Abstract
Background:
We sought to compare the safety and efficacy between two analgesic regimens for patients with head and neck cancer (HNC) undergoing definitive chemoradiation (CRT).
Methods:
This was a prospective, single institution, two-arm, randomized pilot study. Patients with AJCC 7th edition stage II-IV HNC squamous cell carcinoma undergoing CRT were randomized to either arm 1) high dose gabapentin (2700 mg daily) with our institutional standard of care (hydrocodone/acetaminophen progressing to fentanyl as needed) or 2) low dose gabapentin (900 mg daily) with methadone. The primary endpoint was safety and toxicity. Secondary endpoints were pain, opioid requirement, and quality of life (QOL). Differences between arms at multiple time points were compared using a generalized linear mixed regression model with Sidak correction.
Results:
Sixty patients (n=31 in arm 1; n=29 in arm 2) from April, 2015 to August, 2017 were enrolled. There was no difference between arms for adverse events or serious adverse events. Pain was not different between arms. More patients in arm 1 did not require an opioid during treatment (42% vs 7%, p=0.002). Arm 2 had significantly better QOL outcomes across multiple domains, including better overall health (p=0.05), better physical functioning (p=0.04), role functioning (p=0.01), and social functioning (p=0.01).
Conclusions:
High dose prophylactic gabapentin increased the percentage of patients who required no opioid during treatment. Methadone may improve QOL compared to a regimen of short acting opioids and fentanyl. Pain, however, significantly worsened throughout treatment regardless of arm, necessitating further studies to identify a more optimal regimen.
Keywords: Oral mucositis, OMWQ-HN, EORTC QLQ-C30, Patient-report outcomes, Quality of life
Precis
For patients undergoing chemoradiation for head and neck cancer, prophylactic gabapentin with methadone can improve quality of life compared to a gabapentin-based analgesic regimen of short acting opioids and fentanyl.
Introduction
In 2018, there were an estimated 51,540 cases and 10,030 deaths from head and neck cancer (HNC) in the United States1. At least 60% of these patients present with locally advanced, non-metastatic disease2. Concurrent chemotherapy and radiation (CRT) is a standard of care used for inoperable patients and for organ-preservation. Oral mucositis (OM) occurs in greater than 80% of patients undergoing CRT3,4 and causes severe pain, dysphagia, increased aspiration risk, weight loss leading to feeding tube placement, a decrease in quality of life (QOL), and potentially an increase in treatment breaks, hospitalizations and medical care costs5–10. The majority of patients undergoing treatment for HNC report their pain using neuropathic descriptors11.
Prophylactic gabapentin can reduce total opioid requirement and unintentional weight loss, delay feeding tube use and decrease unplanned treatment breaks in patients undergoing CRT for HNC12–16. Combining gabapentin with opioids improves pain relief at lower opioid doses and improves daily activity, mood, sleep and QOL in patients undergoing treatment for HNC17. The dose response of gabapentin is not known. Similarly, although methadone has relatively complex pharmacokinetics, due to its effects on the N-Methyl-D-Aspartate receptor, methadone may be more effective for neuropathic pain18,19.
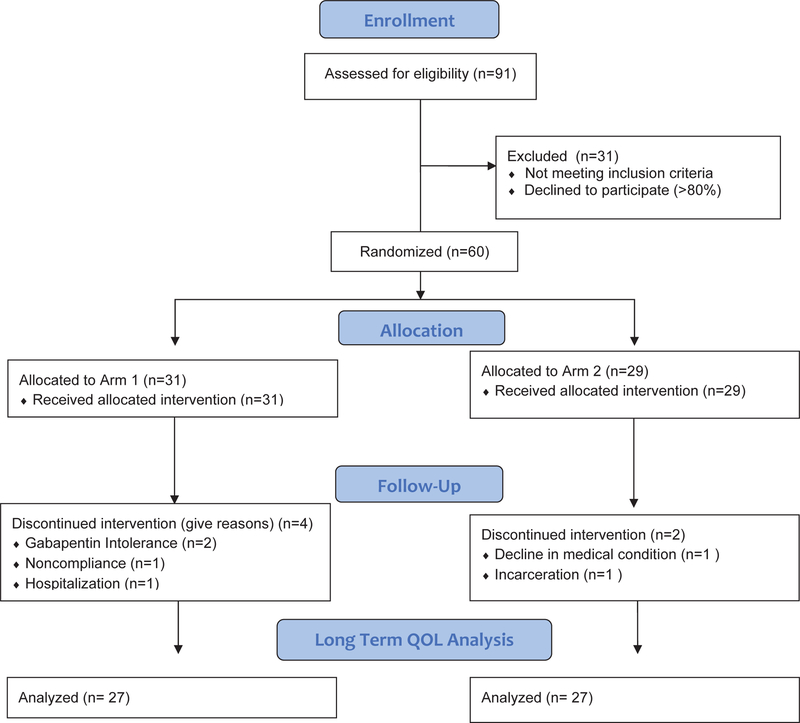
This pilot study (figure 1) compared 2 analgesic regimens allowing evaluation of both 1) the dose effect of prophylactic gabapentin and 2) the efficacy methadone for patients with HNC undergoing CRT by comparing toxicity, patient reported pain, time to first opioid, total opioid requirement, QOL, and nutritional outcomes.
Figure 1.
Consolidated Standard of Reporting Trials (CONSORT) trial diagram. Arm 1: High dose prophylactic gabapentin (2700mg) with hydrocodone/acetaminophen escalating to fentanyl; Arm 2: Low dose gabapentin (900mg) with methadone.
Methods and Materials
This was a prospective randomized pilot study approved by the Roswell Park Comprehensive Cancer Center Institutional Review Board (ClinicalTrials.gov NCT02531906). All patients provided informed consent. Enrollment began April, 2015 and completed August, 2017.
Eligibility Criteria
Eligible patients had AJCC 7th edition Stage II-IV pathologically proven squamous cell carcinoma of the head and neck and were undergoing definitive CRT. Patients were ≥ 18 years of age, had adequate renal function to undergo platinum based chemotherapy, an ECOG performance status ≤ 2, and had the ability to swallow and/or tolerate medications per feeding tube. All patients completed staging workup with CT Neck with contrast and/or PET-CT. Patients were excluded if they had prior radiation or surgery for HNC, had recurrent HNC, or had brain metastases. Patients prescribed medications for chronic pain or neuropathy, those who were under treatment of a pain specialist or substance-abuse program, and patients who were at risk for QTc prolongation (as determined by pharmacist review of concurrent medications known to prolong QTc) were excluded.
Chemoradiation
All patients received definitive radiation with an intensity-modulated radiation therapy (IMRT) regimen (70 Gy in 35 fractions to the primary tumor and 56 Gy to the elective nodes in 35 fractions) with concurrent cisplatin-based chemotherapy (weekly or every 3 weeks), as previously described20,21.
Study Design
Eligible patients were stratified by volume of elective nodal radiation (bilateral vs. unilateral) and randomized (1:1) using permutated blocked design (masked to the investigator. Due to the nature of the intervention, patients and investigators were not blinded.
Prior to or on day 1 of radiation, patients on arm 1 started scheduled gabapentin titrated up to 900 mg three times daily (2700 mg daily total) as tolerated over the course of a minimum of 9 days. Patients were started on 300 mg in the evening on day 1, followed by 300 mg morning and evening on day 2, and three times daily on day 3. Patients were instructed to subsequently increase dose from 300 to 600 mg starting with the evening dose. One of the three daily doses were thus escalated each day by 300 mg until escalation to 900 mg three times daily. Clinically significant subsequent pain was treated per our institutional standard of care which consisted of acetaminophen/hydrocodone 7.5mg/325mg per 15mL elixir taken up to four times per day. A fentanyl transdermal patch was prescribed for long acting pain control after short acting opioids were used three to four times per day, with acetaminophen/hydrocodone for breakthrough. Fentanyl was started at 25mcg/hr and titrated up 100 mcg/hr as needed. Patients in arm 2 started gabapentin 300 mg in the evening on day 1, followed by 300mg morning and evening on day 2, and 3 times daily on day 3. This total dose of 900mg per day was maintained. Clinically significant subsequent pain was treated with methadone 5mg twice daily and titrated up to 15mg twice daily; oxycodone 5 – 10 mg up to every 4 hours was available for breakthrough.
Supportive Treatment
All patients received educational materials and instructed on oral hygiene, hydration and nutrition. Patients were encouraged to gargle with a saline/baking soda mouthwash rinse 20 times per day. Additionally, a compounded elixir of diphenhydramine-xylocaine-antacid 1:1:1 was recommended to be used four times per day for pain.
Assessments
Study data were collected and managed using a secure web application, Research Electronic Data Capture (REDCap), hosted at the Roswell Park Comprehensive Cancer Center22. Characteristics of study subjects and their cancer were collected. The relative frequency of adverse events (AE) or serious adverse events (SAE), regardless if attributable to treatment, were recorded, based on Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. SAEs was determined by the investigator as per National Cancer Institute guidelines23.
The validated, reliable and feasible Oral Mucositis Weekly Questionnaire adapted for head and neck cancer (OMWQ-HN) was administered at baseline and each week during on-treatment visits (OTVs), and on the final week of treatment using a web-based version of the survey that was developed with REDCap24. The OMWQ-HN provides patient-reported responses on domains related to mouth and throat soreness (MTS), overall health (OH), and limitations on daily activities caused by MTS, as previously described24. Patients were also followed through OTVs for analgesic use, weight loss, and feeding tube requirement. Daily opioid use was converted to oral morphine milligram equivalents (MME) according to the Centers for Disease Control conversions25, summated over the course of treatment and reported as total MME.
The European Organization for Research and Treatment Quality of Life Questionnaire-Core 30, version 3.0 (EORTC QLQ-C30) contains a global health scale, five functional scales, and nine symptom scales/items26. The European Organization for Research and Treatment Head and Neck Module (EORTC QLQ-H&N35) contains ten single items and seven subscales27. Raw scores were transformed to a 0–100 scale, with higher score indicating better level of functioning or worse symptoms. Using a web-based version of the surveys that were developed with REDCap, the EORTC QLQ-C30 and QLQ-H&N35 were administered at baseline, end of treatment, and at subsequent follow-up visits which were to occur at 4 weeks, 3 and 4–6 months with optional follow-up at 9 and 12 months, up to 2 years.
Endpoints
The primary endpoint was toxicity as measured by CTCAE version 4.0. Secondary endpoints were pain during CRT as measured by the OMWQ-HN, opioid requirement as measured by MME, and QOL based on the following patient-reported outcomes: OMWQ-HN, EORTC QLQ-C30 and -H&N35. Tertiary outcomes included weight loss and feeding tube requirement.
Statistical Analysis
This study was a pilot trial so the sample size calculations were not fully statistically justified. However, the sample size was selected such that proportions within arm and differences in proportions between arms could be estimated with reasonable precision. A sample size of n=30 per arm would yield 90% confidence intervals (CIs) of width ≤ 0.41 for between-arm differences in proportions of 0.1 to 0.5; and CIs of width ≤ 0.29 for proportions within the treatment arms. For exploratory analyses, two sided tests comparing a quantitative measure (e.g. QOL metrics) between treatment arms would provide 80% power (at α = 0.1) to detect a difference of at least 0.65 standard deviations.
Patient-reported outcomes were compared between groups over the course of treatment from baseline to end-of-treatment for OMWQ-HN and included up to 1 year follow-up for the EORTC QLQ-C30 and -H&N35. Comparisons between groups were also made at the week 3 and end of treatment time points for the OMWQ-HN and at the end of treatment, 4 week, 3, 4–6, 9 and 12 month follow-ups for the EORTC QLC-C30 and -H&N35. The Kruskal - Wallis Test and Fisher’s Exact Test were applied to compare the differences of the demographics and outcomes at individual time points between treatment arms. Logistic Regression and linear regression models (depending on the type of outcome of interest) were further applied to explore the association between outcomes and covariates of interest. Time dependent outcomes were modeled as a function of time, group, their two-way interaction, and a random subject effect (accounting for repeated measures of a given subject across time) using a linear mixed model. The associations between outcomes and group were evaluated using tests about the appropriate contrasts of model estimates. All model assumptions were verified graphically. The Sidak correction was used to adjust for multiple comparisons. SAS version 9.4 (SAS Institute, Cary, NC) was used for statistical analyses. All tests are two-sided and performed at a nominal significance level of 0.05. Results are reported as mean +/− standard deviation (SD) unless otherwise specified.
Results
Between April 2015 and October 2017, 91 patients were screened for eligibility and 60 patients were randomized (n=31 in arm 1; n=29 in arm 2). Of those excluded, >80% patients declined randomization. There was no case of a patient being excluded as a result of concern for QTc prolongation. All patients in each arm completed CRT. Overall, 90% of patients (87% in arm 1 and 93% in arm 2) completed treatment per protocol. The two patients who discontinued protocol-specified analgesic treatment were in arm 2 and were due to gabapentin intolerance; one patient stopped due to nausea and the other had difficulty swallowing the liquid solution (Figure 1). Patient, tumor and treatment characteristics are shown in Table 1. The treatment groups were well balanced. Only one patient required opioids for acute post-biopsy pain prior to initiation of the study and discontinued this prior to enrollment.
Table 1.
Baseline patient, tumor and radiation treatment characteristics
| Arm 1 (n=31) | Arm 2 (n=29) | |
|---|---|---|
| Age (y) | ||
| Median | 61 | 60 |
| Range | 47–75 | 42–77 |
| Gender, n (%) | ||
| Male | 27 (87.1) | 27 (93.1) |
| Female | 4 (12.9) | 2 (6.9) |
| ECOG Performance Status, n (%) | ||
| 0 | 22 (73.3) | 22 (78.6) |
| 1 | 7 (23.3) | 6 (21.4) |
| 2 | 1 (3.3) | 0 |
| Weight (kg) | ||
| Mean +/−SD | 86.4 +/−18 | 90.3 +/−15 |
| Feeding Tube, n (%) | ||
| Yes | 2 (6.5) | 0 |
| No | 29 (94.0) | 29 (100) |
| T stage, n (%) (AJCC 7th ed.) | ||
| T0 | 3 (10) | 3 (10) |
| T1 | 6 (19) | 3 (10) |
| T2 | 8 (26) | 10 (34) |
| T3 | 9 (29) | 10 (34) |
| T4 | 4 (13) | 3 (10) |
| N stage, n (%) (AJCC 7th ed.) | ||
| N0 | 3 (10) | 4 (14) |
| N1 | 6 (19) | 2 (7) |
| N2a | 8 (26) | 8 (28) |
| N2b | 5 (16) | 11 (38) |
| N2c | 4 (13) | 3 (10) |
| N3 | 5 (16) | 1 (3) |
| Overall Clinical Stage, n (%) (AJCC 7th ed.) | ||
| II | 2 (6) | 0 |
| III | 9 (29) | 7 (24) |
| IVA | 14 (45) | 20 (69) |
| IVB | 6 (19) | 1 (3) |
| IVC | 0 | 1 (3) |
| Primary Tumor Site, n (%) | ||
| Nasopharynx | 3 (10) | 1 (3) |
| Oropharynx | 8 (26) | 7 (24) |
| Oral Cavity | 13 (42) | 13 (45) |
| Larynx | 4 (13) | 4 (14) |
| Hypopharynx | 2 (6) | 1 (3) |
| Unknown primary | 1 (3) | 3 (10) |
| Volume of Elective Radiation, n (%) | ||
| Unilateral | 4 (13) | 6 (21) |
| Bilateral | 27 (87) | 23 (79) |
AJCC: American Joint Committee on Cancer.
Toxicity
Tables 2 and 3 show incidence and maximum grade of CTCAE v4.0 AEs and SAEs, respectively, for each arm. For the total cohort, 34/60 (57%) patients had a grade 3+ AE and 18/60 (30%) patients had an SAE. Maximum grade 3+ AE occurred in 15/31 (48%) of patients in arm 1 compared to 19/29 (66%) in arm 2 (p=0.20). The majority of patients had AEs due to mucosal inflammation with 12/15 (80%) and 13/19 (68%) in arm 1 and arm 2, respectively. 11/31 (35%) of patients in arm 1 had a maximum grade 3+ SAE compared to 7/29 (24%) in arm 2 (p=0.41). Two patients in arm 1 had a grade 5 SAE; one cardiac arrest occurring 1 week after completing CRT and one patient died after 8 fractions of CRT due to unknown causes with no autopsy performed.
Table 2.
Incidence and grade of CTCAE v4.0 Adverse Events (AEs)
| System Organ Class | Preferred Term | Arm 1 (n=31) | Arm 2 (n=29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| Blood and lymphatic system disorders | Lymphopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Gastrointestinal disorders | Abdominal discomfort | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Dysphagia | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Nausea | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Odynophagia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Oral pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Stomatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Vomiting | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| General disorders and administration site conditions | Mucosal inflammation | 0 | 0 | 11 | 1 | 0 | 0 | 0 | 12 | 1 | 0 |
| Pain | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Injury, poisoning and procedural complications | Radiation skin injury | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Investigations | Lymphocyte count decreased | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutrophil count decreased | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| White blood cell count decreased | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metabolism and nutrition disorders | Decreased appetite | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nervous system disorders | Tremor | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | Oropharyngeal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Skin and subcutaneous tissue disorders | Erythema | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any AE- Maximum Grade Seen (Total) | 0 | 0 | 13 | 2 | 0 | 0 | 0 | 18 | 1 | 0 | |
CTCAE: Common Toxicity Criteria for Adverse Events
Table 3.
Incidence and grade of CTCAE v4.0 Serious Adverse Events (SAEs)
| System Organ Class | Preferred Term | Arm 1 (n=31) | Arm 2 (n=29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| Blood and lymphatic system disorders | Febrile neutropenia | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cardiac disorders | Cardiac arrest | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | Diarrhea | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysphagia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vomiting | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| General disorders and administration site conditions | Death | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Product adhesion issue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Pyrexia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | |
| Systemic inflammatory response syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Infections and infestations | Bronchitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pneumonia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metabolism and nutrition disorders | Dehydration | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Psychiatric disorders | Depression | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Renal and urinary disorders | Renal failure acute | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | Hypoxia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Vascular disorders | Embolism | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any SAE- Maximum Grade Seen (Total) | 0 | 0 | 9 | 0 | 2 | 0 | 0 | 6 | 1 | 0 | |
CTCAE: Common Toxicity Criteria for Adverse Events
Patient-Reported Pain and Opiate Requirement
All patients had a worsening in MTS and pain over the course of CRT (Table 4 and 5, p<0.01). There was no significant difference in MTS (p=0.87) or pain (p=0.87) between arms or reported impact on daily activities due to MTS, although there was a near significance favoring arm 2 for less impact of MTS on sleeping (p=0.06). Arm 2 had 64% less total narcotic requirement compared to arm 1 (580 +/−409 vs. 1629 +/−1849 MME, p=0.11). Patients in arm 1 required first opioid at 22.6 days compared to 18.2 calendar days (p=0.09). In arm 1 and 2, 13/31 (42%) and 2/29 (7%) patients, respectively, never required any opioids during treatment (p=0.002). After initiating and increasing hydrocodone to 3 to 4 times per day on arm 1, 6/31 (19%) patients required escalation to fentanyl. On arm 2, 6/29 (21%) patients required oxycodone for breakthrough. No patient required more than 20 mg daily of methadone. No patients required hospitalization for pain control. At 4 week follow-up, 9/29 (31%) patients in arm 1 remained on an opioid compared to 5/29 (17%) in arm 2 (p=0.36). All patients were successfully tapered off opioid medication by 8 week follow-up.
Table 4.
Generalized linear mixed regression analysis for OMWQ-HN patient reported outcomes, (mean +/−SD).
| Baseline | Mid-Treatment (Week 3) | End of Treatment (Week 7) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Arm 1 (n=31) | Arm 2 (n=29) | Arm 1 (n=29) | Arm 2 (n=29) | Arm 1 (n=23) | Arm 2 (n=21) | ||
| OMWQ-HN | |||||||
| OHa | 71 +/−28 | 86 +/−14 | 78 +/−15 | 71 +/−20 | 55 +/−19 | 63 +/−19 | 0.05 |
| MTSa | 18 +/−26 | 13 +/−22 | 34 +/−27 | 42 +/−27 | 46 +/−24 | 48 +/−27 | 0.87 |
| MTSb | 0.4 +/−0.7 | 0.4 +/−0.8 | 2.4 +/−1.1 | 2.2 +/−1.0 | 2.0 +1.1 | 2.1 +/−1.1 | 0.51 |
| Limitations due to MTSC | |||||||
| Swallowing | 0.4 +/−1.0 | 0.6 +/−1.2 | 1.3 +/−1.2 | 1.6 +/−1.2 | 1.6 +/−1.2 | 1.4 +/−1.1 | 0.72 |
| Drinking | 0.3 +/−1.0 | 0.5 +/−1.2 | 0.9 +/−1.2 | 0.8 +/−1.1 | 1.7 +/−1.4 | 1.6 +/−1.1 | 0.60 |
| Eating | 0.5 +/−1.2 | 0.5 +/−1.1 | 1.0 +/−1.3 | 1.6 +/−1.3 | 2.2 +/−1.5 | 1.9 +/−1.3 | 0.97 |
| Talking | 0.4 +/−1.0 | 0.4 +/−0.9 | 0.8 +/−1.2 | 0.8 +/−1.2 | 1.7 +/−1.2 | 1.6 +/−1.2 | 0.58 |
| Sleeping | 0.5 +/−1.0 | 0.2 +/−0.8 | 0.8 +/−1.2 | 0.7 +/−1.2 | 1.5 +/−1.2 | 0.8 +/−1.2 | 0.06 |
OMWQ-HN: oral mucositis weekly questionnaire-head and neck cancer; OH: overall health; MTS: mouth and throat soreness.
Raw OH and MTS higher scores indicate better level of functioning or worse symptoms.
MTS: 0=no soreness 1=a little soreness 2= moderate soreness 3=quite a lot of soreness 4=extreme soreness.
Limitations due to MTS: 1=not at all 2=a little 3=quite a bit 4=very much
Table 5.
Generalized linear mixed regression analysis for EORTC QLQ-C30 and H&N35 patient reported outcomes, (mean +/−SD).
| Baseline | End of Treatment (Week 7) | 4 week follow-up | 3 month follow-up | 4–6 month follow-up | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 (n=30) | Arm 2 (n=28) | Arm 1 (n=30) | Arm 2 (n=26) | Arm 1 (n=25) | Arm 2 (n=26) | Arm 1 (n=26) | Arm 2 (n=25) | Arm 1 (n=22) | Arm 2 (n=23) | |||
| EORTC QLQ-C30 | ||||||||||||
| Functional scalesa | ||||||||||||
| Global Health | 75 +/−21 | 84 +/−16 | 53 +/−24 | 58 +/−21 | 62 +/−18 | 72 +/−18 | 72 +/−20 | 76 +/−17 | 76 +/−19 | 82 +/−20 | 0.15 | |
| Physical functioning | 90 +/−22 | 97 +/−7 | 73 +/−24 | 82 +/−22 | 81 +/−16 | 86 +/−14 | 80 +/−25 | 90 +/−11 | 87 +/−17 | 90 +/−17 | 0.04 | |
| Role functioning | 87 +/−28 | 96 +/−12 | 52 +/−33 | 65 +/−35 | 57 +/−27 | 75 +/−28 | 65 +/−34 | 84 +/−21 | 81 +/−24 | 86 +/−23 | 0.01 | |
| Emotional functioning | 75 +/−21 | 85 +/−17 | 77 +/−23 | 78 +/−25 | 77 +/−24 | 81 +/−24 | 78 +/−24 | 81 +/−18 | 80 +/−20 | 85 +/−18 | 0.33 | |
| Cognitive functioning | 92 +/−20 | 93 +/−12 | 77 +/−25 | 83 +/−22 | 86 +/−19 | 90 +/−18 | 85 +/−23 | 93 +/−12 | 88 +/−18 | 88 +/−12 | 0.31 | |
| Social functioning | 86 +/−22 | 94 +/−15 | 48 +/−37 | 64 +/−28 | 57 +/−28 | 69 +/−27 | 71 +/−34 | 88 +/−16 | 82 +/−25 | 91 +/−14 | 0.01 | |
| Symptom scales/itemsb | ||||||||||||
| Fatigue | 81 +/−21 | 88 +/−15 | 47 +/−25 | 53 +/−26 | 55 +/−25 | 69 +/−20 | 62 +/−30 | 76 +/−17 | 72 +/−23 | 77 +/−23 | 0.06 | |
| Nausea and vomiting | 2 +/−7 | 2 +/−6 | 26 +/−27 | 28 +/−29 | 13 +/−18 | 10 +/−16 | 8 +/−17 | 3 +/−9 | 5 +/−11 | 1 +/−4 | 0.76 | |
| Pain | 7 +/−13 | 13 +/−17 | 42 +/−29 | 41 +/−27 | 26 +/−22 | 17 +/−16 | 17 +/−21 | 13 +/−22 | 9 +/−18 | 12 +/−16 | 0.87 | |
| Dyspnea | 13 +/−24 | 6 +/−13 | 16 +/−24 | 12 +/−23 | 12 +/−23 | 8 +/−17 | 9 +/−20 | 13 +/−22 | 6 +/−13 | 9 +/−18 | 0.59 | |
| Insomnia | 23 +/−27 | 16 +/−19 | 48 +/−35 | 24 +/−32 | 31 +/−32 | 27 +/−28 | 28 +/−26 | 21 +/−25 | 17 +/−25 | 16 +/−24 | <0.01 | |
| Appetite loss | 10 +/−23 | 8 +/−17 | 63 +/−35 | 55 +/−28 | 35 +/−33 | 35 +/−36 | 23 +/−30 | 21 +/−29 | 11 +/−16 | 17 +/−36 | 0.56 | |
| Constipation | 7 +/−14 | 8 +/−20 | 18 +/−27 | 40 +/−33 | 16 +/−24 | 22 +/−23 | 12 +/−25 | 11 +/−19 | 3 +/−10 | 4 +/−12 | 0.07 | |
| Diarrhea | 8 +/−17 | 1 +/−6 | 21 +/−28 | 4 +/−14 | 11 +/−23 | 3 +/−9 | 6 +/−16 | 0 +/−0 | 2 +/−7 | 1 +/−7 | <0.01 | |
| Financial difficulties | 11 +/−20 | 17 +/−26 | 23 +/−32 | 24 +/−32 | 24 +/−26 | 30 +/−34 | 23 +/−31 | 19 +/−27 | 26 +/−29 | 17 +/−24 | 0.86 | |
| EORTC QLQ-H&N35 | ||||||||||||
| Symptom scales/itemsb | ||||||||||||
| Pain | 14 +/−16 | 12 +/−13 | 42 +/−24 | 42 +/−22 | 27 +/−21 | 24 +/−15 | 17 +/−15 | 17 +/−15 | 11 +/−15 | 9 +/−9 | 0.92 | |
| Swallowing | 11 +/−21 | 4 +/−10 | 59 +/−26 | 43 +/−27 | 38 +/−27 | 27 +/−25 | 32 +/−30 | 15 +/−21 | 24 +/−25 | 18 +/−27 | <0.01 | |
| Senses problems | 8 +/−16 | 8 +/−14 | 44 +/−25 | 47 +/−21 | 34 +/−22 | 40 +/−20 | 30 +/−24 | 25 +/−16 | 27 +/−22 | 33 +/−27 | 0.81 | |
| Speech problems | 17 +/−27 | 8 +/−15 | 64 +/−31 | 26 +/−31 | 43 +/−31 | 30 +/−25 | 28 +/−24 | 12 +/−14 | 21 +/−28 | 14 +/−15 | 0.02 | |
| Social eating | 9 +/−15 | 5 +/−10 | 56 +/−33 | 39 +/−21 | 45 +/−29 | 31 +/−24 | 29 +/−27 | 20 +/−24 | 35 +/−33 | 21 +/−27 | 0.02 | |
| Social contact | 5 +/−10 | 2 +/−5 | 32 +/−31 | 18 +/−21 | 24 +/−23 | 13 +/−18 | 14 +/−22 | 5 +/−7 | 7 +/−16 | 5 +/−8 | <0.01 | |
| Sexuality | 20 +/−30 | 15 +/−24 | 57 +/−37 | 44 +/−37 | 29 +/−30 | 39 +/−39 | 25 +/−34 | 24 +/−29 | 22 +/−30 | 20 +/−22 | 0.34 | |
| Teeth | 9 +/−17 | 5 +/−15 | 6 +/−13 | 6 +/−13 | 9 +/−23 | 4 +/−11 | 6 +/−13 | 9 +/−18 | 12 +/−24 | 7 +/−17 | 0.96 | |
| Opening mouth | 10 +/−20 | 2 +/−9 | 26 +/−32 | 15 +/−22 | 20 +/−29 | 6 +/−16 | 13 +/−19 | 8 +/−15 | 21 +/−28 | 9 +/−15 | 0.05 | |
| Dry mouth | 20 +/−27 | 12 +/−19 | 56 +/−31 | 51 +/−27 | 53 +/−35 | 55 +/−23 | 53 +/−30 | 64 +/−27 | 59 +/−37 | 54 +/−26 | 0.98 | |
| Sticky saliva | 13 +/−26 | 4 +/−11 | 79 +/−26 | 64 +/−28 | 68 +/−30 | 54 +/−30 | 45 +/−31 | 43 +/−23 | 50 +/−35 | 39 +/−28 | 0.04 | |
| Coughing | 30 +/−30 | 20 +/−21 | 59 +/−30 | 51 +/−32 | 47 +/−26 | 35 +/−22 | 36 +/−28 | 29 +/−24 | 30 +/−23 | 25 +/−27 | 0.18 | |
| Feeling ill | 9 +/−15 | 6 +/−13 | 36 +/−30 | 35 +/−31 | 21 +/−23 | 12 +/−23 | 10 +/−25 | 4 +/−11 | 8 +/−14 | 1 +/−7 | 0.40 | |
| Yes/Noc | ||||||||||||
| Pain killers | 20 +/−41 | 32 +/−48 | 67 +/−48 | 92 +/−27 | 56 +/−51 | 50 +/−51 | 15 +/−37 | 16 +/−37 | 14 +/−35 | 4 +/−21 | 0.06 | |
| Nutritional supplement | 33 +/−48 | 25 +/−44 | 67 +/−48 | 77 +/−43 | 52 +/−51 | 62 +/−50 | 39 +/−50 | 44 +/−51 | 46 +/−51 | 30 +/−47 | 0.74 | |
| Feeding tube | 7 +/−25 | 0 +/−0 | 47 +/−51 | 23 +/−43 | 48 +/−51 | 23 +/−43 | 46 +/−51 | 12 +/−33 | 50 +/−51 | 26 +/−45 | 0.16 | |
| Weight loss | 33 +/−48 | 11 +/−32 | 87 +/−35 | 77 +/−43 | 60 +/−50 | 65 +/−49 | 35 +/−49 | 28 +/−46 | 18 +/−40 | 13 +/−34 | <0.01 | |
| Weight gain | 10 +/−31 | 39 +/−50 | 13 +/−35 | 19 +/−40 | 24 +/−44 | 39 +/−50 | 23 +/−43 | 40 +/−50 | 27 +/−46 | 52 +/−51 | 0.01 | |
EORTC QLQ-C30: European Organization for Research and Treatment Quality of Life Questionnaire-Core 30, version 3.0; EORTC QLQ-H&N35: European Organization for Research and Treatment Quality of Life Questionnaire-Head and Neck Module.
Raw functional scale higher scores indicate better level of functioning.
Symptom scale higher scores indicate worse symptoms, 1=not at all 2=a little 3=quite a bit 4=very much, linearly transformed to scale 0–100.
0=no 1=yes, linearly transformed to scale 0–100.
Quality of Life Domains
Tables 4 and 5 show the comparison between arms using the generalized linear mixed regression analysis. Throughout CRT, patients in both arms had a significant worsening in OH (Table 4, p<0.01), global health scale (p<0.01) and all functional scales (p<0.01), with the exception of emotional functioning (p=0.68). While there were differences between arms at baseline, these differences were accounted for with the use of the linear mixed regression model. During CRT, arm 2 had significantly better OH on the OMWQ-HN vs. arm 1 (p=0.05). Arm 1 OH did not change from baseline to week 3 (p=0.92), suggesting a delay in OH decline.
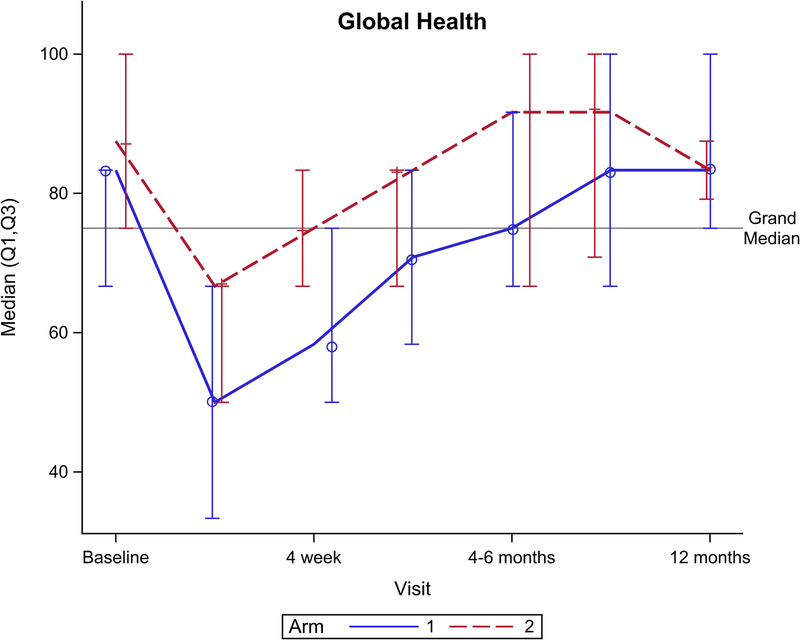
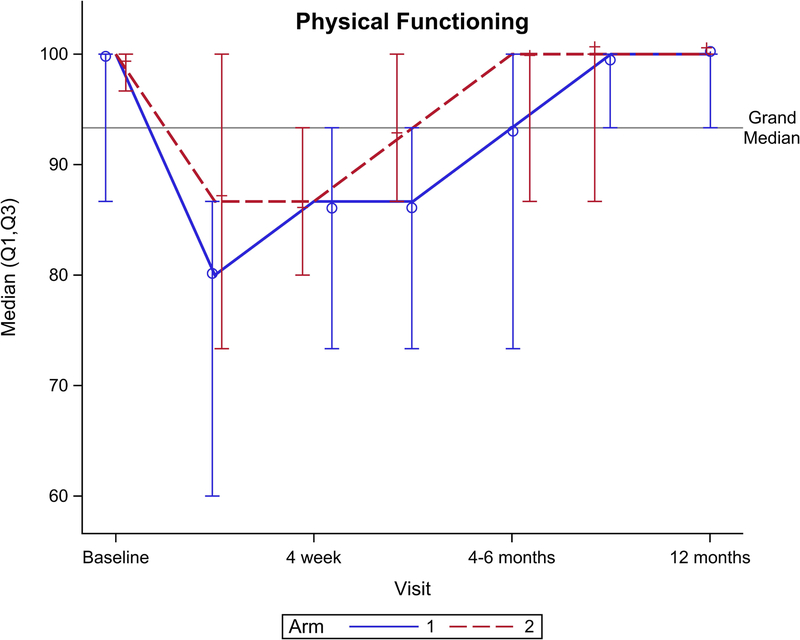
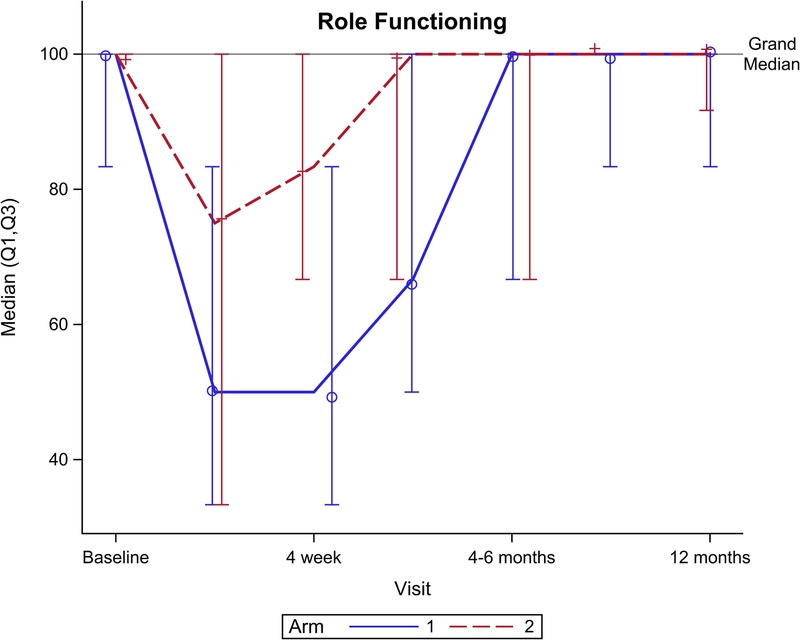
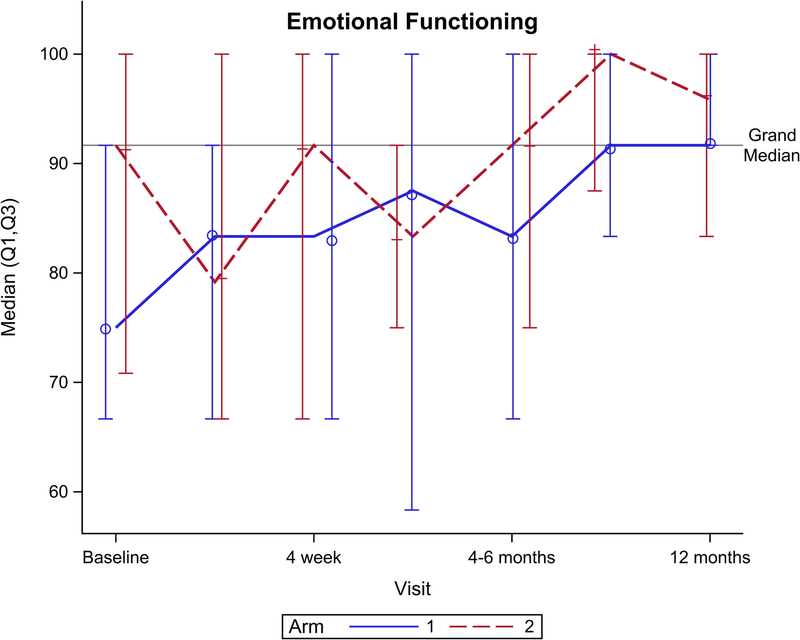
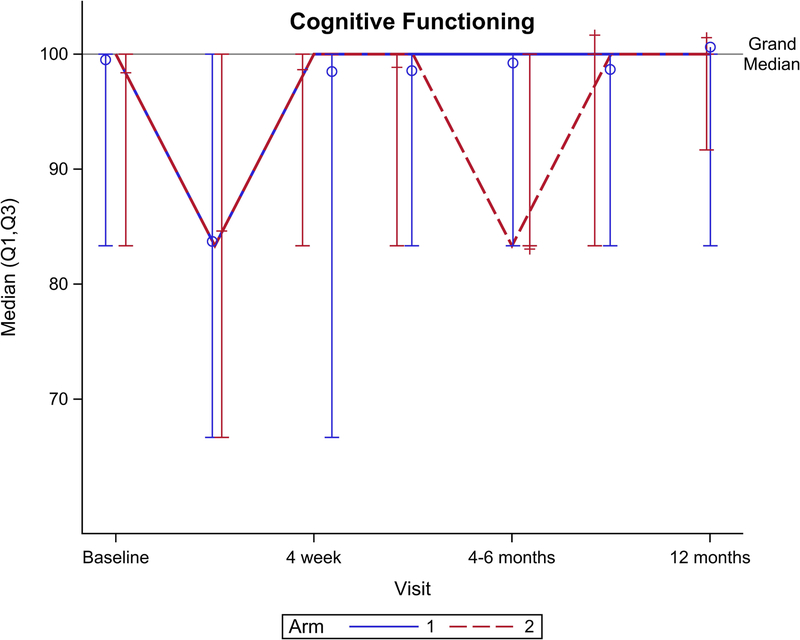
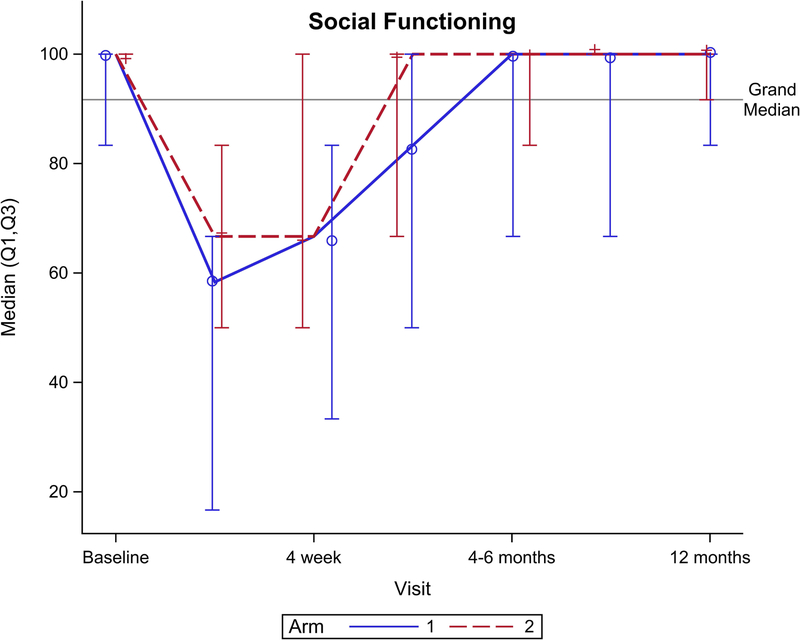
Figure 2 shows EORTC QLQ-C30 QOL outcomes for the global health scale and five functional domains. Though no significant difference was seen for the global health scale over total follow-up (Figure 2A, p=0.15), there was a significant benefit favoring arm 2 seen at 4 weeks follow-up (p=0.049). Notably, global health scale returned to baseline after CRT at: 4 weeks for arm 2 vs. 4–6 months after CRT for arm 1. From baseline to 1 year follow-up, arm 2 had better physical functioning (Figure 2B, p=0.04), role functioning (Figure 2C, p=0.01), and social functioning (Figure 2F, p=0.01) compared to arm 1. There was no significant difference between arms for cognitive (Figure 2E, p= 0.31) or emotional (Figure 2D, p=0.33) functioning.
Figure 2.
EORTC QLQ-C30 scores by arm. (A) Global health scale, (B) Physical functioning, (C) Role functioning, (D) Emotional functioning, (E) Cognitive functioning, (F) Social functioning.
Comparing QOL outcomes using the EORTC QLQ-H&N35, arm 2 had less insomnia (p<0.01) coinciding with a near significance in decreased fatigue (p=0.06). Arm 1 had worse diarrhea (p<0.01) and arm 2 was nearly significant for worse constipation (p=0.07) though, clinically, neither of these were notable. Arm 2 also had significantly better swallowing (p<0.01), fewer speech problems (p=0.02), less trouble with social eating (p=0.02), less trouble with social contact (p<0.01), less difficulty opening the mouth (p=0.05), less sticky saliva (p=0.04), less patient-reported feeding tube use (p<0.01), and greater patient-reported weight gain (p=0.01) compared to arm 1. There were no significant differences between the two arms in any domain of the EORTC QLQ-C30 or -H&N35 after the first 3 months of follow-up.
Nutritional Outcomes
There was no significant difference between arms in end-of-treatment weight (79.8 +/−16 vs. 84.4 +/−15 kgs, p=0.59) or weight loss (6.6 +/−4.5 vs. 5.9 +/−4.0 kgs, p=0.54) for arm 1 and arm 2, respectively. At completion of CRT, 9/29 (31%) of patients in arm 1 required placement of a feeding tube compared to 4/29 (14%) in arm 2 (p=0.21).
Discussion
This study shows that for patients undergoing CRT for HNC, prophylactic gabapentin (900mg daily) combined with methadone is consistently associated with better patient-reported QOL outcomes (see Table 4 and 5 and Figure 2) and significantly faster return of the global health scale to baseline following CRT (4 weeks for arm 2 vs. 4–6 months for arm 1 (p=0.049)) compared to a regimen combining 2700mg prophylactic gabapentin with an institutional standard of care. There were no differences in adverse events, pain, or weight loss outcomes between the arms over the course of treatment.
Studies have shown outpatient methadone to be efficacious in reducing cancer pain28,29. Two trials have specifically evaluated HNC patients. Haumann et al. in a randomized controlled trial of 52 patients showed that methadone was superior to fentanyl for neuropathic pain due to HNC30. A subsequent randomized controlled trial of 82 patients showed noninferiority of methadone to fentanyl specifically for HNC patients with radiation-induced pain; the authors also found less need to escalate opioids with methadone31.
A prior retrospective analysis has shown that outpatient administration of methadone in cancer patients is considered safe and has a similar side effect profile to other opioids32. Recently, a prospective study of 145 patients with advanced cancer treated in an outpatient palliative care clinic with rotation to methadone found a significant improvement in pain relief without an increase in opioid toxicity33. In 2013, a federally convened expert panel developed a consensus statement on methadone induction and stabilization. The comprehensive report offers specific recommendations for initiating methadone treatment and concludes methadone can be safely prescribed so long as the risks are recognized, there is adequate patient education and there is a plan of intervention at signs of toxicity34. Notably, this report assumes a typical starting dose of 10–30mg per day. Our study showed a benefit for patients at the lowest therapeutic threshold of 5 mg twice per day and patients did not require more than 20mg per day at any point in CRT.
Our study demonstrates a clear benefit of methadone across a wide range of QOL domains. Less insomnia and less fatigue reported in the methadone arm may lead to these broad improvements. Methadone has a relatively long duration of analgesia, allowing it to be dosed less frequently35. Consequently, methadone may improve sleep without interruption (Table 4, sleeping p=0.06). This is consistent with a pilot trial of methadone in post-herpetic neuralgia patients who had less allodynia and an improvement in sleep36. Also, fatigue tended to be less with methadone (Table 4, p=0.06), and fatigue is known to reduce QOL in cancer patients and is associated with treatment discontinuation37,38. The benefits seen in arm 2 do not appear to be related to any improvement in worst patient-reported pain. Consistent with Elting et al., we found the opioid analgesics on both arms to be inadequate to prevent the worsening pain and decline in QOL throughout CRT39.
Given the body of work demonstrating the benefit of gabapentin for patients undergoing radiation for HNC, patients were likely deriving benefits from gabapentin in both arms14,16. Gabapentin was well tolerated with only 2/60 (3%) of patients discontinuing treatment due to intolerance to gabapentin, and both of whom were in the low dose gabapentin arm, suggesting dose is not limiting tolerance. This pilot study was not powered to detect a difference between arms in weight loss or feeding tube requirement. The results of this study are consistent, however, with previous studies showing gabapentin can improve swallowing function, nutritional status, QOL and reduce opioid requirement in patients undergoing radiation and CRT for HNC12,13,16,17. In this study, it appears the higher dose of gabapentin had its greatest benefit in the early weeks of CRT as arm 1 OH did not change from baseline to week 3 (p=0.92), which coincided with a 4 day delay in first opioid use (22.6 vs 18.2 days, p=0.09).
Additionally, more patients in arm 1 did not require an opioid during treatment (42% vs 7%, p=0.002); this suggests a favorable effect of increasing gabapentin dose on reducing opiate requirement. Interestingly, despite 42% of patients on arm 1 needing no opioids (zero MME,) the patients on arm 2 with methadone had 64% less total narcotic requirement (580 +/−409 vs. 1629 +/−1849 MME, p=0.11.) Therefore, methadone compared to hydrocodone followed by fentanyl likely is even more effective than suggested by the current data.
Limitations
This was a small pilot study that failed to show improvement in pain or the primary endpoint of toxicity. Due to the exploratory nature of the QOL comparisons, this study was not powered to detect a difference; thus, the true effect size may be smaller than seen here and a larger confirmatory study is needed. Moreover, the study was underpowered to demonstrate, even with 64% reduction of MME, that opiate requirement was significantly reduced in arm 2. The MME of methadone is known to change with dose; however, in the narrow range needed on this study (less than 20 mg daily) the conversion factor is stable25. There was a declining rate of survey completion rates at week 7 and in follow-up, therefore a non-response bias cannot be excluded and the QOL findings may be limited to patients who are more likely to participate. In order to address this limitation, we analyzed week 6 outcomes which had a participation rate of 97 and 100% in both arms, and found the comparison between arms to be consistent with the results of the generalized linear mixed regression analysis across all OMWQ-HN scores. Follow-up after one year was not included due to small sample size; though all patients are being followed regularly as per standard of care. Because there was no methadone-only arm, we cannot definitively conclude the benefits of methadone would be appreciated without concurrent gabapentin. Moreover, there may be differences between arms that could be attributed to the dose-effect of gabapentin, rather than the opiate. It would be difficult to assess these differences without a randomized trial and we have not found equipoise to exclude gabapentin from our institutional standard for HNC patients undergoing CRT. There are concerns regarding the generalizability of our findings. While not generally considered addictive, gabapentin has recently been suggested to have abuse potential40. Though no recorded cardiac toxicity was noted with methadone in this study, high doses of methadone have been associated with fatal arrhythmias secondary to QT interval prolongation41,42. Therefore, analgesic strategy should be individualized for each patient with consideration of comorbidities and concurrent medications.
Future Directions
Both pain and QOL have been shown to be predictors of survival outcomes in patients undergoing treatment for HNC43,44 perhaps due to the need for treatment breaks; prolonged treatment (≥ 57 days) is associated with an 8-fold increase in locoregional progression20. In the United States, overdose deaths involving opioids has increased 4.1 fold from 2002 to 2016, with a 22 fold increase in deaths predominately due to fentanyl and fentanyl analogues. Deaths involving methadone have been stable or declined during this timeframe45. Our study suggests methadone may reduce total opiate requirement compared to those patients treated per our fentanyl-based analgesic regimen. With the aim of improving QOL in HNC patients, a further benefit of methadone is to reduce both overall opiate requirement as well as short-acting opioids and fentanyl availability. Additional advantages include low cost and a reduction of the euphoric effects of concurrent narcotic use46.
In this context, we have made high dose (3600mg daily) gabapentin and methadone the standard arm in our current study (clinicaltrials.gov NCT 03574792), with randomization to with or without the serotonin and norepinephrine reuptake inhibitor, venlafaxine, which when combined with gabapentin has been shown to improve pain and QOL in patients with diabetic neuropathy47.
Conclusions
High dose prophylactic gabapentin increased the percentage of patients who required no opioid during treatment. Methadone may improve QOL compared to a regimen of short acting opioids and fentanyl. Pain significantly worsened throughout treatment regardless of arm; thus, further studies are needed to optimize the analgesic regimen.
Acknowledgements
We would like to thank Debbie Neimanis for her tireless efforts as the clinical trial nurse on this project. Adam Oberkircher and Kelsey Bezon contributed greatly to the care of these patients. Alex Ostrowski and Mary Platek helped set up the Redcap Database. Maryann Mikucki assisted with critical review of the final manuscript.
Research support
The study utilized the Biostatistics and Statistical Genomics shared resource supported by Roswell Park’s Cancer Center Support Grant from the NCI (P30CA016056).
Footnotes
Conflict of interest
All authors declare that they have no competing interests.
References
- 1.Society AAC. Cancer Facts & Figures 2018. 2018. [Google Scholar]
- 2.Network NCC. Head and Neck Cancers Version 2.2018. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed January 3, 2019.
- 3.Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106(2):329–336. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110–1120. [DOI] [PubMed] [Google Scholar]
- 5.Trotti A Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47(1):1–12. [DOI] [PubMed] [Google Scholar]
- 6.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–262. [DOI] [PubMed] [Google Scholar]
- 7.Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers. The patient’s perspective. Cancer Nurs. 2002;25(6):461–467; quiz 468–469. [DOI] [PubMed] [Google Scholar]
- 8.Nonzee NJ, Dandade NA, Patel U, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis : results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113(6):1446–1452. [DOI] [PubMed] [Google Scholar]
- 9.Kramer S, Newcomb M, Hessler J, Siddiqui F. Prophylactic versus reactive PEG tube placement in head and neck cancer. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2014;150(3):407–412. [DOI] [PubMed] [Google Scholar]
- 10.Strom T, Trotti AM, Kish J, et al. Risk factors for percutaneous endoscopic gastrostomy tube placement during chemoradiotherapy for oropharyngeal cancer. JAMA otolaryngology-- head & neck surgery. 2013;139(11):1242–1246. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JB, Wilkie DJ, Fischer DJ, Kim YO, Villines D. Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol. 2009;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar Ad V, Weinstein G, Dutta PR, Chalian A, Both S, Quon H. Gabapentin for the treatment of pain related to radiation-induced mucositis in patients with head and neck tumors treated with intensity-modulated radiation therapy. Head Neck. 2010;32(2):173–177. [DOI] [PubMed] [Google Scholar]
- 13.Bar Ad V, Weinstein G, Dutta PR, et al. Gabapentin for the treatment of pain syndrome related to radiation-induced mucositis in patients with head and neck cancer treated with concurrent chemoradiotherapy. Cancer. 2010;116(17):4206–4213. [DOI] [PubMed] [Google Scholar]
- 14.Dong T, Reed A, Jones GC, Scoble D, Deeken J, Bajaj GK Retrospective Analysis of Prophylactic Gabapentin on Pain and Weight Loss in Patients Undergoing Radiation Therapy for Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):893–894. [Google Scholar]
- 15.Sharp H, Morris JC, Van Waes C, Gius D, Cooley-Zgela T, Singh AK. High incidence of oral dysesthesias on a trial of gefitinib, Paclitaxel, and concurrent external beam radiation for locally advanced head and neck cancers. American journal of clinical oncology. 2008;31(6):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starmer HM, Yang W, Raval R, et al. Effect of gabapentin on swallowing during and after chemoradiation for oropharyngeal squamous cell cancer. Dysphagia. 2014;29(3):396–402. [DOI] [PubMed] [Google Scholar]
- 17.Bar Ad V Gabapentin for the treatment of cancer-related pain syndromes. Rev Recent Clin Trials. 2010;5(3):174–178. [DOI] [PubMed] [Google Scholar]
- 18.Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol. 1998;56(5):553–559. [DOI] [PubMed] [Google Scholar]
- 19.Manfredonia JF. Prescribing methadone for pain management in end-of-life care. J Am Osteopath Assoc. 2005;105(3 Suppl 1):S18–21. [PubMed] [Google Scholar]
- 20.Platek ME, McCloskey SA, Cruz M, et al. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck. 2013;35(5):684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung-Kee-Fung SD, Hackett R, Hales L, Warren G, Singh AK. A prospective trial of volumetric intensity-modulated arc therapy vs conventional intensity modulated radiation therapy in advanced head and neck cancer. World J Clin Oncol. 2012;3(4):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute NC. NCI Guidelines for Investigators: Adverse Event Reporting Requirement for DCTD (CTEP and CIP) and DCP INDs and IDEs. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/aeguidelines.pdf.
- 24.Epstein JB, Beaumont JL, Gwede CK, et al. Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer. 2007;109(9):1914–1922. [DOI] [PubMed] [Google Scholar]
- 25.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 26.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 27.Bjordal K, de Graeff A, Fayers PM, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer. 2000;36(14):1796–1807. [DOI] [PubMed] [Google Scholar]
- 28.Peirano GP, Mammana GP, Bertolino MS, et al. Methadone as first-line opioid treatment for cancer pain in a developing country palliative care unit. Support Care Cancer. 2016;24(8):3551–3556. [DOI] [PubMed] [Google Scholar]
- 29.Mercadante S, Casuccio A, Agnello A, Barresi L. Methadone response in advanced cancer patients with pain followed at home. J Pain Symptom Manage. 1999;18(3):188–192. [DOI] [PubMed] [Google Scholar]
- 30.Haumann J, Geurts JW, van Kuijk SM, Kremer B, Joosten EA, van den Beuken-van Everdingen MH. Methadone is superior to fentanyl in treating neuropathic pain in patients with head-and-neck cancer. Eur J Cancer. 2016;65:121–129. [DOI] [PubMed] [Google Scholar]
- 31.Haumann J, van Kuijk SMJ, Geurts JW, et al. Methadone versus Fentanyl in Patients with Radiation-Induced Nociceptive Pain with Head and Neck Cancer: A Randomized Controlled Noninferiority Trial. Pain Pract. 2018;18(3):331–340. [DOI] [PubMed] [Google Scholar]
- 32.Parsons HA, de la Cruz M, El Osta B, et al. Methadone initiation and rotation in the outpatient setting for patients with cancer pain. Cancer. 2010;116(2):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porta-Sales J, Garzon-Rodriguez C, Villavicencio-Chavez C, Llorens-Torrome S, Gonzalez-Barboteo J. Efficacy and Safety of Methadone as a Second-Line Opioid for Cancer Pain in an Outpatient Clinic: A Prospective Open-Label Study. Oncologist. 2016;21(8):981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxter LE Sr., Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7(6):377–386. [DOI] [PubMed] [Google Scholar]
- 35.Leppert W The role of methadone in cancer pain treatment--a review. International journal of clinical practice. 2009;63(7):1095–1109. [DOI] [PubMed] [Google Scholar]
- 36.Teixeira MJ, Okada M, Moscoso AS, et al. Methadone in post-herpetic neuralgia: A pilot proof-of-concept study. Clinics (Sao Paulo). 2013;68(7):1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank-Stromborg M, Wright P. Ambulatory cancer patients’ perception of the physical and psychosocial changes in their lives since the diagnosis of cancer. Cancer Nurs. 1984;7(2):117–130. [PubMed] [Google Scholar]
- 38.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21(1):23–36. [PubMed] [Google Scholar]
- 39.Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113(10):2704–2713. [DOI] [PubMed] [Google Scholar]
- 40.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137(6):501–504. [DOI] [PubMed] [Google Scholar]
- 42.Cruciani RA, Sekine R, Homel P, et al. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29(4):385–391. [DOI] [PubMed] [Google Scholar]
- 43.Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, Duffy SA. Quality of life scores predict survival among patients with head and neck cancer. J Clin Oncol. 2008;26(16):2754–2760. [DOI] [PubMed] [Google Scholar]
- 44.Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EY. Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain. 2014;15(10):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prevention CfDCa. Overdose Death Rates. August. 2018; https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- 46.Lowinson JHPJ, Salsitz E, Joseph H, Marion IJ, Dole VP. . Methadone Maintenance In: Lowinson JH. Substance abuse: a comprehensive textbook. 1997:pp. 405–415. [Google Scholar]
- 47.Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscul Dis. 2001;3(2):53–62. [DOI] [PubMed] [Google Scholar]