Summary
Background:
Nonalcoholic fatty liver disease (NAFLD) is associated with an increased risk of cardiovascular disease. It is not well understood, however, which individuals with NAFLD are at highest risk for cardiovascular disease.
Aims:
Our aim was to determine the factors associated with incident cardiovascular events in a prospective cohort of individuals with biopsy-proven NAFLD without pre-existing cardiovascular disease.
Methods:
From 2011–2018, adults with biopsy-proven NAFLD without cardiovascular disease were enrolled in a tissue repository and were followed prospectively to the first recorded date of incident cardiovascular disease, death, or the end of follow-up (11/1/2018). Competing risks analysis was performed to identify predictors of incident cardiovascular disease.
Results:
After a median follow-up time of 5.2 years, 26/285 (9.1%) individuals experienced an incident cardiovascular event. Advanced fibrosis (stage 3–4) on biopsy was a significant predictor of incident cardiovascular disease, and this persisted on multivariable analysis after considering relevant covariates, including cardiovascular risk scores, which were not independent predictors. Of the non-invasive indicators of fibrosis, the NAFLD fibrosis score was the only independent predictor of cardiovascular disease. Other histologic features, including steatohepatitis, were not associated with incident cardiovascular disease.
Conclusions:
In adults with biopsy-proven NAFLD, advanced fibrosis on biopsy and higher NAFLD fibrosis score were significant and independent predictors of incident cardiovascular disease, even after considering traditional risk factors and cardiovascular risk scores. These findings should be considered when evaluating NAFLD patients for primary prevention of cardiovascular disease, and further evaluation into the link between advanced fibrosis and cardiovascular disease is needed.
Keywords: atherosclerosis, cardiovascular risk, hepatic fibrosis, non-alcoholic steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in the world, affecting an estimated 25% of the population, and its prevalence continues to grow.1 NAFLD is characterized by the accumulation of fat in the liver in the absence of excessive alcohol intake, and it encompasses a spectrum ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) to liver fibrosis and cirrhosis.
Beyond its hepatic manifestations, NAFLD is closely linked with cardiovascular disease (CVD), which confers significant morbidity and mortality in this population. More individuals with NAFLD die from CVD than from liver-related complications, and NAFLD has been identified as an independent risk factor for CVD.2–5 It is therefore important to identify and modify the cardiovascular risk in these patients; however, it is not well understood which patients with NAFLD are at highest risk for cardiovascular events or whether cardiovascular risk varies across the spectrum of NAFLD.
Cardiovascular risk stratification has important clinical implications, including the decision to implement primary prevention with statins and other agents. In the general population, risk scores such as the Framingham Risk Score (FRS) or the Atherosclerotic Cardiovascular Disease (ASCVD) Pooled Cohort Equations Risk Score help guide these decisions.6, 7 The FRS for Coronary Heart Disease was validated for use in NAFLD patients in one study, though this study lacked liver histology, thus it remains unknown whether NAFLD-specific features such as the presence of steatohepatitis or fibrosis influence cardiovascular risk.8
Existing data regarding the impact of NAFLD severity on cardiovascular risk is both conflicting and limited. A recent meta-analysis found that more severe NAFLD was associated with a higher risk of CVD, but only one of the included studies used histologic data.5 Few studies examining the link between NAFLD and CVD have been performed in biopsy-characterized cohorts. Some of these have suggested that only histologic evidence of NASH confers increased cardiovascular mortality, while others have not demonstrated this relationship.9–12 Other studies, like the one in the aforementioned meta-analysis, have identified fibrosis as the best predictor of long-term survival, with increased cardiovascular mortality in patients with advanced fibrosis.2, 3, 5 Yet, data on the relationship between fibrosis and CVD has also been inconsistent, and many of these prior studies were limited by either a retrospective design, a small number with advanced fibrosis, or inclusion of individuals with pre-existing CVD.13, 14 Furthermore, nearly all of these studies assessed cardiovascular mortality; few evaluated the development of incident fatal and non-fatal CVD as an outcome.
Therefore, to better understand the determinants of cardiovascular risk in patients with NAFLD, we conducted this study of a prospective, biopsy-characterized cohort of individuals with NAFLD without pre-existing CVD and with long-term clinical follow-up. We aimed to identify predictors of incident CVD and to examine whether histologic or clinical features are NAFLD-specific risk factors for incident cardiovascular events.
Materials and Methods
Study Population and Data Collection
From 2011–2018, adults with biopsy-proven NAFLD were enrolled prospectively in the Massachusetts General Hospital NAFLD tissue repository. Individuals with decompensated cirrhosis, prior bariatric surgery, pre-existing CVD or a cardiovascular event within six months of enrollment, or less than six months of follow-up time were excluded from this study (Figure S1). Liver biopsies were performed for standard clinical indications and were interpreted by experienced hepatopathologists. All subjects had histologically-confirmed NAFLD defined by validated histologic criteria, and the modified Brunt system was used for fibrosis staging.15 Baseline demographic, clinical, and laboratory data was collected. Non-invasive fibrosis scores, including the NAFLD fibrosis score (NFS), fibrosis-4 (FIB-4) index, aspartate aminotransferase (AST) to platelet ratio index (APRI), AST/alanine aminotransferase (ALT) ratio, and Model for End Stage Liver Disease incorporating sodium (MELD-Na) were computed from baseline laboratory data and calculated according to their published algorithms (Supplement).16–20 The NFS, FIB-4, and APRI were also categorized according to established cut-offs for the presence or absence of significant fibrosis: NFS >0.676 and <−1.455, FIB-4 >2.67 and <1.3, and APRI >0.7.20–22 Advanced fibrosis was defined as stage three or four fibrosis on biopsy.
Baseline cardiovascular risk scores were also calculated. The Framingham General Cardiovascular Risk Score estimates 10-year rates of cardiovascular events, including coronary heart disease, cerebrovascular disease, peripheral vascular disease, and congestive heart failure, to quantify risk in patients free of CVD.6 The ASCVD risk score similarly estimates 10-year primary cardiovascular risk, though the events are limited to nonfatal myocardial infarction, coronary heart disease death, and stroke.7 Both the baseline FRS and ASCVD risk scores were computed (Supplement).6, 7 For patients outside of the age range of the risk scores (ages 20–79 and 40–79, respectively), the lower or upper age limit was substituted accordingly. An FRS ≥10% or an ASCVD risk score ≥7.5% was considered increased risk for CVD.6, 7
Follow-up and Outcomes
Patients were followed prospectively from enrollment to the first recorded date of incident CVD, liver transplant, bariatric surgery, death, or the end of follow-up (11/1/2018), whichever came first. The primary outcome was incident CVD, which was defined as: a new diagnosis of coronary artery disease, congestive heart failure, peripheral vascular disease, stroke, transient ischemic attack, or a major adverse cardiac event, which included myocardial infarction, coronary revascularization, or cardiac-related death. These diagnoses were made according to clinical criteria, and the outcomes were identified through physician-led review of the electronic medical record.
Statistical Analysis
Descriptive statistics were calculated for the demographic and clinical characteristics. Comparisons were performed using chi-square tests or Fisher exact tests for categorical variables and t-tests or Wilcoxon rank-sum tests for continuous variables, depending on the normality of the distribution. Competing risks analysis using the Fine and Gray model was performed to identify independent predictors of incident CVD, with non-CVD death treated as a competing outcome. Single predictor and multivariable subhazard ratios were estimated by the cumulative incidence function. Covariates considered included demographics, comorbidities, laboratory values, histologic characteristics, non-invasive fibrosis scores, and cardiovascular risk scores. The indicators of fibrosis (i.e. fibrosis stage and non-invasive scores) were considered in separate models, as were the cardiovascular risk scores. Predictors with p<0.10 in univariable analyses were entered and retained in the multivariable models at a p<0.05 level of significance using a backward selection process. Collinearity was tested for among all included variables. To specifically evaluate the relationship between advanced fibrosis, cardiovascular risk scores, and incident CVD, a separate set of models was built to adjust each indicator of fibrosis for cardiovascular risk scores.
Data was missing in less than 5% of cases, with the exception of cholesterol levels, which were missing in 8.4% (24/285) and international normalized ratio (INR), which was missing in 7.0% (20/285). Missing data was imputed from the median value. All analyses were performed using SAS® Studio software, Version 3.71 (SAS Institute, Cary, NC) and a p-value less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 296 patients with biopsy-proven NAFLD and without CVD were enrolled and followed prospectively. Of these, 285 had at least six months of follow-up as of 11/1/2018 and were included in the final cohort (Figure S1). Baseline demographic and clinical characteristics are shown in Table 1. The cohort was predominantly female (56.5%), white (73.3%), and never-smokers (63.5%). The median age was 52 years and most were middle-aged, though ages ranged from 18 to 81. The majority had hypertension (58.6%) and dyslipidemia (52.6%), while a smaller subset had diabetes (30.9%), most of whom were non-insulin-dependent (72.7%). One-third were on a statin or taking aspirin. On liver biopsy, 82.5% had findings consistent with steatohepatitis and 69.8% had fibrosis, with nearly 20% with advanced fibrosis (stage 3–4). The overall follow-up of the cohort was a median of 5.2 years (interquartile range [IQR] 2.4–6.8 years; range 0.5–8.7 years).
Table 1.
Baseline demographic and clinical characteristics.
| Cardiovascular event (n=26) |
No event (n=259) |
p-value | |
|---|---|---|---|
| Age, years | 59 (48–67) | 52 (42–60) | 0.01 |
| Female sex | 65.4% (17) | 55.6% (144) | 0.34 |
| Race/ethnicity | 0.25 | ||
| Hispanic | 3.8% (1) | 16.2% (42) | |
| Non-Hispanic white | 88.5% (23) | 71.8% (186) | |
| Non-Hispanic black | 3.8% (1) | 1.5% (4) | |
| Asian | 3.8% (1) | 7.0% (18) | |
| Other/not specified | 0 | 3.5% (9) | |
| Smoking status | 0.05 | ||
| Current | 19.2% (5) | 9.3% (24) | |
| Former | 38.5% (10) | 25.1% (65) | |
| Never | 42.3% (11) | 65.6% (170) | |
| BMI, kg/m2 | 32.8 (27.9–38.4) | 31.7 (28.3–36.3) | 0.57 |
| Comorbidities | |||
| Hypertension | 76.9% (20) | 56.8% (147) | 0.047 |
| Dyslipidemia | 53.8% (14) | 52.5% (136) | 0.90 |
| Diabetes | 50.0% (13) | 29.0% (75) | 0.03 |
| Medications | |||
| Aspirin | 38.5% (10) | 27.8% (72) | 0.25 |
| Statin | 34.6% (9) | 33.6% (87) | 0.92 |
| Oral antidiabetic medication | 26.9% (7) | 25.1% (65) | 0.84 |
| Insulin | 15.4% (4) | 7.7% (20) | 0.25 |
| Anti-hypertensive | 69.2% (18) | 50.6% (131) | 0.07 |
| Laboratory values | |||
| AST, U/L | 31 (22–46) | 38 (27–53) | 0.048 |
| ALT, U/L | 30 (21–42) | 49 (30–75) | 0.01 |
| Alkaline phosphatase, U/L | 90 (69–105) | 82 (64–101) | 0.31 |
| Total bilirubin, mg/dL | 0.6 (0.4–0.7) | 0.5 (0.3–0.7) | 0.22 |
| Platelets, x109/L | 224 (183–284) | 228 (192–277) | 0.40 |
| Creatinine, mg/dL | 0.9 (0.8–1.0) | 0.8 (0.7–1.0) | 0.17 |
| INR | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.49 |
| Albumin, g/dL | 4.4 (4.1–4.5) | 4.5 (4.3–4.7) | 0.02 |
| Sodium, mEq/L | 140 (138–141) | 140 (138–141) | 0.84 |
| Total cholesterol, mg/dL | 170 (152–191) | 186 (159–213) | 0.04 |
| HDL cholesterol, mg/dL | 46 (37–52) | 43 (37–51) | 0.28 |
| Non-HDL cholesterol, mg/dL | 106 (83–132) | 138 (115–167) | 0.01 |
| LDL cholesterol, mg/dL | 89 (78–111) | 106 (88–131) | 0.02 |
| Triglycerides, mg/dL | 140 (112–228) | 141 (106–193) | 0.89 |
| Histologic characteristics | |||
| Steatohepatitis | 84.6% (22) | 82.2% (213) | 0.76 |
| Ballooning present | 80.8% (21) | 75.3% (195) | 0.53 |
| Lobular inflammation | 84.6% (22) | 81.1% (210) | 0.66 |
| Fibrosis stage: | 0.01 | ||
| 0 | 34.6% (9) | 29.7% (77) | |
| 1 | 7.7% (2) | 34.4% (89) | |
| 2 | 15.4% (4) | 18.5% (48) | |
| 3 | 23.1% (6) | 9.3% (24) | |
| 4 | 19.2% (5) | 8.1% (21) | |
| Advanced fibrosis (stage 3–4) | 42.3% (11) | 17.4% (45) | 0.01 |
| Risk scores | |||
| NFS | −1.06 (−1.43, 0.80) | −1.70 (−2.61, −0.24) | 0.01 |
| >0.676 | 26.9% (7) | 9.3% (24) | 0.01 |
| <−1.455 | 23.1% (6) | 53.7% (139) | 0.01 |
| FIB-4 | 1.43 (0.88–2.46) | 1.23 (0.83–1.90) | 0.14 |
| >2.67 | 19.2% (5) | 10.5% (30) | 0.34 |
| <1.3 | 42.3% (11) | 55.6% (144) | 0.19 |
| APRI | 0.35 (0.25–0.58) | 0.43 (0.28–0.64) | 0.30 |
| >0.7 | 15.4% (4) | 20.8% (54) | 0.51 |
| AST/ALT ratio | 0.99 (0.82–1.16) | 0.80 (0.65–1.04) | 0.01 |
| MELD-Na score | 8.6 (7.5–9.5) | 7.5 (6.4–9.4) | 0.09 |
| FRS (%) | 13.5 (7.3–21.5) | 8.6 (3.9–15.9) | 0.06 |
| High risk (≥10%) | 25.0% (14) | 22.3% (51) | 0.66 |
| ASCVD risk score (%) | 10.8 (4.2–17.9) | 4.7 (1.7–8.4) | 0.01 |
| High risk (≥7.5%) | 53.6% (30) | 27.1% (62) | 0.01 |
Data expressed as % (n) or median (interquartile range). Abbreviations: ALT, alanine aminotransferase; APRI, AST to platelet ratio index; ASCVD, atherosclerotic cardiovascular disease; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, fibrosis-4; FRS, Framingham risk score; HDL, high-density lipoprotein; INR, international normalized ratio; LDL, low-density lipoprotein; MELD-Na, Model for End-Stage Liver Disease incorporating sodium; NAFLD, non-alcoholic fatty disease; NFS, NAFLD fibrosis score.
Incident Cardiovascular Events
Among the 285 included subjects, 26 (9.1%) experienced an incident cardiovascular event after a median 3.8 years (IQR 1.9–5.0 years; range 0.5–6.9 years): 18 were diagnosed with new coronary artery disease, three of whom had unstable angina or a myocardial infarction requiring revascularization, three developed congestive heart failure (one of whom was concurrently found to have coronary artery disease), five had ischemic strokes, and one had a transient ischemic attack. There were 10 deaths: four due to malignancy (two hepatic and two extra-hepatic), three due to septic shock, one due to respiratory failure, and two without an identified cause. One patient received a liver transplant and eight underwent bariatric surgery.
Patients who experienced an incident cardiovascular event were more likely to be older, have hypertension, diabetes, lower albumin, lower total, non-high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol, as well as lower ALT and AST levels (Table 1). They also had more advanced fibrosis on biopsy and higher NFS, while their MELD-Na scores, FIB-4, and APRI were similar (Table 1). Other biopsy features, including the proportion with steatohepatitis, were similar. While the FRS were similar, the ASCVD risk scores were higher in the incident CVD group, and a significantly greater proportion was considered high-risk. Similar proportions were taking a statin or aspirin.
Predictors of Incident CVD
Significant univariable predictors of incident CVD included age, diabetes, lower ALT levels, higher alkaline phosphatase, higher total bilirubin, lower albumin, lower platelets, lower total, LDL, and non-HDL cholesterol, higher FRS, higher ASCVD risk score, and advanced fibrosis on biopsy (Table 2). Other baseline characteristics were not significantly associated with incident cardiovascular events. In multivariable models considering the covariates in Table 2 (including only one of the cholesterol levels and one cardiovascular risk score), advanced fibrosis, smoking, lower ALT, and lower albumin levels were significantly associated with the development of incident CVD. Neither the FRS nor ASCVD risk score was an independent predictor of incident CVD.
Table 2.
Predictors of incident CVD.
| Univariable | Multivariable | ||
|---|---|---|---|
| Covariate | SHR (95% CI) | p-value | SHR (95% CI) |
| Age, years | 1.05 (1.02–1.08) | 0.01 | |
| Current smoking | 2.22 (0.97–5.11) | 0.06 | 2.40 (1.00–5.73) |
| Diabetes | 2.45 (1.25–4.81) | 0.01 | |
| ALT | 0.97 (0.96–0.99) | 0.01 | 0.98 (0.96–1.00) |
| Alkaline phosphatase | 1.00 (1.00–1.01) | 0.01 | |
| Total bilirubin | 1.76 (1.21–2.55) | 0.01 | |
| Platelets | 0.99 (0.99–1.00) | 0.01 | |
| Albumin | 0.16 (0.08–0.34) | 0.01 | 0.36 (0.16–0.82) |
| Total cholesterol | 0.98 (0.98–1.00) | 0.01 | |
| LDL cholesterol | 0.98 (0.97–0.99) | 0.01 | |
| Non-HDL cholesterol | 0.98 (0.97–0.99) | 0.01 | |
| Advanced fibrosis | 4.48 (2.29–8.79) | 0.01 | 2.86 (1.36–6.04) |
| FRS | 1.04 (1.01–1.08) | 0.02 | |
| ASCVD risk score | 1.05 (1.02–1.08) | 0.01 | |
Covariates shown are predictors with p<0.10 in univariable analyses considered in multivariable models. Total, LDL, and non-HDL cholesterol were collinear and considered separately, though none was significant. Cardiovascular risk scores were also considered in separate models, and neither was significant. Abbreviations: ALT, alanine aminotransferase; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CVD, cardiovascular disease; FRS, Framingham risk score; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty disease; SHR, subhazard ratio.
Of the non-invasive indicators of fibrosis, the NFS, FIB-4, MELD-Na, and AST/ALT ratio were significant predictors of incident CVD in univariable analyses (Table 3). In a multivariable model replacing advanced fibrosis and excluding its component variables (age, BMI, diabetes, AST, ALT, and albumin), the NFS was a significant predictor of CVD (subhazard ratio [SHR] 1.44, 95% confidence interval [CI] 1.22–1.71), along with lower non-HDL cholesterol (SHR 0.99, 95% CI 0.98–1.00) and smoking (SHR 2.39, 95% CI 1.00–5.70). A NFS >0.676 was a significant predictor in a similar model (SHR 3.62, 95% CI 1.73–7.60), along with lower non-HDL cholesterol (SHR 0.99, 95% CI 0.98–1.00) and smoking (SHR 3.08, 95% CI 1.30–7.29), as was a NFS <−1.455 (NFS: SHR 0.22, 95% CI 0.09–0.55; non-HDL: SHR 0.98, 95% CI 0.98–1.00; smoking: SHR 2.40, 95% CI 1.03–5.60). The FIB-4, AST/ALT ratio, and MELD-Na were no longer significant in multivariable models excluding their component variables, and APRI was not a significant predictor of CVD (Table 3).
Table 3.
Non-invasive fibrosis scores and incident CVD.
| Covariate | Univariable SHR (95% CI) | p-value |
|---|---|---|
| NFS | 1.55 (1.32–1.83) | 0.01 |
| >0.676 | 4.61 (2.28–9.32) | 0.01 |
| <−1.455 | 0.18 (0.07–0.43) | 0.01 |
| FIB-4 | 1.33 (1.20–1.48) | 0.01 |
| >2.67 | 2.63 (1.23–5.64) | 0.01 |
| <1.3 | 0.42 (0.21–0.85) | 0.02 |
| APRI | 1.57 (0.95–2.59) | 0.08 |
| >0.7 | 1.31 (0.59–2.90) | 0.51 |
| AST/ALT ratio | 3.15 (1.85–5.39) | 0.01 |
| MELD-Na | 1.09 (1.02–1.16) | 0.01 |
Abbreviations: APRI, AST to platelet ratio index; AST, aspartate aminotransferase; CI, confidence interval; CVD, cardiovascular disease; FIB-4, fibrosis-4 index; MELD-Na, Model for End-Stage Liver Disease incorporating sodium; NFS, NAFLD fibrosis score; SHR, subhazard ratio.
Advanced Fibrosis, Cardiovascular Risk Scores, and Incident CVD
Curves depicting the cumulative incidence of CVD by advanced fibrosis status are shown in Figure 1. When adjusted for the cardiovascular risk scores, advanced fibrosis and the NFS remained significant predictors of incident CVD (Table 4). This was also true when the FRS and ASCVD risk scores were categorized as high risk (≥10% and ≥7.5%, respectively).
Figure 1.
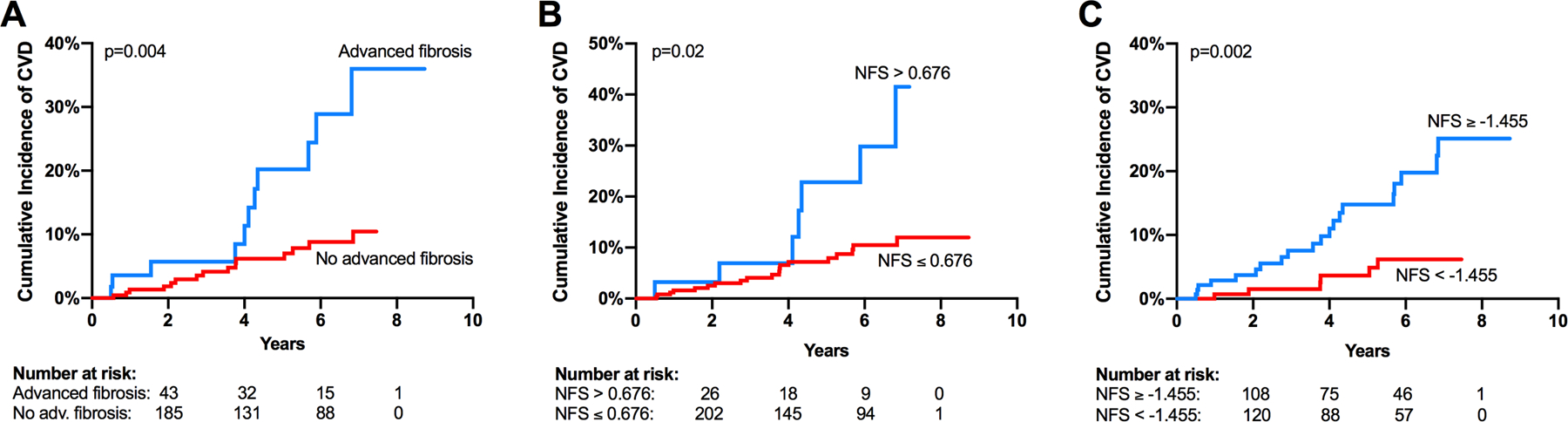
Cumulative incidence curves for incident CVD by advanced fibrosis status (A), NFS greater than or less than or equal to 0.676 (B), and NFS greater than or equal to or less than −1.455 (C). Abbreviations: CVD, cardiovascular disease; NFS, NAFLD fibrosis score.
Table 4.
Advanced fibrosis and NAFLD fibrosis score adjusted for cardiovascular risk scores.
| Advanced fibrosis SHR (95% CI) |
NFS SHR (95% CI) |
NFS >0.676 SHR (95% CI) |
NFS <−1.455 SHR (95% CI) |
|
|---|---|---|---|---|
| Univariable | 4.48 (2.29–8.79) | 1.55 (1.32–1.83) | 4.61 (2.28–9.32) | 0.18 (0.07–0.43) |
| + FRS | 4.02 (2.03–7.98) | 1.54 (1.29–1.83) | 3.91 (1.87–8.20) | 0.19 (0.08–0.49) |
| + High-risk FRS | 4.40 (2.24–8.62) | 1.55 (1.32–1.83) | 4.50 (2.22–9.10) | 0.18 (0.07–0.44) |
| + ASCVD | 3.57 (1.74–7.33) | 1.50 (1.25–1.80) | 3.38 (1.52–7.52) | 0.22 (0.09–0.57) |
| + High-risk ASCVD | 3.25 (1.60–6.60) | 1.45 (1.20–1.74) | 3.16 (1.50–6.62) | 0.26 (0.10–0.67) |
Shown are the hazard ratios for incident CVD in univariable analyses and adjusted for each cardiovascular risk score. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; CVD, cardiovascular disease; FRS, Framingham risk score; HR, hazard ratio; NAFLD, non-alcoholic fatty disease; NFS, NAFLD fibrosis score; SHR, subhazard ratio.
Overall, 19.6% (11/56) with advanced fibrosis on biopsy experienced an incident cardiovascular event compared to 6.6% (15/229) without advanced fibrosis (p=0.01), and the attributable risk of advanced fibrosis for CVD was 66.6%. The patients with advanced fibrosis were older, more often had diabetes, and were more frequently taking antihypertensives (Table S1). They also had higher FRS and ASCVD risk scores, though total, non-HDL, and LDL cholesterol levels were lower. Fewer than one-third were on a statin, and this proportion was similar compared to the non-advanced fibrosis group.
Discussion
Cardiovascular disease is the most common cause of death in patients with NAFLD, but which patients with NAFLD are at highest risk for cardiovascular events is not well understood. In this study, we used a prospective, biopsy-characterized cohort of patients with NAFLD without pre-existing CVD and demonstrated that advanced fibrosis is an independent predictor of incident CVD, accounting for two-thirds of the attributable risk for CVD. Importantly, while many traditional cardiovascular risk factors, including age, diabetes, smoking, and cardiovascular risk scores were significant univariable predictors, they did not attenuate the relationship between advanced liver fibrosis and incident CVD. Thus, our findings build upon prior clinical data indicating that more severe fibrosis is a risk factor for CVD mortality in NAFLD and extend this association to nonfatal, incident CVD.2, 3, 5
The relationship between advanced fibrosis and CVD suggests that the same processes driving progressive NAFLD may also fuel the development of CVD. Multiple potential mechanisms for the link between NAFLD and CVD have been identified, and one model proposes two pathways: one by which cardiovascular events occur via traditional risk factors and another through a more direct linkage, including endothelial dysfunction, systemic inflammation, altered lipid metabolism, insulin resistance, oxidative stress, fetuin-A, and a prothrombotic state, which likely contribute in a complex, interrelated manner.23, 24, 25 The lack of a relationship between steatohepatitis and CVD in our cohort might suggest that drivers or products of fibrogenesis may play a more important role in promoting CVD; however, it is also possible that the cardiovascular consequences of systemic inflammation do not clinically manifest until it has existed long enough to promote the development of advanced fibrosis. In our cohort, the lower albumin and lipid levels as predictors for CVD could support an important role for chronic inflammation. Further studies should be performed to elucidate the mechanistic link between NAFLD with advanced fibrosis and CVD.
Interestingly, a recent study of a cohort with advanced NAFLD identified a higher incidence of cardiovascular events in individuals with stage three fibrosis compared to stage four.26 The authors of this study attributed this finding to lower blood pressure, weight, and cholesterol levels in individuals with stage four fibrosis vs. stage three. Yet, in our cohort, the proportions who developed incident CVD with stages three and four fibrosis were similar (6/30 and 5/26, respectively), and lower cholesterol levels was a significant predictor of CVD. While this study was larger than ours (n=458), it had very few vascular events (14/458 or 3%), which makes these findings somewhat difficult to interpret. Future studies are needed to further investigate whether cardiovascular risk varies among individuals with advanced fibrosis, as this would have important implications for risk stratification.
Of the noninvasive fibrosis scores, the NFS was the only independent predictor of CVD. This finding is consistent with studies demonstrating a relationship between NFS and CVD mortality, and it suggests that the NFS may be a useful indicator of cardiovascular risk.4, 27 Interestingly, advanced fibrosis on biopsy and a high NFS >0.676 did not correlate well in this cohort (less than 50%), so it is possible that these represent slightly different populations. Studies in other populations should be performed to further explore the utility of the NFS as a predictor of CVD, whether this correlates with underlying advanced fibrosis, and, if so, to identify the optimal threshold of NAFLD severity at which further evaluation of cardiovascular risk may be indicated.
Our finding of a significant, independent association between advanced fibrosis and incident CVD has important clinical implications. It persisted despite adjustment for cardiovascular risk scores, suggesting that fibrosis severity, whether determined histologically or through the NFS, may add further prognostic value to cardiovascular risk scores in individuals with NAFLD and should be considered when assessing cardiovascular risk. A similar proportion of patients with advanced fibrosis were taking a statin compared to those without advanced fibrosis, despite more traditional risk factors. This may be due to the historical concern about potential hepatotoxicity of statins, though they have been repeatedly observed to be safe.28 In addition, cholesterol levels were previously an important aspect of the decision to initiate statins, and perhaps because of the lower cholesterol levels in those with advanced fibrosis, statins were not believed to be indicated. Applying the current guidelines retrospectively, a statin was indicated in 70% of individuals with advanced fibrosis, while only 29% were prescribed one, consistent with prior studies demonstrating statin underutilization in patients with NAFLD.29, 30 Ensuring appropriate statin use in patients with NAFLD is important as CVD is the most common cause of death in this population, and the role of statins in reducing the risk of CVD is well-established.31
Beyond advanced fibrosis and the NFS, several other non-traditional CVD risk factors were identified, including lower total, non-HDL, and LDL cholesterol levels. While this is contrary to the established relationship between lipids and CVD, the lower cholesterol levels more likely reflect underlying advanced liver disease and a chronic inflammatory state as opposed to a protective effect. This inverse relationship between LDL level and CVD risk was also observed in a previous study, though it was attributed to statin use.32 In our cohort, however, the lower lipid levels did not appear to be related to statins: they were similar among patients when stratified by statin use, and those with advanced fibrosis had significantly lower total cholesterol, non-HDL, and LDL levels despite a similar proportion taking statins.
This finding suggests that plasma cholesterol levels are not the only CVD risk factor useful in determining whether to initiate primary prevention in patients with NAFLD. While recent cardiovascular guidelines have shifted away from cholesterol goals and toward the use of risk calculators, these algorithms, including the FRS and ASCVD risk score, still incorporate cholesterol levels. Thus, it is possible that these risk scores may underestimate cardiovascular risk in patients with advanced NAFLD. This study was not designed to validate the utility of either cardiovascular risk score within the context of NAFLD, and, while our outcomes were the same as those predicted by the FRS, they were broader than the ASCVD risk score. With those caveats in mind, our results do suggest possible underestimation of cardiovascular risk among those with advanced fibrosis: specifically, our observed event rate of 19.6% after a median follow-up of less than 5 years is nearly double that which is predicted at 10 years by the FRS and ASCVD (11.7% and 8.5%, respectively). While exploratory, these findings nonetheless support further studies to define and validate NAFLD-specific cardiovascular risk scores, particularly in those with advanced fibrosis.
Our study has several strengths. First, it is one of only very few to examine predictors of incident CVD in a well-characterized prospective cohort of patients with biopsy-proven NAFLD and without pre-existing CVD. In addition, we employed physician-led chart review to identify hard clinical outcomes as opposed to relying on ICD codes or administrative registries, which are subject to misclassification. This allowed us to evaluate and confirm all cases of incident fatal and non-fatal CVD, whereas nearly all prior studies have been limited to CVD mortality. Our cohort also encompassed patients across the disease spectrum, with a large proportion (nearly 20%) with advanced fibrosis, and the majority had at least 5 years of follow-up. Limitations include the sample size, though our cohort is one of the largest to date to leverage a highly phenotyped population with biopsy-proven NAFLD and confirmed endpoints, and that our population consisted of patients at a single tertiary care center who had liver biopsies, which may confer selection bias.
In summary, in this prospective cohort of individuals with biopsy-proven NAFLD, advanced fibrosis and higher NFS were associated with an increased risk of incident CVD, even after considering traditional risk factors, including cardiovascular risk scores. Of the traditional risk factors, active smoking was strongest predictor, highlighting the importance of screening for tobacco use and counseling on cessation. A relatively low proportion of the cohort was on a statin for primary prevention of CVD, despite a high burden of CVD risk factors. Appropriate statin utilization should be emphasized in patients with NAFLD in order to reduce the high morbidity and mortality of CVD; moreover, lower lipid levels should not be taken as an indicator of lower CVD risk, as this may signify advanced fibrosis and an increased risk. Current cardiovascular risk scores should be validated in patients with NAFLD, especially those with advanced fibrosis, and efforts to develop NAFLD-specific cardiovascular risk scores should be undertaken. As CVD confers significant morbidity and mortality in the NAFLD population, hepatologists should carefully evaluate these patients for cardiovascular risk and employ efforts to reduce this risk, whether by initiating primary prevention, counseling on tobacco cessation, or other methods of risk reduction.
Supplementary Material
Acknowledgments:
All authors approved the final version of the article, including authorship list.
Grants/financial support: TGS is supported by NIH K23 DK122104 and by the American Association for the Study of Liver Diseases (AASLD) Clinical and Translational Research Award.
Abbreviations:
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase to platelet ratio
- ASCVD
atherosclerotic cardiovascular disease
- AST
aspartate aminotransferase
- CI
confidence interval
- CVD
cardiovascular disease
- FIB-4
fibrosis-4 index
- FRS
Framingham risk score
- HDL
high-density lipoprotein
- INR
international normalized ratio
- IQR
interquartile range
- LDL
low-density lipoprotein
- MELD
Model for End-stage Liver Disease
- MELD
Na, Model for End-stage Liver Disease incorporating Sodium
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NFS
non-alcoholic fatty liver disease fibrosis score
- SHR
subhazard ratio
Footnotes
Conflicts of interest: none.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–54. [DOI] [PubMed] [Google Scholar]
- 4.Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. Journal of Hepatology. 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- 6.D’Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 8.Treeprasertsuk S, Leverage S, Adams LA, et al. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32(6):945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domanski JP, Park SJ, Harrison SA. Cardiovascular disease and nonalcoholic fatty liver disease: does histologic severity matter? J Clin Gastroenterol. 2012;46(5):427–30. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. [DOI] [PubMed] [Google Scholar]
- 11.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–49. [DOI] [PubMed] [Google Scholar]
- 12.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51(2):595–602. [DOI] [PubMed] [Google Scholar]
- 13.Hagstrom H, Nasr P, Ekstedt M, et al. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39(1):197–204. [DOI] [PubMed] [Google Scholar]
- 14.Abeles RD, Mullish BH, Forlano R, et al. Derivation and validation of a cardiovascular risk score for prediction of major acute cardiovascular events in non-alcoholic fatty liver disease; the importance of an elevated mean platelet volume. Aliment Pharmacol Ther. 2019;49(8):1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 16.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95(3):734–9. [DOI] [PubMed] [Google Scholar]
- 17.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. [DOI] [PubMed] [Google Scholar]
- 19.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. [DOI] [PubMed] [Google Scholar]
- 20.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. [DOI] [PubMed] [Google Scholar]
- 21.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–36. [DOI] [PubMed] [Google Scholar]
- 22.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65(2):425–43. [DOI] [PubMed] [Google Scholar]
- 24.Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. [DOI] [PubMed] [Google Scholar]
- 25.Stahl EP, Dhindsa DS, Lee SK, et al. J Am Coll Cardiol. 2019;73(8):948–963. [DOI] [PubMed] [Google Scholar]
- 26.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 2018;155(2):443–57. [DOI] [PubMed] [Google Scholar]
- 27.Simon TG, Corey KE, Cannon CP, et al. The nonalcoholic fatty liver disease (NAFLD) fibrosis score, cardiovascular risk stratification and a strategy for secondary prevention with ezetimibe. Int J Cardiol. 2018;270:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bader T The myth of statin-induced hepatotoxicity. Am J Gastroenterol. 2010;105(5):978–80. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blais P, Lin M, Kramer JR, et al. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci. 2016;61(6):1714–20. [DOI] [PubMed] [Google Scholar]
- 31.Dongiovanni P, Petta S, Mannisto V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705–12. [DOI] [PubMed] [Google Scholar]
- 32.Corey KE, Kartoun U, Zheng H, et al. Using an electronic medical records database to identify non-traditional cardiovascular risk factors in nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111(5):671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


