Abstract
Background:
Prior research on alcohol consumption and pain has yielded inconsistent results regarding the directionality of effects for both consumption-to-pain and pain-to-consumption relations. The present study sought to examine directionality of these relations by testing bidirectional longitudinal associations between consumption and pain interference, a crucial aspect of pain that captures pain-related disability and has been regarded as a valuable measure of treatment outcome. In addition, this study explored possible moderation of these bidirectional longitudinal associations by gender and alcohol use disorder (AUD) symptomatology.
Methods:
Analyses included 29,989 current/former drinkers who were interviewed at both waves (2001 and 2004) of the U.S. National Epidemiological Survey on Alcohol and Related Conditions (NESARC). Analyses used self-report data from both waves on past-year average daily volume of alcohol consumed and past-month pain interference (one item from the Medical Outcomes Study 12-item Short-Form Health Survey [MOS-SF-12]). AUDADIS-IV data from Wave 1 were used to index baseline AUD symptomatology (i.e., symptom count). Cross-lagged panel modeling and multigroup analyses were employed.
Results:
Regarding the consumption-to-pain-interference relation, in general, higher baseline alcohol consumption was associated with lower subsequent pain interference at follow-up. However, among men with higher AUD symptom counts, the opposite pattern emerged, with higher baseline alcohol consumption being significantly related to higher subsequent pain interference at follow-up. Regarding the pain-interference-to-consumption relation, higher baseline pain interference was significantly associated with lower subsequent alcohol consumption at follow-up, and no moderating effects were observed.
Conclusions:
The distinctive patterns of the consumption-to-pain-interference relation observed among men with elevated AUD symptomatology suggest that this relation might be driven by different mechanisms across different groups of individuals. Specifically, the detrimental effect of alcohol on pain interference might emerge at relatively advanced stages of AUD among men, consistent with Koob’s Dark Side of Alcohol Addiction theory in human research.
Keywords: Alcohol Consumption, Alcohol Use Disorder, AUD Symptomatology, Pain Interference, Gender
INTRODUCTION
The co-occurrence of alcohol use disorder (AUD) and chronic pain is common (e.g. Jakubczyk et al., 2015; Von Korff et al., 2005). Given the significant societal costs associated with these conditions (Bouchery et al., 2011; Institute of Medicine, 2011), understanding correlates, mechanisms, and modifying factors underlying their co-occurrence is of rising empirical interest (Egli et al., 2012). Previous studies have reported significant, but differing, relations both from alcohol consumption to pain and from pain to alcohol consumption, with some studies showing positive associations and others showing negative associations (see Ditre et al. [2019] and Zale et al. [2015] for comprehensive reviews). The present study aimed to further explore these alcohol consumption-pain relations across time, and aimed to explore the effects of two relevant moderating factors: gender and AUD symptomatology. Inclusion of these moderators is supported by neurobiological theory suggesting a unique role for AUD in alcohol-pain comorbidity (Borsook et al., 2016; Egli et al., 2012) and prior studies suggesting gender differences in relations between alcohol and pain (e.g., Barry et al., 2013; Zale et al., 2019).
Associations of alcohol and pain-related outcomes.
Prior research has indeed suggested that associations of alcohol consumption on pain experience may change with progression from moderate to excessive consumption. Moderate consumption, when compared to abstinence, has been robustly associated with lower risk for chronic pain conditions and pain-related disability (e.g., Di Giuseppe et al., 2012; Scott et al., 2018). In contrast, there is evidence for a positive association between excessive alcohol consumption and increased pain, although evidence for this relation has been more mixed. A prospective study of older adults has found that problem drinkers experienced higher levels of pain and pain interference (Brennan et al., 2005), and Witkiewitz and Vowles’s (2018) review documented that 16 – 25% of chronic pain patients in treatment reported a history of heavy alcohol use or AUD. In contrast, population-based and clinical studies have linked heavy drinking to reduced pain and pain-related functioning among patients with chronic pain disorders (e.g., MacFarlane et al., 2015; Kim et al., 2013). These mixed findings may result from heterogeneity in samples. Based on our literature review, it appears that studies more often linked alcohol consumption to increased pain when the samples included more problem drinkers or individuals with AUD (e.g. Brennan et al., 2005; Witkiewitz et al., 2015); whereas consumption appeared more likely to be linked to reduced pain in other populations such as chronic pain patients (e.g., Ekholm et al., 2009; MacFarlane et al., 2015).
The apparent moderating impacts of problematic and/or symptomatic drinking on alcohol consumption’s relation to pain may be explained by neurobiological theory describing brain changes that accompany escalation to severe forms of excessive and pathologic drinking. According to the “Dark Side of Alcohol Addiction” (Koob, 2013), the development of AUD may often bring neuroadaptations reflecting down-regulation (i.e., decreased sensitivity) of reward systems and up-regulation (i.e., increased sensitivity) of stress systems – manifestation of allostatic load (Heilig & Koob, 2007). Of key importance for the current study, this model has also been extended to suggest that the same dysregulation of the brain’s reward and stress systems may lead to increased pain sensitization via dysregulation of pain modulation (Borsook et al., 2016; Egli et al., 2012).
Given that recent neurocognitive studies have shown a positive association between AUD severity and alcohol-related neurological dysregulation (e.g., Aloi et al., 2018; Joyner et al., 2016), it is reasonable to suspect that alcohol’s pain-sensitization effects would become increasingly pronounced with increasing AUD severity (e.g., as indexed by AUD symptom count). Further, in addition to increased pain sensitization, other addiction-related neurological impairments (e.g., compromised self-regulatory capacities) would likely exacerbate other negative pain-related outcomes such as pain-related distress and functional disability/interference. Thus, the current study tests the hypothesis that longitudinal associations of alcohol consumption on pain interference may be moderated by AUD severity. Specifically, we predict that alcohol consumption may be associated with decreased pain interference among those with no/low AUD symptoms but may instead increase pain interference among those with medium/high AUD symptoms, and this reversed effect of consumption on increased pain interference will be more pronounced at higher levels of AUD severity (i.e., greater symptom count).
Associations of pain and drinking-related outcomes.
Empirical studies on pain influencing alcohol consumption have also yielded mixed results. An association of pain with less drinking was observed among patients with chronic non-cancer pain (Ekholm et al., 2009). However, this relationship may also vary as a function of heavy/problem-drinking severity. For instance, Brennan and colleagues (2005) reported more use of alcohol as a pain-coping strategy among problem drinkers versus non-problem drinkers. Further, multiple studies have linked pain to higher relapse risk among individuals diagnosed with AUD (Jakubczyk et al., 2015; Witkiewitz et al., 2015). Thus, our literature review suggests that pain-related reductions in drinking may be more commonly observed among pain patients without AUD symptomatology.
The neurobiological theory of addiction described earlier may also explain this apparent moderating effect of AUD symptomatology. Specifically, the neurologic dysregulation that characterizes addiction and often accompanies AUD may also increase the extent that pain generates a stress response “similar” to the stress response triggered by alcohol withdrawal (Jakubczyk et al., 2015; Witkiewitz et al., 2015), perhaps thereby increasing the likelihood that pain and related disability will motivate self-medication via alcohol consumption. In contrast, pain-related reductions in drinking may be more commonly observed among pain patients without a history of AUD, likely reflecting an adaptive pain coping mechanism in which pain patients actively avoid any detrimental interactions of alcohol with pain relievers (Bobo et al., 2013; Ekholm et al., 2009). Thus, the current study tests the hypothesis that longitudinal associations of pain interference on alcohol consumption may be moderated by AUD severity. Specifically, we predict that pain interference may be associated with decreased alcohol consumption among those with no/low AUD symptoms but may instead increase alcohol consumption among those with medium/high AUD symptoms, and this reversed effect of pain interference on increased consumption will be more pronounced at higher levels of AUD severity (i.e., greater symptom counts).
Current Study
To test the bidirectional longitudinal effects between alcohol consumption and pain interference, this study employed cross-lagged panel modeling, using two waves of data spanning three years from the U.S.-representative sample of the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC; Grant et al., 2003). As explained above, we predicted moderation of both effects by AUD-symptom severity (indexed by symptom count). Additionally, our analyses also attended to possible gender moderation, given that previous studies have shown gender differences in alcohol-pain associations (e.g., Barry et al., 2013; Zale et al., 2019). Of particular relevance, a prior study using NESARC data reported that the relation between pain interference and the incidence of AUD diagnoses was significantly moderated by gender, with a positive association between pain interference and AUD onset in men and a negative association between pain interference and AUD onset in women (although the conditional gender-specific effects did not reach statistical significance; Barry et al., 2013). Thus, we hypothesized that the moderating effect of AUD symptomology on the bidirectional relations between alcohol consumption and pain interference would be stronger in men than in women.
An advantage of the NESARC dataset for the current study’s purposes is that it provides representation of the full range of drinking behaviors, in contrast to clinical or treatment-seeking samples that primarily represent more pathologic forms of alcohol involvement. Pain interference, the only pain-related construct assessed in NESARC, measures the extent that physical functioning is affected by pain (i.e. pain-related disability). It has been considered more indicative of treatment progress among chronic pain patients than pain intensity due to the fact that pain interference/physical functioning represents a more downstream outcome of pain conditions than pain intensity (Darnall & Sullivan, 2019; Karayannis, 2019), and thus, it has been argued to provide a broader index of pain relative to pain intensity (Cook et al., 2013). Pain interference is also closely related to central pain sensitization. Assessed by the Brief Pain Inventory (Cleeland & Ryan, 1994), pain interference was found to correlate with central sensitization on pain-related symptoms assessed by the Central Sensitization Inventory (Mayer et al., 2012; Mibu et al., 2019). Finally, NESARC’s assessment of our constructs of interest across two longitudinal waves provides temporal precedence for hypothesis testing, although we emphasize that this study did not aim to draw causal inferences. The above strengths are further enhanced by the representativeness of the NESARC sample and associated advantages regarding generalizability of our findings.
MATERIALS AND METHODS
Sample
NESARC is a nationally representative survey of adults in the U.S. funded by the National Institute on Alcoholism and Alcohol Abuse (Grant et al., 2003). Non-institutionalized residents aged 18 or older were recruited. At the Wave-1 (baseline; 2001–2002) and Wave-2 (follow-up; 2004–2005) assessments, 43,093 and 34,653 participants responded, respectively. The present study included participants who responded at both waves and were current or former drinker at either wave (i.e. lifetime abstainers were excluded; for more on this decision, see Online Supplement 1). Also, four outliers were excluded due to extreme baseline alcohol consumption values. These exclusion criteria yielded a sample of N=29,989 (see Table 1 for descriptive statistics compiled per guidelines from Barry et al. [2012]).
Table 1.
Descriptive statistics for sociodemographic variables
| Sociodemographic variable | Mean (S.D.) |
|---|---|
| Age | 45.1 (16.8) |
| Percent | |
| Sex | |
| Men | 45% |
| Women | 55% |
| Race/Ethnicity | |
| White | 60.9% |
| Black | 17.8% |
| American Indian | 1.7% |
| Asian | 2.3% |
| Hispanic | 17.3% |
| Marital Status | |
| Married | 53.5% |
| Previously married | 23.9% |
| Never married | 22.6% |
| Household Income | |
| Less than $5,000 - $49,999 | 61.4% |
| 50,000 - 200,000 or more | 38.6% |
| Education Levels | |
| Less than high school | 14.4% |
| High school graduate | 28.1% |
| Some college | 31.5% |
| College or higher | 26.0% |
| Employment Status | |
| Full time | 56.2% |
| Part time | 10.5% |
| Not working | 23.9% |
| Missing value | 9.4% |
Measures
Alcohol Consumption was indexed by past-year average daily ethanol consumption, derived from beverage-specific volumes in ounces assessed across four beverage types (cooler, beer, wine, and liquor). Participants reported sizes of drinks, quantity of drinks consumed on a drinking day, and frequency of drinking days per week. The final alcohol consumption variable also adjusted for frequency of and quantity consumed on risky drinking days (defined as > five drinks per day) (for more details, see NIAAA [2004] NESARC Data Notes).
Pain Interference was indexed by one item from the Medical Outcomes Study 12-item Short-Form Health Survey (MOS-SF-12). This item asks participants the extent their engagement in daily activities was impacted by pain in the past four weeks, with response options ranging from (1) “not at all” to (5) “extremely” (Blanco et al., 2016; Karayannis, 2019; Ware et al., 1996).
AUD Symptom Count was a past-year count of the 11 DSM-IV alcohol-abuse and dependence symptoms. These symptoms were assessed via the DSM-IV version of the Alcohol Use Disorder and Associated Disability Interview Schedule (AUDADIS-IV), a structured diagnostic interview administered in NESARC by trained non-clinical interviewers (Grant and Anawait, 2003) that has been shown to yield highly reliable assessments of psychiatric disorders (Grant et al., 2003).
Covariates comprised a rich set of additional variables included in models to adjust for possible confounding and thereby reduce bias in parameter estimates of primary interest. All current covariates have been previously reported to be associated with at least one of this study’s four variables of primary interest (alcohol consumption, pain interference, AUD-symptom count, and gender). These covariates included sociodemographics (age, race/ethnicity, marital status, household income, education level, and employment status), substance use disorders, anxiety disorders, mood disorders, personality disorders, family histories of substance use disorders, depression, antisocial personality disorder, general medical conditions, and stressful life events. Note that gender was also included as a covariate in analyses not testing it as a potential moderator. Previous NESARC studies including similar covariate sets have recommended adoption of this approach as standard practice (e.g. Barry et al., 2013; Blanco et al., 2016). For detailed descriptions of covariates, see Online Supplement 2.
Statistical Analysis
Skewness of average daily alcohol consumption and pain interference was minimized through natural log transformation (see Tables 2). See Tables 3a-3c for prevalences of covariates overall and stratified by gender, AUD symptomatology, and pain interference. All analyses described below followed a sequential model building procedure in Mplus 8 (Muthén & Muthén, 1998–2017) using the MLR estimator to obtain parameter estimates that are robust to non-normality and missing data. Analyses also accounted for NESARC’s primary sampling unit, stratum, and population weights, thereby adjusting for sampling-related nonindependence of observations and deriving U.S.-representative model estimates (via TYPE=COMPLEX with CLUSTER, STRATIFICATION, and WEIGHT specifications).
Table 2.
Four moments of baseline and follow-up alcohol consumption and pain interference stratified by gender after log transformation.
| Before Log Transformation |
After Log Transformation |
|||||
|---|---|---|---|---|---|---|
| Mean | Standard Deviation |
Mean | Standard Deviation |
Skewness | Kurtosis | |
| All respondents | ||||||
| Baseline Alcohol Consumption | 0.39 | 1.15 | −5.40 | 5.33 | −0.70 | −1.05 |
| Baseline Pain Interference | 1.71 | 1.17 | 0.36 | 0.54 | 1.10 | −0.29 |
| Follow-up Alcohol Consumption | 0.38 | 1.13 | −5.37 | 5.34 | −0.72 | −1.05 |
| Follow-up Pain Interference | 1.68 | 1.07 | 0.36 | 0.51 | 1.01 | −0.40 |
| Men | ||||||
| Baseline Alcohol Consumption | 0.61 | 1.45 | −4.47 | 5.35 | −0.94 | −0.68 |
| Baseline Pain Interference | 1.65 | 1.14 | 0.33 | 0.53 | 1.26 | 0.11 |
| Follow-up Alcohol Consumption | 0.59 | 1.50 | −4.51 | 5.39 | −0.93 | −0.72 |
| Follow-up Pain Interference | 1.60 | 1.01 | 0.32 | 0.49 | 1.18 | 0.00 |
| Women | ||||||
| Baseline Alcohol Consumption | 0.21 | 0.77 | −6.16 | 5.18 | −0.57 | −1.24 |
| Baseline Pain Interference | 1.76 | 1.20 | 0.39 | 0.55 | 0.99 | −0.56 |
| Follow-up Alcohol Consumption | 0.20 | 0.66 | −6.07 | 5.19 | −0.60 | −1.22 |
| Follow-up Pain Interference | 1.74 | 1.10 | 0.40 | 0.53 | 0.88 | −0.66 |
Table 3a.
Percentages of all respondents, men, and women who reported psychiatric disorders, general medical conditions, and stressful life events (variables used in model testing)
| All respondents | Men | Women | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Psychiatric disorders | ||||||
| Alcohol use disorder | ||||||
| 0 - 1 symptoms | 89.1 | 84.7 | 92.7 | |||
| 2 - 3 symptoms | 7 | 9.6 | 4.9 | |||
| 4 - 5 symptoms | 2.3 | 3.2 | 1.5 | |||
| ≥6 symptoms | 1.6 | 2.5 | 0.9 | |||
| Substance use disorders | 13.9 | 15.7 | 15.2 | 17.4 | 12.9 | 14.4 |
| Anxiety disorders | 12.3 | 13.5 | 8.6 | 8.9 | 15.3 | 17.3 |
| Mood disorders | 11.1 | 11.2 | 8.2 | 7.7 | 13.5 | 14.1 |
| Personality disorders | 16.0 | 16.3 | 15.7 | |||
| Family history of psychiatric disorders | ||||||
| Substance use disorders | 18.2 | 15.2 | 20.7 | |||
| Depression | 33.5 | 27.7 | 38.4 | |||
| Antisocial personality disorder | 19.0 | 16.5 | 21.1 | |||
| General medical conditions^ | 26.4 | 24.7 | 27.8 | |||
| Stressful life events^ | 64.3 | 60.9 | 67 | |||
General medical conditions and stressful life events were entered as count variables in the models.
However, for the sake of brevity, they are presented as dichotomized variables here to indicate no history vs. any history of a general medical condition or stressful life event.
Table 3c.
Percentages of respondents who reported psychiatric disorders, general medical conditions, and stressful life events, stratified by gender and pain interference groups
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| No or mild pain interference at Baseline |
Moderate to severe pain interference at Baseline |
No or mild pain interference at Baseline |
Moderate to severe pain interference at Baseline |
|||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Psychiatric disorders | ||||||||
| Alcohol use disorder | ||||||||
| 0 - 1 symptoms | 84.6 | 85.0 | 92.7 | 93.3 | ||||
| 2 - 3 symptoms | 9.6 | 8.5 | 4.9 | 4.0 | ||||
| 4 - 5 symptoms | 3.2 | 3.1 | 1.5 | 1.5 | ||||
| ≥6 symptoms | 2.5 | 3.5 | 0.9 | 1.2 | ||||
| Substance use disorders | 15.2 | 17.4 | 20.2 | 22.4 | 13.0 | 14.4 | 17.7 | 18.8 |
| Anxiety disorders | 8.6 | 8.9 | 13.0 | 13.5 | 15.3 | 17.3 | 23.1 | 23.7 |
| Mood disorders | 8.2 | 7.7 | 13.5 | 13.0 | 13.5 | 14.1 | 22.8 | 19.9 |
| Personality disorders | 16.3 | 21.8 | 15.8 | 22.2 | ||||
| Family history of psychiatric disorders | ||||||||
| Substance use disorders | 15.3 | 18.9 | 20.7 | 24.9 | ||||
| Depression | 27.8 | 30.1 | 38.5 | 44.1 | ||||
| Antisocial personality disorder | 16.6 | 19.0 | 21.2 | 26.2 | ||||
| General medical condition^ | 24.8 | 42.2 | 27.9 | 49.6 | ||||
| Stressful life event^ | 61.1 | 70 | 67.3 | 75.3 | ||||
Base Model.
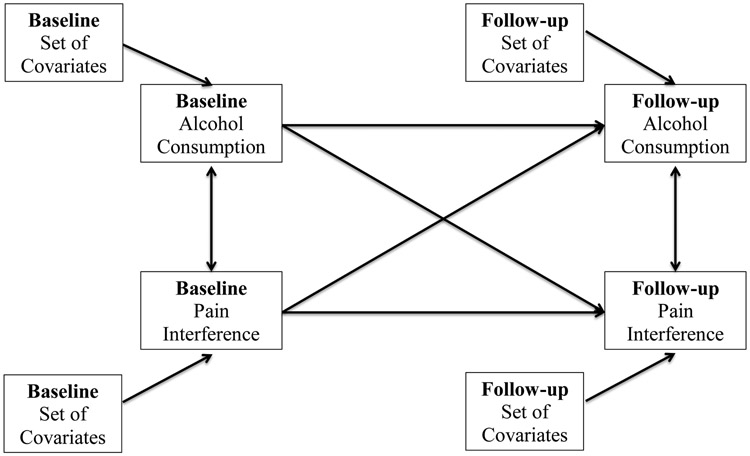
Adjusting for covariates, a cross-lagged panel model (Figure 1) estimated (1) the longitudinal bidirectional cross-lagged associations between alcohol consumption and pain interference, (2) longitudinal stability of alcohol consumption and pain interference, and (3) the cross-sectional relation at baseline and residual cross-sectional relation at follow-up (Burkholder and Harlow, 2003; Sher et al., 1996). Importantly, modeling the stability of consumption and pain interference adjusts for baseline levels of the dependent variables, thereby helping establish temporal precedence in the longitudinal, cross-lagged paths of primary interest (the consumption-to-pain-interference and pain-interference-to-consumption paths).
Figure 1.
A cross-lagged panel model capturing (1) the stability of alcohol consumption and pain interference over time, (2) the adjusted cross-sectional relations at baseline, (3) the bidirectional relations between alcohol consumption and pain interference, and (4) covariance estimates between contemporaneous residuals at follow-up.
Moderator Analyses.
As explained earlier, key hypotheses pertain to possible moderating effects of AUD symptomatology1 on the longitudinal, cross-lagged consumption-to-pain-interference and pain-interference-to-consumption paths. We initially planned to simply treat AUD-symptom-count as a continuous moderator to index AUD-symptom severity, but an examination of the AUD-symptom-count variable revealed that the distribution was predominated by individuals with 0 or 1 symptom(s), with far fewer individuals having values of 2 or more symptoms (akin to a zero-inflated distribution). This degree of skewness and kurtosis can potentially lead to bias in parameter estimates and difficulties in interpreting the findings. This threshold was also selected given its clinical implications. In the NESARC sample, 15% of men and 7% of women met our threshold, which is similar to the percentages of men and women that met DSM-IV criteria for alcohol abuse and/or dependence (13% and 6%, respectively).
Given the described rationales, preliminary tests of moderation attended to possible moderation by AUD-symptom-count as a function of the selected threshold contrasting those with ≤1 symptom(s) (the “inflated group”) versus those with ≥2 symptoms using a multigroup model. As moderation by gender was also of interest, the model also distinguished men from women. Thus, altogether, the multigroup model included four groups: (1) men with ≤1 symptom(s), (2) men with ≥2 symptoms, (3) women with ≤1 symptom(s), and (4) women with ≥2 symptoms (see Table 6). To assess the hypothesized moderating effects of AUD symptoms, Wald χ2 (one-degree-of-freedom) tests (conducted via the Mplus MODEL TEST command) assessed whether the longitudinal cross-lagged consumption-to-pain-interference and pain-interference-to-consumption paths differed significantly by symptom-count group (tested separately in men and women; see Figure 2 and Table 6). To assess the hypothesized moderating effects of gender, additional Wald χ2 tests assessed whether the longitudinal cross-lagged paths differed significantly by gender group (tested separately in ≤1 symptom(s) group and ≥2 symptoms group; see Table 6). If the paths did not differ between the symptom groups for a given gender, then all respondents within that gender were included in subsequent model testing. However, if the paths did differ between the symptom groups for a given gender, then the symptom groups were analyzed separately in subsequent model testing for that gender.
Table 6.
Testing AUD symptom count as a moderator of bidirectional longitudinal cross-lagged relations between alcohol consumption and pain interference: Results from the preliminary tests of moderation using multigroup modeling to contrast those with 0 or 1 symptom(s) (the “inflated group”) versus those with 2 or more symptoms.
| Men (n=12,593) | Women (n=13,834) | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤1 AUD symptom(s) at Baseline (n=10,720) |
≥2 AUD symptoms at Baseline (n=1,873) |
≤1 AUD symptom(s) at Baseline (n=12,799) |
≥2 AUD symptoms at Baseline (n=1,035) |
|||||
| Beta (S.E.) | Two-tailed p-value |
Beta (S.E.) | Two-tailed p-value |
Beta (S.E.) | Two-tailed p-value |
Beta (S.E.) | Two-tailed p-value |
|
| Prospective cross-lagged relations | ||||||||
| Baseline alcohol consumption to follow-up pain interference | −.039 (.010) | < .001 | .049 (.027) | .066 | −.034 (.011) | .002 | −.063 (.029) | .029 |
| Baseline pain interference to follow-up alcohol consumption | −.011 (.009) | .218 | −.051 (.029) | .077 | −.014 (.010) | .152 | .003 (.042) | .938 |
| Temporal stability (i.e., autoregressive paths) | ||||||||
| Baseline alcohol consumption to follow-up alcohol consumption | .584 (.013) | < .001 | .203 (.034) | < .001 | .546 (.012) | < .001 | .249 (.034) | < .001 |
| Baseline pain interference to follow-up pain interference | .305 (.014) | < .001 | .287 (.029) | < .001 | .294 (.012) | < .001 | .344 (.037) | < .001 |
| Cross-sectional relation | ||||||||
| Baseline alcohol consumption with baseline pain interference | −.027 (.013) | .030 | .008 (.027) | .758 | −.051 (.010) | < .001 | −.034 (.037) | .365 |
| Wald χ2 tests | ||||||||
| Tests of two-way interactions by symptom group | ||||||||
| Among men | ||||||||
| Consumption-to-pain-interference path by symptom group | (χ2(1)=4.953 (p=0.026)) | |||||||
| Pain-interference-to-consumption path by symptom group | (χ2(1)=0.972 (p=0.324)) | |||||||
| Among women | ||||||||
| Consumption-to-pain-interference path by symptom group | (χ2(1)=3.265 (p=0.071)) | |||||||
| Pain-interference-to-consumption path by symptom group | (χ2(1)=0.309 (p=0.578)) | |||||||
| Wald χ2 tests | ||||||||
| Tests of two-way interactions by gender group | ||||||||
| Among individuals with ≤1 symptom(s) | ||||||||
| Consumption-to-pain-interference path by gender group | (χ2(1)=0.014 (p=0.907)) | |||||||
| Pain-interference-to-consumption path by gender group | (χ2(1)=0.013 (p=0.910)) | |||||||
| Among individuals with ≥2 symptoms | ||||||||
| Consumption-to-pain-interference path by gender group | (χ2(1)=7.440 (p=0.006)) | |||||||
| Pain-interference-to-consumption path by gender group | (χ2(1)=1.323 (p=0.250)) | |||||||
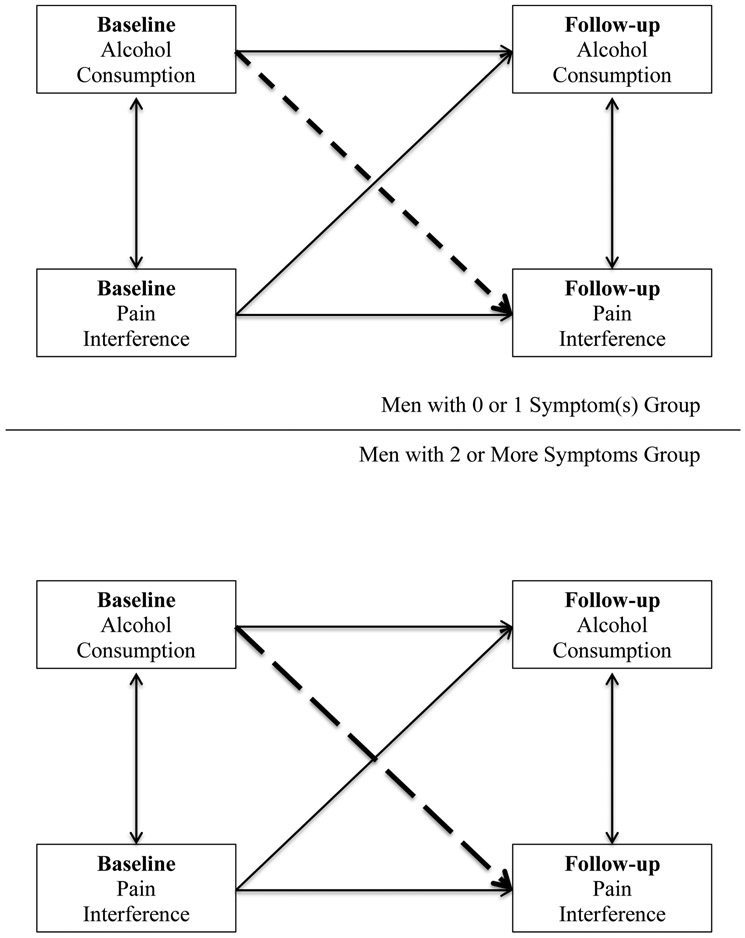
Figure 2.
One example of the multigroup tests comparing whether the association between baseline consumption and follow-up pain interference differed between the 0 or 1 symptom(s) (short-dashed line) vs. 2 or more symptoms group (long-dashed line) among men.
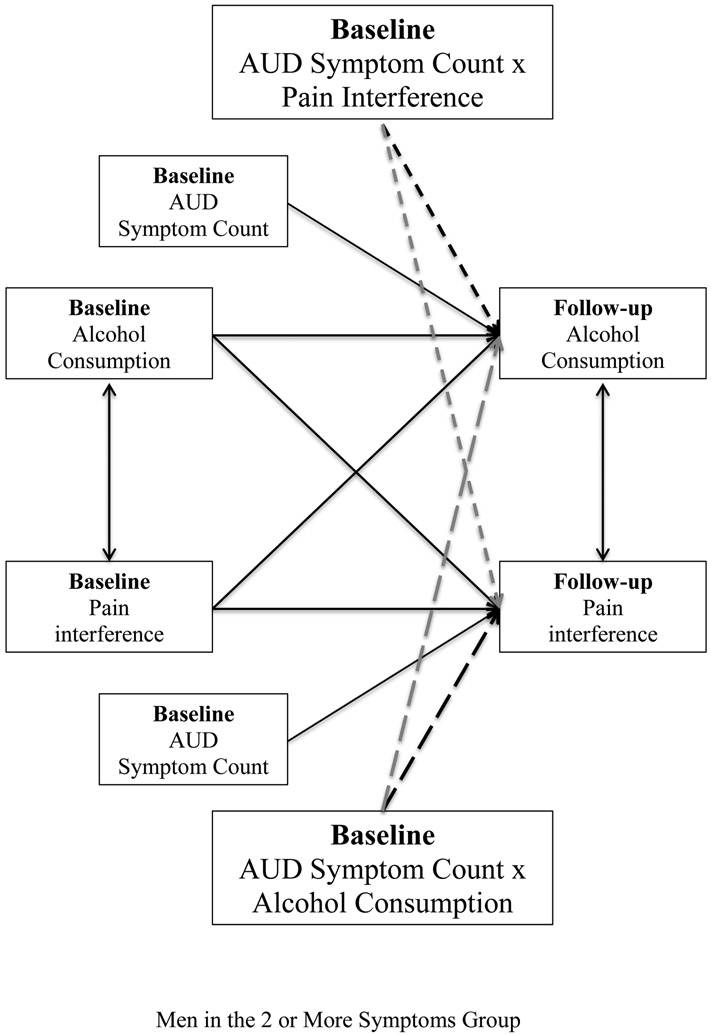
In the final tests of moderation, we created two interaction terms (symptom count x consumption and symptom count x pain interference) by multiplying AUD-symptom-count2 with alcohol consumption and with pain interference at baseline, respectively. These 2-way interaction terms and the main effect of AUD-symptom-count were added to the cross-lagged panel model (see Figure 3). To account for possible gender moderation, we again used a multigroup modeling approach. In this model, the hypothesized moderating effects of AUD symptoms were evaluated based on the significance of the interaction terms (a two-way interaction estimated separately in the two gender groups of this multigroup model). Also, to formally test the possibility that moderation by AUD symptoms may vary by gender, Wald χ2 tests assessed if effects of the interaction terms differed significantly between the two gender groups.
Figure 3.
Moderation model examining the moderation effects of symptom count by consumption (long-dashed line) and symptom count by pain interference (short-dashed line) among men in the 2 or more symptoms group.
RESULTS
Examining the Bidirectional Associations between Alcohol Consumption and Pain Interference from Baseline to Follow-up Assessments.
Zero-order correlations among alcohol consumption and pain interference in log scale across time are provided in Table 4. Base Model. Results of the main effects cross-lagged panel model of alcohol consumption and pain interference that included the described covariates (see Figure 1) are reported in Table 5. First, the adjusted longitudinal cross-lagged relations between alcohol consumption and pain interference were negative and significant. Specifically, higher levels of consumption at baseline were associated with lower levels of pain interference at follow-up (beta=−.039, p<.001), and higher levels of pain interference reported at baseline were related to lower levels of consumption at follow-up (beta=−.013, p=.032). Second, alcohol consumption and pain interference at baseline were significantly associated with themselves at follow-up, demonstrating stability across time (for alcohol consumption, beta=.584, p<.001; for pain interference, beta=.301, p<.001). Third, the adjusted negative cross-sectional relation between alcohol consumption and pain interference was significant at baseline (r=−.039, p<.001).
Table 4.
Zero-order correlations among alcohol consumption and pain interference in log scale across time
| Baseline alcohol consumption |
Baseline pain interference |
Follow-up alcohol consumption |
Follow-up pain interference |
|
|---|---|---|---|---|
| All respondents | ||||
| Baseline alcohol consumption | 1 | |||
| Baseline pain interference | −.115** | 1 | ||
| Follow-up alcohol consumption | .604** | −.141** | 1 | |
| Follow-up pain interference | −.133** | .420** | −.155** | 1 |
| Men below diagonal, Women above diagonal | ||||
| Baseline alcohol consumption | 1 | −.130** | .580** | −.138** |
| Baseline pain interference | −.081** | 1 | −.155** | .433** |
| Follow-up alcohol consumption | .612** | −.109** | 1 | −.169** |
| Follow-up pain interference | −.105** | .398** | −.118** | 1 |
| Men with ≤1 AUD symptom(s) below diagonal, Men with ≥ 2 symptoms above diagonal | ||||
| Baseline alcohol consumption | 1 | .060** | .222** | .089** |
| Baseline pain interference | −.098** | 1 | −0.034 | .374** |
| Follow-up alcohol consumption | .593** | −.123** | 1 | 0.013 |
| Follow-up pain interference | −.118** | .402** | −.134** | 1 |
| Women with ≤1 AUD symptom(s) below diagonal, Women with ≥ 2 symptoms above diagonal | ||||
| Baseline alcohol consumption | 1 | −0.021 | .259** | −0.035 |
| Baseline pain interference | −.137** | 1 | −.075** | .513** |
| Follow-up alcohol consumption | .562** | −.161** | 1 | −.117** |
| Follow-up pain interference | −.144** | .427** | −.172** | 1 |
Correlation is significant at the 0.01 level (2-tailed).
Table 5.
Model results testing bidirectional longitudinal cross-lagged relations between alcohol consumption and pain interference (N = 26,427)
| Beta (S.E.) | Two-tailed p-value |
|
|---|---|---|
| Prospective cross-lagged relations | ||
| Baseline alcohol consumption to follow-up pain interference | −.039 (.007) | < .001 |
| Baseline pain interference to follow-up alcohol consumption | −.013 (.006) | .032 |
| Temporal stability (i.e., autoregressive paths) | ||
| Baseline alcohol consumption to follow-up alcohol consumption | .584 (.010) | < .001 |
| Baseline pain interference to follow-up pain interference | .301 (.009) | < .001 |
| Cross-sectional relation | ||
| Baseline alcohol consumption with baseline pain interference | −.039 (.008) | < .001 |
Examining Moderating Effects of AUD Symptoms and genders on Alcohol-Consumption-to-Pain-Interference Paths.
Preliminary Tests of Moderation (see Table 6):
Multigroup model contrasting those with ≤1 symptom(s) versus ≥2 symptoms. In men, the consumption-to-pain-interference path differed significantly between the ≤1 symptom(s) group versus the ≥2 symptoms group. This moderation by symptom group was such that higher baseline alcohol consumption was significantly associated with lower follow-up pain interference in the ≤1 symptom(s) group but was associated with higher follow-up pain interference in the ≥2 symptoms group at a marginal level (p=.066). In contrast, for women, the consumption-to-pain-interference path did not differ significantly between the symptom-count groups with both groups showing that higher baseline alcohol consumption was significantly associated with lower follow-up pain interference. Multigroup model contrasting men versus women. The consumption-to-pain-interference path did not differ between men and women in the ≤1 symptom(s) group, but it did differ between men and women in the ≥2 symptoms group with men and women respectively showing the described positive and negative relations.
Final Tests of Moderation:
Because the preliminary tests of moderation suggested that men reporting ≤1 symptom(s) significantly differed from men reporting ≥2 symptoms in the consumption-to-pain-interference relation, we conducted separate tests of moderation for these groups. The model focusing on participants with ≥2 symptom(s) and comparing the effect of symptom count (analyzed as a continuous variable ranging from 2–11) x alcohol consumption (2-way interaction) on pain interference across men and women showed that the 2-way interaction terms were significantly different across gender (Wald test value =5.799, p=.016, N=2,908).
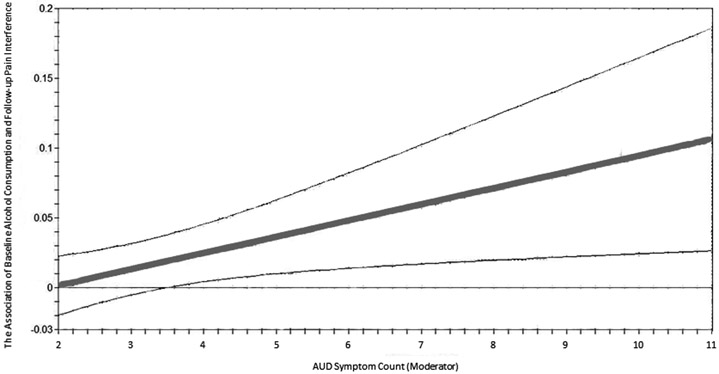
Among men, AUD-symptom-count significantly moderated the association between baseline alcohol consumption and follow-up pain interference (beta of interaction of symptom count x alcohol consumption =.102, s.e.=.045, p=.022, N=1,873). As shown in Figure 4, for individuals with >3 symptoms, zero was not included within the confidence intervals of the association between baseline alcohol consumption and follow-up pain interference. This moderation was such that the association of greater baseline alcohol consumption with higher follow-up pain interference strengthened at higher levels of AUD symptoms. A 1% increase in alcohol consumption was associated with a 0.01% increase in pain interference for men with 2–3 AUD symptoms, a .21% increase in pain interference for men with 4–5 symptoms, and a .41% increase in pain interference for men with ≥6 AUD symptoms (these specific AUD-symptom levels were chosen only to provide illustrative examples of the linear moderation trend). In contrast, the consumption-to-pain-interference path showed no significant difference among men with no vs. one symptom (Wald test value=3.642, p=.056, N=10,720). A negative association between baseline alcohol consumption and follow-up pain interference was found in men with no symptoms (beta=−.030, s.e.=.012, p=.011) or 1 symptom (beta=−.082, s.e.=.025, p=.001).
Figure 4.
The association between alcohol consumption at baseline and pain interference at follow-up as a function of AUD symptom count among men
Among women, AUD symptoms did not significantly moderate the association between baseline alcohol consumption and follow-up pain interference (beta of interaction of symptom count x alcohol consumption =−.045, s.e.=.046, p=.327, N=1,035). Women with ≤1 symptom(s) were excluded in this analysis. However, we also tested the effect of symptom count x alcohol consumption on pain interference in all women, and the results were similar (beta of interaction of symptom count x alcohol consumption=−.068, s.e.=.052, p=.187, N=13,834).
Examining Moderating Effects of AUD Symptoms and gender on Pain-Interference-to-Alcohol-Consumption Paths.
Preliminary Tests of Moderation (see Table 6):
Multigroup model contrasting those with ≤1 symptom(s) versus ≥2 symptoms. When examining differences between the symptom groups in relation of baseline pain interference with follow-up alcohol consumption for men and women, the Wald χ2 tests were consistently non-significant, suggesting similar associations between the symptom groups for both men and women. Multigroup model contrasting men versus women. When examining differences between gender groups in the relation of baseline pain interference with follow-up alcohol consumption for ≤1 symptom(s) and ≥2 symptoms groups, the Wald χ2 tests were also non-significant, suggesting similar associations between gender groups for both ≤1 and ≥2 symptoms groups.
Final Tests of Moderation:
The model including all participants and comparing whether the longitudinal effect of symptom count x pain interference (2-way interaction) on alcohol consumption differed between men and women showed that the 2-way interaction terms were not significantly different across gender groups (Wald test value=1.881, p=.170, N=26,427).
DISCUSSION
Summary of Findings
A series of significant negative associations were revealed between alcohol consumption and pain interference from the cross-lagged panel model in the full sample, which is consistent with the reported zero-order correlations. The cross-sectional relation between consumption and pain interference was negative. Longitudinally, greater consumption assessed at baseline was associated with lower levels of pain interference reported at the three-year follow-up. Greater pain interference reported at baseline was related to lower consumption at follow-up.
For the longitudinal consumption-to-pain-interference path, analyses revealed a gender x AUD-symptom-count interaction on this relation such that AUD-symptom-count modified the association between baseline consumption and follow-up pain interference in men, but not in women. Specifically, men in ≤1 symptom(s) group that reported higher levels of consumption at baseline reported lower levels of pain interference at follow-up, resembling the pattern of results observed in the larger sample. In contrast, men in the ≥2 symptoms group that reported higher levels of consumption at baseline reported greater levels of pain interference at follow-up, and further, this relation grew stronger as the number of reported AUD symptoms increased. Among women, regardless of symptom group, a negative association was found between consumption at baseline and pain interference at follow-up. For the longitudinal pain-interference-to-consumption path, no moderating effects of severity of AUD symptomatology or gender were found and baseline pain interference was negatively associated with alcohol consumption at follow-up.
Interpretations of Findings
Consumption-to-Pain-Interference Path.
To put these findings in the context of the existing literature, extensive research has suggested that moderate drinking is negatively associated with pain and pain-related constructs (Zale et al, 2015). Notably, there have been population-based and clinical studies suggesting that heavy drinking is also associated with reduced pain and physical functioning among patients with chronic widespread pain (MacFarlane et al., 2015) and fibromyalgia (Kim et al., 2013). The series of negative associations found in the present study between consumption and pain interference are consistent with these prior studies. One potential explanation for this finding is that alcohol consumption acts as an effective analgesic among patients without AUD, consistent with pharmacological studies using animal and human models (Campbell, Taylor, & Tizabi, 2007; Mitchell et al., 2012). Nonetheless, caution should be made in drawing such a conclusion given that an untested third variable could be contributing to the observed effect.
As noted, prior studies have found a robust pain-dampening effect of alcohol consumption associated with moderate drinking (e.g. Di Giuseppe et al., 2012), but these studies have not reached a consensus on the nature of the consumption-to-pain relation associated with heavy drinking (e.g. Brennan et al., 2005; MacFarlance et al., 2015). One possible explanation is that only a fraction of heavy drinkers suffer from AUD (Esser et al., 2014)3, which may serve as a moderator of this relation. The conceptualization of allostatic load in Koob’s Dark Side of Addiction theory suggests that neurological dysregulation characterizes an integral facet of the onset and progression of AUD and could be a key element that links AUD and pain chronification (Borsook et al., 2016; Egli et al., 2012) rather than the amount of alcohol consumption per se. Accordingly, we would only expect to observe a positive association between consumption and pain interference among individuals with AUD, and a possible dose effect relationship showing that as the severity of AUD symptomatology increases, the levels of pain interference increase. This is consistent with the findings of the present study when restricting the sample to men as well as prior studies conducted in samples selected for AUD/problem drinkers that have reported a positive relation between alcohol consumption and subsequent pain (e.g., Brennan et al., 2005; Chopra & Tiwari, 2012). Therefore, the current study suggests that separating out the effects of heavy drinking and severity of AUD symptomatology can provide insights into the discrepant results reported in the domain of pain and pain-related disability.
Our findings also highlight the important role of AUD symptom severity in the relation from earlier drinking behavior to later reports of pain-related disability. Recent research has indicated that severity of AUD symptomatology indexed by the Alcohol Use Disorder Identification Test (AUDIT) and DSM-5 symptom count is associated with the degree of neurological dysregulation that has developed in response to disordered drinking (Aloi et al., 2018; Joyner et al., 2016). Our findings further support the hypothesis that this dysregulation is central to the comorbidity of AUD and pain-related disability. Future research may further clarify this phenomenon by assessing AUD severity via different measures and/or capturing AUD severity via a structural equation modeling approach that integrates multiple measures of alcohol dependence and abuse (Moallem et al., 2013). Future studies in AUD-chronic pain comorbidity may also examine the mediating roles of various neurological mechanisms through neurocognitive tasks and neuroimaging techniques, as a better understanding of the specific mechanisms underlying this neurological dysregulation may afford guidance for the development of novel intervention strategies that target populations with comorbid AUD and chronic pain.
Though the above result has important implications for our understanding of the associations between alcohol and pain interference, it is necessary to restate that this moderating effect was only observed for men. It is possible that sample size and a difference in statistical power played a role in the null result for women, given that the subgroup of women with ≥2 AUD symptoms was only about 55% the size of the corresponding subgroup of men. Notably, there were 1035 women ≥2 AUD symptoms in the sample, which provided sufficient power to detect the significant bidirectional consumption-pain interference associations; however, there were only 123 women with 6 or more AUD symptoms, which is where the strongest results were observed for men in the moderation analysis. Thus, reduced statistical power cannot be ruled out as a contributor to the null result for women. Another possible explanation is that the biopsychosocial mechanisms underlying these relations differ in strength or even in kind across genders. For example, pain catastrophizing – negative affect and cognition in reaction to pain manifesting in pessimism and hopelessness – has been found to be positively associated with pain and pain interference (Edwards et al., 2004; Keefe et al., 1989), and is more prevalent among women (Sullivan et al., 2000). In at least one study, pain catastrophizing as a mediational process, has explained observed gender differences in pain-related outcomes (Edwards et al., 2004). Thus, it may be that the anxiolytic effect of alcohol leads to reductions in pain catastrophizing. As a result, the alcohol analgesic effect may be more potent in women compared to men, and could explain why even women with ≥2 AUD symptoms continue to show a negative correlation between alcohol consumption and subsequent pain-related outcomes, such as pain interference.
Pain-Interference-to-Consumption Path.
In our study, increased pain interference at baseline was associated with reduced consumption at follow-up in the full sample. This negative association is consistent with findings from previous studies with individuals who suffered from chronic non-cancer pain (Ekholm et al., 2009) and other gerontological studies (Bobo et al., 2013; Brennan & Soohoo, 2013; Brennan et al., 2011). Plausibly, chronic pain patients and older adults may reduce drinking as part of their health-promoting lifestyle and to avoid alcohol interacting with their medication regimen.
Based on Koob’s theory, we predicted that AUD symptom count would modify this association such that individuals in the ≤1 symptom(s) group would show the observed negative correlation between baseline pain interference and later alcohol consumption, but individuals in the ≥2 symptoms group would show a positive correlation between baseline pain interference and later alcohol consumption. Though not supported in the present study, this hypothesis was informed by prior studies suggesting a positive pain-to-consumption relation when looking at treatment-seeking samples (e.g., Jakubczyk et al., 2015; Witkiewitz et al., 2015). These relations were driven, in part, by individuals that had relapsed in their drinking, and thus, it is plausible that increases in alcohol intake might be elicited by the motivation to cope with withdrawal-related pain and pain interference. In fact, a prior study using NESARC Wave 1 & Wave 2 data found that individuals that completely ceased drinking between the two time points reported significant increases in pain interference compared to individuals that refrained from drinking across both time points (Imtiaz et al., 2018). Further, a second study using NESARC data, showed that individuals in abstinent remission reported greater pain interference than those in non-abstinent remission (Dawson et al., 2008). Together, these studies suggest that complete cessation of drinking might play an important role in elevating pain interference, possibly through the mechanism of withdrawal induced hyperalgesia, which later leads to the increased rates of relapse and increased consumption observed in prior studies (e.g., Jakubczyk et al., 2015; Witkiewitz et al., 2015). Unfortunately, because of the small number of individuals in the 2 or more symptoms group that transitioned from drinker to abstainer between the NESARC surveys (N=198) and the lack of additional time points, we could not evaluate this hypothesis in the present study. Future studies may consider employing alcohol treatment samples with comprehensive, repeated assessments of alcohol and pain phenotypes to test the said explanation.
The present study also failed to find a moderating effect of gender on the pain-interference-to-consumption path. A recent experimental study reported that pain induction led to alcohol craving, and this relation was mediated by negative affect among undergraduates who consumed moderate-to-large amounts of alcohol (Moskal et al., 2018). Previous studies have also suggested that women report higher levels of pain-related anxiety than men (Sullivan et al., 2000), consistent with higher rates of anxiety and mood-related symptoms among women more generally, which is reflected in the prevalence rates of these disorders in the NESARC data (Table 3). Taken together, negative affect may play an important role in the previously reported gender differences in the pain-interference-to-consumption path. Therefore, when the presence/absence of anxiety and mood disorders were partialled out of the models in the present study, this could have reduced our ability to detect gender differences in the pain-interference-to-consumption path. Future epidemiologic and experimental studies could consider including anxiety and mood-related symptoms as moderators in tests of the pain-consumption relation along with gender to further explore this possibility.
Strengths, Limitations, and Future Directions
In addition to the major findings summarized above, this study has a few methodological strengths. First, to our knowledge, this is the first study to examine the bidirectional associations between alcohol consumption and pain interference in drinkers using a nationally representative sample. Second, the present study used a joint model design to simultaneously estimate the consumption-to-pain-interference and pain-interference-to-consumption paths and adjust for parameter bias that can result from estimating these relations separately (Greene, 2003). Third, NESARC provided extensive assessments of sociodemographics, psychiatric disorders, and physical health that allowed us to estimate the consumption-pain interference paths while adjusting for the influences of these potentially confounding variables.
Nonetheless, this study has a few limitations. Most notably, the findings of this study relied on what is a limited picture of the consumption-pain interference relations as it is based on two waves of assessment. Given this two-wave design, linear changes of consumption and pain interference over time are assumed as the temporal resolution required to examine the possibilities of various non-linear patterns of changes in consumption and pain interference (e.g. trajectories resembled logarithmic or spline/piecewise polynomial function, or patterns of fluctuation characterized by rhythmic or irregular ebb and flow) is absent. Importantly, a two-wave design limited us from using models beyond a cross-lagged panel model. A critique of cross-lagged panel models is that they may induce model-bias when the measures show considerable stability over time because it does not partial out the time-invariant (trait-like) component when estimating the change of measures across time (Hamaker et al., 2015). Whereas, a latent state-trait model (Steyer, et al., 1990) would allow us to partition the total variance into a trait-like (stable) and a state-like (evolving) component and produce an unbiased estimate of alcohol consumption and pain interference relations evolving over time, model identification for latent state-trait models requires at least three waves of data. Additionally, the two-wave design prohibits the evaluation of reciprocal models at the within-person level in which pain interference leads to increases in alcohol intake that, in turn, aggravates the experience of pain interference or vice versa. Finally, the establishment of mutual influences between alcohol consumption and pain interference might take longer than three years to develop (e.g., the time between the NESARC Wave 1 & 2 surveys). It is possible that the amount of time required for alcohol consumption to influence pain interference may differ from the amount of time required for pain interference to influence alcohol intake. Thus, multi-wave designs with different time intervals between waves may provide different pictures of the consumption-pain interference relations.
Three additional limitations warrant consideration. First, NESARC only measured one pain-related item, i.e. pain interference that limited generalization to other crucial domains of pain, such as subjective experience of pain intensity, pain cognition, affect, and coping strategies. Including these additional pain constructs would provide a more comprehensive examination of mechanisms underlying the consumption-pain relations. Current findings suggest that future studies may measure constructs of pain-related alcohol expectancies and motives (the expectancy of alcohol’s analgesic effect and intention to use alcohol as pain reliever) to provide further insights into the mechanisms of the consumption-pain relations. Second, it is important to note that the psychiatric diagnoses that were modeled as covariates may lack sensitivity to reveal between-group differences in gender and AUD severity. Specifically, modeling the severity of the other psychiatric disorders, rather than diagnostic status as was done in this study, could enhance the sensitivity of these covariates. Nonetheless, given that the majority of respondents did not experience any symptoms, the symptom count variables were zero inflated, and data were sparse across levels of symptom count in NESARC (Table 3). Thus, diagnostic categories rather than symptom counts were used. Third, ideally, the entire range of AUD symptom count (0–11 symptoms) could be used as one continuous moderator. Unfortunately, the distribution of symptom count was positively skewed with high kurtosis, and the inflated ≤1 symptom(s) group might have obscured meaningful findings. We acknowledge that this methodological weakness limits the clinical implications of our findings. Finally, it should be noted that the standardized effects of the consumption-pain interference associations found in this study were relatively small; the effects may increase if a clinical sample is used.
Conclusions
In summary, the present study found evidence that baseline alcohol consumption was associated with lower levels of pain interference three years later; however, for men with higher numbers of AUD symptoms, this relation was reversed such that they experienced higher levels rather than lower levels of pain interference. Additionally, the present study found that higher levels of baseline pain interference were associated with lower levels of alcohol consumption three years later, and this relation was not moderated by gender or AUD symptom count. Thus, the findings suggest the importance of screening for pain interference among men with AUD, as this group may be more vulnerable to consumption-related reductions in pain functioning that might further reinforce their problem drinking. In contrast, the present study did not yield evidence suggesting that pain interference was associated with subsequent increases in consumption. At a glance, these findings seem to leave open the question of whether it is crucial to screen for lifetime and/or current AUD and heavy drinking behavior among male patients with pain conditions. This seems like a risky proposition, however, as it remains plausible that pain interference related to prior experience of AUD and heavy drinking may be associated with greater likelihood of misusing opioid and other substances as pain relievers. Therefore, personalized pain regimens would likely still benefit from screening of drinking behaviors among pain patients. Overall, we suggest that an integrative treatment approach that targets pain interference as well as alcohol and other substance use disorders may be more efficacious among patients who show alcohol-pain comorbidity, particularly in men.
Supplementary Material
Table 3b.
Percentages of respondents who reported psychiatric disorders, general medical conditions, and stressful life events, stratified by gender and AUD symptom count groups
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤1 AUD symptom at Baseline |
≥2 AUD symptoms at Baseline |
≤1 AUD symptom at Baseline |
≥2 AUD symptoms at Baseline |
|||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Psychiatric disorders | ||||||||
| Substance use disorders | 11.1 | 14.5 | 37.9 | 33.3 | 10.8 | 12.8 | 40.3 | 34.8 |
| Anxiety disorders | 7.5 | 8.1 | 14.4 | 13.3 | 14.4 | 16.7 | 26.2 | 25.1 |
| Mood disorders | 6.5 | 6.8 | 17.6 | 13.1 | 12.1 | 13.3 | 31.3 | 23.6 |
| Personality disorders | 13.9 | 29.2 | 14.2 | 35.2 | ||||
| Family history of psychiatric disorders | ||||||||
| Substance use disorders | 13.9 | 22.7 | 19.8 | 32.0 | ||||
| Depression | 26.1 | 36.6 | 37.2 | 52.8 | ||||
| Antisocial personality disorder | 15.1 | 24.5 | 19.9 | 36.2 | ||||
| General medical condition^ | 25.6 | 19.8 | 28.3 | 20.5 | ||||
| Stressful life event^ | 58.4 | 74.9 | 65.7 | 83.4 | ||||
Acknowledgments
FUNDING SUPPORT
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism: T32AA-013526 & K05-AA-017242 to Kenneth J. Sher, and K99-AA-024236 & R00-AA-024236 to Matthew R. Lee. The authors have no conflict of interest to declare.
Footnotes
In the current study, the term •AUD• refers to both DSM-IV defined alcohol abuse and/or dependence and DSM-5 defined AUD.
In the analysis, AUD symptomatology (i.e. AUD symptom count) was analyzed as a continuous variable rather than as a categorical or an ordinal variable of multiple levels of AUD symptomatology (dummy codes).
The correlations between alcohol consumption and AUD symptom count in the NESARC sample were moderate (0.40 for men in the high symptom count group and 0.34 for all men; 0.32 for all women), suggesting they could exert unique influences on pain interference.
REFERENCES
- Aloi J, Blair KS, Crum KI, Meffert H, White SF, Tyler PM, Thornton LC, Mobley AM, Killanin AD, Adams KO (2018) Adolescents show differential dysfunctions related to Alcohol and Cannabis Use Disorder severity in emotion and executive attention neuro-circuitries. NeuroImage: Clinical 19: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Pilver C, Potenza MN, Desai RA (2012) Prevalence and psychiatric correlates of pain interference among men and women in the general population. J Psychiatr Res 46: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Pilver CE, Hoff RA, Potenza MN (2013) Pain interference and incident mood, anxiety, and substance-use disorders: findings from a representative sample of men and women in the general population. J Psychiatr Res 47: 1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Krueger RF, Hasin DS, Liu S-M, Wang S, Kerridge BT, Saha T, Olfson M (2013) Mapping common psychiatric disorders: structure and predictive validity in the national epidemiologic survey on alcohol and related conditions. JAMA psychiatry 70: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Wall MM, Okuda M, Wang S, Iza M, Olfson M (2016) Pain as a predictor of opioid use disorder in a nationally representative sample. Am J Psychiatry 173: 1189–1195. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Greek AA, Klepinger DH, Herting JR (2013) Predicting 10-year alcohol use trajectories among men age 50 years and older. Am J Geriatric Psychiatry 21: 204–213. [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I (2016) Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 68: 282–297. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD (2011) Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med 41: 516–524. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH (2005) Pain and use of alcohol to manage pain: prevalence and 3‐year outcomes among older problem and non‐problem drinkers. Addiction 100: 777–786. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, SooHoo S, Moos RH (2011) Painful medical conditions and alcohol use: a prospective study among older adults. Pain Med 12: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, SooHoo S (2013) Pain and use of alcohol in later life: prospective evidence from the health and retirement study. J Aging Health 25: 656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder GJ, Harlow LL (2003) An illustration of a longitudinal cross-lagged design for larger structural equation models. Structural Equation Modeling 10: 465–486. [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y (2007) Effects of selective opioid receptor antagonists on alcohol‐induced and nicotine‐induced antinociception. Alcoholism: Clinical and Experimental Research 31: 1435–1440. [DOI] [PubMed] [Google Scholar]
- Chopra K, Tiwari V (2012) Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol 73: 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou K-L, Mackenzie CS, Liang K, Sareen J (2011) Three-year incidence and predictors of first-onset of DSM-IV mood, anxiety, and substance use disorders in older adults: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry 72: 144–155. [DOI] [PubMed] [Google Scholar]
- Cleeland CS and Ryan KM (1994) Pain assessment: Global use of the brief pain inventory. Ann Acad Med Sing 23: 129–138. [PubMed] [Google Scholar]
- Cook KF, Dunn W, Griffith JW, Morrison MT, Tanquary J, Sabata D, Victorson D, Carey LM, MacDermid JC, Dudgeon BJ, Gershon RC (2013) Pain assessment using the NIH Toolbox. Neurology 80(Suppl 3): S49–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, Sullivan MD (2019) On the Importance of Using the Right Metrics for Patient Outcomes and Payment: Pain, Pain Interference, and Physical Function. Pain Med 20: 209–209. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Li T-K, Chou SP, Grant BF (2008) Transitions in and out of alcohol use disorders: their associations with conditional changes in quality of life over a 3-year follow-up interval. Alcohol Alcohol 44: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giuseppe D, Alfredsson L, Bottai M, Askling J, Wolk A (2012) Long term alcohol intake and risk of rheumatoid arthritis in women: a population based cohort study. BMJ 345: e4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, & LaRowe LR (2019) A reciprocal model of pain and substance use: Transdiagnostic considerations, clinical implications, and future directions. Annu Rev Clin Psychol 15: 503–528. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB (2004) Catastrophizing as a mediator of gender differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain 111: 335–341. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S (2012) Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36: 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm O, Grønbæk M, Peuckmann V, Sjøgren P (2009) Alcohol and smoking behavior in chronic pain patients: The role of opioids. European Journal of Pain 13: 606–612. [DOI] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS (2014) Peer Reviewed: Prevalence of Alcohol Dependence Among US Adult Drinkers, 2009–2011. Prev Chronic Dis 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R (2003) The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend 71: 7–16. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, Saha TD, Smith SM, Pulay AJ, Pickering RP (2009) Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry 14: 1051–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant NN, Anawalt BD (2003) Male hypogonadism in the primary care clinic. Primary Care-Clinics in Office Practice 30: 743–764. [DOI] [PubMed] [Google Scholar]
- Greene WH. (2003) Econometric analysis: Pearson Education India. [Google Scholar]
- Hamaker EL, Kuiper RM, Grasman RPPP (2015) A critique of the cross-lagged panel model. Psychol Methods 20: 102–116. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz S, Loheswaran G, Le Foll B, Rehm J (2018) Longitudinal alcohol consumption patterns and health‐related quality of life: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and alcohol review 37: 48–55. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Advancing Pain Research C, and Education. (2011) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jakubczyk A, Ilgen MA, Bohnert AS, Kopera M, Krasowska A, Klimkiewicz A, Blow FC, Brower KJ, Wojnar M (2015) Physical Pain in Alcohol-Dependent Patients Entering Treatment in Poland—Prevalence and Correlates. Journal of studies on alcohol and drugs 76: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner KJ, Pickover AM, Soltis KE, Dennhardt AA, Martens MP, Murphy JG (2016) Deficits in access to reward are associated with college student alcohol use disorder. Alcoholism: Clinical and Experimental Research 40: 2685–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayannis NV (2019) A Focus on the Science of Behavior Change Would Provide a Deeper Understanding of Pain-Related Activity Interference and Ability to Sustain Engagement in Valued Physical Activities. Pain Med 20: 210–211. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Brown GK, Wallston KA, Caldwell DS (1989) Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain 37: 51–56. [DOI] [PubMed] [Google Scholar]
- Kim CH, Vincent A, Clauw DJ, Luedtke CA, Thompson JM, Schneekloth TD, Oh TH (2013) Association between alcohol consumption and symptom severity and quality of life in patients with fibromyalgia. Arthritis Res Ther 15: R42–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013) Addiction is a Reward Deficit and Stress Surfeit Disorder. Frontiers in Psychiatry 4: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GJ, Beasley M, Smith BH, Jones GT, Macfarlane TV (2015) Can large surveys conducted on highly selected populations provide valid information on the epidemiology of common health conditions? An analysis of UK Biobank data on musculoskeletal pain. British journal of pain 9: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, Perez Y, Gatchel RJ (2012) The development and psychometric validation of the central sensitization inventory. Pain Pract 12: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mibu A, Nishigami T, Tanaka K, Manfuku M and Yono S (2019) Difference in the impact of central sensitization on pain-related symptoms between patients with chronic low back pain and knee osteoarthritis. J of Pain Res 12: 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL (2012) Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 4: 116ra116–116ra116. [DOI] [PubMed] [Google Scholar]
- Moallem NR, Courtney KE, Bacio GA, Ray LA (2013) Modeling alcohol use disorder severity: an integrative structural equation modeling approach. Frontiers in psychiatry 4: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal D, Maisto SA, De Vita M, Ditre JW (2018) Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Exp Clin Psychopharmacol 26: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, Muthén B. (1998–2017) Mplus User’s Guide. 8th ed. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) (2004) Wave 1 NESARC Data Notes, Bethesda, MD: NIAAA. [Google Scholar]
- Scott JR, Hassett AL, Schrepf AD, Brummett CM, Harris RE, Clauw DJ, Harte SE (2018) Moderate Alcohol Consumption Is Associated with Reduced Pain and Fibromyalgia Symptoms in Chronic Pain Patients. Pain Med 19: 2515–2527. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Wood PK, Raskin G (1996) Alcohol outcome expectancies and alcohol use: A latent variable cross-lagged panel study. J Abnorm Psychol 105: 561–574. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Auer A (1990) The emotional and cognitive components of trait anxiety: A latent state-trait model. Pers Individ Differ 11: 125–134. [Google Scholar]
- Sullivan MJ, Tripp DA, Santor D (2000) Gender differences in pain and pain behavior: the role of catastrophizing. Cognit Ther Res 24: 121–134. [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R (2005) Chronic spinal pain and physical–mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 113: 331–339. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34: 220–233. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, McCallion E, Vowles KE, Kirouac M, Frohe T, Maisto SA, Hodgson R, Heather N (2015) Association between physical pain and alcohol treatment outcomes: The mediating role of negative affect. J Consult Clin Psychol 83: 1044–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE (2018) Alcohol and Opioid Use, Co‐Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review. Alcoholism: clinical and experimental research 42: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, LaRowe LR, Boissoneault J, Maisto SA, Ditre JW (2019) Gender differences in associations between pain-related anxiety and alcohol use among adults with chronic pain. The American journal of drug and alcohol abuse: 1–9. [DOI] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW (2015) Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev 37: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.