Abstract
Background:
Adolescent alcohol abuse can lead to behavioral dysfunction and chronic, relapsing alcohol use disorder (AUD) in adulthood. However, not all adolescents that consume alcohol will develop an AUD, therefore it is critical to identify neural and environmental risk factors that contribute to increases in susceptibility to AUDs following adolescent alcohol (ethanol) exposure. We previously found that adolescent anesthetic exposure led to strikingly similar behavioral and neural effects as adolescent alcohol exposure. Therefore, we tested the hypothesis that general anesthetic exposure during early adolescence would alter ethanol responses consistent with an exacerbation of the adolescent alcohol phenotype.
Methods:
To test this hypothesis, early adolescent male Sprague-Dawley rats were exposed for a short duration to the general anesthetic isoflurane and tested on multiple ethanol-induced behaviors in mid-late adolescence or adulthood.
Results:
Adolescent rats exposed to isoflurane exhibited decreases in sensitivity to negative properties of ethanol such as its aversive, hypnotic, and socially suppressive effects, as well as increases in voluntary ethanol intake and cognitive impairment. Select behaviors were noted to persist into adulthood following adolescent isoflurane exposure. Similar exposure in adults had no effects on ethanol sensitivity.
Conclusions:
This study demonstrates for the first time that early adolescent isoflurane exposure alters ethanol sensitivity in a manner consistent with an exacerbation of adolescent-typical alcohol responding. These findings suggest that general anesthetic exposure during adolescence may be an environmental risk factor contributing to an enhanced susceptibility to developing AUDs in an already vulnerable population.
Keywords: Adolescence, Alcohol, Anesthesia, Isoflurane, Memory
Introduction
Adolescent alcohol abuse is highly prevalent in the United States, with 24% of underage adolescents reporting binge drinking within the past two weeks (Windle, 2016) and ~15% demonstrating high-intensity binge drinking, defined as use of at least 10-15 drinks per occasion (Patrick et al., 2013). Even more concerning is that individuals who engage in binge-drinking early in adolescence are at significantly greater risk of developing a chronic, relapsing alcohol use disorder (AUD) at some point in their life (Hingson et al., 2006). Excessive alcohol consumption during adolescence leads to dysfunction in the prefrontal cortex that is associated with impairments in higher order cognition, impulse control, and behavioral flexibility in adulthood (for revew: Spear, 2016, Spear, 2018). One potential contributor to high levels of drinking during adolescence is age-specific sensitivities to alcohol: adolescents are insensitive to the alcohol’s aversive cues that protect an organism from excessive intake, while simultaneously being highly sensitive to the rewarding and memory-impairing properties of alcohol compared to adults (Spear, 2000). Although the overwhelming number of individuals that initiate alcohol use and demonstrate binge patterns of alcohol consumption during adolescence is concerning, not all such users develop AUD. Therefore, it is critical to identify precursory environmental and neurobiological factors that further escalate propensity to abuse alcohol during adolescence.
Efforts persist to identify neural and affective traits associated with increased risk for adolescent alcohol use and abuse. For example, human adolescents that basally exhibit reduced levels of grey matter in the prefrontal cortex (PFC) (Cheetham et al., 2017, Cheetham et al., 2014) or lower prefrontal/limbic connectivity (Peters et al., 2017) are at an increased risk to binge-drink during adolescence. At a behavioral level, teens that exhibit reduced inhibitory control or executive function are also at a greater risk (Squeglia et al., 2017, Heinrich et al., 2016). Given the association of anxiety and impulsivity with adolescent alcohol use (Dyer et al., 2019, Smith and Cyders, 2016, Noel, 2014), prevention efforts to reduce these states have been targeted toward such individuals (Conrod et al., 2013). Limited efforts have also attempted to identify external events such as those related to stress (Peltier et al., 2019, Keyes et al., 2012, Enoch, 2011), but the contribution of other environmental factors and individual differences have been little explored.
Adolescent-typical responsiveness to alcohol is evolutionarily conserved. Rigorous preclinical research indicates that adolescent rats are more sensitive than adults to the spatial memory-impairing and appetitive properties of alcohol (ethanol), but less sensitive to ethanol-induced motor-impairing, sedative-hypnotic, and aversive effects (see Spear, 2018, for review). Related, preclinical evidence also indicates that high sociability in males and high baseline levels of anxiety in females increase the likelihood of voluntary ethanol consumption in adolescent rats (Varlinskaya et al., 2015a, Varlinskaya et al., 2015b). Further, exposure to select drugs of abuse during adolescence is an environmental risk factor that may alter ethanol responsiveness. For example, adolescent exposure to THC led to deficits in ethanol-induced working memory impairment that are not observed with ethanol alone, an effect that is developmentally specific (Swartzwelder et al., 2012). Long-lasting cross-tolerance between alcohol and other drugs of abuse, such as nicotine, is also evident in adolescent but not adult animals (Lopez et al., 2001, Boutros et al., 2016). Thus, exposure to psychoactive substances during adolescence may alter subsequent ethanol responsiveness.
Age of anesthetic exposure is an important biological variable. We recently investigated the involvement of a less-considered class of psychoactive compounds – general anesthetics, and their impact on adolescent behavior. One inhalational general anesthetic, isoflurane, is commonly used in humans and preclinical studies (Lin et al., 2017), and is considered one of the safest fluorane compounds used during surgery. We found that exposure to isoflurane during early adolescence led to behavioral alterations analogous to adolescent alcohol exposure (Landin et al., 2019). A single translationally relevant exposure of early adolescent rats to isoflurane resulted in persistent increases in impulsivity, anxiety, and memory impairment into adulthood, along with molecular alterations of truncated maturational pruning associated with increased GABAA receptor extrasynaptic inhibition (Landin et al., 2019). The consequences of adolescent anesthetic exposure are reminiscent of adolescent intermittent ethanol (AIE) exposure effects on memory, impulsivity, and anxiety (see: Spear, 2018) with related increases in sensitivity of extrasynaptic GABAA-R tonic current potentiation (Fleming et al., 2007) and immature dendritic spine density (Trantham-Davidson et al., 2017). These similarities are not surprising given convergent mechanism(s) of action between general anesthetics and ethanol (Hemmings et al., 2005, Kumar et al., 2009), and evidence of transient cross-tolerance in adults (Liang et al., 2007).
Millions of teenagers are exposed to general anesthetics each year, with this exposure occurring during a developmental period associated with high levels of alcohol use and abuse. Given our recent findings (see Landin et al. 2019), it is not clear whether adolescent exposure to general anesthetics in the short term can impact ethanol responding during adolescence, as well as have a potential long-lasting impact into adulthood. The current work examined the hypothesis that prior adolescent general anesthetic exposure would alter subsequent responses to ethanol in a manner consistent with an exacerbation of the adolescent alcohol phenotype, and thus may be a further risk factor for development of AUDs in an already vulnerable adolescent population.
Materials and Methods
Subjects
Early adolescent [postnatal day (P) 24-28] Sprague-Dawley rats were used for most experiments. All experimental rats were male. Dams were obtained from Taconic Biosciences (Rensselaer, NY/USA). For conditioned taste aversion studies, transgenic cFos-LacZ+ rats on a 97to >99% Sprague-Dawley background were used so as to bank brain tissue for future investigations. All adolescent rats were bred in-house and group- or pair-housed after weaning at P21, depending on the experiment. All rats were handled one week before testing and maintained on a standard 12h light–dark schedule with ad libitum access to rat chow and water except where indicated. Testing and sample collection occurred during the light phase. No rats were used for more than one experiment. All experiments were conducted in accordance with the National Institutes of Health Guidelines under Institutional Animal Care and Use Committee-approved protocols at the State University of New York – Binghamton.
Anesthetic Exposure
Rats were exposed to either isoflurane (Southmedic Inc., Barrie, ON/CA) or air (control) during early-adolescence (P25-28) and tested at mid adolescence (P32-35), late adolescence (P46-49), or adulthood (P75). For conditioned taste aversion, adults were exposed on P65 tested on P72. For loss of righting reflex and novel object recognition, adult rats were exposed to isoflurane at P68 and tested at P75 or P89. Control rats were brought into the surgical suite but were only exposed to ambient air. Animals exposed to anesthesia received 40 minutes of 3.0% isoflurane at a 1,000 cc/min flow rate with oxygen as the carrier gas, via VetEquip SOP-Compac 5 (Pleasanton, California, USA) in order to mitigate risk of hypoxemia during anesthetic procedures [for review: (Ehrenfeld et al., 2010)]. Other developmental general anesthetic (GA) exposure models typically utilize lengthy (> 4h) exposures, or multiple exposures [for review: (Loepke and Soriano, 2008)]. The concentration of isoflurane used is approximate to the minimum alveolar concentration in developing male rats (~ 3.2%) for this length of exposure (Lee et al., 2014) and ensures that all subjects achieved immobility during exposure, as concentrations that fail to eliminate responses to noxious stimuli are less than ideal (Antognini and Carstens, 2005).
Conditioned Taste Aversion
Following isoflurane exposure, subjects were isolate-housed and 5 days later tested for ethanol-induced conditioned taste aversion (CTA) as we have done previously (Gore-Langton and Spear, 2018). Rats were initially 50% water-restricted for 24 hours, based on intake during the 24 hours preceding the restriction period. Conditioning was subsequently conducted using a sodium chloride (0.9% NaCl) tastant. Body weights were monitored through the CTA procedure to ensure weights were within 15% of free access age-matched controls. Subjects were kept in their home cages and placed in an experimental room to acclimate for 15 minutes. The conditioning session then began during which subjects were given one hour access to a bottle containing the tastant, after which the bottle was removed and subjects were injected intraperitoneally (i.p.) with either 0 (0.9% saline), 1.5, 2.5 or 3.5 g/kg ethanol (20% v/v in saline) and returned to the colony room with ad libitum access to water. One day later, subjects were again subjected to 50% water deprivation for 24 hours, followed by a test session in which they were again separated and given one hour access to the tastant with no post-test injection. Recorded bottle weights from conditioning and test days were used to calculate baseline consumption and whether CTA (significant reduction in intake relative to conditioning day) occurred.
Loss of Righting Reflex Task
To determine the effect of prior isoflurane exposure on responses to ethanol in a sedative/hypnotic dose range, rats were exposed to isoflurane or air at P28, and separate cohorts were tested for ethanol-induced loss of righting reflex (LORR) in mid-adolescence (P35), late adolescence (P49), or adulthood (P75). On testing day, rats were injected i.p. with a hypnotic dose of ethanol (3.5g/kg, 20% v/v in saline). LORR was defined as the inability of a rat to right itself when placed on its back. Animals remained in a supine position in v-shaped troughs (90° angle, 12.5cm 25.5c m) until the righting reflex was regained – defined as righting itself three times within a 60-second period. LORR duration was calculated by subtracting LORR onset from latency to regain righting reflex. Once the righting reflex was regained, tail blood was collected for blood ethanol content (BEC) analysis (Analox Instruments, Lunenburg, MA).
Novel Object Recognition Task
To examine the impact of adolescent isoflurane exposure on subsequent ethanol-induced memory impairment, we used a novel object recognition (NOR) task as employed elsewhere (Swartzwelder et al., 2012). Rats were exposed to isoflurane or air at P28, and separate cohorts were tested in mid-adolescence (P35) or adulthood (P75). The NOR task consisted of three trials: habituation, familiarization, and recognition. During habituation, rats were placed in a locomotor chamber (AccuScan Instruments, Columbus, OH) enclosed in a cabinet with a small fan for white noise for a 5-minute period without any objects. Rats were then removed and placed back in their respective home cages for 5 minutes. Familiarization was then conducted, during which time rats spent 5 minutes with two identical objects [either two spice jars or two triangular cups] located in the far left and right corners of the locomotion chamber. Immediately following familiarization, rats were injected i.p. with either saline (0.9%) or a 1.0g/kg ethanol, returned to the colony for 24 hours, and then tested for NOR. Rats were then placed in the locomotion chamber containing one familiar object (e.g., spice jar) and one novel object (e.g., cup). Activity chamber assessments were analyzed via laser beam breaks using VersaMax software as we have done previously (Landin et al., 2019). Novel object recognition was calculated as the percent time spent with the novel object over time spent with both objects.
Social Interaction Test
In order to examine the effect of adolescent anesthetic exposure on sensitivity to the socially suppressing effects of ethanol later in life, rats were exposed to isoflurane or air at P24 and social investigation testing occurred 3 weeks later in late adolescence (P46). One day prior to testing (P45), rats were habituated to the testing apparatus for 30 minutes. The Plexiglas test apparatus (designed in-house; 30 × 20 × 20 cm) contained clean pine shavings and was divided into two equally sized compartments by a partition that contained an aperture (7 × 5 cm) to allow movement between compartments. On testing day, rats were injected with either saline or a 1.0g/kg ethanol and placed alone for a 30-minute period in the Plexiglas apparatus, and then exposed to a non-manipulated social partner (non-littermate) of the same age and sex for 10 minutes. The 10-minute test session was video recorded and social investigation was manually scored by an experimenter blind to exposure and drug conditions as in prior work [e.g., (Varlinskaya and Spear, 2009)]. Social investigation was defined as sniffing of any part of the body of the social partner.
Two-Bottle Choice Test
To examine the impact of adolescent anesthetic exposure on ethanol self-administration, rats were exposed to isoflurane or air on P28, and separate cohorts were tested in mid-adolescence (P35) or adulthood (P75) using a two-bottle choice task. The two-bottle choice test has previously been shown to reliably induce voluntary ethanol intake without the need for water deprivation in both adolescents and adults (Broadwater et al., 2013). During this task, rats are able to choose between drinking a sweetened super-saccharin (supersac) solution in one bottle (0.3% sucrose and 0.125% saccharin in water) or 10% ethanol in the same sweetened solution, in a different bottle. Rats were weighed and separated from littermates using a mesh divider in a home cage. Rats were acclimated in the testing room for 15 minutes prior to start of testing. Each of two bottles fitted with stainless steel nozzles were filled with either the ethanol or supersac solutions and weighed. Upon start of testing, the bottles were placed on the metal cage lids and secured in order to prevent movement during the session. Within each test session, bottles remained on the cages for 30 minutes and were subsequently weight after each session to determine the amount of each solution consumed (mL/kg). A total of five testing sessions, conducted every other day, were conducted. Data were averaged across all 5 days of consumption, with consumption expressed as EtOH (grams) per kilogram body weight (g/kg).
Statistical Analyses
Given that all experiments compare isoflurane exposure versus air controls, and rats were tested within separate cohorts at varying ages where known age differences in basal and/or ethanol responding are evident, data were primarily analyzed at each age using an unpaired Student’s t-test with Bonferroni correction where appropriate. For CTA studies, as data were compared as a percentage of saline controls, analyses were conducted using one-way analyses of variance within each exposure and age, and Fishers post hoc tests used for within group dose comparisons. Alpha for all studies was set at p < 0.05.
Results
Effects of adolescent and adult isoflurane exposure on sensitivity to the aversive and hypnotic effects of ethanol.
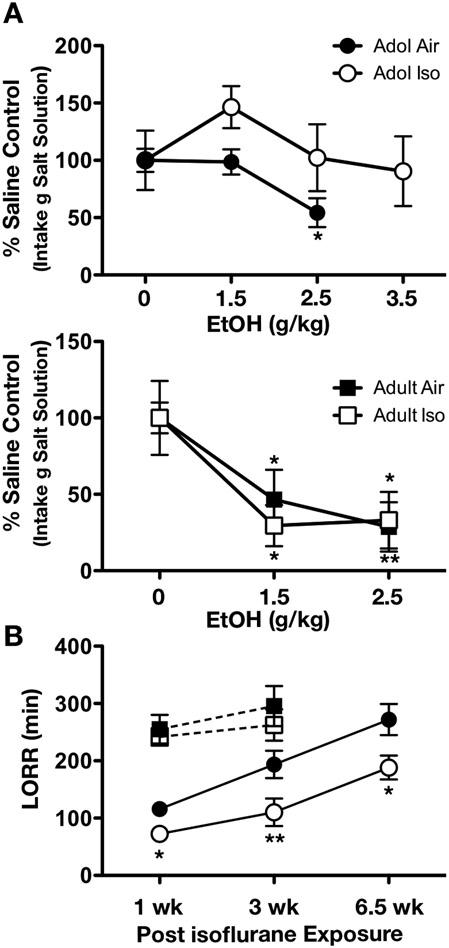
As adolescents are typically insensitive at doses that elicit aversive responding in adults, we first wanted to determine whether this negative association was altered by adolescent general anesthetic exposure. As anticipated, air-exposed adults developed CTA at doses of 1.5 and 2.5 g/kg ethanol (F2,17 = 6.705, p < 0.01, Figure 1A, second panel), whereas CTA was not evident in air-exposed adolescents (P32) until 2.5 g/kg (F2,22 = 5.408, p < 0.05) (Figure 1A, top panel). CTA was also evident in adults exposed to isoflurane at both doses (F2,20 = 4.437, p < 0.05), and adult responses mainly overlapped between isoflurane exposed and air-control subjects. Interestingly, adolescent isoflurane exposure enhanced CTA insensitivity given that these animals did not exhibit CTA at either the 2.5 g/kg or 3.5 g/kg dose of ethanol (F2,27 = 1.220, p = 0.311).
Figure 1.
Adolescent anesthetic exposure reduces aversive ethanol properties. Adolescent air controls exhibited conditioned taste aversion (CTA) at higher ethanol doses. However, isoflurane-exposed adolescents (n = 5-11/group) did not exhibit CTA at any ethanol dose tested. Adults exposed to isoflurane did not differ from air controls (n = 7-9/group), as both exhibited CTA at both ethanol doses (A). Adolescent rats exposed to isoflurane exhibited a reduction in loss of righting reflex time when tested in mid adolescence (n = 3-6/group), late adolescence (n = 4-5/group), and adulthood (n = 6-7/group), compared to air controls. Adults exposed to isoflurane did not differ in loss of righting reflex recovery time compared to controls when tested one (n = 5-7/group) or three (n = 5-6/group) weeks following exposure (B). Data are represented as mean ± SEM. * = p < 0.05, ** = p < 0.01.
We next investigated the short-term impact of early adolescent isoflurane exposure on ethanol’s hypnotic effects. When tested during adolescence, 73.3% of subjects previously exposed to isoflurane failed to lose their righting reflex following a challenge with ethanol dose of 3.5 g/kg (X2 = 10.84, p < 0.01). Of the isoflurane-exposed adolescents that did lose their righting reflex, 43.2% regained the righting reflex faster than air-exposed controls (t7 = 2.971, p < 0.05, Figure 1B). Then we determined whether this reduction in sensitivity to ethanol’s soporific effects was persistent. Rats exposed to isoflurane during adolescence similarly regained their righting reflex more rapidly than air controls when tested 3 weeks post exposure during late adolescence (t7 = 3.173, p < 0.01) or 6.5 weeks later in adulthood (t11 = 2.376, p < 0.05). Decreased time to regain LORR in isoflurane-exposed rats were mainly paralleled by a significant elevation of BECs compared to air-controls, [1 week: control = 174.7±5.4 mg/dl, isoflurane = 195.1±9.3 mg/dl, p< 0.05; 3 weeks: control = 177.0±5.6 mg/dl, isoflurane = 204.8±16.1 mg/dl, p = 0.07; 6.5 weeks: control = 158.1±11.0 mg/dl, isoflurane = 185.1±10.7 mg/dl, p = 0.05), indicating that this effect was likely not due to alterations in pharmacokinetic properties of ethanol. No effects were observed in adults one week or three weeks following isoflurane exposure. Taken together, these findings support that adolescent general anesthetic exposure not only exacerbates blunted aversive responding during adolescence, but that some effects persist into adulthood.
Effects of adolescent and adult isoflurane exposure on ethanol-induced memory impairment.
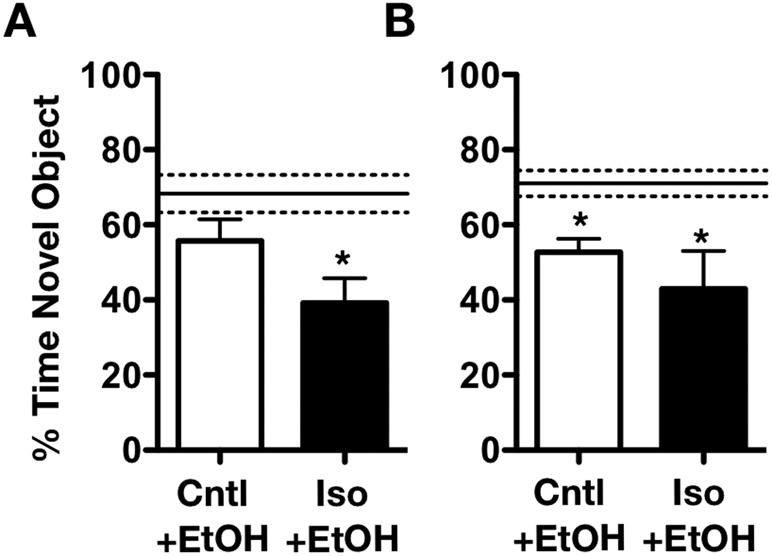
Given the differential ethanol sensitivities observed across adolescent and adults, we next tested the effects of isoflurane exposure on ethanol-induced memory impairment. We previously reported no differences in baseline memory performance one week following adolescent anesthetic exposure (Landin et al., 2019). However, in the present study, adolescent anesthetic-exposed rats challenged with a dose of 1 g/kg ethanol were unable to distinguish the novel object from the familiar object, as indicated by the decrease in time spent exploring the novel object when compared with air-exposed controls (t 11 =2.230, p < 0.05, Figure 2A), whereas air-exposed controls were not affected by this dose. No differences were noted in adults one week following exposure (not shown). Given that reductions in ethanol-induced sedative-hypnotic effects were sustained into adulthood following a one-time isoflurane exposure in adolescence, we next assessed whether persistent impairments in memory performance were evident. A separate cohort of animals exposed to isoflurane during adolescence and tested as adults (P75) also exhibited a similar decrease in novel object exploration following an acute ethanol challenge (t16 = 2.515, p < 0.05; Figure 2B). However, memory impairment in isoflurane-exposed adolescents given an ethanol challenge as adults did not differ from the age-matched isoflurane-exposed rats challenged with saline (not shown – see Landin et al., 2019). Interestingly, air control rats when given an ethanol challenge as adults also had reduced time spent exploring the novel object (t15 = 4.348, p < 0.01, Figure 2B). Taken together, these findings suggest that adolescent general anesthetic exposure exacerbates adolescent sensitivity to ethanol-induced memory impairment.
Figure 2.
Adolescent isoflurane exposure increases memory-impairing effects of ethanol. Only isoflurane-exposed adolescents exhibit a reduction in time spent with the novel object following 1.0g/kg ethanol administration, compared to air-exposed saline controls when tested during adolescence. Air-exposed adolescents did not differ from saline controls (represented by dashed line, n = 5-7; A). When tested in adulthood, air- and isoflurane-exposed adolescents did not differ in the % time spent with the novel object compared to air-exposed saline controls (represented by dashed line, n = 7-9, B). Data are represented as mean ± SEM. * = p < 0.05.
Effects of adolescent ethanol exposure on ethanol-induced social responding.
Adolescent rats typically respond to lower doses of ethanol by social facilitation (i.e., increases in social behavior), with social inhibition evident at higher doses. Low dose social facilitation is no longer evident in late adolescents and adults, with these age groups demonstrating social inhibition al lower ethanol doses relative to early/mid adolescents (Varlinskaya & Spear, 2015). This experiment assessed whether adolescent general anesthetic exposure would decrease age-appropriate sensitivity to ethanol-induced social inhibition three weeks following exposure (P46). As expected, air controls showed a decrease in social investigation following acute ethanol challenge (t18 = 3.105, p <0.01, Figure 3), while isoflurane-exposed rats were insensitive to this effect. These data suggest that early adolescent general anesthetic exposure decreased sensitivity to the socially suppressing effects during late adolescence.
Figure 3.
Adolescent anesthetic exposure reduces ethanol-induced social suppression. As expected, control rats exhibited a decrease in social investigation following 1.0g/kg ethanol exposure compared to rats exposed to saline (as represented by dashed line). However, isoflurane-exposed adolescents did not exhibit the expected ethanol-induced reduction in social investigation relative to saline controls. Data are represented as mean ± SEM. * = p < 0.05, n = 10/group.
Effects of adolescent isoflurane exposure on voluntary ethanol consumption
Lastly, we investigated the impact of adolescent anesthetic exposure on voluntary ethanol consumption using a limited two-bottle choice task at P35 or P75. When tested during adolescence (1 week post-exposure), isoflurane-exposed rats consumed more ethanol than air-exposed controls (t11 = 1.858, p < 0.05; Figure 4A), with no differences evident in super-saccharin consumption (Figure 4B). Rats also did not differ in ethanol preference (Figure 4C). Surprisingly, when tested in adulthood (6.5 weeks post-exposure), isoflurane-exposed rats consumed less ethanol than air-exposed controls (t16 = 1.872, p < 0.05; Figure 4D) while tending to consume more super-saccharin (t16 = 1.506, p = 0.075; Figure 4E). Therefore, adult preference for ethanol was decreased following adolescent isoflurane exposure (t16 = 2.311, p < 0.05, Figure 4F).
Figure 4.
Impact of early adolescent isoflurane exposure on voluntary ethanol consumption in mid-adolescence or adulthood. All data are averaged across 5 drinking days and shown in mL/kg. Isoflurane-exposed adolescents consumed more ethanol than air counterparts (n = 6-7/group) when tested one week following exposure (A). Levels of supersaccharin consumption (B) and % EtOH preference (C) did not differ between groups 1 week later (n=8-10/group). When tested in adulthood, rats exposed to isoflurane during adolescence consumed less ethanol than air-control counterparts (n = 7-11/group; D). Supersaccharin consumption was slightly increased 6.5 weeks later, although not significant (E). % EtOH preference was decreased in isoflurane-exposed adolescents when tested in adulthood (F). Data are represented as mean ± SEM. * = p < 0.05.
Discussion
Adolescents that excessively consume alcohol are at a greater risk to develop chronic, relapsing AUDs at some point in life. Alcohol abuse during adolescence is hypothesized to “lock in” adolescent alcohol phenotypes in adulthood (Spear & Swartzwelder 2014). However, select genetic and environmental risk factors may predispose some adolescents to consume more alcohol than their age-matched counterparts. The present study demonstrates for the first time that a single exposure to general anesthesia during adolescence can alter ethanol responsivity in a manner consistent with an exacerbation of the adolescent-typical responsiveness to alcohol. In particular, adolescent isoflurane exposure further decreased already low adolescent sensitivity to the aversive, sedative/hypnotic and socially suppressing effects of ethanol, increased sensitivity to ethanol-induced memory impairment, and also increased voluntary intake during the adolescent period. Selectively assessed alterations in ethanol sensitivity appeared to persist into adulthood as well. Given the extensive literature demonstrating that these select ethanol sensitivities elevate the risk for development of an AUD, such preclinical findings suggest that adolescent general anesthetic exposure may be a potential risk factor for alcohol abuse in a portion of the population already vulnerable based on age.
The consequences of general anesthetic exposure in infants and children has been well investigated, and have demonstrated consequent cognitive and behavioral abnormalities (see: Lin et al., 2017, for review). However, the impact of such exposures on later developmental periods, including adolescence is very limited, but convergent evidence has been observed with respect to memory and learning (see: Landin et al., 2019, Pavkovic et al., 2018). To our knowledge, the present study is the first to assess the contribution of developmental anesthetic exposure on behaviors related to substance use disorder vulnerability using an animal model, whereas no human data has directly assessed this association. Interestingly, a relatively recent report in JAMA has reported a three-fold increase in persistent opioid use n young individuals who filled perioperative opioid prescriptions after having their third molars removed compared to those that did not (Harbaugh et al., 2018). While this study does note the association of increased prescription refills, it does not differentiate between the initial opiate prescription in naïve individuals relative to the effect of surgical procedure. Long-term human adolescent studies being conducted such as the Adolescent Brain Cognitive Development (ABCD) Study as well the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) could evaluate effects of surgical history, if such information were available. An alternative approach would be to encourage clinicians performing invasive procedures to identify whether younger patients have begun alcohol use or have established a history with alcohol abuse. Preclinically, our findings suggest that researchers investigating adolescent alcohol abuse should consider the impact of anesthetic exposure on ethanol-related behavioral and neural outcomes.
Alterations in ethanol responsiveness following anesthetic exposure were not observed when rats were exposed to isoflurane as adults. For example, we previously observed that adult rats exposed to isoflurane did not exhibit alterations in novel object recognition or inhibitory GABAA receptor expression or function (Landin et al., 2019). In the current study, we observed no changes in ethanol-induced loss of the righting reflex or CTA following adult isoflurane exposure. Therefore, the impact of prior anesthetic exposure on subsequent ethanol responsiveness is likely particularly associated with the labile brain maturational state during adolescence. Given that every behavioral and neurobiological measure we have assessed following adult isoflurane exposure had no effect (Landin et al., 2019, and present findings), we conserved animal numbers and focused largely on the impact of adolescent isoflurane exposure on the brain and behavior rather than direct comparisons of adolescents and adults. The impact of adolescent anesthetic exposure on CTA and social interaction were not assessed into adulthood. While future work can evaluate the persistence of such behaviors, given the similarities between adolescent general anesthetic exposure and AIE, along with CTA being retained following AIE (Saalfield and Spear, 2015), such studies have lower priority. Similarly, as ethanol modulation of social interaction was assessed in late adolescence, it is likely that similar behavioral responses will be observed in adulthood. Conversely, ethanol-induced social facilitation during early adolescence was not assessed due to potential ceiling effects in responding.
The ability for adolescent general anesthetic exposure to elicit insensitivity to ethanol-induced CTA at higher doses was striking. It can be debated as to whether such effects are due to enhanced memory impairment, thus the inability to make the cognitive association between the conditioned stimulus (CS - tastant) and unconditioned stimulus (US - ethanol), especially as adolescents exposed to general anesthetics were less sensitive to ethanol challenge when tested in adolescence. However, ethanol still elicits CTA at doses that would typically be cognitively impairing in both general anesthetic-naïve adolescents and adults, lending greater support to the ability of exposed animals to form CS/US pairings at amnestic doses. Such pairings likely involving engrams less related to traditional learning and memory as evident elsewhere (Saalfield and Spear, 2019, Uematsu et al., 2015). Related, it was surprising to observe that adult control rats also exhibited memory impairment 24 hours following 1.0 g/kg ethanol as prior findings tend to indicated reduced sensitivity in naïve adults (see: Spear & Swartzwelder 2014, for review). Future studies should address ethanol-induced memory impairment utilizing different spatial and non-spatial tasks.
Adolescent general anesthetic exposure increased voluntary ethanol consumption during adolescence without altering supersac intake. Although there was not a statistical difference in ethanol preference in adolescents exposed to isoflurane compared to air-exposed controls, only the isoflurane-exposed group demonstrated an ethanol preference greater than 50%. Previous studies have found that early exposure to a CNS depressant, such as ethanol, lead to higher rates of consumption later in life (Grant and Dawson, 1998, DeWit et al., 2000, Hingson et al., 2006). Enhancement of the inhibitory neural mechanisms responsible for both general anesthetics and ethanol effects may perhaps lead to increases in ethanol intake. For example, increases in voluntary alcohol consumption has been directly linked to increased GABAA-R frequency in the ventral tegmental area (VTA) (Melis et al., 2002). Stress also increased ethanol consumption via GABAA-R activity in the VTA (Ostroumov et al., 2016). Our previously observed increases in extrasynaptic GABAA-Rs following adolescent anesthetic exposure likely contribute to increased intake (Landin et al., 2019), given that knockdown of these receptors has been shown to reduce ethanol intake (Nie et al., 2011). Future studies should identify whether enhancement of extrasynaptic GABAA receptor activity following adolescent GA alters dopaminergic neurons, and whether this is responsible for dysregulating the reward pathway and thus voluntary ethanol consumption.
Given that adolescent general anesthetic exposure seemingly exacerbated adolescent ethanol-induced responses and intake, it was surprising that isoflurane-exposed rats exhibited a decrease in ethanol consumption when tested in adulthood. A reduction in sensitivity to the negative properties of ethanol is typically, but not always, associated with increased likelihood of AUD (Schuckit, 1996). An additional explanation for decreased drinking in isoflurane-exposed rats is that anxiety is persistently elevated into adulthood following general anesthetic exposure (Landin et al., 2019). Therefore, due to neophobia, it may take more sessions and more time per session (> 30 min) to detect differences in isoflurane-exposed rats given that they may approach the bottles more hesitantly. Future studies should assess drinking following adolescent anesthetic exposure utilizing procedures known to produce high levels of ethanol consumption. Further, an important future study would be to identify whether prior isoflurane exposure combined with adolescent ethanol exposure more potently increases voluntary ethanol consumption in adulthood compared to ethanol exposure alone. Given that isoflurane-exposed rats consumed more ethanol than air-exposed counterparts when tested during adolescence, such an approach may be more translationally relevant for development of AUDs in adulthood.
The current study is limited in scope in that we only evaluated the impact of early-mid adolescent exposure that corresponds to approximately 10-18 years of age in humans (Spear, 2015). As we previously investigated and found no impact of adult isoflurane exposure on cognition, anxiety, and GABAA receptor expression and function, there is likely eventual closure to the adolescent anesthetic sensitive period. Further work evaluating the impact of general anesthetic exposure during later adolescent periods or emerging adulthood basally and in response to an acute ethanol challenge are needed. It should also be emphasized that our current findings do not include females. Given that investigation of the adolescent period in the anesthetic field is relatively novel, coupled with our recent findings suggesting the contribution of adaptations to extrasynaptic GABAA receptors that are known to contribute to differential responding across the estrous cycle, our initial efforts were restricted to responses in adolescent males. However, future investigations into female responding are needed, especially given that neonatal studies have found similar (e.g., Jevtovic-Todorovic et al., 2003) and different responses based on sex (e.g., Lee et al., 2014). Finally, although our findings illuminate a potential contributing extrinsic factor to adolescent alcohol use disorder susceptibility, more work is needed to determine whether adaptations in convergent molecular targets for ethanol and anesthetics such as GABAA receptors contribute to changes in adolescent ethanol behaviors.
The minimum alveolar concentration (MAC) for an isoflurane in adults is ~1.6% (Sonner et al., 2007) – that is, the effective concentration at which only 50% of subjects will be unresponsive to a noxious stimulus. The isoflurane concentration employed in the present study was selected to ensure that all subjects would be non-responsive to a noxious stimulus particularly given that exposure to anesthesia during developmental periods may require higher doses for effectiveness than adults (see: Fang et al., 1997). The MAC for developing Sprague-Dawley rats for a similar length of exposure has been reported to be as high as 3.2% at P7 (Lee et al., 2014), approximately twice as high as adults. Concentrations similar to ours have been commonly used in rodent procedures (Howard et al., 2008). It should also be pointed out that isoflurane was delivered using 100% oxygen. While there is some evidence to suggest that oxygen may lead to increases in reactive oxygen species in neonates (Boscolo et al., 2013), it is likely to be less of a contributor in older subjects. Inspiring this concentration of oxygen is likely to have minimal effect on arterial oxygen content due to hemoglobin being close to maximal saturation (Martin and Grocott, 2015). 100% exposure for less than 12 hours is not believed to have detrimental effects in healthy humans (Lumb, 2007). Any potential effects from oxygen appear to be minimal as adults in Landin et al., (2019) and present study were null, and pulse oximetry measure were typically in the min 90% range, but not saturated. Nonetheless, future studies should evaluate reactive oxygen species following adolescent anesthetic exposure.
Overall, the present findings suggest that adolescent isoflurane exposure reduces sensitivity to the aversive properties of ethanol while enhancing sensitivity to the memory-impairing effects of ethanol exposure and voluntary ethanol intake. In doing so, these findings further lend support to general anesthetic exposure during adolescence as a potential environmental risk factor for development of AUDs, and potentially other substance abuse disorders that warrants further investigation.
Acknowledgments
Support:
The work presented in this manuscript was funded by award number P50 AA017823 from the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health. The authors do not have any conflict of interests to declare.
References
- ANTOGNINI JF & CARSTENS E 2005. Measuring minimum alveolar concentration: more than meets the tail. Anesthesiology, 103, 679–80. [DOI] [PubMed] [Google Scholar]
- BOSCOLO A, MILANOVIC D, STARR JA, SANCHEZ V, OKLOPCIC A, MOY L, ORI CC, ERISIR A & JEVTOVIC-TODOROVIC V 2013. Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology, 118, 1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUTROS N, SEMENOVA S & MARKOU A 2016. Adolescent alcohol exposure decreased sensitivity to nicotine in adult Wistar rats. Addict Biol, 21, 826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROADWATER M, VARLINSKAYA EI & SPEAR LP 2013. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol Clin Exp Res, 37, 1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEETHAM A, ALLEN NB, WHITTLE S, SIMMONS J, YUCEL M & LUBMAN DI 2014. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl), 231, 1731–42. [DOI] [PubMed] [Google Scholar]
- CHEETHAM A, ALLEN NB, WHITTLE S, SIMMONS J, YUCEL M & LUBMAN DI 2017. Orbitofrontal Cortex Volume and Effortful Control as Prospective Risk Factors for Substance Use Disorder in Adolescence. Eur Addict Res, 23, 37–44. [DOI] [PubMed] [Google Scholar]
- CONROD PJ, O’LEARY-BARRETT M, NEWTON N, TOPPER L, CASTELLANOS-RYAN N, MACKIE C & GIRARD A 2013. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry, 70, 334–42. [DOI] [PubMed] [Google Scholar]
- DEWIT DJ, ADLAF EM, OFFORD DR & OGBORNE AC 2000. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry, 157, 745–50. [DOI] [PubMed] [Google Scholar]
- DYER ML, EASEY KE, HERON J, HICKMAN M & MUNAFO MR 2019. Associations of child and adolescent anxiety with later alcohol use and disorders: a systematic review and meta-analysis of prospective cohort studies. Addiction, 114, 968–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRENFELD JM, FUNK LM, VAN SCHALKWYK J, MERRY AF, SANDBERG WS & GAWANDE A 2010. The incidence of hypoxemia during surgery: evidence from two institutions. Can J Anaesth, 57, 888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENOCH MA 2011. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl), 214, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG Z, GONG D, IONESCU P, LASTER MJ, EGER EI 2ND & KENDIG J 1997. Maturation decreases ethanol minimum alveolar anesthetic concentration (MAC) more than desflurane MAC in rats. Anesth Analg, 84, 852–8. [DOI] [PubMed] [Google Scholar]
- FLEMING RL, WILSON WA & SWARTZWELDER HS 2007. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol, 97, 3806–11. [DOI] [PubMed] [Google Scholar]
- GORE-LANGTON JK & SPEAR LP 2018. Prenatal ethanol exposure attenuates sensitivity to the aversive effects of ethanol in adolescence and increases adult preference for a 5% ethanol solution in males, but not females. Alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANT BF & DAWSON DA 1998. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse, 10, 163–73. [DOI] [PubMed] [Google Scholar]
- HARBAUGH CM, NALLIAH RP, HU HM, ENGLESBE MJ, WALJEE JF & BRUMMETT CM 2018. Persistent Opioid Use After Wisdom Tooth Extraction. JAMA, 320, 504–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINRICH A, MULLER KU, BANASCHEWSKI T, BARKER GJ, BOKDE ALW, BROMBERG U, BUCHEL C, CONROD P, FAUTH-BUHLER M, PAPADOPOULOS D, GALLINAT J, GARAVAN H, GOWLAND P, HEINZ A, ITTERMANN B, MANN K, MARTINOT JL, PAUS T, PAUSOVA Z, SMOLKA M, STROHLE A, RIETSCHEL M, FLOR H, SCHUMANN G, NEES F & CONSORTIUM I 2016. Prediction of alcohol drinking in adolescents: Personality-traits, behavior, brain responses, and genetic variations in the context of reward sensitivity. Biol Psychol, 118, 79–87. [DOI] [PubMed] [Google Scholar]
- HEMMINGS HC JR., AKABAS MH, GOLDSTEIN PA, TRUDELL JR, ORSER BA & HARRISON NL 2005. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci, 26, 503–10. [DOI] [PubMed] [Google Scholar]
- HINGSON RW, HEEREN T & WINTER MR 2006. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med, 160, 739–46. [DOI] [PubMed] [Google Scholar]
- HOWARD EC, SCHIER CJ, WETZEL JS, DUVAUCHELLE CL & GONZALES RA 2008. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience, 154, 1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEVTOVIC-TODOROVIC V, HARTMAN RE, IZUMI Y, BENSHOFF ND, DIKRANIAN K, ZORUMSKI CF, OLNEY JW & WOZNIAK DF 2003. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci, 23, 876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYES KM, SCHULENBERG JE, O’MALLEY PM, JOHNSTON LD, BACHMAN JG, LI G & HASIN D 2012. Birth cohort effects on adolescent alcohol use: the influence of social norms from 1976 to 2007. Arch Gen Psychiatry, 69, 1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR S, PORCU P, WERNER DF, MATTHEWS DB, DIAZ-GRANADOS JL, HELFAND RS & MORROW AL 2009. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl), 205, 529–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDIN JD, PALAC M, CARTER JM, DZUMAGA Y, SANTERRE-ANDERSON JL, FERNANDEZ GM, SAVAGE LM, VARLINSKAYA EI, SPEAR LP, MOORE SD, SWARTZWELDER HS, FLEMING RL & WERNER DF 2019. General anesthetic exposure in adolescent rats causes persistent maladaptations in cognitive and affective behaviors and neuroplasticity. Neuropharmacology, 150, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE BH, CHAN JT, KRAEVA E, PETERSON K & SALL JW 2014. Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology, 83, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG J, SURYANARAYANAN A, ABRIAM A, SNYDER B, OLSEN RW & SPIGELMAN I 2007. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci, 27, 12367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN EP, LEE JR, LEE CS, DENG M & LOEPKE AW 2017. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol Teratol, 60, 117–128. [DOI] [PubMed] [Google Scholar]
- LOEPKE AW & SORIANO SG 2008. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg, 106, 1681–707. [DOI] [PubMed] [Google Scholar]
- LOPEZ MF, WHITE NM & RANDALL CL 2001. Alcohol tolerance and nicotine cross-tolerance in adolescent mice. Addict Biol, 6, 119–127. [DOI] [PubMed] [Google Scholar]
- LUMB AB 2007. Just a little oxygen to breathe as you go off to sleep…is it always a good idea? Br J Anaesth, 99, 769–71. [DOI] [PubMed] [Google Scholar]
- MARTIN DS & GROCOTT MP 2015. Oxygen therapy and anaesthesia: too much of a good thing? Anaesthesia, 70, 522–7. [DOI] [PubMed] [Google Scholar]
- MELIS M, CAMARINI R, UNGLESS MA & BONCI A 2002. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci, 22, 2074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIE H, REWAL M, GILL TM, RON D & JANAK PH 2011. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A, 108, 4459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOEL X 2014. Why adolescents are at risk of misusing alcohol and gambling. Alcohol Alcohol, 49, 165–72. [DOI] [PubMed] [Google Scholar]
- OSTROUMOV A, THOMAS AM, KIMMEY BA, KARSCH JS, DOYON WM & DANI JA 2016. Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron, 92, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRICK ME, SCHULENBERG JE, MARTZ ME, MAGGS JL, O’MALLEY PM & JOHNSTON LD 2013. Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr, 167, 1019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVKOVIC Z, MILANOVIC D, RUZDIJIC S, KANAZIR S & PESIC V 2018. The influence of propofol anesthesia exposure on nonaversive memory retrieval and expression of molecules involved in memory process in the dorsal hippocampus in peripubertal rats. Paediatr Anaesth, 28, 537–546. [DOI] [PubMed] [Google Scholar]
- PELTIER MR, VERPLAETSE TL, MINEUR YS, PETRAKIS IL, COSGROVE KP, PICCIOTTO MR & MCKEE SA 2019. Sex differences in stress-related alcohol use. Neurobiol Stress, 10, 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS S, PEPER JS, VAN DUIJVENVOORDE ACK, BRAAMS BR & CRONE EA 2017. Amygdala-orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev Sci, 20. [DOI] [PubMed] [Google Scholar]
- SAALFIELD J & SPEAR L 2015. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev Cogn Neurosci, 16, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAALFIELD J & SPEAR L 2019. Fos activation patterns related to acute ethanol and conditioned taste aversion in adolescent and adult rats. Alcohol, 78, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUCKIT MA 1996. Recent developments in the pharmacotherapy of alcohol dependence. J Consult Clin Psychol, 64, 669–76. [DOI] [PubMed] [Google Scholar]
- SMITH GT & CYDERS MA 2016. Integrating affect and impulsivity: The role of positive and negative urgency in substance use risk. Drug Alcohol Depend, 163 Suppl 1, S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONNER JM, WERNER DF, ELSEN FP, XING Y, LIAO M, HARRIS RA, HARRISON NL, FANSELOW MS, EGER EI 2ND & HOMANICS GE 2007. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology, 106, 107–13. [DOI] [PubMed] [Google Scholar]
- SPEAR LP 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev, 24, 417–63. [DOI] [PubMed] [Google Scholar]
- SPEAR LP 2015. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav, 148, 122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP 2016. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neurosci Biobehav Rev, 70, 228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP 2018. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci, 19, 197–214. [DOI] [PubMed] [Google Scholar]
- SQUEGLIA LM, BALL TM, JACOBUS J, BRUMBACK T, MCKENNA BS, NGUYEN-LOUIE TT, SORG SF, PAULUS MP & TAPERT SF 2017. Neural Predictors of Initiating Alcohol Use During Adolescence. Am J Psychiatry, 174, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARTZWELDER NA, RISHER ML, ABDELWAHAB SH, D’ABO A, REZVANI AH, LEVIN ED, WILSON WA, SWARTZWELDER HS & ACHESON SK 2012. Effects of ethanol, Delta(9)-tetrahydrocannabinol, or their combination on object recognition memory and object preference in adolescent and adult male rats. Neurosci Lett, 527, 11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRANTHAM-DAVIDSON H, CENTANNI SW, GARR SC, NEW NN, MULHOLLAND PJ, GASS JT, GLOVER EJ, FLORESCO SB, CREWS FT, KRISHNAN HR, PANDEY SC & CHANDLER LJ 2017. Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology, 42, 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEMATSU A, KITAMURA A, IWATSUKI K, UNEYAMA H & TSURUGIZAWA T 2015. Correlation Between Activation of the Prelimbic Cortex, Basolateral Amygdala, and Agranular Insular Cortex During Taste Memory Formation. Cereb Cortex, 25, 2719–28. [DOI] [PubMed] [Google Scholar]
- VARLINSKAYA EI & SPEAR LP 2009. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res, 33, 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARLINSKAYA EI, TRUXELL EM & SPEAR LP 2015a. Ethanol intake under social circumstances or alone in sprague-dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcohol Clin Exp Res, 39, 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARLINSKAYA EI, TRUXELL EM & SPEAR LP 2015b. Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behav Brain Res, 282, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDLE M 2016. Drinking Over the Lifespan: Focus on Early Adolescents and Youth. Alcohol Res, 38, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]