Abstract
Sex differences in running behaviors between female and male mice occur naturally in the wild. Recent experiments using head‐fixed mice on a voluntary running wheel have exploited analogous locomotor activity to gain insight into the neural underpinnings of a number of behaviors ranging from spatial navigation to decision‐making. It is however largely unknown if sex differences exist between females and males in a head‐fixed experimental paradigm. To address this, we characterized locomotor activity in head‐fixed female and male C57BL/6J mice on a voluntary running wheel. First, we found that over the initial 7‐day period, on average, animals increased both the velocity and the time spent running. Furthermore, we found that female mice habituated to running forward over the initial 2 days of encountering the wheel, while male mice took up to 4 days to habituate to running forward. Taken together, we characterized features of a sexually divergent behavior in head‐fixed running that should be considered in experiments employing female and male mice.
Keywords: C57BL/6J, head‐fixed, running, sex, sex differences
Over a 7‐day period of exposure to a voluntary running wheel, head‐fixed animals significantly increased the velocity and the amount of time with which they ran. During this period, a difference was found in the time female mice run forward as compared to males. The sex‐specific differences were abolished by day 5, at which point both males and females consistently ran in the forward direction. These data suggest sexually dimorphic behaviors occur during early exposure to head‐fixed running.
1. INTRODUCTION
Across multiple species, variability in behavior due to sex differences can be traced to differences in neural circuits (Mowrey & Portman, 2012; Yang & Shah, 2016). In mice, for instance, behaviors as diverse as fear conditioning and navigation on the Morris water maze vary based on the sex of the animal (Gruene, Flick, Flick, Stefano, Shea, & Shansky, 2015; Keeley, Tyndall, Scott, & Saucier, 2013; Roof & Stein, 1999; Yang et al., 2013). Examples of sex‐specific differences in behavior can also be found outside of the domain of fear and learning as well. The distance or the duration that an animal runs vary between females and males both in the wild (Goh & Ladiges, 2015; Lightfoot, Turner, Daves, Vordermark, & Kleeberger, 2004) and when a running wheel is placed in the animal's home cage (Beatty, 1979; Perrigo & Bronson, 1985). Thus, although a number of behaviors studied in laboratory settings may be sexually divergent (An et al., 2011; Beery, 2018; Wald & Wu, 2010; Zucker & Beery, 2010), much of what is known comes from experiments that either exclusively used male mice (Tronson, 2018) or may not have treated sex as an independent variable when both female and male animals were studied (Shansky & Woolley, 2016). For example, a common experiment involves head‐fixing an awake behaving rodent and placing it onto a running wheel to study the circuits involved in sensory processing (Niell, Stryker, & Keck, 2010; Smear, Shusterman, O’Connor, Bozza, & Rinberg, 2011), spatial navigation (Dombeck, Harvey, Tian, Looger, & Tank, 2010; Harvey, Collman, Dombeck, & Tank, 2009) and decision‐making (Abraham et al., 2010; Juavinett, Erlich, & Churchland, 2018; Smear, Resulaj, Zhang, Bozza, & Rinberg, 2013). Despite the ubiquity of this paradigm in systems neuroscience, and the importance of measuring running either as a feature or a confound of experiments, it remains unclear if there are differences between females and males.
To explore this question, we analyzed the running behavior of head‐fixed female and male 2‐ to 3‐month‐old mice over a 7‐day period on a voluntary running wheel. While both females and males increased running over this duration, we saw significant sex differences in the direction that mice ran during the early days of exposure to the running wheel. Within 2 days on the wheel, all female mice ran on average forward, while male mice continued to move on average backward. It was not until day 5 that male mice ran forward. These data suggest sexually dimorphic behaviors occur during early exposure to head‐fixed running.
2. MATERIALS AND METHODS
2.1. Animals
36 C57BL/6J (The Jackson Laboratory) mice, 18 female and 18 male mice, 2–3 months old were utilized for this experiment. Nine female and nine male mice were used for run‐habituation following surgery, and nine female and nine male mice were used for run‐habituation 1 week after surgery. All experiments and procedures were approved by the University Committee on Animal Resources (UCAR) at the University of Rochester Medical Center.
2.2. Head‐fix procedure
Prior to the procedure, animals were dosed with 3.25 mg/kg slow‐release buprenorphine via subcutaneous injection. Animals were anesthetized with 1%–2% vaporized isoflurane in 1.5–2.5 L/min of O2 and then placed in a stereotaxic for surgery (Kopf Instruments, Tujunga, CA, USA). Following a midline incision on the skull, connective tissue was resected and excess skin removed and vetbond (3M) was placed to attach the perimeter skin to the skull. A 3D‐printed headframe was then put into place and was affixed to the skull using Liquid‐Jet dental cement (Lang Dental) taking care to provide enough clearance for the ears. The area was then allowed to dry completely prior to placing the animal into the home cage for recovery. Animals were recovered for 24 hr prior to behavioral habituation.
2.3. Open‐field test
All open‐field testing was conducted in an isolated room with black walls, floor and ceiling to minimize external visual or auditory cues. Animals were placed into a 36‐inch square plexiglass chamber with raised sides, 12 inches in height. An overhead camera was used to track the movement of the animal during the test. Testing was completed before the headframe procedure (baseline), 24 hr after the headframe procedure (headframe) and after the 7 days of running wheel exposure (post‐training). Each test consisted of 5 min of free running. The testing chamber was thoroughly cleaned between each trial to minimize any olfactory cues. Post‐testing analysis was completed using custom MATLAB code (Mathworks).
2.4. Running wheel habituation
Animals were habituated on the running wheel beginning either 24 hr after head‐fix procedure or after seven days of recovery from surgery for seven consecutive days. Mice were weighed daily prior to habituation to ensure that animals did not lose significant body weight (more than 20% of baseline weight in accordance with the policies approved by the University Committee on Animal Resources [UCAR]). Animals were habituated for one hour per day on a cylindrical voluntary running wheel that allowed for both forward and backward running. While animals were monitored remotely with a camera, all habituation took place in darkness, and during habituation, there was no intervention or light input. Habituation was always completed during the animals’ light cycle. Post‐testing analysis was completed using custom MATLAB code.
3. RESULTS
To explore running behavior in head‐fixed animals, we first implanted a 3D‐printed headframe to the skull (Figure 1a, left) that allowed the animal to be placed on a non‐motorized cylindrical running wheel. Following this procedure, animals were given 24 hr to recover prior to seven consecutive days of exposure to the running wheel (Figure 1a, middle). Beginning on day 1, mice were placed on the wheel with their head fixed for 1 hr (Figure 1a, right), allowing them to voluntarily rotate the wheel forward or backward. A representative 15‐min trace from the 1‐hr period for a single animal on day 1 showed epochs of running in the forward direction (positive velocity, Figure 1b) and in the backward direction (negative velocity, Figure 1b). Interspersed between the bouts of running were periods where the animal remained stationary (inset, Figure 1b). To quantify these features of running, we first calculated the velocity over the 7 days of running wheel exposure. We found a significant increase in the overall velocity of running between day 1 and day 7 (N = 18; day 1, 0.92 ± 3.83 cm/s; day 7, 3.92 ± 3.38 cm/s; p = <.001; Wilcoxon rank‐sum test, Figure 1c). Furthermore, across all animals, there was a significant increase in the percentage of time spent running between day 1 and day 7 (N = 18; day 1:6.92 ± 12.15% day 7:32.48 ± 15.95%; p < .0001; Wilcoxon rank‐sum test; Figure 1d). Over the 7‐day habituation period, as the animals grew accustomed to the running wheel, they ran faster and more frequently. After the initial 7‐day exposure period, we observed no increases in either run velocity or duration in animals habituated for more than 20 days (N = 2).
Figure 1.
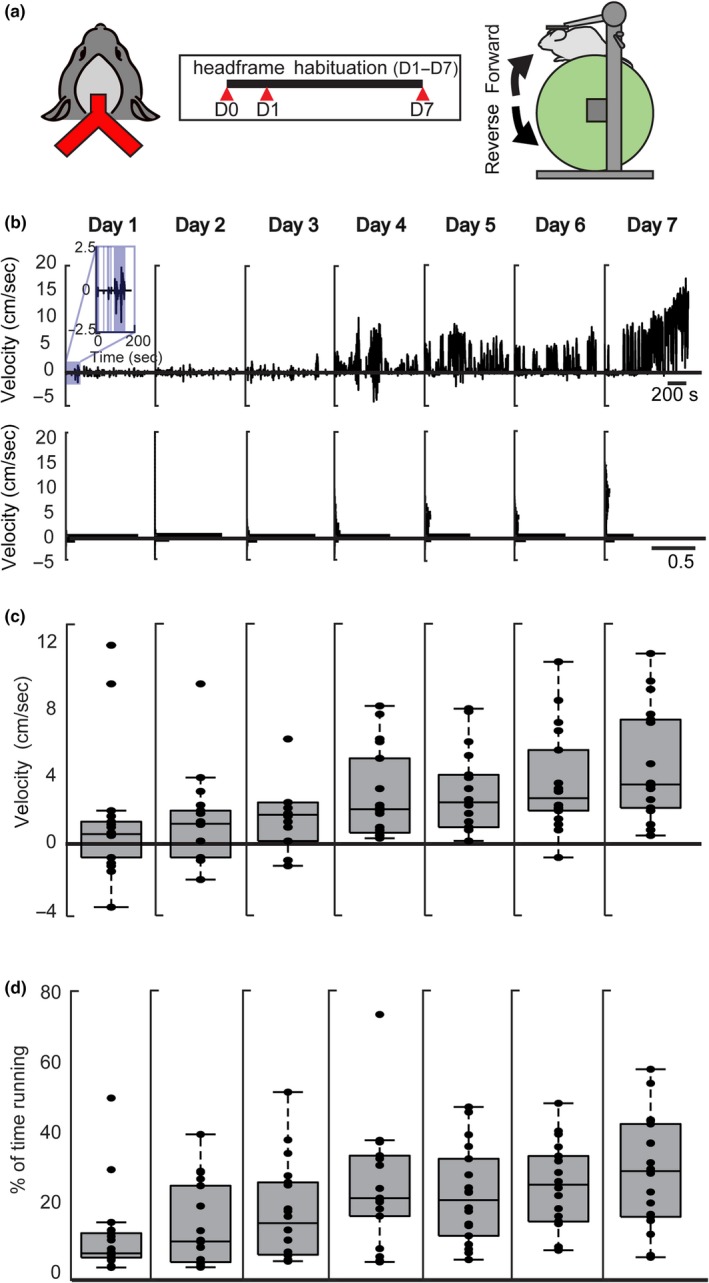
Velocity and percent of time spent running increase during the habituation period. (a, left) Schematic of 3D‐printed headframe affixed to the surface of the skull with wings for head‐fixing during running. (a, middle) Experimental timeline where headframe surgery takes place on day 0, and habituation consists of seven consecutive days beginning 24 hr after surgery (D1‐D7). (a, right) Running wheel schematic where the head‐fixed mouse can run forward (clockwise) or reverse (counterclockwise) on a cylindrical, voluntary running wheel. (b) Example running behavior trace for one animal shows running in both the forward and backward direction. Inset: blue‐shaded areas show epochs of running, and non‐shaded shows epochs where the animal is stationary on the wheel. (c) Velocity analysis for all animals (N = 18). The median velocity for each animal is plotted for each of the 7 days following headframe. Significant increase in velocity between day 1 and day 7 (day 1 median velocity = 0.92 ± 3.83 cm/s, day 7 median velocity = 3.92 ± 3.38 cm/s, p = .0004, Wilcoxon rank‐sum test). Error bars are standard deviation. (d) Percentage of time spent running for all animals (N = 18). The median percentage of time spent running for each animal is plotted for each of the 7 days. Significant increase in the percentage of time spent running between day 1 and day7 (day 1 median time spent running = 6.92 ± 12.15%, day 7 median time spent running = 32.48 ± 15.95%, p = .000072, Wilcoxon rank‐sum test). Error bars are standard deviation
Previous work has identified sex differences in locomotor behavior in wild mice (Goh & Ladiges, 2015; Lightfoot et al., 2004), including differences in locomotor activity when a running wheel is placed in the animal's home cage (Perrigo & Bronson, 1985). To determine whether such differences were also found in head‐fixed mice on a running wheel, we first plotted the distribution of velocity (Figure 2a) excluding epochs where the animal did not move (no movement = activity >−0.5 or <0.5 cm/s) for females (Figure 2a top, N = 9, red) and males (Figure 2a, bottom N = 9, black) across the first 7 days of exposure to the running wheel. Although female mice ran faster (Figure 2b, day 1: N = 9 females, forward velocity = 1.67 ± 3.82 cm/s, reverse velocity = −0.67 ± 0.12 cm/s, N = 9 males, forward velocity = 1.08 ± 2.99 cm/s, reverse velocity = −0.67 ± 1.02 cm/s, forward velocity: p = .78, reverse velocity: p = .51, Wilcoxon rank‐sum test; day 7: N = 9 females, forward velocity = 3.84 ± 3.78 cm/s, reverse velocity = −0.50 ± 0.26 cm/s, N = 9 males, forward velocity = 4.67 ± 2.90 cm/s, reverse velocity = −0.67 ± 0.39 cm/s, forward velocity: p = .88, reverse velocity: p = .12, Wilcoxon rank‐sum test) and more often (Figure 2c, day 1: N = 9 females, percent time running = 8.23 ± 15.10%, N = 9 males, percent time running = 6.34 ± 8.98%, p = .55, Wilcoxon rank‐sum test; day 7: N = 9 females, percent time running = 26.53 ± 16.99%, N = 9 males, percent time running = 33.09 ± 15.87%, p = .80, Wilcoxon rank‐sum test) than male animals over the 7‐day exposure period, consistent with previous work on locomotor activity in home‐cage running wheels (Perrigo & Bronson, 1985), these differences were not significant. To further dissect properties of locomotion during the period, we analyzed not only the time and speed, but the time spent running either forward or backward (Figure 2d). During the first day on the running wheel, no significantly differences were found between females and males (day 1, N = 9 females, percent time females run backward = 20.01% ± 25.20%, N = 9 males, percent time males run backward = 70.45% ± 39.22%, p = .26, Wilcoxon rank‐sum test). On days 2 through 4, however, we found that males spent significantly more time running backward when compared to females (N = 9 females, N = 9 males, day 2: female = 3.68% ± 25.03%, male = 48.72% ± 38.11%, p = .03; day 3: female = 1.17% ± 12.47%, male = 27.19% ± 36.79%, p = .01; day 4: female = 2.50% ± 9.95%, male = 16.82% ± 13.19%, p = .03, Wilcoxon rank‐sum test for all days). By day 5, these sex‐specific differences in head‐fixed running were no longer present.
Figure 2.
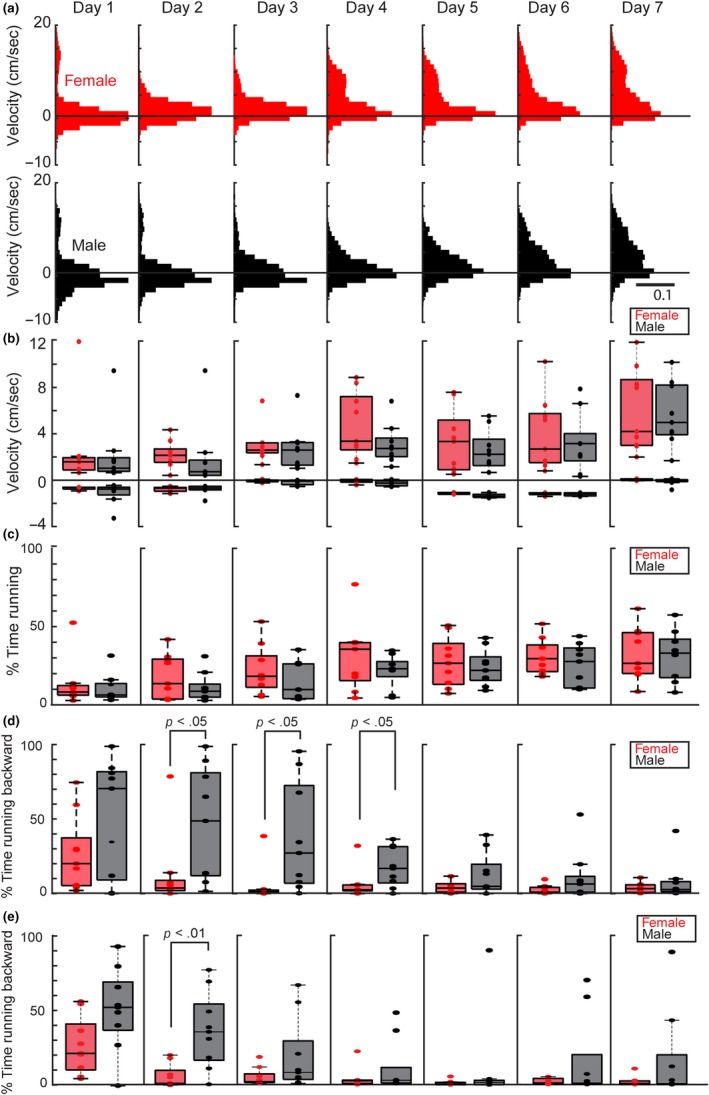
Female mice spend less time running backward compared to male mice. (a) Velocity histogram for females (red, N = 9) and males (black, N = 9) when animals were running (velocities >±0.5 cm/s). The solid black line indicates a velocity of zero. (b) Median velocity traces for females (red, N = 9) and males (black, N = 9) for the 7 days of habituation. Forward velocity and reverse velocity are plotted separately across days. The solid black line indicates a velocity of zero. Error bars are standard deviation. (c) The median percentage of time spent running for females (red, N = 9) and males (black, N = 9) for the 7 days of habituation. The solid black line indicates zero time spent running. Error bars are standard deviation. (d) The median percentage of time spent running in the backward direction for females (red, N = 9) and males (black, N = 9) for the 7 days of habituation. The solid black line indicates zero time spent running reverse. Error bars are standard deviation. Significant differences were seen on days 2, 3 and 4 (day 2, N = 9 females, N = 9 males, female = 3.68% ± 25.03%, male = 48.72% ± 38.11%, p = .03; day 3 female = 1.17% ±12.47%, day 3 male = 27.19% ± 36.79%, p = .01; day 4 female = 2.50% ± 9.95%, day 4 male = 16.82% ± 13.19%, p = .03, Wilcoxon rank‐sum test for all days). (e) The median percentage of time spent running backwards for females (red, N = 9) and males (N = 9) over a 7‐day period following 1 week of recovery. The solid black line indicates zero time spent running backwards. Error bars are standard deviation. Significant differences between males and females were seen on day 2 (day 2: N = 9 females, N = 9 males, day 2: female = 1.45% ± 7.83%, male = 35.42% ± 25.22%, p = .005)
To ensure that these sex differences were not due to differences in post‐operative recovery between males and females, we repeated the headframe procedure and waited 7 days before beginning running wheel habituation (Figure 2e). Consistent with the previous experiment, on day 1 we found that while females spent less time running in reverse as compared to males, (day 1: N = 9 females, percent time females run backward = 22.34% ± 19.03%, N = 9 males, percent time males run backward = 52.94% ± 27.44%), this difference was not statistically significant (p = .09, Wilcoxon rank‐sum test). However, by day 2, female mice spent 1.45% ± 7.83% of the time running backward, significantly less than the time male mice spent running backward (35.42% ± 25.22% N = 9 females, N = 9 males, day 2, p = .005, Wilcoxon rank‐sum test). The trend persisted on days 3 and 4, with female mice running backward less than the male mice (day 3: female = 1.28% ± 6.16%, male = 7.32% ± 24.92%, day 4: female = 2.33% ± 6.98%, male = 2.87% ± 18.14%), but these differences were not statistically significant (day 3: p = .16, Day 4 p = .40, Wilcoxon rank‐sum test for both days). Taken together, these data suggest that sex differences in head‐fixed running during are presented in the early days of habituation and that these differences were presented across different post‐operative recovery times.
To ensure that the differences observed in head‐fixed running were not due to changes in general locomotor activity between female and male mice, we measured animal behavior in an open‐field environment before the headframe procedure (Figure 3a, d), 24 hr following the procedure (Figure 3b, e) and 7 days after the surgery (Figure 3c, f) in both females (N = 8, Figure 3a–c) and males (N = 9, Figure 3d–f). Between Day 1 and Day 7 of the open‐field test, animals were also habituated the running wheel for 1 hr daily. Consistent with previous work (O’Keefe & Dostrovsky, 1971), animals explored both the edges and the center of the open‐field environment (Figure 3a–f, top). Furthermore, both females and males moved around the environment. We quantified this movement by measuring the speed of running (Figure 3a–f, bottom) measuring the run speed before surgery (Figure 3g, top), 24 hr after the headframe procedure (Figure 3g, middle) and 7 days following run habitation (Figure 3g, bottom). Across all three conditions, we found no significant differences between males and females (n = 9 males, N = 8 females, Pre: p = .7, Post: p = .6, 7‐days after: p = .9, Wilcoxon rank‐sum test).
Figure 3.
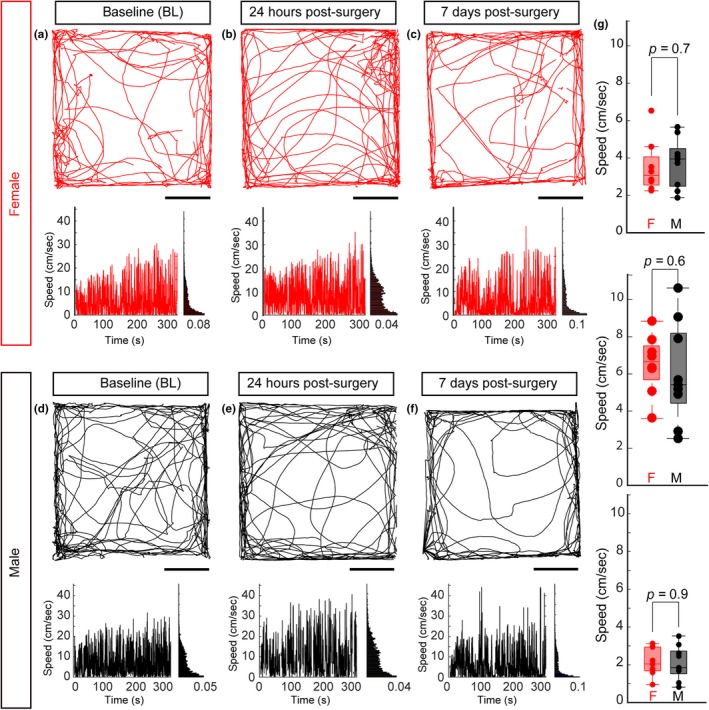
No locomotor differences between female and male mice following headframe implantation. Example trace of a single mouse movement in an open‐field test (a, d) before the headframe implantation, (b, e) 24 hr after the headframe procedure and (c, f) after 7 days of voluntary running wheel exposure for an example female (a–c) and example male (d–f). (a–f, top) The trajectory the animal took over a 5‐min period. Scale bar = 10 inches. (A‐F, bottom, left) Instantaneous speed over time for the trace in the top panel. (Bottom, right) Distribution of the speed over the duration of the 5‐min period. (g) No differences in speed between female and male mice before the headframe implantation (top, N = 9 female, N = 8 male, p = .7, ANOVA), 24 hr after surgery (middle, p = .6, ANOVA) and after 7 days of habituation (bottom, p = .9 ANOVA)
Finally, in addition to the differences we observed in running between females and males, we identified specific features of head‐fixed locomotion that highlighted the diverse ways in which animals habituated to the running wheel over the first 7 days. A plot of normalized position (the cumulative sum of the distance the animal ran over the habituation period divided by the final position of the animal) for two example female (red = day 1, yellow = day 7) and two example male (blue = day 1, green = day 7) mice illustrated three hallmarks in their behavior (Figure 4a). First, on the day when an animal switched from running backward to forward, the moment of this transition was abrupt and included a prolonged bout of forward running. This transition occurred in 7/9 males and 3/9 females, although the day on which it occurred varied across individuals and across both sexes. Second, we observed that once animals ran in a forward direction, they exhibited a stereotypic pattern of locomotion, running forward for brief epochs followed by periods where they remained stationary as illustrated by plots of the acceleration for the four example animals across days 5–7 (Figure 4b, day 5 top row, day 7 bottom row, color corresponding to code in Figure 4a). In each case, periods of forward movement (Figure 4b, black arrows) were interleaved with periods where the animal remained stationary (Figure 4b, gray arrows). Although each individual covered a different distance over the 1‐hr period, the way in which they covered that distance was common across animals. Finally, we found that once an animal transitioned from running backward to running forward, they did not revert to running backward (Figure 4c). We represented this in a change box matrix for all the females and males across the 7 days, where white was a change from running backward on the previous day to running forward, black indicated no change in direction of running, and gray corresponded to a change running from forward to backward.
Figure 4.
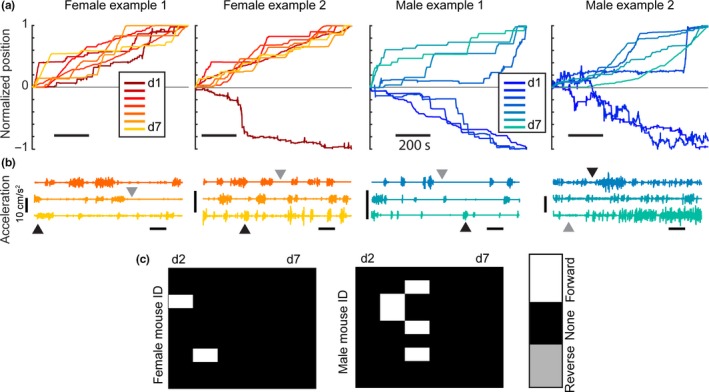
Individual animals display common hallmarks of running behavior. (a) Normalized position for 2 female (red) and 2 male (blue) examples. Normalized position results from the cumulative sum of the distance the animal ran over the habituation period divided by the final position of the animal. Running in the forward direction results in an end position at +1 while running in the reverse direction results in an end position at −1. (b) Acceleration plots for females and males for the last three days of habituation (day 5–7). Black arrows indicate active running while gray arrows indicate periods where the mouse is stationary. (c) Change plots displaying the change in direction between days. Columns represent the difference between the day listed and the day before. Rows are individual animals. The white boxes show a change in running direction from reverse to forward running. The black boxes are no change in direction. The gray boxes are a change from running forward to running in reverse (no gray boxes present)
4. DISCUSSION
In this work, we found that over a 7‐day period of exposure to a voluntary running wheel, head‐fixed animals increased the velocity and the amount of time with which they ran. When we examined sex differences across this period, we observed a significant difference in the time when female mice run forward as compared to males. As a result, female mice had a larger velocity of running on average as compared to male mice, but this difference was not statistically significant. The sex‐specific differences we observed were abolished by day 5, at which point both males and females consistently ran in the forward direction. While anesthesia did influence the magnitude of differences in running after 3 days, we observed that the early sex differences in head‐fixed running persisted on day 2 across different recovery times (either when habituation was done 24 hr following surgery, or after 1 week of recovery time). Additionally, the trend of male mice spending more periods of time running in reverse was presented on days 3 and 4 following 7 days of recovery, suggesting that the sexually dimorphic behaviors associated with run direction early in habitation reflect a general feature of behavioral heterogeneity. On the individual level, several hallmarks of locomotor activity could be observed in both females and males. These included a sudden burst in running as well as epochs of running interleaved with periods of locomotor inactivity. Our findings suggest that some features of the sex differences previously characterized either in free running (Goh & Ladiges, 2015; Lightfoot et al., 2004) or in a running wheel (Perrigo & Bronson, 1985) placed in a rodent's home cage are recapitulated in head‐fixed experiments.
As increased attention is being placed on understanding sex as a biological variable in animal experiments both from investigators and from federal agencies such as the National Institutes of Health (NIH) in the United States (Clayton & Collins, 2014), our results parallel a number of others illustrating how considering sex as an independent variable can reveal important differences in behavior (Gruene, Flick, et al., 2015; Gruene, Roberts, Roberts, Thomas, Ronzio, & Shansky, 2015; Kim et al., 2015; Lin et al., 2011; Yang et al., 2013). For instance, sexually divergent behaviors emerge when Pavlovian fear responses in classical conditioning experiments are analyzed not only in terms of whether an animal freezes or not, but the kind of motion that happens if an animal does move (Gruene, Flick, et al., 2015). Not unlike these experiments, the initial introduction to the head‐fixed running wheel represents a novel environment that the mouse grows accustomed to with increasing days of experience. It is therefore not surprising that sexually divergent strategies for running may emerge over the initial habituation period.
While the underlying mechanisms governing differences in run behavior go beyond the scope of this study, prior work points to a number of targets that may provide context for our results. First, wheel running has often been framed in the context of energy demands, and a number of studies have shown that female mice run more than males and that this increase in running correlates to increased energy expenditure and increased food intake (Perrigo & Bronson, 1985; Tokuyama, Saito, & Okuda, 1982). Wheel running in this context could serve as an experimental proxy for energy allocation, with locomotor effort and food availability pitted against one another (Perrigo & Bronson, 1985). Consequently, it is not surprising that the demands of running and energy allocation may be different between females and males, given that female and male mice may employ different survival and reproductive strategies (Townsend & Calow, 1981). Nor is it surprising that differences in run behavior can be heavily influenced by hormonal regulation (Beatty, 1979; Perrigo & Bronson, 1985). In Swiss albino mice for instance, females are more active than males, but exposing female animals to testosterone early in life reduces their home‐cage running activity (Broida & Svare, 1984). Conversely, castration of males, particularly early in development, increases the amount the animals run (Beatty, 1979; Broida & Svare, 1984), suggesting that hormonal differences can influence running behavior. Interestingly, when paired with a food reward, locomotor activity changed only in males following gonadectomy (Perrigo & Bronson, 1985).
A common theme in these studies is that the timing (age of the animal) of hormonal manipulations plays a major factor in affecting locomotor activity. Not surprisingly then, differences in wheel running between females and males are age‐dependent (Bartling, Al‐Robaiy, Lehnich, Binder, & Hiebl, 2017). At two months of age, females show much higher levels of locomotor activity, but this difference reduces with age (Bartling et al., 2017; Koteja, Garland, Sax, Swallow, & Carter, 1999; Perrigo & Bronson, 1985). In addition to age, behavior can also be strain‐dependent. Castration of C57BL/6J males results in greater decreases in locomotor activity as compared to castration in DBA/2J, for example (Broida & Svare, 1983). Our results are performed in 2‐ to 3‐month‐old C57BL6/J mice, a specific combination of age and strain within a larger space of parameters that likely influence the duration, direction and degree of running. While the age at which we identified head‐fixed differences in running corresponds to the age at which the greatest difference in wheel running has been reported (Bartling et al., 2017), and the strain (C57BL/6J) in which we performed these experiments appear to be the most sensitive to changes in locomotor activity (Broida & Svare, 1983), our results nonetheless highlight the importance of considering sex as a variable in experiments that use mice in a head‐fixed run wheel paradigm.
In behavioral tasks ranging from spatial navigation (Dombeck et al., 2010; Meshulam, Gauthier, Brody, Tank, & Bialek, 2017), to those that investigate how running modulates sensory processing (Niell et al., 2010), a consideration of sex could be important for interpreting results (Shansky & Woolley, 2016) and/or identifying the underlying neural systems that give rise to these behavioral (Beatty, 1979; Perrigo & Bronson, 1985). Finally, our data suggest that the early differences in head‐fixed running could also serve as an experimental framework to investigate the natural diversity of neural circuits involved in fear (Hauner, Howard, Zelano, & Gottfried, 2013; Pibiri, Nelson, Guidotti, Costa, & Pinna, 2008; Yang & Shah, 2016), spatial reasoning (Harvey et al., 2009) and anxiety (An et al., 2011; Ciocchi, Passecker, Malagon‐Vina, Mikus, & Klausberger, 2015). Beyond their relevance to understanding the natural diversity of behaviors (Shansky & Woolley, 2016), the inclusion of female animals in experiments can provide insight into divergent circuits that shape natural behaviors (Tronson, 2018; Yang & Shah, 2016) and the extent to which such differences translate to different vulnerabilities to neurological and psychiatric disorders based on sex (Earls, 1987; The Lancet Neurology, 2019).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relating to the design and findings of this manuscript.
AUTHOR CONTRIBUTIONS
KP supervised this project. EJW performed the experiments. EJW and KP performed the analysis, made the figures and wrote the manuscript.
ACKNOWLEDGEMENTS
KP is supported by NIMH R00 MH101634, NIMH R01 MH113924, NSF CAREER Award, the Cystinosis Research Foundation and the Kilian J. and Caroline F. Schmitt.
Warner EJ, Padmanabhan K. Sex differences in head‐fixed voluntary running behavior in C57BL/6J mice. Eur J Neurosci. 2020;51:721–730. 10.1111/ejn.14654
Edited by Christina Dalla.
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.14654
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, KP, upon reasonable request.
REFERENCES
- Abraham, N. M. , Egger, V. , Shimshek, D. R. , Renden, R. , Fukunaga, I. , Sprengel, R. , … Kuner, T. (2010). Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron, 65, 399–411. 10.1016/j.neuron.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, X.‐L. , Zou, J.‐X. , Wu, R.‐Y. , Yang, Y. , Tai, F.‐D. , Zeng, S.‐Y. , … Broders, H. (2011). Strain and sex differences in anxiety‐like and social behaviors in C57BL/6J and BALB/cJ Mice. Experimental Animals, 60, 111–123. [DOI] [PubMed] [Google Scholar]
- Bartling, B. , Al‐Robaiy, S. , Lehnich, H. , Binder, L. , & Hiebl, B. (2017). Sex‐related differences in the wheel‐running activity of mice decline with increasing age. Experimental Gerontology, 87, 139–147. [DOI] [PubMed] [Google Scholar]
- Beatty, W. W. (1979). Gonadal hormones and sex differences in nonreproductive behaviors in rodents: Organizational and activational influences. Hormones and Behavior, 12, 112–163. 10.1016/0018-506X(79)90017-5 [DOI] [PubMed] [Google Scholar]
- Beery, A. K. (2018). Inclusion of females does not increase variability in rodent research studies. Current Opinion in Behavioral Sciences, 23, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broida, J. , & Svare, B. (1983). Genotype modulates testosterone‐dependent activity and reactivity in male mice. Hormones and Behavior, 17, 76–85. [DOI] [PubMed] [Google Scholar]
- Broida, J. , & Svare, B. (1984). Sex differences in the activity of mice: Modulation by postnatal gonadal hormones. Hormones and Behavior, 18, 65–78. 10.1016/0018-506X(84)90051-5 [DOI] [PubMed] [Google Scholar]
- Ciocchi, S. , Passecker, J. , Malagon‐Vina, H. , Mikus, N. , & Klausberger, T. (2015). Selective information routing by ventral hippocampal CA1 projection neurons. Science, 348, 560–563. [DOI] [PubMed] [Google Scholar]
- Clayton, J. A. , & Collins, F. S. (2014). Policy: NIH to balance sex in cell and animal studies. Nature, 509, 282–283. 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck, D. A. , Harvey, C. D. , Tian, L. , Looger, L. L. , & Tank, D. W. (2010). Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nature Neuroscience, 13, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls, F. (1987). Sex differences in psychiatric disorders: Origins and developmental influences. Psychiatric Developments, 5, 1–23. [PubMed] [Google Scholar]
- Goh, J. , & Ladiges, W. (2015). Voluntary wheel running in mice. Current Protocols in Mouse Biology, 5, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene, T. M. , Flick, K. , Stefano, A. , Shea, S. D. , & Shansky, R. M. (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. Elife, 4, e11352 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene, T. M. , Roberts, E. , Thomas, V. , Ronzio, A. , & Shansky, R. M. (2015). Sex‐specific neuroanatomical correlates of fear expression in prefrontal‐amygdala circuits. Biological Psychiatry, 78, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, C. D. , Collman, F. , Dombeck, D. A. , & Tank, D. W. (2009). Intracellular dynamics of hippocampal place cells during virtual navigation. Nature, 461, 941–946. 10.1038/nature08499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner, K. K. , Howard, J. D. , Zelano, C. , & Gottfried, J. A. (2013). Stimulus‐specific enhancement of fear extinction during slow‐wave sleep. Nature Neuroscience, 16, 1553–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juavinett, A. L. , Erlich, J. C. , & Churchland, A. K. (2018). Decision‐making behaviors: Weighing ethology, complexity, and sensorimotor compatibility. Current Opinion in Neurobiology, 49, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley, R. J. , Tyndall, A. V. , Scott, G. A. , & Saucier, D. M. (2013). Sex difference in cue strategy in a modified version of the Morris water task: Correlations between brain and behaviour. PLoS ONE, 8, e69727 10.1371/journal.pone.0069727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Venkataraju, K. U. U. , Pradhan, K. , Mende, C. , Taranda, J. , Turaga, S. C. C. , … Osten, P. (2015). Mapping social behavior‐induced brain activation at cellular resolution in the mouse. Cell Reports, 10, 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteja, P. , Garland, T. , Sax, J. K. , Swallow, J. G. , & Carter, P. A. (1999). Behaviour of house mice artificially selected for high levels of voluntary wheel running. Animal Behavior, 58, 1307–1318. [DOI] [PubMed] [Google Scholar]
- Lightfoot, J. T. , Turner, M. J. , Daves, M. , Vordermark, A. , & Kleeberger, S. R. (2004). Genetic influence on daily wheel running activity level. Physiological Genomics, 19, 270–276. [DOI] [PubMed] [Google Scholar]
- Lin, D. , Boyle, M. P. , Dollar, P. , Lee, H. , Lein, E. S. , Perona, P. , & Anderson, D. J. (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature, 470, 221–226. 10.1038/nature09736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam, L. , Gauthier, J. L. , Brody, C. D. , Tank, D. W. , & Bialek, W. (2017). Collective behavior of place and non‐place neurons in the hippocampal network. Neuron, 96, 1178–1191. 10.1016/j.neuron.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrey, W. R. , & Portman, D. S. (2012). Sex differences in behavioral decision‐making and the modulation of shared neural circuits. Biology of Sex Differences, 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell, C. M. , Stryker, M. P. , & Keck, W. M. (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron, 65, 472–479. 10.1016/j.neuron.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe, J. , & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Research. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- Perrigo, G. , & Bronson, F. H. (1985). Sex differences in the energy allocation strategies of house mice. Behavioral Ecology and Sociobiology, 17, 297–302. [Google Scholar]
- Pibiri, F. , Nelson, M. , Guidotti, A. , Costa, E. , & Pinna, G. (2008). Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proceedings of the National Academy of Sciences, 105, 5567–5572. 10.1073/pnas.0801853105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof, R. L. , & Stein, D. G. (1999). Gender differences in Morris water maze performance depend on task parameters. Physiology & Behavior, 68, 81–86. [DOI] [PubMed] [Google Scholar]
- Shansky, R. M. , & Woolley, C. S. (2016). Considering sex as a biological variable will be valuable for neuroscience research. Journal of Neuroscience, 36, 11817–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smear, M. , Resulaj, A. , Zhang, J. , Bozza, T. , & Rinberg, D. (2013). Multiple perceptible signals from a single olfactory glomerulus. Nature Neuroscience, 16, 1687–1691. [DOI] [PubMed] [Google Scholar]
- Smear, M. , Shusterman, R. , O’Connor, R. , Bozza, T. , & Rinberg, D. (2011). Perception of sniff phase in mouse olfaction. Nature, 479, 397–400. 10.1038/nature10521 [DOI] [PubMed] [Google Scholar]
- The Lancet Neurology (2019). A spotlight on sex differences in neurological disorders. The Lancet Neurology, 18, 319. [DOI] [PubMed] [Google Scholar]
- Tokuyama, K. , Saito, M. , & Okuda, H. (1982). Effects of wheel running on food intake and weight gain of male and female rats. Physiology & Behavior, 28, 899–903. [DOI] [PubMed] [Google Scholar]
- Townsend, C. R. , & Calow, P. (1981). Physiological ecology. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- Tronson, N. C. (2018). Focus on females: A less biased approach for studying strategies and mechanisms of memory. Current Opinion in Behavioral Sciences, 23, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald, C. , & Wu, C. (2010). Of mice and women: the bias in animal models. Biomedical research, 327, 1571–1572. 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- Yang, C. F. , Chiang, M. C. , Gray, D. C. , Prabhakaran, M. , Alvarado, M. , Juntti, S. A. , … Shah, N. M. (2013). Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell, 153, 896–909. 10.1016/j.cell.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. , & Shah, N. M. (2016). Molecular and neural control of sexually dimorphic social behaviors. Current Opinion in Neurobiology, 38, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker, I. , & Beery, A. K. (2010). Males still dominate animal studies. Nature, 465, 69 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, KP, upon reasonable request.


