American Diabetes Association (ADA) recommends an eye exam every 2 years for those who have no signs of diabetic retinopathy. If there is evidence of diabetic retinopathy, the ADA recommends annual exams.
Nephropathy (Renal Disease)
Nephropathy is a chronic condition characterized by a gradual increase in proteinuria in patients without other conditions that directly cause proteinuria.69,89 Over time, nephropathy may progress to the development of end-stage renal disease and renal transplant. Diabetes is the leading cause of end-stage renal disease, accounting for 4 in every 10 new end-stage renal disease cases. Overall, the incidence and prevalence of end-stage renal disease have increased in recent decades among people with diabetes.78
Metabolic regulation, hypertension, cigarette smoking, obesity, and anemia are all modifiable risk factors for nephropathy.56 Genetic factors also increase risk for diabetic nephropathy.56 In persons with type 2 diabetes, strict metabolic control leads to a significant reduction in the risk of developing microalbuminuria and the risk of progression to persistent proteinuria.32,38,107
Peripheral Neuropathy
Diabetic peripheral neuropathy, a neurodegenerative disease of the peripheral nervous system, is estimated to affect up to 75% of individuals with diabetes.2,19,44,86 Chronic sensorimotor distal symmetric polyneuropathy, the most common DPN,15 can lead to muscle weakness, sensory loss, and pain in the extremities. The predominant early manifestation of DPN is a gradual onset of sensory impairment, including burning and numbness in the feet. Diabetic peripheral neuropathy may go undetected for years due to its gradual progression. Neuropathic pain, present in 1 in 3 people with DPN, can be severe.34,97,106
Diabetic peripheral neuropathy is associated with substantial physical impairments, activity limitations, and reduced quality of life. The presence of DPN increases the risk of foot ulceration and lower extremity amputation10,83 and is associated with greater health care resource use, health care costs, and an inability to work due to physical limitations.19,90 Other potential complications of DPN, such as mobility impairments and falls, can result in significant limitations in activity and participation. Hyperglycemia is the primary risk factor for DPN.86,95 Additional risk factors include older age, longer duration of disease, cigarette smoking, hypertension, elevated triglycerides, higher body mass index, alcohol consumption, and taller height.2,85,95,101,102,112
Lower Extremity Amputations
Nontraumatic lower extremity amputations are associated with high morbidity and mortality among people with diabetes. The 5-year mortality rate after NLEA ranges from 52% to 100%.103 The incidence of NLEA among people with diabetes is estimated to be as high as 704 per 100 000 person-years.77 Individuals with diabetes are 7.4 to 41.3 times more likely to have an NLEA compared to those without diabetes.77
With increased awareness of diabetes-related complications and subsequent implementation of preventive care strategies, the age-adjusted NLEA rates have decreased in the United States, from 70.4 per 10 000 adults with diagnosed diabetes in 1995 to 28.4 per 10 000 in 2010.43 Although overall NLEA rates have decreased, disparities between black and nonblack patients increased between 2007 and 2011, with the amputation-free survival rate being lower among black patients.79 Lower extremity amputation rates also differ significantly by the patient’s geographical location, highlighting the need to address both racial and regional disparities.79
Risk factors for NLEA include increasing age, being male, being African American or Hispanic, having peripheral neuropathy, and having chronic foot ulcers.77 Eighty-five percent of all NLEAs among people with diabetes were preceded by a chronic, nonhealing foot ulcer.83,87 Diabetic foot ulcers are common, with the lifetime risk of a person with diabetes developing an ulcer being as high as 1 in 4.96 Peripheral vascular disease underlies approximately half of all amputations in people with diabetes,83 and is associated with higher mortality after NLEA.103
Musculoskeletal Complications
Musculoskeletal complications associated with diabetes are common. Yet, they may go undetected and impact the ability to participate in physical activity.94 Foot and ankle musculoskeletal complications related to diabetes place the foot at risk for ulceration and amputation. Intrinsic foot muscle deterioration, in the form of reduced muscle volume and increased fat volume, is associated with metatarsophalangeal hyperextension deformity,25 collapse of the midfoot, and decreased foot function during a heel-raise task.48 Metatarsophalangeal hyperextension deformity has a prevalence as high as 85% in those with diabetes and a history of ulcers and amputations.51 Extensive foot joint destruction associated with Charcot neuropathic osteoarthropathy is less common (approximately 1% of people with diabetes) but, when severe, prevents weight bearing.51 Early detection and treatment of musculoskeletal impairments of the foot and ankle may improve the patient’s current and future ability to participate in physical activity and should be addressed. Additionally, physical activity participation may need to be modified when diabetes-associated complications prevent or limit weight-bearing ability. Individuals with diabetes and a history of foot ulcers were found to be 46% less active than control participants matched by age, sex, and body mass index.71
Patients with diabetes are 4 times more likely to have musculoskeletal complications of the shoulder and hand (eg, adhesive capsulitis, Dupuytren’s contracture, and flexor tenosynovitis) compared to those without diabetes.18 There is also an increased prevalence of low back pain in individuals with diabetes.37 Musculoskeletal impairments may be associated with joint mobility limitation and tissue changes, due to the accumulation of nonenzymatic advanced-glycation end products.73,94 Advanced-glycation end-product accumulation results in thicker and stiffer collagen tissues, in particular those with low turnover, such as tendons, skin, and discs,54 thus increasing the risk of contracture and musculoskeletal injury.
TABLE 3 provides comprehensive information to screen for diabetes-associated complications and highlights the physical therapist’s role in patient management. We also provide recommendations that will assist the physical therapist in developing safe and effective programs to promote physical activity and exercise among patients with diabetes and diabetes-associated complications.
TABLE 3.
Screening for Diabetes-Related Complications and Considerations for Patient Management4,9,27,66,84,105
| Screening Items Related to Diabetes and Diabetes-Related Complications | Physical Therapist Role and Responsibility |
|---|---|
| Psychosocial Factors* | |
| Depression • The PHQ-2 may be used as a “first-step” screening. Patients who answer yes to either question should be further evaluated by a mental health provider108 - “During the last 2 weeks, have you been bothered by feeling down, depressed or hopeless?” - “During the last 2 weeks, have you been bothered by little interest or pleasure in doing things?” |
• Annual screening for depression among all patients diagnosed with diabetes. Screening should also be performed at any time depression is suspected • Assist patient in developing an appropriate program to increase physical activity |
| Anxiety disorder • Patients who exhibit anxiety or worries regarding their diabetes-related complications or ability to participate in a lifestyle management program |
• Screen for anxiety disorder and refer for treatment if warranted |
| Disordered eating behaviors (binging, intentional omission of insulin) • Hyperglycemia • Unexplained weight loss |
• Screen for disordered eating behavior, eating disorders, and disrupted eating and refer to appropriate health care providers if warranted |
| Cognitive impairment/dementia | • Screen for cognitive impairments and tailor management to improve understanding and optimize the patient’s ability to adhere to a lifestyle management program. All patients older than 65 y should be screened for cognitive impairments |
| Socioeconomic Factors | |
| Lower social support Poor housing conditions |
• Assess barriers to physical activity participation and problem solve with patient to address barriers • Customize the patient’s activity program based on the patient’s goals and preferred activities • Assist in accessing community resources |
| Diabetes: General | |
| All diabetes-related complications | • Monitor blood pressure and glucose at the initial visit and as indicated at subsequent visits • Educate patient to monitor blood pressure and glucose daily, including response to exercise • Encourage lifestyle modifications to decrease caloric intake and increase physical activity for weight loss • Know medications, their side effects, and their effect on exercise response |
| Cardiovascular | |
| Peripheral vascular disease • Absent dorsal pedis and/or posterior tibial pulse • Capillary refill: ≥4.5 s to refill the nail bed • Color of skin: pale |
• Annual comprehensive foot examination by a diabetes specialist • Educate patient to perform daily foot examinations to look for unnoticed injury and determine need for nail care and moisturizer • If vascular screen is positive for any item, refer for a vascular assessment |
| Vision • Ask about - Changes in vision - Retina damage from microvascular disease - Frequency of receiving an eye exam |
• Refer to ophthalmologist for eye exam if the patient is not receiving annual eye exams and prior to initiating a vigorous exercise program |
| Cardiac autonomic neuropathy • Blood pressure response from supine to stand, drops ≥30 mmHg • Heart rate >100 beats/min after resting 15 min |
• Refer to cardiologist prior to initiating a vigorous exercise program if abnormalities are measured6 |
| Kidney | |
| Ask about • Glomerular filtration rate • Frequency of kidney function assessment (urinary albumin and glomerular filtration rate) |
• Refer to nephrologist or primary care physician if the patient is not receiving regular kidney function screening |
| Peripheral Neuropathy of Feet | |
| Light touch (Semmes-Weinstein monofilaments) • Loss of protective sensation: can’t feel 5.07 monofilament • Absent: can’t feel 6.10 monofilament |
• Annual comprehensive foot examination by diabetes specialist • Educate patient to perform daily foot examinations to look for unnoticed injury and determine need for nail care and moisturizer • If the patient lacks protective sensation, provide education about the need for wearing protective footwear when walking in the home or community and the lack of ability to detect damaging temperatures (cold and hot) |
| Tuning fork (128 Hz) on dorsal great toe interphalangeal joint: time between when the patient and examiner stop feeling vibration • Reduced: ≥10-s difference • Absent: the patient is unable to feel vibration | |
| Biothesiometer • Unable to feel ≤25 V | |
| Achilles reflex • Present with reinforcement: Jendrassik maneuver required • Absent: no reflex with Jendrassik maneuver | |
| Muscle (see below) | |
| Integumentary | |
| Callus: indicator of high pressure and risk of injury Dry/cracked: indicator of autonomic neuropathy and increased risk of skin breakdown |
• See recommendations above for peripheral neuropathy |
| Foot wounds • Neuropathic: plantar surface, area of high pressure, callus • Vascular: lateral surface, poorly perfused, absent pulse |
|
| Musculoskeletal | |
| Joint • Deformity of toes (metatarsophalangeal joint hyperextension) and foot (midfoot collapse); palpation of plantar surface for bony prominences that can be a site of high pressure and skin breakdown24,25,48,49 • Range-of-motion limitations18,59,94 - Hands: prayer sign: the patient is unable to fully extend the fingers when placing the hands together in front of the chest in a prayer position - Shoulders: limited active and passive shoulder flexion range of motion - Ankle/foot: limited ankle dorsiflexion and plantar flexion, decreased extensor digitorum longus length • Ask about current or history of prevalent musculoskeletal injuries in diabetes (frozen shoulder, Dupuytren’s contracture, carpal tunnel, trigger finger) |
• Determine whether the patient has any current pain or significant risk factors for musculoskeletal injury • Examine and address contributing factors to toe and foot deformity, including a short extensor digitorum longus and weak foot intrinsic muscles contributing to metatarsophalangeal joint hyperextension and limited ankle dorsiflexion contributing to foot and toe deformity • Develop regular stretching program to address limited joint mobility of the hands, shoulders, and ankles • Provide exercises specific to limitations in daily functional activities (sit-to-stand, stair and curb ascent and descent, walking speed and endurance) • Develop or connect the patient to community programs to increase physical activity that is appropriately dosed to reduce risk for injury and encourage participation |
| Muscle • Visible atrophy of the thenar/hypothenar eminence of the hand • Loss of muscle strength - Calf and foot: decreased ability to complete full heel raise and lack of plantar flexion of the forefoot on the hindfoot during heel raise48,50 - Hands: decreased grip and/or pinch strength | |
| Function • Slow gait speed (10-m walk time, <12.5 s39) • 2-minute walk test14 (see Bohannon et al14 for age- and sex-specific normative data) • Slow 5-times sit-to-stand (≥10 s70) |
|
Abbreviation: PHQ, Patient Health Questionnaire.
A comprehensive list of measures to assess psychosocial factors is provided in a recent American Diabetes Association position statement111
THE PHYSICAL THERAPIST’S ROLE
As part of the multidisciplinary team, physical therapists should be front-line providers in diabetes prevention and management. Physical therapists should be the provider of choice to assist patients who have been diagnosed with diabetes or who are at risk for diabetes in achieving their physical activity goals. Physical therapists’ education provides both broad and in-depth content covering the pathophysiology of diabetes and associated comorbidities, screening for and treatment of diabetes complications, and prescription of physical activity for individuals with specific and important limitations of the human movement system that moderate physical activity tolerance.
Patients may not be regularly referred to physical therapists for guidance on the development of physical activity programs for chronic conditions, such as diabetes.61 Only 2% of referrals to outpatient physical therapy in the United States were for diabetes as the primary health condition to be treated.61 While it is true that the vast majority of patients seen in outpatient settings have diabetes or are at risk of diabetes,61 these patients are often referred for treatment of a specific impairment or limitation, such as pain or limited mobility. Patients may not be referred to a physical therapist for management of diabetes. Physical therapist–led intervention, including guidance on safe physical activity, should be a key component in the treatment of diabetes. The fact that only 2% of referrals for physical therapy are for management of diabetes perhaps reflects the failure of medical providers to recognize diabetes as an underlying mechanism contributing to many of the diagnosed conditions physical therapists commonly examine and treat (eg, stroke, frozen shoulder, carpal tunnel syndrome, myocardial infarction). Physical therapists have an opportunity to expand practice, provide valuable contributions to the management of diabetes, and counter the global burden of disease.
Physical therapists must consider the impact of diabetes and diabetes risk factors in patients who are referred for other health conditions, such as musculoskeletal pain and mobility limitations, that are frequent among persons with diabetes. Diabetes and associated complications affect the type, duration, intensity, and precautions of physical activity that is prescribed. Screening for diabetes and diabetes risk factors provides an opportunity to promote diabetes prevention and/or management through physical activity, and informs treatment of musculoskeletal pain or mobility limitations. Physical therapists should advocate for regular physical activity as a key component of the treatment of chronic diseases in all patient interactions.98,105
Recommendations for Physical Therapists
The standard for excellent physical therapist–led intervention care within our current model of practice must include screening patients for risk factors for diabetes and diabetes-related complications, and educating each patient about his or her specific risks (TABLE 2). Modifiable risk factors such as physical inactivity, obesity, and hypertension can be directly addressed by physical therapists, by assisting the patient in developing and implementing programs to increase physical activity. The physical therapist should work with the patient to identify and address barriers to exercise. The barriers are often related to comorbidities that require specific accommodations or modifications to the exercise program. TABLE 3 provides key assessment items and recommendations that physical therapists might consider when providing patient education about lifestyle management and physical activity.
Lifestyle Management
Lifestyle management may include diabetes self-management education and support, medical nutrition therapy, physical activity, smoking cessation counseling, and psychosocial care. Components of lifestyle management (eg, diet, physical activity, medication, invitation to smoking cessation courses) may reduce disease severity and improve self-assessed quality of life.40 People who receive diabetes self-management education and support (as a one-to-one interaction, in a group setting, or through internet-based interactions) may experience lower HbA1c, improved quality of life, and lower all-cause mortality risk.6,28 Substantial evidence exists of the benefits of physical activity, which we will discuss in the next section.
Although there is clear benefit to preventive strategies, implementation in routine medical care is often lacking. In 2015, only 54% of people with diabetes attended a self-management class, 63% self-monitored their blood glucose, 62% received an annual eye exam, 72% received an annual foot examination from a professional, 55% performed daily foot self-exams, 71% had their HbA1c checked more than 2 times per year, and 66% participated in physical activity in addition to their daily and work activities.21 Physical therapists can guide patients to implement an effective lifestyle management program. While interviewing the patient to gather the pertinent medical history, determine whether the patient with diabetes has a comprehensive lifestyle management program in place. If so, assess the patient’s ability to follow the program and address any perceived barriers to maintaining this program. If the patient does not have a program in place, communicate with the patient’s health care team and assist in the development of a program by providing appropriate referrals and guiding the development and implementation of physical activity participation. For more information on lifestyle management programs, refer to the ADA6 and the Centers for Disease Control and Prevention website (https://www.cdc.gov/diabetes/prevention/lifestyle-program/experience/index.html).
Physical Activity and Exercise Prescription
Physical activity is an effective “medicine” for diabetes and other chronic diseases.12,28,84,104 Given that patients likely have 2 or more comorbidities,13 the benefit of physical activity may go far beyond that of treating diabetes alone. Benefits of physical activity include improved glucose control, insulin sensitivity, maximum rate of oxygen consumption, and blood pressure.84 The improvements may require modifications to the patient’s pharmacologic management plan. Therefore, the physical therapist should facilitate patient follow-up visits with the primary care physician when needed. Important information to communicate with the physician includes glucose monitoring logs, resting and exercise blood pressure and heart rate, and key physical performance measures (eg, 6- or 2-minute walk test, 5-times sit-to-stand). A full review of the effects of physical activity on diabetes and other chronic diseases is available.84
As a component of lifestyle management, physical therapists should assess the patient’s current level of physical activity and screen to determine the safest and most appropriate regimen for the patient. Recommendations by the ADA include 150 minutes per week of moderate- to vigorous-intensity aerobic activity.9,104 The activities should be spread over 3 days per week, with no more than 2 consecutive days without any activity. In addition, 2 to 3 sessions per week each of resistance exercise and flexibility/balance training are recommended.9 It is also important to decrease the time being sedentary47; therefore, encourage physical activity throughout the day (eg, avoid sitting all day).9,47 Customize the physical activity program to the patient’s specific goals, preferred activities, comorbidities, and risk of complications (TABLE 4).
TABLE 4.
Key Recommendations for Physical Activity Prescription for People With Diabetes and Diabetes-Related Complications4,6,9,27,66,84,105
| Patient Characteristic | Recommendation for Physical Activity Prescription |
|---|---|
| Diabetes: general | • Customize program for patient goals, comorbidities, and complication risk • Prescription of physical activity must take into account baseline activity level in order to avoid injury and maximize long-term compliance • Activity monitors are a useful tool to determine baseline activity and to monitor and encourage activity prescription compliance • Physical activity should be progressed gradually, with the goal to achieve 150 min/wk of moderate- to vigorous-intensity aerobic activity6 • Monitor blood pressure and glucose during and after performing new aerobic and/or resistance exercises • Exercise equipment must accommodate the size and weight of the patient • Decrease sedentary time by getting up and out of the chair every 30 min69 |
| Lower extremity musculoskeletal impairments, neuropathy, and/or a history of foot ulcers/fractures | • Weight-bearing exercise is not contraindicated; however, patients may be more at risk for skin breakdown when there is foot deformity or a history of foot wounds74 • Select activities that minimize lower extremity load, including - Pool-based exercise - Stationary cycling - Elliptical - Seated aerobic activities • If patients have an open sore or injury on their foot, non–weight-bearing exercise is preferred6 |
| Autonomic neuropathy or medication (eg, beta blockers, nitrates, calcium-channel blockers, digoxin, diuretics, ACE inhibitors) that blunts heart rate and blood pressure response to physical activity | • Use rate of perceived exertion in addition to heart rate and blood pressure to monitor exercise response |
| Proliferative and severe nonproliferative diabetic retinopathy | • High-intensity aerobic exercise may be contraindicated due to the potential to exacerbate eye damage6 |
| Obesity | • Resistance training has many of the same benefits as aerobic exercise84 and is often better tolerated by those who are obese or untrained |
Abbreviation: ACE, angiotensin-converting enzyme.
Additional information regarding prescription of physical activity can be found in the Standards of Medical Care in Diabetes published by the ADA,9 and in the joint position statement from the ADA and the American College of Sports Medicine titled “Exercise and Type 2 Diabetes.”26 The resources provided by the ADA and American College of Sports Medicine are consistent with the recently updated Physical Activity Guidelines for Americans105; however, they provide additional specificity for patients with diabetes. Additionally, Kluding et al66 recently published specific recommendations for physical training in people with DPN. An online tool developed by the US Department of Health and Human Services may assist patients to record and monitor their physical activity (https://health.gov/moveyourway/).
The physical therapy profession should consider how we expand our professional reach within our current and future health care system. There are many opportunities for partnering and developing relationships with medical professionals within the established health care system, including endocrinologists, diabetes clinicians, and diabetes educators. There are also community partners and outreach opportunities that would benefit from the skills and knowledge of physical therapists (eg, community-based exercise programs for individuals with diabetes). Physical therapists have the expertise required to safely and effectively prescribe physical activity and oversee exercise in medically complex individuals.
SUMMARY
Diabetes and its complications are major causes of morbidity and mortality and contribute significantly to rising health care costs. The dramatic increase in diabetes, paired with high rates of diabetes complications, will contribute to higher health care costs that are only made worse by high rates of modifiable risk factors, such as obesity and physical inactivity. Physical therapists play a central role in the multidisciplinary health care team in at least 3 ways: (1) providing guidance on physical activity participation that is safe and rewarding to the patient, (2) assessing risk factors for diabetes and diabetes-related complications that modify traditional musculoskeletal exercise prescription, and (3) advocating for regular physical activity as a key component of the treatment of chronic diseases in all patient interactions. ◉
KEY POINTS.
FINDINGS
Physical therapists are key members of the health care team in the treatment of diabetes. Physical therapists should screen for diabetes risk factors and diabetes-related complications that modify traditional musculoskeletal exercise prescription. Physical therapists must also advocate for regular physical activity as a key component in the treatment of chronic diseases in all patient interactions.
IMPLICATIONS
This commentary highlights physical therapists’ role as front-line providers, and provides recommendations for physical therapists in screening for diabetes risk factors and diabetes-related complications, and considerations for patient management.
CAUTION
Given the prevalence of diabetes and diabetes risk factors among patients seen in the outpatient physical therapy setting, it is imperative that physical therapists screen patients for diabetes risk factors and diabetes-related complications, and educate each patient about his or her specific risks. Failure of physical therapists to recognize their role as front-line providers in diabetes prevention and management increases the risk of injury from inappropriate exercise prescription and limits the health potential of patients.
Acknowledgments
Research reported in this publication was, in part, supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the US National Institutes of Health under award number R01DK107809. The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
Footnotes
DATA SHARING: There are no data available.
REFERENCES
- 1.Ackermann RT, Cheng YJ, Williamson DF, Gregg EW. Identifying adults at high risk for diabetes and cardiovascular disease using hemoglobin A1c: National Health and Nutrition Examination Survey 2005–2006. Am J Prev Med. 2011;40:11–17. 10.1016/j.amepre.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162–1167. 10.2337/diacare.20.7.1162 [DOI] [PubMed] [Google Scholar]
- 3.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:804–818. 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41:S7–S12. 10.2337/dc18-S001 [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42:S13–S28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 5. Lifestyle management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42:S46–S60. 10.2337/dc19-S005 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Diagnosis. Available at: https://www.diabetes.org/a1c/diag-nosis. Accessed November 13, 2019.
- 8.American Diabetes Association. Introduction. Diabetes Care. 2006;29 suppl 1:S1–S2.16373929 [Google Scholar]
- 9.American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42:S1–S2. 10.2337/dc19-Sint01 [DOI] [PubMed] [Google Scholar]
- 10.Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233:485–491. 10.1111/j.1365-2796.1993.tb01003.x [DOI] [PubMed] [Google Scholar]
- 11.Arroyo C, Hu FB, Ryan LM, et al. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care. 2004;27:129–133. 10.2337/diacare.27.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis. Eur J Epidemiol. 2015;30:529–542. 10.1007/s10654-015-0056-z [DOI] [PubMed] [Google Scholar]
- 13.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 14.Bohannon RW, Wang YC, Gershon RC. Two-minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch Phys Med Rehabil. 2015;96:472–477. 10.1016/j.apmr.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 15.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. 10.2337/diacare.28.4.956 [DOI] [PubMed] [Google Scholar]
- 16.Boyko EJ, Monteiro-Soares M, Wheeler SGB. Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health; 2018:ch 20. [PubMed] [Google Scholar]
- 17.Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:359–361. 10.15585/mmwr.mm6712a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cagliero E, Apruzzese W, Perlmutter GS, Nathan DM. Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am J Med. 2002;112:487–490. 10.1016/s0002-9343(02)01045-8 [DOI] [PubMed] [Google Scholar]
- 19.Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007;21:306–314. 10.1016/j.jdiacomp.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Diabetes, heart disease, and you. Available at: https://www.cdc.gov/features/diabetes-heart-disease/index.html. Accessed May 15, 2019.
- 21.Centers for Disease Control and Prevention. Diabetes Report Card 2017. Available at: https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf. Accessed November 13, 2019.
- 22.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Available at: https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed November 13, 2019.
- 23.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 24.Cheuy VA, Hastings MK, Commean PK, Mueller MJ. Muscle and joint factors associated with forefoot deformity in the diabetic neuropathic foot. Foot Ankle Int. 2016;37:514–521. 10.1177/1071100715621544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheuy VA, Hastings MK, Commean PK, Ward SR, Mueller MJ. Intrinsic foot muscle deterioration is associated with metatarsophalangeal joint angle in people with diabetes and neuropathy. Clin Biomech (Bristol, Avon). 2013;28:1055–1060. 10.1016/j.clinbiomech.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colberg SR, Albright AL, Blissmer BJ, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Med Sci Sports Exerc. 2010;42:2282–2303. 10.1249/MSS.0b013e3181eeb61c [DOI] [PubMed] [Google Scholar]
- 27.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. 10.2337/dc10-1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conn VS, Hafdahl AR, Mehr DR, LeMaster JW, Brown SA, Nielsen PJ. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007;50:913–921. 10.1007/s00125-007-0625-0 [DOI] [PubMed] [Google Scholar]
- 29.Conte SM, Vale PR. Peripheral arterial disease. Heart Lung Circ. 2018;27:427–432. 10.1016/j.hlc.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 30.Dendup T, Feng X, Clingan S, Astell-Burt T. Environmental risk factors for developing type 2 diabetes mellitus: a systematic review. Int J Environ Res Public Health. 2018;15:78 10.3390/ijerph15010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. 10.2522/ptj.20080020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 33.Diez Roux AV, Jacobs DR, Kiefe CI. Neighborhood characteristics and components of the insulin resistance syndrome in young adults: the Coronary Artery Risk Developoment in Young Adults (CARDIA) study. Diabetes Care. 2002;25:1976–1982. 10.2337/diacare.25.11.1976 [DOI] [PubMed] [Google Scholar]
- 34.Eaton S, Tesfaye S. Clinical manifestations and measurement of somatic neuropathy. Diabetes Rev. 1999;7:312–325. [Google Scholar]
- 35.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care. 1996;19:1097–1102. 10.2337/diacare.19.10.1097 [DOI] [PubMed] [Google Scholar]
- 36.Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am. 2005;89:949–975. 10.1016/j.mcna.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 37.Eivazi M, Abadi L. Low back pain in diabetes mellitus and importance of preventive approach. Health Promot Perspect. 2012;2:80–88. 10.5681/hpp.2012.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldt-Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet. 1986;328:1300–1304. 10.1016/s0140-6736(86)91433-9 [DOI] [PubMed] [Google Scholar]
- 39.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–49. [PubMed] [Google Scholar]
- 40.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. 10.1056/NEJMoa021778 [DOI] [PubMed] [Google Scholar]
- 41.Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes In: National Diabetes Data Group, ed. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:233–258. [Google Scholar]
- 42.Grandinetti A, Kaholokula JK, Chang HK. Delineating the relationship between stress, depressive symptoms, and glucose intolerance [letter]. Diabetes Care. 2000;23:1443–1444. 10.2337/diacare.23.9.1443 [DOI] [PubMed] [Google Scholar]
- 43.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. 10.1056/NEJMoa1310799 [DOI] [PubMed] [Google Scholar]
- 44.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the U.S. adult population ≥40 years of age with and without diabetes: 1999–2000 National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:1591–1597. 10.2337/diacare.27.7.1591 [DOI] [PubMed] [Google Scholar]
- 45.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–2455. 10.1001/jama.2011.812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 47.Hamilton MT, Hamilton DG, Zderic TW. Sedentary behavior as a mediator of type 2 diabetes. Med Sport Sci. 2014;60:11–26. 10.1159/000357332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hastings MK, Mueller MJ, Woodburn J, et al. Acquired midfoot deformity and function in individuals with diabetes and peripheral neuropathy. Clin Biomech (Bristol, Avon). 2016;32:261–267. 10.1016/j.clinbiomech.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hastings MK, Sinacore DR, Woodburn J, et al. Kinetics and kinematics after the Bridle procedure for treatment of traumatic foot drop. Clin Biomech (Bristol, Avon). 2013;28:555–561. 10.1016/j.clinbiomech.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hastings MK, Woodburn J, Mueller MJ, Strube MJ, Johnson JE, Sinacore DR. Kinematics and kinetics of single-limb heel rise in diabetes related medial column foot deformity. Clin Biomech (Bristol, Avon). 2014;29:1016–1022. 10.1016/j.clinbiomech.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holewski JJ, Moss KM, Stess RM, Graf PM, Grunfeld C. Prevalence of foot pathology and lower extremity complications in a diabetic outpatient clinic. J Rehabil Res Dev. 1989;26:35–44. [PubMed] [Google Scholar]
- 52.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. 10.1056/NEJMoa010492 [DOI] [PubMed] [Google Scholar]
- 53.InterAct Consortium. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54:2272–2282. 10.1007/s00125-011-2182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jazini E, Sharan AD, Morse LJ, et al. Alterations in T2 relaxation magnetic resonance imaging of the ovine intervertebral disc due to nonenzymatic glycation. Spine (Phila Pa 1976). 2012;37:E209–E215. 10.1097/BRS.0b013e31822ce81f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. 10.2337/dc06-1842 [DOI] [PubMed] [Google Scholar]
- 56.Jermendy G, Ruggenenti P. Preventing microalbuminuria in patients with type 2 diabetes. Diabetes Metab Res Rev. 2007;23:100–110. 10.1002/dmrr.693 [DOI] [PubMed] [Google Scholar]
- 57.Jiménez-Báez MV, Márquez-González H, Bárcenas-Contreras R, Morales Montoya C, Espinosa-García LF. Early diagnosis of diabetic retinopathy in primary care. Colomb Méd (Cali). 2015;46:14–18. [PMC free article] [PubMed] [Google Scholar]
- 58.Kaye SA, Folsom AR, Sprafka JM, Prineas RJ, Wallace RB. Increased incidence of diabetes mellitus in relation to abdominal adiposity in older women. J Clin Epidemiol. 1991;44:329–334. 10.1016/0895-4356(91)90044-a [DOI] [PubMed] [Google Scholar]
- 59.Kim RP, Edelman SV, Kim DD. Musculoskeletal complications of diabetes mellitus. Clin Diabetes. 2001;19:132–135. 10.2337/diaclin.19.3.132 [DOI] [Google Scholar]
- 60.King KD, Jones JD, Warthen J. Microvascular and macrovascular complications of diabetes mellitus. Am J Pharm Educ. 2005;69:art 87. 10.5688/aj690587 [DOI] [Google Scholar]
- 61.Kirkness CS, Marcus RL, LaStayo PC, Asche CV, Fritz JM. Diabetes and associated risk factors in patients referred for physical therapy in a national primary care electronic medical record database. Phys Ther. 2008;88:1408–1416. 10.2522/ptj.20080129 [DOI] [PubMed] [Google Scholar]
- 62.Klein R, Klein BEK. Epidemiology of ocular functions and diseases in persons with diabetes In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health; 2018:ch 21. [PubMed] [Google Scholar]
- 63.Klein R, Klein BEK, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217–1228. 10.1001/archopht.1994.01090210105023 [DOI] [PubMed] [Google Scholar]
- 64.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–1868. 10.1016/j.ophtha.2008.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. 10.1016/j.ophtha.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kluding PM, Bareiss SK, Hastings M, Marcus RL, Sinacore DR, Mueller MJ. Physical training and activity in people with diabetic peripheral neuropathy: paradigm shift. Phys Ther. 2017;97:31–43. 10.2522/ptj.20160124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2014:CD000990 10.1002/14651858.CD000990.pub3 [DOI] [PubMed] [Google Scholar]
- 68.Ley SH, Schulze MB, Hivert MF, Meigs JB, Hu FB. Risk factors for type 2 diabetes In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health; 2018:ch 13. [Google Scholar]
- 69.Liang S, Zhang XG, Cai GY, et al. Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: a meta-analysis. PLoS One. 2013;8:e64184 10.1371/journal.pone.0064184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Makizako H, Shimada H, Doi T, et al. Predictive cutoff values of the five-times sit-to-stand test and the timed “up & go” test for disability incidence in older people dwelling in the community. Phys Ther. 2017;97:417–424. [DOI] [PubMed] [Google Scholar]
- 71.Maluf KS, Mueller MJ. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech (Bristol, Avon). 2003;18:567–575. 10.1016/s0268-0033(03)00118-9 [DOI] [PubMed] [Google Scholar]
- 72.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. 10.2337/dc08-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monnier VM, Glomb M, Elgawish A, Sell DR. The mechanism of collagen cross-linking in diabetes: a puzzle nearing resolution. Diabetes. 1996;45 suppl 3:S67–S72. 10.2337/diab.45.3.s67 [DOI] [PubMed] [Google Scholar]
- 74.Mueller MJ, Tuttle LJ, LeMaster JW, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94:829–838. 10.1016/j.apmr.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mueller T, Hinterreiter F, Luft C, Poelz W, Haltmayer M, Dieplinger B. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg. 2014;59:1291–1299. 10.1016/j.jvs.2013.11.063 [DOI] [PubMed] [Google Scholar]
- 76.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. 10.2337/dc06-1136 [DOI] [PubMed] [Google Scholar]
- 77.Narres M, Kvitkina T, Claessen H, et al. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic review. PLoS One. 2017;12:e0182081 10.1371/journal.pone.0182081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64. Washington, DC: US Department of Health and Human Services; 2015. [PubMed] [Google Scholar]
- 79.Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: the effects of race and region. Ann Vasc Surg. 2016;30:292–298.e1. 10.1016/j.avsg.2015.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. 10.1016/S0140-6736(11)60614-4 [DOI] [PubMed] [Google Scholar]
- 81.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45 suppl:S5–S67. 10.1016/j.jvs.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 82.Pantalone KM, Hobbs TM, Wells BJ, et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3:e000093 10.1136/bmjdrc-2015-000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–521. 10.2337/diacare.13.5.513 [DOI] [PubMed] [Google Scholar]
- 84.Pedersen BK, Saltin B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25 suppl 3:1–72. 10.1111/sms.12581 [DOI] [PubMed] [Google Scholar]
- 85.Perkins BA, Greene DA, Bril V. Glycemic control is related to the morphological severity of diabetic sensorimotor polyneuropathy. Diabetes Care. 2001;24:748–752. 10.2337/diacare.24.4.748 [DOI] [PubMed] [Google Scholar]
- 86.Pirart J [Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (author’s transl)]. Diabete Metab. 1977;3:97–107. [PubMed] [Google Scholar]
- 87.Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes In: National Diabetes Data Group, ed. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:409–428. [Google Scholar]
- 88.Rewers M, Hamman RF. Risk factors for non-insulin-dependent diabetes In: National Diabetes Data Group, ed. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:179–220. [Google Scholar]
- 89.Roett MA, Liegl S, Jabbarpour Y. Diabetic nephropathy—the family physician’s role. Am Fam Physician. 2012;85:883–889. [PubMed] [Google Scholar]
- 90.Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29:212–217. 10.1016/j.jdiacomp.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 91.Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014;160:1–10. 10.7326/M13-1725 [DOI] [PubMed] [Google Scholar]
- 92.Schootman M, Andresen EM, Wolinsky FD, et al. The effect of adverse housing and neighborhood conditions on the development of diabetes mellitus among middle-aged African Americans. Am J Epidemiol. 2007;166:379–387. 10.1093/aje/kwm190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schulz LO, Bennett PH, Ravussin E, et al. Effects of traditional and Western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care. 2006;29:1866–1871. 10.2337/dc06-0138 [DOI] [PubMed] [Google Scholar]
- 94.Shah KM, Clark BR, McGill JB, Mueller MJ. Upper extremity impairments, pain and disability in patients with diabetes mellitus. Physiotherapy. 2015;101:147–154. 10.1016/j.physio.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw JE, Zimmet PZ. The epidemiology of diabetic neuropathy. Diabetes Rev. 1999;7:245–252. [Google Scholar]
- 96.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. 10.1001/jama.293.2.217 [DOI] [PubMed] [Google Scholar]
- 97.Slyke MP. Painful peripheral diabetic neuropathy: therapeutic approaches. Consult Pharm. 2000;15:544–555. [Google Scholar]
- 98.Stamatakis E, Straker L, Hamer M, Gebel K. The 2018 Physical Activity Guidelines for Americans: what’s new? Implications for clinicians and the public. J Orthop Sports Phys Ther. 2019;49:487–490. 10.2519/jospt.2019.0609 [DOI] [PubMed] [Google Scholar]
- 99.Stamler J, Vaccaro O, Neaton JD, Wentworth D, Multiple Risk Factor Intervention Trial Research Group. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. 10.2337/diacare.16.2.434 [DOI] [PubMed] [Google Scholar]
- 100.Strodl E, Kenardy J. Psychosocial and non-psychosocial risk factors for the new diagnosis of diabetes in elderly women. Diabetes Res Clin Pract. 2006;74:57–65. 10.1016/j.diabres.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 101.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. 10.1056/NEJMoa032782 [DOI] [PubMed] [Google Scholar]
- 102.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28 suppl 1:8–14. 10.1002/dmrr.2239 [DOI] [PubMed] [Google Scholar]
- 103.Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg. 2016;55:591–599. 10.1053/j.jfas.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 104.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. 10.1001/jama.2011.576 [DOI] [PubMed] [Google Scholar]
- 105.US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. [Google Scholar]
- 106.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–973. 10.1007/s001250051477 [DOI] [PubMed] [Google Scholar]
- 107.Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341:1306–1309. 10.1016/0140-6736(93)90816-y [DOI] [PubMed] [Google Scholar]
- 108.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12:439–445. 10.1046/j.1525-1497.1997.00076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. 10.1001/jama.298.22.2654 [DOI] [PubMed] [Google Scholar]
- 110.World Health Organization. Global Report on Diabetes. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 111.Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2126–2140. 10.2337/dc16-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3–22. 10.1016/B978-0-444-53480-4.00001-1 [DOI] [PubMed] [Google Scholar]
- 113.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. 10.1038/414782a [DOI] [PubMed] [Google Scholar]