Abstract
Hydroxychloroquine is a commonly used medication and rarely may result in development of erythema multiforme. This potential cutaneous side effect should be highlighted in information given to patients prior to hydroxychloroquine commencement.
Keywords: antimalarials, erythema multiforme, hydroxychloroquine, severe cutaneous adverse drug reactions
Hydroxychloroquine is a commonly used medication and rarely may result in development of erythema multiforme. This potential cutaneous side effect should be highlighted in information given to patients prior to hydroxychloroquine commencement.
1. CLINICAL IMAGE
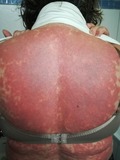
A 60‐year‐old Caucasian woman with a past medical history of essential hypertension and chronic obstructive pulmonary disease presented with a 5‐day history of a widespread pruritic, striking erythematous to purple targetoid eruption on her trunk, neck, upper, and lower limbs (Figures 1 and 2). She also complained of fatigue. She had been started on hydroxychloroquine (HCQ) 200 mg twice daily 19 days prior to presentation for arthritis. Histology of a skin biopsy from the left arm revealed interface dermatitis and eosinophil infiltrates. Hydroxychloroquine was discontinued from the day of admission, and the patient was initially started on treatment with intravenous prednisolone 62.5 mg once daily that was gradually reduced over a 6‐week period. At the time of discharge, 4 weeks later, most of the lesions had subsided and after 8 weeks, her skin appeared normal.
Figure 1.
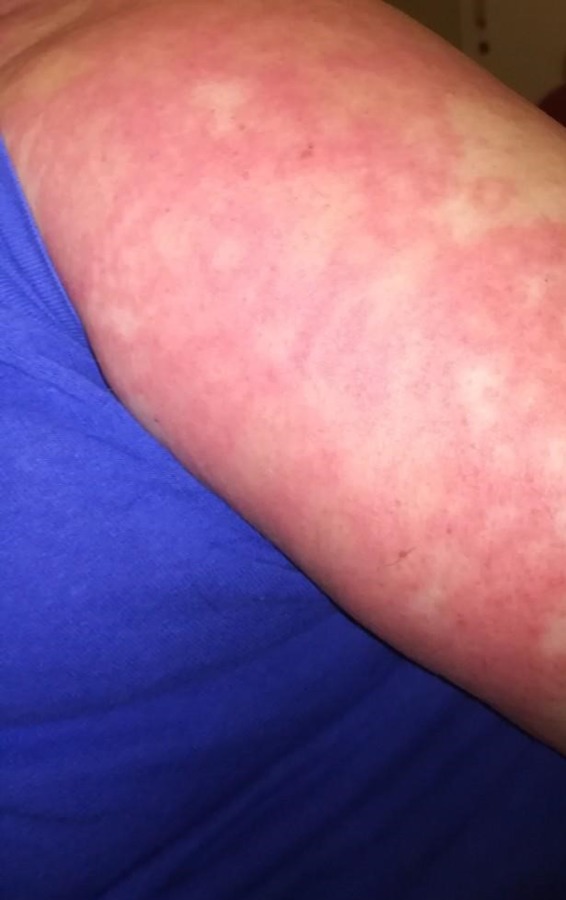
Typical targetoid lesions on the right arm
Figure 2.
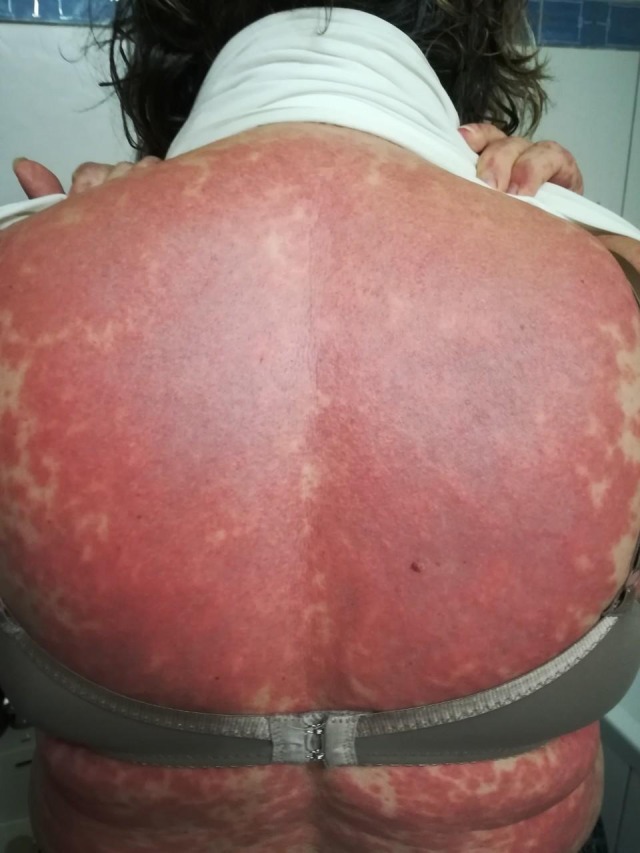
Widespread violaceous to erythematous plaques on the trunk of the patient
1.1. What is your diagnosis?
Hydroxychloroquine‐induced erythema multiforme.
2. DISCUSSION
Severe cutaneous reactions to hydroxychloroquine are uncommon1, 2 However, as in this case, drug hypersensitivity reactions often manifest in skin. Because of the extensive use of HCQ in the treatment of numerous dermatologic and rheumatologic conditions, because no specific therapy is available, and because correct diagnosis generally leads to spontaneous resolution once the causative drug is withdrawn, clinicians should keep the possibility of this rare but severe, extensive, and acute reaction in mind.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
DK: did substantial contributions to conception and design, acquisition, analysis, and interpretation of data, was involved in drafting the manuscript and revising it critically for important intellectual content, and gave final approval of the version to be published. VK, GB, SB, AK, MS, OM, GE, KZ, SEKK, and KK: were involved in drafting the manuscript, revising it critically for important intellectual content, and gave final approval of the version to be published.
CONSENT STATEMENT
Informed written consent was obtained from the patient for publication of her images.
Koumaki D, Koumaki V, Bertsias G, et al. Hydroxychloroquine‐induced erythema multiforme. Clin Case Rep. 2020;8:578–579. 10.1002/ccr3.2648
REFERENCES
- 1. Shipman WD, Vernice NA, Demetres M, Jorizzo JL. An update on the use of hydroxychloroquine in cutaneous lupus erythematosus: a systematic review. J Am Acad Dermatol. 2019;S0190‐9622(19):32387‐32394. [DOI] [PubMed] [Google Scholar]
- 2. Abou Assalie N, Durcan R, Durcan L, Petri MA. Hydroxychloroquine‐induced erythema multiforme. J Clin Rheumatol. 2017;23:127‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]


