Abstract
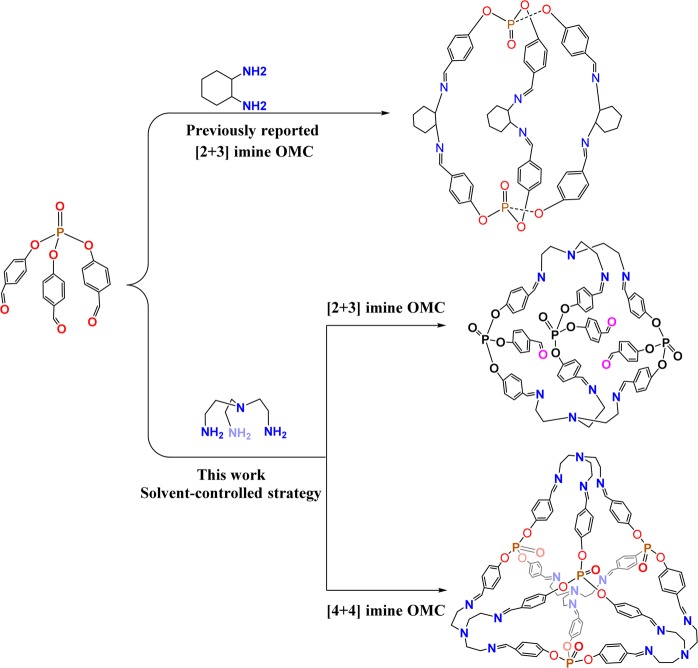
Two flexible subcomponents, namely tris(4-formylphenyl)phosphate and tris(2-aminoethyl)amine, are assembled into a tetrapodal [4 + 4] cage depending on the solvent effect. Single-crystal structure analysis reveals that the caivity is surrounded by four phosphate uints. Good selectivity of CO2 adsorption over CH4 is demonstrated by the gas adsorption experiment.
Subject terms: Structure elucidation, Self-assembly
Introduction
Discrete molecular architectures, especially those organic cage compounds, have received intensive attention in recent years1–8. These intriguing compounds featuring aesthetic geometry and intrinsic cavities display potential applications in gas or organic molecular separation9–14, catalysis15–18, porous liquids19,20 and detection21–23. Synthetic control on the formation of versatile cages with given topologies is crucial before exploring their applications. Due to the characteristic of self-healing, dynamic covalent chemistry has been demonstrated as a powerful approach to synthesize these sophisticated cages from simple precursors. Among them, imine condensation, boronicester or boroxine formation and alkyne metathesis are the most frequently used type of dynamic bond formation24.
Using reversible covalent chemistry to construct organic molecular cages (OMCs), external stimuli, such as solvent, pH, temperature, catalysts, steric and electronic factors, are all worthy of enough attention25. Sometimes, different solvents could govern the self-assembly behavior to form cage products with different geometry. For example, Liu and Warmuth described a solvent-dependent method to selectively synthesize tetrahedral, octahedral and square antiprismatic cages from the same sub-components26. On the other hand, choice of appropriate building blocks or precursors in the self-assembly process is believed as a key and important factor to access the desired and functionalized OMCs27,28. In the family of tritopic building blocks or precursors, 1,3,5-triformylbenzene and triptycene triamine occupy a special status owing to their excellent ability to form various OMCs with different partners1,29–31.
A semi-flexible phosphate based trialdehyde had been proved to be a practicable precursor containing P=O functional site and a functionalized [2 + 3] imine OMC was successfully obtained by our group32. As a part of our ongoing research on OMCs, we aim to further investigate and demonstrate that the semi-flexible precursor could also satisfy the requirements of different geometrical organic cage assembly. So we have changed the amine component from previous ditopic linker (cyclohexanediamine) to a flexible tritopic linker [tris(2-aminoethyl)amine]. For multi-component systems comprising competitive reactions, increasing the number of reactive ending groups and the flexibility of the amine linker is bound to complicate the self-assembly process. To address this challenge, a solvent-controlled method was tried and applied to simplify the self-assembly process towards the desired OMC in this paper.
Results and Discussion
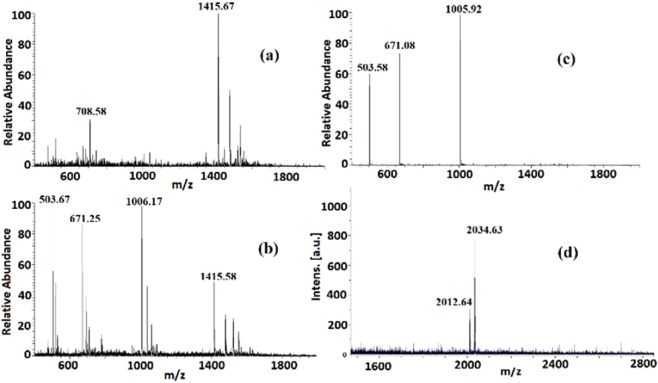
Schiff-base condensation generally has great compatibility with different solvents, and different solvents have large influences on the final molecular crystallization and solid-state crystal packing33,34. Acetonitrile/chloroform (v/v = 5:1) was adopted as a mixed-solvent in the synthesis of previous [2 + 3] phosphate cage just because of its suitability to grow single crystals. Actually, both acetonitrile and chloroform were effective solvents to yield the [2 + 3] cage. However, in the following self-assembly reaction between two tritopic precursors [phosphate trialdehyde and tris(2-aminoethyl)amine], it is found that the type of reaction solvents could dramatically impact the self-assembled outcomes (Fig. 1). In our experiments, acetonitrile as a single solvent for equimolar self-assembly is firstly tried since two tritopic precursors with a 1:1 molar ratio could theoretically form a [4 + 4] molecular cage. Nevertheless, the ESI-MS spectrum does not give the expected [4 + 4] assembled results, in which two positive peaks at m/z = 1415.67 and 708.58 are present corresponding to the full and half peaks of a [2 + 3] molecular cage with three unreacted aldehyde groups (Fig. 2a). Furthermore, the 1H NMR spectrum of this species evidences the existence of the aldehyde group (Fig. S1). The combination of ESI-MS and 1H NMR analyses suggests that one aldehyde group from every phosphate trialdehyde precursor is not involved in the cage construction and six-fold imine condensation produces a credible [2 + 3] cage structure. This half-way self-assembly could be ascribed to the poor solubility for the [2 + 3] cage product in acetonitrile, which tends to precipitate from the reaction solution and terminate the further imine condensation.
Figure 1.
Different OMCs based on the phosphate trialdehyde.
Figure 2.
Solvent Effects on the cage assembly. (a) EIS-MS in acetonitrile; (b) EIS-MS in mixture of acetonitrile and chloroform; (c) EIS-MS in chloroform; (d) MALDI-TOF-MS in chloroform.
Considering that chlorohydrocarbon solvents are often used in the cage syntheses11,16,21–23,35,36, a mixture of 1:1 acetonitrile and chloroform is then used for this reaction. Although the formation of partial [2 + 3] cage still occurs, the successful assembly of a [4 + 4] molecular age is achieved which can be verified by the presence of new ESI-MS peaks at m/z = 1006.17, 671.25 and 503.67 with the isotopic distribution patterns separated by 0.50 ± 0.01, 0.33 ± 0.01 and 0.25 ± 0.01 Da (Figs. 2b and S2), corresponding to 1/2, 1/3 and 1/4 of the molecular weight of the [4 + 4] cage. In order to improve the yield of [4 + 4] cage, pure chloroform is explored, where the ESI-MS spectrum clearly exhibits three positive peaks originating from the [4 + 4] cage excluding the peaks of half-way [2 + 3] cage (Fig. 2c). The formation of [4 + 4] cage was further confirmed by a peak at m/z = 2012.64 in the MALDI-TOF-MS spectrum (Fig. 2d). In addition, 1H NMR spectrum provides reliable proofs for the formation of this [4 + 4] cage, where only one set of signals could be observed for this symmetrical structure (Fig. S3).
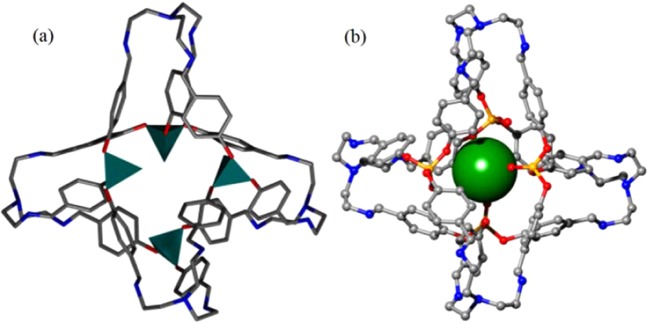
The molecular structure of [4 + 4] cage was further verified by single-crystal X-ray diffraction studies. Slow evaporation of the reaction solution affords the suitable single crystals for the X-ray diffraction determination. Crystallographic analysis shows that this [4 + 4] cage has a tetrapodal structure with symmetry (Fig. 3). The shape of the cage could also be regarded as tetrahedral shape with the tertiary amine nitrogens of the amine linkers as four vertexes. In both [4 + 4] cage and previously reported [2 + 3] cage, the phosphate tetrahedron comprised by four oxygen atoms is very rigid (Fig. 3a). However, three P-O single bonds of the phosphate tetrahedron could be rotated along the axial direction freely, which would result in the three benzene rings pointing toward different orientations during the construction of OMCs with cyclohexanediamine and tris(2-aminoethyl)amine. Herein we use the distance between the O atom of P=O double bond and the centroid of benzene ring to illustrate the discrepancy between the conformation of the cages. This parameter is the same as 3.85 Å for three benzene rings in the previous [2 + 3] cage exhibiting the typical tripod configuration, while it changes to 3.94, 4.10 and 5.13 Å in the current [4 + 4] cage indicating two different orientations in forming a more complicated cage with flexible tris(2-aminoethyl)amine. Unlike the previous [2 + 3] cage, the P=O bonds does not point straight toward the cage centre in this [4 + 4] cage and the window of the cage cavity was partially occupied by the oxygen atoms of the P=O units (Fig. 3b). In addition, a cavity with a 4.0 Å diameter taking into account the van der Waals radii of the atoms is found in the cage, which is comparable with that of some gas molecules such as CO2, CH4 and N237. In addition, the thermal stability of this [4 + 4] cage is evaluated by thermogravimetric analysis (TGA), in which the decomposition temperature is high up to 300 °C (Fig. S4). This work is believed to give a representative example to illustrate the semi-flexible and versatile traits of this phosphate trialdehyde.
Figure 3.
(a) Crystal structure of the cage (phosphate units are represented by four tetrahedrons); (b) the size of the cage (represented by a green ball in a radius of 2.0 Å).
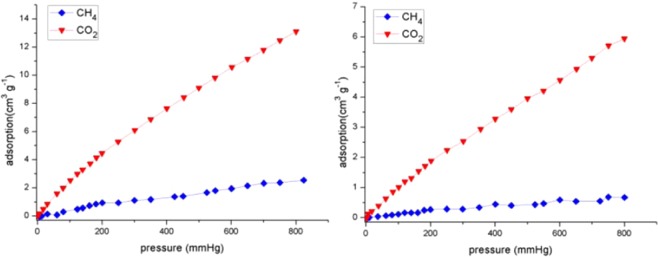
Given that gas sorption phenomena have been revealed on discrete organic molecular solids, gas adsorption properties of this [4 + 4] cage were explored. After thermal activation for 12 h at 80 °C under the high vacuum, the crystalline sample has lost its crystallinity/solvents and became amorphous (Figs. S5 and S6). The N2 adsorption isotherms of the cage at 77 K indicate a low BET surface area (less than 10 m2/g), which is similar to other discrete cage compounds38–41. Gas adsorption experiments at 273 and 298 K reveal that the cage has selective adsorption of CO2 over CH4. As illustrated in Fig. 4, it can absorb 12.46 and 5.71 cm3 g−1 of CO2 at 273 and 298 K and 1.0 bar, whereas small amounts of CH4 uptake can be observed at 1 bar (2.36 and 0.68 cm3 g−1 at 273 and 298 K). The selectivities of CO2 over CH4 estimated from Henry’s constants are 7.10 and 6.52 at 298 and 273 K (Table S2), respectively, which are comparable with other previously reported OMCs (For CO2 vs CH4 adsorption and separation of some selected OMCs, see Table S3). The reason for the high selectivities of CO2 over CH4 is probably due to the presence of polar functional P=O groups for CO2 adsorption12,42,43.
Figure 4.
CO2 (red) and CH4 (blue) adsorption isotherms at 273 (left) and 298 K (right).
Conclusion
In summary, one novel [4 + 4] phosphate OMC can be efficiently synthesized in a one pot reaction through solvent-controlled multicomponent imine condensation between tris(4-formylphenyl)phosphate and tris(2-aminoethyl)amine. It is concluded that acetonitrile is beneficial to the assembly of [2 + 3] halfway cage, while chloroform can promote the full conversion of the functional groups on two reactants. Structurally, this cage possesses one distinctive cavity constituted by four phosphate units. Furthermore, this [4 + 4] phosphate OMC displays good selectivity of CO2 adsorption over CH4. Thus, the strategy we offered here to synthesize the [4 + 4] phosphate cage is believed to be instructive for designing new type functionalized organic cages and host molecules in supramolecular chemistry.
Supplementary information
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21871133), National Natural Science Foundation of Jiangsu Province (No. BK20171334), and Science, Technology and Innovation Commission of Shenzhen Municipality (No. JCYJ20180307153251975).
Author contributions
Gen-Feng Feng synthesized the complexes, performed the characterization and wrote the first draft of the manuscript. Jiao Geng and Fen-Da Feng analyzed the results. Wei Huang designed the experiments and corrected the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61813-6.
References
- 1.Tozawa T, et al. Porous organic cages. Nat. Mater. 2009;8:973–978. doi: 10.1038/nmat2545. [DOI] [PubMed] [Google Scholar]
- 2.Slater AG, Cooper AI. Function-led design of new porous materials. Science. 2015;348:8075. doi: 10.1126/science.aaa8075. [DOI] [PubMed] [Google Scholar]
- 3.Hasell T, Cooper AI. Porous organic cages: soluble, modular and molecular pores. Nat. Rev. Mater. 2016;1:16053. doi: 10.1038/natrevmats.2016.53. [DOI] [Google Scholar]
- 4.Zhang G, Mastalerz M. Organic cage compounds-from shape-persistency to function. Chem. Soc. Rev. 2014;43:1934–1947. doi: 10.1039/C3CS60358J. [DOI] [PubMed] [Google Scholar]
- 5.Jin YH, Zhu YL, Zhang W. Development of organic porous materials through Schiff-base chemistry. CrystEngComm. 2013;15:1484–1499. doi: 10.1039/C2CE26394G. [DOI] [Google Scholar]
- 6.Evans JD, Sumby CJ, Doonan CJ. Synthesis and applications of porous organic cages. Chem. Lett. 2015;44:582–588. doi: 10.1246/cl.150021. [DOI] [Google Scholar]
- 7.Mastalerz M. Porous shape-persistent organic cage compounds of different size, geometry, and function. Acc. Chem. Res. 2018;51:2411–2422. doi: 10.1021/acs.accounts.8b00298. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, et al. From discrete molecular cages to a network of cages exhibiting enhanced CO2 adsorption capacity. Angew. Chem., Int. Ed. 2017;56:7787–7791. doi: 10.1002/anie.201702399. [DOI] [PubMed] [Google Scholar]
- 9.Hasell T, et al. Porous organic cages for sulfur hexafluoride separation. J. Am. Chem. Soc. 2016;138:1653–1659. doi: 10.1021/jacs.5b11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages. Nat. Mater. 2014;13:954–960. doi: 10.1038/nmat4035. [DOI] [PubMed] [Google Scholar]
- 11.Jin YH, et al. A shape-persistent organic molecular cage with high selectivity for the adsorption of CO2 over N2. Angew. Chem., Int. Ed. 2010;49:6348–6351. doi: 10.1002/anie.201001517. [DOI] [PubMed] [Google Scholar]
- 12.Mastalerz M, et al. A salicylbisimine cage compound with high surface area and selective CO2/CH4 adsorption. Angew. Chem., Int. Ed. 2011;50:1046–1051. doi: 10.1002/anie.201005301. [DOI] [PubMed] [Google Scholar]
- 13.Mitra T, et al. Molecular shape sorting using molecular organic cages. Nat. Chem. 2013;5:276–281. doi: 10.1038/nchem.1550. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, et al. A highly C70 selective shape-persistent rectangular prism constructed through one-step alkyne metathesis. J. Am. Chem. Soc. 2011;133:20995–21001. doi: 10.1021/ja210418t. [DOI] [PubMed] [Google Scholar]
- 15.Sun JK, et al. Toward homogenization of heterogeneous metal nanoparticle catalysts with enhanced catalytic performance: soluble porous organic cage as a stabilizer and homogenizer. J. Am. Chem. Soc. 2015;137:7063–7066. doi: 10.1021/jacs.5b04029. [DOI] [PubMed] [Google Scholar]
- 16.Mondal B, et al. Molecular cage impregnated palladium nanoparticles: efficient, additive-free heterogeneous catalysts for cyanation of aryl halides. J. Am. Chem. Soc. 2016;138:1709–1716. doi: 10.1021/jacs.5b13307. [DOI] [PubMed] [Google Scholar]
- 17.Mondal B, Mukherjee PS. Cage encapsulated gold nanoparticles as heterogeneous photocatalyst for facile and selective reduction of nitroarenes to azo compounds. J. Am. Chem. Soc. 2018;140:12592–12601. doi: 10.1021/jacs.8b07767. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, et al. Porous organic cage stabilised palladium nanoparticles: efficient heterogeneous catalysts for carbonylation reaction of aryl halides. Chem. Commun. 2018;54:2796–2799. doi: 10.1039/C7CC09918E. [DOI] [PubMed] [Google Scholar]
- 19.Giri N, et al. Liquids with permanent porosity. Nature. 2015;527:216–220. doi: 10.1038/nature16072. [DOI] [PubMed] [Google Scholar]
- 20.Cooper AI. Porous molecular solids and liquids. ACS Cent. Sci. 2017;3:544–553. doi: 10.1021/acscentsci.7b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acharyya K, Mukherjee PS. A fluorescent organic cage for picric acid detection. Chem. Commun. 2014;50:15788–15791. doi: 10.1039/C4CC06225F. [DOI] [PubMed] [Google Scholar]
- 22.Mondal B, et al. Reversible multistimuli switching of a spiropyran-functionalized organic cage in solid and solution. J. Org. Chem. 2017;82:7783–7790. doi: 10.1021/acs.joc.7b00722. [DOI] [PubMed] [Google Scholar]
- 23.Gupta. M, et al. Benzothiazole integrated into a cryptand for ESIPT-based selective chemosensor for Zn2+ ions. Dalton Trans. 2019;48:7801–7808. doi: 10.1039/C9DT00548J. [DOI] [PubMed] [Google Scholar]
- 24.Mastalerz M. Shape-persistent organic cage compounds by dynamic covalent bond formation. Angew. Chem., Int. Ed. 2010;49:5042–5053. doi: 10.1002/anie.201000443. [DOI] [PubMed] [Google Scholar]
- 25.Meyer CD, et al. Template-directed synthesis employing reversible imine bond formation. Chem. Soc. Rev. 2007;36:1705–1723. doi: 10.1039/b513441m. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Warmuth R. Solvent effects in thermodynamically controlled multicomponent nanocage syntheses. J. Am. Chem. Soc. 2006;128:14120–14127. doi: 10.1021/ja0644733. [DOI] [PubMed] [Google Scholar]
- 27.Ono K, et al. Self-assembly of nanometer-sized boroxine cages from diboronic acids. J. Am. Chem. Soc. 2015;137:7015–7018. doi: 10.1021/jacs.5b02716. [DOI] [PubMed] [Google Scholar]
- 28.Klotzbach S, Beuerle F. Shape-controlled synthesis and self-sorting of covalent organic cage compounds. Angew. Chem., Int. Ed. 2015;54:10356–10360. doi: 10.1002/anie.201502983. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S, et al. Selective gas sorption in a [2 + 3] propeller cage crystal. Chem. Commun. 2011;47:8919–8921. doi: 10.1039/c1cc12460a. [DOI] [PubMed] [Google Scholar]
- 30.Hasell T, et al. Triply interlocked covalent organic cages. Nat. Chem. 2010;2:750–755. doi: 10.1038/nchem.739. [DOI] [PubMed] [Google Scholar]
- 31.Schneider MW, et al. Exo-functionalized shape-persistent [2 + 3] cage compounds: influence of molecular rigidity on formation and permanent porosity. Chem.–Eur. J. 2012;18:4156–4160. doi: 10.1002/chem.201200032. [DOI] [PubMed] [Google Scholar]
- 32.Feng G, et al. Cavity partition and functionalization of a [2 + 3] organic molecular cage by inserting polar P=O bonds. Chem. Commun. 2016;52:9267–9270. doi: 10.1039/C6CC02801B. [DOI] [PubMed] [Google Scholar]
- 33.Hasell T, et al. Controlling the crystallization of porous organic cages: molecular analogs of isoreticular frameworks using shape-specific directing solvents. J. Am. Chem. Soc. 2014;136:1438–1448. doi: 10.1021/ja409594s. [DOI] [PubMed] [Google Scholar]
- 34.Santolini V, et al. Predicting solvent effects on the structure of porous organic molecules. Chem. Commun. 2015;51:15542–15545. doi: 10.1039/C5CC05344G. [DOI] [PubMed] [Google Scholar]
- 35.Ding H, et al. Targeted synthesis of a large triazine-based [4 + 6] organic molecular cage: structure, porosity and gas separation. Chem. Commun. 2015;51:1976–1979. doi: 10.1039/C4CC08883B. [DOI] [PubMed] [Google Scholar]
- 36.Acharyya K, Mukherjee PS. Shape and size directed self-selection in organic cage formation. Chem. Commun. 2015;51:4241–4244. doi: 10.1039/C5CC00075K. [DOI] [PubMed] [Google Scholar]
- 37.Li J-R, et al. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009;38:1477–1504. doi: 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- 38.Jin Y, et al. Highly CO2-selective organic molecular cages: what determines the CO2 selectivity. J. Am. Chem. Soc. 2011;133:6650–6658. doi: 10.1021/ja110846c. [DOI] [PubMed] [Google Scholar]
- 39.Jin YH, et al. Microwave-assisted syntheses of highly CO2-selective organic cage frameworks (OCFs) Chem. Sci. 2012;3:874–877. doi: 10.1039/C1SC00589H. [DOI] [Google Scholar]
- 40.Wang Q-Q, et al. Molecular barrel by a hooping strategy: synthesis, structure, and selective CO2 adsorption facilitated by lone pair–π interactions. J. Am. Chem. Soc. 2017;139:635–638. doi: 10.1021/jacs.6b12386. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, et al. Porous triphenylbenzene-based bicyclooxacalixarene cage for selective adsorption of CO2/N2. Org. Lett. 2016;18:4574–4577. doi: 10.1021/acs.orglett.6b02219. [DOI] [PubMed] [Google Scholar]
- 42.Sumida K, et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012;112:724–781. doi: 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- 43.Schneider MW, et al. Post-modification of the interior of porous shape-persistent organic cage compounds. Angew. Chem., Int. Ed. 2013;52:3611–3615. doi: 10.1002/anie.201208156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.