Abstract
The orexin (hypocretin) system is multifaceted, and regulates sleep-wake cycles, nociception, endocrine function and reward-seeking behavior. We have established an important role for this system in motivation for drugs of abuse. The orexin-1 receptor (Ox1R) antagonist SB334867 (SB) reduces seeking of food and drug reward under conditions of high motivation. There is some evidence that the effects of systemic SB on reward seeking persist beyond the pharmacological availability of the drug, however the time course of these effects is not well characterized, nor is it known whether similar persistent effects are observed following intraparenchymal injections. Here, we used a behavioral economics paradigm, which allows for repeated testing of drug motivation across consecutive days, to examine the persistent effects of acute systemic and local treatment with SB on motivation for the short-acting μ-opioid receptor agonist remifentanil. Systemic injections of SB immediately prior to behavioral testing reduced motivation for remifentanil; this effect was sustained on a subsequent test at 24h, but not on a third test at 48h. When injected into ventral pallidum (VP) the effects of SB were more persistent, with reduced motivation observed for up to 48h. We next made SB injections into VP 24h prior to behavioral testing; this produced effects that persisted for at least 72h post-treatment. Cued reinstatement of extinguished remifentanil seeking was also attenuated by pretreatment with SB 24h earlier. These data indicate that the effects of SB on opioid seeking behavior persist beyond the bioavailability of the compound. These observations have important ramifications for the future clinical use of orexin receptor antagonists for the treatment of addiction.
Keywords: behavioral economics, demand, hypocretin, opiate, reward, ventral pallidum
1. Introduction
Orexin peptides A and B (also known as hypocretin 1 and 2) are produced by hypothalamic neurons (de Lecea et al., 1998; Sakurai et al., 1998b) and are important in numerous functions, including regulating sleep/wake cycles (Mieda, 2017; Ono and Yamanaka, 2017), nociception (Mohammad Ahmadi Soleimani et al., 2015; Roohbakhsh et al., 2018), metabolic and homeostatic functions (Messina et al., 2016; Sassek et al., 2017) as well as for motivation for food and drugs of abuse (Barson, 2018; Borgland et al., 2009; Brodnik et al., 2018; Choi et al., 2010; Harris et al., 2005; James et al., 2017; Mahler et al., 2014a; Moorman et al., 2016; Pantazis et al., 2019; Walker and Lawrence, 2017; Yeoh et al., 2018) and other aspects of addiction such as tolerance and dependency (Ahmadi-Soleimani et al., 2017; Ghaemi-Jandabi et al., 2017). Orexinergic fibers project widely throughout the brain (Baldo et al., 2003; Peyron et al., 1998) to release orexin peptides A and B which act at two G-protein coupled receptors, orexin receptor 1 and 2 (Ox1R and Ox2R) (de Lecea et al., 1998; Hervieu et al., 2001; Marcus et al., 2001; Sakurai et al., 1998a). A significant body of literature shows that administration of Ox1R antagonists, either systemically or locally into reward regions, attenuates a broad range of drug-seeking behaviors (Bentzley and Aston-Jones, 2015; Espana et al., 2010; Fragale et al., 2019; Harris et al., 2005; James et al., 2011; James et al., 2018a; Jupp et al., 2011; Mahler et al., 2013; Moorman et al., 2017; Porter-Stransky et al., 2017; Schmeichel et al., 2017; Smith and Aston-Jones, 2012). Ox2R antagonists are also reported to reduce drug-taking (Brown et al., 2013; Schmeichel et al., 2015), however their effect on cue-driven drug seeking is less robust (Brown et al., 2013; Smith et al., 2009). Such findings have spurred speculation that orexin receptor antagonists, and particularly those that block Ox1R signaling, might represent a novel and effective option for the treatment of addiction (Brodnik et al., 2018; Campbell et al., 2018; James and Aston-Jones, 2017; James et al., 2017; Khoo and Brown, 2014; Perrey and Zhang, 2018; Walker and Lawrence, 2017; Yeoh et al., 2014).
Of the Ox1R antagonists available, SB-334867 (SB) is by far the most commonly used in animal studies, with >400 publications reporting the use of this compound (Perrey and Zhang, 2018). At a dose of 30mg/kg administered systemically, SB is effective at reducing motivated seeking of all drugs tested with limited/no effect on arousal or general motor activity (James et al., 2018c; LeSage et al., 2010; Moorman and Aston-Jones, 2009; Porter-Stransky et al., 2017; Smith and Aston-Jones, 2012). At this dose, peak blood and brain levels are observed 30min post-dosing; high levels are maintained at 4h post-dosing but gradually decline to be virtually non-detectable at 8h (Ishii et al., 2005). Accordingly, studies using SB generally commence behavioral testing 15-30mins after SB administration, and testing is conducted over 1-2h when brain-SB concentrations remain high (Bentzley and Aston-Jones, 2015; Borgland et al., 2006; James et al., 2018c; Smith et al., 2009; Smith et al., 2010; Smith and Aston-Jones, 2012). In studies where SB is delivered locally into discrete brain regions, behavioral testing typically commences within 10min of SB injections (Harris et al., 2007; James et al., 2011; Mahler et al., 2013; Narita et al., 2006).
Despite the relatively short pharmacokinetic profile of SB, two studies reported that the behavioral effects of this compound on feeding behavior are maintained beyond its bioavailability. Both studies showed that the anorexic effects of SB on palatable food or chow intake were maintained at 24h post-acute dosing, an effect that was also accompanied by a significant loss of bodyweight (Haynes et al., 2000; Ishii et al., 2005). One of these studies measured feeding and weight gain at 48h and showed that both indices were normalized at this time point (Ishii et al., 2005). Despite the many studies examining the effect of SB on drug seeking, the persistence of these effects is poorly understood. To our knowledge, only one paper has addressed this question directly. Brodnick et al. (2018) recently reported that systemic injections of SB reduce cocaine self-administration for at least 21h post-delivery, indicating that the anti-drug seeking properties of SB persist beyond its availability. Currently, it is not known how long these effects persist, nor if they extend to local (intracranial) injections of SB. Moreover, it is unclear whether acute SB administration is also associated with a persistent suppression of self-administration for other drugs of abuse, including opioids. Here, we addressed these questions directly by using a behavioral economics (BE) measure of demand/motivation for the short-acting μ-opioid receptor agonist remifentanil. A within-session BE paradigm provides a quantitative measure of both the desired amount of remifentanil at low cost (Q0), and the motivation for this drug as the required effort increases (demand elasticity; α). We chose this approach because inter-individual behavior on this task is highly stable (Bentzley et al., 2014; James et al., 2018a), allowing for repeated testing of drug motivation over consecutive days. Remifentanil is ideally suited for the within-session BE paradigm, as it has a much shorter duration of action than traditional opioids (Glass et al., 1999). Moreover, remifentanil has the potential to be abused (Baylon et al., 2000; Zacny and Galinkin, 1999) and laboratory rats will readily self-administer remifentanil. Indeed, on a progressive-ratio test, rats achieve similar breakpoints for remifentanil as for heroin (Panlilio and Schindler, 2000). In addition, we previously reported that SB, delivered either systemically or directly into caudal ventral pallidum (VP), suppresses motivation for remifentanil on a BE task (Mohammdkhani et al., 2018; Porter-Stransky et al., 2017); thus we could directly compare the persistence of effects across these two distinct routes of administration.
We report that systemic SB reduces motivation for remifentanil on an initial demand test (0.5h) and on a subsequent test at 24h post-treatment, but not on a third test at 48h. The effects of SB were more persistent when administered locally into VP, as reductions in motivation for remifentanil were observed at 0, 24 and 48h post-treatment. We further show that when administered 24h prior to behavioral testing, intra-VP injections of SB are effective at reducing drug motivation, and that these effects persist for 72h. Together our data indicate that SB delivered systemically or locally into VP has effects on remifentanil motivation that persist beyond the pharmacological availability of the compound.
2. Results
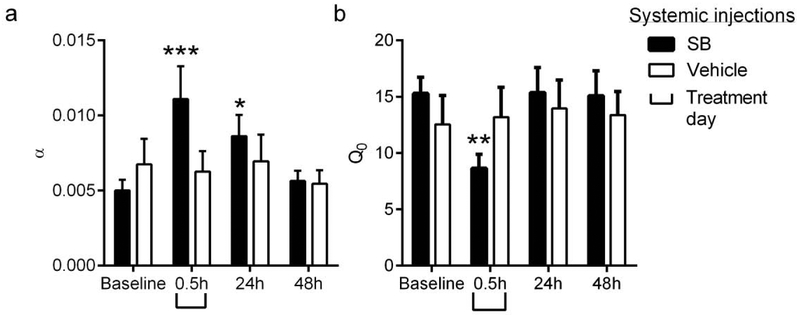
2.1. Experiment 1:
Systemic SB administration (30mg/kg) reduced demand for remifentanil up to 24h post-treatment
We reported elsewhere (Mohammdkhani et al., 2018; Porter-Stransky et al., 2017) that acute systemic treatment with SB (30mg/kg, ip) 0.5h prior to behavioral testing reduced motivation for the short acting μ-opioid receptor agonist remifentanil. Here, we tested the persistence of these effects by analyzing behavior in subsequent BE tests at 24h and 48h post-SB. We focused on the effects of SB on i) demand elasticity (α), which captures how consumption changes in relation to price, where higher a values reflect greater elasticity characterized by a greater reduction in responding as drug price increases; and ii) Q0, a measure of drug intake at null cost. A two-way ANOVA of α values following SB or vehicle treatment revealed a significant ‘Treatment’ × ‘Day’ interaction (Fig. 1a; F3,54=3.585, p=0.0195). Subsequent post-hoc analyses indicated that SB treatment was associated with significantly increased α values (decreased motivation) compared to baseline at 0.5h and 24h (baseline vs. 0.5h, p=0.0003; baseline vs. 24h, p=0.0442), but not 48h (baseline vs. 48h: p=0.9502), following SB treatment (Fig. 1a). Vehicle treatment had no effect on α values at any time point (Fig. 1a). A similar analysis of Q0 values (remifentanil intake at low effort) revealed a ‘Treatment’ × ‘Day’ interaction (Fig. 1b; F3,54=3.473, p=0.0222); post-hoc analyses indicated that Q0 values were significantly reduced at 0.5h (baseline vs. 0.5h: p=0.0013), but not at 24h or 48h (baseline vs. 24h: p>0.05; baseline vs. 48h: p>0.05; Fig. 1b). There was no effect of vehicle on Q0 values (Fig. 1b).
Figure 1. Systemic injections of SB reduced motivation for remifentanil up to 24h post-treatment.
(a) Animals were treated systemically with SB (30mg/kg, ip) or vehicle 0.5h prior to testing on a behavioral economics paradigm for remifentanil demand. BE testing was repeated (with no further SB administration) 24h and 48h later. Compared to baseline values, SB-treated animals exhibited a significant increase in α values (decreased motivation) at 0.5h and 24h post-treatment. Demand elasticity (α) values returned to baseline levels at 48h post-SB administration. There was no change in α values following vehicle treatment, (b) Systemic SB administration reduced remifentanil free consumption at null cost (Q0) at 0.5h only. Bar graphs represent mean ± standard error of mean (SEM). *p<0.05, **p<0.01, ***p<0.001 compared to baseline values.
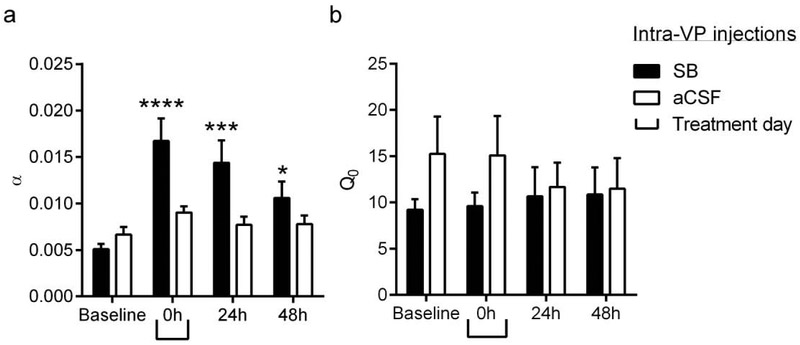
2.2. Experiment 2:
Intra-VP SB microinfusions reduced demand for remifentanil up to 48h post-treatment
We also reported that intra-VP injections of SB immediately prior to behavioral testing reduced demand for remifentanil (Mohammadkhani et al., 2018). Here we tested the persistence of these effects by analyzing data from subsequent BE sessions at 24h and 48h intra-VP SB. A repeated-measures ANOVA of α values following SB/vehicle treatment revealed a significant ‘Treatment’ × ‘Day’ interaction (Fig. 2a; F3,78=4.13, p=0.0090). Subsequent post-hoc analyses revealed that α values were significantly increased (motivation was decreased) at 0h (baseline vs. 0h: p<0.0001), 24h (baseline vs. 24h: p<0.0001) and 48h (baseline vs. 48h: p<0.05) post-SB, reflecting a persistent decrease in motivation for remifentanil (Fig. 2a). There was no effect of aCSF microinjections on α values (Fig. 2a). A similar analysis of Q0 values revealed no effect of SB or aCSF at any time point (F3, 78=0.4549, p=0.714; Fig. 2b).
Figure 2. Intra-VP infusions of SB reduced motivation for remifentanil for at least 48h post-treatment.
(a) Animals received intra-VP microinfusions of SB (1mM) or aCSF immediately prior to testing on a BE paradigm for remifentanil demand (0h). BE testing was repeated (with no further SB administration) at 24h and 48h post-treatment. Compared to baseline values, intra-VP SB treatment was associated with a significant increase in α (decreased motivation) at 0h, 24h and 48h post-treatment. There was no effect of aCSF treatment on α. (b) There was no significant effect of SB on remifentanil consumption at null cost (Q0). Bar graphs represent mean ± standard error of mean (SEM). *p<0.05, ***p<0.001, ****p<0.0001, compared with baseline values.
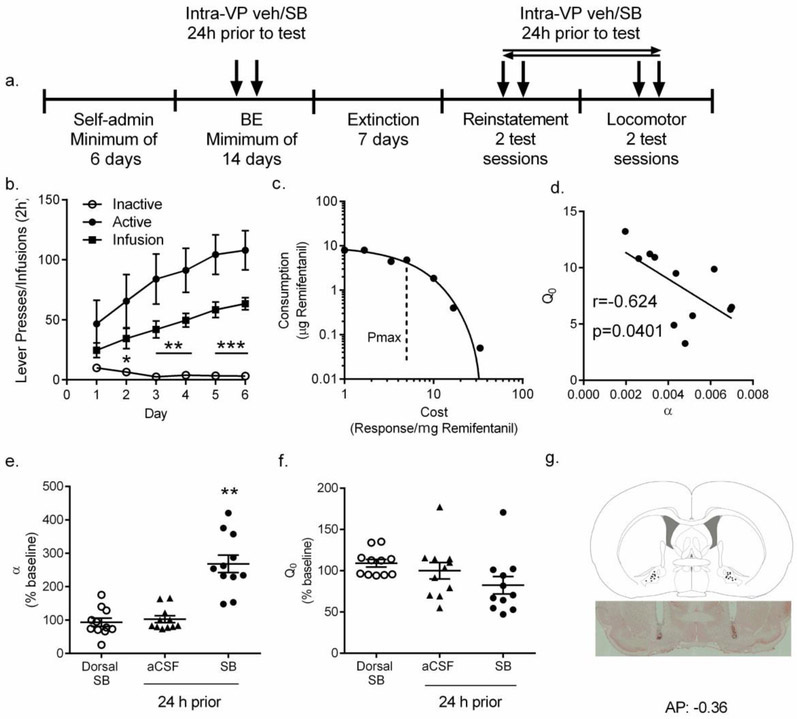
2.3. Experiment 3:
2.3.1. Intra-VP pretreatment with SB (24h prior to testing) reduced demand for remifentanil for at least 72h
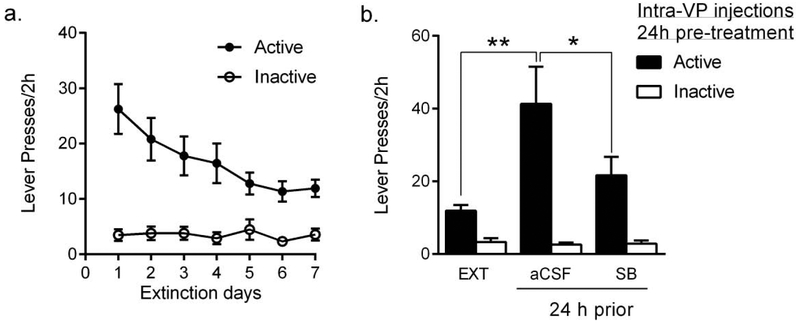
A key question emerging from Experiment 2 was whether plasticity or other changes associated with reduced remifentanil intake in the initial behavioral test following intra-VP SB treatment contributed to reduced motivation for remifentanil in subsequent test sessions. To test this, we trained a new group of rats on the BE paradigm and microinjected SB 24h prior to the first BE test session after training/baseline (Fig. 3a). Similar to the groups used for Experiments 1 and 2 (Mohammdkhani et al., 2018), these rats exhibited a robust preference for the active versus the inactive lever over the final 6d of self-administration training (Fig. 3b; RM ANOVA; F10,131=13.21, p<0.001). Active lever responding increased across training and reached a plateau over the final 3 sessions (Fig. 3b). The total number of remifentanil infusions earned over the training period was similar to that of animals included in Experiments 1 and 2 (Fig. 3b, comparison data shown in Mohammadkhani et al., 2018). Responding on the BE task resulted in accurate demand curve fitting; a representative curve is presented in Fig. 3c. Baseline α and Q0 values were similar to those from animals in Experiments 1 and 2 (also reported in Mohammadkhani et al., 2018). Similar to previous reports (Mohammdkhani et al., 2018; Porter-Stransky et al., 2017), α and Q0 values were negatively correlated (Fig. 3d; r=−0.6243, p=0.0401).
Figure 3. Intra-VP pretreatment with SB 24h prior to testing reduced motivation for remifentanil.
(a) Schematic of behavioral training and testing in Experiment 3 (Figs. 3-5), where intra-VP microinjections of SB or aCSF were made 24h prior to BE testing sessions, (b) Mean behavioral performance during remifentanil self-administration training (n=11). Data show average number of infusions, active and inactive lever presses during the last 6 days of FR1 sessions. Statistical symbols represent comparisons between active and inactive lever responses. (c) A representative demand curve of a single animal during a single remifentanil BE session. Each point represents data from a 10min bin, and the curve was fit using an exponential demand equation using a least sum of squares approach (Bentzley et al., 2014; Porter-Stransky et al., 2017). (d) Remifentanil intake at low cost (Q0) and demand elasticity (α) were negatively correlated, (e) When administered 24h prior to demand testing, intra-VP SB injections significantly increased demand elasticity (α; lowered motivation) for remifentanil, compared to aCSF or microinfusion of SB dorsal to VP. Statistical symbols represent comparison with aCSF control group. (f) Pretreatment with SB had no effect on consumption of remifentanil at null cost (Q0). (g) Representative schematic of VP (top panel; adapted from Paxinos & Watson brain atlas) depicting injection sites from animals in Experiment 3, based on inspection of brain tissue following neutral red stain (representative section shown in lower panel; frontal section, midline at center). The same animals also received control injections of SB 1.8mm immediately dorsal to the injection sites depicted here (‘Dorsal SB’). **p<0.01.
After baseline BE measures were obtained, rats received SB into VP and 24h later they were given a BE test session. Repeated measures ANOVA revealed that microinfusion of SB into VP 24h prior to BE testing significantly increased demand elasticity compared to microinfusion of aCSF or dorsal infusions of SB (Fig. 3e; F2,32=28.92, p=0.0001). Importantly, the effect of SB pretreatment on α values was not due to upward diffusion of SB along the cannulae tract, as α values following dorsal SB infusions were no different to vehicle treatment (Fig 3e; p=0.58). Despite an overall significant effect of SB microinjection into VP on remifentanil free consumption (Q0; Fig. 3f; F2,32=4.268, p=0.0394), post-hoc tests with appropriate corrections failed to detect significant differences between groups (p’s>0.05).
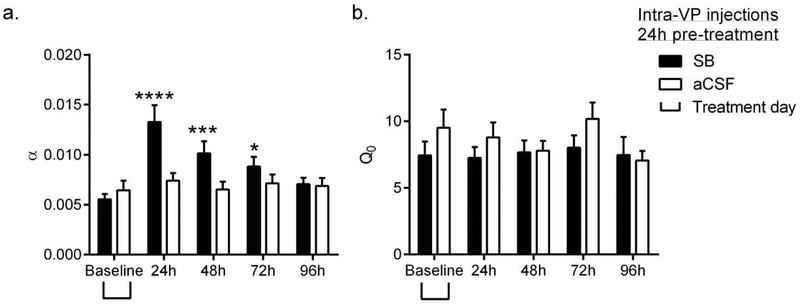
To test the persistence of these effects, we performed a two-way ANOVA of α values from subsequent daily BE tests. This revealed a significant ‘Treatment’ × ‘Day’ interaction (Fig. 4a: F4,80=7.032, p<0.0001), with subsequent posthoc tests indicating that SB pretreatment was associated with significantly higher α values (lower motivation) at 24h (p<0.0001), 48h (p=0.0002) and 72h (p=0.0166) post-treatment (Fig. 4a). There was no effect of SB treatment on Q0 values at any time point (Fig. 4b; F4, 80=1.364, p=0.2539).
Figure 4. Intra-VP pretreatment with SB reduced motivation for remifentanil for up to 72h.
(a) Following BE testing at 24h post intra-VP SB or aCSF treatment (presented in Figures 3e, f), rats were tested (with no further SB administration) on the BE paradigm for an additional 3 sessions at 48h, 72h and 96h after SB. SB pretreatment was associated with a significant increase in α values (decreased motivation) up to 72h post-injection. There was no effect of vehicle treatment. (b) Pretreatment with SB or aCSF had no effect on remifentanil consumption under null cost conditions at any time point. Bar graphs represent mean ± standard error of mean (SEM). *p<0.05, ***p<0.001, ****p<0.0001, compared with baseline values.
2.3.2. Intra-VP pretreatment with SB (24h prior to testing) attenuated cued reinstatement of remifentanil seeking
To test whether intra-VP pre-treatment with SB might also be effective at blocking cued reinstatement at a later time point, we extinguished lever pressing for remifentanil over a period of 7d (Fig. 5a). Animals were then microinjected with either SB or aCSF (counterbalanced) bilaterally into VP, 24h prior to cued reinstatement test sessions. A 2×3 factor repeated measures ANOVA revealed a significant main effect of ‘Treatment’ (F2, 32=7.765, p=0.0018) and ‘Lever’ (F2,32=18.82, p=0.0005), and a significant ‘Treatment’ × ‘Lever’ interaction (F2,32=8.583, p=0.0010). Dunnett post-hoc analyses showed that remifentanil-associated cues reinstated active lever pressing after vehicle pretreatment (vehicle vs. extinction, p=0.0010; Fig. 5b). Pretreatment with SB in VP significantly reduced responding on the active lever during reinstatement (SB vs. vehicle, p=0.0359; Fig. 5b). Cued reinstatement overall was blocked by pretreatment with SB into VP (p=0.4289 versus extinction levels). There was no change in responding on the inactive lever (Fig. 5b; F2, 26=0.2993, p=0.6876) after pretreatment with SB (p=0.91) or vehicle (p=0.60).
Figure 5. Intra-VP pretreatment with SB 24h prior to testing reduced cued reinstatement of remifentanil seeking.
(a) Following BE testing, lever pressing was extinguished in daily sessions for 7d. Data depict the mean number of active and inactive lever presses over these 7d. (b) There was a significant decrease in the number of active lever responses during a 2h cued reinstatement test conducted 24h following intra-VP SB compared to aCSF treatment. There was no effect of treatment on inactive lever responding. Bar graphs represent mean ± standard error of mean (SEM). *p<0.05, **p<0.01. n=9 for all groups.
2.3.3. No effect of intra-VP pretreatment with SB on general locomotor activity
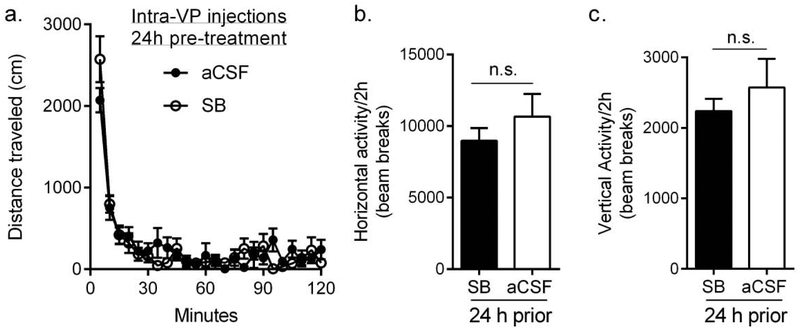
To test for non-specific motoric effects of SB pretreatment, rats were tested for locomotor activity 24h following intra-VP pretreatment with SB or vehicle. Pretreatment with SB did not have a significant effect on the total distance traveled (Fig. 6a; F23,368=1.467, p=0.0779), nor was there an effect on either horizontal (Fig. 6b; p=0.202) or vertical (Fig. 6c; p=0.395) activity over the 2h test sessions, indicating that there was no overall effect of pretreatment with SB on general motor behavior.
Fig 6. Intra-VP pretreatment with SB 24h prior to testing had no effect on general locomotor activity.
(a) Rats displayed no differences in total distance traveled in a test of general locomotor activity when treated with intra-VP SB 24h prior to testing. (b,c) SB treatment also had no effect on horizontal (b) or vertical (c) activity. Bar graphs represent mean ± standard error of mean (SEM). n.s.: not significantly different. n=9 for all groups.
3. Discussion
We tested whether inhibition of remifentanil-seeking by the selective Ox1R antagonist SB persisted beyond the pharmacological availability of the drug. We found that when administered systemically, SB reduced motivation (increased demand elasticity) for remifentanil for up to 24h post-treatment (Experiment 1). When administered locally into VP, the anti-motivational effects of SB persisted for at least 48h (Experiment 2). In a separate group of rats that were treated with SB into VP 24h prior to behavioral testing, we observed reduced motivation for remifentanil in 3 subsequent behavioral tests, indicating that the effects persisted for 72h post-SB administration. We also showed that cued reinstatement of remifentanil seeking was decreased 24h after intra-VP SB (24h prior), without affecting general locomotor activity. Together, these results indicate a persistent effect of SB, both when delivered systemically or intra-VP, on motivated responding for the opioid remifentanil.
We observed reduced motivation for remifentanil at 24h, but not 48h, following systemic SB treatment. This finding aligns with those of Brodnik et al. (2018) who recently tested the persistence of SB effects on cocaine self-administration on a discrete trials-3 schedule of reinforcement. In this paradigm, rats are allowed access to cocaine in 10min trial blocks that are initiated 3 times each hour over each 24h period. This schedule promotes a cyclic pattern of drug taking across the day characterized by high levels of drug intake during the active (lights off) period and low intake during the inactive (lights on) period. The authors reported that when given systemically during the active (lights off) period, SB accelerated the termination of drug taking and delayed the re-initiation of drug taking in the following active period (15-21h following SB delivery). Our data are also consistent with several previous studies which reported that acute treatment with SB (30mg/kg, i.p.) suppressed intake of home cage chow and high fat food at 24h post-dosing, an effect that was also accompanied by a significant loss of body weight over this period (Haynes et al., 2000; Ishii et al., 2005; White et al., 2005). Normal feeding behavior was restored at 48h post-SB treatment (Ishi et al, 2005), which is consistent with the time we observed a normalization of remifentanil-seeking behavior (although body weight deficits are maintained for up to 3d post treatment (Rodgers et al., 2001; White et al., 2005). Together, these findings point to a common time course of SB’s efficacy following systemic administration across several reinforcers, including psychostimulants, opioids and food.
The pharmacokinetics of systemic SB treatment have been well-characterized in adult male rats. At 30mg/kg (i.p.), SB reaches peak plasma and brain concentrations at ~30 min and has a half-life of ~4h (Ishii et al., 2005). At 10mg/kg (i.p.; a dose also commonly used in tests of drug motivation), SB has a terminal elimination half-life of ~0.4h (Porter et al., 2001). As the compound is virtually undetectable in plasma or brain at 2h and 8h post-dosing (10 and 30mg/kg dose, i.p., respectively) (Porter et al., 2001; Ishii et al., 2005), prolonged occupancy at the Ox1R would seem unlikely as the potential mechanism underlying the persistent effects reported here. One hypothesis is that blockade of Ox1R produces enduring neuroadaptations to the Ox1R or downstream signaling pathways involved in motivation (Ishii et al., 2005). Indeed, Brodnik et al. (2018) reported that intra-VTA administration of an alternative Ox1R antagonist, RTIOX-276 (RTI) delivered 24h prior to testing produced a robust reduction in accumbal DA uptake inhibition following a cocaine challenge. These authors also reported a significant reduction in accumbal dopamine transporter (DAT) phosphorylation at 24h post-RTI administration. It is unclear whether such changes could underlie the effects observed here, especially given that accumbal dopamine signaling may be less critical for opioid reward behavior compared to psychostimulants (Gerrits et al., 1994; Gerrits and Van Ree, 1996; Pettit et al., 1984), although see (Corre et al., 2018). Thus, it will be critical for future studies to examine how orexin peptides acting at Ox1R are important for the normal functioning of opioid reward pathways
Many studies utilize local microinjections of SB to examine the role of orexin signaling in specific reward loci. VP is a well-characterized reward structure with major reciprocal connections to the mesolimbic dopamine system, including the nucleus accumbens (NAc) and ventral tegmental area (VTA) (Groenewegen et al., 1993; Haber et al., 1985). Accordingly, VP is a critical node in the brain reward circuit that underlies a range of drug-seeking behaviors, including self-administration and reinstatement behavior (Churchill and Kalivas, 1994; Cooper et al., 2017; Heinsbroek et al., 2017; Mahler et al., 2014b; Smith and Berridge, 2007). VP (particularly caudal VP; targeted here) receives dense innervation from orexin neurons in LH, and both Ox1R and Ox2R are expressed in VP. Ho and Berridge provided the first evidence that orexin acts at VP to mediate reward by showing that intra-VP infusions of orexin-A enhance the hedonic properties of sucrose (Ho and Berridge, 2013). We recently extended these findings to show that blockade of Ox1R signaling in VP reduces motivation for remifentanil without affecting low-effort consumption on an economic demand task that commenced immediately following SB microinjection (Mohammdkhani et al., 2018). Here, we show that these effects persist for at least 48h following treatment. These effects were not attributable to reduced remifentanil intake on the initial BE test, as similar effects were observed following SB pretreatment 24h prior to testing (reduced demand was observed up to 72h under these conditions). Pretreatment with SB 24h prior to testing was also associated with reduced cued reinstatement behavior, but no change in general locomotor activity. Together these findings indicate that the effects of SB on motivated behavior are more persistent following intraparenchymal microinjections compared to systemic injections.
To our knowledge, this is the first study to show a persistent effect of local SB microinjections on reward seeking behavior. Unlike systemic dosing, little is known about the time course of SB availability following intraparenchymal injections, nor is it clear whether this type of administration is associated with persistent neuroadaptations at the site of injection, and how these might relate to reward seeking. VP receives substantial dopaminergic input arising from VTA (Klitenick et al., 1992; Root et al., 2015; Smith and Kieval, 2000), and VTA inputs to VP are activated by drug cues (Mahler and Aston-Jones, 2012). We observed effects of SB up to 72h post-treatment following local injections (compared to 24h post-systemic treatment); this might be related to a higher concentration of SB in critical brain region(s) following the intraparenchymal route of delivery. Future studies should seek to examine whether similar persistent effects are observed following local SB injections into other regions where Ox1R signaling is known to regulate drug behavior, including VTA (James et al., 2011; Mahler et al., 2013; Wang et al., 2009).
Our findings have important implications with respect to the design of studies involving Ox1R antagonists to test the role of Ox1R signaling in reward seeking paradigms. Indeed, care should be taken when interpreting behavioral results from testing conducted up to 24h (for systemic) and 72h (for intracranial) post-administration, as behavior is likely to be influenced by the prior treatment at these time points. Moreover, while only relatively few studies have examined the effect of repeated/chronic SB dosing (Borgland et al., 2006; Zhou et al., 2012), such studies will be important for understanding the potential clinical use of Ox1R antagonists for the treatment of disorders such as addiction. These studies should seek to understand whether the persistent effects, and possible neuroadaptations, associated with acute dosing reported here are additive with repeated dosing.
4. Materials and methods
4.1. Subjects
A total of 35 male Sprague Dawley rats (initial weight 275–300 g; Charles River, Raleigh, NC, USA) were used in these experiments. Data from 24 of these rats were analyzed for other purposes in a separate publication (Mohammadkhani et al., 2018); the remaining 11 rats were prepared exclusively for this study. Rats were single-housed and maintained under a 12 h reverse light/dark cycle (lights off at 08:00 h) in a temperature and humidity-controlled animal facility at Rutgers University. Food and water were available ad libitum. All experimental procedures were approved by the Rutgers Institutional Animal Care and Use Committee and were conducted according to the Guide for the Care and Use of Laboratory Animals. Rats were handled daily after a 3-day acclimation period at the facility; all experiments were performed in the rats’ active (dark) phase.
4.2. Drugs
Remifentanil (obtained from the NIDA Drug Supply Program, National Institute of Drug Abuse) was dissolved in 0.9% sterile saline for intravenous (iv) self-administration. For systemic administration, SB (supplied by the NIDA Drug Supply Program, National Institute of Drug Abuse) was suspended in 10% 2-hydroxypropyl-b-cyclodextrin in sterile water and 2% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA). For intracranial microinjections, SB (1 mM) was suspended in artificial cerebrospinal fluid (aCSF) with agitation, slight heating and sonification as described in our previous study (Mahler et al., 2013). Using this preparation, we avoided microinjections of DMSO or cyclodextrin into VP. Three different groups of rats were treated with SB as follows: In Experiment 1, rats were injected with 30 mg/kg SB or vehicle (i.p.) at a volume of 4.0 ml/kg, 0.5h prior to the first behavioral test (n=10). Rats in Experiment 2 received an intra-VP microinjection of 1mM SB (0.3 μl) immediately prior to the first behavioral test (n=14). In Experiment 3 rats received intra-VP microinjection of 1 mM SB (0.3 μl) 24h prior to the first BE or cued reinstatement test (n=11). A within-subjects design was used whereby each rat received both SB and vehicle; the order was counterbalanced across subjects with a minimum of 2 washout days between tests (details below).
4.3. Intravenous catheter surgery
Following acclimation to the animal facility, rats were anesthetized with a ketamine/xylazine mixture (56.5 and 8.7 mg/kg, i.p., respectively) followed by an injection of an analgesic (rimadyl, 5 mg/kg; s.c.). Rats were then implanted with an indwelling catheter (modified 22 ga cannula, Plastics One) into the jugular vein that exited the body via a biopsy on the back caudal to the mid-scapular region. Catheters were flushed with cefazolin (0.1 ml; 100 mg/ml) and heparin (0.1 ml; 100 U/ml) immediately following surgery, daily beginning 3 days after surgery, and continuing throughout self-administration. Rats were allowed to recover for a minimum of 7 days after surgery before remifentanil self-administration training.
4.4. Stereotaxic surgery
Immediately following catheter implantation, animals in Experiments 2 and 3 were placed in a stereotaxic frame (Kopf, Tujunga, CA, USA) and implanted with bilateral stainless steel guide cannulae (22 gauge, 11 mm, Plastics One, Roanoke, VA, USA) 2mm dorsal to caudal VP (coordinates relative to bregma skull surface in mm: −0.8 posterior, ±2.6 medial–lateral, −7.5 ventral; (Paxinos and Watson, 1998). The cannulae were secured to the skull using jeweler’s screws and dental acrylic; stylets were placed into the guide cannula to prevent occlusion.
4.5. Self-administration training
The self-administration procedure was similar to a recently published study from our laboratory (Porter-Stransky et al., 2017). All self-administration sessions occurred in operant chambers controlled by Med-PC IV software (Med Associates). Each operant chamber was equipped with two levers, a cue light above each lever, a tone generator, a house light, and an infusion pump. A timeline of experimental training and tests is found in Fig. 3a. A response on the active lever resulted in remifentanil infusion (1 μg delivered over 4 sec) paired with a discrete compound cue (auditory tone plus white cue light above the active lever) during self-administration. After each infusion, a 20-sec time-out occurred (signaled by turning off the house light) when additional presses were recorded but did not yield remifentanil or cues. Presses on the inactive lever were recorded but had no consequences. Each session ended after 2h or when 80 infusions were earned, whichever first occurred. Subjects were trained on the FR1 procedure for a minimum of 6 sessions. After earning a minimum of 25 infusions within 2h for at least 3 consecutive sessions, animals advanced to the behavioral economics threshold procedure. Data for self-administration training and BE testing at 0.5h for Experiments 1 and 2 are reported elsewhere (Mohammdkhani et al., 2018).
4.6. Behavioral-economics threshold procedure
Economic demand training for remifentanil was conducted as in a recent study from our laboratory (Porter-Stransky et al., 2017). As previously described (Bentzley et al., 2013), the BE procedure varies the cost of drug by changing the amount of drug infused while maintaining an FR1 schedule of reinforcement. The dose of remifentanil resulting from a response on the active lever decreased every 10 minutes; each 110-minute session tested 11 doses of remifentanil (2, 1, 0.6, 0.3, 0.2, 0.1 0.06, 0.03, 0.02, 0.01 and 0.006 μg/infusion). As in FR1 training, each infusion was accompanied by presentation of a light-tone compound cue; responses on the inactive lever had no consequence. However, unlike the FR1 training procedure, there were no limits on how much drug rats could take in a session nor were there time-out periods in which drug was unavailable. Subjects were trained daily on the BE procedure until responding became stable, defined as ≤20 percent variation in α and Q0 for at least 3 consecutive days. Rats then began treatment and testing, as outlined below.
4.7. Extinction and reinstatement testing
Rats in Experiment 3 underwent extinction training in daily 2h sessions, where lever presses yielded neither remifentanil nor cues. Rats received extinction training for a minimum of 7 days and until they met the criteria of ≤15 active lever presses for at least two consecutive sessions. The following day, rats were tested for cued reinstatement of remifentanil seeking; responses on the active lever during these sessions resulted in presentation of the cues previously paired with remifentanil infusions, but no remifentanil. Rats received at least 2 additional days of extinction (≤15 active lever presses criterion) before undergoing an additional reinstatement test (James et al., 2018b; McGlinchey et al., 2016). The order in which rats received treatment was counterbalanced.
4.8. Locomotor testing
Rats in Experiment 3 were also tested for general locomotor activity, as described previously (James et al., 2018b; McGlinchey et al., 2016). Rats were placed in locomotor chambers (clear acrylic, 42 × 42 × 30 cm) equipped with SuperFlex monitors (Omnitech Electronics Inc, Columbus, OH) containing a 16 × 16 photobeam array for the x/y-axis (horizontal activity) and 16 photobeams for the z-axis (vertical activity). Photobeam breaks were recorded by Fusion SuperFlex software. Rats were given one 2h session to habituate to the chamber. On subsequent test days, rats received intra-cVP injections of SB or vehicle immediately before being monitored for locomotion over a 2h period; total distance traveled, as well as horizontal and vertical activity were recorded. Rats underwent two tests (drug and vehicle treatments), and the order of treatments was counterbalanced.
4.9. Experimental design
4.9.1. Experiment 1: Systemic SB (30mg/kg, ip) administration immediately prior to initial behavioral economic testing for remifentanil.
Rats were trained to self-administer remifentanil and were tested on the BE paradigm until stable α and Q0 values were obtained. The following day, rats were treated systemically with SB or vehicle and tested on the BE paradigm 0.5h later. Rats were re-tested on the BE paradigm in a drug-free state on two additional sessions, 24h apart (24h and 48h post-treatment). Rats were returned to their home cages between testing sessions. Each animal continued on daily BE sessions until α and Q0 values were within ±20% of pre-treatment values (baseline); rats then underwent testing as before with the opposite treatment (SB or vehicle; order counterbalanced). Full analyses of data from the 0.5h test is presented elsewhere (Mohammdkhani et al., 2018) and data presented here represent a re-analysis of behavioral testing on this initial test and during washout days.
4.9.2. Experiment 2: Intra-VP microinfusions of SB immediately prior to testing for remifentanil economic demand.
Local microinfusions of SB are often used to identify important sites of orexin signaling in reward seeking. Such studies have reported that orexin acts at VP to mediate motivational and hedonic properties of reward (Ho and Berridge, 2013; Mohammdkhani et al., 2018); however, these effects have only been tested acutely. Here we sought to test the persistence of these effects by testing rats on the BE task at 0h following an intra-VP microinjection of SB, and then re-testing in a drug-free state 24h and 48h later. Each rat received only a single injection of SB into VP.
The design of this study was identical to that of Experiment 1, except VP microinjections replaced systemic SB injections. On the day prior to testing, rats were acclimated to the infusion procedure by inserting injectors (28g) bilaterally into the guide cannula that protruded 2.0mm below the bottom of the cannulae; injectors were left in place for 1 min (no infusions were made). The next day, we performed control microinfusions of SB to confirm that any behavioral effects were not due to actions at a dorsal site because of diffusion of SB along the cannula tract. Injections were made dorsal to VP using injectors that projected only 0.2 mm below the tip of the guide cannulae (DV: −7.7mm). The following day, rats received bilateral microinfusions (0.3μl/side) of either SB (1mM) or aCSF into VP using 2.0mm injectors. The order of SB and vehicle microinfusions were counterbalanced and administered via polyethylene tubing connected to gastight 10-μl Hamilton syringes (Hamilton, Reno, NV, USA) set in an infusion pump (Model 975, Harvard Apparatus, Holliston, MA, USA). All microinjections were made immediately prior to the first BE test (0h).
Similar to Experiment 1, full analyses of data from the 0h test is presented elsewhere (Mohammdkhani et al., 2018) and data presented here represent a re-analysis of behavioral testing on this initial test and during subsequent washout days.
4.9.3. Experiment 3: Pretreatment with intra-VP SB 24h prior to initial behavioral economic and cued reinstatement testing.
It is possible that reduced remifentanil intake on the initial BE test following acute SB administration (i.e. at 0h) could affect performance on subsequent tests (i.e. at 24 and 48h). To address this, we prepared a new group of animals with VP cannulae and administered intra-VP microinfusions of SB or aCSF, and then underwent BE testing at 24h, 48h, 72h, and 96h post-treatment. Once BE values returned to pre-treatment values, rats were tested under the opposite treatment condition (aCSF vs SB).
Because this is the first study (to our knowledge) to test the effect of pre-treating with SB locally 24h prior to behavioral testing, we also examined the effects on cued reinstatement of remifentanil seeking, as this behavior is well-documented to be orexin-dependent (James et al., 2017). Remifentanil-seeking was extinguished and rats were tested for cued reinstatement behavior (as described above). Intra-VP microinfusions of SB or aCSF (counterbalanced) were delivered 24h prior to reinstatement testing (following the final extinction session).
Rats were also assessed for general locomotor activity; rats received intra-VP microinjections of SB or aCSF 24h prior to motor assessment (described above). The order in which rats were tested on reinstatement and locomotor tests after BE was fully counterbalanced to account for any order effects associated with repeated intra-VP microinjections.
4.10. Localization of injection sites
After the final behavioral test, rats in Experiments 2 and 3 were deeply anesthetized with ketamine/xylazine (56.6/8.7mg/kg) and received bilateral microinfusions of pontamine sky blue (0.3μl) to mark the locations of the injectors. Rats were then decapitated, and brains were flash-frozen in 2-methylbutane and stored at − 80°C. Brains were sectioned into 40 μm-thick sections on a cryostat (Leica CM 3050), mounted, Nissl-stained with neutral red, and cover slipped to localize cannula tracts and verify injection sites. Rats with injectors located in VP between 0.0 and −0.8mm relative to Bregma were included in these analyses.
4.11. Data analysis
Demand curves (example presented in Fig. 3d) were generated for each BE session as previously described (Bentzley et al., 2013; Porter-Stransky et al., 2017). An exponential demand equation (Hursh and Silberberg, 2008) was applied to each animal’s data to generate a demand curve and estimates of preferred remifentanil intake at zero cost (Q0, where the computed demand curve intercepted the ordinate) and demand elasticity (α, the slope of the demand curve). Larger α values indicate greater demand elasticity and are characterized by a greater reduction in responding as drug price increases; this is interpreted as decreased motivation as described in our previous work (Bentzley et al., 2013). Smaller α values indicated less demand elasticity and indicate continued responding for drug despite increases in the cost to obtain drug (i.e., increased motivation). Curve fitting was performed similarly to our previous studies, whereby all data points up until two bins past the point at which maximal responding was observed (Omax) were included in the generation of demand curves. Unlike our previous cocaine studies where data from the first ‘load up’ bin were excluded from analyses, this was unnecessary here because subjects were not observed to ‘load up’ on remifentanil, as has been reported elsewhere (Mohammdkhani et al., 2018; Panlilio et al., 2003; Porter-Stransky et al., 2017).
Parametric and nonparametric statistical analyses were performed in Graphpad Prism version 6. Acquisition of self-administration, individual differences in BE parameters and the effects of SB on locomotor activity were analyzed using repeated measures ANOVA (total distance traveled) and paired t-tests (horizontal/vertical activity). The effects of SB on active/inactive lever responding during reinstatement tests was assessed using separate two-way repeated measures ANOVAs with ‘treatment’ (vehicle, SB) and ‘lever type’ (active, inactive) as the variables. Corrections (Dunnett) were applied to post hoc tests to reduce the risk of Type 1 errors. Linear regression was used to correlate individual Q0 and α values. Data for self-administration training and initial BE testing for Experiments 1 and 2 are reported elsewhere (Mohammdkhani et al., 2018).
We studied persistent effects of the orexin-1 receptor antagonist SB-334867 on opioid motivation
Systemic injections of SB reduced motivation for remifentanil 24h after administration
Intra-pallidal SB reduced motivation for remifentanil up to 72h after administration
Effects of SB on opioid seeking behavior persist beyond the bioavailability of the compound
Acknowledgements
We would like to thank Drs. Kirsten Porter-Stranksy and Brandon Bentzley for their assistance with the remifentanil demand protocol. We would also like to thank Dr. Hannah Bowrey for her helpful guidance in interpreting the data, as well as Nupur Jain for her assistance.
Funding and Disclosure
This work was supported by financial support from the Institute for Research in Fundamental Sciences (IPM; AM), the National Health and Medical Research Council of Australia (CJ Martin Fellowship No. 1072706; MHJ), the National Institute of Drug Abuse (K99 DA045765, MHJ; F31 DA042588, CBP; and R01 DA006214 to GAJ) as well as the Charlotte and Murray Strongwater Endowment for Neuroscience and Brain Health (GAJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadi-Soleimani SM, Azizi H, Gompf HS, Semnanian S, 2017. Role of orexin type-1 receptors in paragiganto-coerulear modulation of opioid withdrawal and tolerance: A site specific focus. Neuropharmacology. 126, 25–37. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE, 2003. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 464, 220–37. [DOI] [PubMed] [Google Scholar]
- Barson JR, 2018. Orexin/hypocretin and dysregulated eating: Promotion of foraging behavior. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylon GJ, Kaplan HL, Somer G, Busto UE, Sellers EM, 2000. Comparative abuse liability of intravenously administered remifentanil and fentanyl. J Clin Psychopharmacol. 20, 597–606. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G, 2013. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology. 226, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G, 2014. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A. 111, 11822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 41, 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A, 2006. Orexin A in the VTA Is Critical for the Induction of Synaptic Plasticity and Behavioral Sensitization to Cocaine. Neuron. 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 29, 11215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, Espana RA, 2018. Hypocretin receptor 1 involvement in cocaine-associated behavior: Therapeutic potential and novel mechanistic insights. Brain Res. [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Marchant NJ, Lawrence AJ, 2018. A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Research. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC, 2010. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 167, 11–20. [DOI] [PubMed] [Google Scholar]
- Churchill L, Kalivas PW, 1994. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J Comp Neurol. 345, 579–95. [DOI] [PubMed] [Google Scholar]
- Cooper S, Robison AJ, Mazei-Robison MS, 2017. Reward Circuitry in Addiction. Neurotherapeutics. 14, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, Luscher C, 2018. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 95, 322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR, 2010. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 31, 336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Pantazis CB, James MH, Aston-Jones G, 2019. The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology. 44, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, Ramsey NF, Wolterink G, van Ree JM, 1994. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology (Berl). 114, 486–94. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Van Ree JM, 1996. Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res. 713, 114–24. [DOI] [PubMed] [Google Scholar]
- Ghaemi-Jandabi M, Azizi H, Ahmadi-Soleimani SM, Semnanian S, 2017. Intracoerulear microinjection of orexin-A induces morphine withdrawal-like signs in rats. Brain Res Bull. 130, 107–111. [DOI] [PubMed] [Google Scholar]
- Glass PS, Gan TJ, Howell S, 1999. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 89, S7–14. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN, 1993. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 57, 113–42. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ, 1985. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 235, 322–35. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G, 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 437, 556–9. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G, 2007. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 183, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR, 2000. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 96, 45–51. [DOI] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC 3rd, Siegel GS, Bobadilla AC, Kupchik YM, Kalivas PW, 2017. Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. J Neurosci. 37, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA, 2001. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 103, 777–97. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC, 2013. An orexin hotspot in ventral pallidum amplifies hedonic 'liking' for sweetness. Neuropsychopharmacology. 38, 1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychological review. 115, 186. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Jeffrey P, Summerfield S, Rodgers RJ, 2005. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 157, 331–41. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV, 2011. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 14, 684–90. [DOI] [PubMed] [Google Scholar]
- James MH, Aston-Jones G, 2017. Orexin/Hypocretin, Central Amygdala, and Escalation of Cocaine Intake. Biol Psychiatry. 81, 552–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2017. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci. 33, 247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, Aston-Jones G, 2018a. Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, McGlinchey EM, Vattikonda A, Mahler SV, Aston-Jones G, 2018b. Cued Reinstatement of Cocaine but Not Sucrose Seeking Is Dependent on Dopamine Signaling in Prelimbic Cortex and Is Associated with Recruitment of Prelimbic Neurons That Project to Contralateral Nucleus Accumbens Core. International Journal of Neuropsychopharmacology. 21, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G, 2018c. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ, 2011. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 1391, 54–9. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Brown RM, 2014. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 28, 713–30. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW, 1992. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 50, 371–86. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA, 2010. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl). 209, 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS, 2012. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 32, 13309–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G, 2013. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl). 226, 687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014a. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 17, 1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G, 2014b. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 17, 577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 435, 6–25. [DOI] [PubMed] [Google Scholar]
- McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G, 2016. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. The Journal of Neuroscience. 36, 8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, De Fusco C, Monda V, Esposito M, Moscatelli F, Valenzano A, Carotenuto M, Viggiano E, Chieffi S, De Luca V, Cibelli G, Monda M, Messina G, 2016. Role of the Orexin System on the Hypothalamus-Pituitary-Thyroid Axis. Front Neural Circuits. 10, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, 2017. The roles of orexins in sleep/wake regulation. Neurosci Res. 118, 56–65. [DOI] [PubMed] [Google Scholar]
- Mohammad Ahmadi Soleimani S, Azizi H, Mirnajafi-Zadeh J, Semnanian S, 2015. Orexin type 1 receptor antagonism in rat locus coeruleus prevents the analgesic effect of intra-LC met-enkephalin microinjection. Pharmacol Biochem Behav. 136, 102–6. [DOI] [PubMed] [Google Scholar]
- Mohammdkhani A, Pantazis CB, Bowrey HE, James MH, Aston-Jones G, 2018. Orexin-1 receptor signaling in ventral pallidum mediates demand for the opioid remifentanil. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G, 2009. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 43, 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G, 2016. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci. 43, 710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G, 2017. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 1654, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T, 2006. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 26, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono D, Yamanaka A, 2017. Hypothalamic regulation of the sleep/wake cycle. Neurosci Res. 118, 74–81. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW, 2000. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl). 150, 61–6. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW, 2003. Variability of drug self-administration in rats. Psychopharmacology (Berl). 167, 9–19. [DOI] [PubMed] [Google Scholar]
- Pantazis CB, James MH, Bentzley BS, Aston-Jones G, 2019. The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addict Biol. e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. A stereotaxic atlas of the rat brain. New York: Academic. [Google Scholar]
- Perrey DA, Zhang Y, 2018. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF, 1984. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl). 84, 167–73. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Bentzley BS, Aston-Jones G, 2017. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 22, 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N, 2001. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 11, 1907–10. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE, 2001. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 13, 1444–52. [DOI] [PubMed] [Google Scholar]
- Roohbakhsh A, Alavi MS, Azhdari-Zarmehri H, 2018. The Orexinergic (Hypocretin) System and Nociception: An Update to Supraspinal Mechanisms. Curr Med Chem. 25, 3917–3929. [DOI] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC, 2015. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 130, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998a. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 92. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998b. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 92, 1 page following 696. [DOI] [PubMed] [Google Scholar]
- Sassek M, Pruszynska-Oszmalek E, Nowak KW, 2017. Orexin A modulates endocrine function and viability of porcine pancreatic islets. J Physiol Pharmacol. 68, 815–821. [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF, 2017. Hypocretin Neurotransmission within the Central Amygdala Mediates Escalated Cocaine Self-Administration and Stress-induced Reinstatement in Rats. Biological psychiatry. 81, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, 2007. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 27, 1594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G, 2009. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. European Journal of Neuroscience. 30, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G, 2010. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 58, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G, 2012. Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 35, 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ, 2000. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 23, S28–33. [DOI] [PubMed] [Google Scholar]
- Walker LC, Lawrence AJ, 2017. The Role of Orexins/Hypocretins in Alcohol Use and Abuse. Curr Top Behav Neurosci. 33, 221–246. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA, 2009. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 65, 857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA, 2005. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 26, 2331–2338. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV, 2014. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh JW, James MH, Adams CD, Bains JS, Sakurai T, Aston-Jones G, Graham BA, Dayas CV, 2018. Activation of lateral hypothalamic group III metabotropic glutamate receptors suppresses cocaine-seeking following abstinence and normalizes drug-associated increases in excitatory drive to orexin/hypocretin cells. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Galinkin JL, 1999. Psychotropic drugs used in anesthesia practice: abuse liability and epidemiology of abuse. Anesthesiology. 90, 269–88. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE, 2012. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 340, 801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]