Abstract
Previous studies demonstrate an association between activation of the maternal immune system during pregnancy and increased risk of neurodevelopmental psychiatric conditions, such as schizophrenia and autism, in the offspring. Relatively recent findings also suggest that the gut microbiota plays an important role in shaping brain development and behavior. Here we show that maternal immune activation (MIA) accomplished by infection with a mouse-adapted influenza virus during pregnancy induced up-regulation of frontal cortex serotonin 5-HT2A receptor (5-HT2AR) density in the adult offspring, a phenotype previously observed in postmortem frontal cortex of schizophrenic subjects. 5-HT2AR agonist-induced head-twitch behavior was also augmented in this preclinical mouse model. Using the novel object recognition (NOR) test to evaluate cognitive performance, we demonstrate that MIA induced NOR deficits in adult offspring. Oral antibiotic treatment of prepubertal mice prevented this cognitive impairment, but not increased frontal cortex 5-HT2AR density or psychedelic-induced head-twitch behavior in adult MIA offspring. Additionally, gut microbiota transplantation from MIA mice produced behavioral deficits in antibiotic-treated mock mice. Adult MIA offspring displayed altered gut microbiota, and relative abundance of specific components of the gut microbiota, including Ruminococcaceae, correlated with frontal cortex 5-HT2AR density. Together, these findings provide a better understanding of basic mechanisms by which prenatal insults impact offspring brain function, and suggest gut-brain axis manipulation as a potential therapeutic approach for neurodevelopmental psychiatric conditions.
Subject terms: Disease model, Microbiome, Schizophrenia
Introduction
Neurodevelopmental psychiatric disorders, including schizophrenia1 and autism2, are severe and usually cause life-long disability. Epidemiological studies have indicated that environmental insults during pregnancy, particularly infection and severe adverse life events, increase the risk of certain psychiatric disorders in offspring. Thus, it has been reported that maternal infection with agents including viruses (influenza and rubella)3,4, bacteria (bronchopneumonia)5 and protozoa (Toxoplasma gondii)6 contribute to the etiologies of neuropsychiatric disorders such as schizophrenia and autism. Converging lines of evidence from humans7 and rodent models8 suggest a link between maternal immune activation (MIA) during pregnancy and abnormalities in offspring brain structure and function. Indeed, rodent9 and non-human primate10 animal models have demonstrated a causal relationship between MIA and neuropathological abnormalities.
There is growing awareness that crosstalk between intestinal bacteria and the CNS is critically important for maintaining homeostasis and plasticity11,12. Three clinical studies demonstrate significant differences in composition of the oropharyngeal13,14 and gut15 microbiome between schizophrenia patients and controls, with similarly altered gut bacterial profiles found in children with autism16,17. Recent preclinical findings also suggest that MIA in mice produces dysbiosis of the intestinal microbiota18–20 and abnormal behavioral phenotypes in offspring18,21. This accumulating evidence supports the existence of altered gut-brain physiological pathways in both MIA models and neurodevelopmental psychiatric conditions. In light of this, antibiotic-induced manipulation of the gut microbiota may serve as a tool to evaluate the contribution of microbiome alterations to the onset of cognitive deficits in mouse prenatal insult models. Other groups have recently reported that prepubertal administration of minocycline – a tetracycline antibiotic also used to inhibit microglial activation – attenuates abnormal behavior in MIA offspring22,23. Nevertheless, molecular mechanisms within the gut-brain axis that are involved in these phenotypes remain largely unclear.
The serotonin 5-HT2A receptor (5-HT2AR) is a family A G protein-coupled receptor (GPCR) involved in mechanisms related to cognition, perception and sensorimotor gating24,25. We previously showed up-regulation of the 5-HT2AR in postmortem frontal cortex of untreated schizophrenic subjects26–28 – a pattern of expression that could predispose to certain schizophrenia symptoms. Remarkably, in three independent mouse models of prenatal insults: influenza virus infection29, variable and unpredictable stress8, and immune activation with poly-(I:C)8, we found up-regulation of cortical 5-HT2AR expression in association with behavioral changes indicative of deficits in perception and cognition8,29, findings that others have validated using a variety of prenatal insult models30–32. Using maternal infection with a mouse adapted influenza A/WSN/33 (H1N1) virus as a preclinical MIA model (Fig. 1), here we asked whether manipulation of the gut-brain axis could serve as a novel approach to reduce transition to MIA-induced phenotypes, including dysregulation of frontal cortex 5-HT2AR density and altered performance in behavioral models of psychosis and cognition.
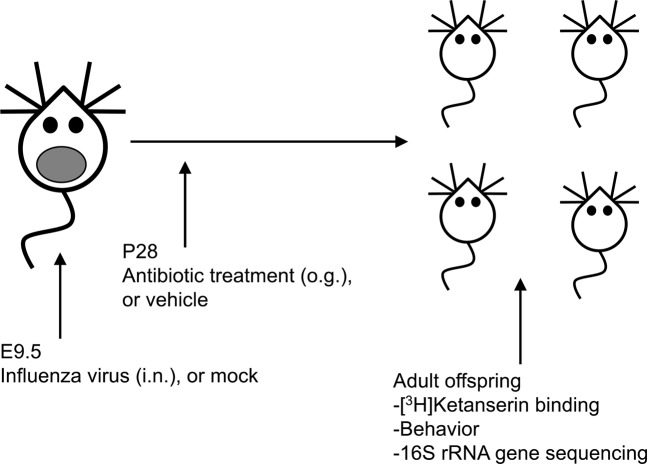
Figure 1.
Schematic representation of a prenatal MIA model. Timed pregnant mice (E9.5) were inoculated (i.n.) with influenza A/WSN/33 (H1N1) virus (5 × 103 pfu), or mock (PBS). After this manipulation, prepubertal (28 d) mice born to influenza virus-infected mothers or mock mothers received a single dose of streptomycin (20 mg), or vehicle by oral gavage. Biochemical, behavioral and 16S rRNA gene sequencing assays were carried out in adult mice.
Results
Prepubertal gut microbiota manipulation does not affect MIA-induced psychosis-related phenotypes later in life
Serotonergic psychedelics, such as lysergic acid diethylamide (LSD) and psilocin, induce in healthy volunteers alterations in perception and sensory processing that show certain similarities to those observed in schizophrenia patients suffering from psychotic episodes33,34. Our previous observations clearly demonstrated that head-twitch behavior represents a mouse behavioral proxy of human psychedelic drug action35,36. Using the psychedelic 5-HT2AR agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) as a pharmacological tool, we already reported that DOI-induced head-twitch behavior was augmented in adult mice born to influenza virus-infected mothers, as compared to control adult mice born to mock-infected mothers29. Consequently, as expected, our findings here show that both head-twitch behavior induced by DOI (0.5 mg/kg) (Fig. 2A) and frontal cortex 5-HT2AR density as measured by radioligand binding assays with the 5-HT2AR antagonist [3H]ketanserin (Fig. 2B) were significantly increased in adult MIA-mice, as compared to adult mice born to mock-infected mothers.
Figure 2.
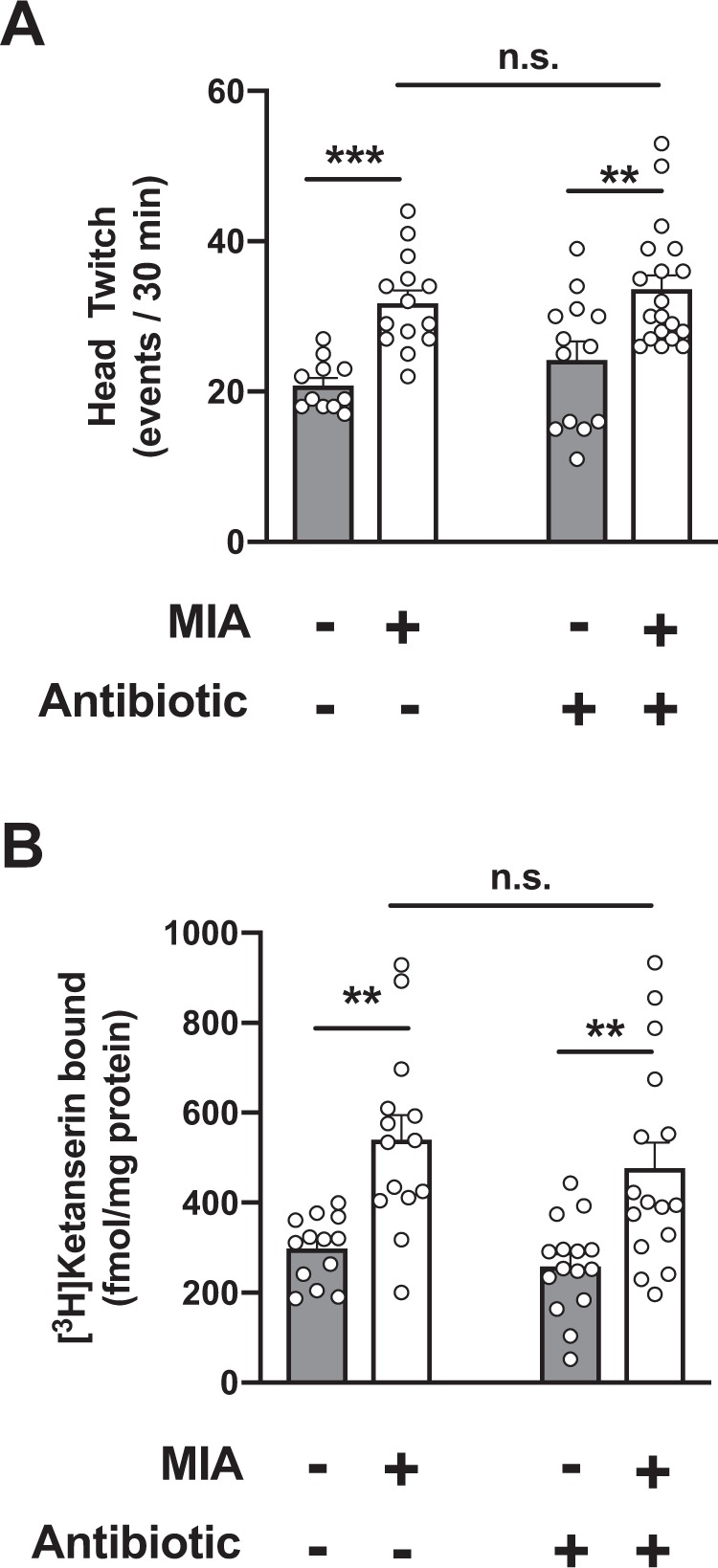
Juvenile mice born to influenza virus-infected mothers (MIA) and controls (mock) received a single dose of antibiotic or vehicle via oral gavage. Head-twitch behavior (A) and [3H]ketanserin binding assays (B) were performed in adult mice. (A) Effect of injection with the psychedelic 5-HT2AR agonist DOI (0.5 mg/kg), or vehicle, on head-twitch behavior (n = 11 – 19). (B) Radioligand binding with the 5-HT2AR antagonist [3H]ketanserin (10 nM) in mouse frontal cortex membrane preparations (n = 13 – 16). Two-way ANOVA (A) F[1,53] = 27.89, p < 0.001; (B) F[1,54] = 27.07, p < 0.001). Bonferroni’s post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant). Data show mean ± s.e.m.
Symptoms of schizophrenia generally first appear during late adolescence or early adulthood1. Unfortunately, results of several clinical studies clearly show that the available antipsychotic medications do not delay or prevent conversion to psychosis or memory deficits in young subjects with initial prodromal symptoms37,38. This underlines the need for new therapeutic strategies to prevent the onset of these psychiatric conditions. Prebiotics are dietary non-digestible fibers that beneficially affect the host’s health mostly by selectively promoting growth and activity of some genera of microorganisms in the colon, generally Lactobacilli or Bifidobacteria39. Previous findings suggest that administration via the drinking water of a specific mixture of non-digestible galacto-oligosaccharides for three weeks prevents the effect of a single injection of lipopolysaccharide on up-regulation of 5-HT2AR immunoreactivity in the mouse frontal cortex40. Based on these findings, together with our previous observations suggesting that up-regulation of frontal cortex 5-HT2AR density is not observed in prepubertal MIA mice8,29, here we tested the extent to which microbiota manipulation during a prepubertal period normalizes MIA-induced alterations in 5-HT2AR expression and 5-HT2AR-dependent behaviors. To do so, mice born to influenza virus-infected mothers during pregnancy or controls received a single dose of antibiotic treatment via oral gavage, or vehicle during the prepubertal period (P28). After this manipulation, mice were housed with normal food and water until they became adult animals (Fig. 1). Increases in head-twitch behavior induced by the psychedelic 5-HT2AR agonist DOI (Fig. 2A) and density of frontal cortex 5-HT2AR when assayed by [3H]ketanserin binding (Fig. 2B) were observed in adult MIA offspring relative to mock offspring regardless of antibiotic treatment at P28.
Prepubertal gut microbiota manipulation prevents MIA-induced deficits in recognition memory
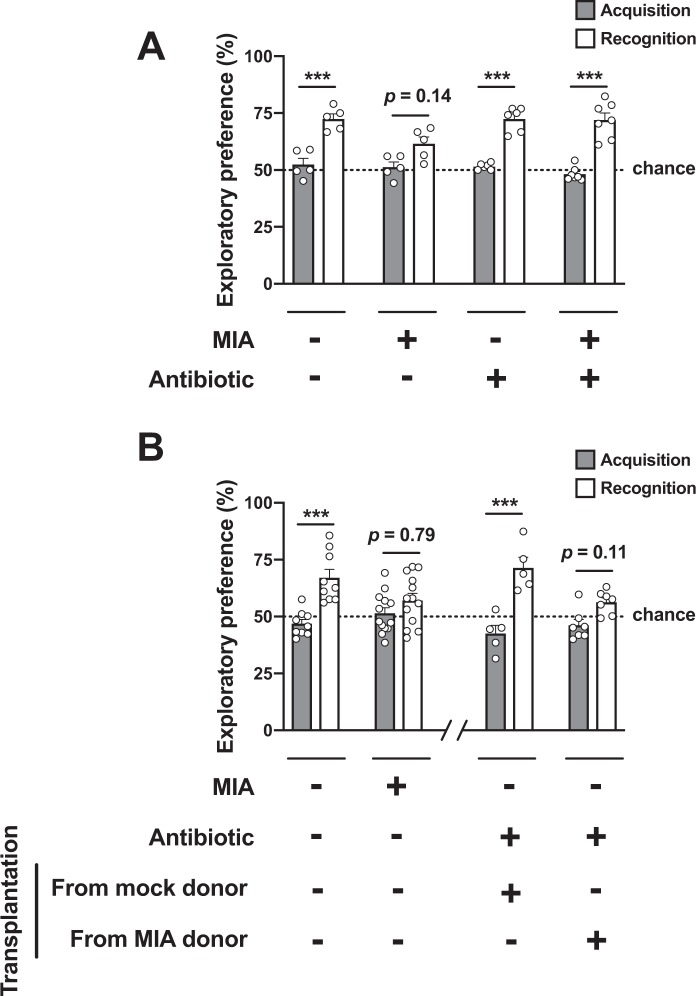
Previous findings including ours suggest that certain models of prenatal insults negatively affect offspring’s cognitive capabilities8,18,21. To test whether prepubertal gut microbiota manipulation modulates MIA-induced cognitive deficits, we evaluated the effect of antibiotic administration via oral gavage at P28 on a novel-object recognition test in adult mice as a measure of cognitive performance. Our results show that novel-object recognition performance was significantly disrupted in adult MIA offspring as compared to adult mock offspring mice when both groups were gavaged with vehicle at P28 (Fig. 3A and Table S1). Importantly, our results also show that prepubertal gut microbiota modulation at P28 prevents the effect of prenatal MIA on adult offspring’s recognition memory performance (Fig. 3A and Table S1).
Figure 3.
(A) Juvenile mice born to influenza virus-infected mothers and controls received a single dose of antibiotic via oral gavage, or vehicle. This paradigm of gut microbiota manipulation prevents MIA-induced cognitive deficits in adult mice. (B) Offspring mice born to influenza virus-infected mothers and controls were allowed to reach adulthood (left side of the panel). Juvenile mice born to control mothers received a single dose of antibiotic via oral gavage, or vehicle. After this manipulation, juvenile mice received fecal microbiota transplantation from adult MIA or mock mice (right side of the panel). Novel object recognition was tested in adult animals (n = 5 – 7 for (A) n = 5 – 13 for (B)). Two-way ANOVA (A) F[1,38] = 135.6, p < 0.001, see also Table S1; (B) (left side of the panel), F[1,40] = 19.76, p < 0.001; (B) (right side of the panel), F[1,20] = 40.14, p < 0.001). Bonferroni’s post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant). Data show mean ± s.e.m.
Microbiota transplantation from MIA mice negatively affects recognition memory
In order to investigate whether the gut microbiota was responsible for triggering cognitive deficits in MIA mice, we transplanted the cecal microbiota from adult MIA mice into young mice born to mock-infected mothers, and tested cognitive capabilities later in life. As before (see Fig. 3A), adult MIA offspring mice show deficits in recognition memory as compared to adult mice born to mock-infected mothers during pregnancy (Fig. 3B). Importantly, adult mice born to mock-infected mothers and transplanted at a prepubertal period with microbiota from adult MIA mice showed behavioral deficits in recognition memory performance (Fig. 3B). This negative effect of gut microbiota manipulation, however, was not observed in adult mice born to mock-infected mothers and transplanted at a prepubertal period with gut microbiota from adult control mice (Fig. 3B).
Influenza virus infection during pregnancy alters adult offspring’s gut microbiota composition, which is rebalanced by juvenile antibiotic treatment
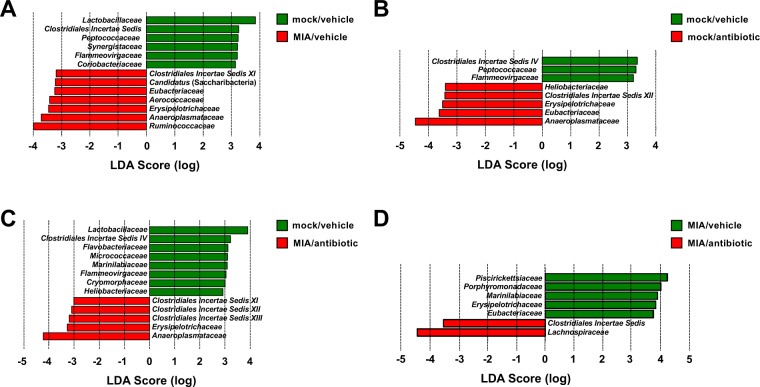
Abnormalities related to the gut microbiota composition have previously been reported in MIA and maternal gestational stress models18,20. To determine the impact of maternal viral infection during the second week of pregnancy on the murine gut microbial community, we surveyed the cecal bacterial composition by 16S rRNA gene sequencing of samples isolated from adult mice born to mothers intranasally exposed to influenza virus, or mock. We used the LEfSe algorithm to define the potential differential bacterial patterns in cecum samples41. Our data show that a total of seven bacterial families were overrepresented in MIA mice – these included Ruminococcaceae, Porphyromonadaceae, Aerococcaceae, and Erysipelotrichaceae (Fig. 4). Similar findings have been observed in the feces of subjects with psychiatric conditions, including schizophrenia, autism and major depressive disorder14,15,42–44. Candidatus (saccharibacteria), an obligate epibiont45, was also more abundant in MIA offspring (Fig. 4). Conversely, adult offspring mice born to mock-infected mothers during pregnancy possessed a gut microbiota enriched in a total of six bacterial families. These included members of the usually beneficial taxa11,46–49 belonging to Lactobacillaceae, Peptococcaceae, and Coriobacteriaceae (Fig. 4). This concept of dysbiosis of the adult gut microbiota induced by maternal infection with a mouse-adapted influenza virus was confirmed by comparison of RDP11 classifier values (Table 1 and Fig. 4).
Figure 4.
Effect of prepubertal antibiotic administration on MIA-induced dysbiosis of the gut microbiota. Juvenile (P28) mice born to influenza virus-infected (MIA) mothers, or mock mothers, received a single dose of antibiotic, or vehicle, via oral gavage at P28. Cecal samples were collected from adult mice and analyzed by linear discriminant analysis effect size (LEfSe). Data are presented in a histogram with Linear Discriminant Analysis (LDA) between groups (n = 9 – 13). (A) mock (juvenile vehicle) vs. MIA (juvenile vehicle). (B) mock (juvenile vehicle) vs. mock (juvenile antibiotic). (C) mock (juvenile vehicle) vs. MIA (juvenile antibiotic). (D) MIA (juvenile vehicle) vs. MIA (juvenile antibiotic).
Table 1.
Relative abundances of bacterial taxa in adult mice born to influenza virus-infected mothers (MIA), or mock, and prepubertally (P28) treated with antibiotics, or vehicle.
| Taxa (family level) | mock – vehicle | MIA – vehicle | mock – antibiotic | MIA – antibiotic | p value |
|---|---|---|---|---|---|
| Erysipelotrichaceae | 0.0022 ± 0.00031 | 0.0067 ± 0.00069 | 0.0056 ± 0.0014 | 0.0046 ± 0.0005 | 0.0005 |
| Eubacteriaceae | 0.00014 ± 0.0001 | 0.0015 ± 0.00038 | 0.0022 ± 0.00056 | 0.0004 ± 0.00022 | 0.0007 |
| Porphyromonadaceae | 0.053 ± 0.0052 | 0.065 ± 0.0069 | 0.045 ± 0.0068 | 0.044 ± 0.0028 | 0.0042 |
| Carnobacteriaceae | 0.00016 ± 0.0002 | N.D. | 0.0011 ± 0.00044 | N.D. | 0.0047 |
| Marinilabiaceae | 0.0020 ± 0.0006 | 0.0044 ± 0.0010 | 0.0017 ± 0.00035 | 0.0005 ± 0.00022 | 0.0073 |
| Coriobacteriaceae | 0.0016 ± 0.00025 | 0.00058 ± 0.0002 | 0.0013 ± 0.00019 | 0.0011 ± 0.00023 | 0.014 |
| Peptococcaceae | 0.0042 ± 0.00070 | 0.0023 ± 0.00031 | 0.0019 ± 0.00048 | 0.0042 ± 0.00066 | 0.014 |
| Clostridiales Incertae Sedis IV | 0.0039 ± 0.00093 | 0.0017 ± 0.00037 | 0.00071 ± 0.00037 | 0.0015 ± 0.00038 | 0.015 |
| Flavobacteriaceae | 0.0021 ± 0.00014 | 0.0021 ± 0.00014 | 0.0016 ± 0.00024 | 0.0015 ± 0.00016 | 0.018 |
| Heliobacteriaceae | 0.0018 ± 0.00014 | 0.0018 ± 0.00022 | 0.0026 ± 0.00048 | 0.0014 ± 0.00019 | 0.022 |
| Lactobacillaceae | 0.024 ± 0.0057 | 0.0094 ± 0.0013 | 0.0129± 0.0025 | 0.0086 ± 0.0019 | 0.023 |
| Clostridiales Incertae Sedis XII | 0.0016 ± 0.00011 | 0.0016± 0.00020 | 0.0026 ± 0.00030 | 0.0021 ± 0.00013 | 0.024 |
| Clostridiales Incertae Sedis XI | 0.00054 ± 0.00021 | 0.0020 ± 0.00054 | 0.00089 ± 0.00023 | 0.0021 ± 0.00051 | 0.025 |
| Anaeroplasmataceae | N.D. | 0.010 ± 0.0035 | 0.055 ± 0.018 | 0.030 ± 0.015 | 0.028 |
| Aerococcaceae | N.D. | 0.00069 ± 0.0002 | 0.00016 ± 0.0001 | 0.00013 ± 0.0001 | 0.033 |
| Candidatus (saccharibacteria) | 0.00062 ± 0.00034 | 0.0024 ± 0.00056 | 0.00055 ± 0.00029 | 0.0029 ± 0.00084 | 0.035 |
| Clostridiales Incertae Sedis | 0.0043 ± 0.0010 | 0.0018 ± 0.00013 | 0.0024 ± 0.00047 | 0.0035 ± 0.00058 | 0.039 |
| Flammeovirgaceae | 0.0010 ± 0.00034 | 0.00015 ± 0.0001 | 0.00013 ± 0.0001 | N.D. | 0.042 |
| Piscirickettsiaceae | 0.00029 ± 0.00019 | 0.00085 ± 0.0003 | 0.055 ± 0.018 | 0.00014 ± 0.0001 | 0.052 |
| Ruminococcaceae | 0.10 ± 0.004 | 0.12 ± 0.004 | 0.11 ± 0.005 | 0.10 ± 0.005 | 0.07 |
| Micrococcaceae | 0.00038 ± 0.00019 | 0.00009 ± 0.003 | 0.00012 ± 0.00036 | N.D. | 0.11 |
| Cryomorphaceae | 0.0010 ± 0.00020 | 0.00079 ± 0.0002 | 0.00096 ± 0.00018 | 0.00056 ± 0.0001 | 0.20 |
| Synergistaceae | 0.0024 ± 0.00014 | 0.0015 ± 0.00028 | 0.0018 ± 0.00049 | 0.0019 ± 0.00037 | 0.23 |
Taxa are listed in order of significance (Kruskal-Wallis test). All values are presented as mean ± s.e.m. N.D., not detected.
We next tested the extent to which treatment of young mice with antibiotics that are not systemically absorbed from the intestine remodels MIA-induced alterations in gut microbiota composition later in life. As above, MIA and mock mice received at P28 a single dose of streptomycin via oral gavage, or vehicle, after which they were allowed access to normal food and water until adulthood. Our data show a relatively low number of alterations in gut microbiota composition of adult offspring born to mock-infected mothers and injected with antibiotic at a prepubertal age, as compared to adult offspring born to mock-infected mothers and injected with vehicle at a prepubertal age (Fig. 4). Examples of these changes induced by prepubertal antibiotic treatment included reduction in Peptococcaceae, as well as augmentation of Erysipelotrichaceae (Table 1, and Figs. 4 and 5).
Figure 5.
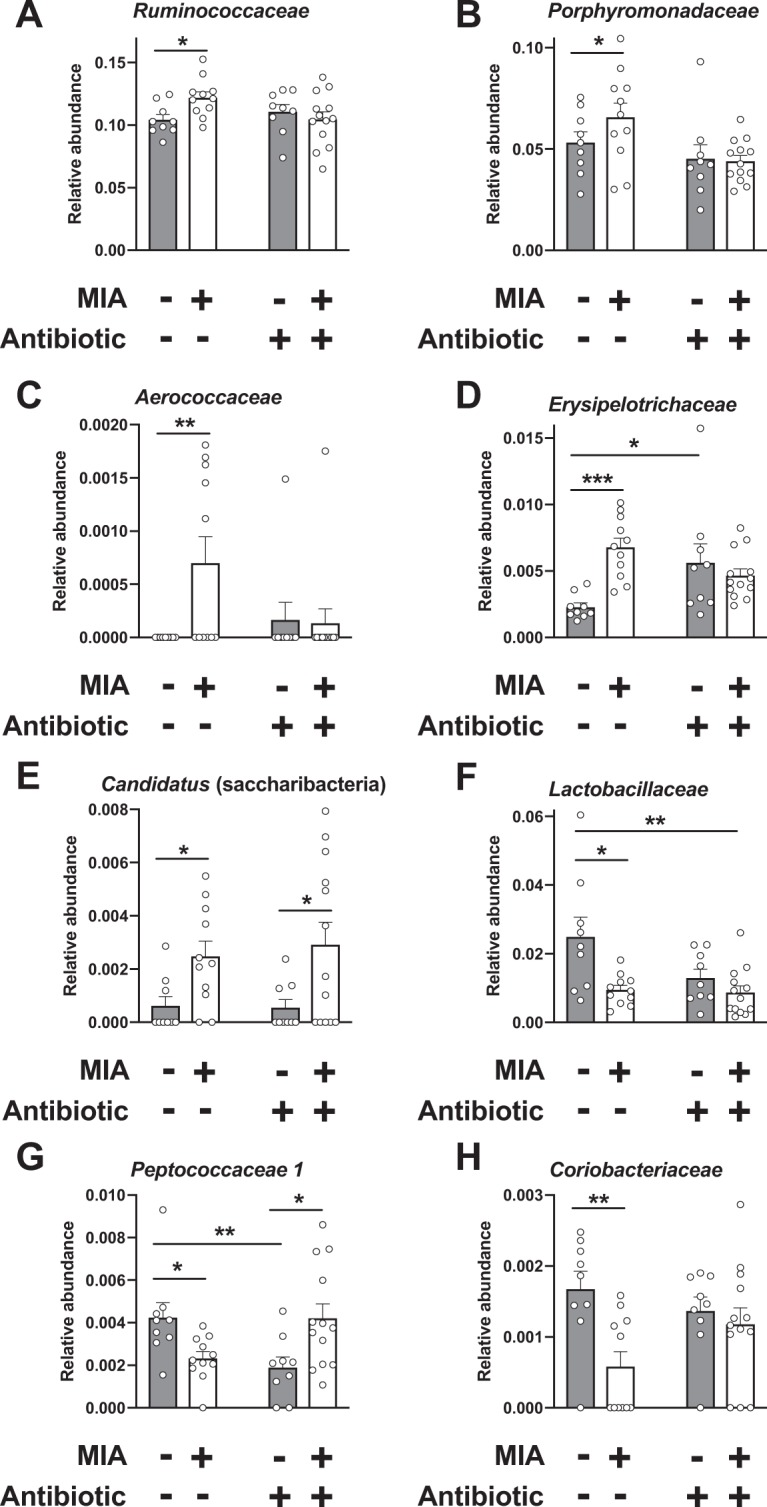
Effect of antibiotic administration or vehicle via oral gavage at P28 on relative abundances at the bacterial family level in mice born to influenza virus-infected (MIA) mothers, or mock mothers (n = 9 – 13). Cecal samples were collected from adult offspring mice. Kruskal-Wallis (see Table 1) followed by uncorrected Dunn’s post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant). Data show mean ± s.e.m.
Our data also suggest the notably novel concept that dysbiosis of the gut microbiota in adult mice born to influenza virus-infected mothers can be partially rebalanced by prepubertal gut microbiota manipulation via antibiotic administration. Thus, potentially pathogenic taxa belonging to Ruminococcaceae, Porphyromonadaceae and Aerococcaceae were not augmented in adult MIA offspring prepubertally treated with antibiotics (Table 1, and Figs. 4 and 5). We also show that the known beneficial family Peptococcaceae, which, as described above, was reduced in adult MIA offspring injected with vehicle at P28 as compared to adult mock offspring injected with vehicle at P28, exhibited an interesting higher relative abundance in adult MIA offspring injected with streptomycin at P28 as compared to adult mock offspring injected with streptomycin at P28 (Table 1, and Figs. 4 and 5). Other alterations induced by prenatal MIA, such as augmentation of Candidatus (saccharibacteria) and Clostridiales Incertae Sedis XI, were unaffected by prepubertal antibiotic treatment (Table 1, and Figs. 4 and 5). Principal coordinate analysis (PCO) shows absence of overall gut microbiota community change in adult MIA offspring injected with vehicle at P28 as compared to the other three experimental groups (Fig. S1), which reinforces the finding about individual species identified in the specific group comparisons.
Abundance of Ruminococcaceae and other taxa correlate with frontal cortex 5-HT2AR density
We next assessed whether the alterations in bacterial family in MIA offspring were linked to the density of the 5-HT2AR in the frontal cortex. Notably, analysis returned a positive correlation between the relative abundance of Ruminococcaceae and frontal cortex 5-HT2AR density (Fig. 6A). Relative abundance of Candidatus (saccharibacteria) also correlated positively with 5-HT2AR density in the mouse frontal cortex (Fig. 6B). Conversely, the trend for a negative correlation between abundance of Lactobacillaceae and density of 5-HT2AR in the mouse frontal cortex was also evident (Fig. 6C).
Figure 6.
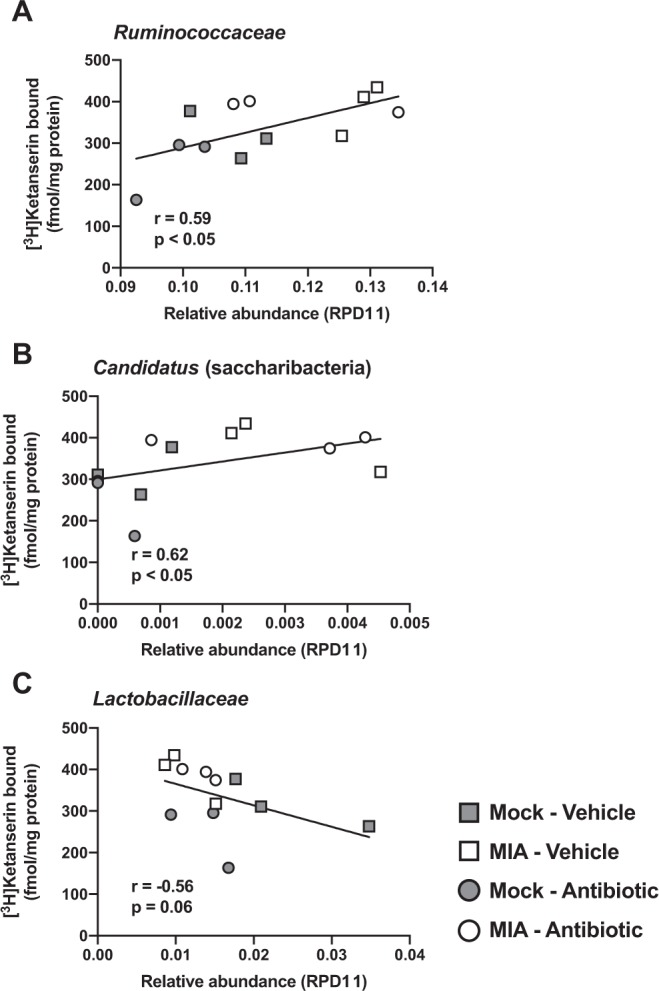
Correlation analysis for the relative abundance of gut bacterial taxa and frontal cortex 5-HT2AR density in adult MIA offspring prepubertally treated with antibiotics, and controls. Microbiome abundances are shown as the mean of relative abundance values from the two mice included in each [3H]ketanserin binding assay. Correlation analysis was conducted using Spearman’s r.
Discussion
Overall, this study investigates the extent to which gut microbiota manipulation at juvenile stages prevents at least part of the negative effects induced by maternal infection with a mouse-adapted influenza virus on behavior models of cognition and expression of the serotonin 5-HT2AR in the mouse frontal cortex. Our current data are consonant with the hypothesis that MIA is associated with altered gut microbiota composition in the offspring, and that antibiotic-induced gut microbiota manipulation at juvenile stages prevents the effects of MIA on cognitive deficits later in life. This conclusion is further supported by our microbiota transplantation techniques showing that fecal microbiota transplantation from adult MIA mice to juvenile controls is sufficient to cause behavioral abnormalities during adulthood. Additionally, although antibiotic administration at P28 was unable to reverse the already reported up-regulation of offspring’s frontal cortex 5-HT2AR density induced by MIA, we show a very strong correlation between certain bacterial taxa, including Ruminococcaceae and Candidatus (saccharibacteria) and expression of this serotonin receptor in the mouse frontal cortex.
Previous findings by other groups show that continuous antibiotic depletion of the gut microbiota induces cognitive deficits in mice. Impairment in the novel object recognition test was reported when adult mice (8–11 weeks) received a cocktail of antibiotics via oral gavage for eleven days50 or when mice received antibiotic treatment from weaning onwards51. The paradigm of gut microbiota manipulation used here is slightly different since we allowed for gut bacterial regrowth following a single antibiotic dose at P28. Accordingly, our findings show a relatively limited effect of prepubertal antibiotic administration in adult mice born to mock-injected mothers, as compared to adult mice born to mock-injected mothers and prepubertally gavaged with vehicle. This absence of negative effects of prepubertal antibiotic treatment is further supported by our behavioral assays. Thus, and opposite to previous findings based on continuous antibiotic administration50,51, our results here show that the novel object recognition test was unaffected in adult mice born to mock-infected mothers that had received prepubertal antibiotic treatment, as compared to adult mice born to mock-infected mothers that had received prepubertal vehicle treatment. Nevertheless, certain bacterial taxa were definitely affected by this particular paradigm of prepubertal gut microbiota manipulation. These included enrichment of taxa implicated in host disease, such as Erysipelotrichaceae. Additional experimentation will be needed to assess the effects of these bacterial taxa on offspring’s physiology and behavior.
With this in mind, however, an interesting observation in our study is that related to the effect of prepubertal gut microbiota manipulation on MIA-induced cognitive deficits in the adult offspring. As previously shown by a number of experimental MIA models8,18,21, maternal infection with a mouse-adapted influenza virus during pregnancy induces deficits in novel object recognition memory. Importantly, a single dose of antibiotic administered via oral gavage at P28 was able to prevent this particular paradigm of deficits in cognitive performance later in life. This behavioral phenotype was observed in parallel with reduction in abundance of components of the gut microbiota that previous work had reported as augmented in fecal samples of patients with schizophrenia, autism and major depressive disorder14,15,42–44 – some of these include, Ruminococcaceae, Porphyromonadaceae, Aerococcaceae, and Erysipelotrichaceae. Still, as above with adult mice born to mock-infected mothers and prepubertally injected with antibiotic, this model of microbiota manipulation at P28 in MIA mice also failed to restore potentially beneficial taxa such as Lactobacillaceae. Whether these, in principle, limitations of prepubertal gut microbiota manipulation are compensated by other families that remain unaffected by prepubertal antibiotic administration in the absence of prenatal MIA, such as Coriobacteriaceae, or become augmented by prepubertal antibiotic administration in MIA mice, such as Peptococcaceae, remains to be investigated.
We also report here that transplantation of gut microbiota from adult MIA mice to prepubertal mock mice is sufficient to induce deficits in recognition memory later in life. This negative effect on novel object recognition is not due to the unwanted effects of the paradigm itself, since it was not observed in mock mice prepubertally receiving gut microbiota transplantation from adult mice born to mock-infected mothers. Our current model of native microbiota “depletion” using a single oral dose of streptomycin was selected based on previous findings by other groups52. Although our current results focused on gut microbiota composition and novel object recognition support this experimental design, additional work will consider the inclusion of treatment with a cocktail of non-absorbable antibiotics targeting both Gram-positive and Gram-negative bacteria.
The 5-HT2AR is a family A GPCR responsible for the majority of effects of psychedelic drugs, including LSD and psilocybin, as well as involved in the mechanism of action of certain antipsychotic medications, such as clozapine and risperidone53. Using the 5-HT2AR antagonist [3H]ketanserin in radioligand binding saturation curves, we previously reported that 5-HT2AR density was increased in postmortem frontal cortex samples (Brodmann area 9) of subjects with antemortem diagnosis of schizophrenia, as compared to control subjects individually matched by gender, age and postmortem delay (time between death and freezing of the samples). Of note, this up-regulation of frontal cortex 5-HT2AR density was observed in schizophrenic subjects tested negative for antipsychotic medication in postmortem toxicological analysis, but not in schizophrenic subjects receiving antipsychotic medication26–28. Although there are many more genes and pathways that may potentially contribute to the risk of schizophrenia, these findings suggest that this particular serotonergic gene may play a key role in the altered cortical processes of schizophrenia. This concept is further supported by recent GWAS studies in schizophrenia patients and controls54.
As discussed above, we previously reported the adult mice born to influenza virus-infected mothers show up-regulation of 5-HT2AR in the frontal cortex29. A similar phenotype has been observed using a variety of prenatal environmental insults8,30–32,55. Here we show that this particular paradigm of prepubertal gut microbiota manipulation did not affect either MIA-induced up-regulation of frontal cortex 5-HT2AR or MIA-induced increase in head-twitch behavior upon psychedelic drug administration. In spite of this, a notable finding of the current study is the strong positive correlation of relative abundance of bacterial taxa such as Ruminococcaceae and Candidatus (saccharibacteria). Frontal cortex 5-HT2AR density was, however, negatively correlated with Lactobacillaceae. Considering the augmentation of Ruminococcaceae previously reported in fecal samples of patients with neurodevelopmental psychiatric conditions43, these findings open a new line of investigation focused on the molecular mechanism underlying this connection between certain components of gut microbiota composition and frontal cortex 5-HT2AR density.
A still open question is the time period in which environmental factors affect gut microbiota composition. Colonization of the gut microbes after birth is critical to the developing newborn immune system, metabolic function and potentially future health. Nevertheless, our previous observations using a cross-fostering experimental approach showed that certain phenotypes induced by prenatal stress during pregnancy, including DOI-induced head-twitch behavior, were increased in prenatally stressed mice that were raised by unstressed surrogate mothers8. A similar concept after cross-fostering has been corroborated by other groups using poly-(I:C)-induced inflammation during pregnancy, also suggesting that maternal gut bacteria during pregnancy promote neurodevelopmental abnormalities in mouse MIA offspring21. Recent work also shows that offspring of germ-free (GF) mice “humanized” with fecal microbiota from individuals with autism spectrum disorder displayed behavioral deficits that include repetitive behavior and decreased sociability56. We are also aware, however, that maternal perturbations induced by prenatal insults do not stop at birth, but can continue throughout lactation, altering the relationship between pups and dams57,58. Further work will be needed to better understand whether MIA-induced maternal perturbations after birth may also affect offspring’s gut microbiota and behavior. In addition, an important limitation of these studies is that they were conducted exclusively in male mice. Future studies including female mice are needed to determine if MIA-induced microbiome and behavioral alterations manifest and respond to antibiotic treatment in a sex-dependent manner.
Metabolomic studies have shown that gut microbial products are found in many extraintestinal tissues, and that molecules derived from the microbiota may influence behavioral phenotypes in mice and humans59–61. Some of these include GPCR ligands that mimic signaling molecules62. The information we provide here is confined to the potential relation between juvenile antibiotic administration on gut microbiota composition and reduction of MIA-induced behaviors. This information will serve as a basis for future studies to test whether metabolites derived from specific bacterial taxa or species directly and/or indirectly modulate 5-HT2AR promoter activity or 5-HT2AR-dependent function. As a long-term goal, our findings may provide the rationale for the development and testing of microbiome-mediated therapeutic strategies and interventions.
Materials and Methods
Animals
Experiments were conducted in accordance with NIH guidelines, and were approved by the Virginia Commonwealth University Animal Care and Use Committee. All efforts were made to minimize animal suffering and the number of animals used. Behavioral testing took place between 9 a.m. and 5 p.m. Animals were housed at 12 h light/dark cycle at 23 °C with food and water ad libitum, except during behavioral testing.
Cells and viruses
Mouse-adapted influenza virus was propagated in Madin-Darby Canine Kidney (MDCK) cells as previously reported29. Viral stocks were stored at –80 °C until viral infections were performed. All experiments with live virus were performed under biosafety level 2 (BSL-2) containment.
Mouse viral infection
Infection of timed pregnant female mice was performed as previously reported29. Briefly, timed pregnant CD1 mice were obtained from Charles River Laboratories. On day 9.5 of pregnancy, mice were anesthetized with ketamine/xylazine before intranasal (i.n.) infection with 5 × 103 plaque-forming units (pfu) of influenza A/WSN/33 (H1H1) virus in 50 µl of PBS. Mock-infected mothers were treated identically but were infused with PBS. Our previous data demonstrate that this sublethal dose of infection causes sickness behavior (lethargy, sleepiness, ruffled fur and lack of grooming), but loss of pregnancy is uncommon29. Day 9.5 of pregnancy was chosen because this is neurodevelopmentally equivalent to the end of the first trimester of human pregnancy63, a critical period during which environmental insults produce a higher risk of schizophrenia and autism in human offspring. Offspring were separated from their mothers after 3 weeks, and males and females were caged separately in groups of three to five. Subsequent experiments were performed in adult (15–20 weeks) male mice, unless otherwise indicated. Litters were separated according to prenatal manipulation after weaning, and divided over several cages64. Three independent cohorts (MIA or mock) of animals were evaluated. Figure 2 includes data from animals in cohorts 1, 2 and 3; Fig. 3 includes data from animals in cohort 2, and Figs. 4–6, Table S1 and Fig. S1 include data from animals in cohort 3. Animals from at least three separate litters were subjected to the different protocols.
Mouse brain samples
Adult male mice were sacrificed by cervical dislocation, and bilateral frontal cortices (bregma 1.90–1.40 mm) were dissected and frozen at –80 °C for radioligand binding assays. Frontal cortex samples were collected at least one week after the last behavioral assay.
Radioligand binding
[3H]Ketanserin binding assays were performed as previously reported29. Briefly, bilateral frontal cortex samples from two mice were pooled together, homogenized using a Teflon-glass grinder, and subjected to the membrane preparation assay. [3H]Ketanserin binding (10 nM) was measured at equilibrium in aliquots of frontal cortex membrane preparations that were incubated at 37 °C for 60 min. [3H]Ketanserin was obtained from PerkinElmer, and nonspecific binding was determined in the presence of 10 µM methysergide (Tocris). Our previous data show that, under these experimental conditions, specific [3H]ketanserin binding is absent in the cortex of 5-HT2AR knockout mice35.
Head-twitch behavior
Head-twitch behavior was tested as previously described35. Briefly, mice were injected (i.p.) with the hallucinogenic 5-HT2AR agonist 1- (2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; 0.5 mg/kg) (Sigma-Aldrich), and 15 min later, they were placed into the center of a Plexiglass cage for 30 min, during which they were videotaped by a video camcorder positioned directly above the cage. Videotapes were scored for head-twitches by an experienced observer blind to prenatal/postnatal manipulations and treatment.
Novel-object recognition (NOR) test
NOR test was assessed as previously reported65,66. Briefly, mice were given a 10-min acquisition trial and a 5-min recognition trial, separated by a 24 h inter-trial return to their home cage. During the acquisition trial, the animals were allowed to explore two different objects (A and B). During the recognition trial, the animals explored a familiar object (A) from the acquisition trial and a novel object (C). Behavior was recorded on video for blind scoring of object exploration. The exploratory preference [100 × (time spent exploring the novel object / total exploration time)] was then calculated for recognition trials.
Microbiota manipulation
Prepubertal (28 d) mice67 born to influenza virus-infected mothers or mock were selected at random for treatment with a single dose of streptomycin (20 mg), or vehicle (distilled water) by oral gavage (200 µl). After this manipulation (Fig. 1), mice were housed with normal drinking water until they became adult animals (15–20 weeks of age).
Fecal transplantation
For microbiota transplantation, young (28 d) mice received a single dose of streptomycin (20 mg), or mock by oral gavage (200 µl). Three days after this paradigm of antibiotic administration, mice went through a fecal microbiota transfer protocol. Donor mice (two adult mice born to influenza virus-infected mothers, and two adult mice born to mock-infected mothers) were placed in empty autoclaved cages (no bedding) and allowed to defecate normally. The fecal content was resuspended in distilled water (10 ml), and centrifuged for 5 min at 200 ×g. Aliquots of the supernatant were stored at –80 °C for transplantation assays. Inoculation (100 µl of fecal content or distilled water) was performed via oral gavage on two consecutive weeks, for a total of 7 times. After this manipulation, mice were housed with normal drinking water until they became adult animals (10–17 weeks of age).
16S rRNA gene sequence analysis
Cecal samples from adult male mice (n = 45 total) were collected and DNA extracted using standard methods, as we have previously reported68,69. After the initial filtering of reads by the Ion Torrent software, we selected reads over 250 bp for further processing. We then used the RDP11 Bayesian Classifier to annotate taxa with bootstrap values over 60. Taxa were annotated as unknown if the bootstrap value was below 60. Finally, we removed taxa that were below 0.1% to exclude rare taxa. After this quality filtering, we obtained 1,319,338 high quality reads for further analysis of bacterial composition, with a mean of 31,412.80 reads per sample, ranging from 19,262 to 61,783. Microbiome relative abundances as compositional data were ranged from zero to one. Changes in microbial composition between different experimental groups were assessed using RDP11 (Ribosomal Database Project) Bayesian analysis70, QIIME (Quantitative Insights into Microbial Ecology) pipeline71, and linear discriminant analysis effect size (LEfSe)41. Principal coordinate analysis (PCO) based on Bray-Curtis dissimilarity was performed following standard methods, as we have previously reported68,69.
Statistical analysis
Statistical significance of experiments involving three or more groups across two or more experimental conditions was assessed by two-way or three-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of relative abundance values was assessed by nonparametric Kruskal-Wallis followed by uncorrected Dunn’s post hoc test. Correlation analysis between [3H]ketanserin binding and relative abundance values (mean of relative abundance values from the two mice included in each [3H]ketanserin binding assay) was conducted using the Spearman’s r. The level of significance was chosen at p = 0.05. All data are presented as mean ± standard error of the mean (s.e.m).
Supplementary information
Supplementary Information - Table S1 and Figure S1.
Acknowledgements
The authors would like to thank Jeremy Seto for his early help in microbiota experiments. NIH R01 MH084894 (J.G.-M.), NIH R01 MH111940 (J.G.-M.), NIH R21 TR002024 (J.S.B), VA Merit Review 2I0CX001076 (J.S.B), and NIH F30 MH116550 (J.M.S.) participated in the funding of this study. This work was also partially funded by CRIP (Center for Research on Influenza Pathogenesis), and NIAID-funded Center of Excellence for Influenza Research and Surveillance (CEIRS, contract HHSN272201400008C to A.G.-S.). D.I. was a recipient of a Grain-in-Aid for Scientific Research (19K07332) from JSPS.
Author contributions
J.M.S., J.L.M. and J.G.-M. conceived the project, designed experiments, and analyzed data. J.M.S. and J.G.-M. wrote the manuscript. J.M.S. and J.L.M. performed experiments. J.G.-M. supervised the research. D.I. helped with behavior assays. D.J.K., supervised by J.S.B., helped with microbiota manipulation assays. M.S. and S.S.D., supervised by P.M.G., performed 16S rRNA gene sequence analysis. R.M.-M., supervised by A.G.-S., generated A/WSN/33 (H1N1) viral particles. M.G.D. helped with biostatistical analysis. All authors discussed the results and commented on the manuscript.
Data availability
Datasets generated during the course of the current study are available, upon reasonable request, from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61635-6.
References
- 1.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- 3.Yudofsky SC. Contracting schizophrenia: lessons from the influenza epidemic of 1918–1919. JAMA. 2009;301:324–326. doi: 10.1001/jama.2008.980. [DOI] [PubMed] [Google Scholar]
- 4.Brown AS, et al. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/S0006-3223(01)01068-X. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown AS, et al. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 8.Holloway T, et al. Prenatal Stress Induces Schizophrenia-Like Alterations of Serotonin 2A and Metabotropic Glutamate 2 Receptors in the Adult Offspring: Role of Maternal Immune System. J Neurosci. 2013;33:1088–1098. doi: 10.1523/JNEUROSCI.2331-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kentner AC, et al. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology. 2019;44:245–258. doi: 10.1038/s41386-018-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauman MD, et al. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75:332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Nallar E, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yolken RH, et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr Bull. 2015;41:1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y. et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr Res (2018). [DOI] [PubMed]
- 16.De Angelis M, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PloS ONE. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son JS, et al. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PloS ONE. 2015;10:e0137725. doi: 10.1371/journal.pone.0137725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim JS, Lim MY, Choi Y, Ko G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol Brain. 2017;10:14. doi: 10.1186/s13041-017-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasarevic E, Howard CD, Misic AM, Beiting DP, Bale TL. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 2017;7:44182. doi: 10.1038/srep44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S. et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature (2017). [DOI] [PMC free article] [PubMed]
- 22.Giovanoli S, et al. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl Psychiatry. 2016;6:e772. doi: 10.1038/tp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu F, Zheng Y, Liu Y, Zhang X, Zhao J. Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic-polyribocytidilic acid. Psychiatry Res. 2014;219:680–686. doi: 10.1016/j.psychres.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 24.Bekinschtein P, Renner MC, Gonzalez MC, Weisstaub N. Role of medial prefrontal cortex serotonin 2A receptors in the control of retrieval of recognition memory in rats. J Neurosci. 2013;33:15716–15725. doi: 10.1523/JNEUROSCI.2087-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morici, J. F. et al. 5-HT2a receptor in mPFC influences context-guided reconsolidation of object memory in perirhinal cortex. Elife7 (2018). [DOI] [PMC free article] [PubMed]
- 26.Gonzalez-Maeso J, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muguruza C, et al. Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2013;23:852–864. doi: 10.1016/j.euroneuro.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muguruza C, et al. Evaluation of 5-HT2A and mGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: effect of antidepressant treatment. Neuropharmacology. 2014;86:311–318. doi: 10.1016/j.neuropharm.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno JL, et al. Maternal Influenza Viral Infection Causes Schizophrenia-Like Alterations of 5-HT2A and mGlu2 Receptors in the Adult Offspring. J Neurosci. 2011;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akatsu, S., Ishikawa, C., Takemura, K., Ohtani, A. & Shiga, T. Effects of prenatal stress and neonatal handling on anxiety, spatial learning and serotonergic system of male offspring mice. Neurosci Res (2015). [DOI] [PubMed]
- 31.Malkova NV, Gallagher JJ, Yu CZ, Jacobs RE, Patterson PH. Manganese-enhanced magnetic resonance imaging reveals increased DOI-induced brain activity in a mouse model of schizophrenia. Proc Natl Acad Sci USA. 2014;111:E2492–2500. doi: 10.1073/pnas.1323287111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wischhof L, Irrsack E, Dietz F, Koch M. Maternal lipopolysaccharide treatment differentially affects 5-HT and mGlu2/3 receptor function in the adult male and female rat offspring. Neuropharmacology. 2015;97:275–288. doi: 10.1016/j.neuropharm.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 34.Schmid, Y. et al. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry (2015). [DOI] [PubMed]
- 35.Gonzalez-Maeso J, et al. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Hanks JB, Gonzalez-Maeso J. Animal models of serotonergic psychedelics. ACS Chem Neurosci. 2013;4:33–42. doi: 10.1021/cn300138m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGlashan TH, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 38.Yung AR, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32. doi: 10.1016/S0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 39.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savignac HM, et al. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-beta levels in male mice. Brain Behav Immun. 2016;52:120–131. doi: 10.1016/j.bbi.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng P, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 43.Coretti L, et al. Gut Microbiota Features in Young Children With Autism Spectrum Disorders. Front Microbiol. 2018;9:3146. doi: 10.3389/fmicb.2018.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plaza-Díaz Julio, Gómez-Fernández Antonio, Chueca Natalia, Torre-Aguilar María, Gil Ángel, Perez-Navero Juan, Flores-Rojas Katherine, Martín-Borreguero Pilar, Solis-Urra Patricio, Ruiz-Ojeda Francisco, Garcia Federico, Gil-Campos Mercedes. Autism Spectrum Disorder (ASD) with and without Mental Regression is Associated with Changes in the Fecal Microbiota. Nutrients. 2019;11(2):337. doi: 10.3390/nu11020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takenaka R., Aoi, Y., Ozaki, N., Ohashi, A. & Kindaichi, T. Specificities and Efficiencies of Primers Targeting Candidatus Phylum Saccharibacteria in Activated Sludge. Materials (Basel)11 (2018). [DOI] [PMC free article] [PubMed]
- 46.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 47.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 48.Felice, V. D. & O’Mahony, S. M. The microbiome and disorders of the central nervous system. Pharmacol Biochem Behav (2017). [DOI] [PubMed]
- 49.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 50.Frohlich EE, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desbonnet L, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Willing BP, Vacharaksa A, Croxen M, Thanachayanont T, Finlay BB. Altering host resistance to infections through microbial transplantation. PloS ONE. 2011;6:e26988. doi: 10.1371/journal.pone.0026988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichols DE. Psychedelics. Pharmacol Rev. 2016;68:264–355. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Consortium. S. W. G.O.T.P.G Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, et al. Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience. 2015;301:363–374. doi: 10.1016/j.neuroscience.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Sharon G, et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell. 2019;177:1600–1618 e1617. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darnaudery M, Dutriez I, Viltart O, Morley-Fletcher S, Maccari S. Stress during gestation induces lasting effects on emotional reactivity of the dam rat. Behav Brain Res. 2004;153:211–216. doi: 10.1016/j.bbr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Vanbesien-Mailliot CC, et al. Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrinology. 2007;32:114–124. doi: 10.1016/j.psyneuen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 59.De Vadder F, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 60.Guo CJ, et al. Discovery of Reactive Microbiota-Derived Metabolites that Inhibit Host Proteases. Cell. 2017;168:517–526 e518. doi: 10.1016/j.cell.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen L. J. et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature (2017). [DOI] [PMC free article] [PubMed]
- 63.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ibi D, et al. Antipsychotic-induced Hdac2 transcription via NF-kappaB leads to synaptic and cognitive side effects. Nat Neurosci. 2017;20:1247–1259. doi: 10.1038/nn.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de la Fuente Revenga M, et al. HDAC2-dependent Antipsychotic-like Effects of Chronic Treatment with the HDAC Inhibitor SAHA in Mice. Neuroscience. 2018;388:102–117. doi: 10.1016/j.neuroscience.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bajaj JS, et al. Elderly patients have an altered gut-brain axis regardless of the presence of cirrhosis. Sci Rep. 2016;6:38481. doi: 10.1038/srep38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajaj JS, et al. Gut Microbiota Alterations can predict Hospitalizations in Cirrhosis Independent of Diabetes Mellitus. Sci Rep. 2015;5:18559. doi: 10.1038/srep18559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information - Table S1 and Figure S1.
Data Availability Statement
Datasets generated during the course of the current study are available, upon reasonable request, from the corresponding author.