Abstract
Non-restorative sleep is a key diagnostic feature of the musculoskeletal pain disorder fibromyalgia, and is robustly associated with poor physical functioning, including activity interference. However, the mechanisms through which non-restorative sleep elicits activity interference among individuals with fibromyalgia at the within-person level remain unclear. The present study tested the following three-path mediation model, using data gathered from a 21-day electronic daily diary in 220 individuals with fibromyalgia: previous night non-restorative sleep → morning pain catastrophizing → afternoon pain severity → end-of-day activity interference. Results of multilevel structural equation modeling supported the three-path mediation model. Previous night’s non-restorative sleep and morning pain catastrophizing were also directly related to end-of-day activity interference. Previous night non-restorative sleep did not significantly predict afternoon pain severity while controlling for the effect of morning pain catastrophizing. Greater non-restorative sleep during the previous night and a higher level of morning pain catastrophizing appear to serve as risk factors for experiencing greater daily pain and activity interference later in the day. These findings point to the potential utility of targeted interventions that improve both sleep quality and pain catastrophizing to help individuals with chronic pain engage in important daily activities despite experiencing pain.
Keywords: Chronic pain, fibromyalgia, activity interference, physical functioning, pain catastrophizing, sleep, mediation, multilevel modeling, daily diary
Introduction
Fibromyalgia (FM) is a chronic widespread musculoskeletal pain condition that predominantly affects women and interferes with individuals’ daily functioning58,80. Repeated activity interference can substantially limit one’s successful adjustment to chronic pain through the development of negative self-schema and reduction of rewards that stem from engaging in meaningful and enjoyable activities23,26. Hence, a central target of chronic pain management is helping individuals increase their engagement in and performance of important daily activities14.
One of the key diagnostic and clinical features of FM that is modifiable and has implications in modulating individuals’ daily functioning is non-restorative sleep, a subjective perception of poor sleep quality and being unrefreshed upon awakening63,76. More than 90% of individuals with FM report experiencing non-restorative sleep5,70, and ample evidence indicates that poor sleep quality is robustly associated with activity interference and decreased daily goal pursuit2,5,32,41. However, the intra-day processes that link non-restorative sleep with activity interference among individuals with FM are not well understood. Identifying the underlying mechanisms can inform our efforts to develop targeted intervention strategies to diminish the negative impact of non-restorative sleep on daily functioning of individuals with FM.
Both experimental and observational studies have repeatedly demonstrated that sleep can modulate individuals’ pain sensations and perception20,34,57. Greater pain experience, in turn, is considered one of the strongest precursors of activity interference33,54. Combined, these findings suggest that non-restorative sleep may promote heightened pain-related activity interference. However, the factors mediating the relation between non-restorative sleep and heightened pain experience have been largely unexplored, especially beyond the laboratory. Pain catastrophizing, a salient maladaptive pain coping style characterized by rumination, helplessness, and magnification65, is a promising candidate as a mediator of this relation. Previous studies have found that individuals with FM who are poor sleepers report high levels of pain catastrophizing37, and that poorer sleep quality predicts higher pain catastrophizing24,77. This may be because poor sleep quality induces biased attentional and interpretative processes that can perpetuate and/or exacerbate pain sensitivity25,51,52. Hence, when an individual experiences non-restorative sleep, he or she may be more likely to manifest higher pain catastrophizing. Pain catastrophizing, in turn, is a potent predictor of various pain-related experiences, including pain severity and activity interference50. In fact, using a cross-lagged model, Campbell and her colleagues found that pain catastrophizing preceded and contributed to subsequent changes in the pain experience of patients with FM8. When individuals experience higher than usual non-restorative sleep, they may be more likely to catastrophize, which in turn, may precipitate increased pain.
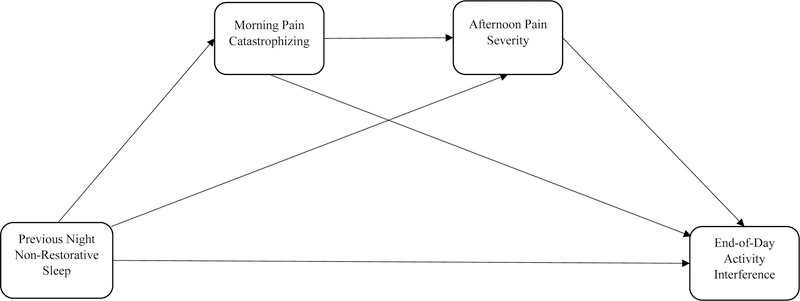
In the present study, we proposed an intra-day process model that links the relation between non-restorative sleep and activity interference. Although the associations between components (i.e., non-restorative sleep, pain catastrophizing, pain severity, and activity interference) have been examined previously, a model that integrates all these factors simultaneously at the within-person level has not been explored. Investigating this intra-day process model can yield a better understanding of “how” previous night non-restorative sleep is associated with activity interference during the day, providing additional insight for researchers and clinicians who aim to help individuals with FM improve their daily physical function. Specifically, in the present study we tested a model in which morning pain catastrophizing and afternoon pain severity are sequential mediators of the prospective association between morning reports of the previous night’s non-restorative sleep and end-of-day activity interference. We hypothesized that on days when individuals report greater than usual non-restorative sleep for the prior night, they experience more activity interference at the end of the day through higher morning pain catastrophizing and afternoon pain severity. This hypothesized intra-day process model is tested by means of a three-path mediation model67, which is illustrated in Figure 1. Lastly, we conducted sensitivity analyses to evaluate if this model is robust from individual differences including overall sleep quality, depression status, and pain management medication (i.e., opioids and tricyclics) use that may modulate individuals’ daily experience of sleep, pain, catastrophizing, and physical function.
Figure 1.
Visual illustration of hypothesized multilevel three-path mediated model.
Method
This is a secondary data analysis of a parent study which collected daily diary data at both pre-and post-intervention trials. The focus of the parent study was to compare the benefits of cognitive, acceptance-based, and educational approaches for functional and affective health in individuals with fibromyalgia. The main intervention outcome paper is currently in preparation. There have been a number of studies that were published based upon the pre-intervention diary data69,78,79,83. However, none of these papers overlap with the aims of the present study.
Participants
Participants were recruited from the Phoenix, Arizona metropolitan area as part of a larger randomized clinical trial for fibromyalgia (FM) between 2008 and 2013. Recruitment method included physician referrals, newspaper advertisements, online positing, fliers posted in local physicians’ offices, and FM support groups. Inclusion criteria were: 1) ages between 18 and 72 years; 2) speaks English; 3) have had pain lasting three or more months in at least four quadrants of the body, or in two quadrants of the body with substantial sleep disturbance and fatigue; and 4) report pain in at least 11 of 18 tender points during a physical examination at a home visit, consistent with diagnostic criteria for fibromyalgia established by the American College of Rheumatology81. Exclusion criteria were: 1) pursuing litigation related to pain; 2) participating in a psychosocial treatment for pain or mood disturbance; and 3) having autoimmune or neuropathic pain conditions. Participants were not allowed to engage in psychological treatment for mood and/or pain symptoms throughout the duration of the study. However, they were not refrained from using some other complementary and alternative medicines (e.g., acupuncture) or physical therapy during the study. Two hundred twenty participants who consented to participate, met the inclusion and exclusion criteria, and participated in pre-intervention daily diary assessment were used in the present study.
Procedure
Participant screening and survey data assessment.
Individuals who expressed interest in the study were first screened by phone to determine their eligibility. Those who screened to be eligible then received a tender point examination performed by a trained nurse during a home visit. For this exam, a dolorimeter was used to administer 4 kilograms of pressure to 18 tender point and 3 control point sites on the body. Once enrolled into the study, those participants who signed the informed consent form received an initial packet of questionnaires that measured their pain-related experiences and physical and psychosocial health. Trained research staff members also conducted phone interviews to assess participants’ history of depression, trauma, and other significant life events. All the study procedures were approved by the Institutional Review Board at Arizona State University prior to initiating the study.
Daily diary assessment.
Prior to starting the pre-intervention diary assessment, a research staff member met with participants individually to provide them with a cell phone and instructions on how to complete the diary through the phone. During the 21-day diary, an automated phone system (i.e., Interactive Voice Response system) called each participant on their phone four times a day and delivered audio recorded questions. Participants were asked to indicate their responses to questions using the phone keypad. The four assessment time points per day were: 1) in the early-morning, 20 minutes after a wake up time specified by the participant (the time that participant set for the early-morning call was consistent across the 21 days and the call assessed only sleep quality); 2) in the late-morning at 11:00 am (subsequently referred to as “morning”); 3) in the afternoon at 4:00 pm; and 4) at the end of the day shortly before going to bed. If a participant missed a call, he or she was instructed to call into the system within three hours to complete the assessment. Participants were not allowed to call into the system to complete the early-morning sleep survey after 10:30 AM. Participants’ calls were regularly monitored by research staff members and if a participant missed all diary assessments two days in a row, a research staff contacted the individual to address any barriers to completing diary. Participants were paid $2/day for completing diaries with a bonus of $1/day for a 50% or greater diary completion rate.
Measures
Day-level variables
Previous Night’s Non-Restorative Sleep:
Each morning, 20 minutes after participants’ usual wake up time, they were asked to rate the quality of their sleep on the previous night on a 101-point scale (0 = “extremely poor sleep” to 100 = “extremely good sleep”), and how refreshed they felt upon awakening on a 101-point scale (0 = “not at all refreshed” to 100 = “extremely refreshed”). These sleep diary items are analogous to those in the PROMIS Sleep Disturbance scale6. They were reverse-coded so as to indicate that higher values represent greater non-restorative sleep. The within-person correlation between the two items was .60, and we computed the mean of these two items.
Morning Pain Catastrophizing:
Participants were asked to rate the degree to which they experienced pain catastrophizing (i.e., “you felt your pain was so bad you couldn’t stand it anymore”) in the past two to three hours on a five-point numerical scale ranging from 1 (not at all) to 5 (completely). The item was adapted from the Pain Catastrophizing Scale66.
Afternoon Pain Severity:
Participants were asked to rate their average level of pain in the past two to three hours using the standard Numerical Rating Scale27 that ranges from 0 (no pain) to 100 (pain as bad as it can be). Note that the pain severity rating was also measured in the morning and was used as a covariate in our model.
End-of-Day Activity Interference:
End-of-day activity interference was measured by four items drawn from SF-36 Role Physical subscale75. Participants were asked to rate the following items using a three-point numerical scale ranging from 1 (no) to 3 (yes, very much): (1) “Did you cut down on the amount of time spent on work or other activities?”, (2) “Did you accomplish less than you would have liked?”, (3) “Were you limited in the kind of work or other activities you did?”, and (4) “Did you have difficulty performing work or other activities?” We computed means of these four items. The within-person reliability of these items was 0.74.
Person-level variables for sensitivity analyses
Poor Sleepers vs. Non-Poor Sleepers.
By averaging daily non-restorative sleep assessments across 21-days, we categorized participants into poor sleepers vs. non-poor sleepers. Those we scored more than 1.5 standard deviation above the mean of average non-restorative sleep (i.e., > 65.11 out of 100) classified as poor sleepers (n = 37), and the remaining 177 participants were categorized as non-poor sleepers.
Individuals with Clinical Level Depression vs. Non-Clinical Level Depression.
The Hamilton Depression Inventory-Short Form (HDI-SF; Reynolds & Kobak, 1995) was used to examine participants’ clinical level depression status. There are 15 items in HDI-SF and scores can range between 0 and 52; scores greater than 13 are thought to signify clinical level of depression. Based upon this clinical cutoff, we classified participants into either clinical level depression group (n = 66) or non-depressed group (n = 124). HDI-SF is known for its strong reliability and validity53. Cronbach’s alpha for the HDI-SF was .88.
Tricyclics and Opioid Medication Use.
Participants were asked to list the tricyclics and opioid medications they were currently using. A total 21 participants reported using tricyclics and 109 reported using opioids.
Data Analytic Plan
Prior to conducting the main analyses, diary completion rates, descriptive statistics including means, standard deviations, and intra-class correlations (ICCs) were calculated. As our data structure was hierarchical (i.e., repeated daily assessments nested in each participant), a multilevel structural equation model (MSEM) was employed to test the hypothesized model. MSEM allows for testing complex multilevel models such as the three-path mediation model evaluated in the current study43,49. As it is possible that the effect of morning pain catastrophizing on afternoon pain severity is confounded by the participants’ experience of morning pain severity, morning pain severity (i.e., auto-correlation) was included as a control variable in the final model.
MSEM partitions the within-and between-person level variances, and therefore, path coefficients are directly interpreted at the corresponding levels of analysis49. In the present study, we report the results of only the within-person level model because (1) the research questions and hypotheses of the present study are all at the within-person level, and (2) the associations between study variables in model at the between-person level are all cross-sectional, i.e., they represent average of 21-day diary assessments.
In terms of centering, all within-person predictors in MSEM were person-mean centered, which is the conventional method employed in multilevel modeling19. The person-centered scores represent day-to-day deviations from an individual’s own mean score over the entire 21-day diary period for that variable. Hence, person-centered predictors can address research questions such as, “On days when individuals experience greater than their usual level of non-restorative sleep, do they experience higher levels of morning pain catastrophizing?”
All analyses were conducted by Mplus version 8.045. To test the three-path mediated effect, we used the joint significance test. According to Taylor et al’s simulation study67, the joint significance test provides the best control of Type I error rates and adequate statistical power. We were not able to use the percentile bootstrap method that provides 95% confidence limits of a three-path mediated effect because this function is not yet available in the MSEM framework. Use of the joint significance test for examining the three-path mediated effect requires three paths to be statistically significant at an alpha level of 0.05. These paths are: (1) the path from the independent variable to the first mediator (i.e., previous night non-restorative sleep → morning pain catastrophizing), (2) the path from the first mediator to the second mediator (i.e., morning pain catastrophizing → afternoon pain severity), and (3) the path from the second mediator to the dependent variable (i.e., afternoon pain severity → end-of-day activity interference). If all three paths are significant at p-value less than 0.05, then a significant three-path mediated effect can be inferred.
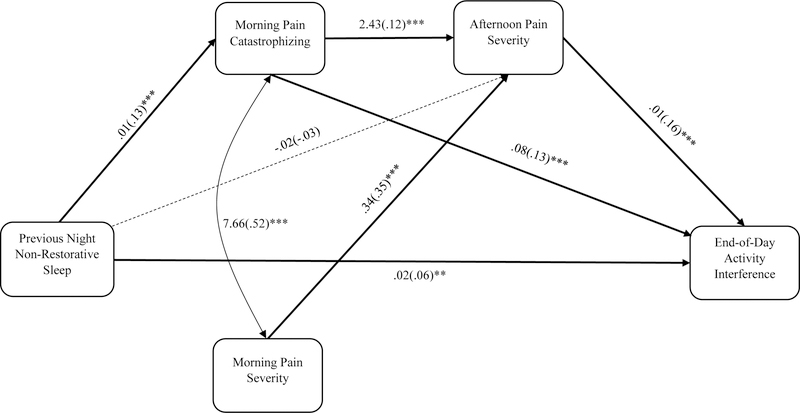
Model fit was evaluated by using comparative fit index (CFI), root mean square of approximation (RMSEA), and the standardized root mean squared residual (SRMR). Values greater than 0.90 for CFI, less than 0.08 for RMSEA, and below 0.10 for SRMR indicate an acceptable model fit7,31. We used the full information maximum likelihood (FIML) under the missing at random assumption to handle missing data38. FIML is an advanced and one of the most powerful methods to handle missing data. Instead of imputing missing values, FIML estimates a maximum likelihood function for each individual case using all the observed data and provide the most plausible model parameters17. FIML is superior to other conventional missing data strategies including listwise deletion, pairwise deletion, and mean imputation, and it provide unbiased regression estimates under MAR assumption18. In calculating effect sizes, we used R2 (variance explained by predictors). Unfortunately, Mplus statistical software cannot calculate how each of the predictors (i.e., exogenous variables) contribute to the R-square of the dependent (i.e., endogenous) variables. As an alternative, we provided standardized regression path coefficients for each of the paths in Figure 2.
Figure 2.
Morning pain catastrophizing and afternoon pain severity as sequential mediators of the relationship between previous night non-restorative sleep and end-of-day activity interference. Note. Both unstandardized and standardized regression coefficients are provided. Values in brackets are standardized path coefficients. *p < .05, **p < .01, ***p < .001
For sensitivity analyses, we used multiple group analyses. Multiple group analysis44 allows for examining whether regression paths in the within-person model significantly differ by subgroups of individuals with fibromyalgia who exhibit different personal characteristics. There are two major steps in conducting multiple group analysis. First, two models are compared using a chi-square difference test: (1) a configurable model that allows all the within-person level regression paths to be freely estimated across groups, and (2) a constrained model that restricts all paths to be equal across groups. Basically, this analytic approach is similar to the omnibus F-test in ANOVA, which tests the overall difference between groups (factors). Second, if there is a significant chi-square difference between the configurable vs. constrained model, then each regression path is examined to see if there are any significant differences across groups. However, if there is no statistically significant chi-square difference between the two models, we did not proceed to this second step (similar to the conventional approach to ANOVA) as it can significantly elevate Type I errors.
Results
Diary Completion Rates
Overall, participants completed 3,796 of 4,620 (82%) diary assessments possible across 21 days. More specifically, participants completed on average 17.5 days (SD = 4.24) of early-morning assessments of previous night non-restorative sleep, 17.8 days (SD = 4.55) of morning pain catastrophizing, 16.5 days (SD = 5.24) of afternoon pain severity, 17.1 days (SD = 4.87) of end-of-day activity interference.
Sample Characteristics
Table 1 summarizes demographic characteristics of our sample. Participants were predominantly European White, middle-aged women. Approximately 64% of the participants were either married or partnered. About half (53.1%) of the participants were employed and currently working at least part-time. Participants’ median education level was 1 to 3 years of college and their median annual household income level was in the range of $40,000 to $59,999.
Table 1.
Sample characteristics (N = 220)
| Variables | Mean or % (SD) |
|---|---|
| Age (years) | 51.25 (11.02) |
| Gender | |
| Male | 11.2 |
| Female | 87.0 |
| Education | |
| Less than high school | 2.2 |
| Completed high school | 13.0 |
| Post high school | 13.4 |
| 1–3 years of college | 33.2 |
| 4 years of college | 17.5 |
| Post graduate | 17.0 |
| Marital Status | |
| Never married | 8.1 |
| Married/partnered | 55.3 |
| Widowed | 5.8 |
| Divorced | 27.4 |
| Separated | 1.4 |
| Employment | |
| Employed | 53.1 |
| Not working | 46.9 |
| Race/Ethnicity | |
| Caucasian | 78.0 |
| Black/African American | 2.7 |
| Asian | 1.3 |
| Hispanic | 14.3 |
| Native American | 4.0 |
| Native Hawaiian/Pacific Islander | 0.9 |
| Other | 3.6 |
| Income | |
| Under $3,000-$20,999 | 25.6 |
| $21,000-$39,999 | 22.0 |
| $40,000-$59,999 | 17.9 |
| $60,000-$99,999 | 19.7 |
| $100,000 and over | 8.1 |
Preliminary Findings
Table 2 displays descriptive statistics and ICCs. Participants reported moderate levels of previous night non-restorative sleep, morning pain catastrophizing, morning pain severity, and end-of-day activity interference. ICCs ranged from .30 (previous night non-restorative sleep) to .51 (afternoon pain severity), indicating use of multi-level modeling is appropriate given that there was substantial within-person variation. For instance, only 30% of the variation was explained by between-person differences in previous night’s non-restorative sleep and the rest of the variation was explained by within-person changes. All study variables were significantly correlated in expected directions. For instance, greater previous night non-restorative sleep was associated with higher morning pain catastrophizing, afternoon pain severity, and end-of-day activity interference.
Table 2.
Descriptive statistics and bi-variate within-person correlations of study variables
| Variables | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Previous night non-restorative sleep | – | .18** | .06* | .17** |
| 2. Morning pain catastrophizing | – | .48** | .28** | |
| 3. Afternoon pain severity | – | .28** | ||
| 4. End-of-day activity interference | – | |||
| Mean | 51.37 | 2.12 | 50.22 | 2.00 |
| SD | 23.50 | 1.16 | 24.27 | .63 |
| Observed Range | 0–100 | 1–5 | 0–100 | 1–3 |
| ICC | .30 | .45 | .51 | .37 |
Note.
p < .01,
p < .001
Test of the three-path mediation model
Figure 2 presents a summary of the MSEM findings, including the unstandardized and standardized path estimates. Model fit indices showed good fit to the present data, CFI = .98, RMSEA = .05, SRMR-within = .03, and SRMR-between = .05.
On days with greater than average previous night non-restorative sleep, participants reported higher morning pain catastrophizing (Unstandardized B = .11, SE = .01, p < .001). However, previous night non-restorative sleep did not significantly predict afternoon pain severity (B = .47, SE = .30, p = .12), controlling for morning pain catastrophizing. Higher morning pain catastrophizing was associated with higher afternoon pain severity (B = 2.43, SE = .48, p < .001), controlling for morning pain severity and previous night non-restorative sleep. Morning pain catastrophizing also predicted end-of-day activity interference (B = .08, SE = .01, p < .001), over and above afternoon pain severity and previous night’s non-restorative sleep. On days when individuals reported higher afternoon pain severity, they reported greater evening activity interference (B = .01, SE = .00, p < .001), beyond morning pain catastrophizing and previous night non-restorative sleep. Interestingly, previous night’s non-restorative sleep significantly predicted end-of-day activity interference (B = .03, SE = .01, p < .01) even when controlling for morning pain catastrophizing and afternoon pain severity. The joint significance test revealed a significant three-path mediation as predicted: Previous night non-restorative sleep → Morning pain catastrophizing → Afternoon pain severity → End-of-day activity interference.
With regard to effect sizes, previous night non-restorative sleep explained 1.6% variance of morning pain catastrophizing at the within-person level. In terms of afternoon pain severity, a total of 18% of the variance was explained by morning pain severity, morning pain catastrophizing, and previous night non-restorative sleep at the within-person level. Finally, for evening activity interference, 6.1% of the variance was explained by morning pain catastrophizing, afternoon pain severity, and previous night non-restorative sleep.
Findings of sensitivity analyses
Four multiple group analyses were conducted to test potential moderation effects of subgroups [poor sleepers vs. good sleepers; clinically depressed vs. non-depressed groups; individuals using tricyclics vs. non-users; individuals using opioids vs. non-users] on regression paths at the within-person level. Findings of chi-square difference tests showed that the difference of model fit between the freely estimated (configurable model) and the constrained models across these moderators were not statistically significant, [χ2(7) = ranged from .05 to .25, p = 1.00]. These results indicate that overall there were no subgroup differences in the intra-day process model. Thus, we did not proceed to explore whether each of the regression paths differed by these subgroups.
Discussion
In the present study we collected ecologically sensitive daily diary data several times a day from individuals with FM to investigate whether the prospective relation between previous night’s non-restorative sleep and end-of-day activity interference is sequentially mediated through morning pain catastrophizing and afternoon pain severity. As we hypothesized, a statistically significant three-path mediated effect was observed.
More than usual previous night non-restorative sleep was associated with higher morning pain catastrophizing. Previous literature suggests that poor sleep quality can enhance individuals’ threat misinterpretation51,52,56. Thus, when individuals’ sleep is disturbed during the previous night, they may be more likely to interpret pain experience as a salient threat, and therefore, experience increased maladaptive catastrophic thoughts about pain. We also observed a direct effect from previous night’s non-restorative sleep on activity interference at the end-of-day assessment. This robust effect of non-restorative sleep is consistent with a recent meta-analysis suggesting that sleep disturbance is strongly associated with poor self-reported physical functioning3. Regularly assessing daily changes in non-restorative sleep could provide important clinical information on how individuals interpret and react to pain and function on a day-to-day level.
Morning pain catastrophizing predicted afternoon pain severity even after controlling for auto-correlation (i.e., afternoon pain severity regressed on morning pain severity). This finding replicates and further extends previous experimental studies that revealed changes in state pain catastrophizing can precede pain experience and shape individuals’ subsequent pain experience8,9. A daily diary study of individuals with rheumatoid arthritis also reported that more than usual level of pain catastrophizing from the previous day significantly predicted next-day pain severity, while controlling for the previous day’s pain severity64. Importantly, higher morning pain catastrophizing was also associated with greater end-of-day activity interference over and above the effect of afternoon pain severity and previous night’s non-restorative sleep. Copious findings at the between-person level indicate that pain catastrophizing is one of the most important psychosocial factors that modulate individuals’ experience of both pain and pain-related disability15,65. Furthermore, the Fear-Avoidance Model of Chronic Pain—one of the most widely known theoretical frameworks that explains the development and exacerbation of chronic pain— posits pain catastrophizing as a core mechanism underlying pain-induced disability11,35,73. Our within-person intra-day process findings further support previous claims made based upon between-person level findings that helping individuals effectively de-catastrophize momentarily can be an important pain management target28,71,72.
Afternoon pain severity, controlling for morning pain catastrophizing and previous night’s non-restorative sleep predicted activity interference later in the day. Pain is understood to be a salient internal threat signal that distracts individuals from ongoing activities or goal pursuits, and draws selective attention to painful stimuli or related cues12,48. Thus, when pain is elevated, individuals are more likely to experience higher interruption in their engagement in and performance of daily activities. Hence, it may be important to help prevent individuals from experiencing more than typical level of pain during the day. Findings of our intra-day process model indicate that jointly improving previous night’s non-restorative sleep and pain catastrophizing in the morning may potentially mitigate the effect of afternoon pain severity on individuals’ daily physical functioning.
The results of sensitivity analyses were also notable. We found that individual difference factors including overall poor sleep, clinical level of depression, and tricyclics and opioid use did not moderate the link between daily non-restorative sleep and activity interference. In other words, the present intra-day process model was quite robust in relation to these individual differences. Although other potential individual difference variables such as presence of sleep disorders and exercise level need to be tested in the future, our findings indicate the potential that the intra-day process model may be generalizable to quite a wide range of individuals with fibromyalgia.
Implications for Future Studies
Replication of our findings to other chronic pain conditions is needed. Although non-restorative sleep is one of the key diagnostic features of FM, reports of sleep disturbance are quite prevalent for many other chronic pain conditions as well4,68,74. For instance, a recent study revealed that although reports of sleep disturbance were higher in patients with FM than in individuals with osteoarthritis, there was no significant difference in their objective sleep profiles measured by polysomnography84. It is unknown, however, whether the relations between non-restorative sleep and daily pain catastrophizing, pain severity, and activity interference are similar between individuals with FM and those with other chronic pain conditions. Such investigations would provide important information relevant to developing intervention strategies targeted for specific chronic pain conditions.
Our findings also suggest that effective management of daily pain severity and activity interference could be enhanced by improving sleep during the previous night and reducing pain catastrophizing. Evidence-based sleep interventions such as Cognitive Behavioral Therapy for Insomnia (CBTi) have been tested multiple times among various chronic pain samples, providing some evidence that such interventions decrease pain severity, pain catastrophizing, and pain-induced activity interference40,59,60. We suspect that integrating a brief targeted intervention that focuses on reduction of pain catastrophizing could further increase the therapeutic benefit of CBTi on pain-related outcomes. For instance, Darnall and colleagues13 have recently tested a brief (single session; 2–3 hour long) pain catastrophizing intervention that has shown some promise in improving pain-related outcomes. Such investigations could be a viable future research avenue.
The present study focused largely on a cognitive-affective pathway (i.e., pain catastrophizing) that links daily sleep, pain, and activity interference. A potentially fruitful focus for future research would be to evaluate potential biological mechanisms in this link. Some findings suggest that both poor sleep and pain catastrophizing can contribute aberrant glial activation and a subsequent neuroinflammatory state which is characterized by elevation in brain-derived neurotrophic factor (BDNF), interleunkin-1β (IL-1β), and tumor necrosis factor alpha (TNF-α) levels46. In pre-clinical studies, there is some evidence that these pro-inflammatory markers can induce central sensitization29,30, a process characterized by increased neural signaling in the central nervous system that leads to pain hypersensitivity47,82. It has also been hypothesized that central sensitization influences the pathophysiology of fibromyalgia1,55,62. To our knowledge, the associations among non-restorative sleep, pain catastrophizing and pro-inflammatory biomarkers at the day-to-day level have not been well investigated. Such studies would provide a better insight in understanding the development and maintenance of fibromyalgia.
Limitations
There are a number of limitations in our study that should be addressed in future research. First, morning pain catastrophizing was measured by a single diary item that does not capture all domains of pain catastrophizing. Second, our daily sleep diary only measured non-restorative sleep. Inclusion of additional sleep diary items (e.g., bed time, final awake time, out of bed time) and/or using actigraphy to measure various sleep continuity indices (e.g., wake after sleep onset, sleep onset latency, total sleep time, and sleep efficiency) should be considered in future studies. Additionally, ambulatory electroencephalographic sleep monitoring may be used to assess sleep architecture in the home environment21,36,39, which may offer other clinically important findings. Third, we did not measure the experience of early morning pain catastrophizing and pain severity immediately after participants’ awakening. Reports of previous night’s non-restorative sleep may have been influenced by participants’ early morning pain severity and catastrophizing level. Fourth, as is quite common for daily diary studies10,22,43, the effect sizes in the present study were small overall. Future studies need to investigate whether these small effects in the within-day or within-person context can become more clinically meaningful as they accrue over time (i.e., months or years later). Finally, a number of person-level factors that may modulate the present intra-day process model were not considered. For instance, it has been suggested that sleep disorders such as chronic insomnia, sleep apnea, and restless leg syndrome can influence the perception of sleep and are associated with higher pain sensitivity16,42,61. Some other individual difference factors such as exercise level may have also emerged as a moderating variable of our intra-day process model. Future studies need to assess these important between-person moderating variables and evaluate whether the present model is generalizable across various individuals with FM.
Conclusion
In spite of these limitations, the present study has some notable strengths. This is one of the few studies that thoroughly examined the intra-day process model that explains the relationship between non-restorative sleep and activity interference among individuals with FM. The sample size was quite large, and participants were assessed four times per day for 21 days with overall good diary completion rates. We also used a state-of-the-art statistical approach (i.e., MSEM and three-path mediation) to evaluate our hypothesized model. Our study demonstrated that on days when individuals with FM experienced greater non-restorative sleep during the previous night, they reported greater end-of-day activity interference, mediated at least partially through higher morning pain catastrophizing and afternoon pain severity. Findings of our study emphasize the importance of evaluating daily non-restorative sleep among individuals with FM. Our intra-day process model also points out a potential need for developing accessible pain interventions that efficiently target improving daily sleep quality and attenuating pain catastrophizing to help individuals with FM more fully engage in their important daily activities despite having pain.
Highlights.
Daily non-restorative sleep and activity interference are sequentially mediated
Greater non-restorative sleep is associated with higher morning pain catastrophizing
Morning pain catastrophizing significantly predicts afternoon pain severity
Higher afternoon pain severity is related to greater end-of-day activity interference
Non-restorative sleep and catastrophizing also uniquely predict activity interference
Perspectives:
This study provides a better understanding of how non-restorative sleep is associated with daily activity interference among individuals with fibromyalgia. An intervention that targets attenuating non-restorative sleep and pain catastrophizing may help improve daily physical functioning of this population.
Acknowledgments
Source of Funding
Funding for this research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR053245 awarded to MCD) and National Institute of Neurological Disorders and Stroke (T32-NS070201 to CJM for postdoctoral training).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abeles AM, Pillinger MH, Solitar BM, Abeles M: Narrative review: the pathophysiology of fibromyalgia. Ann Intern Med; 146:726–34, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Affleck G, Tennen H, Urrows S, Higgins P, Abeles M, Hall C, Karoly P, Newton C: Fibromyalgia and women’s pursuit of personal goals: A daily process analysis. Heal Psychol; 17:40–7, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Afolalu EF, Ramlee F, Tang NKY: Effects of sleep changes on pain-related health outcomes in the general population: A systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev; 39:82–97, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argoff CE: The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain; 23:15–22, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bigatti SM, Hernandez AM, Cronan TA, Rand KL: Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Care Res; 59:961–7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Johnston KL, Shablesky-Cade MA, Pilkonis PA: Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep; 33:781–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne BM: Structural equation modeling with AMOS, EQS, and LISREL: Comparative approaches to testing for the factorial validity of a measuring instrument. Int J Test; 1:55–86, 2001. [Google Scholar]
- 8.Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, Edwards RR, Fontaine KR: Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther; 14:R231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR: Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: a cross-lagged panel analysis among healthy, pain-free participants. J Pain; 11:876–84, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Conner TS, Tennen H, Zautra AJ, Affleck G, Armeli S, Fifield J: Coping with rheumatoid arthritis pain in daily life: within-person analyses reveal hidden vulnerability for the formerly depressed. Pain; 126:198–209, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P: Fear-avoidance model of chronic pain. Clin J Pain; 28:475–83, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Van Damme S, Crombez G, Lorenz J: Pain draws visual attention to its location: experimental evidence for a threat-related bias. J Pain; 8:976–82, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Darnall BD, Sturgeon JA, Kao M-C, Hah JM, Mackey SC: From catastrophizing to recovery: a pilot study of a single-session treatment for pain catastrophizing. J Pain Res; 7:219–26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J: Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain; 113:9–19, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Edwards RR, Iii COB, Bathon J, Haythornthwaite JA: Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum; 55:325–32, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR, Quartana PJ, Allen RP, Greenbaum S, Earley CJ, Smith MT: Alterations in pain responses in treated and untreated patients with restless legs syndrome: associations with sleep disruption. Sleep Med; 12:603–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enders C, Bandalos D: The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model; 8:430–57, 2001. [Google Scholar]
- 18.Enders CK: Applied missing data analysis. Guilford press; 2010. [Google Scholar]
- 19.Enders CK, Tofighi D: Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods; 12:121–38, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Finan PH, Goodin BR, Smith MT: The association of sleep and pain: an update and a path forward. J Pain; 14:1539–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finan PH, Richards JM, Gamaldo CE, Han D, Leoutsakos JM, Salas R, Irwin MR, Smith MT: Validation of a wireless, self-application, ambulatory electroencephalographic sleep monitoring device in healthy volunteers. J Clin Sleep Med; 12:1443–51, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H: COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain; 152:300–7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fordyce WE: Behavioral methods for chronic pain and illness. Mosby St. Louis; 1976.
- 24.Goodin BR, Fillingim RB, Machala S, McGuire L, Buenaver LF, Campbell CM, Smith MT: Subjective sleep quality and ethnicity are interactively related to standard and situation-specific measures of pain catastrophizing. Pain Med; 12:913–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton NA, Atchley RA, Karlson CW, Taylor D, McCurdy D: The role of sleep and attention in the etiology and maintenance of fibromyalgia. Cognit Ther Res; 36:81–93, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen MP, Karoly P: Control beliefs, coping efforts, and adjustment to chronic pain. J Consult Clin Psychol; 59:431–8, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MP, Karoly P, Braver S: The measurement of clinical pain intensity: a comparison of six methods. Pain; 27:117–26, 1986. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MP, Turner JA, Romano JM: Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol; 69:655–62, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ji R-R, Chamessian A, Zhang Y-Q: Pain regulation by non-neuronal cells and inflammation. Science; 354:572–7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R: Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci; 28:5189–94, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kline RB: Software review: Software programs for structural equation modeling: Amos, EQS, and LISREL. J Psychoeduc Assess Sage Publications Sage CA: Thousand Oaks, CA; 16:343–64, 1998. [Google Scholar]
- 32.Kothari DJ, Davis MC, Yeung EW, Tennen HA: Positive affect and pain : mediators of the within-day relation linking sleep quality to activity interference in fibromyalgia. Pain; 156:540–6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krebs EE, Carey TS, Weinberger M: Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med; 22:1453–8, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lautenbacher S, Kundermann B, Krieg J-C: Sleep deprivation and pain perception. Sleep Med Rev; 10:357–69, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS: The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J Behav Med; 30:77–94, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Levendowski DJ, Ferini-Strambi L, Gamaldo C, Cetel M, Rosenberg R, Westbrook PR: The accuracy, night-to-night variability, and stability of frontopolar sleep electroencephalography biomarkers. J Clin Sleep Med; 13:791–803, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liedberg GM, Björk M, Börsbo B: Self-reported nonrestorative sleep in fibromyalgia– relationship to impairments of body functions, personal function factors, and quality of life. J Pain Res; 8:499–505, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little RJA, Rubin DB: Statistical analysis with missing data. John Wiley & Sons; 2014. [Google Scholar]
- 39.Lucey BP, Mcleland JS, Toedebusch CD, Boyd J, Morris JC, Landsness EC, Yamada K, Holtzman DM: Comparison of a single- channel EEG sleep study to polysomnography. J Sleep Res; 25:625–35, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez MP, Miró E, Sánchez AI, Díaz-Piedra C, Cáliz R, Vlaeyen JWS, Buela-Casal G: Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med; 37:683–97, 2014. [DOI] [PubMed] [Google Scholar]
- 41.McCracken LM, Iverson GL: Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag; 7:75–9, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Moldofsky H: Sleep and pain. Sleep Med Rev; 5:385–96, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Mun CJ, Thummala K, Davis MC, Karoly P, Tennen H, Zautra AJ: Predictors and social consequences of daily pain expectancy among adults with chronic pain. Pain; 158:1224–33, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthén B, Asparouhov T: Latent variable analysis with categorical outcomes: Multiple-group and growth modeling in Mplus. Mplus web notes; 4:1–22, 2002. [Google Scholar]
- 45.Muthen LK, Muthen BO: Mplus User’s Guide. eighth. Muthen & Muthen, 2017. [Google Scholar]
- 46.Nijs J, Loggia ML, Polli A, Moens M, Huysmans E, Goudman L, Meeus M, Vanderweeën L, Ickmans K, Clauw D: Sleep disturbances and severe stress as glial activators: key targets for treating central sensitization in chronic pain patients? Expert Opin Ther Targets; 21:817–26, 2017. [DOI] [PubMed] [Google Scholar]
- 47.Nijs J, Torres-Cueco R, van Wilgen P, Lluch Girbés E, Struyf F, Roussel N, Van Oosterwijck J, Daenen L, Kuppens K, Vanderweeen L: Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician; 17:447–57, 2014. [PubMed] [Google Scholar]
- 48.Pearce J, Morley S: An experimental investigation of the construct validity of the McGill Pain Questionnaire. Pain; 39:115–21, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Preacher KJ, Zyphur MJ, Zhang Z: A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods; 15:209–33, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Quartana PJ, Campbell CM, Edwards RR: Pain catastrophizing a critical review. Expert Rev Neurother; 9:745–58, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ree MJ, Harvey AG: Interpretive biases in chronic insomnia: An investigation using a priming paradigm. Behav Ther; 37:248–58, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Ree MJ, Pollitt A, Harvey AG: An investigation of interpretive bias in insomnia: an analog study comparing normal and poor sleepers. Sleep; 29:1359–62, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds WM, Kobak KA: Reliability and validity of the Hamilton Depression Inventory: A paper-and-pencil version of the Hamilton Depression Rating Scale Clinical Interview. Psychol Assess; 7:472–83, 1995. [Google Scholar]
- 54.Rudy TE, Kerns RD, Turk DC: Chronic pain and depression: toward a cognitive-behavioral mediation model. Pain; 35:129–40, 1988. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt-Wilcke T, Clauw DJ: Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol; 7:518–27, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Schuh-Hofer S, Wodarski R, Pfau DB, Caspani O, Magerl W, Kennedy JD, Treede R-D: One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain; 154:1613–21, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Sivertsen B, Lallukka T, Petrie KJ, Steingrímsdóttir ÓA, Stubhaug A, Nielsen CS: Sleep and pain sensitivity in adults. Pain; 156:1433–9, 2015. [DOI] [PubMed] [Google Scholar]
- 58.Sluka KA, Clauw DJ: Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience; 338:114–29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, McInrue E, Han D, Leoutsakis J, Haythornthwaite JA: Cognitive–behavioral therapy for insomnia in knee osteoarthritis: A randomized, double- blind, active placebo–controlled clinical trial. Arthritis Rheumatol; 67:1221–33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith MT, Haythornthwaite JA: How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev; 8:119–32, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA: Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep; 32:779–90, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staud R: Biology and therapy of fibromyalgia: pain in fibromyalgia syndrome. Arthritis Res Ther; 8:208, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stone KC, Taylor DJ, McCrae CS, Kalsekar A, Lichstein KL: Nonrestorative sleep. Sleep Med Rev; 12:275–88, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Sturgeon JA, Zautra AJ: State and trait pain catastrophizing and emotional health in rheumatoid arthritis. Ann Behav Med; 45:69–77, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan M, Thorn B, Haythornthwaite J, Keefe K, Martine M, Bradley L, Lefebvre J: Theoretical perspectives on the relation between catastrophising and pain. Clin J Pain; 17:52–64, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Sullivan MJL, Bishop SR, Pivik J: The Pain Catastrophizing Scale: Development and validation. Psychol Assess; 7:524–32, 1995. [Google Scholar]
- 67.Taylor AB, MacKinnon DP, Tein J-Y: Tests of the three-path mediated effect. Organ Res Methods; 11:241–69, 2008. [Google Scholar]
- 68.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ: Comorbidity of chronic insomnia with medical problems. Sleep; 30:213–8, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Taylor SS, Davis MC, Yeung EW, Zautra AJ, Tennen HA: Relations between adaptive and maladaptive pain cognitions and within-day pain exacerbations in individuals with fibromyalgia. J Behav Med; 40:458–67, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theadom A, Cropley M, Humphrey K-L: Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res; 62:145–51, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Thorn BE, Boothby JL, Sullivan MJL: Targeted treatment of catastrophizing for the management of chronic pain. Cogn Behav Pract; 9:127–38, 2002. [Google Scholar]
- 72.Turner JA, Jensen MP, Romano JM: Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain; 85:115–25, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Vlaeyen JW, Linton SJ: Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain; 85:317–32, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Wallen GR, Minniti CP, Krumlauf M, Eckes E, Allen D, Oguhebe A, Seamon C, Darbari DS, Hildesheim M, Yang L: Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry; 14:207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ware JE Jr, Sherbourne CD: The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care; 30:473–83, 1992. [PubMed] [Google Scholar]
- 76.Wilkinson K, Shapiro C: Nonrestorative sleep: Symptom or unique diagnostic entity? Sleep Med; 13:561–9, 2012. [DOI] [PubMed] [Google Scholar]
- 77.Wilt JA, Davin S, Scheman J: A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients. Scand J pain; 10:122–9, 2016. [DOI] [PubMed] [Google Scholar]
- 78.Wolf LD, Davis MC: Loneliness, daily pain, and perceptions of interpersonal events in adults with fibromyalgia. Heal Psychol; 33:929–37, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf LD, Davis MC, Yeung EW, Tennen HA: The within-day relation between lonely episodes and subsequent clinical pain in individuals with fibromyalgia: mediating role of pain cognitions. J Psychosom Res; 79:202–6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfe F, Clauw DJ, Fitzcharles M, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB: The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res; 62:600–10, 2010. [DOI] [PubMed] [Google Scholar]
- 81.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P: The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum; 33:160–72, 1990. [DOI] [PubMed] [Google Scholar]
- 82.Woolf CJ: Central sensitization: implications for the diagnosis and treatment of pain. Pain; 152:S2–15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeung EW, Davis MC, Aiken LS, Tennen HA: Daily social enjoyment interrupts the cycle of same-day and next-day fatigue in women with fibromyalgia. Ann Behav Med; 49:411–9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeung WK, Morgan K, Mckenna F: Comparison of sleep structure and psychometric profiles in patients with fibromyalgia, osteoarthritis and healthy controls. J Sleep Res; 27:292–300, 2018. [DOI] [PubMed] [Google Scholar]