Abstract
Background
Electrocautery ablation (EA) is a common treatment modality for anal high-grade squamous intraepithelial lesions (HSIL) but its effectiveness is understudied. This study aimed to determine ablation outcomes and to identify clinicopathological factors associated with post-ablation recurrence.
Methods
330 people living with HIV (PLWH) with de novo intra-anal HSIL treated with EA from 2009 to 2016 were studied retrospectively. Using long-term surveillance high-resolution anoscopy biopsy data, treatment failures were classified as local recurrence (HSIL at the treated site upon surveillance) or overall recurrence (HSIL at treated or untreated sites). The associations of these outcomes with clinical factors were analyzed using Cox proportional hazards models.
Results
88% of participants were men who have sex with men, median age was 45.5 years (range 35–51) and 49% had multiple index HSILs (range 2–6). At a median of 12.2 months post-ablation (range 6.3–20.9), 45% had local recurrence while 60% had overall recurrence. Current cigarette smoking, HIV viremia (HIV-1 RNA ≥100 copies/mL) and multiple index HSILs were predictive of local recurrence. Overall recurrence was more common in current smokers and those with multiple index lesions. In multivariable models that included HPV genotypes, baseline and persistent infection with HPV16/18 were significantly associated with both local and overall recurrence.
Conclusions
EA is an effective treatment modality for anal HSIL in PLWH but recurrence rates are substantial. Multiple index HSILs, HIV viremia, smoking and both baseline and persistent infection with HPV16/18 negatively impact treatment success. Ongoing surveillance is imperative to capture recurrence early and improve long-term treatment outcomes.
Keywords: HIV, Anal cancer precursors, HSIL, Electrocautery ablation, Outcomes, Recurrence
Precis:
Electrocautery ablation is an effective modality for the treatment of anal high-grade squamous intraepithelial lesions (HSIL) in people living with HIV, but recurrence rates are substantial. Multiple index HSILs, HIV viremia, smoking and HPV16/18 are risk factors for post-ablation HSIL recurrence.
INTRODUCTION
The incidence of human papillomavirus (HPV)-related anal squamous cell carcinoma in the United States has risen by ~2.2% per year over the last decade with 8,300 new cases projected in 2019.1, 2 Anal high-grade squamous intraepithelial lesions (HSIL) are the immediate cancer precursors and are highly prevalent among people living with HIV (PLWH), particularly among men who have sex with men (MSM) and women.3–6 Given the paucity of data on the natural history of anal HSIL and its treatment outcomes, there has been ongoing debate whether HSIL treatment is justified and cost-effective.7, 8 With guidance from a prospective clinical trial still years away,9 specialized anal dysplasia clinics are screening and treating anal HSIL proactively in high-risk populations, aiming to eradicate these precursors in order to prevent malignant transformation.10, 11
Treatment options for anal HSIL include topical immune modulators, chemotherapeutics, surgical excision, and targeted ablation using cryotherapy or thermocoagulation.12, 13 Among these options, high-resolution anoscopy (HRA)-guided electrocautery ablation (EA) has gained popularity as a fast, office-based procedure that produces favorable results with a low rate of complications. EA destroys individual lesions by inducing localized tissue necrosis to the depth of the submucosa while sparing adjacent benign-appearing tissue.14 EA has been shown to be superior to topical immune modulators or chemotherapeutics for the treatment of anal HSIL.15 In a retrospective study, adding HPV vaccine to HSIL treatment (adjuvant HPV vaccination) improved treatment outcomes among HIV uninfected MSM and a mathematical modeling study found adjuvant vaccination to be cost-effective;16, 17 however, a recent randomized clinical trial did not confirm such synergy among PLWH.18
Studies on ablation efficacy have been heterogeneous in cohort characteristics and surveillance strategies, yet HSIL clearance following infrared coagulation (IRC) and/or EA has been consistently high, ranging from 53% to 87% in HIV infected and uninfected MSM.19–24 However, HSIL recurs frequently following ablation and necessitates ongoing surveillance and repeated treatments.25
In this study, we have summarized our experience using EA combined with HRA surveillance to manage anal HSIL in a large real-world cohort of PLWH, the majority of whom were MSM. Our objectives were to determine ablation effectiveness and to identify key clinicopathological factors associated with post-ablation HSIL recurrence.
MATERIALS AND METHODS
Patient selection
Institutional Review Board approval was first obtained from the Icahn School of Medicine. The Mount Sinai anal dysplasia database was searched from January 2009 to December 2016 for PLWH referred for anal cancer screening either with or without previously obtained anal cytology who met the following inclusion criteria: (1) De novo, biopsy-proven intra-anal canal HSIL, (2) electrocautery ablation within 6 months of diagnosis, and (3) one or more surveillance HRAs with biopsy following ablation. Patients with a history of anal cancer or prior HSIL treatment were excluded. Electronic medical records were reviewed for clinical characteristics such as age, gender, race/ethnicity, history of AIDS diagnosis (as evidenced by nadir CD4+ T-cell count <200 cells/mm3 or clinical evidence of AIDS), HIV-1 RNA level and CD4+ T-cell count within 6 months prior to HRA, as well as smoking history.
High-resolution anoscopy and biopsy
All patients underwent digital anorectal exam (DARE) and HRA at initial and follow-up visits. Unless previously obtained, anal cytology samples were collected immediately prior to HRA. Author M.G. performed all HRA and biopsy procedures using previously described techniques.26 Following treatment with 3% acetic acid and Lugol’s iodine, the squamocolumnar junction, distal anal canal, and anal margin were visualized under 15x magnification to look for abnormal vascular patterns and other potential signs of HSIL or cancer, including ulceration, mass effect, and mucosal friability. Areas suspicious for HSIL or cancer were biopsied. If no suspicious mucosal changes were identified, then no biopsy was taken and the patient was scored as having a ‘benign’ examination. Random biopsies of healthy-appearing tissue were not performed during this study.
Electrocautery ablation
Author M.G. performed all electrocautery ablation procedures using a hyfrecator (ConMed Corporation, Utica, NY). Under HRA guidance, index HSILs were identified and the hyfrecator was used to ablate the lesions after achieving local anesthesia by using 1% lidocaine hydrochloride with epinephrine 1:100,000. The hyfrecator was used at a setting of 15 Watts. Lesions were fulgurated and debrided with blunt and sharp dissection to healthy tissue and submucosal vessels were coagulated.23 All HSILs detected at baseline were ablated concomitantly during the same treatment visit.
Classification of ablation outcomes
The anal canal was divided into octants and biopsy sites recorded as anterior, right anterior, right lateral, right posterior, posterior, left posterior, left lateral, or left anterior. To account for potential mucosal shifts in between HRA procedures, lesions from any three adjacent octants were considered the same location for the purpose of surveillance analyses. Based on surveillance HRA and biopsy results, ablation outcomes were classified as overall recurrence (any HSIL detected upon follow-up) or no recurrence (no evidence of HSIL). Recurrence was then further sub-classified as local recurrence or metachronous recurrence according to lesion location. Local recurrence was defined as HSIL recurring in the same location as the index lesion. Metachronous recurrence was defined as HSIL recurring in a location independent from the index lesion. For time-to-event analyses we calculated time to recurrence by measuring the date of ablation until the occurrence of the outcome of interest, i.e., a recurrence event; subjects with no recurrence were censored at their latest surveillance HRA. For recurrence events we also collected data on the number of HSIL lesions detected upon surveillance HRA.
Pathology review
All biopsies were processed following standard histological protocol, serially sectioned into 6 levels, and stained with Hematoxylin and Eosin. Surgical pathologists at the Mount Sinai Hospital rendered diagnoses based on standard morphological criteria for low-grade squamous intraepithelial lesions (LSIL) and HSIL. Consensus review was conducted by two or more pathologists in ~80% of the biopsies to confirm histological diagnoses. P16 immunohistochemistry was used in selected cases (~40% of the biopsies) to grade morphologically ambiguous lesions wherein strong and diffuse positive immunoreactivity supported the diagnosis of HSIL.27
Results of HPV genotyping from liquid cytology fluid were obtained from the pathology database and were limited to samples collected concurrently or within 3 months of index HRA beginning in February 2012 (HPV testing was performed in 92% of cases after this date.). We also collected data on HPV status at each surveillance HRA. Oncogenic HPV subtype analysis was performed using the Roche Cobas® 4800 HPV kit (Roche Diagnostics, Indianapolis, Indiana, USA) capable of detecting 14 types of high-risk HPV (16, 18, and other types including 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). HPV status was categorized as no high risk HPV, HPV 16/18 with or without other high-risk HPV or non-16/18 high-risk HPV.
Statistical analysis
We first used descriptive statistics to summarize the baseline characteristic of the study population. To estimate the difference in HSIL recurrence on follow-up by patient characteristics, we used the Wilcoxon test for continuous variables (age and CD4+ T-cell count measures) and the chi-square test for categorical variables. We used Kaplan-Meier methods to estimate the cumulative risk of two primary outcomes: local and overall HSIL recurrence. We also compared cumulative risk of recurrence by baseline HPV status; the difference in risk was compared using the log-rank test. Hazard ratios (HR) and associated CI were computed by fitting multivariable Cox proportional hazard regression models to evaluate significant predictors from univariate testing, adjusted for demographic factors and other potential confounders. We first evaluated the association of HIV viremia (HIV RNA level <100 copies/mL versus ≥100 copies/mL to differentiate between viral “blips” and more significant viremia28; obtained within 6 months prior to index HRA), smoking status and HSIL burden at baseline (solitary versus multiple) with local and overall recurrence. As HPV typing was only available after early 2012, we fitted separate multivariable models to include baseline HPV infection status excluding subjects seen prior to February 2012. As HPV16/18 status was predictive of the outcomes of interest in univariate and multivariable analyses, we fitted additional models including persons with longitudinal HPV information to assess the impact of HPV16/18 persistence on HSIL recurrence by categorizing HPV16/18 infection as always negative, intermittent (positive at some surveillance HRAs) or persistent (positive at all surveillance HRAs). There was missing data for baseline HPV status (8% in models excluding persons included prior to the start of routine testing), smoking status and race/ethnicity (<3%). We employed multiple imputation methods in multivariable models to account for missing data (including baseline HPV status); these results are presented and did not differ significantly from complete case analyses. All analyses were performed using STATA Version 15 (Stata Corporation, College Station, TX).
RESULTS
Patient Characteristics
A total of 330 PLWH met inclusion criteria. The median age at index HSIL diagnosis was 45.5 years (interquartile range 35–51); 88% were MSM, 12% were women and 28% were current smokers (Table 2). At baseline, 51% of patients were found to have a solitary index HSIL while 49% harbored 2 to 6 HSILs. Among patients with baseline HPV genotyping results (n=268), oncogenic HPV types were detected in 93%, including 48% who tested positive for HPV16 and/or 18 with or without other high-risk types, and 45% who tested positive for only non-16/18 high-risk types. All participants were prescribed antiretroviral therapy during the study period and had a median CD4+ T-cell count of 633 cells/μl. 82% of subjects had HIV-1 RNA <100 copies/mL with 6 months of index HSIL diagnosis.
Table 2.
Comparison of baseline demographic and clinical characteristics between ablation outcome groups
| Patient characteristics | Baseline cohort characteristics (n=330) | Outcomes on any follow-up | P-value** | |
|---|---|---|---|---|
| No HSIL recurrence (n=132) | Overall HSIL Recurrence (n=198) | |||
| Age, years, median, (IQR) | 45.5 (35–51) | 46 (35.5–52) | 45 (34–53) | 0.7 |
| MSM, n (%) | 290 (88) | 118 (89) | 172 (87) | 0.4 |
| Race/Ethnicity, n (%) | ||||
| White | 124 (38) | 42 (32) | 82 (41) | 0.5 |
| Black | 65 (20) | 28 (21) | 37 (19) | |
| Hispanic | 105 (32) | 47 (36) | 58 (29) | |
| Other | 28 (9) | 11 (8) | 17 (9) | |
| Unknown | 8 (2) | 4 (3) | 4 (2) | |
| Smoking Status, n (%) | ||||
| Current Smoker | 93 (28) | 25 (19) | 68 (34) | 0.02 |
| Former Smoker | 81 (25) | 36 (27) | 45 (23) | |
| Never Smoker | 153 (46) | 69 (52) | 84 (42) | |
| Unknown | 3 (1) | 2 (2) | 1 (<1) | |
| AIDS Diagnosis, n (%) | 131 (40) | 56 (42) | 75 (38) | 0.4 |
| HIV RNA, n (%) | ||||
| >100 copies/ml | 59 (18) | 16 (12) | 43 (22) | 0.03 |
| CD4+ T-cell count, median cells/ul, (IQR) | 633 (459–828) | 610 (443–771) | 647 (460–869) | 0.3 |
| Follow-up HRA Examinations, median (range) | 1 (1–8) | 2 (1–8) | 1 (1–4) | <0.001 |
| Lesion Burden at Baseline, n (%) | ||||
| Solitary | 167 (51) | 57 (43) | 110 (56) | 0.03 |
| Multiple | 163 (49) | 75 (57) | 88 (44) | |
| High Risk HPV Types, n (%) | ||||
| Total # | 268 | 106 | 162 | |
| Negative | 19 (7) | 16 (15) | 3 (2) | < 0.001 |
| HPV 16/18 | 128 (48) | 43 (41) | 85 (52) | |
| Other HR HPV* | 121 (45) | 47 (44) | 74 (46) | |
IQR: Interquartile range; HPV: Human Papillomavirus; HSIL: High-grade squamous intraepithelial lesion; MSM: Men who have sex with men
Other HR HPV types include HPV-31/33/35/39/45/51/52/56/58/59/66/68
P-values for comparisons of “No HSIL on Any Follow-up” versus “Overall HSIL Recurrence” columns
Ablation Outcomes and Predictors
The median follow-up after ablation was 12.2 months (interquartile range 6.3–20.9). Overall, 148 (45%) patients had local recurrence at the ablated site (Table 1). 142 (43%) patients developed metachronous lesions. Overall, 198 patients (60%) experienced recurrent HSIL. None of the patients progressed to invasive cancer during the study period. Among patients who experienced HSIL recurrence, 67% were found to have a solitary lesion on surveillance HRA, 28% had two lesions, and 5% had three or more lesions. In unadjusted analyses (Table 2), overall HSIL recurrence following EA was significantly associated with HIV RNA >100 copies/mL (p=0.03), multiple index lesions at baseline (p=0.03), and infection by high-risk HPV types (p<0.001). A greater proportion of patients with HSIL recurrence were also current smokers (34% vs. 19%; p=0.02). HSIL recurrence was highest for patients infected by HPV16/18 (52%), followed by those infected by other high-risk HPV types (46%) and was lowest for those with undetectable high-risk HPV types (2%, p<0.001). We found no statistically significant difference in post-ablation recurrence by age, race, AIDS diagnosis and CD4+ T-cell count.
Table 1.
Post-ablation recurrence at 12 months median follow-up (n=330)
| Recurrence | N (%; 95% CI) |
|---|---|
| Local | 148 (45%; 95% CI: 39%–50%) |
| Metachronous | 142 (43%; 95% CI: 38%–49%) |
| Overall | 198 (60%; 95% CI: 54%–65%) |
Local Recurrence: HSIL detected in the location of the index lesion upon follow-up;
Metachronous Recurrence: HSIL in a location different from the index lesion upon follow-up;
Overall Recurrence: A combined outcome of local recurrence and metachronous lesions (the first two rows)
95% CI: 95% confidence interval
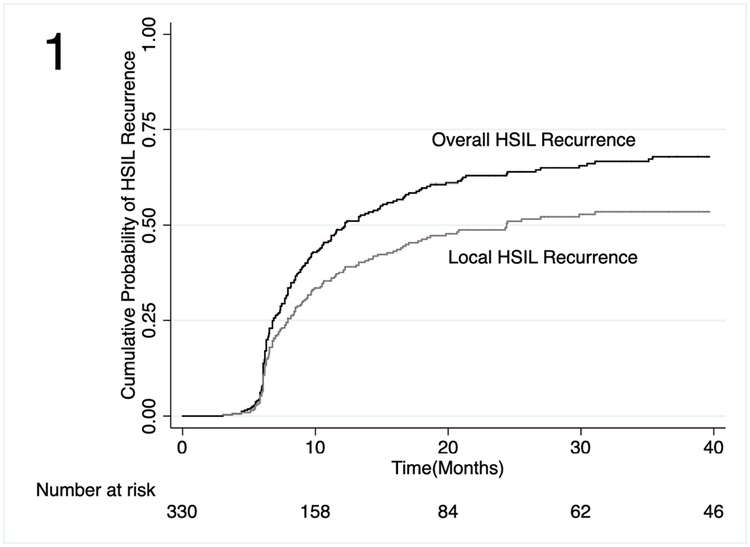
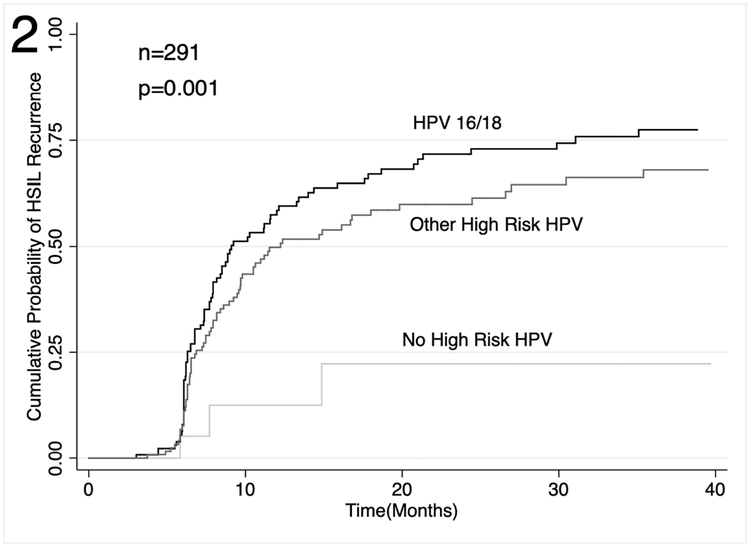
Unadjusted time-to-event analyses showed a cumulative probability of local HSIL recurrence of 8% at 6 months (Figure 1; 95% confidence interval [CI]: 5%−12%), 38% (95% CI: 33%−44%) at 12 months and 53% (95% CI: 47%−60%) at 36 months. Cumulative probability of overall HSIL recurrence was 50% (95% CI: 44%−55%) at 12 months and 68% (95% CI: 62%−74%) at 36 months. In unadjusted time-to-event analyses of overall HSIL recurrence by baseline HPV status, HPV16/18 was associated with the greatest risk of HSIL recurrence (Figure 2; p=0.001).
Figure 1.
Cumulative probability of overall and local recurrence following electrocautery ablation of anal HSIL among PLWH.
Figure 2.
Cumulative probability of overall HSIL recurrence following electrocautery ablation of anal HSIL among PLWH by baseline HPV status.
In multivariable analyses (Table 3), local HSIL recurrence after EA was independently associated with HIV infection with viremia (hazard ratio [HR] 1.5; 95% CI: 1.0–2.2), as was current smoking (HR 1.7; 95% CI: 1.1–2.4, compared to never having smoked) and the presence of multiple baseline lesions (HR 1.8; 95% CI: 1.3–2.5). Current smoking and multiple baseline lesions were also significantly associated with overall HSIL recurrence. In subgroup analyses, when comparing outcomes by high-risk HPV infection at baseline (n=291), detection of HPV 16/18 (HR 4.7 for local and 5.6 for overall recurrence) or other high-risk HPV types (HR 3.4 for local and 4.3 for overall recurrence) were independently associated with an increased risk of HSIL recurrence, compared to subjects with negative high-risk HPV test (each p<0.05). HPV genotyping results on surveillance were available for 184 patients. In adjusted analyses of patients with longitudinal HPV genotyping results, persistent HPV16/18 infection was associated with both local recurrence (HR 2.3; 95% CI: 1.4–3.7) and overall HSIL recurrence (HR 2.0; 95% CI: 1.3–3.1).
Table 3:
Multivariable analysis of predictors of post-ablation local and overall recurrence
| Entire Study Cohort (N=330) | HPV Testing Eligible Cohort (N=291) | Longitudinal HPV16/18 Cohort (N=184) | ||||
|---|---|---|---|---|---|---|
| HR for Local Recurrence (95% CI) | HR for Overall Recurrence (95% CI) | HR for Local Recurrence (95% CI) | HR for Overall Recurrence (95% CI) | HR for Local Recurrence (95% CI) | HR for Overall Recurrence (95% CI) | |
| Age | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) |
| Female | 0.9 (0.4–2.0) | 1.3 (0.8–2.1) | 0.9 (0.4–1.9) | 1.2 (0.7–2.2) | 0.8 (0.3–2.1) | 0.8 (0.3–2.1) |
| Race/Ethnicity | ||||||
| White | Reference | Reference | Reference | Reference | Reference | Reference |
| Black | 0.7 (0.5–1.2) | 0.6 (0.4–1.0) | 0.9 (0.5–1.5) | 0.7 (0.4–1.1) | 1.1 (0.5–2.2) | 0.7 (0.4–1.2) |
| Hispanic | 0.8 (0.5–1.2) | 0.7 (0.5–1.0) | 0.9 (0.6–1.5) | 0.8 (0.5–1.2) | 1.2 (0.7–2.2) | 0.7 (0.4–1.2) |
| Other | 1.1 (0.6–2.0) | 0.8 (0.5–1.4) | 1.0 (0.5–2.0) | 0.8 (0.4–1.2) | 1.7 (0.6–4.7) | 0.8 (0.3–2.0) |
| Smoking Status | ||||||
| Never Smoker | Reference | Reference | Reference | Reference | Reference | Reference |
| Former Smoker | 1.1 (0.7–1.7) | 1.0 (0.7–1.4) | 1.0 (0.7–1.7) | 0.9 (0.6–1.4) | 1.1 (0.6–2.0) | 1.2 (0.7–1.9) |
| Current Smoker | 1.7 (1.1–2.4) | 1.8 (1.3–2.5) | 1.3 (0.9–2.1) | 1.7 (1.2–2.5) | 1.2 (0.7–2.2) | 1.8 (1.1–2.9) |
| HIV Viremia | ||||||
| Non-viremic | Reference | Reference | Reference | Reference | Reference | Reference |
| Viremic | 1.5 (1.0–2.2) | 1.3 (0.9–1.8) | 1.3 (0.9–2.0) | 1.2 (0.8–1.7) | 1.9 (1.1–3.3) | 1.4 (0.8–2.3) |
| Lesion Burden at Baseline | ||||||
| Solitary | Reference | Reference | Reference | Reference | Reference | Reference |
| Multiple | 1.8 (1.3–2.5) | 1.4 (1.1–1.9) | 1.8 (1.2–2.6) | 1.3 (0.9–1.8) | 1.7 (1.1–2.8) | 1.3 (0.9–2.0) |
| High Risk HPV Types | ||||||
| No High Risk HPV | -- | -- | Reference | Reference | -- | -- |
| HPV16/18 | -- | -- | 4.7 (1.1–19.5) | 5.6 (1.7–17.9) | -- | -- |
| Other HR HPV | -- | -- | 3.4 (0.8–14.1) | 4.3 (1.3–13.7) | -- | -- |
| HPV 16/18 Longitudinal Positivity | ||||||
| Always HPV 16/18 Negative | Reference | Reference | ||||
| Intermittent HPV 16/18 Positivity | 1.9 (0.6–6.3) | 2.2 (0.8–6.3) | ||||
| Persistent HPV 16/18 Positivity | 2.3 (1.4–3.7) | 2.0 (1.3–3.1) | ||||
HR: Hazard ratio; HPV: Human papillomavirus
Persistent HPV 16/18 Positivity: HPV16/18 was detected in both baseline and follow-up cytology samples.
Intermittent HPV 16/18 Positivity: HPV16/18 was detected only once, either at baseline or follow-up.
Always HPV 16/18 Negative: HPV 16/18 was neither detected at baseline nor at follow-up.
DISCUSSION
In this retrospective study, we evaluated the effectiveness of electrocautery ablation as an initial treatment for anal HSIL. Our cohort comprised 330 PLHW, predominantly MSM, with a de novo diagnosis of intra-anal HSIL. After a single ablation treatment, 62% of study participants were HSIL-free at the ablation site 12 months following treatment and 47% maintained remission at the treated HSIL site 36 months post EA. Although overall post-ablation HSIL recurrence was substantial (50% at 12 months and 68% at 36 months), no subject progressed to cancer during the study period. We further demonstrated that HSIL burden at baseline, being an active smoker, HIV viremia and HPV16/18 infection had a strongly negative impact on treatment effectiveness, implying that more vigilant post-treatment surveillance may be warranted for patients with these risk factors.
Our study is in agreement with others regarding the effectiveness of ablation in eradicating anal HSIL. Cranston et al reported a 64% HSIL clearance rate among 68 HIV infected MSM treated with IRC22. Recently, Goldstone et al published the first randomized, prospective trial comparing IRC ablation to active monitoring in 120 HIV infected participants with 1–3 anal HSILs.29 Complete index HSIL clearance was 62% for participants treated with IRC compared to 30% in the active monitoring group at one-year follow-up, a result that demonstrates a clear treatment benefit in patients with limited disease as well as the potential for spontaneous regression. A decision-analytic model that was developed based on SEER data has also affirmed the cost-effectiveness of HSIL treatment, particularly for HIV infected MSM ≥ 38 years old.8
Index HSIL clearance rates when treated with EA are similarly high, but there remains significant concern over response durability and disease recurrence. We observed an overall HSIL recurrence rate of 50% within 1 year and 68% within 3 years of ablation, underscoring the need for ongoing, active surveillance following initial ablation. Our findings are consistent with the largest retrospective study on long-term outcomes, one that used a variety of ablative techniques (laser, IRC and EA)21. The authors estimated the probability of recurrence within 1, 2 and 3 years as 53%, 68% and 77% for HIV-infected patients, with slightly lower estimates for HIV uninfected subjects (49%, 57% and 66%).
High post-ablation recurrence is largely attributed to the targeted treatment approach whereby only visibly abnormal tissue is ablated under HRA guidance. Microscopic residual disease that is left untreated may pave the way for recurrence and multiple index HSILs likely exacerbate that risk. This is supported by our finding that a high volume of baseline disease is a significant risk factor for recurrence. In addition, some experts speculate that ablative therapy might promote activation of latent HPV in non-dysplastic tissue surrounding ablated HSIL sites and thereby catalyze recurrence.29 Reassuringly, while 49% of subjects in our cohort had multiple index HSILs (range 2–6) prior to EA, two thirds of all recurrences presented as a solitary lesion, suggesting a reduction of disease volume by ablative therapy.
Importantly, none of the study participants who underwent EA of anal HSIL progressed to anal cancer after a median follow-up of 12.2 months. Two recently published papers report that anal HSIL progresses to invasive anal cancer at rates of 1.3% to 1.9% per year.30, 31 Despite slight variations in patient cohort characteristics and longer median follow-up in these series, one would expect to see 4–6 patients progress to cancer within 12.2 months by adopting similar progression rates to our cohort. The latest practice guidelines for colon and rectal surgeons give only a weak recommendation for both screening and surveillance of populations at risk for anal dysplasia.32 In contrast, our data strongly suggests that for PLWH whose anal HSIL is treated and properly surveilled, the progression risk to cancer is diminished, despite significant post-EA recurrence rates.
HIV infection is considered one of the major risk factors for post-ablation recurrence. In our cohort, a 50% increase in local HSIL recurrence rates was associated with HIV viremia, even after adjusting for potential confounders. Nevertheless, in contrast to previous evidence, we did not find any association between prior severe immunosuppression (AIDS diagnosis or nadir CD4 count <200 cells/mm3) and EA outcomes.25 Since most viremic patients in our study had relatively robust CD4 counts, this finding implies that low-level or intermittent viremia leads to subtle immunosuppression or other immunological disturbances that may be more conducive for HPV infection to persist and progress.33 This is corroborated by previous work our group has published on the immune microenvironment of anal HSILs. We found that anal HSILs among PLWH harbored excess mucosa-infiltrating CD8+ T lymphocytes, and this was significantly associated with ablation resistance34. HIV infection may disrupt the anal mucosa, thereby facilitating HPV infection35. Furthermore, the HIV tat protein has been shown to increase expression of the HPV oncoprotein E6 and to reduce activity of the tumor suppressor gene p53, providing a direct link between an HIV viral protein and the HPV carcinogenic pathway36.
Active cigarette smoking is an established risk factor for persistent anogenital HPV infection, HSIL and anal cancer.6, 37, 38 Furthermore, anal oncogenic HPV viral loads have been shown to be significantly higher in smokers compared with non-smokers.39 Consistent with these observations, both local and overall HSIL recurrence in our cohort were associated with being a current smoker.
There is limited data on the impact of specific high-risk HPV types on HSIL treatment outcomes. In this study, we found that high-risk HPV infection, particularly persistent infection with HPV16/18, conferred significant risk of post-ablation HSIL recurrence. We have recently reported that baseline HPV16/18 infection was associated with an increased likelihood of progression from anal LSIL to HSIL compared to baseline infection with non-16/18 high-risk HPV types40. Taken together, our findings underscore the importance of HPV16 and 18 in anal carcinogenesis, suggesting a potential role of HPV genotyping for risk-stratification in anal cancer screening.41, 42 Studying the role of high-risk HPV infection and HIV-related local immune disturbance in the continuum of disease progression, screening effectiveness, HSIL treatment and continued surveillance, may help formulate more targeted anal cancer prevention algorithms that will maximize the value of screen-and-treat approaches.
As previously reported, the quality of HRAs and technical proficiency of ablative treatments are subject to a lengthy learning process with significant interoperator variability.43, 44 Quality can vary even within the same operator depending on a variety of factors: patients’ anxiety and discomfort can render the exam challenging, rushed or abbreviated. Anatomic challenges such as pre-existing scar tissue, post-operative or radiation changes, hemorrhoids, bleeding and/or mucosal prolapse can have a significant impact upon the quality of the exam. Such factors likely affect the detection rate of HSIL as well as ablation efficacy, thereby influencing overall recurrence rates.
Our study has several strengths. It is one of the largest clinical datasets published to date assessing treatment effectiveness of EA for anal HSIL in PLWH and factors associated with treatment outcomes. Despite its retrospective design, it utilized a meticulous, longitudinal database capturing clinical and epidemiological variables and is unique in containing high-risk HPV infection and clinical HIV data. A single operator with significant experience performed all HRAs and ablative treatments, preventing interoperator variability. Lastly, we utilized HRA-guided biopsy as a surveillance strategy, providing definitive histopathological confirmation of any recurrence. As for notable limitations, treatment adverse events were not systematically captured. Furthermore, size and morphology of index HSILs were not recorded and may have had an impact on recurrence risk.
In summary, our study corroborates that EA is an effective treatment for anal HSIL in PLWH and achieves high index HSIL clearance rates. HSIL recurrence is a considerable downside of the targeted ablative approach. HIV viremia, smoking, HPV16/18 infection and the number of index HSILs have a negative impact on treatment success. Careful, ongoing surveillance by means of HRA and biopsy is imperative to capture recurrence early and to improve long-term treatment outcomes.
Funding sources:
Keith Sigel receives funding through NCI K07CA180782
Footnotes
Conflicts of Interest: Ashish A. Deshmukh received consulting fee from Merck on unrelated projects.
REFERENCES
- 1.Institute NC. SEER Cancer Factsheets: Anal Cancer. Available from URL: http://seer.cancer.gov/statfacts/html/anus.html. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69: 7–34. [DOI] [PubMed] [Google Scholar]
- 3.Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. International Journal of Cancer. 2014;134: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 4.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 5.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncology. 2012;13: 487–500. [DOI] [PubMed] [Google Scholar]
- 6.Gaisa M, Ita-Nagy F, Sigel K, et al. High Rates of Anal High-Grade Squamous Intraepithelial Lesions in HIV-Infected Women Who Do Not Meet Screening Guidelines. Clinical Infectious Diseases. 2017;64: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orchard M, Roman A, Parvaiz AC. Anal intraepithelial neoplasia--is treatment better than observation? International Journal of Surgery (London, England). 2013;11: 438–441. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh AA, Chiao EY, Cantor SB, et al. Management of precancerous anal intraepithelial lesions in human immunodeficiency virus-positive men who have sex with men: Clinical effectiveness and cost-effectiveness. Cancer. 2017;123: 4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkhalter JE, Atkinson TM, Berry-Lawhorn J, et al. Initial Development and Content Validation of a Health-Related Symptom Index for Persons either Treated or Monitored for Anal High-Grade Squamous Intraepithelial Lesions. Value in Health. 2018;21: 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palefsky JM. Screening to prevent anal cancer: Current thinking and future directions. Cancer Cytopathology. 2015;123: 509–510. [DOI] [PubMed] [Google Scholar]
- 11.Nathan M, Sheaff M, Fox P, Goon P, Gilson R, Lacey C. Early treatment of anal intraepithelial neoplasia. BMJ. 2011;343: d7717. [DOI] [PubMed] [Google Scholar]
- 12.Fox PA. Treatment options for anal intraepithelial neoplasia and evidence for their effectiveness. Sex Health. 2012;9: 587–592. [DOI] [PubMed] [Google Scholar]
- 13.Megill C, Wilkin T. Topical therapies for the treatment of anal high-grade squamous intraepithelial lesions. Seminars in Colon & Rectal Surgery. 2017;28: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terlizzi JP, Goldstone S. Ablative therapies for the treatment of anal high-grade squamous intraepithelial lesions. Seminars in Colon & Rectal Surgery. 2017;28: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richel O, de Vries HJ, van Noesel CJ, Dijkgraaf MG, Prins JM. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncology. 2013;14: 346–353. [DOI] [PubMed] [Google Scholar]
- 16.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clinical Infectious Diseases. 2012;54: 891–898. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32: 6941–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkin TJ, Chen H, Cespedes MS, et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clinical Infectious Diseases. 2018;67: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Diseases of the Colon and Rectum. 2008;51: 829–835; discussion 835–827. [DOI] [PubMed] [Google Scholar]
- 20.Stier EA, Goldstone SE, Berry JM, et al. Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. Journal of Acquired Immune Deficiency Syndromes. 2008;47: 56–61. [DOI] [PubMed] [Google Scholar]
- 21.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: recurrence and incidence of cancer. Diseases of the Colon and Rectum. 2014;57: 316–323. [DOI] [PubMed] [Google Scholar]
- 22.Cranston RD, Hirschowitz SL, Cortina G, Moe AA. A retrospective clinical study of the treatment of high-grade anal dysplasia by infrared coagulation in a population of HIV-positive men who have sex with men. International Journal of STD and AIDS. 2008;19: 118–120. [DOI] [PubMed] [Google Scholar]
- 23.Marks DK, Goldstone SE. Electrocautery ablation of high-grade anal squamous intraepithelial lesions in HIV-negative and HIV-positive men who have sex with men. Journal of Acquired Immune Deficiency Syndromes. 2012;59: 259–265. [DOI] [PubMed] [Google Scholar]
- 24.Burgos J, Curran A, Landolfi S, et al. The effectiveness of electrocautery ablation for the treatment of high-grade anal intraepithelial neoplasia in HIV-infected men who have sex with men. HIV Medicine. 2016;17: 524–531. [DOI] [PubMed] [Google Scholar]
- 25.Burgos J, Curran A, Landolfi S, et al. Risk factors of high-grade anal intraepithelial neoplasia recurrence in HIV-infected MSM. AIDS. 2017;31: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 26.Jay N, Berry JM, Miaskowski C, et al. Colposcopic Characteristics and Lugol’s Staining Differentiate Anal High-Grade and Low-Grade Squamous Intraepithelial Lesions During High Resolution Anoscopy. Papillomavirus Res. 2015;1: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Archives of Pathology and Laboratory Medicine. 2012;136: 1266–1297. [DOI] [PubMed] [Google Scholar]
- 28.Fidler S, Olson AD, Bucher HC, et al. Virological Blips and Predictors of Post Treatment Viral Control After Stopping ART Started in Primary HIV Infection. Journal of Acquired Immune Deficiency Syndromes. 2017;74: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstone SE, Lensing SY, Stier EA, et al. A randomized clinical trial of infrared coagulation ablation versus active monitoring of intra-anal high-grade dysplasia in HIV-infected adults: An AIDS Malignancy Consortium trial. Clinical Infectious Diseases. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cajas-Monson LC, Ramamoorthy SL, Cosman BC. Expectant Management of High-Grade Anal Dysplasia in People with HIV: Long-term Data. Diseases of the Colon and Rectum. 2018;61: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 31.Lee GC, Kunitake H, Milch H, et al. What Is the Risk of Anal Carcinoma in Patients With Anal Intraepithelial Neoplasia III? Diseases of the Colon and Rectum. 2018;61: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart DB, Gaertner WB, Glasgow SC, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (Revised 2018). Diseases of the Colon and Rectum. 2018;61: 755–774. [DOI] [PubMed] [Google Scholar]
- 33.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Gaisa MM, Wang X, et al. Differences in the Immune Microenvironment of Anal Cancer Precursors by HIV Status and Association With Ablation Outcomes. Journal of Infectious Diseases. 2018;217: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tugizov SM, Herrera R, Chin-Hong P, et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barillari G, Palladino C, Bacigalupo I, Leone P, Falchi M, Ensoli B. Entrance of the Tat protein of HIV-1 into human uterine cervical carcinoma cells causes upregulation of HPV-E6 expression and a decrease in p53 protein levels. Oncology Letters. 2016;12: 2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaccarella S, Herrero R, Snijders PJ, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. International Journal of Epidemiology. 2008;37: 536–546. [DOI] [PubMed] [Google Scholar]
- 38.Bertisch B, Franceschi S, Lise M, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. American Journal of Epidemiology. 2013;178: 877–884. [DOI] [PubMed] [Google Scholar]
- 39.Wieland U, Hellmich M, Wetendorf J, et al. Smoking and anal high-risk human papillomavirus DNA loads in HIV-positive men who have sex with men. International Journal of Medical Microbiology. 2015;305: 689–696. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Sigel K, Gaisa MM. Human Papillomavirus Genotypes Predict Progression of Anal Low-Grade Squamous Intraepithelial Lesions. Journal of Infectious Diseases. 2018;218: 1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infectious Diseases. 2018;18: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambursky JA, Terlizzi JP, Goldstone SE. Testing for Human Papillomavirus Strains 16 and 18 Helps Predict the Presence of Anal High-Grade Squamous Intraepithelial Lesions. Diseases of the Colon and Rectum. 2018;61: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 43.Hillman RJ, Gunathilake MP, Jin F, Tong W, Field A, Carr A. Ability to detect high-grade squamous anal intraepithelial lesions at high resolution anoscopy improves over time. Sex Health. 2016;13: 177–181. [DOI] [PubMed] [Google Scholar]
- 44.Siegenbeek van Heukelom ML, Marra E, Cairo I, et al. Detection Rate of High-Grade Squamous Intraepithelial Lesions as a Quality Assurance Metric for High-Resolution Anoscopy in HIV-Positive Men. Diseases of the Colon and Rectum. 2018;61: 780–786. [DOI] [PubMed] [Google Scholar]