Abstract
Rearing insects is expected to dramatically increase during the next few years, and this will be associated with generating high quantities of frass (insect excreta). It is necessary to find solutions allowing the efficient valorization of these by-products before a major upscaling of the industry takes place. Therefore, this study aims at investigating the fertilizer potential of frass. A pot experiment was established and soil was amended either with mealworm (Tenebrio molitor L.) frass (10 Mg ha−1), with mineral fertilizer (NPK) at equivalent nutrient level to frass or with a mixture of 50% NPK and 50% frass. Changes of soil properties and growth and nutrient uptake by barley (Hordeum vulgare L.) were then analyzed. Due to its rapid mineralization and the presence of nutrient in a readily-available form, we found that frass is as efficient as mineral NPK fertilizer to improve biomass and N, P and K uptake by barley. Compared to mineral fertilizer, water soluble P concentration is five times lower in the presence of frass, which prevents P from loss and sorption onto soil constituents. More importantly, BIOLOG EcoPlate reveals that addition of frass stimulates soil microbial activity, especially when it is mixed with mineral fertilizer, suggesting a synergistic effect between both amendments. Taken together, our results indicate that frass has a great potential to be used as a partial or a complete substitute for mineral NPK fertilizer. This is especially relevant in the context of a reduced availability of mineral fertilizers while being consistent with circular economy’s principles.
Subject terms: Agroecology, Element cycles, Biogeochemistry
Introduction
Meeting an ever increasing food demand while reducing agriculture’s negative environmental impact is one of the greatest challenges of the twenty-first century1,2. Societal transition and industrial transformation are developing to ensure sustainable food production for 2.3 billion more people in the next four decades. With the demand for nutritionally crucial proteins and meat products which is expected to increase from current levels by more than 75% in 2050, we need to search for alternatives to conventional protein sources if we want to restrict agricultural land use and avoid biodiversity losses and environmental degradation3,4. The use of natural resources must also be reduced by adopting an eco-industrial development approach which aims at closing economic and ecological loops of resource flows5.
Rearing insects for mass consumption is increasingly sparking interest due to their high nutritional value and, most importantly, their resource efficiency when converting organic matter, especially waste, into protein6–9. As recently reviewed by Dicke10, insect production has a much smaller ecological footprint in terms of land and water use and greenhouse warming potential compared to the production of chicken, pigs and cattle which is related to its much lower feed to meat conversion ratio. In addition to be an alternative source of proteins for humans and animals, insect farming has also been considered to produce biofuel, some rare lipids and chitin, which has a great economical value due to its application in food, cosmetics, pharmaceuticals, textile etc.11.
There have been major efforts to promote the use of insects as a protein source, spurred in large part by the Food and Agriculture Organization (FAO) of the United Nations work examining the multiple dimensions of insect rearing as an important future food source and a viable option for alleviating food insecurity12. In European Union, novel food regulation 2015/2283 (in application since 1st January 2018) clarified the legal status of insects and their derived products. Several EU members (e.g. Austria, Belgium, Denmark, Finland and Netherlands) now authorize companies to produce and sell insects as food while others (France and Germany) have partially legalized their production and commercialization13. These incentive policies have boosted the implementation of insect producers. For example, Ÿnsect, a French insect farming startup, has recently raised $125 million in Series C funding to develop their activities, which is the largest early-stage agtech funding deal on record in Europe (https://agfunder.com/). Overall, in EU, it is expected that the number of jobs generated by the development of this sector will increase from a few hundreds to a few thousands by 202514.
Despite being highly efficient in converting biowaste into biomass, insect production itself also yields a waste stream consisting in moulting skins (exuviae) and, more importantly, insect faeces (“frass”)10. In natural conditions, it is well known that frass deposition to soil has a great impact on soil fertility due to its high nutrient and labile C content15–17. Therefore, several companies have already decided to sell frass as a fertilizer10,18. Even though some farmers have reported beneficial effects of frass to plants19, there is however currently very limited information on the ability of frass produced by insect farms to improve soil fertility and, ultimately, plant growth20. As stressed by Berggren et al.3, the fertilizer potential of frass needs thus urgent research before a major upscaling of the industry takes place. This would be also relevant given the urgent need to find cost-effective and environmental-friendly alternatives to conventional mineral fertilizers whose the production relies on fossil fuels and finite resources21–23.
Here, we evaluate the potential of frass from mealworm (Tenebrio molitor L.) as a fertilizer by investigating its impact on nutrient availability, soil biological activity and crop (barley; Hordeum vulgare L.) growth.
Results and Discussion
Frass characterization
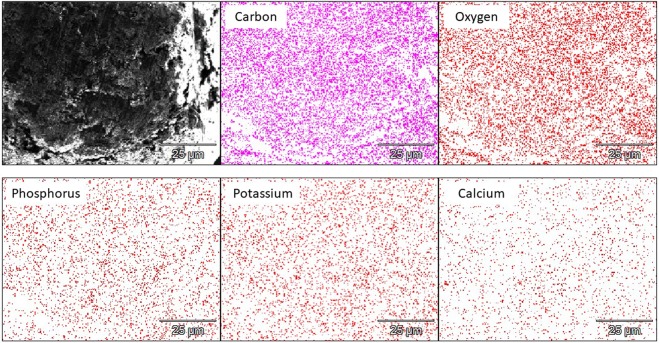
Chemical characterization of frass (Table 1) revealed that it had concentrations of N, K and P as high as those found in farmyard manure and, especially, poultry manure24, which confirms its high fertilizer potential. By contrast to conventional mineral fertilizer, frass also contained small concentrations of micronutrients (i.e. Cu and Zn), which may be further beneficial for crops. Moreover, SEM image of frass (Fig. 1) revealed that frass had a layered structure while EDS mapping (Fig. 2) showed a uniform distribution of nutrients (P, K and Ca) within the frass organic matter (represented by the C and O maps), suggesting the absence of isolated mineral phases which might potentially drive nutrient release by frass. Thus, the release of nutrients by frass after its incorporation into soil should be homogenous and possibly more extended than fertilizers in which nutrients are unevenly distributed25. The C fractionation in frass (Table 1) was also similar to that of poultry manure26, with high soluble C fraction and low cellulose-like and lignin-like fractions. This high fraction of labile C is in agreement with previous findings and may be related to the high digestion of cellulose and lignin compounds by mealworm larvae27,28.
Table 1.
Chemical characteristics of frass.
| Organic C g kg−1 | Total N g kg−1 | Total K g kg−1 | Total P g kg−1 | Total Cu mg kg−1 | Total Zn mg kg−1 |
|---|---|---|---|---|---|
| 393 | 50 | 17 | 20 | 10 | 94.2 |
| pH | EC dS m−1 | Soluble fraction %Corg | Hemicellulose-like fraction %Corg | Cellulose-like fraction %Corg | Lignin-like fraction %Corg |
| 5.8 | 5.3 | 49.3 | 31 | 15.2 | 4.4 |
Figure 1.
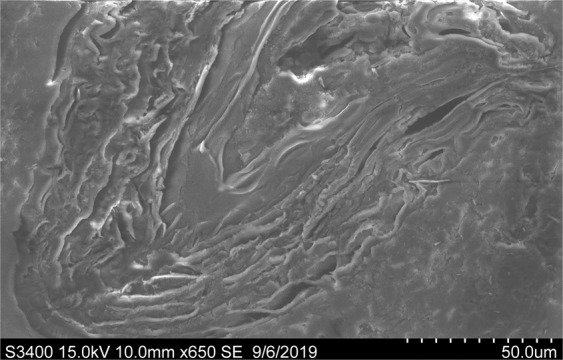
Scanning electron micrograph of frass.
Figure 2.
SEM-EDS analysis of the frass surface.
Frass mineralization
In line with results from natural ecosystems showing that insect frass was rapidly decomposed in an early decomposition process17,29, our data showed that frass from mealworm farm was quickly mineralized after its incorporation into the soil (Fig. 3). After 7 days of incubation, 37% of total organic carbon (TOC) was mineralized. Then, a slower but continuous C mineralization occurred, reaching 56% of TOC after 91 days. Nitrogen mineralization had a similar pattern: a rapid mineralization just after frass application (37% of total N after 17 days of incubation) was followed by a slower but continuous N mineralization (55% of total N at the end of the incubation period). In terms of mineralization, the mealworm frass used in this study compares well with frass from other insects. For instance, Lovett and Ruesink17 reported that gypsy moth frass was highly decomposed in a 120-day incubation experiment, with the greatest mineralization in the first 10 days. The mealworm frass used in this study had a high labile C concentration (Table 1) and this likely stimulated microbial growth, resulting in high rate of C and N mineralization16,30,31. This is in agreement with Rothé et al.26 who found that the mineralization pattern of poultry manure, which had a similar chemical composition than frass, was intense in the early stages of incubation due to its high labile C concentration.
Figure 3.
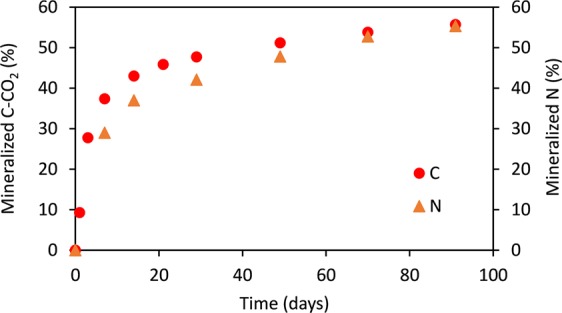
Carbon and nitrogen mineralization dynamics of frass.
Microbial metabolic activity and diversity
Microbial metabolic activity and diversity were assessed through the measurement of average well color development (AWCD) and S and H indices in BIOLOG EcoPlate (Fig. 4). AWCD reflects the oxidative capacity of soil microorganisms and may be used as an indicator of soil microbial activity32. Organic amendments are source of available energy for soil microorganisms. As a result, the supply of organic matter to soil through different amendments generally stimulates microbial activity and increases the microbial community functional potential, which can be reflected by an increase of AWCD and R and H indices32. Similar to other organic amendments, the incorporation of frass, either at 50% or 100%, resulted in an increase of the AWCD and/or S indices compared to the NPK treatment. These results are consistent with Zhong et al.33 who reported that AWCD and diversity indices were increased by the application of organic manure alone or with NPK compared to the NPK applied alone, and reflects that the addition of organic C, alone or in combination with NPK, led to C utilization pattern shifts and increased soil microbial functional diversity. Compared to the control, the lack of improvement of S index in the frass treatment might be explained by the relatively low rate of frass application in the present study (i.e. 10 Mg ha−1). Indeed, Gomez et al.32 showed that, although organic amendments increased AWCD at both 10 and 20 Mg ha−1 application rate, S index was only improved in the 20 Mg ha−1 treatment due to higher C availability for microorganisms. However, S and H indices were interestingly the highest in the 50NPK/50Frass treatment. According to Hu et al.34, higher diversity in soil amended with a combination of both organic and mineral fertilizers is due to a larger number of C sources under this treatment: the presence of the organic amendment provided direct C sources while the addition of NPK provided indirect C sources by stimulating the release of root exudates. Since the functioning of ecosystems strongly relies on soil biodiversity35,36, the added benefits resulting from the combined use of frass and NPK on soil microbial functional diversity suggests that a partial substitution of mineral fertilizer by frass could be an enticing option for the sustainable management of agroecosystems. It must however be noted that our study only focused on soil microorganisms. The next challenge will be therefore to investigate the effect of frass on soil fauna as it also plays a key role on the regulation of ecosystem functioning37.
Figure 4.
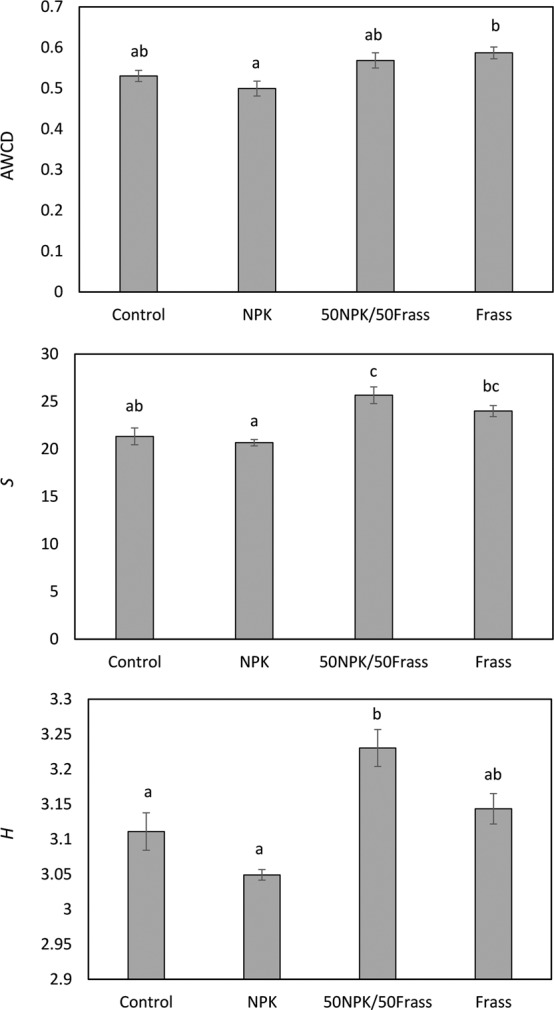
Average well-color development (AWCD), Richness (S) and Shannon-Weaver index (H) of metabolized substrates in BIOLOG EcoPlate after frass application. Values are average (n = 3) ± standard error. Columns with same letter do not differ significantly at the 5% level.
Soil properties
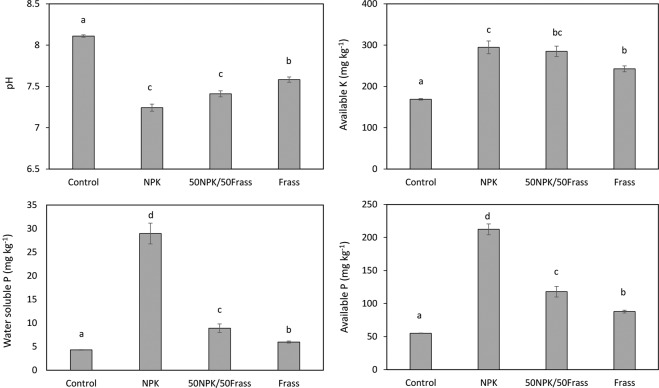
Although the soil pH of the frass treatment was not as low as that of the NPK treatment, the addition of frass significantly decreased soil pH, most likely due to the slightly acidic nature of frass (Table 1) as well as to its rapid decomposition which led to the production of CO2 and organic acids (Fig. 5). The fast nitrification, as reported in the incubation experiment (Fig. 3), may be an additional source of protons in the frass treatment38. Even though all the fertilizer treatments added the same total amounts of P and K, the highest available K and P concentrations were measured in the NPK treatment which was obviously due to the supply of these nutrients in a soluble form (Fig. 5). However, available K and P concentrations were greater in the frass treatment than in the control, suggesting that frass provided nutrients in a readily available form. The lower K and P concentrations in the presence of frass compared to the NPK treatment is in agreement with other studies comparing organic and mineral fertilizer over similar short experimental duration39. For instance, Houben et al.22 found that, after 100 days, available P concentration in a soil amended with sewage sludge was 50% lower than that in the presence of a mineral fertilizer. However, after one year, the authors found that both sewage sludge and mineral fertilizers were equally effective to improve P availability due to the continuous mineralization of sludge. Compared to mineral fertilizer, Andrews et al.40 found that compost increased K availability by 11 and 20% after 1 and 3 years, respectively, which was attributed to the K supply by compost and the increase of exchange sites due to organic matter added. These findings stress the need for longer-term experiment to better constrain the kinetics of nutrient release by frass. The available K and P concentrations when 50% of the NPK were substituted by 50% frass (50NPK/50Frass treatment) were similar and lower, respectively, than when NPK is used alone but higher compared to the complete NPK substitution by frass. This suggests that available K and P concentration in the 50NPK/50Frass treatment reflects both the rapid K and P supply by the conventional fertilizer and the slightly slower K and P supply by frass, as previously found for other organic amendments41. In addition, the supply of soluble organic carbon by frass might improve P availability by reducing sorption of P supplied by mineral fertilizer due to complexation with Fe, Al and Ca, which prevents P precipitation, and competition with inorganic P for the same sorption sites42. On the other hand, P and K supply by mineral fertilizer may promote the release of P and K from frass by stimulating its mineralization43, which is consistent with the elevated microbial metabolic activity and diversity shown by this treatment.
Figure 5.
pH and concentrations of water soluble P and available K and P in soil. Values are average (n = 4) ± standard error. Columns with same letter do not differ significantly at the 5% level.
Biomass and nutrient uptake
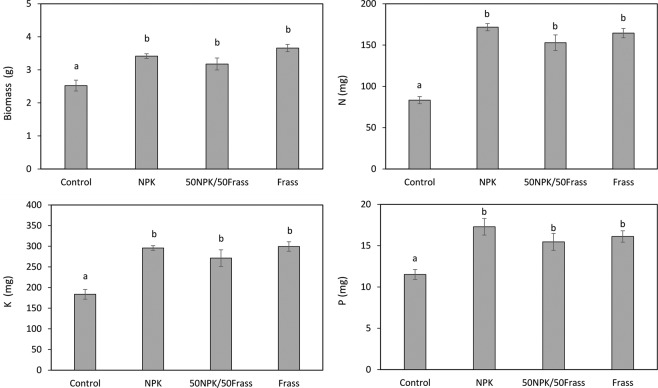
Despite having lower available P or K and P concentrations, the 50NPK/50Frass and frass treatment, respectively, were as effective as the NPK treatment to improve barley biomass and nutrient uptake (Fig. 6), indicating a high effectiveness of N, K and P contained in frass. This may be explained by the rapid frass mineralization and the presence of N, K and P in a readily available form which gradually supplied nutrient to plants throughout the growing period44. It is also likely that barley was able to mobilize nutrients from less available pool, as demonstrated by Nobile et al.23 who reported that, due to high specific root length and great carboxylate release, barley acquired as much P from sludge as from mineral fertilizer.
Figure 6.
Biomass and concentrations of N, K and P of harvested barley. Values are average (n = 4) ± standard error. Columns with same letter do not differ significantly at the 5% level.
Compared to sole application of either NPK or frass, the combination of half doses of both did not significantly impact plant biomass and nutrient uptake, suggesting no interaction effects between both amendments (Fig. 6). Mixing organic amendment with mineral fertilizer is known to improve plant growth because organic matter help retaining soluble nutrients added by mineral fertilizer, preventing them from leaching45. Under the current pot experiment conditions where irrigation is controlled, the possibility of nutrients losses via leaching is very low and this most likely explained the lack of synergistic effect between frass and NPK46. In field experiment should therefore be carried out to confirm the ability of frass to reduce leaching of nutrients released by mineral fertilizer. On the other hand, the similar effect of frass on biomass and nutrient uptake as compared to NPK suggests that frass acted as a nutrient source and could be used as a partial or complete substitute of mineral fertilizer. Interestingly, despite having much lower water soluble P concentrations (Fig. 5), treatments with frass induced similar P uptake than the NPK treatment (Fig. 6). Water soluble P is known to be closely related to P loss in runoff and leaching waters47,48 and is therefore considered as an important index for soil P status in the environment49. On the other hand, water soluble P in NPK can be rapidly fixed in less available forms in the soil due to strong sorption on soil constituents50. Since P uptake by plants was not impaired by the lower water soluble P concentration in frass treatments, it appears that P in frass may be mineralized and released during the incubation and became available for plant uptake and/or be protected by organic substances from sorption to sparingly available forms, as reported for other organic amendments51. Thus, by limiting water soluble P concentration without impacting P uptake by plants, frass prevents P from loss and sorption while acting as an effective fertilizer. One perspective of this work will be to test the effect of frass on the growth and nutrient uptake of other crop species in order to determine to what extent the ability of frass to supply high amount of nutrients to plants is mediated by the strategies of plants to mobilize and acquire these nutrients.
Conclusion
Insect production is expected to dramatically grow in the next few years due to the increasing need of finding alternative sources of protein. Given the « zero waste » context and the need to contribute to the circular economy, it is necessary to capitalize on all components of the insects, including their frass. This study indicates that frass has a great potential to be used as a partial or a complete substitute of mineral NPK fertilizer. Indeed, due to its rapid mineralization and its high content in readily-available nutrient, frass has similar effectiveness to supply N, P and K and sustain biomass production than NPK fertilizer. In addition, compared to mineral fertilizer, water soluble P concentration is up to five times lower in the presence of frass, which prevents P from loss and sorption onto soil constituents. Most importantly, the presence of frass may increase microbial metabolic activity and diversity, suggesting a better soil functioning, especially when frass is combined with mineral fertilizer.
As this was a greenhouse study, further in situ researches are required because temporal mineralization in controlled conditions may be different from mineralization in field due to e.g. differences of moisture, temperature and soil and crop biodiversity, thereby affecting possibly the temporal release of nutrient for plants. Nonetheless, our findings suggest that the forecasted growing amount of frass generated in the near future might constitute a sustainable resource for managing NPK nutrition in cropping system and a promising alternative to conventional fertilizer.
Materials and methods
Frass
Frass from mealworm (Tenebrio molitor L.) was provided in the form of powder by Ÿnsect (ŸnFrass, Paris, France), an industrial company farming this insect at the large-scale. The mealworms were fed exclusively on local agricultural raw materials (wheat bran) authorized by French and European regulations for farm animal feeds. The frass was hygienized (70 °C, 60 minutes) and used without any chemical input, making it a fertilizer compatible with organic farming and not subject to any specific restrictions. Moisture content was 12.4% (103 °C; 4 h). Frass was analyzed for pH and electrical conductivity in a 1:5 frass: water suspension (w/v), organic C content52, total nitrogen (N) content53, phosphorus (P), potassium (K), copper (Cu) and zinc (Zn) concentrations54. Four biochemical fractions were determined using a modified Van Soest method55: soluble (SOL), hemicellulose-like (HCE), cellulose-like (CEL) and lignin-like (LIG).
SEM-EDS characterization of frass
The inner morphology of frass was investigated on polished section of frass particles impregnated with epoxy using a scanning electron microscope (SEM; Hitachi S3400N). The SEM was equipped with an energy-dispersive X-ray spectrometer (EDS; Thermo Ultradry) for element detection and element mapping was carried out on frass particles mounted on double-sided adhesive carbon tape. Voltage was set at 20 kV and counting time was 200 seconds.
Soil
The study soil was sampled in Beauvais (Northern France) and was classified as a Haplic Luvisol56. A total mass of 100 kg was obtained by composite sampling (0–10 cm depth) from a cultivated land. After sampling, the soil was air-dried, crushed and sieved at 2 mm. Particle size analysis using the pipette method57 revealed that the soil was a silt loam (USDA classification) with 16% sand, 67% silt, and 17% clay. Chemical characterization of the soil was carried out using the procedures described in Houben et al.58 and revealed that organic C was 1.54%, total N was 0.18%, the cation exchange capacity (CEC) was 12.5 cmolc kg−1 and pH was 7.8. Available concentrations as assessed using the acetate ammonium-EDTA extraction58 were Ca 3869 mg kg−1, Mg 101 mg kg−1, K 292 mg kg−1, P 72 mg kg−1.
Description of treatments
The frass was applied to the soil at a rate of 10 Mg dry matter ha−1 (hereafter called “Frass” treatment). A mineral control adding the same quantity of N, P and K as in the Frass treatment was achieved by mixing the soil with appropriate amount of inorganic nutrients (NH4NO3, KH2PO4 and KCl) in solution (hereafter called “NPK”). Untreated soil (hereafter called “control”) and another treatment adding 50% frass plus 50% NPK were also performed (hereafter called “50NPK/50Frass”).
Incubation experiment: C and N mineralization
The kinetics of frass mineralization were followed during laboratory incubations of control and frass treatments, based on the French normalization59. An amount equivalent to 25 g of dry soil mixture was incubated in 1.2 L hermetic jars kept in a dark room at 28 °C. The experiment was conducted in four replicates and lasted 91 days. The water content of the mixtures was adjusted at field capacity with demineralized water and controlled during the incubation period. In each glass jar, C mineralized as CO2 was trapped in 10 mL of 0.5 mol L−1 NaOH, which was replaced after 1, 3, 7, 14, 21, 28, 49, 70 and 91 days of incubation. The C-CO2 trapped in NaOH was determined by titration of the residual NaOH after precipitation of the carbonate with barium chloride. Similar incubation was carried out for N mineralization. Mineral N (N-NH4+ and N-NO3−) was extracted by shaking the mixtures for 1 h with 100 mL 1 mol L−1 KCl after 0, 7, 14, 28, 56 and 91 days of incubation60. The mineral N in the extracts was analysed by colorimetry on a continuous flow analyser (Skalar, The Netherlands). As recommended by Doublet et al.60, the dynamics of C mineralisation in soil was calculated by subtracting C-CO2 mineralised in the control treatment to C-CO2 mineralised from the frass treatment and the results were expressed as a percentage of the total organic C (TOC) in frass. The release of mineral N from frass was calculated by subtracting the mineral N measured in the control treatment from the mineral N in the frass treatment.
Pot experiment
A pot experiment was conducted to determine the effect of frass on the nutrient availability for plants. Plastic plant pots were filled with 3500 g of each mixture in four replicates. Before sowing, th pots were placed in a controlled dark room and the mixtures were equilibrated during two weeks at 80% water holding capacity. After the equilibration period, the pots were transferred to a greenhouse glass and were arranged according to a randomized design. In each pot, 8 seeds of barley (Hordeum vulgare L.) were sown. After 10 days, the plants were thinned to two plants per pot. The trials were conducted under controlled greenhouse conditions (temperature 18–25 °C, 16 h photoperiod) with daily sprinkler watering to maintain the soil moisture at field capacity. After 9 weeks, shoots were harvested with ceramic scissors, dried (60 °C; 72 h), weighed and crushed. The content of P and K in aerial parts was analysed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES; Jarrell Ash) after aqua regia digestion. The content of N in aerial parts was analysed using the Dumas combustion method.
Soil analyses
After the harvest, water-soluble phosphorus concentration in soil (soil:water 1:60; w-v) was determined as described by Sissingh61. The available K and P concentrations were determined using the ammonium acetate-EDTA soil test58. Soil pH was measured in H2O (soil:water 1:5; w-v). Community-level physiological profiles (CLPPs) based on the ability of microorganisms to oxidise various substrates were assessed using BIOLOG EcoPlates (BIOLOG Co., Hayward, USA). Each 96-well plate consisted of three replicates, each one comprising 31 sole C sources and a water blank. Five grams of soil collected at the end of the pot experiment, were shaken with 45 ml of sterile 0.85% NaCl for 30 min at 200 rpm and then diluted to 1:1000. Each plate well was inoculated with 150 µL of the dilution and the plates were incu bated at 25 °C. Color development for each well was obtained in terms of optical density (OD) at 590 nm using an automated plate reader. Average well colour development (AWCD) was calculated as a measure of microbial functional diversity. AWCD was calculated as:
where R is the absorbance of the control well (containing water instead of C source) and Ci is the absorbance of plate well inoculated with C source i. Richness (S), as the number of oxidized C substrates and the Shannon-Weaver index (H) were calculated using an OD of 0.25 as threshold for positive response32. Shannon-Weaver index was calculated as follows:
where
Kinetic curves suggested that after 72 h incubation time, the wells with the most active microbial communities reached the asymptote of color development. Therefore, this point was considered as the optimal incubation time for further statistical analyses, as suggested by Doan et al.62.
Statistical analyses
All recorded data were analysed using descriptive statistics (mean ± standard error) and normality was determined using the Shapiro-Wilk test. The data were subjected to one-way ANOVA and Tukey’s post-hoc test to compare treatments, which had a normal distribution. Data without normal distribution were subjected to the Kruskall-Wallis test and Mann-Whitney post-hoc test. All statistical analyses were performed using R software version 3.5.063 and the package Rcmdr64.
Acknowledgements
We thank the “ASET 158” students, Céline Roisin, Aurore Coutelier and Vincent Hervé for technical assistance. Sébastien Potel is gratefully acknowledged for help in SEM-EDS analyses.
Author contributions
D.H., G.D., M-P.F. and A-M.D. conceived and designed the experiments. D.H. and A-M.D. conducted the experiments and analyzed the data. D.H. wrote the manuscript with inputs from G.D., M-P. F. and A-M.D. All authors reviewed the manuscript.
Competing interests
We declare that the research was funded by a private corporation, Ÿnsect; however, we ensure the research is free of bias.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478:337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 2.Godfray HCJ, et al. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 3.Berggren Å, Jansson A, Low M. Approaching ecological sustainability in the emerging insects-as-food industry. Trends Ecol. Evol. 2019;34:132–138. doi: 10.1016/j.tree.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Tittonell P. Ecological intensification of agriculture — sustainable by nature. Curr. Opin. Environ. Sustain. 2014;8:53–61. doi: 10.1016/j.cosust.2014.08.006. [DOI] [Google Scholar]
- 5.Deutz P, Gibbs D. Industrial ecology and regional development: eco-industrial development as cluster policy. Reg. Stud. 2008;42:1313–1328. doi: 10.1080/00343400802195121. [DOI] [Google Scholar]
- 6.de Castro RJS, Ohara A, Aguilar JG, dos S, Domingues MAF. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018;76:82–89. doi: 10.1016/j.tifs.2018.04.006. [DOI] [Google Scholar]
- 7.Surendra KC, Olivier R, Tomberlin JK, Jha R, Khanal SK. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy. 2016;98:197–202. doi: 10.1016/j.renene.2016.03.022. [DOI] [Google Scholar]
- 8.Varelas V, Langton M. Forest biomass waste as a potential innovative source for rearing edible insects for food and feed – A review. Innov. Food Sci. Emerg. Technol. 2017;41:193–205. doi: 10.1016/j.ifset.2017.03.007. [DOI] [Google Scholar]
- 9.Wegier A, Alavez V, Pérez-López J, Calzada L, Cerritos R. Beef or grasshopper hamburgers: The ecological implications of choosing one over the other. Basic Appl. Ecol. 2018;26:89–100. doi: 10.1016/j.baae.2017.09.004. [DOI] [Google Scholar]
- 10.Dicke M. Insects as feed and the Sustainable Development Goals. J. Insects Food Feed. 2018;4:147–156. doi: 10.3920/JIFF2018.0003. [DOI] [Google Scholar]
- 11.Gortari, M. C. & Hours, R. A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 16, (2013).
- 12.Van Huis, A. et al. Edible insects: future prospects for food and feed security. (Food and Agriculture Organization of the United Nations, 2013).
- 13.Mancini, S., Moruzzo, R., Riccioli, F. & Paci, G. European consumers’ readiness to adopt insects as food. A review. Food Res. Int. 10.1016/j.foodres.2019.01.041. (2019). [DOI] [PubMed]
- 14.Derrien, C. & Boccuni, A. Current Status of the Insect Producing Industry in Europe. In Edible Insects in Sustainable Food Systems (eds. Halloran, A., Flore, R., Vantomme, P. & Roos, N.) 471–479 (Springer International Publishing, 10.1007/978-3-319-74011-9_30. 2018).
- 15.Frost CJ, Hunter MD. Insect Canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology. 2004;85:3335–3347. doi: 10.1890/04-0003. [DOI] [Google Scholar]
- 16.Kagata H, Ohgushi T. Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Popul. Ecol. 2012;54:75–82. doi: 10.1007/s10144-011-0281-6. [DOI] [Google Scholar]
- 17.Lovett GM, Ruesink AE. Carbon and nitrogen mineralization from decomposing gypsy moth frass. Oecologia. 1995;104:133–138. doi: 10.1007/BF00328577. [DOI] [PubMed] [Google Scholar]
- 18.Payne Clr, et al. Insects as food and feed: European perspectives on recent research and future priorities. J. Insects Food Feed. 2016;2:269–276. doi: 10.3920/JIFF2016.0011. [DOI] [Google Scholar]
- 19.Halloran A, Roos N, Eilenberg J, Cerutti A, Bruun S. Life cycle assessment of edible insects for food protein: a review. Agron. Sustain. Dev. 2016;36:57. doi: 10.1007/s13593-016-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poveda J, et al. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019;142:110–122. doi: 10.1016/j.apsoil.2019.04.016. [DOI] [Google Scholar]
- 21.Faucon M-P, et al. Advances and perspectives to improve the phosphorus availability in cropping systems for agroecological phosphorus management. Adv. Agron. 2015;134:51–79. doi: 10.1016/bs.agron.2015.06.003. [DOI] [Google Scholar]
- 22.Houben D, et al. Response of phosphorus dynamics to sewage sludge application in an agroecosystem in northern France. Appl. Soil Ecol. 2019;137:178–186. doi: 10.1016/j.apsoil.2019.02.017. [DOI] [Google Scholar]
- 23.Nobile C, et al. Phosphorus-acquisition strategies of canola, wheat and barley in soil amended with sewage sludges. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson FA, Chambers BJ, Smith KA. Nutrient composition of poultry manures in England and Wales. Bioresour. Technol. 1996;58:279–284. doi: 10.1016/S0960-8524(97)86087-7. [DOI] [Google Scholar]
- 25.da Cruz DF, Bortoletto-Santos R, Guimarães GGF, Polito WL, Ribeiro C. Role of polymeric coating on the phosphate availability as a fertilizer: Insight from phosphate release by castor polyurethane coatings. J. Agric. Food Chem. 2017;65:5890–5895. doi: 10.1021/acs.jafc.7b01686. [DOI] [PubMed] [Google Scholar]
- 26.Rothé M, Darnaudery M, Thuriès L. Organic fertilizers, green manures and mixtures of the two revealed their potential as substitutes for inorganic fertilizers used in pineapple cropping. Sci. Hortic. 2019;257:108691. doi: 10.1016/j.scienta.2019.108691. [DOI] [Google Scholar]
- 27.Li L, Zhao Z, Liu H. Feasibility of feeding yellow mealworm (Tenebrio molitor L.) in bioregenerative life support systems as a source of animal protein for humans. Acta Astronaut. 2013;92:103–109. doi: 10.1016/j.actaastro.2012.03.012. [DOI] [Google Scholar]
- 28.Wang H, et al. Insect biorefinery: a green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels. 2017;10:304. doi: 10.1186/s13068-017-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madritch MD, Donaldson JR, Lindroth RL. Canopy herbivory can mediate the influence of plant genotype on soil processes through frass deposition. Soil Biol. Biochem. 2007;39:1192–1201. doi: 10.1016/j.soilbio.2006.12.027. [DOI] [Google Scholar]
- 30.Lovett GM, et al. Insect Defoliation and Nitrogen Cycling in Forests. BioScience. 2002;52:335. doi: 10.1641/0006-3568(2002)052[0335:IDANCI]2.0.CO;2. [DOI] [Google Scholar]
- 31.Zimmer M, Topp W. The role of coprophagy in nutrient release from feces of phytophagous insects. Soil Biol. Biochem. 2002;34:1093–1099. doi: 10.1016/S0038-0717(02)00044-5. [DOI] [Google Scholar]
- 32.Gomez E, Ferreras L, Toresani S. Soil bacterial functional diversity as influenced by organic amendment application. Bioresour. Technol. 2006;97:1484–1489. doi: 10.1016/j.biortech.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Zhong W, et al. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil. 2010;326:511–522. doi: 10.1007/s11104-009-9988-y. [DOI] [Google Scholar]
- 34.Hu J, et al. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soils Sediments. 2011;11:271–280. doi: 10.1007/s11368-010-0308-1. [DOI] [Google Scholar]
- 35.Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- 36.Faucon M-P, Houben D, Lambers H. Plant functional traits: soil and ecosystem services. Trends Plant Sci. 2017;22:385–394. doi: 10.1016/j.tplants.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Bardgett, R. D. & Wardle, D. A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change. (Oxford Series in Ecology and Evolution, 2010).
- 38.van Breemen N, Mulder J, Driscoll CT. Acidification and alkalinization of soils. Plant Soil. 1983;75:283–308. doi: 10.1007/BF02369968. [DOI] [Google Scholar]
- 39.Srivastava PK, et al. Effects of combined application of vermicompost and mineral fertilizer on the growth of Allium cepa L. and soil fertility. J. Plant Nutr. Soil Sci. 2012;175:101–107. doi: 10.1002/jpln.201000390. [DOI] [Google Scholar]
- 40.Andrews SS, et al. On-farm assessment of soil quality in California’s Central Valley. Agron. J. 2002;94:12–23. doi: 10.2134/agronj2002.0012. [DOI] [Google Scholar]
- 41.Palm, C. A., Myers, R. J. K. & Nandwa, S. M. Combined Use of Organic and Inorganic Nutrient Sources for Soil Fertility Maintenance and Replenishment. In Replenishing Soil Fertility in Africa (eds. Buresh, R. J., Sanchez, P. A. & Calhoum, F.) 193–217 (SSSA, American Society of Agronomy, 1997).
- 42.Regelink IC, Weng L, Lair GJ, Comans RNJ. Adsorption of phosphate and organic matter on metal (hydr)oxides in arable and forest soil: a mechanistic modelling study. Eur. J. Soil Sci. 2015;66:867–875. doi: 10.1111/ejss.12285. [DOI] [Google Scholar]
- 43.Yang L, Li T, Li F, Lemcoff JH, Cohen S. Fertilization regulates soil enzymatic activity and fertility dynamics in a cucumber field. Sci. Hortic. 2008;116:21–26. doi: 10.1016/j.scienta.2007.11.001. [DOI] [Google Scholar]
- 44.Adediran JA, Taiwo LB, Akande MO, Sobulo RA, Idowu OJ. Application of organic and inorganic fertilizer for sustainable maize and cowpea yields in Nigeria. J. Plant Nutr. 2005;27:1163–1181. doi: 10.1081/PLN-120038542. [DOI] [Google Scholar]
- 45.Yang Y, et al. Leaching behavior of phosphorus in sandy soils amended with organic material. Soil Sci. 2008;173:257–266. doi: 10.1097/SS.0b013e31816d1edf. [DOI] [Google Scholar]
- 46.Saha A, Basak BB, Gajbhiye NA, Kalariya KA, Manivel P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crops Prod. 2019;140:111607. doi: 10.1016/j.indcrop.2019.111607. [DOI] [Google Scholar]
- 47.Renneson M, et al. Degree of phosphorus saturation in agricultural loamy soils with a near-neutral pH. Eur. J. Soil Sci. 2015;66:33–41. doi: 10.1111/ejss.12207. [DOI] [Google Scholar]
- 48.Vadas PA, Kleinman PJA, Sharpley AN, Turner BL. Relating soil phosphorus to dissolved phosphorus in runoff: a single extraction coefficient for water quality modeling. J. Environ. Qual. 2005;34:572–580. doi: 10.2134/jeq2005.0572. [DOI] [PubMed] [Google Scholar]
- 49.Maguire RO, Sims JT. Soil testing to predict phosphorus leaching. J. Environ. Qual. 2002;31:1601–1609. doi: 10.2134/jeq2002.1601. [DOI] [PubMed] [Google Scholar]
- 50.Huang X-L, Chen Y, Shenker M. Dynamics of phosphorus phytoavailability in soil amended with stabilized sewage sludge materials. Geoderma. 2012;170:144–153. doi: 10.1016/j.geoderma.2011.11.025. [DOI] [Google Scholar]
- 51.Kahiluoto H, Kuisma M, Ketoja E, Salo T, Heikkinen J. Phosphorus in manure and sewage sludge more recyclable than in soluble inorganic fertilizer. Environ. Sci. Technol. 2015;49:2115–2122. doi: 10.1021/es503387y. [DOI] [PubMed] [Google Scholar]
- 52.AFNOR. NF EN 13039. Soil improvers and growing media - Determination of organic matter content and ash. (AFNOR, 2011).
- 53.AFNOR. NF EN 13654-1. Soil improvers and growing media - Determination of nitrogen - Part 1: modified Kjeldahl method. (AFNOR, 2002).
- 54.AFNOR. NF EN 13650. Soil improvers and growing media - Extraction of aqua regia soluble elements. (AFNOR, 2002).
- 55.AFNOR. Norme XP U 44-162. Amendements organiques et supports de culture - Caracterisation de la matière organique par fractionnement biochimique et estimation de sa stabilité biologique. (AFNOR, 2009).
- 56.IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015 International soils classification system for naming soils and creating legends for soil maps. World Resources Reports No. 106. in (ed. FAO) 192 (2015).
- 57.AFNOR. NF X31-107. Soil quality - Particle size determination by sedimentation - Pipette method. (AFNOR, 2003).
- 58.Houben D, Meunier C, Pereira B. & Sonnet, P. Predicting the degree of phosphorus saturation using the ammonium acetate–EDTA soil test. Soil Use Manag. 2011;27:283–293. [Google Scholar]
- 59.AFNOR. FD-U-44-163 Soil Improvers and Growing Media - Characterization of Organic atter by Potential Mineralization of Carbon and Nitrogen. (AFNOR, 2016).
- 60.Doublet J, Francou C, Poitrenaud M, Houot S. Influence of bulking agents on organic matter evolution during sewage sludge composting; consequences on compost organic matter stability and N availability. Bioresour. Technol. 2011;102:1298–1307. doi: 10.1016/j.biortech.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 61.Sissingh HA. Analytical technique of the Pw method, used for the assessment of the phosphate status of arable soils in the Netherlands. Plant Soil. 1971;34:483–486. doi: 10.1007/BF01372800. [DOI] [Google Scholar]
- 62.Doan TT, Jusselme DM, Lata J-C, Van Nguyen B, Jouquet P. The earthworm species Metaphire posthuma modulates the effect of organic amendments (compost vs. vermicompost from buffalo manure) on soil microbial properties. A laboratory experiment. Eur. J. Soil Biol. 2013;59:15–21. doi: 10.1016/j.ejsobi.2013.08.005. [DOI] [Google Scholar]
- 63.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. (ISBN3-900051-07-0 https://www.R-project.org, 2017).
- 64.Fox J. The R Commander: A Basic-Statistics Graphical User Interface to R. J. Stat. Softw. 2005;14:1–42. [Google Scholar]