Abstract
Purpose:
To determine the impact of renal function trajectory, defined as the change in renal function over time before and after renal artery stent placement, on long-term risk for renal replacement therapy (RRT) and mortality.
Materials and methods:
Estimated glomerular filtration rate (eGFR) 6–12 months prior to renal artery stent placement, at the time of intervention, and 6–12 months after intervention, was determined. A total of 398 patients were included in the study. The effect of eGFR change before and after renal artery stent placement was calculated. Cox proportional hazard ratio was used to determine the risk for RRT and all-cause mortality.
Results:
The risk for RRT was significantly influenced by eGFR change from the time of intervention to follow-up at 6–12 month after treatment (P=0.02). Furthermore, among patients with a post-intervention eGFR ≤ 40 ml/min/1.73m2, for every 1 unit of eGFR increase there was a significant decrease in RRT and all-cause mortality (P < 0.001 and P<0.001, respectively). Secondary parameters that increased RRT risk included diabetes at the time of intervention (P=0.03), increased baseline proteinuria (P<0.001) and stage 4 chronic kidney disease (CKD) (P=0.01) and stage 5 CKD (P=0.003). Multivariate analysis demonstrated higher all-cause mortality rates among patients with diabetes at time of intervention (P=0.009).
Conclusion:
Post-intervention eGFR trajectory improvement approaching 40 ml/min/1.73m2 was associated with decreased RRT and mortality risk. These findings suggest that patients with advanced CKD and renal artery stenosis may benefit from revascularization regardless of their pre-intervention renal function measurement.
Introduction
The role of renal artery stent placement for renovascular hypertension is evolving. Early literature demonstrated revascularization of hemodynamically significant renal artery stenosis was an effective therapy for refractory hypertension (1, 2). Subsequent studies including the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) and Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trials found that renal artery stent placement did not confer additional benefit over medical therapy for renovascular hypertension or renal function improvement (3, 4). As a result, the indications for renal artery revascularization have become more focused and include medically refractory hypertension, recurrent pulmonary edema, and rapidly declining renal function (5).
Data on the impact of renal function trajectory prior to renal artery stent placement on post-intervention kidney function is limited. Watson et al reported that renal artery stent placement improved or stabilized renal function and preserved kidney size in 33 patients at a mean follow-up time of 18 months (6). Another study with 40 patients (22 treated medically and 18 treated with stent placement) by Arthurs and colleagues compared renal function trajectory over 24 months prior to intervention with renal function trajectory for 48 months after intervention. They showed that revascularization significantly slowed the rate of renal function decline whereas renal function decline accelerated in a control group treated with medical therapy alone (7). The current study aimed to evaluate the effect of renal artery stent placement on renal replacement therapy (RRT) and all-cause mortality based on renal function change from 6–12 months pre-intervention to the time of intervention, and from the time of intervention to 6–12 months post-intervention.
Materials and methods
Approval from the institutional review board was obtained for this retrospective single institution cohort study. All patients who underwent renal artery stent placement between January 1996 and June 2016 were reviewed. In total, 1225 patients were identified with 807 patients excluded for missing data, largely pre-intervention renal function 6–12 months prior to renal artery intervention. An additional 20 patients were excluded because they declined to participate in the study. The final cohort consisted of 398 patients. Mean patient age was 73.4 ± 8.6 years with 206 (51.8 %) males and 192 (48.2%) females. Bilateral renal artery stenosis was treated in 162 (41%) patients. Patient characteristics at the time of intervention are presented in Table 1.
Table 1.
Patient characteristics at time of intervention.
| (N=398) | |
|---|---|
| Age, years, Mean (SD) | 73.4 (8.6) |
| Sex | |
| Female | 192 (48.2%) |
| Male | 206 (51.8%) |
| Estimated glomerular filtration rate, Mean (SD), ml/min/1.73m2 | |
| 6–12 months pre intervention | 48.3 (18.7) |
| At time of intervention | 42.5 (17.8) |
| 6–12 months post intervention | 45.5 (20.1) |
| Proteinuria, Mean (SD), mg/24hrs | 428.5 (888.1) |
| Comorbidities, n (%) | |
| Diabetes Mellitus | 138 (34.7) |
| Hypertension | 387 (97.0) |
| Hyperlipidemia | 331 (83.2) |
| Coronary artery disease | 267 (67.1) |
| Statin | 320 (80.4) |
| Medications, n (%) | |
| Angiotensin-converting enzyme inhibitor/Angiotensin II receptor blocker | 351 (88.2) |
| Calcium Channel Blocker | 321 (80.7) |
| Beta Blocker | 335 (84.2) |
| Aspirin | 350 (87.9) |
| Clopidogrel | 264 (66.3) |
| Warfarin | 156 (39.2) |
| Insulin | 102 (25.6) |
Procedure
Patients were referred to the Intervention Radiology Division with the intention of renal artery stent placement if Doppler ultrasound demonstrated a main renal artery peak systolic velocity >180 cm/s or a renal-to-aortic ratio >3.5. Antihypertensive and statin medications were continued until the day of the procedure in all patients. Intravascular access was obtained from the common femoral artery. The renal arteries were selected and angiograms were performed. Identified stenoses ≥50% by visual assessment were treated with angioplasty followed by balloon-mounted bare-metal stent placement. The mean stent diameter was 5.9 ± 0.9 mm. Embolic protection was not utilized. All patients were prescribed aspirin after stent placement unless they had an aspirin allergy.
Measured outcomes
The primary outcome was the impact of estimated glomerular filtration rate (eGFR) on the incidence of RRT, which includes hemodialysis and transplant, and mortality. The 24-hour proteinuria, medications and comorbidities including chronic kidney disease (CKD), coronary artery disease, diabetes mellitus, and smoking history were analyzed as possible factors contributing to RRT and mortality. The need for RRT was obtained by querying the United States Renal Data System. Mortality information was obtained by querying the United States Social Security Death Index and health system medical record up to December 31, 2017.
Estimated glomerular filtration rate was calculated from serum creatinine levels and patient demographic information using the Modification of Diet in Renal Disease equation (8). The eGFR 6–12 months prior to renal artery stent placement, at the time of intervention, and 6–12 months after intervention was determined. Chronic kidney disease stages were determined based on the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group classification (9).
Statistical analysis
Descriptive statistics for patient characteristics are reported as number (percentage) for discrete variables and as mean (SD) for continuous variables. Estimates for overall survival and for survival free of need for RRT were calculated using the Kaplan Meier method. The functional form of post-intervention eGFR was examined using a smoothing spline curve for the outcomes of overall survival and need for RRT. eGFR post-intervention indicated a linear association with overall survival and RRT, though limited to those patients with an eGFR <40 ml/min/1.73m2. In patients with an eGFR >40 ml/min/1.73m2 there was no indication of a change in the eGFR association for either outcome. A univariate Cox model was used to assess the association of eGFR and patient and disease characteristics with need for RRT and overall survival. A multivariate Cox model was also used to assess variables contributing to overall survival. However, a multivariate analysis for RRT was not performed due to the low number of occurrences in the study. The alpha-level was set at 0.05 for statistical significance. All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
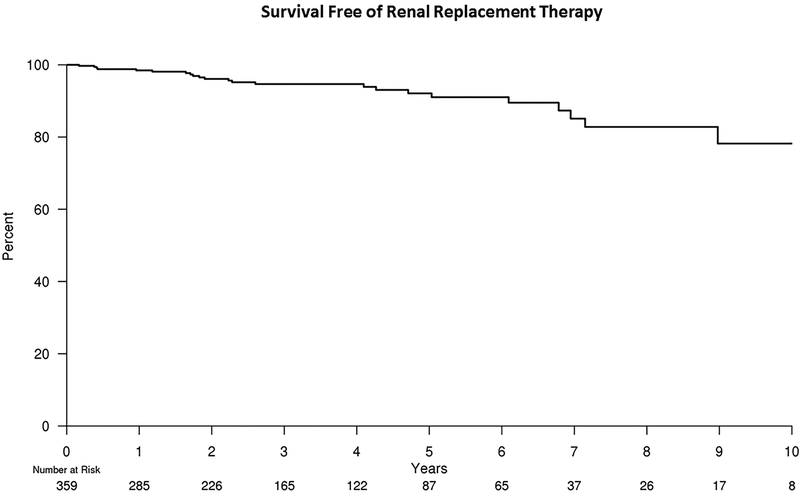
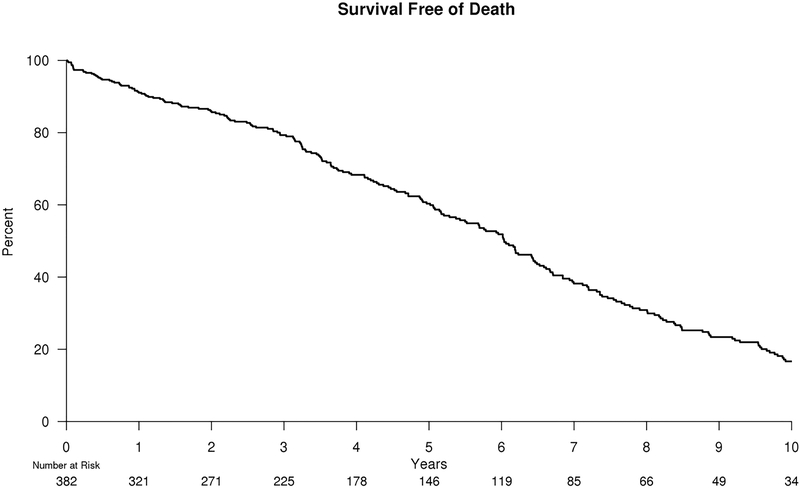
Of the 398 patients who underwent renal artery stent placement 14 patients started RRT, 16 patients died, and 9 were lost to follow-up within 1 year of intervention. The analyses for RRT-free survival and mortality risk began after the 6–12 month post-intervention renal function was determined. The median follow-up time was 6.8 years for RRT and 11.0 years for mortality. A total of 23 patients eventually went on to RRT following post-intervention eGFR evaluation. The RRT free-survival at 5 years was 92.1% (95% confidence interval [CI], 88.0%–96.1%). Regarding mortality, a total of 248 patients died after 6–12 month post-intervention eGFR evaluation with a 5-year mortality risk of 60.3% (95% CI, 54.9%–66.3%). Kaplan-Meier estimates for freedom from the need for RRT and all-cause mortality are illustrated in Figures 1 and 2, respectively.
Figure 1.
Kaplan-Meier estimates for freedom from the need for RRT after renal artery stent placement. Fourteen patients were started on RRT, 16 patients died and 9 were lost to follow-up within 1 year of intervention resulting in an initial number at risk of 359 patients.
Figure 2.
Kaplan-Meier estimates for freedom from all-cause mortality after renal artery stent placement. Sixteen patients died within 1 year of intervention resulting in initial number at risk of 382 patients.
Mean pre-intervention, at intervention, and post-intervention eGFR measured 48.3 ± 18.7 ml/min/1.73m2, 42.5 ± 17.8 ml/min/1.73m2, and 45.5 ± 20.1 ml/min/1.73m2, respectively. The eGFR trajectory from 6–12 months pre-intervention to time of intervention was not significantly associated with risk for RRT or all-cause mortality (P = 0.47 and P = 0.45, respectively). However, the eGFR trajectory from intervention to 6–12 months post-intervention was significantly associated with the freedom from RRT (Hazard ratio [HR] 0.96; 95% CI 0.92–0.99; P = 0.02). This change was not associated with freedom from all-cause mortality (HR 0.99; 95% CI 0.98–1.00; P = 0.21).
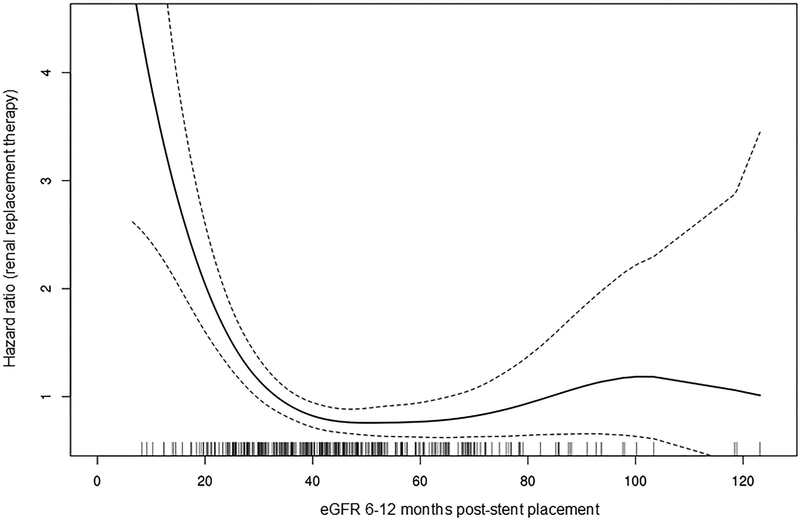
The functional form of eGFR post-intervention with need for RRT was examined. Among patients with a post-intervention eGFR ≤40 ml/min/1.73m2, for a 1 unit eGFR increase up to 40 ml/min/1.73m2, there was a significant decrease in RRT risk (HR 0.87; 95% CI 0.83–0.91; P < 0.001) (Figure 3) as well as all-cause mortality risk (HR 0.95; 95% CI 0.94–0.97; P < 0.001). However, patients with eGFR > 40 ml/min/1.73m2 at 6–12 months post-intervention did not experience lower incidences of RRT or death.
Figure 3.
Risk for RRT as a function of 6–12 months post-intervention eGFR. Each vertical bar at the bottom of the figure represents an individual patient. Note the risk decreases as eGFR approaches 40 ml/min/1.73m2.
Baseline comorbidities and medication analyses with regard to RRT and death outcomes were assessed (Table 2 and Table 3, respectively). Variables associated with higher rates of RRT included diabetes at the time of intervention (P=0.03); increased baseline proteinuria (P<0.001); and stage 4 and stage 5 CKD, relative to stage 1/2/3A (P=0.01 and P=0.003, respectively). Higher mortality rates were observed at univariate analysis in patients with diabetes at time of intervention (P=0.005); increased baseline proteinuria (P=0.01); and stage 4 CKD relative to stage 1/2/3A (P=0.005). A multivariate analysis for mortality was performed including eGFR 6–12 months after intervention, CKD stage, baseline proteinuria, bilateral versus unilateral intervention, ACE inhibitor, angiotensin receptor blocker, clopidogrel, diabetes, coronary artery disease, and cerebrovascular disease. In the final parsimonious model, diabetes was the only comorbidity associated with increased mortality (HR 1.42; 95% CI 1.09–1.86; P = 0.009), while mortality decreased for every 1 unit increase in eGFR up to 40 ml/min/1.73m2 among patients with post-interventional eGFR <40 ml/min/1.73m2 (HR 0.95; 95 % CI 0.94–0.97; P < 0.001).
Table 2.
Univariate Cox Models, predictors for freedom from renal replacement therapy
| Hazard Ratio | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|
| Change, eGFR from pre-intervention to intervention* | 1.01 | 0.98 | 1.05 | 0.47 |
| Change, eGFR from intervention-post-intervention** | 0.96 | 0.92 | 0.99 | 0.02 |
| eGFR post-intervention † | 0.87 | 0.83 | 0.91 | <0.001 |
| CKD stage | ||||
| 1/2/3A | 1.00 | |||
| 3B | 1.29 | 0.37 | 4.47 | 0.69 |
| 4 | 4.32 | 1.35 | 13.80 | 0.01 |
| 5 | 13.58 | 2.46 | 75.13 | 0.003 |
| Proteinuria, per 100 mg | 1.09 | 1.06 | 1.12 | <0.001 |
| Bilateral intervention | 2.60 | 1.12 | 6.07 | 0.03 |
| ACEI/ARB | 1.50 | 0.35 | 6.41 | 0.59 |
| Clopidogrel | 1.75 | 0.71 | 4.31 | 0.23 |
| Diabetes | 2.55 | 1.11 | 5.84 | 0.03 |
| Coronary artery disease | 2.85 | 0.96 | 8.42 | 0.06 |
| Stroke | 0.66 | 0.20 | 2.21 | 0.50 |
Per 1 ml/min/1.73m2 eGFR trajectory increase for 6–12 months leading up to renal artery stent placement.
Per 1 ml/min/1.73m2 eGFR trajectory increase from renal artery stent placement up to 6–12 months follow-up.
eGFR trajectory from renal artery stent placement up to 6–12 months follow-up among patients with eGFR <40. Among patients with an eGFR at post-intervention <40 ml/min/1.73m2, the hazard ratio is for a one unit increase in eGFR, up to 40 ml/min/1.73m2.
CKD=chronic kidney disease; ACEI=angiotensin-converting-enzyme inhibitor; ARB=angiotensin receptor blocker; CI=confidence interval Impact of renal function trajectory on dialysis and mortality risk after renal artery revascularization1
Table 3.
Univariate Cox models, predictors for freedom from all-cause mortality.
| Hazard Ratio | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|
| Change, eGFR pre-intervention - baseline* | 1.01 | 0.99 | 1.02 | 0.45 |
| Change, eGFR baseline-post-intervention** | 0.99 | 0.98 | 1.00 | 0.21 |
| eGFR post-intervention † | 0.95 | 0.94 | 0.97 | <0.001 |
| CKD stage | ||||
| 1/2/3A | 1.00 | |||
| 3B | 0.92 | 0.68 | 1.25 | 0.60 |
| 4 | 1.61 | 1.15 | 2.23 | 0.005 |
| 5 | 1.55 | 0.82 | 2.93 | 0.18 |
| Proteinuria, per 100 mg | 1.02 | 1.00 | 1.04 | 0.01 |
| Bilateral intervention | 1.19 | 0.92 | 1.54 | 0.18 |
| ACEI/ARB | 0.97 | 0.66 | 1.44 | 0.89 |
| Clopidogrel | 1.28 | 0.97 | 1.67 | 0.08 |
| Diabetes | 1.46 | 1.12 | 1.90 | 0.005 |
| Coronary artery disease | 1.28 | 0.96 | 1.71 | 0.09 |
| Stroke | 1.06 | 0.77 | 1.44 | 0.73 |
eGFR trajectory for 6–12 months leading up to renal artery revascularization.
eGFR trajectory from renal artery stent placement up to 6–12 months follow-up.
eGFR trajectory from renal artery stent placement up to 6–12 months follow-up among patients with eGFR <40 ml/min/1.73m2. Among patients with an eGFR at post-intervention <40 ml/min/1.73m2, the hazard ratio is for a one unit increase in eGFR, up to 40 ml/min/1.73m2.
CKD=chronic kidney disease; ACEI=angiotensin-converting-enzyme inhibitor; ARB=angiotensin receptor blocker; CI=confidence interval.
Discussion
Renal artery stent placement has been shown to effectively improve renovascular hypertension and stabilize renal function, but improvement in eGFR is limited (4, 10). In this study, patients had a modest eGFR improvement after renal artery stent placement. However, this small improvement, particularly in patients with advanced CKD, may be significant for reducing RRT and mortality risk. Among patients with stage 3B–5 CKD, eGFR improvement toward 40 ml/min/1.73m2 at 6–12 months after intervention was associated with a decreased risk of both RRT and all-cause mortality.
The rates of RRT and all-cause mortality were 5.8% and 62.3%, respectively. In contrast, the rates of these variables in the stenting plus medical therapy arm of the CORAL trial were 3.5% and 13.7%, respectively. The mean eGFR in the CORAL trial was 58.0 ± 23.4 ml/min/1.73m2 compared to 42.5 ± 17.8 ml/min/1.73m2 in the current study at the time of intervention (4). The findings in the present study indicate that for patients with advanced CKD, the risk for RRT and all-cause mortality will decrease with each unit of eGFR increase. Patients with renal insufficiency from renal artery stenosis and intact compensatory mechanisms such as sustained hypertension may benefit more from revascularization (11). This may explain the lack of benefit from renal artery stent placement that was demonstrated in the CORAL trial where patients had better baseline kidney function as well as questionable anatomic and no hemodynamic assessment of renal artery stenosis significance (12).
Renal function may improve in approximately half of patients who undergo renal artery revascularization while about one-third of patients may experience progression of kidney disease (13). Patients with lower baseline eGFR or rapidly declining kidney function may have a greater response to stent placement compared to patients with normal or near-normal kidney function as was seen in previous randomized control trials (11, 14, 15). The rate of progression to RRT after renal stent placement has been reported to be approximately 10–20 % among patients with chronic kidney disease (16, 17). A study by Thatipelli et al found that among 16 RRT patients with renal artery stenosis, 8 patients were liberated from RRT and remained free from RRT over an average follow-up time of 564 days (18). In that study, 24-hour proteinuria, eGFR prior to intervention, and renal size measured at ultrasound were determined to be predictors for RRT discontinuation. In the present study, elevated 24-hour proteinuria, diabetes and stage 4 CKD increased RRT risk.
Patients with renovascular hypertension and CKD secondary to renal artery stenosis may have higher rates of mortality related to cardiovascular events (19). High-grade 24-hour proteinuria, CKD stage 3b, 4 or 5, carotid disease and diabetes are associated with increased risk for all-cause mortality after renal artery stent placement (15, 20, 21). Higher-grade proteinuria is associated with elevated risk for acute kidney injury after renal artery intervention. Data from the CORAL trial also found that patients who received renal artery stents with lower urine albumin to creatinine ratios had better event-free survival, lower cardiovascular and renal mortality, less progressive renal failure, and better overall survival (22). In the current study, only diabetes was a significant risk factors for increased all-cause mortality in the multivariate model. Furthermore, an eGFR of 40 ml/min/1.73m2 may have utility as a target for optimizing intervention and medical therapy goals to improve outcomes in patients with renal artery stenosis.
A few limitations should be considered. This was a non-randomized retrospective study. Consequently, clinical follow-up and medical therapy were not standardized. In order to capture 398 patients with data 6–12 months prior intervention, at intervention, and 6–12 months post intervention, 1225 patient had to be reviewed. The large number of excluded patients introduces selection bias. This study determined eGFR levels at single time points before and after intervention, and the patient’s clinical status at the time of renal function assessment may have had an effect on the calculated eGFR. The male-to-female ratio was incidentally almost equivalent, which is not representative of the male-predominant prevalence of atherosclerosis in the general population and represents a bias of the study (23). Renal artery stenoses were initially identified by ultrasound criteria and treated if there was at least 50% luminal narrowing of the vessel angiographically. However, renal artery diameter and pressure gradients to further evaluate stenosis severity were not measured. Other treatment factors such as the use of anti-lipid medications such as statins, and reintervention were not evaluated. Statins have been shown to reduce renal artery restenosis rates and confer renal protection benefits over conventional lipid-lowering therapy (24, 25). Lastly, the lack of a control group makes it difficult to assess the effect of renal artery stent placement on RRT and all-cause mortality.
In conclusion, the risk for RRT decreased based on renal function improvement after intervention, but renal function within 12 months prior to intervention did not impact these risks. Most importantly, post-intervention eGFR trajectory improvement approaching 40 ml/min/1.73m2 was associated with decreased RRT and death risk. These findings suggest that patients with advanced CKD and renal artery stenosis may benefit from revascularization regardless of their pre-intervention renal function outlook.
Disclosures:
Sanjay Misra receives funding from National Institutes of Health Grant HL098967 from the National Heart, Lung, and Blood Institute and DK107870 from National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gill KS, Fowler RC. Atherosclerotic renal arterial stenosis: clinical outcomes of stent placement for hypertension and renal failure. Radiology. 2003;226(3):821–6. [DOI] [PubMed] [Google Scholar]
- 2.Soulez G, Therasse E, Qanadli SD, Froment D, Leveille M, Nicolet V, et al. Prediction of clinical response after renal angioplasty: respective value of renal Doppler sonography and scintigraphy. AJR Am J Roentgenol. 2003;181(4):1029–35. [DOI] [PubMed] [Google Scholar]
- 3.Investigators TA. Revascularization versus Medical Therapy for Renal-Artery Stenosis. N Engl J Med. 2009;361(20):1953–62. [DOI] [PubMed] [Google Scholar]
- 4.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis. N Engl J Med. 2014;370(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings CG, Houston JG, Severn A, Bell S, Mackenzie IS, MacDonald TM. Renal Artery Stenosis—When To Screen, What To Stent? Curr Atheroscler Rep. 2014;16(6):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of Renal Artery Stenting on Renal Function and Size in Patients with Atherosclerotic Renovascular Disease. Circulation. 2000;102(14):1671. [DOI] [PubMed] [Google Scholar]
- 7.Arthurs Z, Starnes B, Cuadrado D, Sohn V, Cushner H, Andersen C. Renal artery stenting slows the rate of renal function decline. J Vasc Surg. 2007;45(4):726–32. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis J, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130(6):461–70. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 10.Korsakas S, Mohaupt MG, Dinkel HP, Mahler F, Do DD, Voegele J, et al. Delay of dialysis in end-stage renal failure: Prospective study on percutaneous renal artery interventions. Kidney International.65(1):251–8. [DOI] [PubMed] [Google Scholar]
- 11.Milewski K, Fil W, Buszman P, Janik M, Wanha W, Martin T, et al. Renal Artery Stenting Associated With Improvement in Renal Function and Blood Pressure Control in Long-Term Follow-Up. Kidney Blood Press Res. 2016;41(3):278–87. [DOI] [PubMed] [Google Scholar]
- 12.Sos TA, Mann SJ. Did Renal Artery Stent Placement Fail in the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) Study or Did the CORAL Study Fail Renal Artery Stent Placement? The CORAL Roll-in Experience and the CORAL Trials. Journal of Vascular and Interventional Radiology. 2014;25(4):520–3. [DOI] [PubMed] [Google Scholar]
- 13.Balk E, Raman G, Chung M, et al. Effectiveness of management strategies for renal artery stenosis: A systematic review. Ann Intern Med. 2006;145(12):901–12. [DOI] [PubMed] [Google Scholar]
- 14.Modrall JG, Timaran CH, Rosero EB, Chung J, Arko FA, Valentine RJ, et al. Predictors of outcome for renal artery stenting performed for salvage of renal function. J Vasc Surg. 2011;54(5):1414–21.e1. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Zhang D, Haller S, Kanjwal K, Colyer W, Brewster P, et al. Determinants of renal function in patients with renal artery stenosis. Vasc Med. 2011;16(5):331–8. [DOI] [PubMed] [Google Scholar]
- 16.Beutler JJ, Van Ampting JMA, Ven PJVD, Koomans HA, Beek FJA, Woittiez A-JJ, et al. Long-Term Effects of Arterial Stenting on Kidney Functionfor Patients with Ostial Atherosclerotic Renal Artery Stenosis and Renal Insufficiency. J Am Soc Nephrol. 2001;12(7):1475–81. [DOI] [PubMed] [Google Scholar]
- 17.Paulsen D, Kløw NE, Rogstad B, Leivestad T, Lien B, Vatne K, et al. Preservation of renal function by percutaneous transluminal angioplasty in ischaemic renal disease. Nephrol Dial Transplant. 1999;14(6):1454–61. [DOI] [PubMed] [Google Scholar]
- 18.Thatipelli M, Misra S, Johnson CM, Andrews JC, Stanson AW, Bjarnason H, et al. Renal Artery Stent Placement for Restoration of Renal Function in Hemodialysis Recipients with Renal Artery Stenosis. J Vasc Interv Radiol. 2008;19(11):1563–8. [DOI] [PubMed] [Google Scholar]
- 19.Kashyap VS, Sepulveda RN, Bena JF, Nally JV, Poggio ED, Greenberg RK, et al. The management of renal artery atherosclerosis for renal salvage: Does stenting help? J Vasc Surg. 2007;45(1):101–8. [DOI] [PubMed] [Google Scholar]
- 20.Misra S, Khosla A, Allred J, Harmsen WS, Textor SC, McKusick MA. Mortality and Renal Replacement Therapy after Renal Artery Stent Placement for Atherosclerotic Renovascular Disease. J Vasc Interv Radiol.27(8):1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TP, Cooper CJ, Pencina KM, D’Agostino R, Massaro J, Cutlip DE, et al. Relationship of Albuminuria and Renal Artery Stent Outcomes: Results From the CORAL Randomized Clinical Trial (Cardiovascular Outcomes With Renal Artery Lesions). Hypertension. 2016;68(5):1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016;118(4):535–46. [DOI] [PubMed] [Google Scholar]
- 24.Corriere MA, Edwards MS, Pearce JD, Andrews JS, Geary RL, Hansen KJ. Restenosis after renal artery angioplasty and stenting: incidence and risk factors. J Vasc Surg. 2009;50(4):813–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng M, Dong H, Jiang X, Che W, Zou Y, Zhang Y, et al. A randomized unblinded trial to compare effects of intensive versus conventional lipid-lowering therapy in patients undergoing renal artery stenting. J Cardiol. 2019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]