Abstract
Background and Aims
Epicardial adipose tissue (EAT), the visceral fat depot of the heart, is a modifiable cardio-metbolic risk factor and therapeutic target. Semaglutide and dulaglutide, glucagon-like peptide-1 (GLP-1) receptor agonists, are indicated for the treatment of type 2 diabetes mellitus (T2DM). GLP-1 receptor agonists have recently shown to reduce cardiovascular risk. Epicardial adipose tissue expresses GLP-1 receptors (GLP-1Rs). GLP-1 receptor agonist liraglutide is known to significantly decrease EAT thickness. However, the effects of GLP-1 receptor agonists semaglutide and dulaglutide on EAT thickness are unknown.
Materials and Methods
We performed a 12-week, controlled, parallel study in 80 subjects with T2DM and obesity. Patients received either semaglutide, up to 1 mg subcutaneous (sc) weekly, or dulaglutide, up to 1.5 mg sc weekly, as the standard of care in addition to their usual medication regimen. Twenty subjects with T2DM and obesity were started on metformin and a diet and served as the control group. Ultrasound-measured EAT thickness was measured at baseline and at the 12-week follow-up.
Results
Epicardial adipose tissue thickness significantly decreased in both semaglutide and dulaglutide groups (P < 0.001) after 12 weeks, accounting for a 20% reduction. There was no EAT reduction in the metformin group. Body mass index (BMI) and HbA1c improved in all groups without reaching statistical significance. Epicardial adipose tissue thickness reduction was significantly greater (P < 0.01) with the higher doses of semaglutide (1 mg) and dulaglutide (1.5 mg), respectively.
Conclusion
Weekly administration of either GLP-1 receptor agonists semaglutide or dulaglutide causes a rapid, substantial, and dose-dependent reduction in EAT thickness.
Keywords: epicardial fat, epicardial adipose tissue, semaglutide, dulaglutide
Epicardial fat is a therapeutic target and a cardio-metabolic risk factor. Weekly GLP-1 receptor agonists semaglutide and dulaglutide as add-in therapy reduce ultrasound-measured epicardial fat thickness by 20% after 12 weeks.
Visceral fat accumulation is a key component of type 2 diabetes mellitus (T2DM). Visceral fat quantification and reduction represents an effective approach to identify high-risk patients and reduce their cardio-metabolic risk. Epicardial adipose tissue (EAT) is the visceral fat of the heart, with unique anatomical, functional, and genetic properties [1–4]. Epicardial adipose tissue is higher in subjects with T2DM and metabolic syndrome, independently of traditional body fat indicators [5–7]. Epicardial adipose tissue can be easily measured with standard ultrasound and independently reflects intra-abdominal visceral fat and myocardial triglyceride content [7–10]. Given its rapid metabolism, organ fat specificity, and simple measurability, EAT serves as a modifiable therapeutic target for medications modulating the adipose tissue, such as the glucagon-like peptide-1 (GLP-1) receptor agonists [11]. In addition to providing control of diabetes mellitus, GLP-1 receptor agonists have shown to provide weight loss and cardiovascular protective effects [12–15]. Clinical trials found that GLP-1 receptor agonists as a class reduced major adverse cardiovascular events [12–15]. We recently showed that add-on therapy with liraglutide, a once-daily GLP-1 receptor agonist, significantly decreased EAT thickness by almost 30% after 12 weeks in subjects with T2DM [16]. A milder reduction of EAT thickness was also reported after 12 weeks of treatment with exenatide, a once weekly GLP-1 receptor agonist [17]. Remarkably, human EAT expressed the GLP-1 receptor (GLP-1R) gene and protein, suggesting that EAT reduction can be mediated by a direct effect and activation of GLP-1R [18]. Long-acting GLP-1 receptor analogs, such as semaglutide and dulaglutide, are convenient and effective therapeutic options given the ease of weekly administration and the benefit of weight loss [19]. However, to date, there are no data on the effects of either semaglutide or dulaglutide on visceral fat. The roles of semaglutide and dulaglutide, respectively, on EAT are also unknown. Hence, in this study, we sought to evaluate the effects of weekly semaglutide and dulaglutide on EAT thickness in overweight/obese type 2 diabetics. Epicardial adipose tissue served as a marker to explore the effects of these weekly GLP-1 receptor agonists on visceral adiposity.
1. Materials and Methods
A. Study Design
This was a 12-week parallel group study in a total of 80 patients: 60 overweight/obese (body mass index [BMI] ≥ 27 kg/m2) type 2 diabetic subjects inadequately controlled on current diabetes treatment and 20 overweight/obese individuals newly diagnosed with T2DM who served as the control group. Patients were treated with the standard of care during routine clinical practice. Data were obtained from electronic medical records.
B. Patients
Subjects were screened among those routinely referred to the University of Miami Division of Diabetes, Endocrinology, and Metabolism outpatient clinic. Data were collected from electronic medical records.
There were 3 study groups: 30 patients who received semaglutide in addition to their current diabetes regimen, 30 patients who received additional dulaglutide, and 20 patients who were started on metformin and received dietary recommendations.
Patients were allocated to either semaglutide or dulaglutide based on preference or medical insurance coverage. All patients received diabetes education as part of the standard of care and were advised to continue their usual physical activity. The study was approved by local institutional review board (IRB # 20190944) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study has been registered in www.clinicaltrials.gov (NCT04200625).
C. Inclusion and Exclusion Criteria
Patients were started on semaglutide or dulaglutide on the basis of the following inclusion criteria: type 2 diabetes, BMI ≥ 27 kg/m2, and age ≥ 18 years old. Exclusion criteria were the following: type 1 diabetes; concurrent use of dipeptidyl peptidase 4 (DPP-4) inhibitors or other GLP-1 agonist receptors; a history of diabetic ketoacidosis; or known contraindications to GLP-1 receptor agonists, such as a previous history of pancreatitis or medullary thyroid carcinoma, a personal or family history of multiple endocrine neoplasia type 2, acute infective diseases, cancer or chemotherapy, use of systemic corticosteroids within the 3 months prior this study, a known or suspected allergy to semaglutide or dulaglutide excipients or related products, pregnancy, breastfeeding, or the intention of becoming pregnant.
D. Study Procedures
D-1. Medication administration
Injections were self-administered weekly, subcutaneously in the abdomen at any time of day irrespective of meals. Injections were administered on the same day of the week. Semaglutide was started at the dose of 0.25 mg for the first 4 weeks and then titrated up to the maintenance dose (0.5 or 1.0 mg once weekly). Fifteen patients achieved the 1.0 mg dose. Patients were started on dulaglutide, 0.75 mg, for the first 4 weeks and then continued at the same dosage or titrated up to 1.5 mg once weekly. Fifteen patients achieved the 1.5 mg dose.
Controls were started on metformin at the dose of 500 mg once or twice daily, depending on tolerability. Once maintenance doses were reached, they were not changed during the course of the study. Adherence to the drugs was assessed by a monthly phone call or e-mail correspondence with the patients. Patients were instructed to maintain the same dosages of their other diabetic medications during the 12 weeks of the study.
E. Ultrasound measurement of the epicardial fat thickness.
Each patient underwent an onsite transthoracic ultrasound measurement of EAT thickness at baseline and at the 12-week follow-up visit. Epicardial adipose tissue thickness was measured according to the method first described and validated by Iacobellis et al [5, 6]. Briefly, EAT was identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium. Epicardial adipose tissue thickness was measured perpendicularly on the free wall of the right ventricle at end-systole in 3 cardiac cycles. The parasternal long-axis view allowed for the most accurate measurement of EAT on the right ventricle, with optimal cursor beam orientation in each view. Maximum EAT thickness was measured at the point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus, used as the anatomical landmark for this view. The average value of 3 cardiac cycles was calculated and used for analysis. Intraobserver reproducibility of the measurement of EAT was assessed by the intraclass correlation coefficient. The echocardiographer performing the follow-up measurement was blinded to the baseline EAT thickness value.
2. Physical examination.
All anthropometric measures were routinely obtained by the nurse or physician assistant at each visit. Height (in cm) and weight (in kg) were measured and BMI was automatically calculated as weight in kilograms divided by the square of height in meters (kg/m2). Blood pressure was measured in the seated position using an automated cuff and digital readout. Resting heart rate was also measured at each visit.
A. Laboratory tests.
HemoglobinA1c (HbA1c) and the fasting laboratory analysis of a comprehensive metabolic panel and lipid profile were obtained from all individuals at each visit as the standard of care. Fasting c-peptide was calculated in the two GLP-1 receptor agonist groups at baseline. Patients were advised to monitor their capillary glucose once to twice daily.
B. Statistical Analysis
The primary outcome of the study was a reduction in EAT thickness on semaglutide or dulaglutide compared to control. The secondary outcome was a change in HbA1c during treatment. Our group previously showed a statistically and clinically significant change in EAT thickness in 54 patients treated with liraglutide, a once-daily GLP-1 receptor agonist [17]. Based on these data, we assumed that a difference of at least 1 mm in EAT thickness was clinically and statistically significant. Hence, using a significance level of P < 0.05 and 90% power, the current study sample size was sufficient to detect a statistically significant difference in EAT between the treatments. Continuous variables were considered as means with their standard deviations (SDs) or medians. A two-sample t-test with a 95% confidence interval (CI) for difference was performed to evaluate differences between the baseline and 12 weeks within each group. Nonparametric tests were used to calculate changes in EAT and other study variables between groups. Relations between study variables were calculated using simple linear regression analysis. Two-tailed P < 0.05 indicates statistical significance. The intraclass correlation coefficient of the EAT measurement was 0.90, indicating good reproducibility and reliability. Statistical analysis was performed using IBM SPSS Statistics 26, IBM Corp, Armonk, NY.
C. Results
3. Baseline
Main baseline clinical characteristics of the patients are summarized in Table 1. Study groups were quite homogenous. There were no differences in EAT, age, ethnicity, c-peptide, lipids, blood pressure, heart rate, other diabetes drugs, lipid lowering and blood pressure medications, renal or liver function tests, and diabetes history between the semaglutide and dulaglutide groups. However, HbA1c was higher in the dulaglutide group than in the semaglutide group (< 0.01).
Table 1.
Baseline Study Variables
| Semaglutide n = 30 | Dulaglutide n = 30 | Metformin n = 20 | P | |
|---|---|---|---|---|
| Age (years) | 57 ± 10 | 55 ± 7.8 | 44 ± 12 | < 0.01 |
| Male (n) | 21 | 13 | 8 | |
| Ethnicity | ||||
| White | 17 | 18 | 9 | |
| Hispanic | 10 | 9 | 9 | |
| African American | 3 | 3 | 2 | |
| DM Hx (ys) | 7 ± 4 | 7 ± 4 | 2 ± 1 | < 0.01 |
| Weight (kg) | 105 ± 14 | 106 ± 21 | 94 ± 26 | ns |
| BMI (Kg/m2) | 34.3 ± 5 | 36.5 ± 6 | 33.5 ± 6 | ns |
| HbA1c (%) | 7.3 ± 1.2 | 8.2 ± 1.2 | 5.8 ± 0.6 | < 0.01 |
| c-peptide (ng/ml) | 3.8 ± 1.9 | 2.9 ± 1.8 | na | ns |
| SBP (mmHg) | 132 ± 13 | 127 ± 18 | 123 ± 15 | ns |
| DBP (mmHg) | 79.5 ± 8 | 76.8 ± 13 | 77.5 ± 9 | ns |
| HR (bpm) | 79 ± 10 | 83 ± 13 | 75 ± 13 | ns |
| LDL (mg/dl) | 99 ± 41 | 87 ± 33 | 116 ± 36 | ns |
| HDL (mg/dl) | 45 ± 16 | 43 ± 15 | 47 ± 11 | ns |
| TG (mg/dl) | 130 ± 65 | 125 ± 60 | 135 ± 50 | ns |
| EAT (mm) | 9.5 ± 2.6 | 9.2 ± 2.2 | 7.1 ± 2.1 | < 0.01 |
| Other diabetes meds | Metformin (30); SGLT2i (5); insulin (1) | Metformin (30); SGLT2i (6); insulin (3) | none | |
| Statins | 24 | 25 | 4 | |
| ACE-I or ARB | 18 | 19 | 4 |
Data are expressed as mean ± SD, or as actual number.
Abbreviations: ACE-I, ACE inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; DM hx, diabetes history; EAT, epicardial adipose tissue; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein cholesterol; HR, heart rate; LDL, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SGLT2i, sodium glucose co-transporter 2 inhibitor; TG (triglycerides).
When compared to controls, EAT thickness was significantly higher at baseline in patients on semaglutide and dulaglutide (P < 0.01) despite no statistically significant difference in the BMI between the 3 groups. Patients in the GLP-1 receptor agonists groups were also older, with a longer duration of diabetes and with a higher HbA1c than controls, as expected. Physical activity did not differ between the groups, as most of the patients were sedentary to moderately active. Although the diet varied according to the different ethnicities and habits of the patients, no significant differences in carbohydrates or calorie intake between the groups were detected at baseline.
4. Intervention
Changes in study variables are reported in Table 2. Each group had 2 patients lost to follow-up. Statistical analysis was performed only on patients who completed the 12-week study.
Table 2.
Changes of the Study Variables in the 3 Groups During the 12 Weeks
| Semaglutide Baseline n = 30 | 12 Weeks n = 28 | P | Dulaglutide Baseline n = 30 | 12 Weeks n = 28 | P | Metformin Baseline n = 20 | 12 Weeks n = 18 | P | |
|---|---|---|---|---|---|---|---|---|---|
| BMI (Kg/m2) | 34.3 ± 5 | 33.8 ± 4 | ns | 36.5 ± 6 | 34 ± 5 | ns | 33.5 ± 6 | 32.1 ± 4 | ns |
| HbA1c (%) | 7.3 ± 1.2 | 6.9 ± 1.2 | ns | 8.2 ± 1.2 | 7.7 ± 1.1 | ns | 5.8 ± 0.6 | 5.9 ± 0.9 | ns |
| SBP (mmHg) | 132 ± 13 | 131 ± 14 | ns | 127 ± 18 | 126 ± 16 | ns | 123 ± 15 | 127 ± 17 | ns |
| DBP(mmHg) | 79.5 ± 8 | 81 ± 8 | ns | 76.8 ± 13 | 78 ± 9 | ns | 77.5 ± 9 | 76 ± 10 | ns |
| HR (bpm) | 79 ± 10 | 81 ± 11 | ns | 83 ± 13 | 79 ± 15 | ns | 75 ± 13 | 77 ± 16 | ns |
| LDL (mg/dl) | 99 ± 41 | 78 ± 30 | ns | 87 ± 33 | 78 ± 41 | ns | 116 ± 36 | 89 ± 17 | < 0.05 |
| HDL(mg/dl) | 45 ± 16 | 44 ± 16 | ns | 43 ± 15 | 42 ± 13 | ns | 47 ± 11 | 55 ± 23 | ns |
| EAT (mm) | 9.5 ± 2.6 | 7.5 ± 2 | < 0.01 | 9.3 ± 2.2 | 7.7 ± 2.2 | < 0.01 | 7.1 ± 2.1 | 7.1 ± 2.2 | ns |
Table 2 shows the cumulative effects of semaglutide and dulaglutide regardless of the medication dosage. Data are expressed as mean ± SD.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; EAT, epicardial adipose tissue; HbA1c, hemoglobin A1c; HDL, high density lipoprotein cholesterol; HR, heart rate; LDL, low density lipoprotein cholesterol; SBP, systolic blood pressure.
Epicardial adipose tissue thickness decreased significantly in both the semaglutide and dulaglutide groups after the 12 weeks (P < 0.01 for both), accounting for an approximate 20% reduction in both groups, whereas there was no significant reduction in EAT thickness in the metformin group. Table 2 showed the cumulative effects of semaglutide and dulaglutide regardless of the medication dosage.
Body mass index decreased in all groups without reaching statistical significance, reflecting an approximately 5%, 6%, and 4% reduction in the semaglutide, dulaglutide, and metformin groups, respectively. HbA1c and LDL were also reduced in the semaglutide and dulaglutide groups without reaching enough statistical significance. Blood pressure and heart rate were overall unchanged during the study.
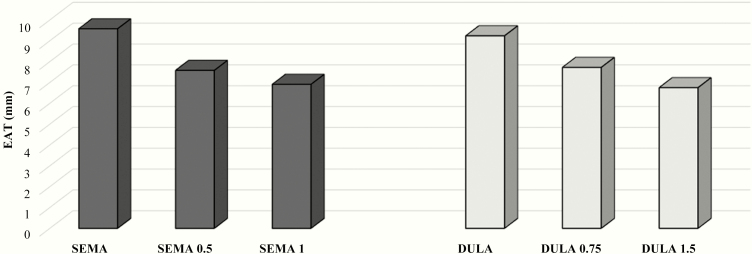
The effects of the different doses of semaglutide and dulaglutide on EAT thickness were then analyzed. Fifteen patients in the semaglutide group and 15 patients in the dulaglutide group reached the maximum medication dosage: 1 mg for semaglutide and 1.5 mg for dulaglutide, respectively. Epicardial adipose tissue showed a further statistically significant reduction in these patients, P < 0.01 in both groups (Fig. 1). Body mass index reduction was higher in patients receiving the maximum medication dosages (approximately 12% in both groups). HbA1c reduction was statistically higher in those receiving semaglutide, 1 mg, as compared to patients using dulaglutide, 1.5 mg, once a week (< 0.01).
Figure 1.
EAT reduction in the semaglutide and dulaglutide groups after 12 weeks, according to the medication dosage.
Legend: EAT (epicardial fat thickness); SEMA (semaglutide) and DULA (dulaglutide) bars indicate baseline EAT. SEMA 0.5 and DULA 0.75 bars indicate EAT after 12-week treatment with semaglutide, 0.5 mg, and dulaglutide, 0.75 mg, once weekly, respectively. SEMA 1 and DULA 1.5 bars indicate EAT after 12-week treatment with semaglutide, 1 mg, and dulaglutide, 1.5 mg, once weekly, respectively.
In a univariate analysis in the semaglutide group, the change in BMI from baseline to 12 weeks was the strongest predictor of change in HbA1c. In the dulaglutide group, neither a change in EAT thickness or a change in BMI correlated with change in HbA1c over the course of the study.
A. Safety
Six patients (2 from each group) were lost to follow-up. Four patients allocated to either dulaglutide or semaglutide withdrew after 2 to 4 weeks, as they were experiencing nausea. Two patients who were started on metformin discontinued the medication because of diarrhea or bloating. Most of the patients were able to tolerate the maximum dosage of the drugs in each group. No serious adverse events occurred and no patients required hospitalization during the study.
Discussion
In this study, we showed for the first time that 2 once-weekly GLP-1 receptor agonists, semaglutide and dulaglutide, each induced a significant reduction of ultrasound-measured EAT thickness. We also found that the reduction in EAT thickness was dose-dependent in both the semaglutide and dulaglutide groups.
This study was designed to reflect a real-life clinical scenario. Weekly GLP-1 receptor agonists, semaglutide and dulaglutide, are effective and convenient therapeutic options to improve glycemic control, induce weight loss, and reduce cardiometabolic risk in type 2 diabetics. This study shows, for the first time, that semaglutide and dulaglutide each significantly reduce EAT thickness over 12 weeks.
EAT has shown to be a modifiable and measurable cardiovascular risk factor that is thicker in subjects with diabetes, owing to its strong correlation with visceral adiposity and insulin resistance [7–9]. Due to its peculiar anatomical location and proinflammatory transcriptome, EAT plays a key role in the development and progression of atherosclerosis and coronary artery disease [20]. Epicardial adipose tissue has been associated with fatal and nonfatal coronary events regardless of the traditional cardiovascular risk factors [21]. Remarkably, diabetic EAT has a peculiar proatherogenic transcriptome, as recently reported [22].
Our group previously showed that daily GLP-1 receptor agonist liraglutide added to metformin induced a large (approximately 30%) and rapid (after 12 weeks) reduction of EAT thickness in subjects with diabetes and obesity [16]. Hence, findings of this current study are parallel, as epicardial fat thickness significantly reduced in size, although by a smaller but still impressive 20% after the 12-week treatment with semaglutide or dulaglutide. This may imply a class effect of GLP-1 receptor agonists on EAT. The higher EAT shrinkage that was observed with liraglutide [16] could be simply due to the different formulation or other factors that could make EAT less responsive, such as worse glucose control and a longer duration of diabetes. Epicardial adipose tissue activity can be downregulated in advanced chronic diseases, as we observed in severe coronary artery disease [3].
Consistent with the SUSTAIN 7 trial [19], patients lost weight on either semaglutide or dulaglutide. However, the extent of weight loss was smaller as compared to the reduction in EAT thickness (approximately 5% versus 20%), suggesting a possible independent effect of the medications.
To support this hypothesis, we have recently demonstrated that human EAT expressed GLP-1R gene and protein [18]. RNA-sequencing and quantitative real-time RT-PCR were performed to evaluate the presence of GLP-1R in EAT obtained from subjects with diabetes and coronary artery disease who were candidates for an elective coronary artery bypass graft [18]. Immunofluorescence clearly confirmed the presence of GLP-1R protein within EAT, whereas the signal was absent in the subcutaneous fat sample obtain from the same patient [18]. GLP-1 receptor agonists effects may therefore be specific to visceral fat, including EAT. The mechanisms behind the fat reduction in response to the GLP-1R activation are unclear. It has been also suggested that GLP-1 promotes EAT preadipocyte differentiation, improves insulin sensitivity, and stimulates EAT thermogenesis and adipocyte browning [23–25]. Interestingly, a correlation between epicardial fat GLP-1R and genes encoding for brown fat activity and fatty acid oxidation has been recently reported [26]. It is plausible that the GLP-1 effect could be specific to visceral fat. One study showed that exenatide, a first generation weekly GLP-1 receptor, induced the reduction of EAT and hepatic triglyceride content [27]. GLP-1 receptor is expressed in the adipose tissue and mRNA, and protein expressions are increased in epicardial and other visceral fat [18, 24, 28, 29]. The drug-induced browning effect on EAT certainly warrants further investigation.
Also consistent with the SUSTAIN 7 trial, we found that the quantity of change in outcomes was dependent on the medication dose [19]. In fact, EAT decreased more when both medications were titrated to the maximum dose, although we did not observe any meaningful difference between the higher dose of semaglutide compared to the higher dose of dulaglutide. Weight loss was also substantially higher with semaglutide, 1 mg, and dulaglutide, 1.5 mg, respectively.
As excessive EAT is well-correlated to higher cardiovascular risk, we can speculate that our findings can provide a potential explanation on the mechanisms behind the cardio-protective effects of semaglutide and dulaglutide [18]. However, this hypothesis should be evaluated further with larger studies.
Conclusion
In conclusion, we showed for the first time a substantial, rapid, and dose-dependent reduction of ultrasound-measured EAT thickness with both semaglutide and dulaglutide. Our results may open new avenues in the clinical use of weekly GLP-1 analogues that may go beyond the current indications. Given its peculiar properties and rapid responsiveness, ultrasound-measured EAT may serve as a routine therapeutic target for medications modulating adipose tissue in patients with T2DM and obesity.
Study Limitations
This was not a randomized clinical trial and no cardiovascular outcomes were evaluated. A randomized clinical trial would be desirable. However, these preliminary data could have an immediate application. Our study could serve as pilot for a future randomized clinical trial. Some results may have reached statistical significance with a larger sample size. Although its advantages overcome the disadvantages, echocardiography measurement may have some limitations. Echocardiographic EAT is a linear measurement at a single location and therefore may not reflect the variability of fat thickness or total EAT volume as measured by computed tomography. No other visceral fat depots were measured in this study. However, EAT thickness by ultrasound has been shown to nicely correlate with waist circumference and intra-abdominal, intrahepatic, and intracardiac fat and serve as a good surrogate marker of visceral adiposity [1]. Despite the study groups having similar demographic characteristics, we cannot rule out the presence of confounding variables. Hence, the study results should be interpreted with caution.
Acknowledgments
Financial Support: None.
Clinical Trial Information: ID # NCT04200625
Additional Information
Disclosure Summary: The authors declare no conflict of interest.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11(6):363–371. [DOI] [PubMed] [Google Scholar]
- 2. Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22(11):450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAninch EA, Fonseca TL, Poggioli R, et al. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity (Silver Spring). 2015;23(6):1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–543. [DOI] [PubMed] [Google Scholar]
- 5. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–9; quiz 1417. [DOI] [PubMed] [Google Scholar]
- 6. Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–310. [DOI] [PubMed] [Google Scholar]
- 7. Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163–5168. [DOI] [PubMed] [Google Scholar]
- 8. Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Metaanalysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111(1):73–78. [DOI] [PubMed] [Google Scholar]
- 9. Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90(11):6300–6302. [DOI] [PubMed] [Google Scholar]
- 10. Malavazos AE, Di Leo G, Secchi F, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010;105(12):1831–1835. [DOI] [PubMed] [Google Scholar]
- 11. Iacobellis G. Epicardial fat: a new cardiovascular therapeutic target. Curr Opin Pharmacol. 2016;27:13–18. [DOI] [PubMed] [Google Scholar]
- 12. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;S2213-8587(19)30249. [DOI] [PubMed] [Google Scholar]
- 13. Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerstein HC, Colhoun HM, Dagenais GR, et al. ; REWIND Investigators Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. [DOI] [PubMed] [Google Scholar]
- 15. Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 16. Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring). 2017;25(2):311–316. [DOI] [PubMed] [Google Scholar]
- 17. Morano S, Romagnoli E, Filardi T, et al. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: an ultrasonography study. Acta Diabetol. 2015;52(4):727–732. [DOI] [PubMed] [Google Scholar]
- 18. Iacobellis G, Camarena V, Sant DW, Wang G. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm Metab Res. 2017;49(8):625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pratley RE, Aroda VR, Lingvay I, et al. ; SUSTAIN 7 investigators Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. [DOI] [PubMed] [Google Scholar]
- 20. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466. [DOI] [PubMed] [Google Scholar]
- 21. Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61(13):1388–1395. [DOI] [PubMed] [Google Scholar]
- 22. Camarena V, Sant D, Mohseni M, et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr Metab Cardiovasc Dis. 2017;27(8):739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor–or not? Endocrinol. 2013;154(1):4–8. [DOI] [PubMed] [Google Scholar]
- 24. Yang J, Ren J, Song J, et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int J Mol Med. 2013;31(6):1429–1435. [DOI] [PubMed] [Google Scholar]
- 25. Beiroa D, Imbernon M, Gallego R, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63(10):3346–3358. [DOI] [PubMed] [Google Scholar]
- 26. Dozio E, Vianello E, Malavazos AE, et al. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: a target to modulate cardiovascular risk? Int J Cardiol. 2019;292:218–224. [DOI] [PubMed] [Google Scholar]
- 27. Dutour A, Abdesselam I, Ancel P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18(9):882–891. [DOI] [PubMed] [Google Scholar]
- 28. Vendrell J, El Bekay R, Peral B, et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinol. 2011;152(11):4072–4079. [DOI] [PubMed] [Google Scholar]
- 29. Jendle J, Nauck MA, Matthews DR, et al. ; LEAD-2 and LEAD-3 Study Groups Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11(12):1163–1172. [DOI] [PubMed] [Google Scholar]