Abstract
Aberrant NF-κB activation is a hallmark of most B-cell malignancies. Recurrent inactivating somatic mutations in the NFKBIE gene, which encodes IκBε, an inhibitor of NF-κB-inducible activity, are reported in several B-cell malignancies with highest frequencies in chronic lymphocytic leukemia and primary mediastinal B-cell lymphoma, and account for a fraction of NF-κB pathway activation. The impact of NFKBIE deficiency on B-cell development and function remains, however, largely unknown. Here, we show that Nfkbie-deficient mice exhibit an amplification of marginal zone B cells and an expansion of B1 B-cell subsets. In germinal center (GC)-dependent immune response, Nfkbie deficiency triggers expansion of GC B-cells through increasing cell proliferation in a B-cell autonomous manner. We also show that Nfkbie deficiency results in hyperproliferation of a B1 B-cell subset and leads to increased NF-κB activation in these cells upon Toll-like receptor stimulation. Nfkbie deficiency cooperates with mutant MYD88 signaling and enhances B-cell proliferation in vitro. In aged mice, Nfkbie absence drives the development of an oligoclonal indolent B-cell lymphoproliferative disorders, resembling monoclonal B-cell lymphocytosis. Collectively, these findings shed light on an essential role of IκBε in finely tuning B-cell development and function.
Subject terms: B-cell lymphoma, Chronic lymphocytic leukaemia, B cells, Oncogenes
Introduction
The NF-κB signaling pathway plays essential roles in cell survival, differentiation, proliferation, inflammation, and immune regulation1. Aberrant NF-κB activation is frequently observed in several B-cell malignancies2–6. This activation can result from interactions with the tumor microenvironment7–9 or as a result of somatic mutations affecting NF-κB family members or upstream signaling components, such as CD79B, BIRC3, CARD11, MYD88, TNFAIP3/A20, or inhibitors of kappa B (IκBs; refs. 3,5,6,10–13). The inactivation of IκB genes is recurrently observed in human B-cell malignancies. The NF-κB inhibitor I-kappa-B-epsilon (NFKBIE) gene, which encodes IκBε, is targeted by a recurrent 4-bp truncating mutation in addition to rare point mutations in 5–10% of chronic lymphocytic leukemia (CLL)2,3,14–16 and various B-cell lymphomas (BCLs), including diffuse large B-cell lymphoma (DLBCL; 5%), mantle cell lymphoma (5%), splenic marginal zone lymphoma (1.8%), and primary mediastinal B-cell lymphoma (PMBCL; 23% of patients)12,17. NFKBIE mutations are enriched among advanced stage CLL and associated with poor-prognostic outcome, suggesting that they might be involved in disease progression2,3,12,17. Compared to NFKBIE-wild-type (WT) patients, NFKBIE-mutated CLL cells showed reduced IκBε protein levels and decreased p65 inhibition, along with increased phosphorylation and nuclear translocation of p65 (ref. 2).
The NF-κB transcription factor family members are maintained as inactive homo- or heterodimers in the cytoplasm by IκB, including the classical IκB protein family: IκBα, IκBβ and IκBε (ref. 1). IκBε is an inhibitor of inducible NF-κB activity, which traps the Rel proteins in the cytoplasm at the resting state1,18,19. Its expression is higher in primary murine splenic B cells than in T cells and has a specific role in the late retro-control of B-cell response to external stimuli, including signaling through the B-cell receptor (BCR) and Toll-like receptors (TLRs)20–22. IκBε is more expressed in IgM+ B cells than in IgG+ B cells and presents a slower turnover than IκBα and IκBβ, following in vitro activation of primary splenic B cells with LPS or a combination of anti-IgM and CD40L (ref. 21). IκBε shows preferential specificity for NF-κB Rel homodimers, with respect to other NF-κB transcription factor subunits21. IκBε-deficient B cells present higher expression and activity of Rel at early time points of activation with IgM and IL4 compared to WT cells20–22.
To get more insights into the role of NFKBIE in normal and malignant B-cell differentiation, we studied Nfkbie-deficient mice and showed that the loss of Nfkbie results in marginal zone B (MZB) and B1 cells expansion, and a higher sensitivity to T-cell-dependent and -independent stimulation. We also show that Nfkbie deficiency cooperates with mutant MYD88 signaling and causes enhanced B-cell proliferation. In aged mice, Nfkbie absence drives development of an oligoclonal indolent B-cell lymphoproliferative disorders, resembling monoclonal B-cell lymphocytosis (MBL).
Materials and methods
Additional information can be found in the Supplemental Methods.
Mice
Inactivated Nfkbie allele on a mixed Sv129xDBA-2xC57BL/6 J background has been described previously;23 >20 back-crosses were performed on the C57BL/6 J background to give rise to a pure congenic Nfkbie−/− C57BL/6 J strain. Mutant mice in this pure C57BL/6 J background were housed in the animal facility of the Gustave Roussy Institute (SCEA, Gustave Roussy, Villejuif, France), crossed with WT C57BL/6 J mice and age-matched animals of selected genotypes were sacrificed at the indicated times. Bone marrow transplantation were performed as described24,25. Animal experiments were conducted in compliance with the Gustave Roussy Institutional guidelines and authorized by the Direction Départementale des Services Vétérinaires du Val de Marne.
Mice immunization
For the analysis of germinal center (GC) formation in T-cell-dependent immune response, Nfkbie-deficient and WT age-matched 2–3-month-old mice were immunized with sheep red blood cells (SRBCs; 1 × 108 cells per mouse) via intraperitoneal injection. Mice were sacrificed 10 days after immunization and spleens were analyzed for GC B cells and plasma cells.
For in vivo TLR9 activation, CpG ODN (Invivogen) was administered intraperitoneally at a dose of 333 μg per mouse in 200 μL phosphate-buffered saline. Mice were sacrificed 10 days after immunization; inguinal lymph nodes and spleens were analyzed for GC B cells.
In vitro generation of induced GC B cells
Splenic untouched resting murine B cells were first isolated from 2 to 3-month-old animals using the mouse CD43 (Ly-48) B-cell isolation kit (MicroBeads, Miltenyi Biotec) according to the manufacturer’s instructions (yield > 90% CD19+B220+). MZB and FOB cells were flow-sorted as described in the “Cell isolation and culture” section. 40LB-cells expressing CD40L and producing BAFF were a kind gift of Pr. D. Kitamura26 and cultured in DMEM media (Invitrogen) with 10% FBS and penicillin/streptomycin.
For B-cell culture, RPMI-1640 medium (Invitrogen) was supplemented with 10% FBS, 0,055 mM 2-ME, 10 mM HEPES, 1 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin (invitrogen). Purified B cells (110 × 103 cells per well) were cultured in a six-well plate in the presence of 40LB-cells (0.5 × 106 cells per dish) that had been irradiated with 80 Gy X-ray. rIL-4 (1 ng/ml; Peprotech) was added to the primary culture for 4 days. Cells were harvested at day 4 (D4) and processed for flow cytometric analysis.
MBL diagnosis
The appearance of a B220lowCD19+CD5+ population and its percentage among peripheral blood mononuclear cells (PBMCs) were used for initial MBL-like disease diagnosis. The diagnostic criterion for MBL in mice was arbitrary defined as the appearance of oligoclonal/monoclonal B220lowCD19+CD5+ cells, constituting over 5% of PBMCs without a detectable increase in total white blood cell count.
Genescan analysis
cDNA from sorted Nfkbie-deficient B cells (B220lowCD19+) and WT B cells (B220+CD19+) was PCR amplified using VH family primers (VH1, VH2, VH3, VH5, VH6, and VH7 as indicated) and a common JH FAM-conjugated primer to assess IgH CDR3 diversity27. PCR products were run on a 3130xl sequencer (Applied Biosystems) and data were analyzed using the Peakscanner software (Applied Biosystems).
Retroviral infection and in vivo cell transfer
MYD88WT and that containing L265P mutation (MYD88L265P) cDNA were synthesized by GenScript and subcloned into MSCV-eGFP backbone. Viral particles and transduction procedures were described previously3.
Statistical analysis
Statistical significance of differences between the results was assessed using a two-tailed unpaired Student’s t-test with Welch’s correction, performed using Prism (GraphPad software, version 5.03). Statistically significant p values: *p values < 0.05; **p values < 0.01 and ***p values < 0.005. Error bars displayed throughout the paper represent s.e.m. or s.d. as indicated in figure legends. No statistical method was used to predetermine sample size. No blinding and no randomization of samples were applied. No data was excluded.
Results
Nfkbie−/− mice show amplification of MZB and B1 B cells
Initial analyses of immune cells in Nfkbie−/− mice on a mixed genetic background showed only very subtle phenotypic consequences on thymic lymphoid T-cell subsets23. Our analysis showed that, compared to 5–8-month-old WT, aged-matched Nfkbie−/− mice on a pure C57BL/6 J background presented an increase in cell number and frequency of a population expressing low levels of the B220 marker (B220low) in spleen, thymus, lymph nodes, peritoneal cavity, and peripheral blood (Fig. 1a). Detailed immunophenotyping in spleen and peritoneal cavity was compatible with a B1 B-cell population: CD19+, B220low/−, CD43+, CD23−, CD21−, CD93−, IgDlow, and IgM+ (Supplementary Fig. 1a).
Fig. 1. Nfkbie−/− mice present an expansion of B1a B cells and a biased differentiation pathway toward MZB.
a Upper panel: representative plots of FACS analysis of the CD19+B220low population in spleen, peritoneal cavity (PerC), lymph node (LN), thymus, and peripheral blood of Nfkbie+/+, Nfkbie+/−, and Nfkbie−/− mice. Lower panel: percentages and absolute cell numbers of the CD19+B220low population in spleen and PerC, and percentages of the CD19+B220low population in peripheral blood. Each symbol represents one mouse. b Analysis of the B1 B-cell subset, B1a (CD19+B220lowCD5+) and B1b (CD19+B220lowCD5−), partitioning in spleen and PerC of Nfkbie+/+ and Nfkbie−/− mice (n = 5). Each symbol represents one mouse. c Representative FACS plots of IgM and CD5 expression on the CD19+B220low cells in PerC of Nfkbie+/+ (n = 5) and Nfkbie−/− mice (n = 5). d Percentages and absolute cell numbers of CD19+B220low IgMlow B cells in the PerC of Nfkbie+/+, and Nfkbie−/− of 2, 7–9 and 12–16-month-old mice (n = 5). Each symbol represents one mouse. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant.
Analysis of CD5 expression showed that both B1a (CD5+) and B1b (CD5−) subsets were increased in spleen of Nfkbie−/− mice, whereas only the B1a cell population augmented in peritoneal cavity (Fig. 1b). Surface IgM expression on B1 cells was higher in Nfkbie−/− than in WT splenic cells, in contrast to peritoneal ones (Supplementary Fig. 1b). Based on their surface IgM expression levels, peritoneal cavity Nfkbie−/− (B220lowCD19+CD5+) B1a cells were subdivided into IgMhigh and IgMlow B1a subsets (Fig. 1c). The IgMlow B1a B-cell population increased with age in Nfkbie−/− mice (Fig. 1d).
B2 populations flow cytometry analysis revealed lower frequency and numbers of mature follicular B (FoB) cells, defined as CD19+B220+CD23+CD21+, and increased frequency and numbers of MZB cells, defined as CD19+B220+CD23lowCD21hi, in Nfkbie−/− mice with respect to WT (Supplementary Fig. 1d), as reported20. Nfkbie−/− MZB cells displayed significantly higher IgM expression than WT MZB cells, unlike FoB cells WT and Nfkbie−/− cells (Supplementary Fig. 1c).
Together, these data indicate that the absence of Nfkbie affects mature B-cell subsets differentiation and leads to expansion of MZB and B1a B cells. These B-cell subsets are known to mediate the innate functions of the B lineage. Both populations are particularly sensitive to variations in NF-κB activity and strongly influenced by BCR specificity and strength of signaling28–30.
Nfkbie deficiency affects the frequency of the B1 B-cell progenitor and the transition from transitional B cells to mature B cells
We next analyzed in detail hematopoietic differentiation, including B-cell development, in the bone marrow of 2-month-old KO mice. Proportions of LSK cells, myeloid (CMP, GMP, and MEP), and common lymphoid progenitor were comparable between WT, Nfkbie+/− and Nfkbie−/− mice (Supplementary Fig. 2a–c). Analysis of early B-cell differentiation stages, including pre-pro-B (CD19−B220+CD43+), pre-B (CD19+B220+CD43−), and pro-B (CD19+B220+CD43+), showed no significant alteration of the percentages and numbers of each population (Supplementary Fig. 2d, f). Analysis of Hardy fractions did not reveal any abnormalities in Nfkbie−/− mice either (Supplementary Fig. 2e, g), indicating that loss of Nfkbie does not impact early B-cell development in the bone marrow.
We then evaluated the frequency and numbers of B1 B-cell progenitors (Lin−CD93+CD19+B220-/low) in mutant bone marrow31. Significantly higher frequency of Lin−CD93+CD19+B220−/low cells in 2-month-old Nfkbie−/− mice were observed compared to those of WT or Nfkbie+/− mice, with a trend for Nfkbie−/− B1 progenitor cell number increases (Fig. 2a).
Fig. 2. Nfkbie−/− deficiency alters the frequency of B1 progenitor cells and the transition from transitional to mature B cells.
a Bone marrow B1 B-cell progenitor analysis of 2-month-old mice. Upper panel: representative plots of FACS analysis of B1 (Lin−IgM−CD93+B220−CD19+) B-cell progenitor. Lower panel: percentage and absolute cell numbers of the bone marrow Lin−IgM−CD93+B220−CD19+ population. Each symbol represents one mouse. Nfkbie+/+ (n = 5), Nfkbie+/− mice (n = 6) and Nfkbie−/− mice (n = 6). b Analysis of total B cells (CD19+), B1 (CD19+B220low) and B2 (CD19+B220+) subset distribution in the spleen and peritoneal cavity. Each symbol represents one mouse. Nfkbie+/+ (n = 5), Nfkbie+/− mice (n = 6), and Nfkbie−/− mice (n = 6). c Analysis of splenic transitional (T1 (CD19+B220+CD93+IgMhiCD23−) and T2 (CD19+B220+CD93+IgMhiCD23+)) B cells, MZB cells and FoB cells distribution. Each symbol represents one mouse. Nfkbie+/+ (n = 5), Nfkbie+/− mice (n = 6), and Nfkbie−/− mice (n = 6). Data are mean ± SEM. *p < 0.05, **p < 0.01; ns, not significant.
To check the balance between B1 and B2 cells, we analyzed their proportions in the spleen and peritoneal cavity. Percentages of total CD19+ B cells increased significantly in peritoneal cavity but remained stable in the spleen of Nfkbie−/− mice, as compared to WT animals (Fig. 2b). Percentages of splenic and peritoneal B2 cells (CD19+B220+) were also similar between both genotypes (Fig. 2b); however, percentages of B1 cells were significantly higher in Nfkbie−/− mice. These data indicate that there is no shift toward the B1 cell lineage in mutant mice and that the increase in B1 cell numbers might result from the increase of the B1 B-cell progenitor cells.
We next analyzed peripheral splenic B2 B-cell populations from 2-month-old mutant mice. Immature transitional B-cells frequencies and numbers were unaffected by Nfkbie deficiency, whereas decrease of mature FoB-cell population and increase of MZB cells observed in older mice were already present (Fig. 2c). Additional analyses of non-B-cell lineage did not show the reported CD44− CD25+(DN3) thymocytes decrease (Supplementary Fig. 2h), which might therefore result from the mixed genetic background of the mutant mice23. No other abnormality of major hematopoietic lineages was observed in 2-month-old mice (Supplementary Fig. 2i) or older Nfkbie−/− (Supplementary Fig. 2j) mice.
To assess whether higher B1 and MZB B-cell numbers were due to increased proliferation or reduced apoptosis, we investigated ex vivo proliferation and apoptosis. No significant differences in the proportion of Ki67+ or viable MZB, FoB, B1 B cells between Nfkbie−/− and WT mice were detected (Supplementary Fig. 3a, b). As the frequency and cell numbers of splenic transitional B cells were equivalent between Nfkbie−/− and WT mice, these findings suggest that Nfkbie deficiency biases the differentiation of transitional B cell into MZB cell fate.
Overall, these data indicate that Nfkbie is important for follicular versus MZB cell fate decision and that its loss may affect the size of the B1 B-cell progenitor compartment.
Biased differentiation toward MZB cell and expansion of B1 B-cell subsets in absence of Nfkbie is cell-autonomous
To investigate whether Nfkbie deficiency-associated changes were cell-autonomous, we performed competitive bone marrow reconstitutions (Fig. 3a for scheme). FACS analysis in peripheral blood showed that recipients of WT CD45.2+ cell had a stable reconstitution with ~30% donor cells, whereas there was a steady increase in the percentage of donor cells in recipients of Nfkbie+/− and Nfkbie−/− CD45.2+ cells that reached statistical significance at 22 weeks with over 40% of donor cells (Fig. 3b).
Fig. 3. Biased differentiation of mature B-cell subsets in absence of Nfkbie is cell-autonomous.
a Scheme of the competitive BM reconstitution assay. b Percentage of CD45.2+ (donor cells) in peripheral blood of CD45.1 recipient chimeric mice along time after adoptive transfer (n = 5). c Percentage of CD45.2+ B, T, and myeloid cells in the spleen of CD45.1 recipient chimeric mice 7 months post transfer. Each symbol represents one mouse (n = 5). d Analysis of peripheral splenic CD45.2 donor B-cell populations in recipient chimeric mice 7 months post transfer. Left panel: follicular (FoB: CD23+CD21+), marginal zone (MZB: CD23lowCD21high), and immature B cells (IM: CD23−CD21−) distribution. Right panel: splenic B1 B-cell percentage. Each symbol represents one mouse (n = 5). Data are mean ± SEM. *p < 0.05, **p < 0.01; ns, not significant.
Analysis of the distribution of cell lineages showed that Nfkbie+/− and Nfkbie−/− LSK (Lin−Sca1+Kit+) cell transfer resulted in a greater proportion of CD19+ B cells than that of WT cells in recipient mice (Fig. 3c). Consistent with our initial observations, at 22 weeks Nfkbie−/− cells contributed to a significantly higher proportion of splenic MZB cells and B220lowCD19+ B1 cells than WT ones (Fig. 3d). These data establish the cell-autonomous nature of the Nfkbie-deficient-associated phenotypes.
Aged Nfkbie−/− mice develop a MBL-like phenotype
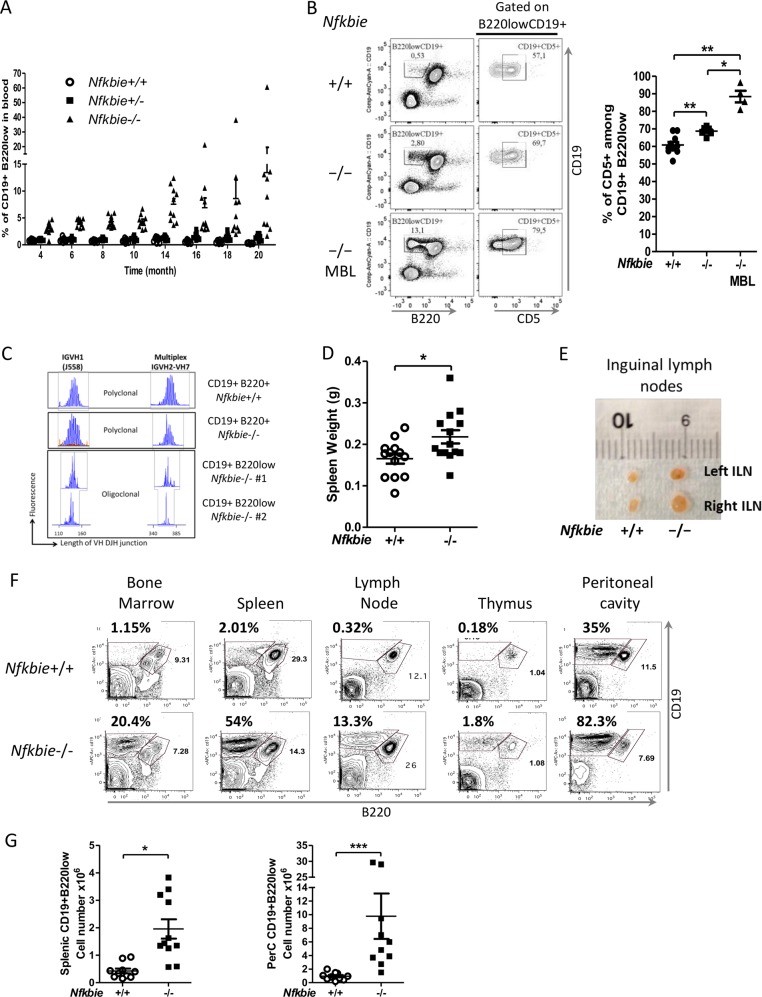
To assess the long-term impact of Nfkbie deficiency, we monitored monthly a cohort of ten Nfkbie−/−, ten Nfkbie+/− mice, and ten control WT mice for the presence of the B220lowCD19+ population in blood. After 12 months, five out of ten (50%) Nfkbie−/− mice started to develop MBL (defined as over 5% of B220lowCD19+ within PBMCs; Fig. 4a). Cytological analysis of blood smears from MBL developing Nfkbie−/− mice showed larger lymphocytes with more abundant cytoplasm around the nucleus (Supplementary Fig. 4a). Nfkbie−/− mice that developed MBL-like disease showed a clear expansion of CD5+ cells among the B220lowCD19+ population (Fig. 4b). Analysis of IGH gene rearrangements revealed an oligoclonal pattern of the B220lowCD19+ population in Nfkbie−/− mice (Fig. 4c). However, total white blood cell counts were not significantly elevated in 20-month-old Nfkbie−/− mice compared with those of control mice (Supplementary Fig. 4b) neither was survival rate of these aged mutant animals (not shown). A single Nfkbie−/− mouse, devoid of MBL, developed a disseminated tumor in subcutaneous tissues in the cervical area, resulting in a swollen neck. Flow cytometric analysis of tumor-infiltrating cells showed a dominant population of B220+CD19+CD5− B-cell population (data not shown). Nevertheless, old Nfkbie−/− mice (>12 months) showed a mild splenomegaly (Fig. 4d) and 60% (6/10) of mice presented enlarged lymph nodes (Fig. 4e). They had no systemic symptoms and flow cytometry, and cell count analysis revealed significant multiorgan infiltration of B220lowCD19+ cells (Fig. 4f, g).
Fig. 4. Aged Nfkbie−/− mice develop a MBL-like phenotype.
a Peripheral blood was monitored for the presence of CD19+B220low B cells starting at 4 months. Each symbol represents one mouse (n = 10). b Right panel: percentage of CD5+ cells among CD19+B220low population in the peripheral blood of 16-month-old mice. Each symbol represents one mouse. Left panel: representative plots of FACS analysis of the CD19+B220low B cells and of the expression of CD5 on the CD19+B220low population in the peripheral blood of Nfkbie+/+ (n = 10), Nfkbie−/− without MBL(−/−) (n = 6), and Nfkbie−/− with MBL (−/−MBL) (n = 4). c Analyses of BCR rearrangement clonality in sorted Nfkbie+/+ CD19+B220+ B cells, and Nfkbie−/− CD19+B220+ and CD19+B220low B cells of two different 14-month-old mice (#1 and #2). V–J junctions were PCR amplified from cDNA of sorted B cells using VH family primers (VH1 and multiplexed PCR of VH2, VH3, VH5, VH6, and VH7) and FAM-conjugated consensus JH primer to assess the IgH CDR3 diversity. d Spleen weight of old (>12 months) Nfkbie+/+ (n = 13) and Nfkbie−/− mice (n = 14). Each symbol represents one mouse. e Representative image from littermate Nfkbie+/+ and Nfkbie−/− old mice showing enlarged inguinal lymph nodes of Nfkbie−/− mice. f Representative flow cytometry profiles of CD19+B220low infiltration in bone marrow, spleen, lymph nodes, thymus, and peritoneal cavity of 18-month-old Nfkbie−/− mice with MBL (n = 5) and age-matched Nfkbie+/+ mice. g Absolute cell numbers of CD19+B220low B cells in the spleen and peritoneal cavity of old Nfkbie+/+ and Nfkbie−/− mice (n = 11). Each symbol represents one mouse. Data are mean ± SEM. *p < 0.05, **p < 0.01.
Nfkbie-deficient B1a cells hyper-proliferate in response to TLR stimulation and exhibit enhanced NF-κB signaling
We then investigated the impact of Nfkbie deficiency on the proliferative response of splenic and peritoneal B-cell subsets to T-cell independent stimuli, such as TLR agonists. These stimuli are known to induce NF-κB activity in B cells1,8,20–22,32. FACS-sorted splenic B-cell subsets, FoB (CD19+B220+CD23+CD21+), MZB (CD19+B220+CD23lowCD21hi), and B1 (CD19+B220low) cells were stimulated with anti-IgM, LPS, or CpG oligodeoxynucleotides, and cell division was measured by CFSE dilution and cell count after 72 h of culture. We found that splenic B1 (Fig. 5a) and MZB (Supplementary Fig. 5a) cells lacking Nfkbie displayed increased proliferation rate in response to LPS and CpG compared with WT B cells. Furthermore, Nfkbie−/− MZB cells showed significantly higher proliferation rate in response to IgM stimulation compared to WT MZB cells (Supplementary Fig. 5a). There was no significant difference in FoB cells proliferation in response to LPS, CpG, and anti-IgM between mutant and WT cells (Supplementary Fig. 5b). As WT B1 cells, Nfkbie−/− B1 cells did not respond to anti-IgM stimulation (data not shown). This confirms that MZB and B1 and to a lesser extent FoB cells require IκBε to limit their proliferative response in a B-cell-intrinsic manner.
Fig. 5. In B cells, IκBε sets the proliferation threshold in response to TLR or T-dependent antigen stimulations.
a Sorted splenic B1 cells were labeled with CFSE and cultured in the presence of LPS or CpG. Cell division was measured by CFSE dilution at 72 h. Percentages of cells that underwent at least one division and cell numbers at 72 h are shown. Each symbol represents one mouse (n = 3). b Sorted peritoneal Nfkbie+/+ B1a, Nfkbie−/− B1a IgMhi, and Nfkbie−/− B1a IgMlow cells were labeled with CFSE, cultured in the presence of LPS or CpG, and analyzed as in a. Each symbol represents one mouse. Nfkbie+/+ (n = 4), Nfkbie+/− mice (n = 5), and Nfkbie−/− mice (n = 5). c Sorted peritoneal Nfkbie+/+ B1a, Nfkbie−/− B1a IgMhi, and Nfkbie−/− B1a IgMlow cells were cultured in complete medium without any stimulant for 24 h. Spontaneous cell death was assessed by sytox staining. Each symbol represents one mouse. Nfkbie+/+ (n = 4), Nfkbie+/− mice (n = 5), and Nfkbie−/− mice (n = 5). d Analysis of phosphorylated p65 (Ser536) in sorted Nfkbie−/− and Nfkbie+/+ B1a B cells at T0 and after treatment with LPS for 15 min and 3 h. Each symbol represents one mouse (n = 3). e Analysis of intracellular and nuclear localization of cRel in sorted B1a Nfkbie−/− and Nfkbie+/+ B cells at T0 and after treatment with LPS for 15 min, 3 h, and 18 h. To show nuclear localization of cRel, cytoplasm was removed from cRel-stained cells with nuclear isolation media (HBSS media with 1 mM EDTA and 0.5% NP-40). Each symbol represents one mouse (n = 3). f Sorted Nfkbie−/− and Nfkbie+/+ B1a B cells were stimulated with LPS and treated for 72 h with serial dilutions of IT-901. At 72 h viable cell number was determined by cell count and trypan blue exclusion assay. The percentage of inhibition was calculated relative to vehicle-treated cells number. 0 stands for vehicle (DMSO). Each symbol represents one mouse (n = 3). g Sorted Nfkbie−/− and Nfkbie+/+ B1a B cells were stimulated with LPS and treated for 72 h with serial dilutions of IT-901. Apoptosis was evaluated by Annexin V–PE and sytox staining. Percentages of double-negative (i.e., Annexin V-negative and sytox-negative) living cells are shown. 0 stands for vehicle (DMSO). Each symbol represents one mouse (n = 3). Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
We next focused our analysis on B-cell subsets from the peritoneal cavity. Peritoneal B2 B cells were sorted as CD19+B220+. Similarly to splenic FoB cells, increased proliferation of Nfkbie−/− peritoneal B2 B-cells in response to stimuli did not reached statistical significance when compared with WT cells (Supplementary Fig. 5c).
In Nfkbie−/− mice, peritoneal B1a B cells were sorted as two populations, B1a IgMhi and B1a IgMlow, while in Nfkbie+/+ mice B1a B cells were sorted as one single population expressing high levels of IgM (Fig. 1c). Analysis of IgM expression levels between Nfkbie+/+ B1a cells, Nfkbie−/− B1a IgMhi showed no significant difference (Supplementary Fig. 5d). Similarly to WT cells, Nfkbie−/− B1a IgMhi and IgMlow cells did not respond to anti-IgM stimulation (data not shown). Nfkbie−/− B1a IgMhi cells showed higher proliferation in response to LPS or CpG stimulation compared to WT B1a B cells (Fig. 5b), whereas Nfkbie−/− B1a IgMlow cell response was similar to that of WT B1a B cells and significantly inferior to that of Nfkbie−/− B1a IgMhi (Fig. 5b).
Spontaneous cell death assessment of sorted WT B1a cells, Nfkbie−/− B1a IgMhi and IgMlow subsets was quantified after 24 h ex vivo culture without any stimuli using sytox blue staining. A minor fraction of Nfkbie−/− B1a IgMhi cells underwent cell death in such ex vivo culture (Fig. 5c). However, WT B1a cells and Nfkbie−/− B1a IgMlow cells showed a significant higher death ratio than Nfkbie−/− B1a IgMhi cells (Fig. 5c).
We next sought to investigate how IκBε deficiency impacts NF-κB activation in CD5+B1a B cells through analyzing phosphorylated p65 (RelA) levels and cRel nuclear localization by flow cytometry33,34. We found significantly higher levels of p65 phosphorylation and cRel nuclear translocation in LPS-stimulated Nfkbie−/− B1a B cells compared to those of their WT counterparts (Fig. 5d, e). No difference was observed between Nfkbie−/− and WT B1a B cells in the expression levels of cRel (Fig. 5e) and p65 (not shown) in quiescent cells or after LPS stimulation. These data indicate that IκBε deficiency in CD5+B1a B cells leads to increased NF-κB activation upon stimulation.
Since IκBε deficiency leads to enhanced NF-κB activation, we hypothesized that Nfkbie-deficient B1a B cells would be differentially sensitive to NF-κB inhibition. We thus treated Nfkbie−/− and WT B1a B cells with increasing concentration of the NF-κB inhibitor IT-901 (ref. 34), in presence or absence of LPS stimulation. Cells were analyzed for the growth inhibition and apoptosis induction. We found that IT-901 inhibited cell growth of both Nfkbie−/− and WT B1a B cells in a dose-dependent manner; however, the IC50 value was higher for Nfkbie−/− B1a B cells (3 µM) compared to that of WT B1a B cells (2.4 μM; Fig. 5f). At 4 µM, no significant difference in cell growth inhibition was observed (Fig. 5f). Analysis of apoptosis showed a significant difference in survival between Nfkbie−/− and WT B1a B cells when cells where vehicle treated or IT-901 treated at 0.5 µM, 1 µM, and 2 µM, whereas such difference was lost at 4 µM and 8 µM (Fig. 5g). These data show that Nfkbie−/− B1a B cells are generally less sensitive to NF-κB activity inhibition than WT B1a B cells.
Overall these data demonstrate that IκBε is critical to limit B-cell proliferation in response to stimuli through the control of p65 phosphorylation and cRel nuclear translocation. They also establish a link between IgM expression levels and proliferation of Nfkbie-deficient B cells.
Nfkbie deficiency enhances GC B-cell proliferation
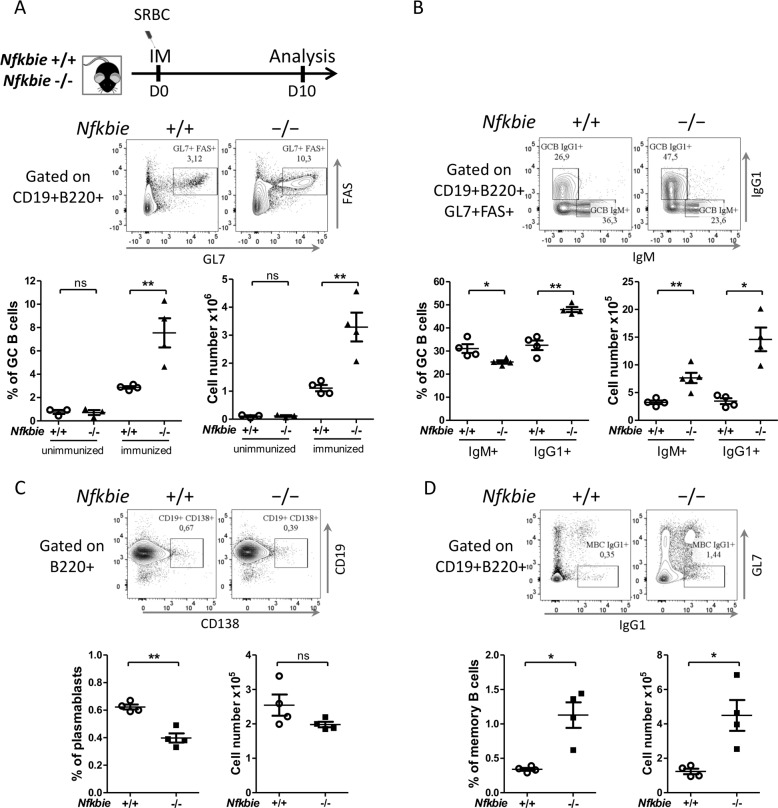
GC reaction is necessary for maturation of the humoral immune response, including production of high-affinity plasma cells and memory B-cells. We explored the impact of Nfkbie absence in GC by immunizing mice with SRBCs. FACS analysis revealed an increase in both percentages and absolute cell numbers of GC B cells in Nfkbie−/− mice compared with those of WT mice (Fig. 6a). Frequency of follicular T-helper cells remained similar in both genotypes (data not shown). In Nfkbie−/− mice, IgG1+ GC B cells were significantly increased and IgM+ GC B cells were significantly decreased (Fig. 6b). Percentages but not absolute cell numbers of plasmablasts/plasma cells were reduced in Nfkbie−/− mice compared to those of WT mice (Fig. 6c). Nfkbie−/− mice had significantly higher percentages and cell numbers of GL7-IgG1+ memory B cells (Fig. 6d). These data suggest that IκBε plays a role in the generation/proliferation of GC B cells, isotype switching, and terminal differentiation of GC B cells.
Fig. 6. Nfkbie deficiency enhances GC B-cell formation.
a Scheme of the immunization protocol. Mice were intraperitoneally immunized with SRBC on day 0 (D0) and analyzed 10 days afterward (D10). Representative flow cytometry analysis of splenocytes from SRBC-immunized mice of the indicated genotypes. Gates depict germinal center (GC) B cells (B220+CD19+CD95(Fas)+GL7+). Percentages and absolute cell numbers of GC B cells in the spleen of immunized mice are shown. Each symbol represents one mouse (n = 4). b Flow cytometry plots showing IgM and IgG1 expression by GC (B220+CD19+CD95(Fas)+GL7+) B cells. Histograms show percentages of IgG1+ and IgM+ Nfkbie+/+, and Nfkbie−/− GC B cells. Each symbol represents one mouse (n = 4). c Splenic plasma cells generation after SRBC immunization of Nfkbie+/+ and Nfkbie−/− mice; shown as FACS profiles (upper panel) and quantitation (lower panel). Each symbol represents one mouse (n = 4). d Memory B cells formation after SRBC immunization of Nfkbie+/+ and Nfkbie−/− mice; shown as FACS profiles (upper panel) and quantitation (lower panel). Each symbol represents one mouse (n = 4). Data are mean ± SEM. *p < 0.05, **p < 0.01; ns, not significant.
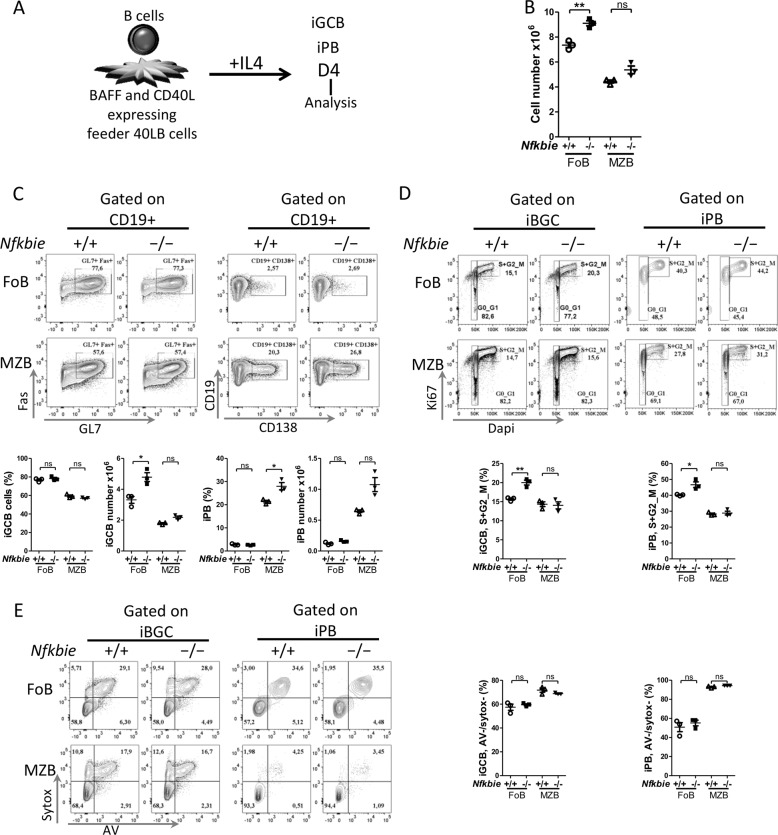
To establish the cell-autonomous nature of this phenotype, we sorted naive B cells from Nfkbie−/− and Nfkbie+/+ mice, and compared their capacity to generate GC B cells in vitro under culture conditions that mimic the GC reaction26 (Fig. 7a for scheme). To avoid the confounding effect of Nfkbie deficiency in biasing mature B-cell subsets repartition, we sorted B cells as separate FoB and MZB populations. At day 4 (D4), we observed a significant increase in the total cell numbers of Nfkbie−/− FoB cells compared to Nfkbie+/+ FoB cells (Fig. 7b); however, differences between Nfkbie−/− and Nfkbie+/+ MZB cells did not reach statistical significance (Fig. 7b). Flow cytometry analysis showed significantly increased numbers of in vitro-induced GC B cells (iGCB; CD19+GL7+CD95+) derived from Nfkbie−/− vs Nfkbie+/+ FoB cells but not for MZB cells at D4 (Fig. 7c). In vitro-induced plasmablast (iPB) population (CD19+CD138+) was higher in Nfkbie−/− compared to Nfkbie+/+ MZB cells at D4 (Fig. 7c).
Fig. 7. Nfkbie deficiency induces germinal center generation and amplification in vitro.
a Scheme of the germinal center B cells (iGCB) and plasmablasts (iPB) in vitro culture system. b Total number of live Nfkbie+/+ and Nfkbie−/− cells cultured on 40LB with IL4 and analyzed on day 4 (D4). Each symbol represents one mouse (n = 3). c Flow cytometry plots show live iGCB cells (CD19+GL7+FAS+) and iPB (CD19+CD138+) generated from FoB and MZB cells. Histograms present percentages (upper) and absolute cell numbers (lower) of iGCB cells and iPB on day 4. Each symbol represents one mouse (n = 3). d Representative cell cycle profiles of iGCB cells (left) and iPB cells (right), labeled with Dapi and anti-Ki67, and analyzed by flow cytometry at day 4 (D4). Numbers indicate percentages of cells in the gated populations. Histograms show percentages of the iGCB cells (left) and iPB (right) in the S and G2/M phases of the cell cycle on day 4. Each symbol represents one mouse (n = 3). e Flow cytometry plots show representative apoptosis analysis of iGCB cells (left) and iPB cells (right), labeled with Annexin V and sytox, and analyzed by flow cytometry at day 4 (D4). Numbers indicate percentages of cells in the gated populations. Histograms show percentages of viable (defined as Annexin V− and sytox−) iGCB cells (left) and iPB (right) on day 4. Each symbol represents one mouse (n = 3). Data are mean ± SEM. *p < 0.05, **p < 0.01; ns, not significant.
We further investigated iGCB cell number changes in this system by analyzing cell cycle and apoptosis. iGCB cells derived from Nfkbie−/− FoB cells displayed a significant increase of S and G2/M phases as compared to iGCB cells derived from Nfkbie+/+ FoB cells (Fig. 7d). No such difference was observed for MZB cells (Fig. 7d). Analysis of the apoptosis rate showed no difference between Nfkbie−/− and Nfkbie+/+ in both FoB and MZB cells subsets (Fig. 7e).
These in vitro data mirror the in vivo results and indicate that Nfkbie deficiency in B cells induces expansion of GC B cells through increasing proliferation of GC B cells in a B-cell-autonomous fashion.
To demonstrate the direct impact of Nfkbie deficiency on proliferation via cell cycle, we used a CRISPR/Cas9 approach and successfully generated three knockout Nfkbie clones of the pro-B-cell line Ba/F3 (Supplementary Fig. 6a). We confirmed the knockout of Nfkbie gene at the protein level in the three clones (Supplementary Fig. 6a). Proliferation survival and cell cycle analysis were performed in control and edited cells in the presence of IL-3 at 24 h, 48 h, and 72 h. Nfkbie knockout clones exhibited increased proliferation when compared to WT cells (Supplementary Fig. 6b). Nfkbie knockout clones showed a decrease in the percentages of cells in G0/G1 phase, and an increase in S and G2/M phases as compared to controls (Supplementary Fig. 6c). Analysis of apoptosis failed to detect any changes between knockout and WT cells (Supplementary Fig. 6d). Altogether, these data indicate that the effect of Nfkbie deficiency on proliferation of Ba/F3 cells is due to an increased cell cycle entry and is apoptosis independent.
Nfkbie loss cooperates with MYD88 signaling
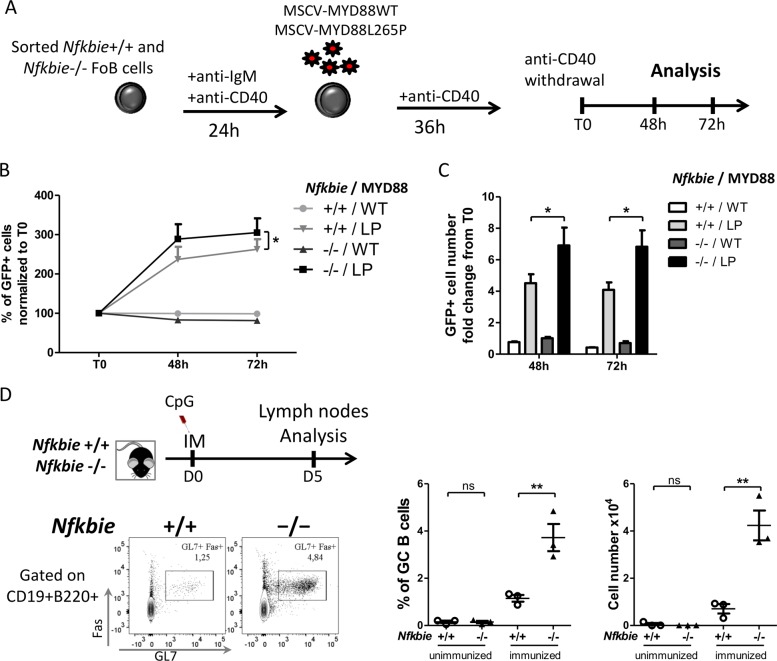
MYD88 codes for an proximal signaling adaptor downstream of IL-1R and mammalian TLRs. Mutations in MYD88, essentially the missense p.L265P mutation, are observed in a wide range of B-cell malignancies8,10,13,35–39. They have been shown to activate the JAK and NF-κB pathways36. Since both NFKBIE and MYD88 mutations may control TLR signaling and co-occur in human B-cell malignancies (Table 1), we used mutant MYD88 to document the interactions between MYD88 and Nfkbie mutations in primary B cells. Purified WT or Nfkbie−/− follicular (CD19+B220+CD23+CD21+) B cells were activated with anti-IgM and anti-CD40, transduced with MYD88 (WT) or MYD88L265P (LP) retrovirus, and cultured with anti-CD40 for 36 h and the proportion of fluorescent cells was monitored in the culture, as described40 (Fig. 8a for scheme). As already reported40, expression of mutant MYD88 conferred a growth advantage over B cells expressing WT constructs. However, ectopic expression of MYD88LP conferred a significant growth advantage to the Nfkbie-deficient-transduced cells in comparison to other genotypes (Fig. 8b). Nfkbie-deficient-MYD88LP-transduced cells showed an increased cell number compared to other conditions (Fig. 8c). This indicates that targeting the TLR-NFKB axe endows the double-mutant cell with a growth advantage over the other cells.
Table 1.
Co-occurrence of NFKBIE and MYD88 mutations in several human B-cell malignancies.
| Number of MYD88-mutated patient | Number of NFKBIE-mutated patient | Number of MYD88 and NFKBIE-mutated patient | B-cell neoplasm | Reference |
|---|---|---|---|---|
| 172 | 37 | 5 | DLBCL | Reddy et al.11 |
| 8 | 4 | 1 | rDLBCL | Morin et al.17 |
| 1 | 1 | 1 | PCDLBCL-LT | Fox et al.37 |
| 15 | 27 | 1 | CLL | Nadeu et al.39 |
| 16 | 1 | 1 | WM | Roos-Weil et al.35 |
DLBCL diffuse large B-cell lymphoma, rDLBCL relapsed diffuse large B-cell lymphoma, PCDLBCL-LT primary cutaneous diffuse large B-cell lymphoma-leg type, CLL chronic lymphocytic leukemia, WM Waldenström macroglobulinemia.
Fig. 8. Actively mutated MYD88 cooperate with Nfkbie deficiency in B cell.
a Scheme for retrovirally introducing wild type (WT) and mutant MYD88 (L265P) into activated Nfkbie+/+ and Nfkbie−/− mature splenic B cells. B cells were activated by anti-IgM with anti-CD40 for 24 h. They were then infected with a retrovirus expressing MYD88WT-GFP or MYD88L265P-GFP, and cultured with anti-CD40 for 36 h. Afterward, cells were washed to withdraw anti-CD40 (time (T) 0) and GFP-positive-cell percentages b and absolute numbers c were monitored at 48 h and 72 h. b Percentages of GFP-positive cells normalized to T0. Results are mean ± SD of three independent experiments performed in duplicate. c Fold changes of absolute cell numbers of GFP-positive cells normalized to T0. Data are mean ± SD of three independent experiments performed in duplicate. d Representative flow cytometry analysis of lymph node from CpG-injected mice of the indicated genotypes. Gates depict germinal center (GC) B cells (B220+CD19+CD95(Fas)+GL7+). The percentage of GC B cells and their cell number in the lymph node are shown. Each symbol represents one mouse (n = 3). Data are mean ± SEM. *p < 0.05.
MYD88 is the canonical adaptor for signaling pathways downstream of the members of the TLR family, including TLR9. Genetic or pharmacological inactivation of TLR9 was shown to inhibits MYD88L265P-induced proliferation in murine and human B-cell lines40,41. TLR9 is an essential component of the oncogenic BCR signaling supercomplex (MyD88-TLR9-BCR) identified in ABC-DLBCL and leads to NF-κB activation41. Therefore, to examine the cooperation between Nfkbie loss and MYD88 signaling in vivo, we immunized mice intraperitoneally with the TLR9 ligand, CpG oligodeoxynucleotides, and analyzed inguinal lymph nodes 5 days later for GC B-cell formation. We observed a significant higher GC B-cell frequency and absolute cell numbers in Nfkbie−/− compared to Nfkbie+/+ mice (Fig. 8d). In the spleen, there was no induction of GC B-cell generation as the percentage of GC B cells was similar to unimmunized mice (data not shown). These data indicate that signaling through TLR9/MYD88 may cooperate with Nfkbie deficiency to promote GC B-cell formation and proliferation.
Discussion
The prevalent inactivation of NFKBIE in human BCL and the link between polymorphisms in the NFKBIE gene, and human autoimmune and infectious diseases42,43 suggest a role for IκBε in B-cell development, function, and transformation. To address this question, we analyzed in detail young and aging Nfkbie-deficient mice. Our data indicate that IκBε activity is required for correct B-cell development on the one hand, and for limiting B-cell activation induced by stimuli triggered by pathogens, such as TLR ligands and for restricting proliferation of GC B cells on the other hand. In addition, Nfkbie deficiency induces MZB cell amplification —in line with previous observations20—due to a higher differentiation of transitional B cells toward MZB cells over the FoB-cell pathway. In B cells, IκBε provides negative regulation upon TLR stimulation by controlling NF-κB activation through limiting nuclear translocation of Rel-containing NF-κB dimers (p65 and cRel)2,20–22. In LPS-stimulated IκBε-deficient CD5+B1a B cells, we observed stronger p65 phosphorylation and cRel nuclear translocation, suggesting enhanced NF-κB activity in these cells. This was further supported by NF-κB activity inhibition experiments, which uncovered a decreased sensitivity to drug-induced apoptosis and cell proliferation inhibition of IκBε-deficient B1a B cells, with respect to their WT counterparts.
NF-κB activity downstream BCR and cytokine receptors signaling plays critical roles in the commitment of transitional B cells into follicular or MZB cell fate29 and in the development of mature B1 B cells28,30. Mice deficient in components of the alternative NF-κB pathway (e.g., NF-κB2) or of the classical NF-κB pathway (e.g., NF-κB1 and Rel), present a drastic reduction in the number of FoB and MZB cells, and B1 B cells, respectively28. In keeping with its negative regulator function20,21, absence of Nfkbie elicits elevated NF-κB activity downstream BCR and cytokine receptors2,20–22 that likely accounts for the bias in mature B-cell development observed here.
We also uncovered that Nfkbie activity is required for correct development and function of B1 B cells. B1 cells might differentiate from fetal liver and adult bone marrow B1 progenitors31. The bone marrow of Nfkbie−/− mice contains more B1 progenitors than WT mice. However, we did not observe increased proliferation or survival of the B1 B cells directly isolated from Nfkbie−/− and WT mice. These findings suggest a role for IκBε in the generation or expansion of B1 progenitor pool. BCR signaling plays an instructive role in B1a cell lineage determination and maintenance28,30,44,45. In addition, recent studies showed that BCR signaling cooperates with TLR signaling in controlling expansion and activation of B1a B cells46–49. NF-κB is one of the key transcription factors activated by BCR and TLR signaling. Consequently, increase of NF-κB activation upon BCR and TLR signaling likely drives the expansion of B1a cell compartment observed in the Nfkbie-deficient mice.
Consistent with IκBε being required to limit B-cell activation20,22, our findings revealed that Nfkbie deficiency enhances proliferation of MZB and B1a IgMhi B cells in response to the TLR4 and TLR9 ligand, LPS, and CpG oligodeoxynucleotides respectively. B1a cells of Nfkbie-deficient mice show a significant downregulation of IgM expression in peritoneal B1a cells, with respect to WT and presented two B220lowCD19+CD5+B1a populations with different levels of IgM expression (B1a IgMhi and B1a IgMlow). Nfkbie−/− B1a IgMlow population showed impaired proliferation response to TLRs stimuli and reduced ex vivo survival compared to B1a IgMhi cells, in agreement with the key role of BCR in B1 cells expansion and activation28,30,45–49. Downregulation of IgM on B cells contributes to a physiological B-cell immune tolerance mechanism called anergy, and serves to restrain their response to endogenous/self-antigens and to chronic BCR stimulation50. Our results strongly suggest that the B1a IgMlow population is phenotypically and functionally anergic as defined by in vivo downregulation of surface IgM expression, CD5 expression, in vitro hyporesponsiveness to TLR-dependent stimuli and ex vivo-reduced survival50–54. Importantly, these properties are hallmark features of human CLL44,54, in which NFKBIE is mutated in ~10% of cases2,3,15,16. Recent work has uncovered cooperation between BCR and TLR in response to innate mitogenic stimuli. BCR-deficient B cells show impaired proliferation in response to LPS or CpG, indicating that correct BCR surface expression and signaling are critical for B-cell proliferation in response to such innate immune stimuli48,49. However, whether regaining IgM surface expression reverses anergic functions in these cells, as reported for autoreactive anergic cell model studies55, needs to be tested.
Nfkbie-deficient mice develop several features of human lymphoproliferative B-cell disorders, in particular, MBL, a low-penetrance premalignant stage13,56. Firstly, Nfkbie deficiency in mice leads to the expansion of an oligoclonal CD19+CD5+B-cell subset, and in humans, mature CD19+CD5+B cells, show high transcriptional and functional similarity to CLL B cells and are candidate for being a normal cellular counterpart of CLL B cells13,45,54. Secondly, Nfkbie-deficient B1a cells exhibited increased survival, proliferation, and enhanced NF-κB activity in response to stimulation consistently with the observations made for NFKBIE-mutated human CLL B-cells2. Thirdly, B220lowCD19+CD5+B cells expanded dramatically in the peripheral blood of old Nfkbie-deficient mice and infiltrated several organs, including spleen and lymph nodes. Finally, the lymphoproliferative disease developed by Nfkbie-deficient mice is indolent and has low penetrance, resembling the mostly indolent course of MBL and CLL, diseases of elderly population. Aging is associated with activation of innate immune cells, including a subset of B1a B cells, due to the increased auto- and pathogen-derived antigen availability, age-associated immune dysfunction, and age-induced gut permeability45,57,58. These age-associated changes could participate in the generation of MBL-like and/or CLL-like malignancies45,54,59. In this perspective, the indolent MBL development in Nfkbie−/− mice might largely be attributed to the increased survival and hyperproliferation of B1a IgMhi B cells, in response to immune stimulation that would be more available with age and the anergic nature of B1a IgMlow B cells and their hyporesponsiveness to stimulation. Indeed, in mice, MBL and CLL develops from B1a CD5+B cells and several study demonstrate a crucial role for BCR expression, reactivity, and signaling in the malignant transformation of these cells45,60–62.
NF-κB activity plays an important role in the regulation of GC reaction32,63. Conditional deletion of Rel and Rela in GC B cells revealed that Rel is required to maintain GC populations, whereas Rela promoted Blimp-1-mediated plasma cell development63. Our data show that under T-cell-dependent immunization condition, Nfkbie deficiency promotes the excessive generation of GC B cells but not TFH cells in vivo. The amplification of GC B cells seems to be caused by an increased proliferation of Nfkbie-deficient GC B cells, suggesting a role for IκBε in controlling proliferation of GC B cells. In line with these findings, it was recently shown that activating mutations in CARD11 (observed in ~5% of DLBCL (ref. 5)), a NF-κB activator protein downstream BCR signaling, increases GC formation and GC B-cell proliferation through enhanced cycling64. GC B cells represent the origin of several BCLs including DLBCL (ref. 32). Deregulated NF-κB signaling is a hallmark of GC-derived B-cell malignancies3,5,6,8. Mutations in NFKBIE gene are detected in ~5% of DLBCL and 23% of PMBCL (refs. 12,17). Our results provide a functional link between Nfkbie mutations and GC-derived BCL. As pointed above, the absence of Nfkbie induces a bias of B2 B-cell development toward MZB cells. MZB B cells are the normal cellular counterpart of splenic marginal zone lymphoma, a BCL in which NFKBIE is also mutated in ~3% of cases12. Our data strongly suggest that IκBε plays an essential role in regulating proliferation and differentiation of the B cells from which these lymphomas derive.
In CLL and several BCL, aberrant NF-κB activation is the result of genetic alterations in pathways leading to NF-κB activation and aberrant stimulation from the microenvironment (through BCR, CD40, and TLR)2,3,5,6,8,10–12,32. In vivo injection of the TLR9 ligand, CpG oligodeoxynucleotide, increased generation, and proliferation of Nfkbie−/− GC B cells compared to that of WT ones. Accordingly, MYD88L265P mutation, a mutation observed across many subtypes of human lymphoid malignancies, increased proliferation of Nfkbie−/− cells compared to that of WT B cells in vitro. In line with these findings, in Waldenström macroglobulinemia and DLBCL human cell lines, inactivation of TNFAIP3 (encoding A20, a negative regulator of NF-κB signaling) also cooperates with MYD88LP mutation to enhance NF-κB activation and resistance to BCR signaling inhibitor, ibrutinib65,66. Together, these observations demonstrate that inactivation of Nfkbie in mice predisposes to MBL and CLL, and upon collaboration with oncogenic events, it might promote BCL development.
Supplementary information
Acknowledgements
We thank the members of the Gustave Roussy Platforms, including Patrick Gonin for excellent mouse care, and Philippe Rameau for assistance with FACS cell sorting. We thank Dr. Daisuke Kitamura for 40LB cell line. This work is supported by the INSERM, Institut National du Cancer (2013-PLBIO-09, 2016- PLBIO-068 and INCa-DGOS-INSERM 12551), Fondation pour la Recherche Médicale (FRM), Association Laurette Fugain and équipe labelisée Ligue Nationale Contre le Cancer. S. Aoufouchi is supported by Comité Val-d’Oise de la Ligue contre le cancer. W. Darwiche is supported by a Fondation de France postdoctoral fellowship (No 00078457). H. Ghamlouch is a recipient of grants from Region Ile de France, Fondation ARC pour la recherche sur le cancer and Fondation de France (No 00067113).
Author contributions
VDV, DRW, FNK, OAB and HG designed the overall study and interpreted data. VDV, DRW, LS, EM, YL, WD, ZA, FD, SM, TM, SA and HG performed, analyzed and interpreted the experiments. VDV and HG generated figures. DRW, VDV, OAB and HG wrote the manuscript with input from SM.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Veronique Della-Valle, Damien Roos-Weil
Contributor Information
Olivier A. Bernard, Email: olivier.bernard@inserm.fr
Hussein Ghamlouch, Email: hussein.ghamlouch@hotmail.com.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41408-020-0305-6).
References
- 1.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansouri L, et al. Functional loss of IkappaBepsilon leads to NF-kappaB deregulation in aggressive chronic lymphocytic leukemia. J. Exp. Med. 2015;212:833–843. doi: 10.1084/jem.20142009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damm F, et al. Acquired initiating mutations in early hematopoietic cells of CLL patients. Cancer Discov. 2014;4:1088–1101. doi: 10.1158/2159-8290.CD-14-0104. [DOI] [PubMed] [Google Scholar]
- 4.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compagno M, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okosun J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat. Genet. 2014;46:176–181. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herishanu Y, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansouri L, Papakonstantinou N, Ntoufa S, Stamatopoulos K, Rosenquist R. NF-kappaB activation in chronic lymphocytic leukemia: a point of convergence of external triggers and intrinsic lesions. Sem. Cancer Biol. 2016;39:40–48. doi: 10.1016/j.semcancer.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Oppezzo P, Dighiero G. “Role of the B-cell receptor and the microenvironment in chronic lymphocytic leukemia”. Blood Cancer J. 2013;3:e149. doi: 10.1038/bcj.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz R, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy A, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171:481–494 e415. doi: 10.1016/j.cell.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansouri L, et al. Frequent NFKBIE deletions are associated with poor outcome in primary mediastinal B-cell lymphoma. Blood. 2016;128:2666–2670. doi: 10.1182/blood-2016-03-704528. [DOI] [PubMed] [Google Scholar]
- 13.Ghamlouch H, Nguyen-Khac F, Bernard OA. Chronic lymphocytic leukaemia genomics and the precision medicine era. Br. J. Haematol. 2017;178:852–870. doi: 10.1111/bjh.14719. [DOI] [PubMed] [Google Scholar]
- 14.Domenech E, et al. New mutations in chronic lymphocytic leukemia identified by target enrichment and deep sequencing. PLoS ONE. 2012;7:e38158. doi: 10.1371/journal.pone.0038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose-Zerilli MJ, et al. Longitudinal copy number, whole exome and targeted deep sequencing of ‘good risk’ IGHV-mutated CLL patients with progressive disease. Leukemia. 2016;30:1301–1310. doi: 10.1038/leu.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungstrom V, et al. Whole-exome sequencing in relapsing chronic lymphocytic leukemia: clinical impact of recurrent RPS15 mutations. Blood. 2016;127:1007–1016. doi: 10.1182/blood-2015-10-674572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin RD, et al. Genetic landscapes of relapsed and refractory diffuse large B-cell lymphomas. Clin. Cancer Res. 2016;22:2290–2300. doi: 10.1158/1078-0432.CCR-15-2123. [DOI] [PubMed] [Google Scholar]
- 18.Whiteside ST, Epinat JC, Rice NR, Israel A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF-kappa B activity. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simeonidis S, Liang S, Chen G, Thanos D. Cloning and functional characterization of mouse IkappaBepsilon. Proc. Natl Acad. Sci. USA. 1997;94:14372–14377. doi: 10.1073/pnas.94.26.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves BN, et al. IkappaBepsilon is a key regulator of B cell expansion by providing negative feedback on cRel and RelA in a stimulus-specific manner. J. Immunol. 2014;192:3121–3132. doi: 10.4049/jimmunol.1302351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doerre S, et al. Reductions in I kappa B epsilon and changes in NF-kappa B activity during B lymphocyte differentiation. J. Immunol. 2005;174:983–991. doi: 10.4049/jimmunol.174.2.983. [DOI] [PubMed] [Google Scholar]
- 22.Clark JM, et al. Inhibitor of kappa B epsilon (IkappaBepsilon) is a non-redundant regulator of c-Rel-dependent gene expression in murine T and B cells. PLoS ONE. 2011;6:e24504. doi: 10.1371/journal.pone.0024504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memet S, et al. IkappaBepsilon-deficient mice: reduction of one T cell precursor subspecies and enhanced Ig isotype switching and cytokine synthesis. J. Immunol. 1999;163:5994–6005. [PubMed] [Google Scholar]
- 24.Quivoron C, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Mouly E, et al. B-cell tumor development in Tet2-deficient mice. Blood Adv. 2018;2:703–714. doi: 10.1182/bloodadvances.2017014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nojima T, et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat. Commun. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 27.Walter JE, et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J. Exp. Med. 2010;207:1541–1554. doi: 10.1084/jem.20091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen GK, Adori M, Karlsson Hedestam GB. NF-kappaB signaling in B-1 cell development. Ann. NY Acad. Sci. 2015;1362:39–47. doi: 10.1111/nyas.12800. [DOI] [PubMed] [Google Scholar]
- 29.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. 2009;9:767–777. doi: 10.1038/nrc2759. [DOI] [PubMed] [Google Scholar]
- 30.Graf R, et al. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363:748–753. doi: 10.1126/science.aau8475. [DOI] [PubMed] [Google Scholar]
- 31.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 32.Klein U, Heise N. Unexpected functions of nuclear factor-kappaB during germinal center B-cell development: implications for lymphomagenesis. Curr. Opin. Hematol. 2015;22:379–387. doi: 10.1097/MOH.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doan LL, Tanner MK, Grimes HL. Intranuclear staining of proteins in heterogeneous cell populations and verification of nuclear localization by flow cytometric analysis. J. Immunol. Methods. 2003;279:193–198. doi: 10.1016/S0022-1759(03)00184-4. [DOI] [PubMed] [Google Scholar]
- 34.Shono Y, et al. Characterization of a c-Rel inhibitor that mediates anticancer properties in hematologic malignancies by blocking NF-kappaB-controlled oxidative stress responses. Cancer Res. 2016;76:377–389. doi: 10.1158/0008-5472.CAN-14-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos-Weil D, et al. A recurrent activating missense mutation in waldenstrom macroglobulinemia affects the DNA binding of the ETS transcription factor SPI1 and enhances proliferation. Cancer Discov. 2019;9:796–811. doi: 10.1158/2159-8290.CD-18-0873. [DOI] [PubMed] [Google Scholar]
- 36.Ngo VN, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox, L. C. et al. Molecular mechanisms of disease progression in primary cutaneous diffuse large B-cell lymphoma, leg type during ibrutinib therapy. Int. J. Mol. Sci.19 (2018). [DOI] [PMC free article] [PubMed]
- 38.Martinez-Trillos A, et al. Mutations in TLR/MYD88 pathway identify a subset of young chronic lymphocytic leukemia patients with favorable outcome. Blood. 2014;123:3790–3796. doi: 10.1182/blood-2013-12-543306. [DOI] [PubMed] [Google Scholar]
- 39.Nadeu F, et al. Clinical impact of the subclonal architecture and mutational complexity in chronic lymphocytic leukemia. Leukemia. 2018;32:645–653. doi: 10.1038/leu.2017.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JQ, Jeelall YS, Beutler B, Horikawa K, Goodnow CC. Consequences of the recurrent MYD88(L265P) somatic mutation for B cell tolerance. J. Exp. Med. 2014;211:413–426. doi: 10.1084/jem.20131424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phelan JD, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560:387–391. doi: 10.1038/s41586-018-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myouzen K, et al. Functional variants in NFKBIE and RTKN2 involved in activation of the NF-kappaB pathway are associated with rheumatoid arthritis in Japanese. PLoS Genet. 2012;8:e1002949. doi: 10.1371/journal.pgen.1002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundbo LF, et al. Genetic variation in NFKBIE is associated with increased risk of pneumococcal meningitis in children. EBioMedicine. 2015;3:93–99. doi: 10.1016/j.ebiom.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ntoufa S, Vilia MG, Stamatopoulos K, Ghia P, Muzio M. Toll-like receptors signaling: a complex network for NF-kappaB activation in B-cell lymphoid malignancies. Sem. Cancer Biol. 2016;39:15–25. doi: 10.1016/j.semcancer.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Hayakawa K, et al. B cells generated by B-1 development can progress to chronic lymphocytic leukemia. Ann. NY Acad. Sci. 2015;1362:250–255. doi: 10.1111/nyas.12768. [DOI] [PubMed] [Google Scholar]
- 46.Kreuk, L. S. et al. B cell receptor and Toll-like receptor signaling coordinate to control distinct B-1 responses to both self and the microbiota. eLife8 (2019). [DOI] [PMC free article] [PubMed]
- 47.Savage, H. P. et al. TLR induces reorganization of the IgM-BCR complex regulating murine B-1 cell responses to infections. eLife8 (2019). [DOI] [PMC free article] [PubMed]
- 48.Otipoby KL, et al. The B-cell antigen receptor integrates adaptive and innate immune signals. Proc. Natl Acad. Sci. USA. 2015;112:12145–12150. doi: 10.1073/pnas.1516428112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweighoffer E, Nys J, Vanes L, Smithers N, Tybulewicz VLJ. TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK. J. Exp. Med. 2017;214:1269–1280. doi: 10.1084/jem.20161117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 52.Hippen KL, Tze LE, Behrens TW. CD5 maintains tolerance in anergic B cells. J. Exp. Med. 2000;191:883–890. doi: 10.1084/jem.191.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darwiche W, Gubler B, Marolleau JP, Ghamlouch H. Chronic lymphocytic leukemia B-cell normal cellular counterpart: clues from a functional perspective. Front. Immunol. 2018;9:683. doi: 10.3389/fimmu.2018.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat. Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 56.Kostopoulos IV, et al. Clonal evolution is a prognostic factor for the clinical progression of monoclonal B-cell lymphocytosis. Blood Cancer J. 2017;7:e597. doi: 10.1038/bcj.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holodick NE, Rothstein TL. B cells in the aging immune system: time to consider B-1 cells. Ann. NY Acad. Sci. 2015;1362:176–187. doi: 10.1111/nyas.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bodogai, M. et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med.10 (2018). [DOI] [PMC free article] [PubMed]
- 59.Kikushige Y, Miyamoto T. Hematopoietic stem cell aging and chronic lymphocytic leukemia pathogenesis. Int. J. Hematol. 2014;100:335–340. doi: 10.1007/s12185-014-1651-6. [DOI] [PubMed] [Google Scholar]
- 60.Hayakawa K, et al. Early generated B1 B cells with restricted BCRs become chronic lymphocytic leukemia with continued c-Myc and low Bmf expression. J. Exp. Med. 2016;213:3007–3024. doi: 10.1084/jem.20160712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayakawa K, et al. Loss of a chromosomal region with synteny to human 13q14 occurs in mouse chronic lymphocytic leukemia that originates from early-generated B-1 B cells. Leukemia. 2016;30:1510–1519. doi: 10.1038/leu.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayakawa K, et al. Early generated B-1-derived B cells have the capacity to progress to become mantle cell lymphoma-like neoplasia in aged mice. J. Immunol. 2018;201:804–813. doi: 10.4049/jimmunol.1800400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heise N, et al. Germinal center B cell maintenance and differentiation are controlled by distinct NF-kappaB transcription factor subunits. J. Exp. Med. 2014;211:2103–2118. doi: 10.1084/jem.20132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wray-Dutra MN, et al. Activated CARD11 accelerates germinal center kinetics, promoting mTORC1 and terminal differentiation. J. Exp. Med. 2018;215:2445–2461. doi: 10.1084/jem.20180230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wenzl K, et al. Loss of TNFAIP3 enhances MYD88L265P-driven signaling in non-Hodgkin lymphoma. Blood Cancer J. 2018;8:97. doi: 10.1038/s41408-018-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braggio E, et al. Identification of copy number abnormalities and inactivating mutations in two negative regulators of nuclear factor-kappaB signaling pathways in Waldenstrom’s macroglobulinemia. Cancer Res. 2009;69:3579–3588. doi: 10.1158/0008-5472.CAN-08-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.