Abstract
Primary central nervous system (CNS) marginal zone B‐cell lymphoma (MZBCL) arising from the dural meninges is a rare but indolent disease. This malignancy can present in various ways, hence making it difficult to diagnose. Biopsy results dictate an appropriate treatment plan, which commonly consists of a combination of surgical resection, whole brain radiotherapy and systemic therapy.
Keywords: central nervous system lymphomas, dural lymphoma, marginal zone B‐cell lymphoma, meningeal lymphoma
Primary central nervous system (CNS) marginal zone B‐cell lymphoma (MZBCL) arising from the dural meninges is a rare but indolent disease. This malignancy can present in various ways, hence making it difficult to diagnose. Biopsy results dictate an appropriate treatment plan, which commonly consists of a combination of surgical resection, whole brain radiotherapy and systemic therapy.

1. INTRODUCTION
Marginal zone B‐cell lymphoma (MZBCL) of the dura can present with a variety of neurological symptoms. Diagnostic imaging is often misleading, as MZBCL can resemble other pathological conditions such as subdural hematomas or meningiomas. Treatment is tailored toward immunohistological findings on biopsy. Given the paucity of cases, there is no standard approach to management. Varying combinations of surgical resection, radiation, and chemotherapy have shown favorable rates of response. If cells expressing CD20 + are present, rituximab as a single agent may be sufficient for treatment, although the addition of whole brain radiation therapy (WBRT) should also be considered. The objective herein is to outline a case of a primary central nervous system (CNS) marginal zone B‐cell lymphoma (MZBCL) arising from the dural meninges in a patient who presented with stroke‐like symptoms, in order to describe the clinical and biological features of primary dural lymphoma in this context. In this case, initial imaging was consistent with what was thought to be an acute subarachnoid hemorrhage (SAH) (Figure 1). However, surgical biopsy demonstrated MZBCL, and further immunehistochemistry studies revealed mainly CD20 + B cells and no co‐expression of BCL6.A review of lymphomas involving the CNS including their clinical features, radiographic findings, and immunohistology, as well as our management plan for this case, is provided throughout the manuscript. Patients with MZBCL of the dura can present with various complications, including headaches, seizures, dis‐coordination, weakness, or numbness. Some cases of MZBCL have mimicked the appearance of subdural hematomas1, 2 and meningiomas3, 4 on imaging tests. Biopsy is required for diagnosis, with the utmost importance for further immunohistochemical analysis, in order to tailor treatment directed against this malignancy.
Figure 1.
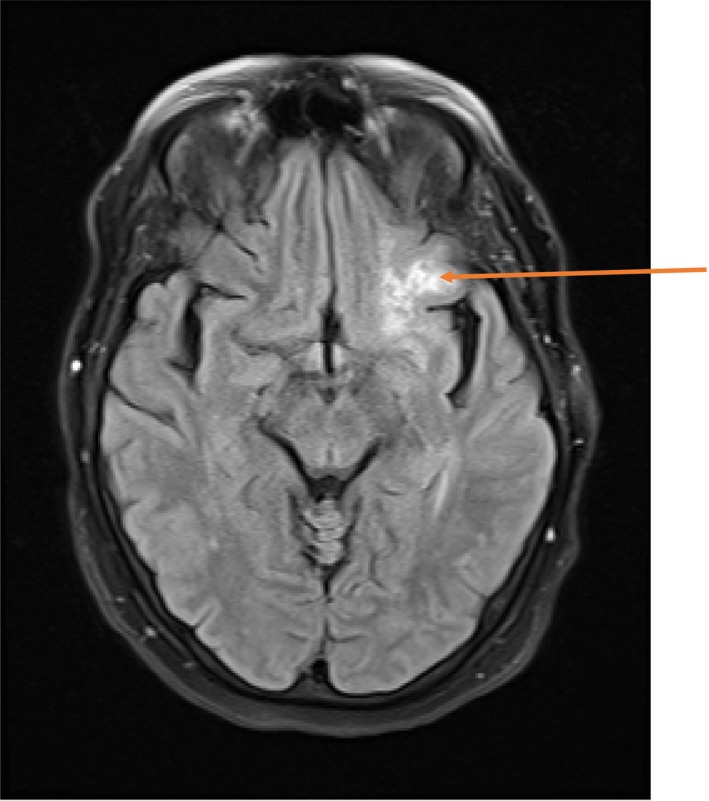
On FLAIR, there is curvilinear hyperintensity in the left central sulcus (orange arrow) suggesting subarachnoid hemorrhage or given history of sarcoidosis, focal meningeal neurosarcoidosis, or possibly leptomeningeal spread of malignancy
Review of literature demonstrates very few cases reported of marginal zone B‐cell lymphomas (MZBCL) involving the dura. It is an uncommon subset of primary central nervous system (CNS) lymphoma.5 MZBCL appears to arise from the meninges, thus contributing to its common misdiagnosis as a meningioma.6 Absence of disease outside of the CNS is a major detail within the clinical definition of primary CNS lymphoma. Within the 2016 classification revisions of lymphoid neoplasms by the World Health Organization (WHO) is a type of an entity classified as mature B‐cell neoplasm, called extranodal marginal zone lymphoma of mucosa‐associated lymphoid tissue (MALT lymphoma).7 MZBCL is encompassed within this subset of lymphoid malignancies.7
It is a low‐grade, indolent disease, which classically lacks invasion of the brain parenchyma. MZBCL is further characterized by responsiveness to treatment and overall favorable prognosis.8, 9
2. CASE REPORT
A 72‐year‐old woman with a medical history significant for sarcoidosis, paroxysmal atrial fibrillation, on apixaban, obstructive sleep apnea, hypertension, hyperlipidemia, and non‐insulin–dependent type 2 diabetes mellitus, presented to the emergency department with sudden onset involuntary movements of her right arm and hand, which was followed by acute onset left‐sided facial droop, dysarthria, and expressive aphasia.
Initially, stroke was suspected; therefore, she underwent computed tomography (CT) imaging of the head without contrast. This showed a curvilinear hyperdensity in the left central sulcus region, left subinsular region, and inferior frontal operculum worrisome for an acute subarachnoid hemorrhage (SAH). CT angiography of the head and neck did not reveal overt vascular abnormalities. Further investigation with angiograms of the carotid and vertebral arteries successfully confirmed the absence of vascular lesions or aneurysms that may have accounted for hemorrhage. Noted in particular, there was no active extravasation of contrast evident during the study. Magnetic resonance imaging (MRI) of the brain showed curvilinear hyperintensity filling along the left central sulcus without evidence of acute blood within the sulcus (Figure 1). The MRI also revealed a second area of focal parenchymal abnormality located anterior to the left frontal horn, with subtle mass effect and associated vasogenic edema. Cerebrospinal fluid (CSF) analysis from lumbar puncture did not reveal overt xanthochromia. Given these findings, intracerebral hemorrhage was felt less likely to be the cause of the patient's presentation, thus raising concern for an underlying inflammatory or neoplastic process.
Due to concerns of an underlying malignancy, the decision was made to pursue a left frontal craniectomy with stereotactic biopsy of the abnormal leptomeningeal region within the left frontal lobe. Preliminary results from pathology revealed lymphocytic infiltration of the meninges, suggestive of either a lymphoma or inflammatory process. Final pathological results with molecular analysis supported a diagnosis of B‐cell lymphoma. The lymphocyte population was comprised of mainly of CD20 + B cells (Figure 2) and few scattered CD3 + T cells (Figure 3). The B cells had no co‐expression of CD5, CD10, nor BCL6. Studies also showed that they were negative for CD43 and TdT. The proliferation marker MIB1 was positive in <10% of lymphocytes, which was indicative of a low proliferation index. Cyclin D1 and in situ hybridization for EBER were negative, while PCR studies were positive for clonal rearrangement of IgH and Kappa light chain.
Figure 2.

CD20 is a B‐cell marker and highlights the prominent B‐cell population
Figure 3.
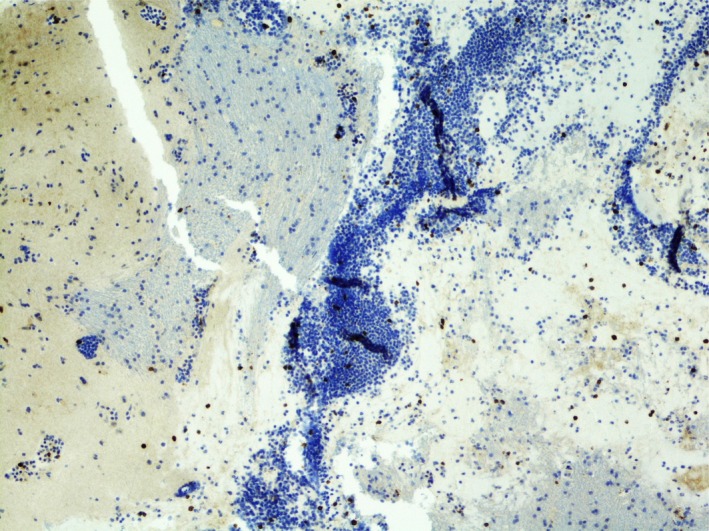
CD3 is a T‐cell marker and most of the lymphocytes are negative
After medical optimization, the patient was discharged home on dexamethasone and antiseizure prophylaxis, with instructions to continue care for her lymphoma with hematology/oncology as an outpatient. During follow‐up, a whole‐body fludeoxyglucose positron emission tomography (FDG PET) scan showed no evidence of FDG avidity nor pathologic lymphadenopathy outside of the CNS. The patient also underwent bone marrow aspirate and biopsy which effectively ruled out bone marrow involvement from her lymphoma. She then began treatment with whole brain radiation therapy (WBRT), completing a total dose of 2400 cGy over 12 fractions. Radiation therapy was well tolerated and was accompanied by several doses of memantine to mitigate possible neurocognitive effects. Following WBRT, the patient developed significant fatigue, ageusia, and episodic headaches without neurological deficits. Nevertheless, she subsequently began systemic treatment with single‐agent rituximab, 375 mg/m2 once weekly, for a total duration of 4 weeks.
Surveillance brain MRI demonstrated persistent enhancement of the central sulcus as well as FLAIR hyperintensity of the orbitofrontal region, despite unequivocal improvement. Findings suggested a low level of residual disease. White matter FLAIR hyperintensities that were new were thought to be radiation induced.
A few months after completing treatment, the patient returned to the hospital with confusion, weakness, and malaise. She underwent initial evaluation with CT of the head which showed no acute intracranial abnormalities to explain her symptoms. An electroencephalogram showed absence of seizure‐like activity. After an extensive workup, her symptoms were ultimately believed to be attributable to polypharmacy from her multitude of home medications.
A repeat MRI of the brain performed 10 months after completion of systemic treatment remained unchanged as compared to prior MRI, and with no evidence of active intracranial disease.
The patient continues to be followed regularly in the outpatient setting with medical oncology and neurosurgery. She has shown no clinical evidence of disease recurrence to date, having completed therapy greater than 12 months ago.
3. DISCUSSION
Primary lymphomas of the CNS (PCNSL) are a rare, aggressive type of extranodal non‐Hodgkin's lymphoma (NHL) with overall poor prognosis. PCNSL exclusively affects the CNS brain parenchyma, meninges, spinal cord, cranial nerves, and intraocular structures. PCNSL represent 3% of intracranial neoplasms in immunocompetent patients.10 A relatively higher incidence is observed among those who are immunocompromised, most commonly reported within groups of patients with acquired immune deficiency syndrome (AIDS) as well as solid organ transplant recipients.11 Further classification shows that MZBCL of the dura is a very uncommon subset of primary CNS lymphoma. Only 10% of all dural lymphomas are estimated to be low‐grade lymphomas such as MZBCL.
Central nervous system involvement from lymphoma arising elsewhere in the body is exceedingly rare, a condition termed secondary CNS lymphomas (SCNSL). SCNSL most commonly affects the leptomeninges, accounting for 60% of cases; however, cases of brain parenchymal as well as ophthalmic involvement have been reported. As with most other metastatic cancers, it is uncommon to see solitary isolated brain parenchymal lesions from SCNSL.10
Immunohistopathological diagnosis via brain biopsy is fundamental to establish the diagnosis of PCNSL. In selected cases, CSF cytology with flow cytometry, vitrectomy, or chorioretinal biopsy may be sufficient for diagnosis.12 PCNSL and SCNSL share a similar histology, thus making it difficult to establish a standardized method to distinguish between these two entities based on biopsy findings alone. It is thought that microRNA expression profiles obtained from CSF samples may be a useful biomarker, but this is not yet standard of care.10 Further investigation via molecular analysis is pertinent for diagnosing the subtype of PCNSL, as well as determining next steps in management.
The majority of primary intracranial lymphomas are known to be aggressive lymphomas, most commonly diffuse large B‐cell lymphomas (DLBCL).13 Most PCNS DLBCL have a postgerminal center or an activated B‐cell immunophenotype. They express CD19, CD20, and CD79a antigens. The prognostic importance of other biomarkers such as B‐cell CLL/lymphoma 6 (BCL6) and 2 (BCL2), melanoma‐associated antigen (mutated) 1 (MUM1)/interferon regulatory factor 4 (IRF4) remains unclear, but they are reported to be positive in many cases.14
Treatment strategies for PCNSL continue to be explored. Newer studies have demonstrated that high‐dose methotrexate (MTX)‐based induction chemotherapy combined with other chemotherapeutic agents is highly effective, which has now become the standard of care in newly diagnosed PCNSL.11 Intrathecal chemotherapy (ie, methotrexate or cytarabine) has also been studied for treatment of PCNSL as well as leptomeningeal carcinomatosis prophylaxis, but results regarding its benefit remain unclear.11 In addition to evaluating treatment strategies for PCNSL, current studies show that administration of two or more cycles of induction chemotherapy with high‐dose MTX and/or cytarabine might be beneficial for the treatment of SCNSL.
Targeted therapies, such as rituximab, which have been proven to cross the blood‐brain barrier should be implemented given their proven benefit in clinical outcomes.15 A study performed by Ruhstaller et al measured rituximab concentrations in the CSF and compared it to blood concentrations after its infusion. They concluded that rituximab has adequate blood‐brain barrier (BBB) penetration and remains active within the CNS.16 Other studies also support rituximab penetration within CNS: On one hand due to the fact that some tumors may cause blood‐barrier disruption, thus increasing antibody delivery to CNS, and on the other hand, rituximab has a long plasma half‐life and binds and accumulates in target B cells.17
Primary lymphomas of the CNS are known to be radiosensitive; however, WBRT alone is insufficient to adequately control the disease.11 Many patients, especially the elderly, are affected by neurotoxicity, to the point where cognitive impairment may be severe. Studies suggest that avoiding WBRT in the initial treatment phase of PCNSL may be an acceptable option, as not receiving WBRT may not reduce overall survival.11 There are ongoing trials evaluating the usefulness of rituximab and consolidative treatment with chemotherapy, with or without autologous stem cell transplantation, or low‐dose WBRT in patients with PCNSL.11
Primary dural lymphoma (PDL) is a rare subtype of PCNSL that originates in the dura, can involve the epidural or subdural space, and does not infiltrate the brain parenchyma.18 This explains its predilection for areas with large amounts of meningeal cells. In fact, 62.5% of cases involve the leptomeninges.8 Despite some published case reports and case series on PDL, the precise incidence of PDL is unknown.8
The main characteristics and differences between primary and secondary CNS lymphomas, in contrast to primary dural lymphomas, can be found in Table 1.
Table 1.
Differences between primary, secondary CNS lymphomas, and primary dural lymphomas
| PCNSL | SCNSL | PDL | |
|---|---|---|---|
| Types |
90% of intracranial lymphomas are aggressive, most commonly DLBCL 10% of all dural lymphomas are low‐grade lymphomas, lymphoblastic, T‐cell, Burkitt's lymphomas, and intraparenchymal marginal zone lymphoma |
Involves leptomeninges (60% of cases), the brain parenchyma, and few cases involve the eyes |
It is a rare subtype of PCNSL Originates in the dura matter and can involve the epidural or subdural space. Does not affect the brain parenchyma |
| Incidence |
Represents 2% of primary CNS tumors in the United States 30 Annual incidence in the US is approximately 1400 new cases each year 30 |
Occurs in <1% of indolent and <5% of aggressive systemic lymphomas when CNS prophylaxis is given 32 May occur in up to 50% of cases of Burkitt's/lymphoblastic lymphoma or AIDS‐related lymphoma when no prophylaxis is given 32 |
Unknown. There are only case reports/case series published |
| Diagnosis |
On imaging, solitary mass is seen in 70% of cases, commonly in the supratentorial region, with a tendency to affect the periventricular white matter In immune‐suppressed patients, multiple lesions are seen twice as often |
Neither MRI nor histology itself is able to distinguish between PCNSL and SCNSL. Systemic lymphoma must be excluded in the case of all histologically proven brain lymphoma lesions |
Either a single or multiple extra‐axial lesions that are diffusely enhancing are seen on imaging. 95% of images revealed a dural tail |
| Characteristics | Immunocompetent patients with DLBCL have secondary involvement of the CNS in 1%‐10% of the cases | Extranodal lymphoma, especially involvement of the kidneys and/or adrenal glands, testes, and female reproductive tract, as well as extensive marrow involvement, has been demonstrated to be a site‐specific additional risk factor for SCNSL |
More common in women Symptoms are nonspecific: headaches, meningeal irritation, seizures or epilepsy, scalp swelling, and symptoms of cranial nerve involvement |
| IHC staining |
Most PCNS DLBCL express CD19, CD20, and CD79a antigens. 10% ‐ 20% are CD10+, and 50% ‐ 80% are BCL6 and BCL2 positive 95% stain positive for MUM‐1 PCNSL shows somatic hypermutation of genes such as BCL6, MYC, PIM1, and PAX5 31 |
Unclear |
Immunological staining is generally positive for CD20, CD22, CD19 and CD79a and PAX‐5. Tumor cells are positive for BCL2 50% of cases are CD43 positive |
| Treatment |
High‐dose methotrexate (MTX)‐based induction chemotherapy combined with other chemotherapeutic agents It is known to be radiosensitive |
High‐dose chemotherapy with autologous stem cell transplant is feasible and effective Two or more cycles of induction chemotherapy (high‐dose MTX and/or cytarabine) oral targeted therapies or therapies such as rituximab have shown promising results |
Most patients undergo surgical treatment, radiotherapy or chemotherapy; or a combination thereof. >50% patients undergo surgical resection. In single site disease, surgery + focal relatively low‐dose radiotherapy In systemic disease, R‐CHOP + maintenance Rituximab |
| Prognosis | Aggressive behavior and overall poor outcome | Very poor prognosis |
Indolent disease with a good prognosis PDLs have a 5‐year overall survival rate is >86% |
Primary dural lymphoma has been described as a low‐grade MZBCL, whereas most other types of CNS lymphomas are high‐level grade such as DLBCL. The 2016 WHO lymphoma classification recognizes three different subcategories of marginal zone lymphomas (MZL): marginal zone lymphoma of mucosa‐associated lymphoid tissue (MZL‐MALT), splenic marginal zone lymphoma (SMZL), and nodal marginal zone lymphoma (NMZL); MZL‐MALT is noted for being the most represented type among the three 3, whereas NMZL is the least.8
Mucosa‐associated lymphoid tissue lymphomas are a rare type of low‐grade B‐cell neoplasms that occur in a variety of extranodal sites. They are seen mainly in individuals between ages 40 and 50, and are four to five times more predominant in women as compared to men.19, 20 These tumors commonly present with nonspecific symptoms including headaches, meningeal irritation, seizures, scalp swelling, and signs of cranial nerve involvement.8
They can sometimes occur in nonlymphatic organs that contain small lymphoid tissue clusters in mucosal and nonmucosal layers, such as the dura mater, skin, or orbital tissues.19 MALT lymphoma has also been described to involve the lung, bladder, salivary glands, conjunctiva, and lacrimal glands.20
The CNS is nearly absent of mucosa‐associated lymphoid tissue.9 However, it has been hypothesized that there are biological similarities between the arachnoid membrane meningothelial cells and epithelial cells in tissues from which MALT lymphomas typically arise, thus providing a possible explanation for how MALT lymphomas present within the CNS.21, 22
When MALT lymphomas involve the CNS, they are most commonly located within the dura mater. They are frequently described on diagnostic imaging as being either dural‐based or leptomeningeal, without any evidence of brain parenchymal infiltration or systemic dissemination.19 These malignancies can appear on imaging as either single or multiple extra‐axial lesions that are diffusely enhancing, with 95% of studies reporting a dural tail.18
Not surprisingly, due to the similar radiological findings between MALT lymphomas and meningiomas, many cases are initially mistaken for the latter and are less frequently identified as a subdural hematoma.1, 23, 24 Erroneous diagnoses can delay adequate treatment when isolated PDLs are mistaken for meningiomas.24 Yet, the diagnosis remains challenging. Epidural hematoma, meningioma, hemangiopericytoma, meningeal metastasis, and meningeal sarcoma should always be within the differential diagnosis when there are equivocal findings on initial imaging. Ferguson et al suggest ways to distinguish meningiomas from MALT lymphomas based on an assessment of the malignancy's location and behavior, as lymphomas tend to localize within the dural margin and infiltrate, into the skull base foramina as well as spaces outside of the cranial vault.25 Other differential diagnoses of dural‐based lesions are complex, including neurosarcoidosis, aspergillosis, dural metastasis, postradiation or postsurgical dural reaction, chloromas, and schwannomas.
Interestingly, studies have demonstrated that patients with sarcoidosis have a higher incidence of lymphomas, a disease manifestation termed sarcoidosis‐lymphoma syndrome.26
Radiographic imaging is not sufficient to reach a diagnosis. Clinical information such as the patient's age, personal medical history, and identification of risk factors for lymphoma is increasingly important. Other diagnostic considerations include metastases, and inflammatory or infectious granulomatous disease such as Wegener's disease, Rosai‐Dorfman disease, syphilis, tuberculosis, or other secondary lymphoproliferative lesions.19 For example, dural MALT lymphomas have been associated with chronic inflammatory states such as hepatitis C and autoimmune diseases.9, 25
A confirmatory diagnosis is obtained through pathological analysis via histology and/or cytology.9 Neoplastic cells spread diffusely and are found adjacent to the meningeal tissue. Cells can appear as monocytoid with occasional differentiation into plasmacytic cells and less commonly large lymphoid cells. These cells have a low proliferation index, corresponding with an indolent course.18 Immunological staining is generally positive for CD20, CD22, CD19 and CD79a and PAX‐5. Tumor cells are positive for BCL2, and half of the cases are CD43 positive.14
Primary dural lymphoma usually have a very favorable prognosis as compared to PCNSL with parenchymal involvement, or systemic lymphoma with CNS metastasis. PDL is very seldom the first identifying lesion that leads to the diagnosis of systemic lymphoma. PDL has a 5‐year overall survival rate of >86%.18 Regardless, an optimal therapeutic approach has yet to be established. This is due to the paucity of cases, and thus, there remains a lack of large, randomized controlled trials available.25 Therefore, most patients undergo either surgical treatment, radiation therapy, chemotherapy, or various combinations of these treatment modalities without an established standardized approach.
Important to note, more than half of the patients are treated with surgical resection, as these lesions are often mistaken for a meningiomas. When there is a single site of disease involvement, surgery followed by focal low‐dose radiotherapy has shown excellent response rates, as MZBCL have proven to be radiosensitive.4
The exact utility of chemotherapy in reducing risk of disease recurrence remains uncertain. However, cases of patients who have systemic disease treated with combination rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone (R‐CHOP) followed by maintenance rituximab demonstrate an improvement in outcomes.8 Rituximab is commonly used for the treatment of systemic marginal zone lymphoma. However, analysis of its efficacy for the treatment of dural‐based MZBCL is limited.20
High‐dose methotrexate followed by WBRT carries a high risk of cognitive impairment, extensive neurotoxicity, and development of progressive leukoencephalopathy. Therefore, this treatment regimen should be avoided in cases of PDL, particularly when other in less invasive treatment options are highly effective.11 Some experts opt for surgical resection, followed by chemotherapy with rituximab and bendamustine, excluding the use of radiation therapy.20
Regardless of therapeutic approach, long‐term active surveillance is required, given that patients with PDL are at an increased risk for disease recurrence years after the initial diagnosis and treatment.
Current treatment options for early‐stage nodal MZL and localized extranodal MALT lymphoma include surgical resection, radiation therapy, and single‐agent immune or targeted therapy (eg, ibrutinib).27, 28
In cases of MALT lymphoma, the frequency of methylated genes increases as throughout disease progression.29 Interestingly, hypomethylating agents have not been extensively studied within this clinical context. Those with MALT lymphoma not responding to or not eligible for locally directed therapy have been effectively treated with the use of rituximab. For cases of resistance to rituximab, a secondary anti‐CD20 + agent, obinutuzumab, can be used as an alternative therapy.28
As our understanding of the molecular pathways involved in pathogenesis continues to grow, so will the development of more specific and effective therapies targeted against these malignancies.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
NL‐L: contributed to preparation, creation, and/or presentation of the published work, specifically writing the initial draft, and literature review. LD, SDA, and MK: contributed to critical review, commentary, or revision. JS.H: contributed to preparation, creation, and/or presentation of the published work, specifically writing the initial draft.
Lopetegui‐Lia N, Delasos L, Asad SD, Kumar M, Harrison JS. Primary central nervous system marginal zone B‐cell lymphoma arising from the dural meninges: A case report and review of literature. Clin Case Rep. 2020;8:491–497. 10.1002/ccr3.2680
REFERENCES
- 1. Gocmen S, Gamsizkan M, Onguru O, Sefali M, Erdogan EJ. Primary dural lymphoma mimicking a subdural hematoma. Clin Neurosci. 2010;17(3):380‐382. [DOI] [PubMed] [Google Scholar]
- 2. Goetz P, Lafuente J, Revesz T, Galloway M, Dogan A, Kitchen NJ. Primary low‐grade B‐cell lymphoma of mucosa‐associated lymphoid tissue of the dura mimicking the presentation of an acute subdural hematoma. Case report and review of the literature. Neurosurg. 2002;96(3):611‐614. [DOI] [PubMed] [Google Scholar]
- 3. Pavlou G, Pal D, Bucur S, Chakrabarty A, van Hille PT. Intracranial non‐Hodgkin's MALT lymphoma mimicking a large convexity meningioma. Acta Neurochir (Wien). 2006;148(7):791‐793. [DOI] [PubMed] [Google Scholar]
- 4. Villeneuve A, Rubin F, Bonfils P. Meningeal marginal zone B‐cell lymphoma: the meningioma trap. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(2):131‐132. [DOI] [PubMed] [Google Scholar]
- 5. Eichler AF, Batchelor TT. Primary central nervous system lymphoma: presentation, diagnosis and staging. Neurosurg Focus. 2006;21(5):E15. [DOI] [PubMed] [Google Scholar]
- 6. Tu PH, Giannini C, Judkins AR, et al. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low‐grade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005;23:5718‐5727. [DOI] [PubMed] [Google Scholar]
- 7. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de la Fuente MI, Haggiagi A, Moul A, et al. Marginal zone dural lymphoma: the memorial sloan kettering cancer center and University of Miami experiences. Leuk Lymphoma. 2017;58(4):882‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayanambakkam A, Ibrahimi S, Bilal K, Cherry MA. Extranodal marginal zone lymphoma of the central nervous system. Clin Lymphoma Myeloma Leuk. 2018;18(1):34‐37.e8. [DOI] [PubMed] [Google Scholar]
- 10. Baraniskin A, Chomiak M, Ahle G, et al. MicroRNA‐30c as a novel diagnostic biomarker for primary and secondary B‐cell lymphoma of the CNS. J Neurooncol. 2018;137(3):463‐468. [DOI] [PubMed] [Google Scholar]
- 11. Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han CH, Batchelor TT. Primary CNS lymphoma. Continuum (Minneap Minn). 2017;23(6):1601‐1618. [DOI] [PubMed] [Google Scholar]
- 13. Shenkier TN, Blay JY, O'Neill BP, et al. Primary CNS lymphoma of T‐cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol. 2005;23:2233‐2239. [DOI] [PubMed] [Google Scholar]
- 14. Sahm F, Reuss DE, Giannini CWHO. 2016 classification: changes and advancements in the diagnosis of miscellaneous primary CNS tumours. Neuropathol Appl Neurobiol. 2018;44(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 15. Schmitz N. Wu HS advances in the treatment of secondary CNS lymphoma. J Clin Oncol. 2015;33(33):3851‐3853. [DOI] [PubMed] [Google Scholar]
- 16. Ruhstaller TW, Amsler U, Cerny T. Rituximab: active treatment of central nervous system involvement by non‐Hodgkin's lymphoma? Ann Oncol. 2000;11(3):374‐375. [DOI] [PubMed] [Google Scholar]
- 17. Lampson A. Monoclonal antibodies in neuro‐oncology. mAbs. 2011;3(2):153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lv ZW, Cheng KL, Tian HJ, Han XM. Primary diffuse large B‐cell lymphoma of the dura with skull and scal involvement: a case report and brief review of literature. Oncol Lett. 2016;11(6):3583‐3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sebastián C, Vela AC, Figueroa R, Marín MÁ, Alfaro J. Primary intracranial mucosa‐associated lymphoid tissue lymphoma. A report of two cases and literature review. Neuroradiol J. 2014;27(4):425‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okimoto RA, Perry A, Rubenstein JL. 77‐year‐old woman with a dural‐based mass. Marginal zone B‐cell lymphoma (MZBCL). Brain Pathol. 2015;25(1):111‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar S, Kumar D, Kaldjian EP, et al. Primary low‐grade B‐cell lymphoma of the dura: a mucosa associated lymphoid tissue‐type lymphoma. Am J Surg Pathol. 1997;21:81‐87. [DOI] [PubMed] [Google Scholar]
- 22. Park I, Huh J, Kim JH, Lee SW, Ryu MH, Kang YK. Primary central nervous system marginal zone B‐cell lymphoma of the basal ganglia mimicking low‐grade glioma: a case report and review of the literature. Clin Lymphoma Myeloma. 2008;8(5):305‐308. [DOI] [PubMed] [Google Scholar]
- 23. Spina V, Rossi D. Molecular pathogenesis of splenic and nodal marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30(1‐2):5‐12. [DOI] [PubMed] [Google Scholar]
- 24. Partovi S, Karimi S, Lyo JK, Esmaeili A, Tan J, Deangelis LM. Multimodality imaging of primary CNS lymphoma in immunocompetent patients. Br J Radiol. 2014;87:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferguson SD, Musleh W, Gurbuxani S, Shafizadeh SF, Lesniak MS. Intracranial mucosa‐associated lymphoid tissue (MALT) lymphoma. J Clin Neurosci. 2010;17(5):666. [DOI] [PubMed] [Google Scholar]
- 26. Yang K, Algird AR, Lu JQ. Dural‐based marginal zone lymphoma in a patient with sarcoidosis. World Neurosurg. 2019;122:569‐572. [DOI] [PubMed] [Google Scholar]
- 27. Tadmor T, Polliack A. Nodal marginal zone lymphoma: clinical features, diagnosis, management and treatment. Best Pract Res Clin Haematol. 2017;30(1‐2):92‐98. [DOI] [PubMed] [Google Scholar]
- 28. Zinzani PL, Broccoli A. Possible novel agents in marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30(1‐2):149–157. [DOI] [PubMed] [Google Scholar]
- 29. Arribas AJ, Bertoni F. Methylation patterns in marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30(1‐2):24‐31. [DOI] [PubMed] [Google Scholar]
- 30. Lukas RV, Stupp R, Gondi V, Raizer JJ. Primary central nervous system lymphoma‐PART 1: epidemiology, diagnosis, staging, and prognosis. Oncology (Williston Park). 2018;32(1):17‐22. [PubMed] [Google Scholar]
- 31. Rubenstein JL, Gupta NK, Mannis GN, LaMarre AK, Treseler P. How i treat CNS lymphomas. Blood. 2013;122(14):2318‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;12(14):733‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]


