Abstract
The present study compares the net portal appearance of dietary iron (Fe) and selenium (Se) after meals containing different sources and levels of these minerals. Twelve pigs (55.1 ± 3.7 kg) were used in a cross-over design to assess the 11-h net portal-drained viscera (PDV) flux of serum Fe and Se after ingestion of boluses containing inorganic (I) or organic (O) dietary Fe and Se at industry average (A; 200 and 0.6 mg, respectively) or high (H; 400 and 1.2 mg, respectively) levels. Arterial serum Fe concentrations increased by an average of 158% within 6 h post-meal and gradually decreased thereafter (P < 0.001). Values were greater (P < 0.001) for I than for O until 6 h post-meal and greater (P ≤ 0.001) for A than for H from 4 to 8 h post-meal. For the whole post-prandial period (11 h), arterial serum Fe concentrations tended (P = 0.06) to be greater for I than for O and were lowest for HO (P ≤ 0.03). Net PDV flux of Fe tended to be greater for AI than for AO (P ≥ 0.07). Cumulative appearance of Fe in PDV serum (% of dietary intake) was greater for I than for O (2.43 vs. −0.76%; P = 0.02) and A tended to be greater than H (1.96 vs. −0.29 %; P = 0.09) until 3 h post-meal, but these effects further faded out (P ≥ 0.43). Arterial serum Se concentration decreased for all treatments (average of 7%) from premeal values (P < 0.001), and this was more pronounced for O than for I (P = 0.03). Irrespective of treatment, net PDV flux of Se was positive (different from 0, P ≤ 0.03) during the first 90 min post-meal, decreased to negative minimum values (different from 0, P = 0.03) at 5 h post-meal, and was not different from 0 thereafter (P ≥ 0.11). Cumulative appearance of Se in PDV serum (% of dietary intake) was greater for I than for O (20.0 vs. −3.8%; P = 0.04) only at 45 min post-meal. In conclusion, both dietary Fe and Se absorption are limited to the early post-meal period. Whereas for Fe, the level effect is in accordance with the known negative correlation between its dietary concentration and percentage of intestinal absorption, this was not the case for dietary Se. The postabsorptive availability of dietary I was greater than O for both minerals and, particularly for Fe, at low levels.
Keywords: dietary level, intestinal absorption, iron, pigs, selenium, source
Introduction
Adequate trace mineral supplementation is essential in livestock nutrition. Among them, iron (Fe) and selenium (Se) are particularly important due to their crucial functions in animals’ health (Di Silvestro, 2005; Kurokawa and Berry, 2013).
Flohr et al. (2016) reported that industrial supplementation levels of dietary iron (mainly inorganic sources) average 76.1 mg/kg for growing/finishing pigs. However, according to National Research Council (2012), grains commonly used in pigs’ diets generally have substantial levels of native Fe (organic nonheme source) that potentially cope (depending on its bioavailability) with the requirements of growing pig (40 to 60 mg/kg). It implies that the total ingested Fe (native plus supplemental levels) may be much higher than the pigs’ needs. Although Fe is an essential trace element, at high levels it can promote the formation of reactive oxygen species that induce lipid peroxidation, DNA damage, and cell death (Bresgen and Eckl, 2015; Wessling-Resnick, 2017). Mechanisms of Fe absorption differ according to dietary source (Gulec et al., 2014), but the homeostatic control at the enterocyte level regulates postabsorptive availability (Conrad et al., 1987; Wessling-Resnick, 2017) and may have an important impact on Fe fate. The mechanisms of Fe homeostasis are well known (Ganz and Nemeth, 2012), but the limited knowledge on the postabsorptive availability of different levels and sources of dietary Fe in pigs limit the optimization of supplementation strategies.
Dietary Se derives from inorganic or organic sources. Dietary organic Se, native in feedstuff, is actively absorbed through amino acid transport mechanisms (McDowell, 2003) and deposited into proteins following the metabolism of methionine (Schrauzer, 2003) or, under the regulation of the transsulfuration pathway, synthesizes selenoproteins (Gonzalez-Flores et al., 2013). Dietary inorganic Se is passively absorbed by simple diffusion (Wolffram et al., 1989) and, by short-cutting transsulfuration reactions, rapidly synthesizes selenide that is the substrate for selenoproteins (Windisch, 2002). The lack of a regulatory mechanism for the synthesis of selenide (highly toxic metabolite) from inorganic Se makes the range between its adequate and toxic level very narrow. The current regulation for Se supplementation allows up to 0.3 mg/kg in the diet of pigs (Food and Drug Administration, 2019), but greater levels may be potentially beneficial for the antioxidant and immune systems (Surai, 2006). The lack of information on the intestinal fate of Se and the postabsorptive availability of its sources and levels complicate these debates and hamper possible higher supplemental Se approaches.
The present study compares the net portal-drained viscera (PDV) flux of Fe and Se in growing pigs fed diets containing different sources and levels of these minerals. It aims to determine the impact of source and level of dietary Fe and Se on their postabsorptive availability.
Materials and Methods
The experimental procedures followed the guidelines of the Canadian Council on Animal Care (2009) and were approved by the Institutional Animal Care Committee of the Sherbrooke Research and Development Centre (Quebec, Canada). All animals were cared for according to the recommended code of practice of the National Farm Animal Care Council (2014).
Animals, surgeries, and postoperative care
Twelve Yorkshire-Landrace × Duroc gilts were selected at 30 kg of BW and fed ad libitum a conventional basal diet for growing pigs (Table 1) until surgery. Average BW at surgery was 55.1 ± 3.7 kg at 100.1 ± 3.7 d of age. The surgery procedure has been described by Hooda et al. (2009). Briefly, a catheter was inserted in the portal vein at approximately 2.5 cm before its entry into the liver and an ultrasonic flow probe was installed around the portal vein 1.0 cm distal to the catheter. Another catheter was inserted through the carotid artery up to the junction between the carotid and subclavian arteries.
Table 1.
Composition of basal and semipurified diets
| Ingredients | Amount, % |
|---|---|
| Basal diet1 | |
| Corn | 56.3 |
| Soybean meal 48% | 20.5 |
| Wheat | 15.0 |
| Distillers dried grain with solubles | 5.8 |
| Macro-premix | 2.4 |
| Limestone | 1.2 |
| Salt | 0.3 |
| Monocalcium phosphate | 0.5 |
| l-Lysine | 0.19 |
| Methionine | 0.01 |
| Mineral and vitamin premix2 | 0.2 |
| Semipurified diet3 | |
| Cornstarch | 71.4 |
| Casein | 17.2 |
| Sucrose | 11.4 |
1Analyzed values were as follows: protein, 16.8%; Ca, 0.83%; P, 0.49%; Fe, 288.3 mg/kg; Se, 0.3 mg/kg; Zn, 199.0 mg/kg; Cu, 40.7 mg/kg; and Mn, 43.1 mg/kg. Metabolizable energy was estimated at 3,082 kcal/kg.
2Supplied, per kg of feed: Mn, 27 mg; Zn, 137 mg; Fe, 99 mg; Cu, 26 mg; I, 1 mg; Se, 300 μg; vitamin A, 3020 IU; vitamin D3, 900 IU; vitamin E, 40 IU; vitamin K, 1.5 mg; thiamine, 2.0 mg; riboflavin, 3.5 mg; niacin, 20 mg; pantothenic acid, 15 mg; folic acid, 0.5 mg; pyridoxine, 2.0 mg; biotin, 50 μg; choline, 100 mg; and vitamin B12, 20 μg.
3Analyzed values were as follows: protein, 16.4 %; Ca, 0.03 %; P, 0.11 %; Fe, 26.6 mg/kg; Se, <0.1 mg/kg; Zn, 11.3 mg/kg; Cu, 2.3 mg/kg; and Mn, 1.2 mg/kg. Metabolizable energy was estimated at 3,868 kcal/kg.
After surgery, animals were penned individually (1 m × 1.8 m) and fed twice daily with increasing amounts of the conventional growing-phase diet described above up to a total of 1.6 kg/d. Seven to 10 d after surgery, when animals fully recovered appetite and normal growth rate, they were gradually adapted during 3 to 5 d to a metabolic cage (with free access to water) and to the consumption of a semipurified diet (up to 0.8 kg/d; Table 1) containing cornstarch (Produits alimentaires Berthelet, QC, Canada), casein (C-7078, Sigma–Aldrich, St Louis, MO), and sucrose (S-8501, Sigma–Aldrich).
Treatments
The above-semipurified diet was used to provide a feed matrix devoid of significant amounts of endogenous trace minerals. On experimental days, treatments were served within a bolus of 100 g of sweetened jelly to induce a rapid consumption (<15 min) just prior to a meal (0.8 kg/d) of semipurified diet. According to a cross-over experimental design, each animal received 4 dietary treatments containing Fe and Se from inorganic (I; FeSO4 and Na2SeO3) or organic (O; Bioplex, Alltech Inc., Nicholasville, KY) sources both providing elemental Fe and Se at levels slightly higher than the industry average (A) at 200 and 0.6 mg, respectively, or more than twice higher (H) at 400 and 1.2 mg, respectively, in a 2 × 2 factorial arrangement (AI, HI, AO, and HO).
Daily amounts (based on a feed intake of 2.0 kg/d) of supplemental dietary Fe and Se for A levels were based on levels currently used by the pig industry in North America (Flohr et al., 2016). For both I and O sources, boluses also contained 2 levels (A and H) of 3 other trace elements commonly incorporated in trace mineral premixes, respectively, 200 and 400 mg of Zn, 20 and 40 mg of Cu, and 8 and 16 mg of Mn. This premix preparation allowed maintaining constant ratios among these 5 trace minerals irrespective of their levels (A or H) of ingestion.
Blood collection and analysis
On experimental days, pigs were moved to metabolic cages and blood samples (4 mL) were collected simultaneously from the 2 catheters 5 min before the oral bolus containing 1 of the 4 treatments, every 45 min for the first 3 h after bolus, and every hour for the following 8 h for a total of 11 postprandial hours. Portal blood flow was recorded continuously during 11 h using a flowmeter (Transonic 400-series; Ithaca, NY) and the WinDaq software (DATAQ Instruments Inc., Akron, OH). After samples collection, pigs were offered the conventional growing-phase diet described above to complete their usual daily allowance according to their BW. Between experimental days, animals were moved back to their respective pens and fed the conventional growing-phase diet described above.
Immediately after sampling, arterial and PDV blood were transferred from syringes into EDTA-treated tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ) and trace element-free tubes BD Hemogard Closure (Vacutainer, Becton Dickinson). Packed cell volume was measured in duplicate on fresh PDV blood by microcentrifugation. Aliquots of arterial and PDV blood were frozen for hemoglobin determination according to the method of Drabkin (Manet, 1969). After at least 4 h of decantation at room temperature, arterial and PDV serum were collected after centrifugation of blood at 1,800 × g for 10 min at 4 °C and frozen at −20 °C for Fe and Se determination. Measurements of Fe were performed using the QuantiChrom Iron Assay Kit (DIFE-250; Cedarlane, Ontario, Canada), and Se was measured using a fluorimetric method adapted by Giguère et al. (2005) from the technique of Sheehan and Gao (1990). Average intra- and interassay CV were 3.7% and 5.3% for Fe and 3.0% and 3.3% for Se. For both minerals, a CV equal or smaller than 5% between duplicate was accepted.
Calculations and statistical analysis
Out of 48 postprandial serum profiles, 2 profiles for AI, 1 profile for HI, and 1 profile for HO were not collected because of portal catheter malfunctioning. The remaining 44 postprandial serum profiles were completely collected. Net PDV fluxes of Fe and Se were calculated for each sampling time as described by Girard et al. (2001). Positive net flux indicates the release of the mineral from PDV, whereas negative net flux indicates uptake of mineral by PDV. Arterial concentrations and net PDV fluxes of Fe and Se recorded 5 min before the meal were used as basal levels (t0). Blood and serum PDV flows (L/min) as well as arterial concentrations of Fe and Se (mg/L), serum arterial and PDV fluxes of Fe and Se (mg/min), porto-arterial differences of serum Fe and Se (µg/L), net PDV flux of serum Fe and Se (µg/min), and cumulative appearance of Fe and Se in PDV serum (expressed in % of dietary intake) were analyzed for each sampling time using the mixed procedure of SAS (SAS Inst., Inc., Cary, NC; Littell et al., 1996) according to a cross-over design in which pigs, periods, and treatments (2 sources and 2 levels) were included in the model along with repeated measures in time (unequally spaced). For blood and serum PDV flows, arterial concentrations, and serum PDV fluxes of Fe and Se, the time effect considered the whole postprandial period (660 min). For porto-arterial differences of Fe and Se, net PDV fluxes of Fe and Se, and cumulative appearance of Fe and Se in PDV serum, t0 was removed from statistics. Because time intervals were unequally spaced, the following covariance structures were compared: space (power), space (Gaussian), space (exponential), space (linear), space (linear logarithmic), space (spherical), ante-dependence, and unstructured. For each variable, only the statistical analysis with the best-fit statistic value was considered. When the treatment effect was significant, multiple comparisons between treatments were performed using a t-test. Results are reported as least square means ± SEM. Differences were considered significant at P ≤ 0.05 and tendencies at 0.05 < P ≤ 0.10.
Results and Discussion
Analytical concentrations of Fe and Se in the basal and semipurified diets are presented in Table 1. The actual intakes of Fe and Se for each experimental meal corresponded to 225.2 and 0.7 mg for AI, 423.8 and 1.3 mg for HI, 246.7 and 0.6 mg for AO, and 466.8 and 1.2 mg for HO. The present A levels are 20% to 25% higher than the industry levels reported by Flohr et al. (2016).
Portal-drained viscera serum flux was not affected by treatments (P ≥ 0.84; Table 2). Prebolus (t0) average value was 0.83 ± 0.03 L/min. This level rapidly increased to 1.09 ± 0.03 L/min between 45 and 90 min post-bolus followed by a gradual decrease to 0.78 ± 0.03 L/min at the end of the sampling period (time effect; P < 0.001; Supplementary File 1). These values are similar to those previously reported by Hooda et al. (2009, 2010) and by this laboratory (Matte et al., 2010, 2012, 2017; Dalto et al., 2018, 2019) using ultrasonic perivascular blood flow measurements in growing pigs.
Table 2.
Average portal-drained viscera (PDV) serum flow, iron and selenium arterial serum concentration, porto-arterial difference, net PDV serum flux, and cumulative appearance of iron and selenium in PDV serum after 11 postprandial hours according to dietary treatments1
| Item | AI | HI | AO | HO | SEM | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Source | Level | Source × level | ||||||
| PDV serum flow, L/min | 0.90 | 0.89 | 0.91 | 0.90 | 0.05 | 0.84 | 0.84 | 0.99 |
| Arterial serum Fe, mg/L | 2.01 | 2.02 | 1.97 | 1.57 | 0.13 | 0.06 | 0.13 | 0.10 |
| Fe porto-arterial difference, µg/L2 | 7.72 | −11.73 | −22.22 | −13.57 | 14.64 | 0.27 | 0.71 | 0.33 |
| Net PDV serum Fe flux, µg/min3 | 11.45 | −15.21 | −25.01 | −7.41 | 12.11 | 0.23 | 0.70 | 0.07 |
| Cumulative appearance of Fe in PDV serum, % of dietary intake3 | 0.77 | −3.42 | −9.24 | −1.83 | 3.49 | 0.23 | 0.64 | 0.12 |
| Arterial serum Se, mg/L | 0.14 | 0.15 | 0.14 | 0.14 | 0.01 | 0.19 | 0.46 | 0.60 |
| Se porto-arterial difference, µg/L2 | 0.18 | −0.44 | −0.63 | −0.35 | 0.66 | 0.57 | 0.69 | 0.49 |
| Net PDV serum Se flux, µg/min2 | 0.57 | −0.33 | −0.25 | −0.38 | 0.58 | 0.44 | 0.36 | 0.44 |
| Cumulative appearance of Se in PDV serum, % of dietary intake2 | 27.26 | −27.88 | −49.90 | −19.34 | 61.86 | 0.58 | 0.84 | 0.50 |
1Dietary treatments: AI, 200 mg Fe and 0.6 mg Se; HI, 400 mg Fe and 1.2 mg Se; AO, 200 mg Fe and 0.6 mg Se; HO, 400 mg Fe and 1.2 mg Se.
2Values not statistically difference from zero (P ≥ 0.12).
3Only AO was statistically difference from zero (P ≤ 0.04).
Iron
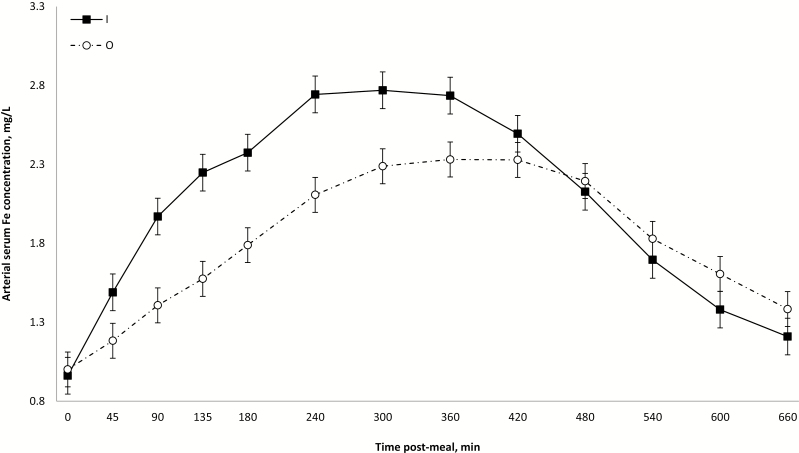
Across treatments, arterial serum Fe gradually increased from premeal values (0.98 ± 0.08 mg/L) to a maximum between 5 and 7 h post-meal (2.53 ± 0.08 mg/L) followed by a gradual decrease to 1.30 ± 0.08 mg/L at the end of the sampling period (time effect; P < 0.001; Supplementary File 2). These time effects are comparable to those reported by Blachier et al. (2007) despite the different routes of administration between their study and the present one (oral vs. intraduodenal). Arterial serum Fe concentrations tended to be lower for O than for I (P ≤ 0.06), and this was particularly marked for HO (interaction source × level; P = 0.10; Table 2). It is known that Fe absorption occurs throughout the small intestine but mostly in the duodenum (Gulec et al., 2014). Wheby and Crosby (1963) reported short-term (within 4 to 6 h) and long-term transport (up to 24 h) of Fe after its absorption by enterocytes. Considering the present positive cumulative appearance of Fe in PDV serum across treatments during the first 3 h post-meal, the present increase in arterial serum Fe within the first 5 to 7 h post-meal is consistent with the presence of a short-term transport of Fe.
In terms of intestinal fate of dietary Fe, nonheme Fe is mainly present in the ferric (Fe3+) form and is insoluble at pH > 3. By binding to gastric mucin, Fe3+ remains soluble and available for absorption at neutral pH (Conrad et al., 1991). In the intestine, Fe3+ must be reduced to Fe2+ (ferrous Fe) to bind, at the apical membrane of enterocytes (gut lumen), the membrane Fe2+-specific divalent metal transporter-1 (DMT1; Gulec et al., 2014). In the enterocyte, Fe2+ binds ferritin, an intracellular Fe storage protein (Wessling-Resnick, 2017) that is modulated by Fe repletion (Conrad et al., 1987). At the basolateral membrane of enterocytes, ferroportin transfers Fe to the portal circulation (Gulec et al., 2014). This last transfer can be regulated by the postintestinal Fe metabolism as increasing levels of plasma transferrin (extracellular Fe binding-protein) stimulates hepatic hepcidin synthesis and release which induces degradation of enterocyte ferroportin (Nemeth et al., 2004). Although hepatic hepcidin is considered as the main regulator of Fe homeostasis (Ganz and Nemeth, 2012), another regulatory mechanism controlled at the enterocyte level by ferritin is known to respond primarily to dietary Fe levels (Wessling-Resnick, 2017). According to Galy et al. (2013), when dietary Fe is ingested as a bolus, the rapid increase in Fe content at the intestinal lumen triggers the intracellular synthesis of ferritin (intracellular pool of Fe). High levels of ferritin within enterocytes induce the internalization of ferroportin and DMT1 and, consequently, contribute to generate a mucosal block of Fe by reducing the efflux of excessive absorbed Fe at the basolateral membrane of enterocytes as well as by controlling further enterocyte absorptive capacity at the apical membrane (Galy et al., 2013). In addition to this regulation of Fe flow through enterocytes by ferritin, excessive Fe sequestered by this storage protein may return to the intestinal lumen through the enterocytes turnover where it is eventually excreted in feces (Donovan et al., 2005). This route of Fe excretion may not be negligible because approximately 20% to 25% of the intestinal mucosa is renewed daily (Van der Flier and Clevers, 2009).
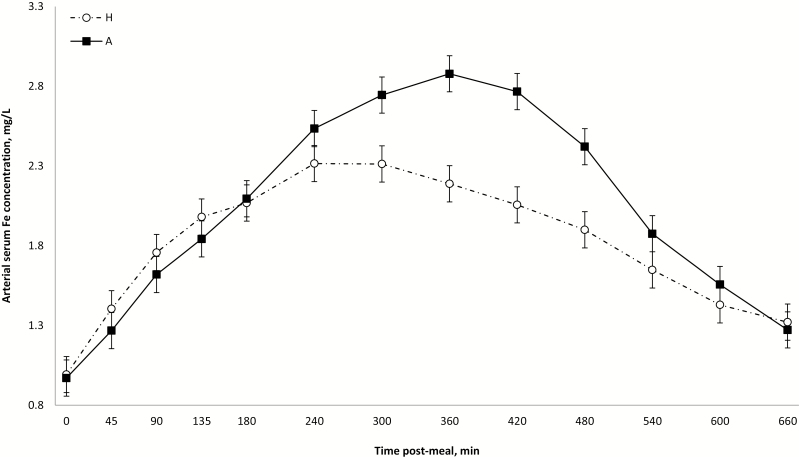
Besides the abovementioned source response on arterial serum Fe concentrations until 6 h post-meal, there was also a transient response of dietary levels in favor of A over H from 4 to 8 h post-meal; all these effects disappeared thereafter (interactions source × time and level × time; P < 0.001; Figs. 1 and 2). The specificity of DMT1 for Fe2+ implies that irrespective of the source of nonheme Fe (inorganic or organic proteinate), a common free ionic substrate (Fe2+) is transported across the apical membrane of the enterocytes. In this sense, the effect of different Fe sources on their arterial serum concentration would be dependent on their capacity in generating Fe2+ that will ultimately modulate intracellular Fe levels and, consequently, homeostasis mechanisms. Studies in mammals have shown that amino acids may improve Fe absorption (Van Campen, 1973; Taylor et al., 1986) by forming unstable Fe chelates that serve as Fe donors to mucin at neutral pH (Conrad et al., 1991). In piglets, Yu et al. (2000) reported that several indicators of Fe metabolism support a greater bioavailability of dietary nonheme Fe proteinate (120 ppm) compared with dietary Fe sulfate, but, in particular for blood plasma Fe, after an overnight fasting period, no source effect was detected. Taken together from the abovementioned effects of amino acids on Fe intestinal absorption and from the results from Yu et al. (2000), one would normally anticipate a better intestinal absorption of the present dietary O compared with I. The higher intracellular levels of Fe expected from O than I would trigger greater intracellular sequestration of Fe and could explain the paradoxical present lower release in portal vein during the early postmeal period. Nevertheless, considering that DMT1 mediates mucosal influx of different metals, it cannot be ruled out that high levels of others minerals present in H diets may have interfered with the absorption of Fe in the present study when compared with Yu et al. (2000). According to Shawki et al. (2015), Fe binds DMT1 in preference to zinc, copper, and manganese. Therefore, considering that HO and HI were not similarly affected, it appears that the source of dietary Fe has definitely a role to play in the process of intestinal absorption of Fe in pigs but the exact involved mechanisms remains to be elucidated.
Figure 1.
Average arterial serum iron concentrations (mg/L) within 660 postprandial minutes according to iron source, presented as LS means ± SEM. Source × time interaction (P < 0.001).
Figure 2.
Average arterial serum iron concentrations (mg/L) within 660 postprandial minutes according to iron level, presented as LS means ± SEM. Level × time interaction (P < 0.001).
Net PDV flux of Fe across the whole postprandial period was not affected by source or level (P ≥ 0.23; Table 2). The absence of level effect (P = 0.70) on the net PDV flux of Fe implies that a large part of dietary H (400 mg) was significantly unabsorbed and/or retained within enterocytes compared with dietary A (200 mg). Considering the expected higher Fe intestinal absorption from O than I (see above), it cannot be excluded that similar net PDV flux between dietary sources may be the consequence of a greater retention of Fe from dietary O within enterocytes, which is in line with the proposed greater activation of homeostatic mechanisms by O than I. In fact, a tendency for interaction source × level (tendency at P ≥ 0.07; Table 2) was detected for net PDV flux of Fe in which AI was greater than AO (P = 0.04), whereas HI and HO were intermediate and not different between them (P = 0.64). Whereas for dietary A levels the systemic regulation is apparently activated in response to the postabsorptive uptake of Fe sources (similar arterial concentrations for AI and AO with different net PDV flux), it was the opposite for dietary H levels where similar net PDV fluxes are associated to different arterial concentrations for HI and HO. For the later, the stimulus-triggering mechanisms of homeostatic regulation appears to be at the intraluminal and/or intraenterocyte level because net PDV flux of Fe was similar among HO and HI, but this equilibrium was disturbed further at the systemic level, resulting in different arterial concentrations.
The cumulative appearance of Fe in PDV serum (expressed in % of dietary intake) was affected by source (P = 0.02) and tended to be affected by level (P = 0.09) after 3 h post-meal (Supplementary File 3). Value for inorganic Fe (2.43 ± 0.94%) differed from zero (P = 0.02) and was greater than O (−0.76 ± 0.89 %) that did not differ from zero (P = 0.40). For dietary levels, values for A (1.96 ± 0.92 %) differed from zero (P = 0.04) and were greater than H (−0.29 ± 0.91 %) that did not differ from zero (P = 0.76). Although both source and level effects faded out and were no longer detectable at 11 h post-meal (P ≥ 0.23), average values for dietary O at −5.54 ± 2.39% were different from zero (P = 0.03), but not those for I at −1.33 ± 2.51 % (P = 0.60). For Fe levels, across the whole postprandial period, average values for A (−4.23 ± 2.45%) were similar to H (−2.63 ± 2.44 %), but whereas A tended to be different from zero (P = 0.10), H was not (P = 0.29). Considering that Fe is mainly absorbed in the duodenum (Gulec et al., 2014) and the mean gastric emptying time in pigs is 3 h (Wilfart et al., 2007; Strathe et al., 2008), cumulative portal appearance at the early postmeal period is probably more reliable to reflect treatment responses than after 11 h. In fact, the early postprandial response to dietary levels is in line with Braude et al. (1962) who reported that the efficiency of Fe absorption is inversely proportional to its dietary intake. Nevertheless, across treatments, this short-term (<4 h) postabsorptive appearance of Fe is small whatever the dietary sources or levels. In this way, it is noteworthy to mention that these values, derived from serum samples, might be slightly underestimated because nearly 20% of dietary Fe is found in red blood cells within 40 min post-meal (Furugouri, 1974). In addition, as mentioned above in this section, it cannot be ruled out that the use of boluses instead of a regular multifeeding regimen may have affected results. More precise measurements of net PDV flux of Fe would require labeled Fe.
Selenium
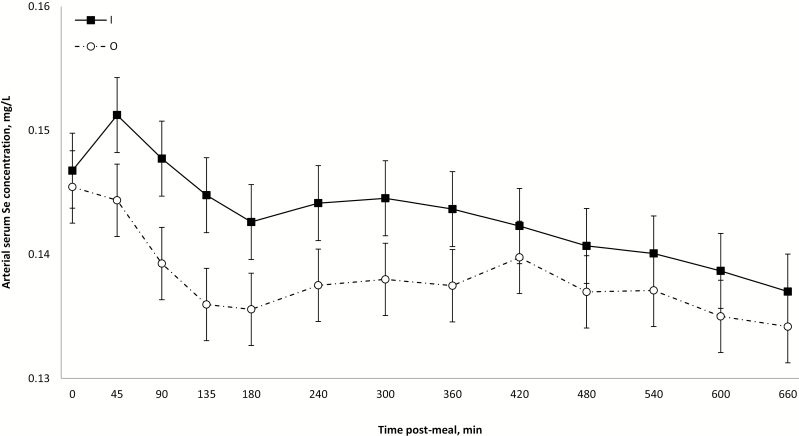
No treatment effect (P ≥ 0.19) was detected on arterial serum Se concentration (Table 2). Arterial levels gradually decreased from premeal values (0.146 ± 0.002 mg/L) to a minimum at 11 h post-meal (0.136 ± 0.002 mg/L; time effect; P < 0.001; Supplementary File 4). Arterial levels for O decreased faster than for I during the whole postprandial period (interaction source × time; P = 0.03; Fig. 3). Such a continuous decrease of postmeal concentrations of serum Se suggests that this mineral is either not absorbed or rapidly deposited/utilized in body tissues. The former is unlikely because studies on Se availability using other methods have reported values of 50% to 90% (Fairweather-Tait et al., 2010). Independent of metabolic differences between Se sources, it is known that whole blood Se levels are responsive to dietary Se (Fortier et al., 2012; Dalto et al., 2015, 2016), whereas serum Se increases significantly after long-term supplementation at nutritional levels (Mahan and Kim, 1996; Yoon and McMillan, 2006) or after short-term supranutritional supplementation (Kim and Mahan, 2001). Although serum Se represents a readily available pool of Se and contains about 75% of Se found in whole blood (Reilly, 2004), the continuous decline during the postprandial period suggests that the present dietary supplementations were below the levels needed to saturate Se blood deposits (red blood cells). In the present study, arterial serum Se levels were never greater than prebolus values. In contrast to the present results, a preliminary and exploratory study from this laboratory (unpublished data) using the same surgical approach showed that arterial serum Se concentration constantly increased during 10 h post-meal in pigs supplemented with 6 mg/kg of dietary Se (10 times the present levels). These conflicting results may suggest the possible existence of a mechanism of Se retention that would be controlled by intracellular Se saturation. Such mechanism of Se saturation has been proposed to exist in the liver (Oster et al., 1988). Because the gastrointestinal tract is a key source of reactive oxygen species (Bhattacharyya et al., 2014), a significant part (about 23%) of the whole body transsulfuration of methionine for the synthesis of glutathione occurs at the intestinal level (Riedijk et al., 2007). Considering that the metabolism of O Se (Se-methionine) is interchangeable with that of methionine (Schrauzer, 2003), it cannot be excluded that this tissue might have a significant requirement of Se (synthesis of antioxidant selenoenzymes) and, consequently, may be a major contributor to the systemic retention/utilization of dietary Se. Therefore, it can be hypothesized that enterocytes would take up and retain available Se (systemic and dietary) to cope with intestinal antioxidant requirements (among others), and after these basal intracellular Se needs are met (whatever after short-term supranutritional supplementation or long-term nutritional supplementation), Se would be released to the portal circulation.
Figure 3.
Average arterial serum selenium concentrations (mg/L) within 660 postprandial minutes according to selenium source, presented as LS means ± SEM. Source × time interaction (P = 0.03).
The present source × time effect on arterial serum Se is not in line with the recognized greater tissue deposition of O compared with I Se (Svoboda et al., 2008; Fortier et al., 2012; Dalto et al., 2015, 2016). However, considering that serum Se is a readily available pool, the greater tissue deposition of O Se could occur at the expense of serum Se levels. Unpublished data from this laboratory using pigs supplemented with 6 mg/kg of dietary Se (10 times the present levels) show that whole blood Se levels for O and I were similar during the first 3 h post-meal followed by greater O concentrations compared with I for the remaining 7 h of experimental period, suggesting a possible deposition of serum Se into red blood cells. Net PDV flux of Se was not affected by treatments (P ≥ 0.36; Table 2). Values were positive during the first 90 min (2.72 ± 1.01 µg/min) post-meal but rapidly decreased reaching a minimum at 5 h post-meal (−2.13 ± 0.91 µg/min) followed by a gradual increase until the end of the experimental period (0.55 ± 0.91 µg/min; time effect; P < 0.001; Supplementary File 5). Independent of sources or levels, Se appears to be strictly regulated within enterocytes, suggesting an important role of the intestinal tissue on the homeostasis of this mineral. These results support the abovementioned hypothesis of a possible mechanism of intestinal Se retention controlled by intracellular Se saturation.
The cumulative appearance of Se in PDV serum (expressed in % of dietary intake) was affected by source (P = 0.04) at 45 min post-meal in which I (20.0 ± 8.10 %) differed from 0 (P = 0.02) and was greater than O at −3.8 ± 7.83% (not different from zero, P = 0.63; Supplementary File 6). However, this effect further faded out and was no longer detected at 11 h post-meal (P = 0.58) where none of the values differed from zero (P ≥ 0.43). High values of absorption (20%) at 45 min indicate that the absorption and postabsorptive release of I was rapid and limited to the early postmeal period, whereas O, based on the above discussion, may have been retained by the intestinal tissue. Based on the fact that Se was measured only in serum (readily available pool) in the present study and considering the important use of Se by the intestinal tissue, it cannot be ruled out that the present approach of Se portal appearance was not sensitive enough for dietary Se at levels used in common feeding practices.
Conclusions
Under the present experimental conditions, the postabsorptive availability of dietary Fe and Se was limited to the early postmeal period and was greater for I than for O.
The short-term postabsorptive availability of dietary Fe was low for both sources, but the mechanisms regulating Fe homeostasis by O remain to be determined.
For Se, besides the very rapid intestinal absorption for I, the present study showed that the intestinal tissue may play a crucial role in systemic Se homeostasis. The proposed existence of a mechanism of intestinal Se retention controlled by intracellular Se saturation deserves further investigation.
Supplementary Data
Supplementary data are available at Journal of Animal Science online.
Supplementary File 1. Average portal-drained viscera serum flux (L/min) within 660 post-prandial minutes across treatments, presented as LS means ± SEM. Time effect (P < 0.001).
Supplementary File 2. Average arterial serum iron concentration (mg/L) within 660 post-prandial minutes, across treatments, presented as LS means ± SEM. Time effect (P < 0.001).
Supplementary File 3. Cumulative net portal-drained viscera appearance of iron (% of dietary intake) within 660 post-prandial minutes, according to dietary treatments, presented as LS means ± SEM. Source effect at 180 and 240 minutes (P ≤ 0.02). Inorganic Fe was or tended to be different from zero at 180 and 240 minutes (P ≤ 0.07). Except for inorganic Fe at 180 (P = 0.02) and 240 (P = 0.07) minutes post-meal, all other values were not different from zero (P ≥ 0.14).
Supplementary File 4. Average arterial serum selenium concentration (mg/L) within 660 post-prandial minutes, across treatments, presented as LS means ± SEM. Time effect (P < 0.001).
Supplementary File 5. Average net portal-drained flux of selenium (μg/min) within 660 post-prandial minutes, across treatments, presented as LS means ± SEM. Time effect (P < 0.001).
Supplementary File 6. Cumulative net portal-drained viscera appearance of selenium (% of dietary intake) within 660 post-prandial minutes, according to dietary treatments, presented as LS means ± SEM. Source effect at 45 minutes (P = 0.04). Inorganic Fe was different from zero at 45 minutes (P = 0.02).
Acknowledgments
The authors are grateful to M. Guillette, I. Audet, and V. Noël for their technical assistance and to the animal care team under supervision of M. Turcotte.
Glossary
Abbreviations
- DMT1
divalent metal transporter-1
- I
inorganic
- O
organic
- PDV
portal-drained viscera
Funding
This study was supported by Alltech Biotechnology Center, Nicholasville, KY, USA, and Agriculture and Agri-Food Canada.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Bhattacharyya A., Chattopadhyay R., Mitra S., and Crowe S. E.. . 2014. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94:329–354. doi: 10.1152/physrev.00040.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F., Vaugelade P., Robert V., Kibangou B., Canonne-Hergaux F., Delpal S., Bureau F., Blottière H., and Bouglé D.. . 2007. Comparative capacities of the pig colon and duodenum for luminal iron absorption. Can. J. Physiol. Pharmacol. 85:185–192. doi: 10.1139/y07-007 [DOI] [PubMed] [Google Scholar]
- Braude R., Chamberlain A. G., Kotarbinska M., and Mitchell K. G.. . 1962. The metabolism of iron in piglets given labelled iron either orally or by injection. Br. J. Nutr. 16:427–449. doi: 10.1079/bjn19620043 [DOI] [PubMed] [Google Scholar]
- Bresgen N., and Eckl P. M.. . 2015. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 5:808–847. doi: 10.3390/biom5020808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC). 2009. Guide to the care and use of experimental animals. CCAC, Ottawa, Canada. [Google Scholar]
- Conrad M. E., Parmley R. T., and Osterloh K.. . 1987. Small intestinal regulation of iron absorption in the rat. J. Lab. Clin. Med. 110:418–426. [PubMed] [Google Scholar]
- Conrad M. E., Umbreit J. N., and Moore E. G.. . 1991. A role for mucin in the absorption of inorganic iron and other metal cations. A study in rats. Gastroenterology 100:129–136. doi: 10.1016/0016-5085(91)90592-9 [DOI] [PubMed] [Google Scholar]
- Dalto D. B., Audet I., Girard C. L., and Matte J. J.. . 2018. Bioavailability of vitamin B12 from dairy products using a pig model. Nutrients 10:1134–1144. doi: 10.3390/nu10091134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalto D. B., Audet I., Lapointe J., and Matte J. J.. . 2016. The importance of pyridoxine for the impact of the dietary selenium sources on redox balance, embryo development, and reproductive performance in gilts. J. Trace Elem. Med. Biol. 34:79–89. doi: 10.1016/j.jtemb.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Dalto D. B., Audet I., and Matte J. J.. . 2019. Impact of dietary zinc:copper ratio on the postprandial net portal appearance of these minerals in pigs. J. Anim. Sci. 97:3938–3946. doi: 10.1093/jas/skz238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalto D. B., Roy M., Audet I., Palin M. F., Guay F., Lapointe J., and Matte J. J.. . 2015. Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidative stress induced by oestrus in pubertal pig. J. Trace Elem. Med. Biol. 32:21–29. doi: 10.1016/j.jtemb.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Di Silvestro R. A. 2005. Iron. In: Di Silvestro, R. A. editor. Handbook of minerals as nutritional supplements. Washington, DC: CRC Press; p. 89–128. [Google Scholar]
- Donovan A., Lima C. A., Pinkus J. L., Pinkus G. S., Zon L. I., Robine S., and Andrews N. C.. . 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1:191–200. doi: 10.1016/j.cmet.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait S. J., Collings R., and Hurst R.. . 2010. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 91:1484S–1491S. doi: 10.3945/ajcn.2010.28674J [DOI] [PubMed] [Google Scholar]
- Flohr J. R., DeRouchey J. M., Woodworth J. C., Tokach M. D., Goodband R. D., and Dritz S. S.. . 2016. A survey of current feeding regimens for vitamins and trace minerals in the US swine industry. J. Swine Health Prod. 24:290–303. [Google Scholar]
- Food and Drug Administration (FDA). 2019. Food additives permitted in feed and drinking water of animals: Selenomethionine hydroxy analogue. Fed. Reg. 84: 7991–7993. [Google Scholar]
- Fortier M. E., Audet I., Giguère A., Laforest J. P., Bilodeau J. F., Quesnel H., and Matte J. J.. . 2012. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J. Anim. Sci. 90:231–240. doi: 10.2527/jas.2010-3340 [DOI] [PubMed] [Google Scholar]
- Furugouri K. 1974. Kinetics in iron metabolism in piglets. J. Anim. Sci. 38:1249–1256. doi: 10.2527/jas1974.3861249x [DOI] [PubMed] [Google Scholar]
- Galy B., Ferring-Appel D., Becker C., Gretz N., Gröne H. J., Schümann K., and Hentze M. W.. . 2013. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep. 3:844–857. doi: 10.1016/j.celrep.2013.02.026 [DOI] [PubMed] [Google Scholar]
- Ganz T., and Nemeth E.. . 2012. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère A., Fortier M.-E., and Matte J. J.. . 2005. Rapid, sensitive and versatile determination of selenium in different biological samples. Can. J. Anim. Sci. 85: 533–536. doi: 10.4141/a05-044 [DOI] [Google Scholar]
- Girard C. L., Lapierre H., Desrochers A., Benchaar C., Matte J. J., and Rémond D.. . 2001. Net flux of folates and vitamin B12 through the gastrointestinal tract and the liver of lactating dairy cows. Br. J. Nutr. 86:707–715. doi: 10.1079/bjn2001472 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores J. N., Shetty S. P., Dubey A., and Copeland P. R.. . 2013. The molecular biology of selenocysteine. Biomol. Concepts 4:349–365. doi: 10.1515/bmc-2013-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulec S., Anderson G. J., and Collins J. F.. . 2014. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 307:G397–G409. doi: 10.1152/ajpgi.00348.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda S., Matte J. J., Vasanthan T., and Zijlstra R. T.. . 2010. Dietary oat beta-glucan reduces peak net glucose flux and insulin production and modulates plasma incretin in portal-vein catheterized grower pigs. J. Nutr. 140:1564–1569. doi: 10.3945/jn.110.122721 [DOI] [PubMed] [Google Scholar]
- Hooda S., Matte J. J., Wilkinson C. W., and Zijlstra R. T.. . 2009. Technical note: An improved surgical model for the long-term studies of kinetics and quantification of nutrient absorption in swine. J. Anim. Sci. 87:2013–2019. doi: 10.2527/jas.2008-1423 [DOI] [PubMed] [Google Scholar]
- Kim Y. Y., and Mahan D. C.. . 2001. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 79:942–948. doi: 10.2527/2001.794942x [DOI] [PubMed] [Google Scholar]
- Kurokawa S., and Berry M. J.. . 2013. Selenium: Role of the essential metalloid in health. In: Sigel A., Sigel H., Sigel R.K.O, editors, Interrelations between essential metal ions and human diseases. Springer, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R. C., Milliken G. A., Stroup W. W., and Wolfinger R. D.. . 1996. SAS system for mixed models. SAS Institute Inc., Cary, NC. [Google Scholar]
- Mahan D. C., and Kim Y. Y.. . 1996. Effect of inorganic or organic selenium at two dietary levels on reproductive performance and tissue selenium concentrations in first-parity gilts and their progeny. J. Anim. Sci. 74:2711–2718. doi: 10.2527/1996.74112711x [DOI] [PubMed] [Google Scholar]
- Manet L. 1969. Techniques usuelles de biologie clinique. Hématologie. Éditions médicales Flammarion, Paris, France, p. 39. [Google Scholar]
- Matte J. J., Girard C. L., and Guay F.. . 2017. Intestinal fate of dietary zinc and copper: Postprandial net fluxes of these trace elements in portal vein of pigs. J. Trace Elem. Med. Biol. 44:65–70. doi: 10.1016/j.jtemb.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Matte J. J., Guay F., and Girard C. L.. . 2012. Bioavailability of vitamin B12 in cows’ milk. Br. J. Nutr. 107:61–66. doi: 10.1017/S0007114511002364 [DOI] [PubMed] [Google Scholar]
- Matte J. J., Guay F., Le Floc’h N., and Girard C. L.. . 2010. Bioavailability of dietary cyanocobalamin (vitamin B12) in growing pigs. J. Anim. Sci. 88:3936–3944. doi: 10.2527/jas.2010-2979 [DOI] [PubMed] [Google Scholar]
- Mcdowell L. R. 2003. Minerals in animal and human nutrition. 2nd ed.Elsevier Sci., Amsterdam, The Netherlands. [Google Scholar]
- National Farm Animal Care Council (NFACC). 2014. Code of practice for care and handling of pigs. NFACC and Canadian Pork Council, Ottawa, Canada. [Google Scholar]
- National Research Council (NRC). 2012. Nutrient requirements of swine. 11th ed.National Academic Press, Washington, DC. [Google Scholar]
- Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., and Kaplan J.. . 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. doi: 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- Oster O., Schmiedel G., and Prellwitz W.. . 1988. The organ distribution of selenium in German adults. Biol. Trace Elem. Res. 15:23–45. doi: 10.1007/bf02990125 [DOI] [PubMed] [Google Scholar]
- Reilly C. 2004. Seleinum. In: The nutritional trace metals. Blackwell Publ., Oxford, UK. [Google Scholar]
- Riedijk M. A., Stoll B., Chacko S., Schierbeek H., Sunehag A. L., van Goudoever J. B., and Burrin D. G.. . 2007. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. USA 104:3408–3413. doi: 10.1073/pnas.0607965104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauzer G. N. 2003. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 47:73–112. doi: 10.1016/s1043-4526(03)47002-2 [DOI] [PubMed] [Google Scholar]
- Shawki A., Anthony S. R., Nose Y., Engevik M. A., Niespodzany E. J., Barrientos T., Öhrvik H., Worrell R. T., Thiele D. J., and Mackenzie B.. . 2015. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 309:G635–G647. doi: 10.1152/ajpgi.00160.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan T. M., and Gao M.. . 1990. Simplified fluorometric assay of total selenium in plasma and urine. Clin. Chem. 36:2124–2126. [PubMed] [Google Scholar]
- Strathe A. B., Danfaer A., and Chwalibog A.. . 2008. A dynamic model of digestion and absorption in pigs. Anim. Feed Sci. Technol. 143: 328–371. doi: 10.1016/j.anifeedsci.2007.05.018 [DOI] [Google Scholar]
- Surai P. F. 2006. Selenium in pig nutrition. In: Surai P. F., editor, Selenium in nutrition and health. Nottingham Univ. Press, Nottingham, UK. p. 454–459. [Google Scholar]
- Svoboda M., Ficek R., and Drabek J.. . 2008. Efficacy of selenium from Se-enriched yeast on selenium transfer from sows to piglets. Acta Vet. Brno 77: 515-521. doi: 10.2754/avb200877040515 [DOI] [Google Scholar]
- Taylor P. G., Martínez-Torres C., Romano E. L., and Layrisse M.. . 1986. The effect of cysteine-containing peptides released during meat digestion on iron absorption in humans. Am. J. Clin. Nutr. 43:68–71. doi: 10.1093/ajcn/43.1.68 [DOI] [PubMed] [Google Scholar]
- Van Campen D. 1973. Enhancement of iron absorption from ligated segments of rat intestine by histidine, cysteine, and lysine: Effects of removing ionizing groups and of stereoisomerism. J. Nutr. 103:139–142. doi: 10.1093/jn/103.1.139 [DOI] [PubMed] [Google Scholar]
- Van der Flier L. G., and Clevers H.. . 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71:241–260. doi: 10.1146/annurev.physiol.010908.163145 [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M. 2017. Iron: Basic nutritional aspects. In: Collins J., editor, Molecular, genetic, and nutritional aspects of major and trace minerals. Academic Press, Cambridge, MA. [Google Scholar]
- Wheby M. S., and Crosby W. H.. . 1963. The gastrointestinal tract and iron absorption. Blood 22:416–428. [PubMed] [Google Scholar]
- Wilfart A., Montagne L., Simmins H., Noblet J., and Milgen J. V.. . 2007. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br. J. Nutr. 98:54–62. doi: 10.1017/S0007114507682981 [DOI] [PubMed] [Google Scholar]
- Windisch W. 2002. Interaction of chemical species with biological regulation of the metabolism of essential trace elements. Anal. Bioanal. Chem. 372:421–425. doi: 10.1007/s00216-001-1117-6 [DOI] [PubMed] [Google Scholar]
- Wolffram S., Berger B., Grenacher B., and Scharrer E.. . 1989. Transport of selenoamino acids and their sulfur analogues across the intestinal brush border membrane of pigs. J. Nutr. 119:706–712. doi: 10.1093/jn/119.5.706 [DOI] [PubMed] [Google Scholar]
- Yoon I., and McMillan E.. . 2006. Comparative effects of organic and inorganic selenium on selenium transfer from sows to nursing pigs. J. Anim. Sci. 84:1729–1733. doi: 10.2527/jas.2005-311 [DOI] [PubMed] [Google Scholar]
- Yu B., Huang W.-J., and Chiou P. W.-S.. . 2000. Bioavailability of iron from amino acid complex in weanling pigs. Anim. Feed Sci. Tech. 86:39–52. doi: 10.1016/S0377-8401(00)00154-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.