Abstract
Depth of invasion (DOI) can be calculated preoperatively by MRI, and whether MRI-determined DOI can predict prognosis as well as whether it can be used as an indicator of neck dissection in cT1N0 tongue squamous cell carcinoma (SCC) remains unknown. The main goal of the current study was to answer these unknowns. A total of 151 patients with surgically treated cT1N0 tongue SCC were retrospectively enrolled, and MRI-determined DOI was measured based on T1-weighted layers with a 3.0T scan. The Chi-square test was used to evaluate the association between clinical pathologic variables and neck lymph node metastasis, and the factors that were significant in the Chi-square test were then analyzed in a multivariate logistic regression analysis model to determine the independent predictors. The main study endpoints were locoregional control (LRC) and disease-specific survival (DSS), and the Kaplan-Meier method (log-rank test) was used to calculate the LRC and DSS rates. The factors that were significant in univariate analysis were then analyzed in the Cox model to determine the independent prognostic factors. A value of p < 0.05 was considered significant, and all statistical analyses were performed with SPSS 20.0. Occult neck lymph node metastasis was noted in 26 (17.2%) patients, and the ROC curve indicated that the optimal cutoff value of MRI-determined DOI was 7.5 mm for predicting neck lymph node metastasis, with a sensitivity of 86.9%. The factors of lymphovascular invasion, MRI-determined DOI, pathologic DOI, and pathologic tumor grade were significantly associated with the presence of neck lymph node metastasis in univariate analysis, and further logistic regression analysis confirmed the independence of lymphovascular invasion, MRI-determined DOI, and pathologic DOI in predicting neck lymph node metastasis. The 5-year LRC and DSS rates were 84% and 90%, respectively. Cox model analysis suggested the MRI-determined DOI was an independent prognostic factor for both LRC and DSS. Therefore, elective neck dissection is suggested if MRI-determined DOI is greater than 7.5 mm in cT1N0 tongue SCC, and MRI-determined DOI ≥ 7.5 mm indicates additional risk for disease recurrence and cancer-related death.
Subject terms: Oral cancer detection, Cancer imaging, Oral cancer detection, Surgical oncology, Risk factors
Introduction
Tongue squamous cell carcinoma (SCC) is the most common malignancy in the oral cavity, and despite advances in diagnosis and treatment, its prognosis has not significantly improved. Locoregional recurrence is the most frequent failure pattern within 2 years after treatment1. Increasing evidence indicates that neck lymph node metastasis is one of the most important prognostic factors in tongue SCC1, but unfortunately, these positive lymph nodes are usually occult or subclinical at the initial treatment in early-stage tongue SCC. Owing to a wide range of occult metastasis rates2,3, either elective neck dissection (END) or the watchful waiting policy has been the favored treatment for cT1N0 tongue SCC4,5. Investigators favoring END have commented that it allows accurate disease staging and decision making for the need for adjuvant therapies, and the resection of metastatic lymph nodes can potentially reduce the recurrence risk6,7; however, the main concern according to the traditional watchful waiting policy is the associated surgical complications, which include shoulder dysfunction and over-treatment, in patients with no pathologic metastases8. Considering that there is no accurate diagnostic procedure for staging the neck preoperatively, elective management of the neck in cT1N0 tongue SCC has been the subject of much debate during the past 3 decades and continues to be controversial.
Depth of invasion (DOI) has now been added in the newest edition of the AJCC tumor-node-metastasis staging system9, and abundant literature has shown a significant relationship between DOI and neck lymph node metastasis10–12; however, data regarding pathologic DOI usually cannot be obtained by frozen section or incisional biopsy, and DOI might have a limited role in benefiting decision making regarding neck treatment preoperatively. There are various radiology methods, including CT, MRI, PET-CT, and PET-MRI, available for clinical evaluation, but MRI is the most widely used to evaluate soft tissue disease, and current evidence has reported the reliability of MRI in measuring DOI13,14 as well as the prognostic value of MRI-determined tumor thickness in tongue SCC15,16. MRI-determined DOI is significantly different from MRI-determined tumor thickness, but whether MRI-determined DOI has the same effect as MRI-determined tumor thickness and whether it can be used as an indicator of END in cT1N0 tongue SCC remain unknown; therefore, the main goal of the current study was to clarify these unknowns.
Results
Demographic and pathologic data
There were 151 patients (111 males and 40 females) enrolled in total, and the mean age was 57.1 (range: 30–78) years. There were 102 (67.5%) smokers and 61 (40.4%) drinkers. Flap reconstruction was performed in 32 (21.2%) patients: 25 submental island flaps and 7 radial forearm flaps. Perineural invasion and lymphovascular invasion were present in 23 (15.2%) and 19 (12.6%) patients, respectively. Pathologic tumor grades were characterized as low in 75 patients, intermediate in 51 patients, and high in 25 patients. Negative margins were achieved in all patients. Tumor growth patterns of ulcer type, invasive type, and exogenous type were noted in 72 (47.7%), 20 (13.2%), and 59 (39.1%) patients, respectively.
Cervical metastasis data
Positive neck lymph nodes were noted in 26 (17.2%) patients; there was one positive lymph node in 15 patients, two positive lymph nodes in 7 patients, and three positive lymph nodes in 4 patients. There was no extracapsular spread in any of the positive lymph nodes.
Image analysis and DOI data
At the time of MRI-determined DOI calculation, two radiologists were blinded to each other; if the measurement results were not consistent, the two observers would discuss and solve the divergence together. Figure 1 depicts the interobserver variation, and the interobserver reliability was excellent (ICC = 0.934). The mean MRI-determined and pathologic DOIs were 6.9 (range: 2–13) mm and 4.2 (range: 1.0–10.0) mm, and the difference was significant (p < 0.001). ROC analysis revealed that the optimal cutoff value of MRI-determined DOI was 7.5 mm for predicting neck lymph node metastasis, with the area under the curve being 0.848; specificity: 82.0%; and sensitivity: 86.9% (Fig. 2).
Figure 1.
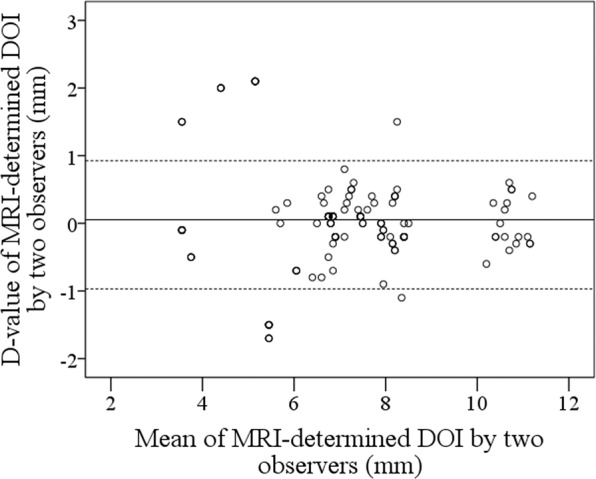
Bland-Altman plots comparing the interobserver variation (ICC = 0.934).
Figure 2.
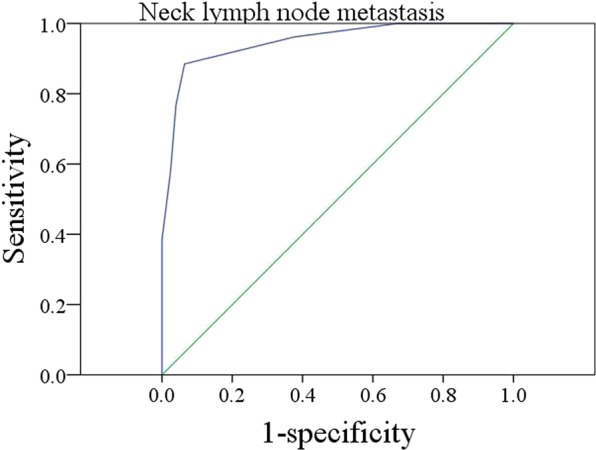
ROC analysis of the optimal cutoff value of MRI-determined DOI for predicting neck lymph node metastasis.
Predictors for occult cervical metastasis
As described in Table 1, the factors of lymphovascular invasion (p = 0.015), MRI-determined DOI (p = 0.007), pathologic DOI (p = 0.008), and pathologic tumor grade (p = 0.034) were significantly associated with the presence of neck lymph node metastasis. Further logistic regression analysis confirmed the independence of lymphovascular invasion (p = 0.022, 2.475 [1.233–4.997]), MRI-determined DOI (p = 0.009, 2.978 [1.574–7.332]), and pathologic DOI (p < 0.001, 3.112 [1.812–9.668]) in predicting neck lymph node metastasis.
Table 1.
Univariate and multivariate analysis of predictors for neck lymph node metastasis.
| Variables | Neck lymph node metastasis | Univariate | Logistic regression | ||
|---|---|---|---|---|---|
| Positive | Negative | p | p | OR [95% CI] | |
| Age | |||||
| ≥57 | 16 | 74 | |||
| <57 | 10 | 51 | 0.825 | ||
| Sex | |||||
| Male | 20 | 91 | |||
| Female | 6 | 34 | 0.665 | ||
| Smokers | |||||
| Yes | 18 | 84 | |||
| No | 8 | 41 | 0.841 | ||
| Drinkers | |||||
| Yes | 11 | 50 | |||
| No | 15 | 75 | 0.827 | ||
| Perineural invasion | |||||
| Yes | 6 | 17 | |||
| No | 20 | 108 | 0.221 | ||
| Lymphovascular invasion | |||||
| Yes | 7 | 12 | |||
| No | 19 | 113 | 0.015 | 0.022 | 2.475 [1.233–4.997] |
| Pathologic tumor grade | |||||
| Low | 8 | 67 | |||
| Intermediate + high | 18 | 58 | 0.034 | 0.110 | 2.414 [0.894–6.114] |
| MRI-determined DOI* | |||||
| ≥7.5 mm | 12 | 26 | |||
| <7.5 mm | 14 | 99 | 0.007 | 0.009 | 2.978 [1.574–7.332] |
| Pathologic DOI | |||||
| >5.0 mm | 13 | 30 | |||
| ≤5.0 mm | 13 | 95 | 0.008 | <0.001 | 3.112 [1.812–9.668] |
| Tumor growth pattern | |||||
| Ulcer type | 12 | 60 | |||
| Invasive type | 5 | 15 | |||
| Exogenous type | 9 | 50 | 0.599 | ||
*DOI: depth of invasion.
Survival data
During our follow-up with a mean time of 70.4 (range: 8–103) months, 30 patients underwent adjuvant radiotherapy, and 6 patients also underwent adjuvant chemotherapy. Locoregional recurrence occurred in 22 patients, of whom 16 patients had cervical metastasis at the time of initial treatment, and disease-related death occurred in 13 patients.
The 5-year LRC rate was 84%. With regard to the prognostic factors for LRC, as described in Table 2, the factors of perineural invasion (p = 0.016), lymphovascular invasion (p = 0.009), MRI-determined DOI (p < 0.001), pathologic DOI (p < 0.001), and neck lymph node metastasis (p = 0.004) were significantly related to LRC. Further, the Cox model indicated the independence of lymphovascular invasion (p = 0.016, 2.007 [1.274–5.732]), MRI-determined DOI (p < 0.001, 2.842 [1.449–7.264]), neck lymph node metastasis (p = 0.035, 1.745 [1.152–4.221]) and pathologic DOI (p < 0.001, 3.246 [1.679–8.336]) in predicting LRC. In patients with MRI-determined DOI ≥ 7.5 mm, the 5-year LRC rate was 68%; in patients with MRI-determined DOI < 7.5 mm, the 5-year LRC rate was 90%, and the difference was significant (Fig. 3, p < 0.001). In the further subgroup analysis of 125 pN0 patients, in 30 patients with MRI-determined DOI ≥ 7.5 mm, the 5-year LRC rate was 87%; in 95 patients with MRI-determined DOI < 7.5 mm, the 5-year LRC rate was 98%, and the difference was significant (Fig. 4, p = 0.01).
Table 2.
Prognostic factors for the locoregional control survival in patients with T1 tumors.
| Variables | Univariate | Cox model | |
|---|---|---|---|
| Log-rank test | p | RR [95% CI] | |
| Age | 0.634 | ||
| Sex | 0.187 | ||
| Smokers | 0.334 | ||
| Drinkers | 0.227 | ||
| Neck lymph node metastasis | 0.004 | 0.035 | 1.745 [1.152–4.221] |
| Perineural invasion | 0.016 | 0.114 | |
| Lymphovascular invasion | 0.009 | 0.016 | 2.007 [1.274–5.732] |
| Pathologic tumor grade | 0.095 | ||
| MRI-determined DOI | <0.001 | <0.001 | 2.842 [1.449–7.264] |
| Pathologic DOI | <0.001 | <0.001 | 3.246 [1.679–8.336] |
| Tumor growth pattern | 0.397 | ||
| Adjuvant treatment | 0.572 | ||
Figure 3.
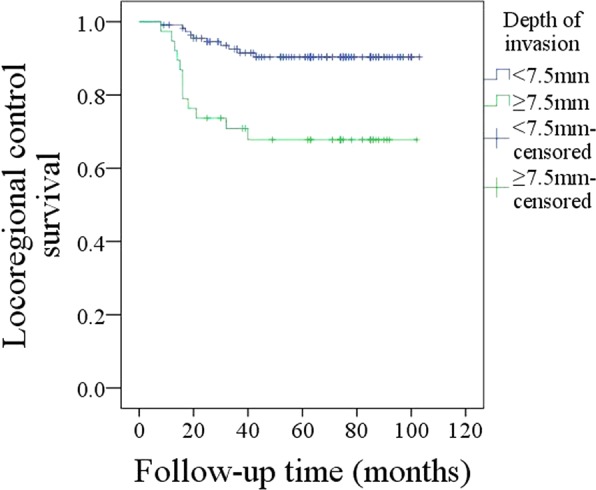
Comparison of locoregional control survival in patients with different MRI-determined depths of invasion (p < 0.001).
Figure 4.
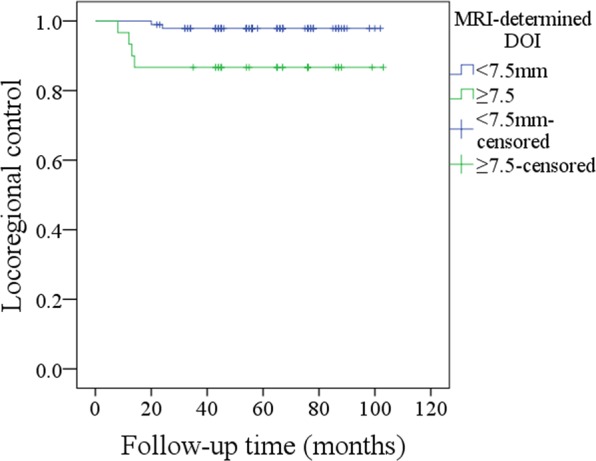
Comparison of locoregional control survival in pN0 patients with different MRI-determined depths of invasion (p = 0.01).
The 5-year DSS rate was 90%. With regard to the prognostic factors for DSS, as described in Table 3, the factors of MRI-determined DOI (p < 0.001), pathologic DOI (p < 0.001), and neck lymph node metastasis (p = 0.008) were significantly related to LRC. Further, the Cox model indicated the independence of MRI-determined DOI (p < 0.001, 2.441 [1.635–5.994]), pathologic DOI (p < 0.001, 3.002 [1.753–6.885]), and neck lymph node metastasis (p = 0.005, 2.665 [1.442–5.322]) in predicting DSS. In patients with MRI-determined DOI ≥ 7.5 mm, the 5-year DSS rate was 73%; in patients with MRI-determined DOI < 7.5 mm, the 5-year DSS rate was 96%, and the difference was significant (Fig. 5, p < 0.001).
Table 3.
Prognostic factors for the disease-specific survival in patients with T1 tumors.
| Variables | Univariate | Cox model | |
|---|---|---|---|
| Log-rank test | p | RR [95% CI] | |
| Age | 0.241 | ||
| Sex | 0.387 | ||
| Smokers | 0.841 | ||
| Drinkers | 0.458 | ||
| Neck lymph node metastasis | 0.008 | 0.005 | 2.665 [1.442–5.322] |
| Perineural invasion | 0.089 | ||
| Lymphovascular invasion | 0.110 | ||
| Pathologic tumor grade | 0.175 | ||
| MRI-determined DOI | <0.001 | <0.001 | 2.441 [1.635–5.994] |
| Pathologic DOI | <0.001 | <0.001 | 3.002 [1.753–6.885] |
| Tumor growth pattern | 0.422 | ||
| Adjuvant treatment | 0.631 | ||
Figure 5.
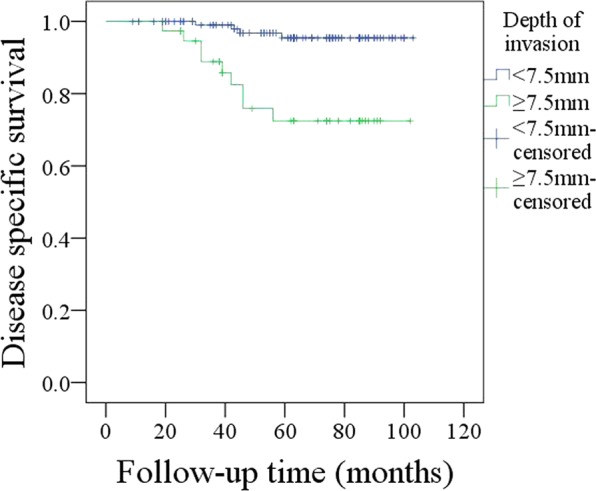
Comparison of disease-specific survival in patients with different MRI-determined depths of invasion (p < 0.001).
Discussion
The most valuable finding in the current study was that MRI-determined DOI was significantly associated with the presence of neck lymph node metastasis, which added a nearly 3-fold risk of neck lymph node metastasis if MRI-determined DOI was greater than 7.5 mm, and MRI-determined DOI was an independent prognostic factor for LRC and DSS. This finding might provide preoperative benefits for neck management in cT1N0 tongue SCC, and neck dissection is strongly suggested if preoperative MRI-determined DOI is greater than 7.5 mm.
The feasibility of measuring DOI by MRI has been widely analyzed13,14,16,17. Murakami et al.13 compared the interrater reliability of different methods of DOI measurement by MRI and found that the axial invasive portion method had excellent interrater reliability. The data in the current study were also obtained with the axial invasive portion method. Lam et al.18 described that the tumor thickness measured on T1-sequence MRI was 0.8 mm greater than that measured in pathological sections but was 2 mm greater on T2 sequences than that measured in pathological sections on average. A similar finding was also noted by Preda et al.19. T1-weighted images are more accurate than T2-weighted images for measuring DOI. DOI in T2-weighted images can be overestimated owing to inflammation and surrounding tissue edema. Therefore, in the current study, the MRI-determined DOI was obtained based on T1-weighted sequences to increase our reliability. On the other hand, Park et al.14 reported that compared to the data measured in postoperative pathological sections, the DOI measured on T1-weighted MRI was 1.5 mm greater, but the mean difference between MRI-determined DOI and pathologic DOI was 2.7 mm in the current study, which is slightly higher than previous findings14,18,19. A possible explanation is that only T1 stage tumors were included for analysis, and a relatively high extent of tissue shrinkage exists in small tumors. On the other hand, unlike our study, some of the abovementioned measurements were obtained in contrast-enhanced T1-weighted images, and the use of contrast medium might also affect the reliability of measurements; however, in our cancer center, the primary site of tongue SCC is usually evaluated with nonenhanced MRI.
The presence of neck lymph node metastasis is an important prognostic factor for head and neck SCC1,3. END is usually an important component of the primary operation, but owing to the wide range of occult metastasis rates in cT1N0 tongue SCC7, the neck management of cT1N0 tongue SCC has been debated over the years and remains controversial. The ideal treatment for patients with cT1N0 tongue SCC must strike a balance between the possible surgical morbidity and optimal oncological outcomes. The common principle is that if the rate of occult metastasis is greater than 20%, cN0 neck nodes should be treated11,20. In the current study, the overall occult metastasis rate was 17.2%, but all patients underwent END. There are at least three aspects for the explanation of this phenomenon. First, the high requirement of routine follow-up for the wait-and-see policy was usually not achievable by our patients, as described by our previous studies21,22. Patients in our cancer hospital usually come from low-income families and remote districts. Second, there is abundant evidence indicating that patients who do not undergo prophylactic therapy of the clinically N0 neck nodes usually have a low salvage rate of disease recurrence2–5. Third, as the most important factor, there are no reliable predictors for occult neck lymph node metastasis from previous studies.
A number of researchers have aimed to explore the potential predictors of occult neck lymph node metastasis. Tumor budding has been defined as the presence of small clusters of cancer cells or isolated single cancer cells, suggesting a highly aggressive biologic behavior and a great possibility of migrating to the adjacent stroma. Xie et al.23 described that tumor budding intensity was significantly associated with occult lymph node metastasis. Tumor cell proliferation, microvascular regeneration, and tumor metastasis can be promoted by a systemic inflammatory response. Furthermore, the neutrophil-to-lymphocyte ratio (NLR) in peripheral blood is an accurate and reliable inflammatory marker. A high NLR has been significantly related to worse survival in many kinds of solid cancers24. Abbate et al.25 first demonstrated a higher risk for occult neck lymph node metastasis when the pretreatment NLR was greater than 2.93. Loganathan et al.26 recently reported that END should be considered when the tumor thickness exceeds 5 mm based on the significant relationship between tumor thickness and occult neck lymph node metastasis. Other analyzed variables included perineural invasion, lymphovascular invasion, and pathologic DOI27,28. However, data regarding pathologic factors usually cannot be obtained preoperatively, and owing to being easily influenced by infection or inflammation, the pretreatment NLR is nonspecific. Therefore, additional accurate indicators are needed.
As discussed above, MRI-determined DOI can be reliably calculated preoperatively, and our results demonstrated high predictive value of MRI-determined DOI ≥ 7.5 mm for identifying occult metastasis with a sensitivity of 86.9%. In another study by Jung et al.29, the authors noted that there was a significant association between nodal metastasis and MRI-determined DOI with a cutoff value of 10.5 mm in T1-weighted images, but the authors failed to provide information about the sensitivity, and the variation from our results could be explained by the different calculation methods used for the cutoff value. The potential mechanism for our interesting finding is presented as follows: MRI-determined DOI can indirectly reflect pathologic DOI, as the mean difference between MRI-determined DOI and pathologic DOI was 2.7 mm in the current study; therefore, an MRI-determined DOI cutoff value of 7.5 mm would indicate a pathologic DOI cutoff value of 5.0 mm. Extensive studies have reported that the neck lymph node metastasis risk increases apparently with pathologic DOI > 5.0 mm10,11,27,28.
Prognostic factors for tongue SCC have been extensively analyzed, and the widely accepted risk factors include disease stage, tumor differentiation, perineural invasion, lymphovascular invasion, neck lymph node status, pathologic DOI, and so on1,12,16,22,26,30. Similar findings were also noted in the current study. However, the significance of MRI-determined DOI in the survival of tongue SCC remains unknown. This is the first study to describe a significant association between MRI-determined DOI and prognosis. MRI-determined DOI ≥ 7.5 mm indicates a higher risk of disease recurrence and cancer-related death. The potential mechanism might be explained by the fact that greater MRI-determined DOI indicates greater pathologic DOI, and the negative effect of pathologic DOI on prognosis has been widely suggested. Tam et al.12 recently reported that DOI was an independent predictor for both overall survival and DSS. Similar findings were also presented by Iida et al.31 and Jung et al.29.
Almost all the studies regarding MRI-determined DOI are focused on evaluating the association between MRI-determined DOI and pathologic DOI. We hope our study will benefit neck management in patients with cT1N0 tongue SCC and help to identify additional methods to improve its progression.
There were some limitations in the current study: the statistical power was decreased by the inherent bias in a retrospective study, we had a small sample size, and additional large randomized control trials are needed to clarify the question.
In summary, there is a significant relationship between MRI-determined DOI and occult neck lymph node metastasis in cT1N0 tongue SCC, and elective neck dissection and adjuvant therapy are suggested if MRI-determined DOI is greater than 7.5 mm; MRI-determined DOI ≥ 7.5 mm indicates additional risk for disease recurrence and cancer-related death.
Materials and methods
Ethical consideration
Our study was approved by the Zhengzhou University institutional research committee. Written consent for medical research was obtained from all patients at the time of initial treatment. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patient selection and data collection
From January 2010 to December 2016, the medical records of adult (≥18 years old) patients with surgically treated tongue SCC were reviewed. The enrolled patients had to meet the following criteria: the disease was primary; the disease was re-staged as cT1N0M0 according to the 7th AJCC classification followed by ultrasound, CT, and MRI examinations; data regarding MRI could be obtained; and data regarding the follow-up could be obtained. Information including age, sex, tumor growth pattern, adverse pathologic characteristics, and follow-up of the enrolled patients was extracted and analyzed. Drinkers were defined as those who consumed at least one alcoholic drink per day for at least 1 year, and smokers were defined as those who smoked on a daily basis or had quit smoking for less than 5 years1.
MRI-determined DOI was measured based on T1-weighted layers with a 3.0T scan 13,14 it was defined as the vertical distance between the deepest point of tumor infiltration and the simulated normal mucosal junction (Fig. 6). For exogenous tumors, the part above the mucosal surface was neglected, and for ulcerative tumors, the invaginated part was added17. The MRI-determined DOI was measured by at least two radiologists with 10 years of working experience.
Figure 6.
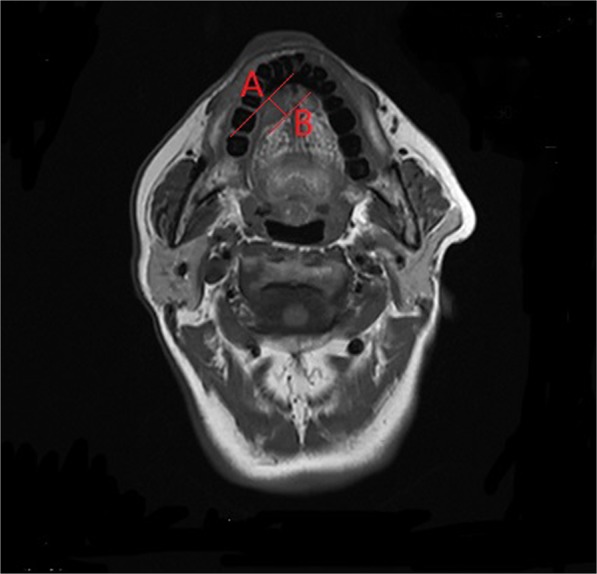
Measurement of the MRI-determined depth of invasion (distance between A and B points) based on the adjacent normal mucosal junction to the deepest infiltration point.
All pathologic sections were re-reviewed by at least two pathologists, and perineural invasion was considered to be present if tumor cells were identified within the perineural space and/or nerve bundle; lymphovascular infiltration was positive if tumor cells were noted within the lymphovascular channels32. The pathologic DOI was measured from the level of the adjacent normal mucosa to the deepest point of tumor infiltration, regardless of the presence or absence of ulceration9.
MRI examination
MRI scanning (SIEMENS Prasma, 3.0T) was performed by several technicians within one week before surgery. The scanning protocol included T1 (TR: 697 ms, TE: 7.1 ms) and T2 (TR: 4070 ms, TE: 112 ms) axial, coronal, and sagittal sequences along with T2 axial and coronal sequences with fat suppression (FS) (TR: 4400 ms, TE: 112 ms). No contrast medium was used during the MRI scan. The images were reconstructed with the thickness of a 1.0-mm slice.
Treatment principle
In our cancer center, END (levels I–III) was routinely performed in tongue SCC patients with the exception of those with very early-stage disease. Primary tumor excision was usually performed without lip splitting, and the mouth floor tissue was usually preserved unless lingual lymph node metastasis was reported by frozen section. After therapy, the patients were examined every 3 months during the first year, every 6 months during the second year, and once per year after the second year1. Once there was suspicion of disease recurrence, aspiration biopsy or incisional biopsy combined with other examinations was performed.
Data analysis
The MRI-determined and pathologic DOIs were compared using paired Student’s t tests. The ROC curve was used to determine the optimal cutoff of the MRI-determined DOI for predicting neck lymph node metastasis. The interobserver reliability was compared by Bland-Altman plots. The intraclass correlation coefficients (ICCs) were analyzed. An ICC value <0.4 indicated poor agreement, a value varying from 0.4 to 0.75 indicated fair to good agreement, and a value greater than 0.75 indicated excellent agreement. The Chi-square test was used to evaluate the association between clinical pathologic variables and neck lymph node metastasis, and the factors that were significant in the Chi-square test were then analyzed in a multivariate logistic regression analysis model to determine the independent predictors. The primary outcomes were locoregional control (LRC) and disease-specific survival (DSS). The survival time of LRC was calculated from the date of surgery to the date of local or regional recurrence or the last follow-up, and the survival time of DSS was calculated from the date of surgery to the date of cancer-related death or the last follow-up. The Kaplan-Meier method (log-rank test) was used to calculate the LRC and DSS rates. The factors that were significant in univariate analysis were then analyzed in the Cox model to determine the independent prognostic factors. A value of p < 0.05 was considered significant, and all statistical analyses were performed with SPSS 20.0.
Supplementary information
Author contributions
Study design and manuscript writing: C.M.-X., J.H.-Y., L.Q.-K., X.X.-Z., L.F.-W., X.J.-C. and H.L.-L. Study selection and data analysis: C.M.-X., J.H.-Y. and L.Q.-K. Study quality evaluation: C.M.-X., L.F.-W., X.J.-C., H.L.-L. and Q.Y. Manuscript revision: C.M.-X., J.H.-Y., L.Q.-K., X.X.-Z., L.F.-W., X.J.-C. and H.L.-L. All authors have read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article. The primary data can be obtained from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chunmiao Xu and Junhui Yuan.
Supplementary information
is available for this paper at 10.1038/s41598-020-61474-5.
References
- 1.Fang Q, et al. Value of lingual lymph node metastasis in patients with squamous cell carcinoma of the tongue. Laryngoscope. 2019;129:2527–2530. doi: 10.1002/lary.27927. [DOI] [PubMed] [Google Scholar]
- 2.Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head. Neck. 1997;19:583–588. doi: 10.1002/(SICI)1097-0347(199710)19:7<583::AID-HED4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Mücke T, et al. Incidence and outcome for patients with occult lymph node involvement in T1 and T2 oral squamous cell carcinoma: a prospective study. BMC Cancer. 2014;14:346. doi: 10.1186/1471-2407-14-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Z, Li JN, Li CZ, Guo CB. Elective neck dissection versus observation in the management of early tongue carcinoma with clinically node-negative neck: a retrospective study of 229 cases. J. Craniomaxillofac Surg. 2014;42:806–810. doi: 10.1016/j.jcms.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, et al. Elective neck dissection versus wait-and-watch policy for oral cavity squamous cell carcinoma in early stage: A Systematic Review and Meta-Analysis based on survival data. J. Oral. Maxillofac. Surg. 2019;77:2154–2167. doi: 10.1016/j.joms.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Lim, Y. C., et al Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: elective neck dissection versus observation. 116, 461–465 (2006). [DOI] [PubMed]
- 7.Abu-Ghanem S, et al. Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 2016;142:857–865. doi: 10.1001/jamaoto.2016.1281. [DOI] [PubMed] [Google Scholar]
- 8.Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am. J. Surg. 1989;158:309–313. doi: 10.1016/0002-9610(89)90122-0. [DOI] [PubMed] [Google Scholar]
- 9.Lydiatt WM, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 10.Mair MD, et al. Depth of invasion, size and number of metastatic nodes predicts extracapsular spread in early oral cancers with occult metastases. Oral. Oncol. 2018;81:95–99. doi: 10.1016/j.oraloncology.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Mitani S, et al. Anatomic invasive depth predicts delayed cervical lymph node metastasis of tongue squamous cell carcinoma. Am. J. Surg. Patho. 2016;40:934–942. doi: 10.1097/PAS.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 12.Tam S, Amit M, Zafereo M, Bell D, Weber RS. Depth of invasion as a predictor of nodal disease and survival in patients with oral tongue squamous cell carcinoma. Head. Neck. 2019;41:177–184. doi: 10.1002/hed.25936. [DOI] [PubMed] [Google Scholar]
- 13.Murakami R, et al. Reliability of MRI-derived depth of invasion of oral tongue cancer. Acad. Radiol. 2019;26:e180–e186. doi: 10.1016/j.acra.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Park JO, et al. Diagnostic accuracy of magnetic resonance imaging (MRI) in the assessment of tumor invasion depth in oral/oropharyngeal cancer. Oral. Oncol. 2011;47:381–386. doi: 10.1016/j.oraloncology.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Okura M, et al. Tumor thickness and paralingual distance of coronal MR imaging predicts cervical node metastases in oral tongue carcinoma. AJNR Am. J. Neuroradiol. 2008;29:45–50. doi: 10.3174/ajnr.A0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu H, et al. Predicting the prognosis of oral tongue carcinoma using a simple quantitative measurement based on preoperative MR imaging: tumor thickness versus tumor volume. AJNR Am. J. Neuroradiol. 2015;36:1338–1342. doi: 10.3174/ajnr.A4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao MH, et al. Accuracy of magnetic resonance imaging in evaluating the depth of invasion of tongue cancer. A prospective cohort study. Oral. Oncol. 2019;91:79–84. doi: 10.1016/j.oraloncology.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Lam P, et al. Correlating MRI and histologic tumor thickness in the assessment of oral tongue cancer. AJR Am. J. Roentgenol. 2004;182:803–808. doi: 10.2214/ajr.182.3.1820803. [DOI] [PubMed] [Google Scholar]
- 19.Preda L, et al. Relationship between histologic thickness of tongue carcinoma and thickness estimated from preoperative MRI. Eur. Radiol. 2006;16:2242–2248. doi: 10.1007/s00330-006-0263-9. [DOI] [PubMed] [Google Scholar]
- 20.Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch. Otolaryngol. Head. Neck Surg. 1994;120:699–702. doi: 10.1001/archotol.1994.01880310005001. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Yuan S, Fang Q, Sun Q. Natural history of untreated squamous cell carcinoma of the head and neck. Clin. Otolaryngol. 2019;44:200–203. doi: 10.1111/coa.13260. [DOI] [PubMed] [Google Scholar]
- 22.Cui, M. et al. Prognostic Value of a Family History of Oral Tongue Squamous Cell Carcinoma: A Matched-Pair Study. Laryngoscope, 10.1002/lary.28471 (2019). [DOI] [PubMed]
- 23.Xie N, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J. Oral. Pathol. Med. 2015;44:266–272. doi: 10.1111/jop.12242. [DOI] [PubMed] [Google Scholar]
- 24.Fang Q, Liu F, Seng D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manag. Res. 2019;11:1081–1085. doi: 10.2147/CMAR.S192788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbate V, et al. Iaconetta G6, Califano L. Pre-treatment Neutrophil-to-Lymphocyte Ratio as a predictor for occult cervical metastasis in early stage (T1-T2 cN0) squamous cell carcinoma of the oral tongue. Surg. Oncol. 2018;27:503–507. doi: 10.1016/j.suronc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Loganathan P, Sayan A, Hsu DWK, Paraneetharan S, Ilankovan V. Squamous cell carcinoma of the anterior tongue: is tumour thickness an indicator for cervical metastasis? Int. J. Oral. Maxillofac. Surg. 2017;46:407–412. doi: 10.1016/j.ijom.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Lim SC, et al. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin. Cancer Res. 2004;10(1 Pt 1):166–172. doi: 10.1158/1078-0432.CCR-0533-3. [DOI] [PubMed] [Google Scholar]
- 28.Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol. Head. Neck Surg. 2004;131:472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Jung J, et al. Significant invasion depth of early oral tongue cancer originated from the lateral border to predict regional metastases and prognosis. Int. J. Oral. Maxillofac. Surg. 2009;38:653–660. doi: 10.1016/j.ijom.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Dai L, Fang Q, Li P, Wu J, Zhang X. Secondary Squamous Cell Carcinoma of the Oral Cavity after Nasopharyngeal Carcinoma. Cancer Res. Treat. 2020;52:109–116. doi: 10.4143/crt.2019.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iida Y, et al. Depth of invasion in superficial oral tongue carcinoma quantified using intraoral ultrasonography. Laryngoscope. 2018;128:2778–2782. doi: 10.1002/lary.27305. [DOI] [PubMed] [Google Scholar]
- 32.Skulsky SL, et al. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines In Oncology. Head. Neck. 2017;39:578–594. doi: 10.1002/hed.24580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The primary data can be obtained from the corresponding author.


