Abstract
Context
Rapid bone density loss starts during the menopause transition (MT). Whether other components of bone strength deteriorate before the final menstrual period (FMP) remains uncertain.
Objective
To discern whether trabecular bone score (TBS) declines during the MT.
Design
An 18-year longitudinal analysis from the Study of Women’s Health Across Nation.
Setting
Community-based cohort.
Participants
A total of 243 black, 164 Japanese, and 298 white, initially pre- or early perimenopausal women, who experienced their FMP.
Main Outcome Measures
TBS, an indicator of bone strength.
Results
Multivariable mixed effects regressions fitted piecewise linear models to repeated measures of TBS as a function of time before or after the FMP; covariates were age at FMP, race/ethnicity, and body mass index. Prior to 1.5 years before the FMP, in the referent individual (a white woman with age at FMP of 52.2 years and body mass index of 28.0 kg/m2), TBS evidenced no change (slope 0.12% per year, P = 0.2991). TBS loss began 1.5 years before the FMP, declining by 1.16% annually (P < 0.0001). Starting 2 years after the FMP, annual rate of TBS loss lessened to 0.89% (P < 0.0001). In the 5 years before through the 5 years after the FMP, in the referent individual, total TBS decline was 6.3% (P < 0.0001), but black participants’ total TBS loss was 4.90% (P = 0.0008, difference in black and white 10-year change). Results for Japanese did not differ from those of white women.
Conclusions
The occurrence of an MT-related decline in TBS supports the thesis that this period is particularly damaging to skeletal integrity.
Keywords: menopause, trabecular bone score, epidemiology, cohort, longitudinal
Mounting evidence indicates that menopause-related threats to women’s bone health begin during the menopause transition (MT), not in postmenopause. A cogent picture of the biology of menopause-related bone loss is emerging. Initially, approximately 2 years before the final menstrual period (FMP), a marked increase in bone turnover occurs (1). Then, at about 1.5 years after the FMP, bone turnover plateaus. The temporal pattern of increased bone turnover portends an accelerated decline in bone mineral density (BMD), which starts about year later, 1 year before the FMP (2–4). Unfavorable alterations in other indicators of bone strength and resistance to fracture, composite strength indices and hip structural analysis also emerge in relation to the MT (5, 6). Femoral neck (FN) bone remodeling begins approximately 1 year before the FMP, characterized by an increase in FN diameter, as would be expected in cortical bone (7, 8). But the gain in FN bone diameter does not counterbalance the degree of FN BMD loss; taken together, these 2 opposing forces result in an average 0.7% annual decline in FN strength during the MT, estimated using composite strength indices, which quantify bone strength relative to the loading demand) (5, 8).
Whether decline in trabecular bone score (TBS), a recently developed marker of bone strength, is a component of the shifting physiology and anatomy of bone during the MT remains unknown. Higher TBS values correspond to better structured, stronger bone; conversely, lower TBS values signal less well-constructed, weaker bone. In postmenopausal women and men aged 40 and older, TBS predicts fractures independently of BMD and most clinical risk factors (9, 10). In a large multinational meta-analysis, TBS remained significantly related to incident major osteoporotic fracture and hip fracture after accounting for the Fracture Risk Assessment Tool (FRAX) (9–11). One cross-sectional study of 619 US women examined the relation between age and TBS; it found little change in TBS before age 45, but a negative association with age greater than that (12). Although this analysis suggests that MT plays a role in TBS decline (congruent with the known effect of the MT on BMD loss), it could not directly test that question (2–4; 12).
To examine the possible MT-TBS link, a longitudinal TBS study containing detailed information about the MT is required. Thus, the aim of this study is to assess whether the MT influences TBS, using 18 years of longitudinal data from the Study of Women’s Health Across the Nation (SWAN). We hypothesize that there will be a menopause-related decline in TBS, the trajectory of which will mirror the patterns previously observed for BMD, composite strength indices, and hip structural analysis (2, 4, 6, 8). Specifically, we propose that TBS will be stable until a few years before the FMP, at which time decline will start and TBS loss will decelerate, but not stop, in postmenopause. To test this hypothesis, we modeled longitudinal change in TBS in relation to the number of years before or after the FMP. We also examined whether race/ethnicity and/or body mass index (BMI) influenced the rate of menopause-related TBS decline (if present).
Materials and Methods
Study sample
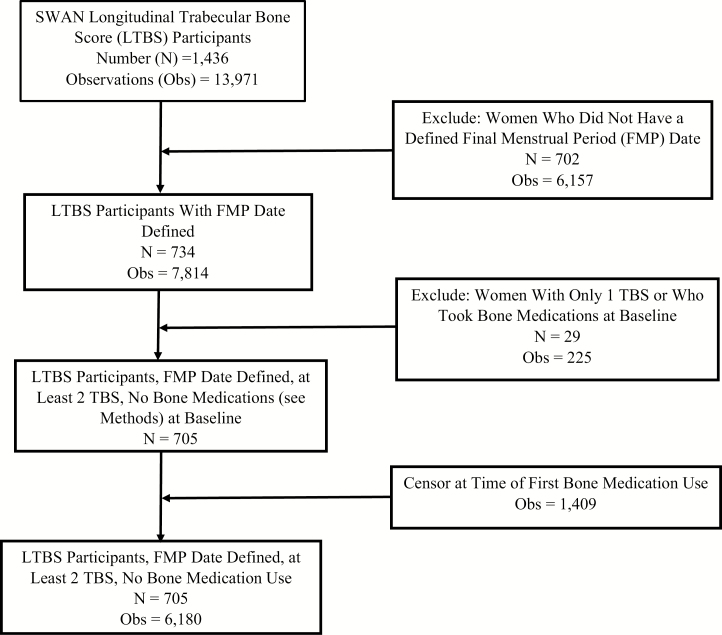
SWAN is a multisite cohort study of the MT and mid-life (13). At entry, women were between 42 and 52 years of age, had an intact uterus and at least 1 intact ovary, were not taking hormone therapy, had at least 1 menstrual period in the 3 months before screening, and self-identified as a member of 1 of 5 ethnic/racial groups. Beginning in 1996, participants were enrolled in 7 US cities: Boston, MA; Chicago, IL; Detroit, MI; Pittsburgh, PA; Los Angeles, CA; Newark, NJ; and Oakland, CA (baseline N = 3302). All sites enrolled white women, and Boston, Chicago, Detroit, and Pittsburgh enrolled black women. Los Angeles, Newark, and Oakland enrolled Japanese, Hispanic, and Chinese participants, respectively. Chicago and Newark centers did not measure BMD. The SWAN bone cohort consists of 2352 women. The Boston, Detroit, and Los Angeles sites began the SWAN study using Hologic 4500A model densitometers; because TBS can only be measured using scans acquired on a 4500A or newer densitometer, women in the bone cohort from these 3 sites constituted the eligible SWAN TBS Study sample (N = 1436). The current study includes TBS data from baseline through follow-up visit 13, which ended in 2013. Analysis eligibility criteria were having a known FMP date and at least 2 TBS measurements after accounting for baseline exclusions. Bone active agents (hormone therapy, tamoxifen, raloxifene, osteoporosis treatments, and GnRH agonists) were exclusions, resulting in an analysis sample size of 705 (Fig. 1). Sites obtained institutional review board approval and participants gave written, informed consent.
Figure 1.
Derivation of the analysis sample for TBS in relation to the FMP. Participants are from the SWAN-TBS. Abbreviations: FBS, final menstrual period; SWAN, Study of Women’s Health Across the Nation; TBS, trabecular bone score.
Outcomes
The outcome, TBS, is a textural parameter derived from lumbar spine (LS) BMD scans. SWAN-TBS sites initially measured BMD using Hologic 4500A instruments (Hologic, Inc., Waltham, MA) and then upgraded to Discovery models. Each site scanned volunteers on their old and new machines to permit BMD and TBS cross-calibrations. A standard BMD quality-control program, conducted in collaboration with Synarc, Inc. (Newark, CA), included daily phantom measurements, SWAN site cross-calibration with a circulating anthropomorphic spine standard, local site review of all scans, central review of scans that met problem-flagging criteria, and central review of a 5% random sample of scans.
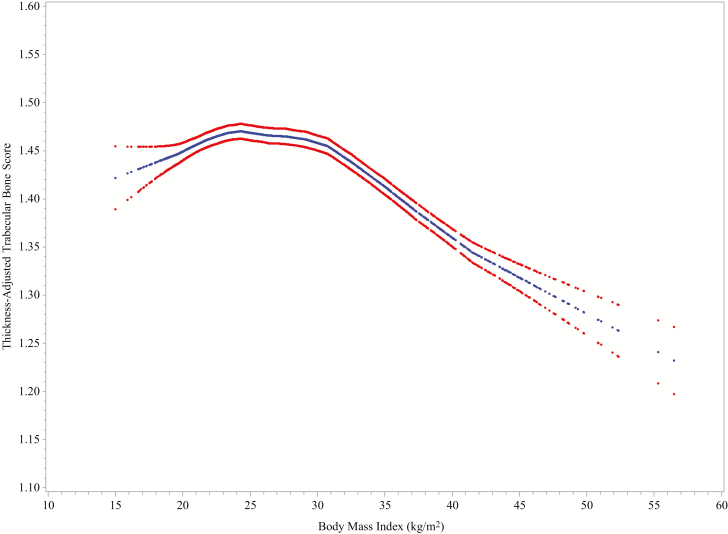
To acquire TBS measures, we retrieved LS BMD scans from SWAN baseline through SWAN follow-up visit 13. We initially used TBS iNsight Software, version 2.3.0 (Med-Imaps, Pessac, France). The TBS software (iNsight v3.0 and older) accounts for between-participant soft-tissue variability by correcting for soft tissue in the TBS algorithm, using BMI to estimate the soft-tissue correction. However, on scans acquired using Hologic densitometers, even after BMI-based correction, there remains a negative correlation between TBS and BMI (14). SWAN-TBS initially acquired the BMI-corrected TBS (version 2.3.0) and found a residual strong negative relation between BMI and TBS (data not shown). Med-Imaps subsequently derived a new soft-tissue correction that uses directly measured soft-tissue thickness (computed by the dual-energy x-ray absorptiometry [DXA] device) rather than using BMI as a surrogate for it (15). This new beta version of TBS is termed tissue “thickness-corrected TBS” (TBS_TH). Because TBS_TH directly adjusts for thickness, BMI does not enter into the computational algorithm. SWAN-TBS reran all scans using the beta version of the TBS_TH software. Shevroja et al examined the relation between TBS_TH and BMI in a study of 1331 women with mean age 64.6 (±7.5) years and mean BMI of 25.9 (±4.5) kg/m2 (16). They reported no correlation between BMI and TBS_TH. Unlike the results reported by Shevroja et al, SWAN-TBS found a tripartite cross-sectional relation between TBS_TH and BMI: positive up to a BMI of ~24 kg/m2, “flat” between 24 and 31 kg/m2, and negative when BMI was greater than 31 (Fig. 2). Our longitudinal analyses, therefore, account for this nonlinear relation between TBS_TH and BMI in our study sample by using BMI splines (see the Data Analysis section). When we use the term TBS in the remainder of this report, we are referring to TBS_TH.
Figure 2.
LOESS plots of TBS_TH in relation to BMI at baseline, in the Study of Women’s Health Across the Nation Trabecular Bone Score Study (N = 1375). Blue curves illustrate mean values; cross-sectional, 95% confidence intervals are indicated by the red curves. We accounted for the nonlinear relation between BMI and TBS_TH by using BMI splines in our models (see the Data Analysis section). Abbreviations: BMI, body mass index; LOESS, locally estimated scatterplot smoothing; TBS_TH, thickness-corrected trabecular bone score.
Primary predictor
The primary exposure is the number of years before or after the FMP that TBS was assessed. We compute time before or after FMP using the month and year of the FMP and the month and year of each TBS measure; it is negative if TBS is measured before the FMP date and positive if measured after. SWAN designates the FMP date as the last menstrual bleeding date reported during the visit immediately before the first visit when the participant was classified as postmenopausal (ie, reported 12 months of amenorrhea).
Other predictors
Age (years), self-defined race/ethnicity (black, Japanese, white), menstrual bleeding patterns, and use of medication that affects bone density (yes/no, time varying) were obtained using annual, standardized interviews. Weight (kilograms, time varying) and height (meters) were assessed at each visit, using calibrated scales and stadiometers. BMI (weight in kg/[height in m]2) was calculated.
Data analysis
Using means (standard deviations) for continuous variables and numbers (percentages) for categorical variables, we summarized characteristics of 3 relevant samples: participants in the SWAN bone cohort (all 5 SWAN bone study sites), women from the 3 bone sites included in the TBS study (SWAN-TBS cohort), and those in the analysis sample.
To quantify change in TBS relative to FMP time, we used a stepwise approach: (1) nonparametric, locally estimated scatterplot smoothing (LOESS)-based identification of the functional form of the TBS trajectory in relation to FMP time; (2) piecewise linear regression to define optimal knot placement for the parametric TBS trajectory; and (3) piecewise linear regression with fixed knots to estimate rate of change in TBS within each segment and the associations between hypothesized predictors and TBS slopes (17).
We normalized TBS measurements to each woman’s own baseline TBS, for 2 reasons: (1) to remove between-women variation in starting level of TBS when examining within-woman change in TBS using LOESS plots and (2) to estimate mean change in TBS over time as percent of starting TBS lost (or gained) per year. LOESS plots suggested piecewise linear trajectories with 3 segments and 2 changes in slope, or knots (Fig. 2). In steps 2 and 3, we used mixed effects linear regression to fit piecewise–linear growth curves to repeated measurements of baseline-normalized values of TBS as a function of FMP time, using linear splines with 2 fixed knots. To account for within-woman correlation between repeated observations, we included random effects for the intercept and 3 slopes (which allows them to vary from woman to woman).
In step 2, we tested model adequacy and suitability of candidate knot locations by running null models with only random effects and no fixed effects. We evaluated knot selection by examining the change in the explained proportion of within-woman variance (pseudo R2) when each of the knots was varied (in 6-month intervals) around the candidate locations suggested by the LOESS plots. Knots placed at FMP time minus 1.5 years and plus 2.0 years minimized the unexplained variance, suggesting 3 distinct segments in the TBS trajectory: FMP time <-1.5 years, -1.5 to 2 years, and >2 years, which we term the premenopause, MT, and postmenopause segments, respectively. We formally tested for a change in slope at the 2 knots before adjusting for covariates; the slope change was statistically significant at both knots (P < 0.0001).
Last (step 3), we added, as fixed effects on the intercept and 3 slopes (1 for each of the 3 TBS trajectory segments), age at FMP, race/ethnicity, and BMI splines to the mixed effects models. This model evaluates how each covariate influences the rate of change in TBS outcome during each of the 3 TBS trajectory segments. BMI changed over time. We therefore modeled BMI measured at the time of each longitudinal TBS measurement affecting current TBS, BMI at the baseline visit affecting the premenopause TBS slope, BMI at the visit closest to FMP minus 1.5 years affecting the TBS slope in MT, and BMI at the visit closest to FMP plus 2 years affecting the postmenopause TBS slope. Because the relation between BMI and TBS was nonlinear, we modeled BMI as a continuous predictor and allowed for different effects within 3 BMI ranges, with cut-points based on the LOESS plot of baseline TBS and BMI (Fig. 2). The ranges for the BMI splines were: low, <24; mid, 24 to 31, and high, >31. The final model also adjusted for a SWAN TBS study site as a fixed effect on intercept and each of 3 slopes. Twenty-four women began taking bone active medications after study baseline but before their second TBS measure and were therefore censored because having a minimum of 2 TBS numbers was required. Thus, 681 participants were represented in the multivariable model; they had no missing data for any covariates. We conducted analyses in SAS version 9.2 and specified a 2-sided alpha of 0.05 for statistical significance.
Definition of the referent
To test whether race/ethnicity had independent association with change in TBS over time, we stipulated white (the largest race/ethnicity group) as the referent group, allowing us to test the effects of race/ethnicity on TBS, having accounted for age at FMP and BMI in the entire sample. We modeled additive effects of the 3 predictors (race/ethnicity, age at FMP, and BMI); thus, the associations of age at FMP and BMI are presumed to be the same in all race/ethnicity groups. As referent values in all race/ethnicity groups, we used the entire study sample’s mean age at FMP (52.2 years) and mean BMI (28.0 kg/m2).
Results
Participant characteristics
The analytic sample (N = 705) consisted of 228 black (34%), 164 Japanese (23%), and 298 white (42%) women [Table 1]. The analysis sample average age at baseline was 46.75 years (standard deviation [SD], 2.7 years), age at FMP was 52.2 years (SD, 2.9 years), and baseline BMI was 28.0 kg/m2 (SD, 7.3 kg/m2). Average BMI was greatest among black women (31.2 kg/m2) and least among Japanese women (22.8 kg/m2); mean BMI in white women was intermediate (28.1 kg/m2). Characteristics of the analytic sample were similar to those of the entire SWAN-TBS cohort (N = 1436) and to the parent SWAN bone cohort (N = 2532).
Table 1.
Characteristics of Participants in the SWAN Bone Cohort, the SWAN-TBS Cohort, and the Analytic Sample
| Participant Characteristicsa | SWAN Bone Cohortb (N = 2352) | SWAN-TBS Cohortc (N = 1436) | Analytic Sampled (N = 705) |
|---|---|---|---|
| SWAN clinical site | |||
| Boston, MA | 435 (18) | 432 (30) | 228 (32) |
| Detroit, MI | 530 (23) | 529 (37) | 241 (34) |
| Los Angeles, CA | 476 (20) | 475 (33) | 236 (33) |
| Davis, CA | 455 (19) | NA | NA |
| Pittsburgh, PA | 456 (19) | NA | NA |
| Race/ethnicity | |||
| Black | 659 (28) | 499 (35) | 243 (34) |
| Japanese | 270 (11) | 270 (19) | 164 (23) |
| White | 1173 (50) | 667 (46) | 298 (42) |
| Chinese | 250 (11) | NA | NA |
| Age, y | 46.4 (2.7) | 46.5 (2.8) | 46.5 (2.7) |
| Age at final menstrual period | NA | NA | 52.2 (2.9) |
| Body mass index, kg/m2 | |||
| Overall | 27.5 (6.9) | 28.1 (7.3) | 28.0 (7.3) |
| Black | 31.2 (7.2) | 31.5 (7.5) | 31.2 (7.5) |
| White | 27.6 (6.6) | 27.8 (6.9) | 28.1 (7.1) |
| Japanese | 22.7 (3.7) | 22.7 (3.7) | 22.8 (3.5) |
| Chinese | 23.2 (3.9) | NA | NA |
| Lumbar spine BMD, g/cm2 | |||
| Overall | 1.08 (0.14) | 1.08 (0.14) | 1.08 (0.14) |
| Black | 1.14 (0.15) | 1.14 (0.14) | 1.14 (0.14) |
| White | 1.06 (0.13) | 1.06 (0.13) | 1.06 (0.13) |
| Japanese | 1.02 (0.12) | 1.03 (0.12) | 1.03 (0.12) |
| Chinese | 1.04 (0.13) | NA | NA |
| Lumbar spine TBSe | |||
| Overall | NA | 1.44 (0.09) | 1.44 (0.09) |
| Black | NA | 1.41 (0.09) | 1.42 (0.09) |
| White | NA | 1.44 (0.09) | 1.44 (0.09) |
| Japanese | NA | 1.48 (0.07) | 1.48 (0.07) |
Values in table are means (standard deviation) for continuous variables and numbers (percentages) for categorical variables.
BMD, bone mineral density; NA, not available; SWAN, Study of Women’s Health Across the Nation; TBS, trabecular bone score.
aAll data are from SWAN and SWAN-TBS study baseline.
bWomen were recruited into the SWAN bone cohort at 5 SWAN sites. Most women joined the SWAN bone cohort at SWAN baseline, but women could enroll in the bone study through the third SWAN study visit.
cSWAN-TBS was conducted using data from the Boston, Detroit, and Los Angeles SWAN sites. These sites used Hologic 4500 (or later model) instruments from the outset of SWAN. TBS cannot be computed using older Hologic models.
dThe analytic sample was limited to participants from the TBS cohort with known final menstrual period dates. For exclusion criteria, see the Methods section and Fig. 1 (derivation of study sample).
eMeasured using thickness-corrected TBS. See the Methods section for details.
We acquired TBS readings from 97% of SWAN bone cohort participants at the 3 eligible SWAN sites. Median number of visits per woman was 11 (interquartile range, 7, 13), the modal number was 13 (with a maximum of 14). Average follow-up duration was 12.0 years (range, 15.7 years).
Mean trajectory of TBS relative to the FMP date
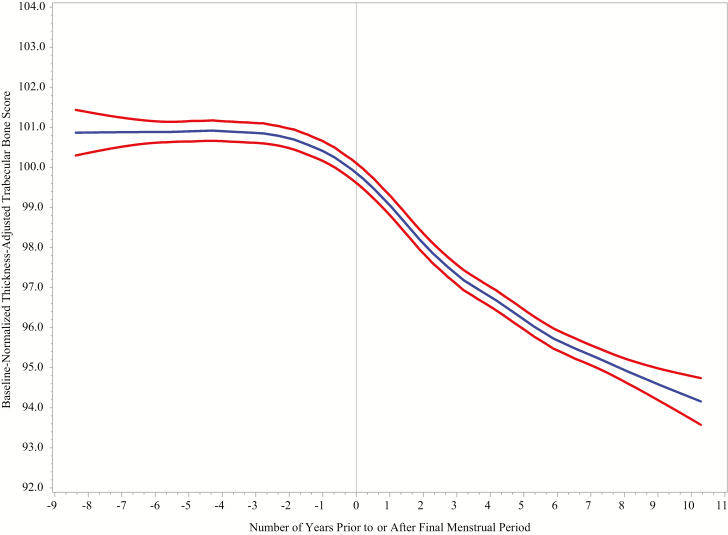
Figure 3 is a LOESS plot of mean TBS as a function of FMP time (ie, number of years before or after the FMP, where FMP time is 0 on the day of the FMP, negative on dates before FMP, and positive on dates after FMP). At ~1.5 years before the FMP, the LOESS plot suggested no decline in TBS; onset of decline appeared at ~1.5 years before the FMP. Approximately 2 years after FMP, the LOESS indicated that TBS decline lessened. TBS trajectories appeared linear within each of these 3 intervals; therefore, we proposed a 3-segment model of change in TBS relative to FMP time. We tested this hypothesized model by fitting piecewise-linear growth curves with 3 linear segments, using mixed effects linear models and formal testing of knot locations, which confirmed that the best knot locations were at FMP time -1.5 years and +2 years.
Figure 3.
LOESS plots of baseline normalized values of TBS_TH in relation to time before or after the FMP in the analysis sample. Participants are from the Study of Women’s Health Across the Nation Trabecular Bone Score Study. Blue curves illustrate mean values. Cross-sectional, 95% confidence intervals are indicated by the red curves. Number of observations is 6831. To minimize outlier influence, the plot is truncated at the extremes of FMP-time, 8 years before FMP (5th percentile) and 10 years after FMP (95th percentile). The LOESS plot presents a cross-section at each time; thus, it is influenced by the sample composition at each cross-section and by between-women differences. Therefore, the LOESS plot is used only to develop a hypothesis about the functional form of the relation between the exposure (FMP time) and the outcome (TBS_TH). Abbreviations: LOESS, locally estimated scatterplot smoothing; FMP, final menstrual period; TBS_TH, thickness-corrected trabecular bone score.
TBS rates of change in referent SWAN participant
In Table 2, the first row of data shows the annualized rate of change in TBS (ie, the TBS slope) during each time interval for the referent woman. The referent is aged 52.2 years at FMP and has a BMI of 28.0 kg/m2 (these are the mean age and BMI values based on the complete analysis sample [ie, including all racial/ethnic groups]). The referent’s race/ethnicity, a categorical variable, is stipulated as white. In the referent, average annual rate of TBS change during the premenopause was 0.12% (95% confidence intervals [CI], -0.11% to 0.34%) and the rate of TBS change in the MT was -1.16% (95% CI, -1.58% to -0.74%). The nonoverlapping CIs of the premenopausal slope and the MT slope indicate that they are statistically significantly different from each other. During postmenopause, the annual rate of change in TBS averaged -0.89% (95% CI, -1.17% to -0.61%). CIs for the MT slope and the postmenopause slope overlap. Thus, one must calculate the slope difference formally assess whether the MT slope and the postmenopause slope are statistically different. The difference (postmenopause slope minus MT slope) is +0.27% and the 2 slopes are not statistically different from each other. Ten-year absolute TBS loss, computed for the 5 years through 5 years after the FMP was 6.3% (95% CI, 7.9%-4.8%).
Table 2.
The Influence of Race/Ethnicity, Age at the FMP, and BMI on Annualized Rates of Change in TBS in the SWAN TBS Study: Results of Mixed Effects Linear Regression
| Adjusted Associations With Annualized Rates of Change in TBS (% per year) During Each Interval Before and After the FMP, with 95% CIsa,b,c | Adjusted Associations with 10-y TBS change: 5 y before to 5 y after FMP | |||
|---|---|---|---|---|
| Premenopause 9 y to 1.5 y Before FMP | Menopause Transition 1.5 y Before to 2.0 y After FMP | Postmenopause 2.0 y to 9 y After FMP | ||
| Referent individuald | 0.12% (-0.11%, 0.34%) | -1.16% (-1.58%, -0.74%) | -0.89% (-1.17%, -0.61%) | -6.3% (-7.9%, -4.8%) |
| Race/ethnicity | ||||
| Black | -0.10% (-0.25%, 0.04%) | 0.27% (0.03%, 0.50%) | 0.26% (0.12%, 0.40%) | 1.4% (0.6%, 2.1%) |
| Japanese | -0.03% (-0.23%, 0.17%) | 0.03% (-0.32%, 0.38%) | -0.09% (-0.32%, 0.14%) | -0.3% (-1.5%, 0.9%) |
| Age at FMP (per y) | -0.016% (-0.04%, 0.005%) | -0.044% (-0.08%, -0.009%) | 0.016% (-0.01%, 0.04%) | -0.16% (-0.3%, -0.04%) |
| BMI (per kg/m2 increment) | ||||
| If BMI < 24 kg/m2 | 0.008% (-0.03%, 0.04%) | 0.014% (-0.05%, 0.08%) | -0.02% (-0.07%, 0.03%) | 0.02% (-0.2%, 0.3%) |
| If BMI 24-31 kg/m2 | 0.031% (0.009%, 0.05%) | 0.042% (0.001%, 0.08%) | 0.050% (0.02%, 0.08%) | 0.41% (0.3%, 0.6%) |
| If BMI > 31 kg/m2 | -0.040% (-0.06%, -0.02%) | 0.009% (-0.02%, 0.04%) | 0.014% (-0.00%, 0.03%) | -0.07% (-0.2%, 0.04%) |
Abbreviations: BMI, body mass index; CI, confidence interval; FMP, final menstrual period; SWAN, Study of Women’s Health Across the Nation; TBS, trabecular bone score.
aEstimated associations of slopes and 10-y cumulative change with race/ethnicity, age at FMP, and BMI. In addition to age at FMP, race/ethnicity, and BMI (time varying), the model also adjusts for SWAN study site and includes random effects for the intercept and the 3 slopes. Random effect standard deviations for the 3 slopes were 0.42%, 0.77%, and 0.39% per year. Standard deviation of the unmodeled residual error was 2.43%.
bEstimates shown in bold font are statistically significantly different from 0.
cValues shown in bold font are statistically significantly different from zero.
dModel-predicted slopes (percentage of baseline level lost per year) and cumulative 10-y change around the FMP for the referent individual (white, age at FMP = 52.2 years, BMI = 28.0 kg/m2).
Race/ethnicity and age-at-FMP effects on TBS change rates
To obtain the TBS change rates in nonwhite women and for those whose FMP did not occur at 52.2 years, we add the effect size estimates shown in Table 2 (for either race/ethnicity or age at FMP) to the slopes in the referent woman. (Table 2 estimates for race/ethnicity and age-at-FMP are relative, rather than absolute, slopes.) Black women manifested less TBS decline than the referent sample (Table 2): their TBS slope in MT was -0.89% (95% CI, -1.35% to -0.44%) and in the postmenopausal interval it was -0.63% (95% CI, -0.92% to -0.34%). The cumulative loss of TBS in the 10-year span bracketing the FMP was also less in black women, 5.0% (95% CI, 3.33%-6.63%). Japanese-specific slopes for change in TBS did not differ from those of the referent sample during any of the 3 intervals (P > 0.4 for each of the 3 slope comparisons). Greater age at FMP accentuated the annual drop in TBS during the MT segment (0.044% additional annualized loss for each year later FMP date). Aggregated over the 10-year period bracketing the FMP, TBS decline is 0.16% greater per each year increase in age at FMP.
BMI effects on rates of change in TBS
Because the cross-sectional association between BMI and TBS was positive when BMI < 24 kg/m2, negative when BMI > 31 kg/m2, and 0 slope in the BMI range of 24 to 31 kg/m2 (Fig. 2), we included 3 BMI splines in the multiply adjusted, piecewise-linear growth curve model. BMI spline cut points correspond to the values at which the relation between BMI and TBS changed direction in the cross-sectional analysis. In longitudinal analysis, when BMI was <24 kg/m2, increasing BMI did not influence the rate of change in TBS before, during, or after the MT nor did it have an impact on the 10-year cumulative loss in TBS (Table 2). When BMI was in the mid-range (24-31 kg/m2), greater BMI lowered the rate of TBS loss in each interval: for each 1-unit increment in BMI, the rate of premenopausal TBS loss was 0.031% less, TBS loss was 0.042% less during the MT, and postmenopausal TS loss was 0.050% less. In aggregate, 10-year TBS loss was diminished by 0.41% for each 1-unit increment in BMI. In the highest BMI range (>31 kg/m2), during premenopause only, higher BMI worsened the rate of TBS loss (-0.040% more loss per BMI unit). However, the cumulative, 10-year loss of TBS was not significantly affected by BMI in the high BMI range (P = 0.2).
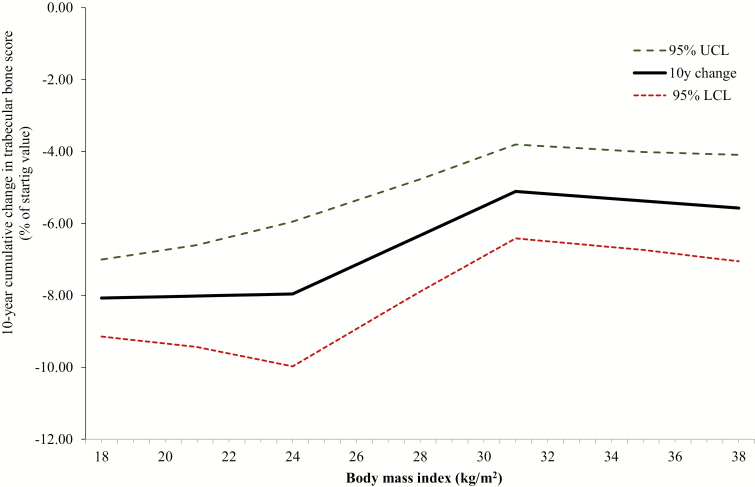
Figure 4 illustrates the influence of BMI on cumulative change in TBS in the period spanning 5 years before through 5 years after the FMP. Within the mid-range of BMI (24-31 kg/m2), the 10-year total TBS loss is lessened as BMI becomes larger: cumulative loss ranges from a minimum of 5.11% (when BMI equals 31) to a maximum of 7.6% (at a BMI of 24 kg/m2). When BMI is less than 24, cumulative 10-year TBS loss does not vary with greater BMI: TBS loss is constant across the low BMI range, at approximately 8%. Similarly, collective 10-year TBS decline was not statistically significantly influenced by BMI values in the range of 31 kg/m2 or more. Within the high BMI spectrum, TBS loss is approximately 5.3% at all BMI values.
Figure 4.
Cumulative change in thickness-adjusted trabecular bone score during the 10-year interval spanning 5 years before through 5 years after the final menstrual period as function of body mass index, Study of Women’s Health Across the Nation Trabecular Bone Score Study. The solid black line illustrates the mean value of 10-year change across each of 3 body mass index ranges (<24, 24-31, and >41); dashed lines describe the 95% confidence interval. Model is adjusted for race, age at final menstrual period, and study site (see the Data Analysis section). Abbreviations: LCL, lower confidence limit; UCL, upper confidence limit.
Discussion
This study described the longitudinal pattern of change in TBS throughout an 18-year time span bracketing the FMP. Using data from a multiracial/ethnic cohort, we quantified the TBS trajectory in a referent woman (white, with overall sample average BMI and age at FMP) and explicitly tested whether rates of TBS change in black or Japanese women differed from those of white women. In the referent woman, before the onset of the MT, TBS was stable (its 0.12% annual slope was not significantly different from 0). TBS decline began 1.5 years before the FMP, averaging 1.16% per year, a 10-fold change in rate. Two years after the FMP, the rate of TBS decline slowed, but did not stop, averaging 0.89% annually. Black women lost less TBS than did white women, but TBS change in Japanese women did not differ from that of white women. The effect of BMI on TBS change varied by BMI level; it was prominent in the mid-range of 24 to 31 kg/m2.
Our hypothesis, that the MT would be accompanied by an accelerated decline in TBS, followed by a slower rate in postmenopause, was upheld. We anticipated this pattern based on our prior SWAN analyses of other bone strength indicators and a few prior longitudinal studies that inferred, but did not formally test, that the MT played a role in TBS loss (4, 6, 8, 18–20). One study that assessed TBS in 29 women once during premenopause and again 3 years later when they were postmenopausal found a cumulative drop of TBS of 4.6% (~1.8% yearly decline) (18). Apart from that, studies of TBS in relation to menopause are confined to postmenopausal women (19, 20). The Manitoba Bone Density Program obtained TBS longitudinally in 1150 postmenopausal women with mean initial age 62 years; after average follow-up of 3.3 years, the annual drop in TBS was 0.3% (19). In an older (mean age, 77 years) sample of osteoporotic women constituted from the placebo arm of a randomized controlled trial, cumulative rates of TBS loss were 0.36%, 0.45%, and 0.49% at 1, 2, and 3 years of follow-up, respectively (20). The pattern of results from these initial explorations—a higher rate of TBS loss in the pre- to postmenopause study and a slower loss rate characterizing the 2 postmenopause-only studies—appears consistent with SWAN-TBS findings (18–20). Our study, designed specifically to identify the relation between the MT and TBS, makes a substantive contribution by estimating the longitudinal rate of TBS change using multiple, serial observations to gauge change in TBS relative to the date of the FMP.
TBS loss rates during the MT and postmenopause were similar in white and Japanese women, but black women experienced less TBS decline. The TBS trajectory of black SWAN participants aligns with lower fracture rates in US black compared to US white women, and may contribute to the fracture differential between these 2 groups (21). Although fracture rates in US Asian women are also less than those of US white women, MT-related TBS losses were similar in Japanese and white women in SWAN; thus, differential rate of loss in this marker of bone strength does not correspond to the Japanese-white fracture discord (21).
Despite being thickness-adjusted (direct adjustment for soft-tissue attenuation of x-rays), our baseline TBS values were not independent of BMI. The acknowledged TBS technical constraint imposed by BMI was originally addressed by including BMI (a surrogate for tissue thickness) in the TBS algorithm (version 2.1) and by optimizing the TBS algorithm for a BMI range of 15 to 37 kg/m2 (22). This approach remediated the negative BMI-TBS association when DXA scans were obtained with GE Lunar devices, but it did not succeed for Hologic-acquired scans (14, 23). Therefore, a new TBS_TH algorithm was developed that adjusts for tissue thickness measured using DXA. Applying the thickness-adjusted TBS algorithm to LS scans acquired with Hologic DXA devices, Shevroja et al reported no correlation between BMI and TBS (16). In contrast, the SWAN-TBS cross-sectional TBS-BMD relation was positive for BMI less than ~24 kg/m2, negative for BMI greater than ~31 kg/m2, and flat in between these 2 BMI values. In SWAN-TBS, average BMI is 28 kg/m2 and ~19 and ~43 kg/m2 mark the 5th and 95th percentiles of the distribution. Compared with the study by Shevroja et al, the greater BMI values of our participants may have enabled us able to observe depression of TBS by BMI because many SWAN-TBS participants had high BMI. However, the low BMI range appears to be reasonably represented in both the SWAN-TBS and the Shevroja analysis; why these studies found dissimilar relations between low-range BMIs and thickness-adjusted TBS is not apparent.
The influence of BMI on the trajectory of TBS across the MT was inhomogeneous. In longitudinal analysis, the low BMI range (<24 kg/m2) evidenced no association between higher BMI and change in TBS over time. For mid-range BMI (24-31 kg/m2), greater BMI was related to lesser TBS decline during all MT stages. Conversely, within the topmost BMI grouping (>31 kg/m2), higher BMI had a small negative effect on TBS during premenopause, but its magnitude was insufficient to increase the cumulative 10-year loss in the high BMI range. Our results are consonant with previous investigations of the relations among BMI, BMD, and fracture, which theorize that the protective effect of greater BMI is lost when the level of BMI exceeds obesity levels (24–27). Beneficial effects of BMI on bone, such as more biomechanical bone loading or greater postmenopausal estrogen levels, may dominate in the BMI midrange (24, 27, 28). But negative BMI effects on bone, such as a detrimental effect of immobility or greater levels of endogenous inflammation, may exert more influence at high BMI levels, accounting for the negative relation between BMI and TBS in premenopausal women with severe obesity (25, 27, 29).
The occurrence of an MT-related decline in TBS further advances the thesis that this part of the life course is particularly damaging to skeletal integrity. For many years, investigators have asked whether the rapid BMD loss that happens during the MT has structural implications (4, 30–32). We find that TBS declines more rapidly in the MT than in the postmenopause phase. This, coupled with the cumulative drop in TBS during the ~4 years bracketing the FMP of ~0.64 SD, suggests that the answer is yes. TBS quantifies an aspect of bone strength that is not synonymous with microarchitecture, but, independent of BMD, it predicts fracture in postmenopausal women and men (9–11). We do not contend that TBS is an independent predictor of fracture when measured during the MT or in early postmenopause; we did not test this hypothesis here (nor have others examined it). The inference that lower levels of TBS are unfavorable relies on prior reports (9–11). Future work from SWAN-TBS will examine the fracture predictive capacity of TBS in our cohort.
Study limitations include unavoidable imprecision in identifying the timing of the acceleration deceleration of TBS loss, especially the latter, which is much less distinct. To counter possible change point misspecification, we computed cumulative, 10-year change rates. SWAN upgraded Hologic densitometer models, but in vivo calibration protocols permitted cross-site and within-site longitudinal standardization of BMD and TBS. We used a beta version of thickness-adjusted TBS, which lessened but did not eliminate, the cross-sectional relation between BMI and TBS. However, our use of BMI splines in longitudinal analysis mitigated against confounding by the nonlinear TBS-BMD association evident in cross-section. Ours is a community-based sample; results may not be generalizable to the US population. Strengths of this work include its long duration of follow-up, during which we acquired up to 13 serial assessments of TBS and covariates, ability to study TBS change among women from 3 racial/ethnic groups, use of thickness-adjusted TBS, meticulous attention to the analytic treatment of BMI, and reliance on an FMP time approach, which provides a more precise understanding of how outcomes relate to menopause than does than an analysis that relies clinical MT stages (2–4).
In conclusion, this study newly discloses a period of accelerated TBS loss surrounding the FMP, adding another facet to our understanding of the threats to bone strength during the MT. It also reveals that race/ethnicity and BMI have independent effects on MT-related TBS loss.
Acknowledgments
The authors thank Franck Michelet, PhD, for developing the cross-calibration equations presented in the Appendix. The authors also thank the study staff at each site and all the women who participated in SWAN.
Financial Support: The SWAN TBS Study is funded by R01AG026463. The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants R01AG026463, U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and 5R01AG026463).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, principal investigator (PI) 2011-present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999-present; Robert Neer, PI 1994-1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009-present; Lynda Powell, PI 1994-2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011-present, Rachel Wildman, PI 2010-2011; Nanette Santoro, PI 2004-2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994-2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012-2016; Sherry Sherman 1994-2012; Marcia Ory 1994-2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012-present; Kim Sutton-Tyrrell, PI 2001-2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995-2001.
Steering Committee: Susan Johnson, current chair; Chris Gallagher, former chair.
Author Contributions: Obtaining funding (J.C., G.A.G., A.S.K.); participant recruitment and enrollment (G.A.G., M.H.H., J.S.F., S.H.); data calibration, management, and cleaning (G.A.G., M.H.H., W.H., A.S.K., D.H.); analytic design (G.A.G., A.S.K.); statistical analysis (G.A.G., M.H.H., W.H., A.S.K.).; primary manuscript drafting (G.A.G., A.S.K.); and critical review and revision of manuscript (all authors).
Glossary
Abbreviations
- BMI
body mass index
- BMD
bone mineral density
- CI
confidence interval
- DXA
dual-energy x-ray absorptiometry
- FMP
final menstrual period
- FN
femoral neck
- LOESS
locally estimated scatterplot smoothing
- LS
lumbar spine
- MT
menopause transition
- SD
standard deviation
- SWAN
Study of Women’s Health Across the Nation
- TBS
trabecular bone score
- TBS_TH
thickness-corrected trabecular bone score
Additional Information
Disclosure Summary: Didier Hans is co-owner of the TBS patent and has corresponding ownership shares and position at Medimaps group. All other authors declare no conflicts of interest.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Sowers MR, Zheng H, Greendale GA, et al. Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab. 2013;98(7):2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Recker RR, Lappe JM, Davies KM, Kimmel DB. Change in bone mass immediately before menopause. J Bone Miner Res. 1992;7(8):857–862. [DOI] [PubMed] [Google Scholar]
- 3. Sowers MR, Zheng H, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95(5):2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karlamangla AS, Barrett-Connor E, Young J, Greendale GA. Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int. 2004;15(1):62–70. [DOI] [PubMed] [Google Scholar]
- 6. Nagaraj N, Boudreau RM, Danielson ME, et al. Longitudinal changes in hip geometry in relation to the final menstrual period: Study of Women’s Health Across the Nation (SWAN). Bone. 2019;122:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heaney RP, Barger-Lux MJ, Davies KM, Ryan RA, Johnson ML, Gong G. Bone dimensional change with age: interactions of genetic, hormonal, and body size variables. Osteoporos Int. 1997;7(5):426–431. [DOI] [PubMed] [Google Scholar]
- 8. Ishii S, Cauley JA, Greendale GA, et al. Trajectories of femoral neck strength in relation to the final menstrual period in a multi-ethnic cohort. Osteoporos Int. 2013;24(9):2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–2769. [DOI] [PubMed] [Google Scholar]
- 10. Martineau P, Leslie WD, Johansson H, et al. Clinical utility of using lumbar spine trabecular bone score to adjust fracture probability: the Manitoba BMD cohort. J Bone Miner Res. 2017;32(7):1568–1574. [DOI] [PubMed] [Google Scholar]
- 11. McCloskey EV, Odén A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31(5):940–948. [DOI] [PubMed] [Google Scholar]
- 12. Simonelli C, Leib E, Mossman N, Winzenrieth R, Hans D, McClung M. Creation of an age-adjusted, dual-energy x-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in non-Hispanic US White women. J Clin Densitom. 2014;17(2):314–319. [DOI] [PubMed] [Google Scholar]
- 13. Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000:175–188. [Google Scholar]
- 14. Mazzetti G, Berger C, Leslie WD, et al. ; CaMos Research Group Densitometer-specific differences in the correlation between body mass index and lumbar spine trabecular bone score. J Clin Densitom. 2017;20(2):233–238. [DOI] [PubMed] [Google Scholar]
- 15. De Guio F, Shevroja E, Michelet F, Tran D, Lelong C, Hans D. 2018 trabecular bone score (TBS) integrating a new correction for soft tissue effects based on estimated tissue thickness. J Bone Miner Res. 32 http://www.asbmr.org/education/AbstractDetail?aid=a52f33f4-fdcf-4cc8-b2ad-2dc5fb669ee0. Accessed April 9, 2019. [Google Scholar]
- 16. Shevroja E, Lamy O, Berengere AR, et al. 2018 the impact of a beta trabecular bone score (TBS) algorithm accounting for soft tissue thickness correction on the prediction of incident major osteoporotic fracture (MOF) risk in postmenopausal women: the OsteoLaus study. J Bone Miner Res. 32 http://www.asbmr.org/education/AbstractDetail?aid=01458caa-1def-4d2b-bcb3-3b4caec49f8b. Accessed April 9, 2019. [Google Scholar]
- 17. Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 18. Pedrazzoni M, Casola A, Verzicco I, Abbate B, Vescovini R, Sansoni P. Longitudinal changes of trabecular bone score after estrogen deprivation: effect of menopause and aromatase inhibition. J Endocrinol Invest. 2014;37(9):871–874. [DOI] [PubMed] [Google Scholar]
- 19. Krieg MA, Aubry-Rozier B, Hans D, Leslie WD; Manitoba Bone Density Program Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int. 2013;24(3):1073–1078. [DOI] [PubMed] [Google Scholar]
- 20. Popp AW, Guler S, Lamy O, et al. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: a three-year study. J Bone Miner Res. 2013;28(3):449–454. [DOI] [PubMed] [Google Scholar]
- 21. Cauley JA, Wu L, Wampler NS, et al. Clinical risk factors for fractures in multi-ethnic women: the Women’s Health Initiative. J Bone Miner Res. 2007;22(11):1816–1826. [DOI] [PubMed] [Google Scholar]
- 22. Schacter GI, Leslie WD, Majumdar SR, Morin SN, Lix LM, Hans D. Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: the Manitoba BMD cohort. Osteoporos Int. 2017;28(11):3199–3203. [DOI] [PubMed] [Google Scholar]
- 23. Langsetmo L, Vo TN, Ensrud KE, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group The association between trabecular bone score and lumbar spine volumetric BMD is attenuated among older men with high body mass index. J Bone Miner Res. 2016;31(10):1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–573. [DOI] [PubMed] [Google Scholar]
- 25. Greco EA, Fornari R, Rossi F, et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract. 2010;64(6):817–820. [DOI] [PubMed] [Google Scholar]
- 26. Compston JE, Watts NB, Chapurlat R, et al. ; Glow Investigators Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27(1):1–10. [DOI] [PubMed] [Google Scholar]
- 28. Randolph JF Jr, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. [DOI] [PubMed] [Google Scholar]
- 30. Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004;19(10):1628–1633. [DOI] [PubMed] [Google Scholar]
- 31. Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41(1):111–116. [DOI] [PubMed] [Google Scholar]
- 32. Zaidi M, Turner CH, Canalis E, et al. Bone loss or lost bone: rationale and recommendations for the diagnosis and treatment of early postmenopausal bone loss. Curr Osteoporos Rep. 2009;7(4):118–126. [DOI] [PubMed] [Google Scholar]