Abstract
The process of designing and implementing individualized health-promoting interventions, nutritional or otherwise, is fraught with great difficulty owing to the heterogeneity inherent in factors that influence healthy longevity. This article proposes that careful attention to three principles—life course perspective, U-shaped thinking, and whole organism thinking—creates an attitudinal framework that can be used to reframe biological heterogeneity into the clinically relevant question: Who will benefit? The search for tools to cope with the complexity of this heterogeneity has been dominated by technological advances, including state-of-the-art “-omics” approaches and machine-based handling of “big data.” Here, it is proposed that language precision and nuanced category usage could provide critical tools for coping with heterogeneity, thereby enabling interventionalists to design and implement strategies to promote healthy longevity with greater precision. The lack of a clear understanding of “Who will benefit?” stands as a major obstacle to the design and implementation of nutritional strategies to optimize healthy longevity. This article opens a new dialogue situating the principles of life course perspective, U-shaped thinking, and whole organism thinking, along with cultivating an attitude of language precision at the very core of accelerating creative discovery and refining practical advance in the field of nutrition science.
Keywords: aging, dose response, longevity, neologism, pet dogs, trade-offs
Introduction
More than 60 years ago, the professor of linguistics Wayland Maxfield Parrish wrote that in speechmaking (as in life) it is not failure, but low aim, that is the crime (1971). The same can be said for any scholarly discourse. In the assemblage of the papers from this symposium, our aim should be high. The aim of this article is to attempt an integration of some ideas—principles if you will—that relate to the goal of achieving healthy longevity. If this attempt is successful, the reader will experience these ideas afresh, owing to an original integration not considered before.
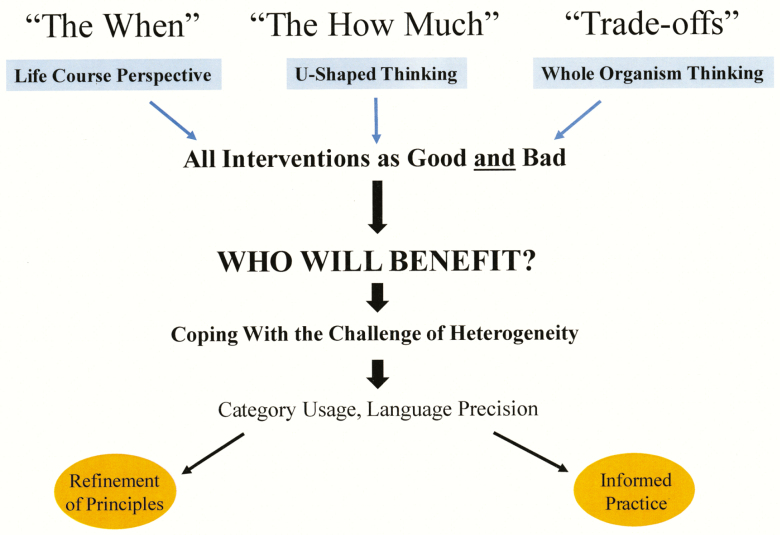
Figure 1 provides the road map for this intended integration. When we engage in the design of interventions—nutritional or otherwise—we are immediately faced with concerns. Three major concerns are: “the when,” “the how much,” and “trade-offs.” Concern for “the when” can be assuaged by the principle of life course perspective—early life events influence adult health outcomes. Life course perspective teaches us to look for critical windows—time periods during the life of the organism when particular interventions will exert the most potent effect. Concern for “the how much” leads us to the principle of U-shaped thinking—the notion that more of “good things” is not necessarily better. In concert with Aristotle’s golden mean, in many situations when it comes to optimizing dose, more is not always better. Concern for “trade-offs” is gainfully addressed by the principle of whole organism thinking, a way of thinking that is increasingly difficult in this age of specialization. If one is offered a pill that can reduce breast cancer or prostate cancer risk in half but doubles the risk for dementia, and one decides the pill is an undesirable option, one has engaged in whole organism thinking—seeking and examining critical trade-offs. In some instances, embracing the principle of whole organism thinking can even spark consideration of trade-offs that extend beyond the individual organism, to include societies and environments.
Figure 1.
The biology of healthy longevity: principles and practice. Attention to three principles—life course perspective, U-shaped thinking, and whole organism thinking—creates an attitudinal framework that reframes biological heterogeneity into the clinically relevant question: Who will benefit? Language precision and nuanced category usage provide critical tools for coping with heterogeneity enabling interventionalists to design and implement nutritional strategies with greater precision.
These three principles converge on a distinctive vantage point—seeing all interventions not as “good” or “bad,” but as both good and bad. Context determines meaning, and no single intervention will be expected to benefit everyone. Prompted by these principles, the central question for interventionalists becomes: Who will benefit? Biology spells heterogeneity. What is needed are tools for coping with, making sense of, this enormous heterogeneity. Two of our most powerful tools for coping with heterogeneity are category usage and language precision. Most scientists and health professionals, however, never receive training in language precision. If we could ramp up our precision with language, we could avoid sloppy category usage, what I have termed naïve substitution (Waters, 2017a). In this way, language precision could provide a deeper discriminating power to identify subtypes of disease and subcategories of treatment response needed to advance informed practice. And if we are courageous enough to develop new words—the process of neologism—to describe our difficulties, our solutions, this could deliver a further refinement of principles. In the sections that follow, a further illustration of these principles is offered in order to show more clearly that, by applying an attitude of language precision, we might cope more effectively with the challenge presented by heterogeneity. This article will intentionally blur the lines between pets and people, drawing several examples from the fields of nutrition, aging, and health promotion in humans, emphasizing cross-species parallels wherever pertinent. It is hoped that with sufficient attention fixed on these principles, we will find ourselves moving away from one-size-fits-all thinking, readied and more disciplined to design and implement nutritional interventions with greater precision, framed in the context of “Who will benefit?”
The When: Life Course Perspective
The pursuit of important questions pertaining to the healthy longevity of pets will benefit from the assemblage of highly cooperative multidisciplinary teams, harnessing the complementary talents of investigators in the fields of animal nutrition and veterinary science. In 2014, I wrote a perspective article published in The Veterinary Journal titled “Longevity in pet dogs: Understanding what’s missing” (Waters, 2014). In the article, which targeted veterinary health professionals, two critical questions were put forward. First, who are we as investigators? No veterinarians receive training in the biology of aging as part of their DVM curriculum, leaving members of the veterinary profession ill-equipped to constructively team up with nutritionists and other scientists to conduct or interpret studies pertaining to the biology of aging. Second, what conceptual framework is guiding us when we are thinking about healthy longevity? This question is an important one because our practice and discovery will rely upon achieving the best conceptual starting points. In the article, I introduced the veterinary profession to the idea of applying a life course perspective to the study of healthy longevity (Waters, 2014). Life course perspective teaches that early life events influence adult health outcomes including longevity (Waters and Kariuki, 2013). The concept of early origins of adult disease, a concept well-known to nutritionists and animal scientists, fits snugly within the notion of life course perspective (Calkins and Devaskar, 2011; Ganu et al., 2012).
Important evidence in support of life course perspective and healthy longevity comes from Vincent Felliti’s work in humans, the ACE study. Felliti studied the health outcomes of a large cohort of adults in the Kaiser Permanente database and collected information on adverse childhood experiences (ACE), such as death of a parent, a brother incarcerated in prison, drug or sexual abuse in the household. Results showed poorer health outcomes and shorter longevity as ACE score increased (Brown et al., 2009). Felliti’s group also showed that adverse childhood experiences were associated with an increased risk of lung cancer (Brown et al., 2010). On the surface, there appears to be a straightforward explanation for this second association. Individuals with adverse childhood experiences are more likely to smoke cigarettes as a coping mechanism and hence the observed increase in the incidence of lung cancer might be considered an expected result. However, the researchers found that the association between adverse childhood experiences and lung cancer held strong even after controlling for differences in smoking exposure. Seemingly, early life events can reset the system in unforeseen ways.
An ovary story
The principle of applying life course perspective to the study of healthy longevity, so impressively supported by the ACE study in humans, raises the question: What early life events influence healthy longevity in pet dogs? Scientists study 100 year-old people—so-called centenarians—to capture clues to healthy longevity. My research group is testing a new idea: The secrets to the biology of healthy longevity can be found by studying the oldest-old dogs. Under my direction, the Gerald P. Murphy Cancer Foundation’s Center for Exceptional Longevity Studies is conducting the first systematic study of the oldest-living dogs in North America. The research has created a database of more than 350 of these exceptional dogs—Rottweilers who have reached 13 years of age, which represents living 30% longer than breed average (equivalent to 100-year-old people). The database contains a rich collection of data obtained from questionnaire on diet, vaccination and medical history, along with biochemical data and a biorepository of plasma, serum, DNA, and necropsy tissues.
For decades, scientists have documented a female longevity advantage in humans—women are four times more likely than men to live to be 100 years old (Terry et al., 2008; Waters, 2011). But scientists do not clearly understand why. We asked the question: Is healthy longevity influenced by ovaries? We believed the answer to this question was within our grasp because in pet dogs, unlike humans, ovaries are frequently and electively removed early in life. Like in women, our studies showed there was a female longevity advantage in Rottweilers—females were twice as likely to reach exceptional longevity as males (Waters et al., 2009). However, this female longevity advantage was completely erased if ovaries were removed during the first 4 years of life. Four months prior to the acceptance of our publication, a physician Dr William Parker published a paper titled “Ovarian Conservation at the Time of Hysterectomy and Long-term Health Outcomes in the Nurses’ Health Study” (Parker et al., 2009). In this study, more than 29,000 women underwent hysterectomy—approximately half of the women retained their ovaries, half of the women had their ovaries removed at the time of uterus removal. Parker’s findings suggested that women who undergo hysterectomy before age 50 should try to retain their ovaries, because retaining ovaries longer was associated with decreased overall mortality, decreased cardiovascular mortality, even a lower risk for lung cancer (Parker et al., 2009). Within 3 months of our published study in dogs, another pertinent study was published (Mason et al., 2009). This was a mouse transplantation study in which young mouse ovaries (harvested from 2-month-old mice) were transplanted into old female mice. Results showed that the transplantation of young mouse ovaries could increase longevity by 13%. Thus, a compelling convergence was witnessed—three different teams of investigators, three different species, with the results pointing to the same conclusion: Ovaries are part of a system that promotes longevity.
At the time, this new idea appeared to fly countercurrent to prevailing wisdom within obstetrics–gynecology circles and contemporary veterinary practice. But is the idea a far-fetched one? Only if one is trapped in a conceptual framework that sees ovaries as reproductive units, not endocrine organs. But ovaries are endocrine organs, and as such, removal of these organs is expected to provoke system-wide consequences. In order to advance the training of the next generation of health professionals, we might contemplate a shift in conceptual framework—ovaries joining the ranks of endocrine organs, such as the thyroid glands, adrenal glands, and insulin-producing pancreas that are judiciously preserved, rather than electively excised. Life course perspective informs us that a priority should be placed on early, intelligent interventions to promote healthy longevity. Early ovariohysterectomy—or any other elective endocrinological disruption—is a doubtful example.
The search for biomarkers
One of the big challenges in the field of aging research has been finding validated biomarkers that can predict successful aging. This effort has been quite frustrating, fraught with difficulty (Bürkle et al., 2015; van Ginneken, 2017; Nelson et al., 2019). Could paying closer attention to life course perspective fit together some of the pieces of the biomarker puzzle? I believe it might be so because of what I refer to as the “flip-flop of physiology” during the life course. Let us consider two illustrative examples. In Scandinavia, Kivipelto and colleagues have conducted important research in people on identifying factors at age 50 that are predictive of the development of Alzheimer’s disease at age 70. Their results indicate that high serum cholesterol at age 50 is strongly associated with an increased risk for Alzheimer’s disease 20 years later (Kivipelto et al., 2001). But, in people who are 70 years old, high cholesterol appears to be protective against Alzheimer’s disease (van Vliet et al., 2009; Zuliani et al., 2010). So is high cholesterol “good” or “bad”? It depends on “the when.”
A second instructive example hinges on the question: Is obesity a predictor of poor survival? Substantial medical evidence indicates that, in persons who are 30, 40, 50, or even 60 years old, obesity is associated with shortened life expectancy (Fontaine et al., 2003; Lung et al., 2019). But growing evidence indicates that with increasing age, fatness loses its sting as a harbinger of death. There are now data to suggest that in advanced age (beyond 80 years in humans), favorable survival may be more closely linked to greater adiposity than to muscle mass (Auyeung et al., 2010). Perhaps it would be instructive to consider why this might be so. Perhaps when grandmother falls at the mailbox, greater adiposity would cushion her fall, reducing her risk of sustaining a hip fracture. Alternatively, after grandmother suffers her fracture and is hospitalized and develops a nosocomial infection, it will be her fat stores that enable her to survive sepsis and return to living independently.
The How Much: U-Shaped Thinking
Why is it so difficult to find “good things” that promote health? Perhaps it is because the public holds an oversimplified view of health promotion. This stance is epitomized by the statement, “Just show me the good things and I will consume as much of them as I can.” But is more really better? Whether you are a disciple of Aristotle and his golden mean or Goldilocks and her affinity for baby bear’s porridge, the message is the same: More is not always better. The anthropologist Gregory Bateson wrote 40 years ago in his thought-provoking book Mind and Nature: A Necessary Unity: “There are no monotone values in biology” (Bateson, 1979). Bateson goes on to develop the idea: “Desired substances, things, patterns, or sequences of experiences that are in some sense ‘good’ for the organism … are never such that more of the something is always better than less of the something. Rather, for all objects and experiences, there is a quantity that has optimum value” (Bateson, 1979).
My research group embraces the wisdom of Bateson’s prescription regarding “the how much” (Waters and Chiang, 2010). In our work, we have articulated the need for what we have termed U-shaped thinking (Waters and Chiang, 2018). The utility of U-shaped thinking can be illustrated by considering a decade of scientific effort attempting to define the optimal dose of the trace mineral selenium for human prostate cancer risk reduction (Waters and Chiang, 2019a).
In 2001, the U.S. National Cancer Institute launched the largest ever prostate cancer prevention trial called SELECT. More than 32,000 men were randomized to receive either selenium, vitamin E, both vitamin E and selenium, or placebo. The expected completion of the trial was in 2012. The primary endpoint was to determine whether daily oral supplementation with these nutrients could decrease prostate cancer incidence. When SELECT was launched, several important questions about selenium and cancer prevention remained unanswered. What is the anticancer mechanism of selenium? What is the most effective dose? My research group viewed this as an exceptional research opportunity because dogs and humans are the only species that develop spontaneous prostate cancer with appreciable frequency, and we had substantial expertise in studying the naturally occurring prostate cancers of pet dogs (Waters et al., 1996, 1998; Waters and Bostwick, 1997; Cornell et al., 2000). We reasoned that the aging dog prostate provided a unique opportunity to study the effect of selenium on prostate cells in an appropriate context. In the aging prostate, this context consists of epithelial cell–stromal cell interactions, oxidative stress, inflammation, declining androgen levels, and stromal senescence. It is unlikely that this complex milieu could be easily recapitulated in a petri dish in the laboratory.
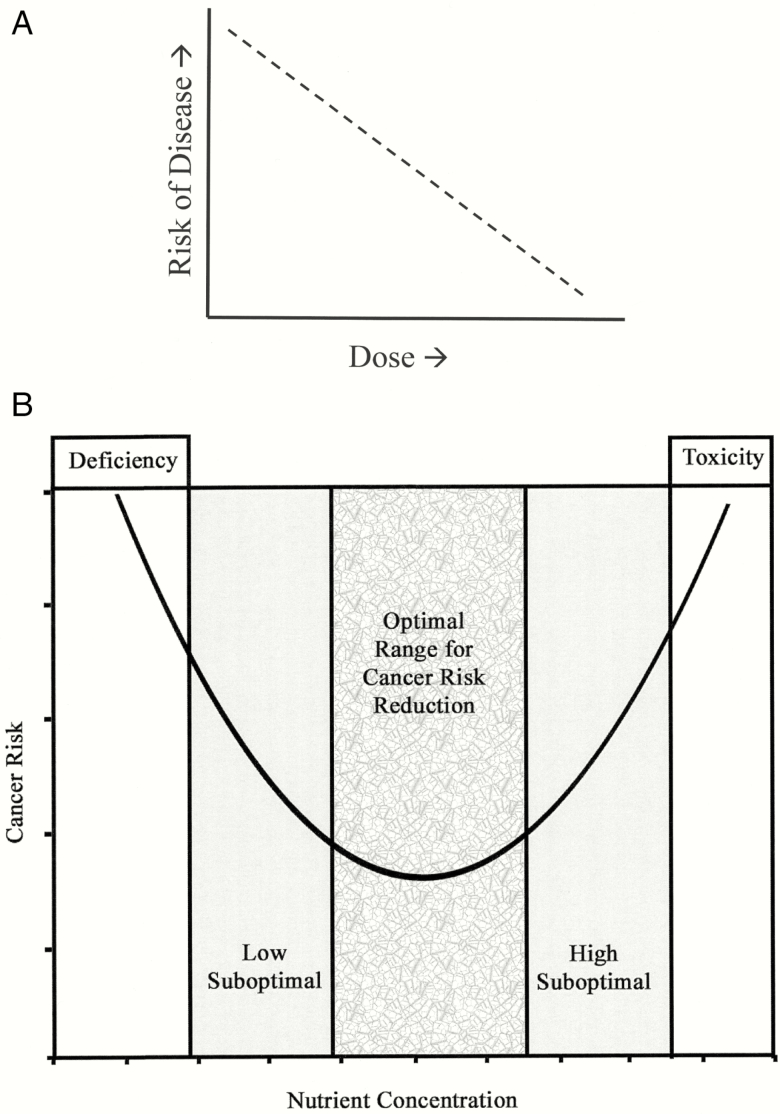
When scientists seek to determine an optimal dose, they analyze dose–response relationships. In Figure 2A, with risk of disease on the vertical axis and dose of intervention on the horizontal axis, the continuous downward slope indicates a linear dose–response, that is, the risk of disease drops continuously with increasing dose of intervention. In 1981, an investigator named Walter Mertz, who was working at the U.S. Department of Agriculture, put forth an idea. Mertz predicted that the biological response between an essential nutrient and a physiologic process is not linear, but instead U-shaped, progressing through regions of deficiency, low suboptimal, and optimal, followed by high suboptimal, and toxicity (Mertz, 1981; Figure 2B). It is not clear that Mertz had very much dose–response data on the association between micronutrients and cancer risk. What he had was an idea—he had a starting point.
Figure 2.
(A) Finding the optimal dose for disease risk reduction. A hypothetical linear relationship is shown between the risk of a particular disease and the dose of a disease preventive agent, consistent with the notion that “more is better”. (B) Dose–response model adapted from Mertz (1981). The U-curve predicts that the biological response to an essential nutrient is characterized by an optimal middle range, consistent with the notion that “more is not necessarily better” (Waters et al., 2005; with permission).
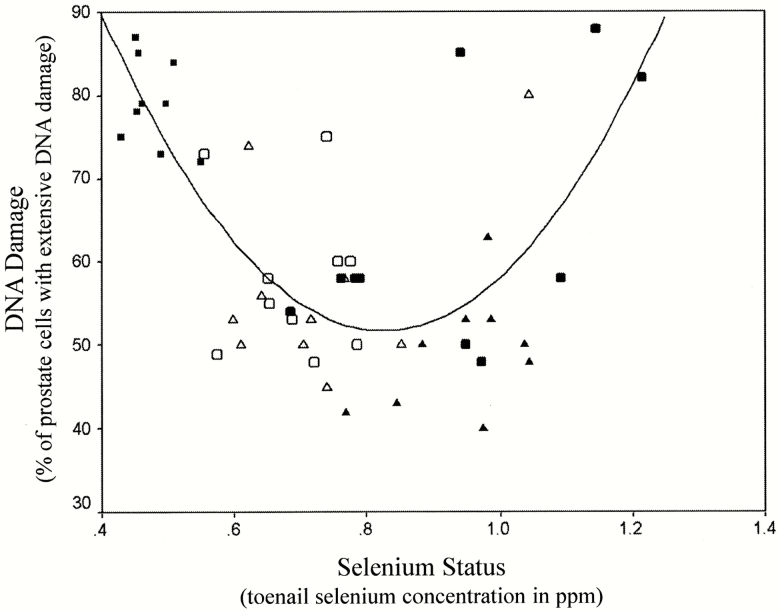
In a randomized feeding trial in dogs published in the Journal of the National Cancer Institute, we showed that daily selenium supplementation significantly decreased DNA damage in the prostate (Waters et al., 2003). Next, we set out to investigate dose–response by probing the relationship between selenium status and DNA damage within the aging prostate. We studied 49 elderly, sexually intact male beagle dogs and assessed selenium status by measuring toenail selenium concentration (conducted by Dr Steven Morris, University of Missouri). DNA damage in the prostate was measured using alkaline comet assay. Our results revealed a U-shaped dose–response relationship between selenium status and prostatic DNA damage in elderly dogs physiologically equivalent to 65-year-old men (Waters et al., 2005; Figure 3). For the first time, our data demonstrated that, for a nutrient and genetic damage in the prostate, Mertz was correct. The implication seemed clear: More selenium is not necessarily better.
Figure 3.
The dog U-curve. The dog U-curve was discovered by studying the dose–response relationship between selenium status and prostatic DNA damage in elderly dogs over a range of selenium concentration achievable in human populations (Waters et al., 2005) (with permission). Each data point represents the result from one dog.
But are the results of these animal studies relevant to the relationship between selenium status and human prostate cancer risk? Our studies were of dogs not men, and used DNA damage rather than cancer as the endpoint. These findings suggested additional selenium could potentially benefit only the subgroup of the population with low selenium levels and that it would not reduce disease in subjects with moderate to high selenium levels. I believe this statement provides a reasonable interpretation of the dog data. But these are not my words. This statement was made by Harvard epidemiologist Walter Willett more than 35 years ago when he published the first cohort study of selenium and cancer risk in humans (Willett et al., 1983).
Is a U-shaped dose–response peculiar to selenium and prostate cancer prevention? If one looks carefully, one can find many examples of U-shaped dose–responses in human biology. For example, consider the relationship between sleep duration and mortality in 82,000 nurses. It is U-shaped—it is undesirable to sleep too little or too much (Patel et al., 2004). Consider the relationship between blood glucose at the time of heart attack and likelihood of survival. The relationship is U-shaped—it is disadvantageous to have too low or too high blood glucose (Pinto et al., 2005). The public is quite familiar with the downside of high cholesterol, but few persons realize that the relationship between serum cholesterol and mortality in men is U-shaped, indicating that low cholesterol as well as high cholesterol is associated with higher mortality (Smith et al., 1992).
Let us return to the SELECT trial. Fast forward 8 years after its inception to 2008 when a press release informed the public: “Prostate cancer prevention study halted. Vitamin E, selenium no help in preventing prostate cancer” (WebMD, 2008). The SELECT trial had been stopped early. In an interim analysis, investigators found no beneficial effect of the supplements on prostate cancer risk and a possible detrimental effect of selenium supplementation on increasing the risk for type 2 diabetes mellitus (Lippman et al., 2009). The disappointing null result of SELECT—the absence of prostate cancer protection—was widely disseminated by the press. “Vitamins can’t fight big killers. Vitamins get an F in cancer prevention” (USA Today, 2009). People were left asking: What happened?
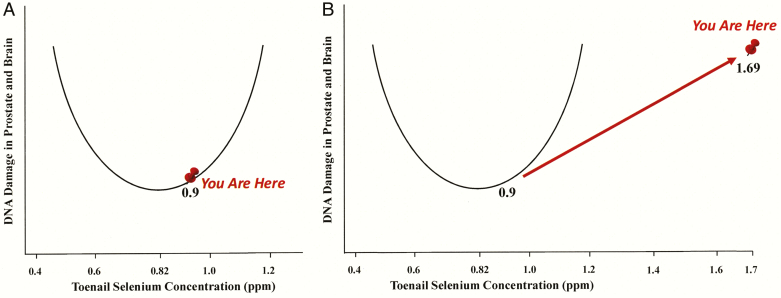
When it comes to interpreting the unexpected results of SELECT, is there a chance we are just lost? When you are lost, you look for a map of the territory (Waters and Chiang, 2018). If you are lost in a shopping mall, you look for a map and when you find on the map “You Are Here” you are no longer lost. Figure 4A shows that if you are the average man in SELECT before supplementation “You Are Here,” already in the trough of the U-curve, residing in the optimal range of selenium status. And if you are the average man in SELECT after selenium supplementation “You Are Here”—at 1.69 ppm toenail selenium concentration, which is equivalent to more than 250 μg/L plasma selenium (Figure 4B). You have been over-supplemented into a potentially dangerous location.
Figure 4.
The dog U-curve: a map to interpret the disappointing results of the selenium and vitamin E cancer prevention trial (SELECT). (A) The dog U-curve predicts that the selenium status of the average man in SELECT prior to selenium supplementation (“You Are Here”) is already in the optimal range for minimizing prostate cancer. (B) The dog U-curve predicts that the selenium status of the average man in SELECT after selenium supplementation (“You Are Here”) clearly exceeds the optimal range for minimizing prostate cancer risk. Adapted from Waters and Chiang (2018).
In a 2008 letter sent to all SELECT study participants, the SELECT findings were summarized as follows: “We now know that selenium and vitamin E do not prevent prostate cancer” (Southwest Oncology Group, 2008). Is that what we really know? Here is what I believe we know: There are no monotone values in biology. And the task of carefully defining population-based dose–response curves—keeping a keen eye out for spotting U-shaped and other non-linear relationships—is a critical step toward achieving the goal of precision nutrition.
My research group is now beginning to investigate diet and dietary supplement usage in pet dogs with exceptional longevity. Unpublished preliminary findings indicate that the relationship between plasma selenium concentration and age- and frailty-adjusted mortality risk in canine centenarians may be U-shaped. To test the extent to which this relationship might be affected by early life events, we will evaluate the impact that age at ovary removal has on the relationship between selenium status and mortality risk. U-shaped thinking teaches us that monolithic, blanket statements about the impact of a particular nutrient on health should be made with great caution. In our everyday verbal exchanges, context determines meaning. Likewise, the optimal dose of an intervention may be quite different depending upon physiological context, which includes the body’s exposure memory to challenges experienced earlier in life (Jones, 2015).
Trade-Offs: Whole Organism Thinking
More than 50 years ago the Swiss physician Paul Tournier framed one of the shortcomings of modern clinical practice: “The need to specialize accords priority to the organ over the organism” (Tournier, 1957). The provocative concept of whole organism thinking helps to steer investigators away from their preoccupation with a favorite organ or favorite disease to consider something more fundamental—trade-offs. Because longevity integrates the incidence and mortality of all diseases, as well as the rate of organismal aging, whole organism thinking urges us to question whether significantly reducing the incidence of a single disease (e.g., a late-onset disease with variable mortality, such as canine mammary cancer) should merit serious consideration as a core principle of any wellness program developed to achieve the goal of overall healthy longevity (Waters et al., 2017). Also, by embracing whole organism thinking, we experience a seismic shift in how we envision interventions—one no longer sees any intervention or life choice, such as taking antioxidant supplements, as “good” or “bad”. Instead, whole organism thinking teaches us to see all interventions as both good and bad (Waters, 2012, 2014).
Though underutilized by nutrition scientists, the concept of trade-offs should be a critical principle when one is considering interventions that can extend healthy life span. Human twin studies (Herskind et al., 1996) suggest that the heritability of longevity is relatively modest, approximately 25–30%, indicating that life choices and environmental exposures including diet may contribute significantly to longevity. Biogerontologists refer to this as the plasticity of longevity. It means that interventions have the potential to positively or negatively influence longevity.
Along with this plasticity, we can expect trade-offs. A study of cancer-suppressing strategies conducted in mice provides a powerful illustration. Tyner et al. (2002) developed a mutant mouse model with exaggerated function of the tumor suppressor p53. The investigators predicted that, in these p53 stuck-in-the-on-position mice, strong tumor suppressive activity would significantly reduce the development of cancers. Since cancer was a major cause of death in wild-type members of this mouse strain, the logical consequence of such cancer suppression would be an extended survival time. What Tyner and colleagues found was remarkable: Profound cancer suppression in these mice came at a cost—accelerated aging. Apparently augmenting the system’s proclivity to purge DNA-damaged cells achieved complete (100%) tumor protection, but also led to an accelerated drop out of cells in critical organs, including bone marrow, liver, and spleen. This was accompanied by signs of accelerated aging; median life span was shortened by 22% (Tyner et al., 2002). There is a lesson here for interventionalists. In biological systems, trade-offs abound, which may lead to unexpected adverse consequences in response to “good things”. Few would have predicted an intervention that could successfully eliminate a major cause of death would also accelerate senescence, considerably shortening overall longevity.
Coping with the Challenge of Biological Heterogeneity
Too often science has been taught as a subject matter—a collection of facts—rather than a method of inquiry we use to make sense of the world. This predicament was addressed more than 100 years ago by John Dewey in a speech delivered to the American Association for the Advancement of Science: “I mean that science has been taught too much as an accumulation of ready-made material with which students are to be made familiar, not enough as a method of thinking, an attitude of mind, after the pattern of which mental habits are to be transformed” (1910; emphasis mine). To Dewey, it is the illumination of method, rather than the claim of a superior significance of facts, that should be instilled in students.
Today, we are concerned about those obstacles that confront nutrition scientists as they attempt to cope with the challenges presented by biological heterogeneity. Perhaps Dewey’s call for seeing science as a method does not go far enough to free us from difficulty. The call that should be sounded today is that science is a method limited by language. The scientific method is a method of perception, and language limits what we can perceive. “We see through our categories”, wrote the general semanticist Wendell Johnson (1956). Words are our starting points—for present practice and future inquiry. This notion is illustrated in Figure 5. According to your categories, is the world flat or round? Are ovaries reproductive units or endocrine organs? Is leukemia a single disease or 15 different diseases? If one sees leukemia as a single disease, it is unlikely that one is very well-equipped to discover another form of leukemia. But if your categories enable you to see leukemia as 15 different diseases, why not discover a 16th?
Figure 5.
Categories are starting points for present practice and future inquiry (see text for explanation).
Clearly, our categories limit what we can discover. Yet, students and faculty in the health sciences are not trained in precision with language and category usage. Instead, training in language precision seems to be reserved for those who will become poets or historians, not scientists or health professionals. Cultivating an attitude of language precision could elevate creative performance in the health sciences, providing a necessary counterbalance to the prevailing technocentric view of medical ascendancy. To make progress, we need what I have termed linguistic readiness—developing an attitude of language precision together with an aptitude for listening with the intent of real change (Waters, 2017a). Our ambitions for rapid advance increasingly point to the need for a new educational prescription—one in which creative excellence in scientific discovery and education is informed and catalyzed by the humanities. Elsewhere, I have written about this bold idea as part of a recent creativity project with colleagues from Australia (Waters, 2017a).
I want to stress here that the power of language extends beyond communication. Consider how language developed. Man needed a word for “moon” so he could think about this object when it was not in his direct sight, for instance, during the daytime. Only later did he need the word for “moon” to tell his colleagues about it. Thus—and this is an important point—language is first a thinking tool, later a means of communication (Langer, 1960). It is quite curious then that we rely upon somebody else’s language—words from our training, words we have borrowed—to describe the experiences we encounter. Clearly, this makes little sense if we are truly trailblazing.
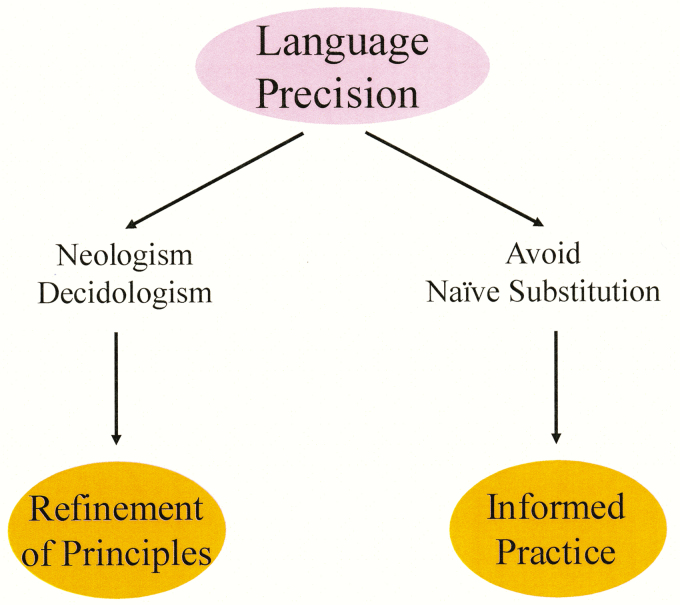
The Art of Sufficient Particularity: Acquiring Tools for Coping with Heterogeneity
The future is pointing toward the implementation of refined nutritional approaches, so-called precision nutrition (Waters et al., 2008; de Toro-Martin et al., 2017; Fröhlich et al., 2018.). Achieving the necessary particularity to identify and exploit interventional targets and to identify new subtypes of disease will demand precision with language. Two aspects of language precision that may deliver the deeper discriminating power that is critical to this aim —the practices of utilizing neologism and avoiding naïve substitution—are explored in the following two sections (Figure 6).
Figure 6.
The art of sufficient particularity: two important aspects of language precision. The practices of using neologism and avoiding naïve substitution are critical for developing the deeper discriminating power to deliver benefits as one navigates biological heterogeneity (see text for explanation).
Neologism
If we train ourselves in unique ways and engage ourselves in pursuing never-before-pursued questions, then an altogether fitting follow-through would be to exploit the power of neologism—the formulation of new words. New words become new tools. The necessity of creating new words has been articulated this way: “The new circumstances under which we are placed call for new words, new phrases, and for the transfer of old words to new objects … necessity obliges us to neologize.” This statement was written more than 200 years ago by Thomas Jefferson (1884; emphasis mine), the person credited with the first use of the term “neologize”.
The practice of neologism can lead to refinement of principles, provoking critical advances achievable only through changes in terminology (Waters, 2017b). Part of the power of neologism is that it reflects ownership—it signals that the worker has thought deeply about the issue at hand. My research group has thought deeply about the role selenium might play as a cancer prevention agent. Conventional wisdom holds that selenium protects cells against genetic damage because it is a component of the enzyme glutathione peroxidase, one of the body’s most powerful antioxidant defenses. But after looking carefully at the data published by experts in this field, it was clear that selenium’s role in cellular protection could not entirely explain its cancer-fighting properties. Based upon studies in dogs and in cell culture, our research posited a new mechanism of how selenium works: Selenium allows the body to selectively sweep away the most damaged cells through apoptosis. A new mechanism had been discovered, new words were needed. Our neologism was homeostatic housecleaning—the ability to selectively sweep away the most damaged cells (Chiang et al., 2009) . And in the spirit of Thomas Jefferson, we introduced homeostatic housecleaning into the scientific literature, prominently displaying the term in the title of a manuscript published in the journal Biofactors (Chiang et al., 2013).
In addition to the strenuous creation of new words, to make surer progress, each of us should consider which words in our domain need to be removed from the prevailing lexicon. Extending the commonsense method asserted by Thomas Jefferson, I have suggested: “Necessity obliges us to decidologize”—to cut out words [decidere (Latin) = to cut off] (Waters, 2017a). If language is indeed the gateway to discovery, it is no surprise that both the art of neologism and the art of decidologism belong in the toolbox of the creative. For example, at the top of my decidologism list is the word “know” because the word provides a false sense of certainty. More importantly, the word “know” serves as a stop sign for further inquiry. It can shift emphasis away from areas that could benefit from further exploration. In its place, my colleagues and I have been quite satisfied with our use of the phraseology “we believe that…” or “we understand that….” Language influences the quality of reflective thinking. And to decidologize is to make a commitment to enhance one’s everyday clarity and performance.
Naïve substitution
In 2015, my colleagues and I published a scientific paper on nutrition and cancer prevention, and in the concluding paragraph we stated:
As scientists and health professionals continue to collectively re-think the role of selenium and other nutrients in cancer prevention, investigators must work to carefully document the form-dependent effects of nutrients. By avoiding a mindset of naïve substitution—seeing one form of nutrient as equivalent to another—we make surer progress toward understanding the implications of our laboratory findings and side-stepping errant assumptions. (Chiang et al., 2015)
As discoverers, each of us needs to avoid the trap that we have termed naïve substitution, the product of insufficient exactitude with language. One might ask: Just how well are scientists staying out of this trap? A consideration here of three examples taken from the human biomedical literature offers an eye-opening perspective (Waters, 2017a).
First, consider the relationship between estrogen and cognitive function. There is a large body of scientific evidence indicating that estrogens promote cognitive function (Tang et al., 1996; Luine, 2014). But when the Women’s Health Initiative conducted a large, randomized clinical trial, estrogen did not delay the onset of dementia (Shumaker et al., 2003). Was this disappointing outcome an unexpected result? Perhaps not, if one considers that the trial did not test estrogen by itself, but instead tested an estrogen–progestogen combination. This is naïve substitution.
Consider the relationship between cognitive function and vitamin E. A 2002 study published in JAMA showed that vitamin E from food was associated with protection against dementia in older persons, but total vitamin E from food and supplements did not seem to be protective (Morris et al., 2002). Perhaps these puzzling-on-the-surface results might be explained by the fact that the major form of vitamin E in food is gamma-tocopherol, whereas the major form of vitamin E found in supplements is alpha-tocopherol. Could these two forms of vitamin E be nonequivalent when it comes to neuroprotection? Yes. In fact, research published 13 years later by the same research group showed that the circulating blood levels of these two forms of vitamin E have drastically different associations with the severity of Alzheimer’s disease pathology in the brain (Morris et al., 2015). Another case of naïve substitution.
Finally, let us consider the relationship between body fat and adverse health consequences. A 2004 study published in the New England Journal of Medicine explored whether women who underwent liposuction—the removal of 9–14 kg of subcutaneous belly fat—would show improvement in risk factors for cardiovascular disease, indicated by changes in blood pressure, inflammation, and lipid profile (Klein et al., 2004). Liposuction was not associated with improvement in any of the risk factors. Perhaps it was because visceral fat—the fat that surrounds abdominal organs—is the most metabolically active fat, far more sinister than subcutaneous fat. Earlier rodent studies had shown that the surgical removal of visceral fat significantly improved health parameters, whereas removal of an equivalent amount of subcutaneous fat had no effect on these parameters (Gabriely et al., 2002). In other words, all fat is not created equal.
What I am proposing here is that an attitude of precision with language can catalyze creative discovery, guide the interpretation of new information, and inform clinical practice. Our understanding of nutrition, health, and aging could benefit from a more nuanced category usage. Naïve substitution is the product of language laxity—leading to unsophisticated, even misleading, categories. The aforementioned liposuction study in women was not a study of the health consequences of body fat. It was a study of the impact of subcutaneous fat. On the surface, reports of disappointing research results like these might appear to have nothing to do with language. Are not these examples just the consequence of loose thinking, improper attention to detail in study design? Here is the point: Loose thinking is loose use of language. This is because our use of words is foundational to the quality of our thinking, not just our ability to communicate those thoughts. The implications of the problem exposed here are non-trivial. Regrettably, the examples provided indicate that language laxity-associated loose thinking can be spotted within the world’s most prestigious medical science journals, the contents of which are relied upon to set the direction of future scientific and medical progress.
Summary and Conclusions
The lack of a clear understanding of “Who will benefit?” stands as a major obstacle to the design and implementation of nutritional strategies to optimize healthy longevity. This article has proposed that a more thoughtful integration of three principles—life course perspective, U-shaped thinking, whole organism thinking—can lead to a richer conceptualization that all interventions should be considered as both good and bad, depending upon physiological context.
What possible insights emerge from adopting this attitudinal framework? First, it reminds us that research is a process, not a thing. Our understandings will always be in a perpetual state of unfinishedness, subject to further refinement. Second, we can expect there to be more questions than answers. And it will be the quality of those questions by which our progress will be benchmarked. Third, we come to realize that in our research studies we never see the whole picture. Our investigations, even the most prized among them, provide us with only glimpses of Nature—not much proof, but instead clues that enable us to more confidently put forth a new set of questions.
My colleagues and I are testing a new idea. We are studying extreme natural biology—Rottweiler dogs with exceptional longevity who live 30% longer than expected—to get clues to the biology of healthy longevity (Buffenstein et al., 2014). Studying these canine centenarians in their homes represents the essential fieldwork needed to advance our understanding of “dog”, no different than heading into the jungle to better understand what it means to be “gorilla”.
Future progress will hinge upon the sharpening of our method. And this sharpening will depend upon the quality of our questions. Questions such as “Is oxidative stress good or bad?” become worthless as one more carefully considers the complexity of adaptive ROS signaling and redox homeostasis (Waters and Chiang, 2019b). Statements such as “Selenium is good for you” can be cast aside as meaningless oversimplifications. Instead, we need to find the courage to confront a new set of questions—questions that might challenge our previous training, our preconceived notions of healthy longevity in pet dogs. “Are ovaries part of a system that promotes longevity?” is one such example. Indeed, a more complete understanding of the relationship between ovaries and longevity might accelerate the discovery of new, tailored nutritional interventions.
Unexpectedly perhaps, this article has proposed that the ability to cope with the challenge of biological heterogeneity—a force that drives the difficulty in finding who will benefit from any prospective intervention—may be contingent upon our capacity for language precision and nuanced category usage. Clearly, emphasis should be placed on training young scientists and health professionals in precision with language. We need “tech readiness”—the ability to apply state-of-the-art technologies, such as “-omics” and the machine-based handling of “big data”. But we also need linguistic readiness (Waters, 2017a). Elsewhere, I have written about this need and about how generational progress in science might be jumpstarted by procuring a deeper appreciation of the limits of perception, thought, and language (Waters, 2017b).
As investigators, we use our principles to carve up Nature into understandable bits. If we are carving up the relationship between nutrition and healthy aging into the when, the how much, and trade-offs, and if we are being agile with new words to express our new difficulties and our attempted solutions, then we will experience our best shot at transforming an integrated understanding of nutrition science into informed practice.
Acknowledgments
This article is based on a presentation titled “Nutrition and Pet Aging: Principles Inform Practice,” presented at the 2019 Annual Meeting of the ASAS and CSAS Companion Animal Symposium I: Nutrition and Health: Companion Animal Applications held on July 9, 2019, at Austin, TX.
Glossary
Abbreviation
- ACE
adverse childhood experiences
Conflict of interest statement
The author discloses no potential conflict of interest.
Literature Cited
- Auyeung T. W., Lee J. S., Leung J., Kwok T., Leung P. C., and Woo J.. . 2010. Survival in older men may benefit from being slightly overweight and centrally obese— a 5-year follow-up study in 4,000 older adults using DXA. J. Gerontol. A. Biol. Sci. Med. Sci. 65:99–104. doi: 10.1093/Gerona/glp099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson G. 1979. Mind and nature: a necessary unity. London: Wildwood House. [Google Scholar]
- Brown D. W., Anda R. F., Felitti V. J., Edwards V. J., Malarcher A. M., Croft J. B., and Giles W. H.. . 2010. Adverse childhood experiences are associated with the risk of lung cancer: a prospective cohort study. BMC Public Health 10:20. doi: 10.1186/1471-2458-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Anda R. F., Tiemeier H., Felitti V. J., Edwards V. J., Croft J. B., and Giles W. H.. . 2009. Adverse childhood experiences and the risk of premature mortality. Am. J. Prev. Med. 37:389–396. doi: 10.1016/j.amepre.2009.06.021 [DOI] [PubMed] [Google Scholar]
- Buffenstein R., Nelson O. L., and Corbit K. C.. . 2014. Questioning the preclinical paradigm: natural, extreme biology as an alternative discovery platform. Aging (Albany, NY). 6:913–920. doi: 10.18632/aging.100704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle A., Moreno-Villanueva M., Benhard J., Blasco M., Zondag G. Hoeijmakers J. H., and Aspinall R.. . 2015. MARK-AGE biomarkers of ageing. Mech. Ageing Dev. 151:2–12. doi: 10.1016/j.mad.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Calkins K., and Devaskar S. U.. . 2011. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 41:158–176. doi: 10.1016/j.cppeds.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang E. C., Bostwick D. G., and Waters D. J.. . 2013. Homeostatic housecleaning effect of selenium: evidence that noncytotoxic oxidant-induced damage sensitizes prostate cancer cells to organic selenium-triggered apoptosis. Biofactors 39:575–588. doi: 10.1002/biof.1106 [DOI] [PubMed] [Google Scholar]
- Chiang E. C., Bostwick D. G., and Waters D. J.. . 2015. Selenium form-dependent anti-carcinogenesis: preferential elimination of oxidant-damaged prostate cancer cell populations by methylseleninic acid is not shared by selenite. Vitam. Miner. 4:1. doi: 10.4172/2376-1318 [DOI] [Google Scholar]
- Chiang E. C., Shen S., Kengeri S. S., Xu H., Combs G. F., Morris J. S., Bostwick D. G., and Waters D. J.. . 2009. Defining the optimal selenium dose for prostate cancer risk reduction: insights from the U-shaped relationship between selenium status, DNA damage, and apoptosis. Dose. Response. 8:285–300. doi: 10.2203/dose-response.09-036.Chiang [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell K. K., Bostwick D. G., Cooley D. M., Hall G., Harvey H. J., Hendrick M. J., Pauli B. U., Render J. A., Stoica G., Sweet D. C., . et al. 2000. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate 45:173–183. doi: [DOI] [PubMed] [Google Scholar]
- de Toro-Martin J., Arsenault B. J., Després J. P., and Vohl M. C.. . 2017. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 9: pii:E913. doi: 10.3390/nu9080913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey J. 1910. Science as subject-matter and as method. Science 787:121–127. [DOI] [PubMed] [Google Scholar]
- Fontaine K. R., Redden D. T., Wang C., Westfall A. O., and Allison D. B.. . 2003. Years of life lost due to obesity. JAMA 289:187–193. doi: 10.1001/jama.289.2.187 [DOI] [PubMed] [Google Scholar]
- Fröhlich H., Balling R., Beerenwinkel N., Kohlbacher O., Kumar S., Lengauer T., Maathuis M. H., Moreau Y., Murphy S. A., Przytycka T. M., . et al. 2018. From hype to reality: data science enabling personalized medicine. BMC Med. 16:150. doi: 10.1186/s12916-018-1122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I., Ma X. H., Yang X. M., Atzmon G., Rajala M. W., Berg A. H., Scherer P., Rossetti L., and Barzilai N.. . 2002. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 51:2951–2958. doi: 10.2337/diabetes.51.10.2951 [DOI] [PubMed] [Google Scholar]
- Ganu R. S., Harris R. A., Collins K., and Aagaard K. M.. . 2012. Early origins of adult disease: approaches for investigating the programmable epigenome in humans, nonhuman primates, and rodents. Ilar J. 53:306–321. doi: 10.1093/ilar.53.3-4.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskind A. M., McGue M., Holm N. V., Sørensen T. I., Harvald B., and Vaupel J. W.. . 1996. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum. Genet. 97:319–323. doi: 10.1007/bf02185763 [DOI] [PubMed] [Google Scholar]
- Jefferson T. 1884. [1813]. Letter to John Waldo. In: Washington H. A., editor, The works of Thomas Jefferson: Being his autobiography, correspondence, reports, messages, addresses, and other writings, official and private Vol. 6 Washington, DC: Taylor & Maury; p. 184–189. [Google Scholar]
- Johnson W. 1956. Your most enchanted listener. New York, NY: Harper. [Google Scholar]
- Jones D. P. 2015. Redox theory of aging. Redox Biol. 5:71–79. doi: 10.1016/j.redox.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M., Helkala E. L., Laakso M. P., Hänninen T., Alhainen K., Soininen H., Tuomilehto J., and Nissinen A.. . 2001. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 322:1447–1451. doi: 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Fontana L., Young V. L., Coggan A. R., Kilo C., Patterson B. W., and Mohammed B. S.. . 2004. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N. Engl. J. Med. 350:2549–2557. doi: 10.1056/NEJMoa033179 [DOI] [PubMed] [Google Scholar]
- Langer S. K. 1960. Philosophy in a new key: a study in the symbolism of reason, rite, and art. Cambridge, MA: Harvard University Press. [Google Scholar]
- Lippman S. M., Klein E. A., Goodman P. J., Lucia M. S., Thompson I. M., Ford L. G., Parnes H. L., Minasian L. M., Gaziano J. M., Hartline J. A., . et al. 2009. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301:39–51. doi: 10.1001/jama.2008.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. N. 2014. Estradiol and cognitive function: past, present and future. Horm. Behav. 66:602–618. doi: 10.1016/j.yhbeh.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung T., Jan S., Tan E. J., Killedar A., and Hayes A.. . 2019. Impact of overweight, obesity and severe obesity on life expectancy of Australian adults. Int. J. Obes. (Lond). 43:782–789. doi: 10.1038/s41366-018-0210-2 [DOI] [PubMed] [Google Scholar]
- Mason J. B., Cargill S. L., Anderson G. B., and Carey J. R.. . 2009. Transplantation of young ovaries to old mice increased life span in transplant recipients. J. Gerontol. A. Biol. Sci. Med. Sci. 64:1207–1211. doi: 10.1093/gerona/glp134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz W. 1981. The essential trace elements. Science 213:1332–1338. [DOI] [PubMed] [Google Scholar]
- Morris M. C., Evans D. A., Bienias J. L., Tangney C. C., Bennett D. A., Aggarwal N., Wilson R. S., and Scherr P. A.. . 2002. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 287:3230–3237. doi: 10.1001/jama.287.24.3230 [DOI] [PubMed] [Google Scholar]
- Morris M. C., Schneider J. A., Li H., Tangney C. C., Nag S., Bennett D. A., Honer W. G., and Barnes L. L.. . 2015. Brain tocopherols related to Alzheimer’s disease neuropathology in humans. Alzheimers Dement. 11:32–39. doi: 10.1016/j.jalz.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Promislow D. E. L., and Masel J.. 2019. Biomarkers for aging identified in cross-sectional studies tend to be non-causative. J. Gerontol. A. Biol. Sci. Med. Sci. doi: 10.1093/Gerona/glz174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W. H., Broder M. S., Chang E., Feskanich D., Farquhar C., Liu Z., Shoupe D., Berek J. S., Hankinson S., and Manson J. E.. . 2009. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet. Gynecol. 113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish W. M. 1971. Our rhetorical world. In: Walter O. M., and Scott R. L., editors, Thinking and speaking: a guide to intelligent oral communication. 2nd ed.New York, NY: Macmillan Company. [Google Scholar]
- Patel S. R., Ayas N. T., Malhotra M. R., White D. P., Schernhammer E. S., Speizer F. E., Stampfer M. J., and Hu F. B.. . 2004. A prospective study of sleep duration and mortality risk in women. Sleep 27:440–444. doi: 10.1093/sleep/27.3.440 [DOI] [PubMed] [Google Scholar]
- Pinto D. S., Skolnick A. H., Kirtane A. J., Murphy S. A., Barron H. V., Giugliano R. P., Cannon C. P., Braunwald E., and Gibson C. M.; TIMI Study Group 2005. U-shaped relationship of blood glucose with adverse outcomes among patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 46:178–180. doi: 10.1016/j.jacc.2005.03.052 [DOI] [PubMed] [Google Scholar]
- Shumaker S. A., Legault C., Rapp S. R., Thal L., Wallace R. B., Ockene J. K., Hendrix S. L., Jones B. N. 3rd, Assaf A. R., Jackson R. D., . et al. ; WHIMS Investigators. 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662. doi: 10.1001/jama.289.20.2651 [DOI] [PubMed] [Google Scholar]
- Smith G. D., Shipley M. J., Marmot M. G., and Rose G.. . 1992. Plasma cholesterol concentration and mortality. The Whitehall Study. JAMA 267:70–76. [PubMed] [Google Scholar]
- Southwest Oncology Group 2008. SELECT participant letter. Available from http://www.cancer.gov/SELECT-participant-letter
- Tang M. X., Jacobs D., Stern Y., Marder K., Schofield P., Gurland B., Andrews H., and Mayeux R.. . 1996. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348:429–432. doi: 10.1016/S0140-6736(96)03356-9 [DOI] [PubMed] [Google Scholar]
- Terry D. F., Sebastiani P., Andersen S. L., and Perls T. T.. . 2008. Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch. Intern. Med. 168:277–283. doi: 10.1001/archinternmed.2007.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier P. 1957. The meaning of persons. Cutchogue, NY: Harper Collins Publishers. [Google Scholar]
- Tyner S. D., Venkatachalam S., Choi J., Jones S., Ghebranious N., Igelmann H., Lu X., Soron G., Cooper B., Brayton C., . et al. 2002. p53 mutant mice that display early ageing-associated phenotypes. Nature 415:45–53. doi: 10.1038/415045a [DOI] [PubMed] [Google Scholar]
- USA Today 2009. Vitamins get “F” in cancer prevention. Available from https://usatoday30.usatoday.com/news/health.2009-01-06.
- van Ginneken V. 2017. Are there any biomarkers of aging? Biomarkers of the brain. Biomed. J. Sci. Tech. Res. 1:193–206. doi: 10.26717/BJSTR.2017.01.000151 [DOI] [Google Scholar]
- van Vliet P., van de Water W., de Craen A. J., and Westendorp R. G.. . 2009. The influence of age on the association between cholesterol and cognitive function. Exp. Gerontol. 44:112–122. doi: 10.1016/j.exger.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Waters D. J. 2011. Aging research 2011: exploring the pet dog paradigm. Ilar J. 52:97–105. doi: 10.1093/ilar.52.1.97 [DOI] [PubMed] [Google Scholar]
- Waters D. J. 2012. The paradox of tethering: key to unleashing creative excellence in the research-education space. Informing Sci. 15:229–245. [Google Scholar]
- Waters D. J. 2014. Longevity in pet dogs: understanding what’s missing. Vet. J. 200:3–5. doi: 10.1016/j.tvjl.2013.11.024 [DOI] [PubMed] [Google Scholar]
- Waters D. J. 2017a. On cultivating an attitude of precision with language: An uncommon prescription for conditioning creative excellence in scientific discovery and education.Text Special Issue 40; Available from http://www.textjournal.com.au/speciss/issue40/Waters.pdf. [Google Scholar]
- Waters D. J. 2017b. As if blackbirds could shape scientists: Wallace Stevens takes a seat in the classroom of interdisciplinary science. Wallace Stevens J. 41:259–269. [Google Scholar]
- Waters D. J., and Bostwick D. G.. . 1997. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J. Urol. 157:713–716. [PubMed] [Google Scholar]
- Waters D. J., Chiang E. C., and Bostwick D. G.. . 2008. The art of casting nets: fishing for the prize of personalized cancer prevention. Nutr. Cancer 60:1–6. doi: 10.1080/01635580701806699 [DOI] [PubMed] [Google Scholar]
- Waters D. J., and Chiang E. C.. . 2010. It’s a U-shaped world: a Batesonian prescription for promoting public health. Et Cetera 67:218–226. [Google Scholar]
- Waters D. J., and Chiang E. C.. . 2018. Five threads: how U-shaped thinking weaves together dogs, men, selenium, and prostate cancer risk. Free Radic. Biol. Med. 127:36–45. doi: 10.1016/j.freeradbiomed.2017.12.039 [DOI] [PubMed] [Google Scholar]
- Waters D. J. and E. Chiang C.. 2019a. Prostate cancer risk reduction achievable through selenium supplementation: looking back to move forward. Acta Sci. Nutr. Health 3:45–50. [Google Scholar]
- Waters D. J. and Chiang E. C.. . 2019b. A matter of context: how an understanding of redox homeostasis informs the consideration of pro-oxidant strategies to target tuberculosis, HIV, and cancer metastasis. Acta Sci. Nutr. Health 3:81–86. doi: 10.31080/ASNH.2019.03.0450 [DOI] [Google Scholar]
- Waters D. J., and Kariuki N. N.. . 2013. The biology of successful aging: Watchful progress at biogerontology’s known-unknown interface. In: Wilmoth, J. M., and K. F. Ferraro, editors, Gerontology: Perspectives and Issues. New York (NY): Springer; p. 19–48. [Google Scholar]
- Waters D. J., Kengeri S. S., Clever B., Booth J. A., Maras A. H., Schlittler D. L., and Hayek M. G.. . 2009. Exploring mechanisms of sex differences in longevity: lifetime ovary exposure and exceptional longevity in dogs. Aging Cell 8:752–755. doi: 10.1111/j.1474-9726.2009.00513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters D. J., Kengeri S. S., Maras A. H., Suckow C. L., and Chiang E. C.. . 2017. Life course analysis of the impact of mammary cancer and pyometra on age-anchored life expectancy in female Rottweilers: implications for envisioning ovary conservation as a strategy to promote healthy longevity in pet dogs. Vet. J. 224:25–37. doi: 10.1016/j.tvjl.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Waters D. J., Patronek G. J., Bostwick D. G., and Glickman L. T.. . 1996. Comparing the age at prostate cancer diagnosis in humans and dogs. J. Natl. Cancer Inst. 88:1686–1687. doi: 10.1093/jnci/88.22.1686-b [DOI] [PubMed] [Google Scholar]
- Waters D. J., Sakr W. A., Hayden D. W., Lang C. M., McKinney L., Murphy G. P., Radinsky R., Ramoner R., Richardson R. C., and Tindall D. J.. . 1998. Workgroup 4: spontaneous prostate carcinoma in dogs and nonhuman primates. Prostate 36:64–67. doi: [DOI] [PubMed] [Google Scholar]
- Waters D. J., Shen S., Cooley D. M., Bostwick D. G., Qian J., Combs G. F. Jr, Glickman L. T., Oteham C., Schlittler D., and Morris J. S.. . 2003. Effects of dietary selenium supplementation on DNA damage and apoptosis in canine prostate. J. Natl. Cancer Inst. 95:237–241. doi: 10.1093/jnci/95.3.237 [DOI] [PubMed] [Google Scholar]
- Waters D. J., Shen S., Glickman L. T., Cooley D. M., Bostwick D. G., Qian J., Combs G. F. Jr, and Morris J. S.. . 2005. Prostate cancer risk and DNA damage: translational significance of selenium supplementation in a canine model. Carcinogenesis 26:1256–1262. doi: 10.1093/carcin/bgi077 [DOI] [PubMed] [Google Scholar]
- WebMD 2008. Prostate cancer prevention study halted Available from http://www.webmed.com/prostate-cancer/news/20081028.
- Willett W. C., Polk B. F., Morris J. S., Stampfer M. J., Pressel S., Rosner B., Taylor J. O., Schneider K., and Hames C. G.. . 1983. Prediagnostic serum selenium and risk of cancer. Lancet 2:130–134. doi: 10.1016/s0140-6736(83)90116-2 [DOI] [PubMed] [Google Scholar]
- Zuliani G., Cavalieri M., Volpato S., Cherubini A., Bandinelli S., Corsi A. M., Lauretani F., Guralnik J. M., Fellin R., and Ferrucci L.. . 2010. Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. The InChianti study. J. Gerontol. A. Biol. Sci. Med. Sci. 65:559–564. doi: 10.1093/Gerona/glg026 [DOI] [PMC free article] [PubMed] [Google Scholar]