Abstract
Neutering is a risk factor for pet obesity, which reduces the quality and length of life. Dietary interventions may serve as preventive and therapeutic options for pet obesity. The objective of this study was to evaluate the effects of specially formulated diets on body weight (BW), body composition, and blood hormones and metabolites of adult female dogs after spay surgery. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to experimentation. Twenty-eight healthy adult intact female Beagles (3.02 ± 0.7 yr; 10.28 ± 0.8 kg; body condition score [BCS]: 4.98 ± 0.57) were used in a longitudinal study. Twenty-four dogs were spayed and randomly allotted to one of three experimental diets: 1) moderate-protein, moderate-fiber diet (control; COSP), 2) high-protein, high-fiber diet (HP-HF), or 3) high-protein, high-fiber diet plus omega-3 and medium-chain fatty acids (HP-HF-O). Four dogs were sham-operated and fed the control diet (COSH). Food intake, BW, BCS, blood hormones and metabolites, body composition (via dual-energy X-ray absorptiometry scans), and voluntary physical activity (via Actical devices) were measured over time. After spay, dogs were fed to maintain BW for 12 wk (restricted phase), then allowed to overeat for 12 wk (ad libitum phase). Change from baseline data was analyzed for treatment, time, and treatment × time effects as well as treatment, feeding regimen, and treatment × feeding regimen effects. During the first 12 wk, HP-HF and HP-HF-O had lower (P < 0.01) blood cholesterol than COSH and COSP. During the second 12 wk, HP-HF and HP-HF-O ate more (P < 0.01) food (g/d) than COSH. BCS change for COSP was greater (P < 0.01) than COSH from week 21 to 24, but HP-HF and HP-HF-O were not different. When comparing data by feeding regimen, HP-HF and HP-HF-O had a greater reduction in serum cholesterol (P < 0.001) than COSH and COSP. During the second 12 wk, all spayed dogs consumed more (P < 0.01) food than COSH. However, COSH, HP-HF, and HP-HF-O had a lower (P < 0.001) increase in BCS than COSP. HP-HF-O and COSH had similar serum leptin during weeks 12 to 24. COSP had higher (P ≤ 0.01) serum C-reactive protein than HP-HF-O. Overall, body fat increase in COSP was greater (P < 0.05) than for COSH at week 24, while HP-HF and HP-HF-O were intermediate. Our results indicate that an HP-HF diet can limit weight gain and body fat increase and attenuate serum cholesterol, triglycerides, and leptin concentrations in dogs after spay surgery.
Keywords: canine nutrition, dietary fiber, high-protein diet, obesity, ovariohysterectomy
Introduction
In humans, obesity is a worldwide health problem, with the global prevalence of obesity nearly tripling from 1975 to 2016. Similar to humans, obesity incidence is increasing in the dog population. Based on the Association for Pet Obesity Prevention data from 2007 to 2018, the incidence of U.S. overweight or obese dogs increased from 43% to 55.8%. Not surprisingly, human obesity is correlated with canine obesity. Ownership-related factors likely play an essential role in obesity risk, including education level, awareness of pet nutrition, and health consciousness (Courcier et al., 2010). Although pet neutering is the most acceptable contraceptive of choice and may be used for treatment and/or prevention of some diseases (Reichler, 2009), this procedure increases the risk of obesity (Linder and Mueller, 2014).
Estrogen mediates the regulation of food intake in the central nervous system and synergizes the effects of other appetite regulatory hormones and neuronal signals (Asarian and Geary, 2006; Yong, 2017). Because spayed dogs lack the estrogen hormone, this appetite suppressant effect is reduced. Additionally, neutering causes a reduction in the resting metabolic rate of animals. These factors create an imbalance between energy consumption and energy expenditure, leading to an accumulation of fat mass and body weight (BW) gain. Clinically obese pets are prone to having several clinical conditions such as chronic inflammation, dyslipidemia, insulin resistance, cardiorespiratory disorders, renal diseases, and cancers, all of which can reduce the quality of life and life expectancy of dogs (German, 2006). Moreover, several studies have shown changes to blood lipid profiles, hormones, and inflammatory and oxidative stress markers as indicators following the metabolic changes in obese subjects (Gayet et al., 2004; Jeusette et al., 2005; Chen et al., 2006; Trayhurn et al., 2008; Webb and Falkowski, 2009; Li et al., 2014; Park et al., 2014). The obese condition not only affects the health status of animals but it also affects the economic status of pet owners due to increased costs of health care for overweight or obese pets (Pet Product News, 2019).
Understanding the physiological and pathological changes that occur after spay surgery with the use of advanced techniques and blood markers may help develop preventive or management strategies that can minimize adverse effects. Presently, there are various preventive and therapeutic options against obesity in companion animals.
The objectives of this study were to feed spayed female dogs to maintain their BW for 12 wk and then feed them ad libitum for an additional 12 wk to determine the effects of high-protein, high-fiber (HP-HF) diets on BW, body composition, voluntary physical activity, blood hormones, metabolites, inflammatory markers, and oxidative stress markers. By feeding dogs to maintain BW prior to and after spay surgery, a secondary objective of this study was to determine the impact of spay surgery on the daily energy requirement of adult female dogs. We hypothesized that the HP-HF diets would limit weight gain and associated metabolic responses after spay surgery as compared with the control diet. We also hypothesized that a high-protein, high-fiber diet supplemented with additional omega-3 and medium-chain fatty acids (HP-HF-O) would be more protective against weight gain and associated metabolic responses after spay surgery as compared to the HP-HF diet.
Materials and Methods
All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to experimentation.
Experimental design
Twenty-eight adult intact female beagles (age: 3.02 ± 0.71 yr, BW: 10.28 ± 0.77 kg; body condition score [BCS]: 4.98 ± 0.57) were used in a longitudinal spay study. The experiment consisted of 29 wk, with a 5-wk baseline phase and a 24-wk post-spay phase.
After a weight maintenance baseline period, where all dogs were fed a dry kibble control diet, all dogs were spayed except for four dogs that were sham-operated and fed the control diet containing a moderate amount of crude protein and dietary fiber throughout the entire study (COSH). Twenty-four spayed dogs were randomly allotted to one of three dry diets: 1) control diet (COSP), 2) HP-HF, and 3) HP-HF-O. All three diets were formulated to meet all Association of American Feed Control Officials (AAFCO, 2016) recommendations for adult dogs at maintenance (Table 1). After spay surgery, dogs were fed at a rate to maintain BW for the first 12 wk (restricted phase). After the first 12 wk, dogs were fed an amount that exceeded (up to 200%) the amount needed to maintain BW for an additional 12 wk to determine food intake and weight gain of each diet if allowed to overeat (ad libitum phase).
Table 1.
Ingredient and chemical composition of experimental diets
| Item | Treatment1 | ||
|---|---|---|---|
| CO | HP-HF | HP-HF-O | |
| Ingredient | % as-is | ||
| Poultry meal (low ash) | 11.36 | 34.16 | 34.16 |
| Soy protein concentrate | 8.00 | 22.00 | 22.00 |
| Barley | 23.00 | 11.00 | 11.00 |
| Beet pulp | 1.00 | 10.00 | 10.00 |
| Brewer’s rice | 38.60 | 8.00 | 8.00 |
| Chicken fat | 10.50 | 5.30 | 4.00 |
| Liquid digest | 3.00 | 3.00 | 3.00 |
| Powder palatant | 2.00 | 2.00 | 2.00 |
| Cellulose | 1.00 | 2.00 | 2.00 |
| Short-chain fructooligosaccharides2 | — | 1.00 | 1.00 |
| Coconut oil3 | — | — | 0.80 |
| Fish oil | — | — | 0.50 |
| Sodium chloride | 0.50 | 0.50 | 0.50 |
| Potassium chloride | 0.45 | 0.45 | 0.45 |
| Mineral premix4 | 0.18 | 0.18 | 0.18 |
| Vitamin premix5 | 0.18 | 0.18 | 0.18 |
| Choline chloride | 0.13 | 0.13 | 0.13 |
| Natural antioxidant6 | 0.10 | 0.10 | 0.10 |
| Analyzed composition | |||
| Dry matter (DM), % | 90.50 | 92.16 | 91.97 |
| %, DM | |||
| Ash | 4.74 | 8.29 | 8.48 |
| Crude protein | 22.31 | 41.94 | 42.91 |
| Acid-hydrolyzed fat | 15.59 | 12.00 | 12.50 |
| Crude fiber | 2.60 | 5.08 | 4.48 |
| Total dietary fiber | 12.10 | 20.90 | 21.00 |
| Insoluble fiber | 6.80 | 13.60 | 14.00 |
| Soluble fiber | 5.30 | 7.30 | 7.00 |
| Nitrogen-free extract7 | 45.26 | 16.87 | 15.11 |
| Gross energy7, kcal/g | 4.61 | 4.66 | 4.64 |
| Metabolizable energy (ME)7, kcal/g | 3.69 | 3.08 | 3.09 |
| Macronutrients on energy basis (% of ME) | |||
| Protein | 21.16 | 47.68 | 48.55 |
| Fat | 35.91 | 33.13 | 34.35 |
| Carbohydrate | 42.93 | 19.18 | 17.10 |
1Treatment: CO, control diet containing a moderate amount of crude protein and dietary fiber, HP-HF, high-protein, high-fiber diet, HP-HF-O, high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids.
2Short-chain fructooligosaccharides: Fortifeed scFOS prebiotic fiber, Ingredion Inc., Westchester, IL USA.
3Coconut oil: Gold label virgin coconut oil, Healthy Traditions, Inc., Blum, TX, USA.
4Provided per kg diet: Mn (as MnSO4), 66.00 mg; Fe (as FeSO4), 120 mg; Cu (as CuSO4), 18.00 mg; Co (as CoSO4), 1.20 mg; Zn (as ZnSO4), 240 mg; iodine (as KI), 180 mg; Se (as Na2SeO3), 0.24 mg.
5Provided per kg diet: vitamin A, 5.28 mg; vitamin D3, 0.04 mg; vitamin E, 120.00 mg; vitamin K, 0.88 mg; thiamin, 4.40 mg; riboflavin, 5.72 mg; pantothenic acid, 22.00 mg; niacin, 39.60 mg; pyridoxine, 3.52 mg; biotin, 0.13 mg; folic acid, 0.44 mg; vitamin B12, 0.11 mg.
6Natural antioxidant: Naturox liquid antioxidant, blend of vegetable oil, natural mixed tocopherols, lecithin, and rosemary extract.
7Nitrogen-free extract = 100 − (ash + crude protein + acid hydrolyzed fat + total dietary fiber); metabolizable energy = 8.5 kcal ME/g fat + 3.5 kcal ME/g protein + 3.5 kcal ME/g nitrogen-free extract; gross energy was measured by bomb calorimetry.
Daily food intake
All dogs were housed individually (1.22 m wide × 1.85 m long) in a humidity- and temperature-controlled room on a 12 h light:12 h dark cycle. Dogs always had free access to fresh water and were fed once a day (0800 hours) throughout the study. The leftover food was weighed every day to calculate the intake.
Body weight and body condition scores
Dogs were weighed and BCS were evaluated twice a week for the first 12 wk after spay surgery and once a week for weeks 13 to 24, with all being performed in the morning before feeding. A 9-point scale BCS system was used (Laflamme, 1997).
Ovariohysterectomy
Food was withheld for at least 8 h before spay surgery. Water was provided until dogs were sedated for the surgery. The operations were done in the Edward R. Madigan Laboratory on the University of Illinois campus. Dogs were pre-medicated with the combination of Ketathesia (ketamine HCl; Henry Schein, Melville, NY; 5.625 mg/kg), Dexdomitor (dexmedetomidine; Zoetis, Parsippany-Troy Hills, NJ; 0.005 mg/kg), and Torbugesic (butorphanol tartrate; Zoetis; 0.225 mg/kg) via intramuscular injection. Metacam oral suspension (meloxicam; Boehringer Ingelheim Vetmedica, Inc., Saint Joseph, MO) was given just prior to anesthesia. Anesthesia was then induced and maintained with 1% to 3% isoflurane in 100% oxygen inhalation. Dogs were positioned in dorsal recumbency, and ovariohysterectomy was performed using standard techniques. Dogs were monitored daily for 5 consecutive days for pain, lethargy, or inappetence, and the incision site was monitored for any sign of infection or dehiscence.
Body composition
Body composition was evaluated by dual-energy x-ray absorptiometry (DEXA: Hologic X-ray Bone Densitometer QDR 4500 Elite Acclaim Series) at the University of Illinois Veterinary Teaching Hospital at weeks 0, 8, 16, and 24. To perform DEXA scans, dogs were sedated by an intramuscular injection of Dexdomitor (0.02 mg/kg) and Torbugesic (0.2 mg/kg) and positioned in sternal recumbency. The four legs, trunk, and head of each dog were scanned individually, and measurements of fat, lean, and bone mineral content were taken in each body region. Body fat percentage was calculated for each part and the entire body.
Voluntary physical activity
Physical activity was measured at weeks 0, 7, 15, and 23 by the use of Actical devices and computer software (Mini Mitter, Bend, OR). During activity monitoring periods, the Actical devices were attached to collars worn around the neck for six consecutive days. Mean activity was presented in activity counts per epoch (epoch length equaled 0.25 min) over the 6-d measurement period during light h (0700 to 1900 hours) and dark h (1900 to 0700 hours).
Complete blood count, serum chemistry, blood hormones and markers, and plasma long-chain fatty acids
At weeks 0, 4, 8, 12, 16, 20, and 24, a single blood sample (up to 20 mL) was collected from each dog via jugular or cephalic puncture. Samples were immediately transferred to appropriate vacutainer tubes. Glass serum tubes with gel for serum separation (#367985 BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ) were used for serum chemistry profiles, hormones (leptin, adiponectin, insulin), C-reactive protein (CRP), superoxide dismutase (SOD), and malondialdehyde (MDA). Plastic whole blood tubes with K2EDTA additive (#367842 BD Vacutainer Plus; Becton Dickinson) were used for a complete blood count. The tubes were centrifuged at 1,200 × g for 10 min at 4 °C for serum collection. Serum chemistry profile and complete blood count were analyzed using a Hitachi 911 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Illinois Veterinary Medicine Diagnostics Laboratory.
The concentrations of serum leptin, adiponectin, insulin, CRP, SOD, and MDA were measured using commercial ELISA kits (leptin: #EZCL-31K, Millipore, Billerica, MA; adiponectin: #LS-F36814, Lifespan Biosciences, Seattle, WA; insulin: #10-1203-01, Mercodia Inc., Winston Salem, NC; CRP: #ab157698, Abcam, Cambridge, UK; SOD: #MBS740341, MyBioSource, San Diego, CA; MDA: #MBS2605193, MyBioSource, San Diego, CA).
Plasma samples were analyzed for fatty acids according to the method of Lepage and Roy (1986) and Masood et al. (2005) using a Thermo Scientific TRACE 1300 gas chromatograph coupled with flame ionization detector to determine individual fatty acid methyl esters. The internal standard (23:0) and external fatty acid methyl ester standards were purchased from Supelco Sigma-Aldrich.
Chemical analysis of diets
All diets were subsampled and ground through a 2-mm screen using a Wiley mill (model 4, Thomas Scientific, Swedesboro, NJ). Samples were analyzed according to procedures of the Association of Official Analytical Chemists (AOAC) for dry matter (DM; 105 °C) and ash (organic matter was calculated from ash) (AOAC, 2006; methods 934.01, 942.05). Crude protein content was calculated from Leco total N values (TruMac N, Leco Corporation, St. Joseph, MI; AOAC, 2006). Total lipid content (acid-hydrolyzed fat) of the samples was determined according to the methods of the American Association of Cereal Chemists (AACC, 1983) and Budde (1952). Gross energy of the samples was measured using an oxygen bomb calorimeter (model 1261, Parr Instruments, Moline, IL). Total dietary fiber (TDF) content was determined according to the method of Prosky et al. (1985).
Statistical analyses
All data were analyzed using the Mixed Models procedure of SAS (version 9.4; SAS Institute, Cary, NC). Daily food intake, daily caloric intake, BW, BCS, serum metabolite, complete blood cell count, blood hormone, inflammatory marker, and oxidative stress marker data were evaluated based on the change from baseline data, which were separated into two phases (restricted phase and ad libitum phase). Body composition and voluntary physical activity data were evaluated based on the change from baseline data for the entire study. These response criteria data were analyzed using repeated measures analysis. In addition to the repeated measures analysis, change from baseline data was also separated into two phases and analyzed as the means of the first 12 wk (restricted phase) and the means of the second 12 wk (ad libitum phase), evaluating differences due to treatment, feeding regimen, and treatment × feeding regimen effects. All results are presented as means ± pooled standard error of the means (SEM). A P-value < 0.05 was considered significant and a P-value < 0.10 was considered a trend.
Results
All baseline data (week 0) were analyzed among groups. The only differences noted among treatments at baseline were serum globulin concentrations and lymphocyte counts (Supplementary Table S1).
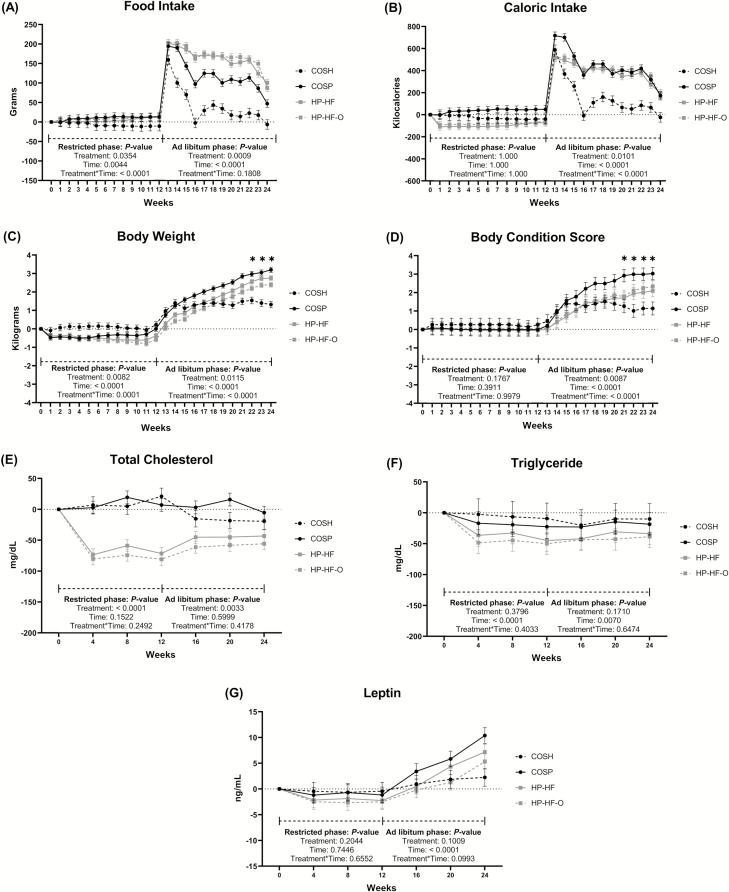
During the first 12 wk, a treatment × time interaction (P < 0.0001) was observed for food intake (g/d) (Figure 1A), but caloric intake (kcal/d) was not different due to time or treatment (Figure 1B). A treatment × time interaction (P < 0.0001) was also observed during the first 12 wk for BW (Figure 1C), but BCS was not affected by treatment (Figure 1D). During the second 12 wk, dogs fed HP-HF or HP-HF-O ate more (P < 0.01) food than sham-operated dogs. Fecal weight (as-is basis) increases were greater (P < 0.01) in HP-HF (136 g/d) and HP-HF-O (116 g/d) dogs than COSH (−12 g/d) and COSP (17 g/d) dogs. During the second 12 wk, treatment × time interactions (P < 0.0001) were observed for caloric intake, BW, and BCS. Change in BW was greater (P < 0.05) for COSP and HP-HF dogs than sham-operated dogs at week 22, while all three groups of spayed dogs had greater (P < 0.01) BW change than sham-operated dogs at weeks 23 and 24. During this phase, the change in BCS of dogs fed COSP was greater (P < 0.01) than sham-operated dogs from week 21 to 24, but dogs fed HP-HF or HP-HF-O were not different (Table 3).
Figure 1.
Change from baseline food intake, caloric intake, body weight, body condition score, blood total cholesterol, triglyceride, and leptin concentration data of adult female dogs fed different diets under restricted or ad libitum feeding regimens after spay surgery. Daily food intake (g/d) (A), caloric intake (kcal/d) (B), weekly body weight (C), weekly body condition score (D), and serum total cholesterol (E), triglyceride (F), and leptin (G) concentrations of dogs from four treatment groups. COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber (black circles discontinued line); COSP, spayed dogs fed the control diet (black circles solid line); HP-HF, spayed dogs fed a high-protein, high-fiber diet (gray squares solid line); HP-HF-O, spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids (gray squares discontinued line). Data are presented as the change from baseline (week 0) least square means ± SEM. The data are separated into two phases: restricted phase (week 1 to 12) and ad libitum phase (week 13 to 24).
Table 3.
Change from baseline caloric intake, body weight, and body condition score data of adult female dogs fed different diets under restricted (R) or ad libitum (A) feeding regimens after spay surgery
| Item | Dietary treatment group1 | P-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COSH | COSP | HP-HF | HP-HF-O | SEM | Treatment | Feeding regimen | Treatment × feeding regimen | |||||
| R | A | R | A | R | A | R | A | |||||
| Change from baseline2 | ||||||||||||
| Daily caloric intake, kcal/d | −27.02cd | 193.02b | 37.28c | 391.26a | −96.53d | 461.23a | −80.77d | 471.36a | 73.567 | 0.0241 | <0.0001 | <0.0001 |
| Body weight, kg | 0.08c | 1.11b | -0.37cd | 2.21a | −0.49d | 2.08a | −0.59d | 1.96a | 0.221 | 0.1878 | <0.0001 | <0.0001 |
| Body condition score3 | 0.25c | 0.93b | 0.04c | 2.23a | −0.03c | 1.51b | 0.09c | 1.44b | 0.493 | 0.0181 | <0.0001 | <0.0001 |
1Dietary treatment group: COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber; COSP = spayed dogs fed the control diet; HP-HF = spayed dogs fed a high-protein, high-fiber diet; HP-HF-O = spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids. Feeding regimen: R, restricted feeding phase, A, ad libitum feeding phase.
2Mean values during restricted feeding phase (change from week 0) and ad libitum phase (change from week 12).
3A 9-point scale body condition scoring system was used (Laflamme, 1997).
a,b,c,dMean values within the same row with unlike superscript letters represent significant treatment × time interactions (P < 0.05). ).
Table 2.
Baseline data of adult female dogs
| Item | Dietary treatment group1 | Reference3 | SEM | P-values | |||
|---|---|---|---|---|---|---|---|
| COSH | COSP | HP-HF | HP-HF-O | Treatment | |||
| Daily food intake, daily caloric intake, body weight, and body condition score | |||||||
| Daily food intake, g/d | 184.3 | 169.6 | 181.3 | 179.5 | — | 9.03 | 0.5579 |
| Daily caloric intake, kcal/d | 680.0 | 625.9 | 668.8 | 662.2 | — | 33.33 | 0.5579 |
| Body weight, kg | 9.92 | 10.18 | 10.33 | 10.21 | — | 0.475 | 0.8126 |
| Body condition score2 | 4.99 | 5.11 | 5.12 | 5.16 | — | 0.311 | 0.9076 |
| Serum chemistry | |||||||
| Total cholesterol, mg/dL | 230.75 | 213.14 | 204.12 | 224.25 | 129 to 297 | 11.745 | 0.4498 |
| Triglycerides, mg/dL | 66.75 | 65.64 | 73.50 | 81.88 | 32 to 154 | 30.577 | 0.7997 |
| Blood hormones | |||||||
| Leptin, ng/mL | 3.87 | 4.16 | 4.57 | 4.87 | — | 1.700 | 0.8930 |
| Plasma long-chain fatty acids | |||||||
| Myristic acid, C14:0 | 6.62 | 5.90 | 6.05 | 5.75 | — | 0.617 | 0.8063 |
| Palmitic acid, C16:0 | 476.54 | 463.42 | 477.84 | 461.64 | — | 41.328 | 0.9730 |
| Stearic acid, C18:0 | 586.73 | 564.17 | 614.43 | 594.88 | — | 36.078 | 0.8075 |
| Oleic acid, C18:0 | 346.43 | 341.45 | 354.01 | 347.24 | — | 43.179 | 0.9877 |
| Linoleic acid, C18:2n6 | 817.48 | 795.04 | 797.11 | 809.40 | — | 55.889 | 0.9923 |
| Gamma-linolenic acid, C18:3n6 | 9.29 | 8.96 | 10.84 | 9.83 | — | 0.723 | 0.3133 |
| Alpha-linolenic acid, C18:3n3 | 7.90 | 8.19 | 7.70 | 8.01 | — | 0.842 | 0.9793 |
| Arachidonic acid, C20:4n6 | 706.88 | 656.40 | 721.90 | 690.82 | — | 48.096 | 0.8102 |
| Eicosapentaenoic acid, C20:5n3 | 5.17 | 5.22 | 5.85 | 5.89 | — | 0.869 | 0.7915 |
| Docosahexaenoic acid, C22:6n3 | 13.78 | 14.06 | 14.70 | 14.71 | — | 2.236 | 0.9583 |
| Body composition | |||||||
| Lean muscle mass, g | 6,361 | 6,168 | 6,745 | 6,407 | — | 210.6 | 0.1727 |
| Fat mass, g | 3,027 | 3,328 | 3,003 | 3,169 | — | 248.5 | 0.7097 |
| Fat percentage, % | 31.55 | 34.03 | 30.46 | 32.24 | — | 1.599 | 0.4430 |
| Bone mineral content, g | 202.6 | 213.2 | 229.6 | 209.7 | — | 13.93 | 0.3279 |
| Voluntary physical activity (counts/epoch)4 | |||||||
| Daily activity | 44.59 | 42.10 | 41.09 | 48.11 | — | 5.814 | 0.6242 |
| Light activity | 59.19 | 62.70 | 59.67 | 68.54 | — | 8.560 | 0.7262 |
| Dark activity | 30.00 | 21.42 | 22.52 | 27.68 | — | 3.691 | 0.2489 |
| Light:dark activity ratio | 2.26 | 2.98 | 2.74 | 2.56 | — | 0.290 | 0.4632 |
1Dietary treatment group: COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber; COSP, spayed dogs fed the control diet; HP-HF, spayed dogs fed a high-protein, high-fiber diet; HP-HF-O, spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids.
2A 9-point scale body condition scoring system was used (Laflamme, 1997).
3University of Illinois Veterinary Diagnostic Laboratory reference ranges.
4Mean activity was represented as activity counts per epoch (epoch duration, 15 s).
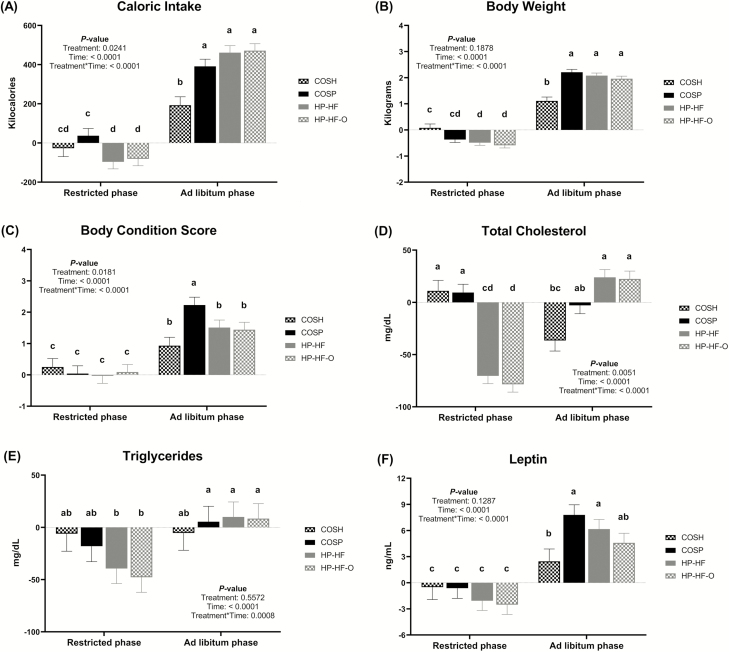
When comparing data by feeding regimen (restricted vs. ad libitum phase), treatment × feeding regimen interactions (P < 0.0001) were observed for change in food and caloric intake, BW, and BCS (Figure 2). During the first 12 wk, food intake was not different among groups, but COSP dogs had a greater increase (P < 0.05) in caloric intake than HP-HF and HP-HF-O dogs (Figure 2A). During the second 12 wk, all dogs consumed more (P < 0.001) food and calories and gained more (P < 0.001) weight than they did during the first 12 wk (Figure 2B). During this phase, dogs fed HP-HF or HP-HF-O ate more (P < 0.01) food than dogs fed COSP, with all spayed dogs consuming more (P < 0.01) food than sham-operated dogs (data not shown). During the second 12 wk, all spayed dogs had greater (P < 0.001) caloric intake than sham-operated dogs. During the first 12 wk, dogs fed HP-HF or HP-HF-O had greater reductions (P < 0.05) in BW than sham-operated dogs. During the second 12 wk, all spayed dogs gained more (P < 0.0001) weight than sham-operated dogs. During this time, sham-operated dogs and those fed HP-HF or HP-HF-O had a lower (P < 0.001) increase in BCS than COSP dogs (Figure 2C).
Figure 2.
Feeding regimen changes in caloric intake, body weight, body condition score, blood total cholesterol, triglyceride, and leptin concentration data of adult female dogs fed different diets under restricted or ad libitum feeding regimens after spay surgery. Daily caloric intake (kcal/d) (A), weekly body weight (B) and body condition score (C), serum total cholesterol (D), triglycerides (E), and leptin concentration (F) of dogs from four treatment groups: COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber (black and white bars); COSP. spayed dogs fed the control diet (black bar); HP-HF, spayed dogs fed a high-protein, high-fiber diet (gray bar); HP-HF-O, spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids (gray and white bars). Data are presented as the change from baseline (week 0) least square means ± SEM.
Although some statistical differences were noted, serum metabolites remained within reference ranges for all dogs (Supplementary Table S2). Therefore, most data will not be discussed in the text. Of note, the reduction in serum cholesterol was greater (P < 0.01) in HP-HF and HP-HF-O dogs than in COSH and COSP dogs (Figure 1E). Serum triglycerides were different (P < 0.05) due to time during the first 12 wk but not due to treatment (Figure 1F). During the second 12 wk, a treatment effect (P < 0.05) was observed for serum cholesterol. The increase in serum cholesterol in the second 12 wk was greater (P < 0.05) in HP-HF and HP-HF-O dogs than sham-operated dogs. Furthermore, serum triglycerides were affected by time (P < 0.05).
When comparing data by feeding regimen, treatment × feeding regimen interactions were observed for cholesterol and triglycerides. During the first 12 wk, HP-HF and HP-HF-O dogs had a greater reduction in serum cholesterol (P < 0.001) than sham-operated or COSP dogs (Figure 2D). During the second 12 wk, however, HP-HF and HP-HF-O dogs had a greater (P < 0.001) increase in serum cholesterol than COSH dogs. Serum triglycerides were not different among COSH or COSP dogs across the entire study, but HP-HF and HP-HF-O dogs had greater (P < 0.001) reduction in serum triglycerides during the first 12 wk than the second 12 wk (Figure 2E). Although some statistical differences were observed, complete blood cell counts were within the reference ranges for all dogs, except for total white blood cell counts, which were slightly lower than the reference range in HP-HF dogs (Supplementary Table S3).
Of the hormones, inflammatory markers, and oxidative stress markers measured, serum adiponectin was affected by time (P < 0.001) during the first 12 wk, but no other differences were observed (data not shown). During the second 12 wk, serum leptin concentrations were increased (P < 0.001) with time and tended to be different due to treatment (P = 0.10; Figure 1G). During the second 12 wk, serum CRP was affected by a treatment × time interaction (P < 0.05; data not shown). Other serum hormones and inflammatory and oxidative stress markers were not affected by treatment, time, or treatment × time.
When comparing these data by feeding regimen, serum leptin and insulin were affected by treatment × feeding regimen interaction. Serum insulin was not different among treatment groups during the first 12 wk. However, dogs fed COSP and HP-HF had a reduction (P < 0.001) in insulin concentrations during the first 12 wk and an increase during the second 12 wk. Serum leptin was not different among treatment groups during the first 12 wk. Serum leptin was greater (P < 0.05) in all groups during the second 12 wk compared with the first 12 wk and was greater (P < 0.05) in dogs fed COSP and HP-HF compared with sham-operated dogs (Figure 2F). Interestingly, HP-HF-O dogs had no difference (P > 0.05) in serum leptin compared with sham-operated dogs during this time. Serum adiponectin was affected by feeding regimen (P < 0.001; data not shown). Serum CRP tended to be different (P = 0.09) due to feeding regimen and was different due to treatment, with COSP dogs having higher (P ≤ 0.01) CRP concentrations than dogs fed HP-HF-O (data not shown). The other serum hormones and markers were not altered by treatment, time, or treatment × feeding regimen.
Treatment × time interactions were observed for plasma myristic acid, palmitic acid, oleic acid, linoleic acid, alpha-linolenic acid, and docosahexaenoic acid (DHA) concentrations (Table 4). Dogs fed HP-HF-O had the greatest increase (P < 0.001) in plasma myristic acid concentrations at week 24 when compared with other groups at any time points. A similar response was noted for plasma DHA, which had the greatest increase (P < 0.0001) in HP-HF-O dogs at week 24. Dogs fed the HP-HF-O diet also had a greater increase (P < 0.0001) in plasma DHA at week 12 than all other treatments, which had a lower increase or reduction in plasma DHA compared with baseline. From baseline to week 12, plasma palmitic acid concentrations had a greater decrease (P < 0.05) in dogs fed HP-HF compared with COSP and COSH dogs. Similarly, from baseline to week 12, plasma linoleic acid concentrations had a greater decrease (P < 0.01) in dogs fed HP-HF or HP-HF-O compared with those fed COSH or COSP. Treatment effects were observed for plasma stearic acid, γ-linolenic acid, arachidonic acid, and eicosapentaenoic acid (EPA) concentrations. Dogs fed HP-HF or HP-HF-O had a greater reduction in plasma stearic acid (P < 0.01) and γ-linolenic acid (P < 0.05) concentrations compared with sham-operated and COSP dogs. Similarly, dogs fed HP-HF or HP-HF-O tended to have a greater (P < 0.06) reduction in plasma arachidonic acid concentrations compared with sham-operated and COSP dogs. Lastly, dogs fed HP-HF-O had a greater increase (P < 0.0001) in plasma EPA concentrations than dogs fed other treatments.
Table 4.
Change from baseline plasma long-chain fatty acids of adult female dogs fed different diets under restricted or ad libitum feeding regimens after spay surgery
| Item | Dietary treatment group1 | P-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COSH | COSP | HP-HF | HP-HF-O | SEM | Treatment | Time | Treatment × Time | |||||
| Week 12 | Week 24 | Week 12 | Week 24 | Week 12 | Week 24 | Week 12 | Week 24 | |||||
| Change from baseline2: long-chain fatty acids, µg/mL | ||||||||||||
| Myristic acid, C14:0 | −1.02bc | −1.73bc | −0.88bc | −0.53bc | −2.98cd | −1.19b | −0.09bc | 2.40a | 0.745 | 0.0159 | 0.0004 | 0.0005 |
| Palmitic acid, C16:0 | −5.23a | −49.24ab | −30.95a | −47.49a | −203.70b | −140.89ab | −154.09ab | −110.35ab | 40.258 | 0.0127 | 0.3843 | 0.0295 |
| Stearic acid, C18:0 | 62.81 | 1.94 | −6.8 | 17.94 | −248.76 | −123.97 | −195.12 | −117.69 | 40.626 | 0.0030 | 0.0769 | 0.0664 |
| Oleic acid, C18:1n9 | −4.59 | −38.31 | −30.65 | −29.31 | −147.21 | −96.28 | −139.64 | −112.73 | 43.664 | 0.1087 | 0.2116 | 0.0568 |
| Linoleic acid, C18:2n6 | −2.86a | −120.16ab | 18.30a | −110.94a | −370.89b | −294.83ab | −341.24b | −284.81ab | 50.447 | 0.0006 | 0.1893 | 0.0014 |
| Gamma-linolenic acid, C18:3n6 | −0.41 | −0.88 | −0.11 | −0.10 | −3.69 | −1.40 | −3.82 | −3.08 | 0.774 | 0.0245 | 0.2103 | 0.2302 |
| Alpha-linolenic acid, C18:3n3 | −0.41ab | −1.60ab | −0.63ab | −1.52ab | −2.87b | −0.56a | −2.68ab | −1.25ab | 0.834 | 0.8289 | 0.2396 | 0.0025 |
| Arachidonic acid, C20:4n6 | 131.06 | 49.52 | 9.59 | 77.43 | −261.46 | −75.14 | −223.53 | −127.53 | 58.425 | 0.0579 | 0.0629 | 0.1132 |
| Eicosapentaenoic acid, C20:5n3 | −1.77 | −0.69 | −0.38 | 0.25 | −0.55 | 1.17 | 14.59 | 24.68 | 1.731 | <0.0001 | 0.0016 | 0.5290 |
| Docosahexaenoic acid, C22:6n3 | 0.81c | −0.17c | −2.92c | −0.43c | −6.54c | −1.65c | 27.20b | 52.01a | 4.449 | <0.0001 | <0.0001 | <0.0001 |
1Dietary treatment group: COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber; COSP, spayed dogs fed the control diet; HP-HF, spayed dogs fed a high-protein, high-fiber diet; HP-HF-O, spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids.
2Mean values during restricted feeding phase (change from week 0 to week 12) and ad libitum phase (change from week 0 to week 24).
a,b,c,dMean values within the same row with unlike superscript letters represent significant treatment × time interactions (P < 0.05).
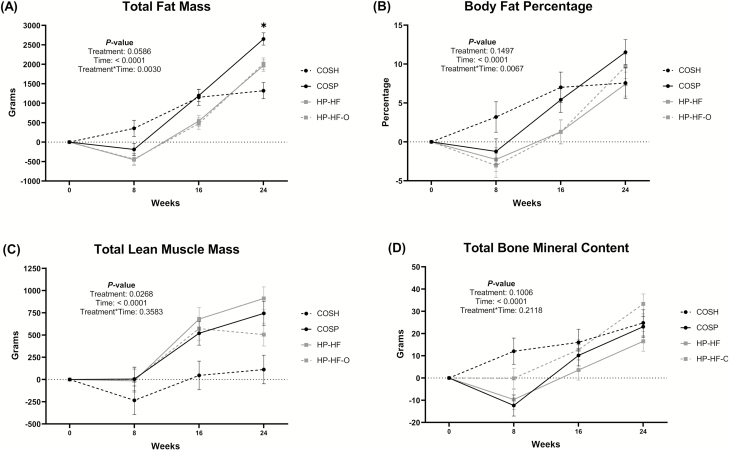
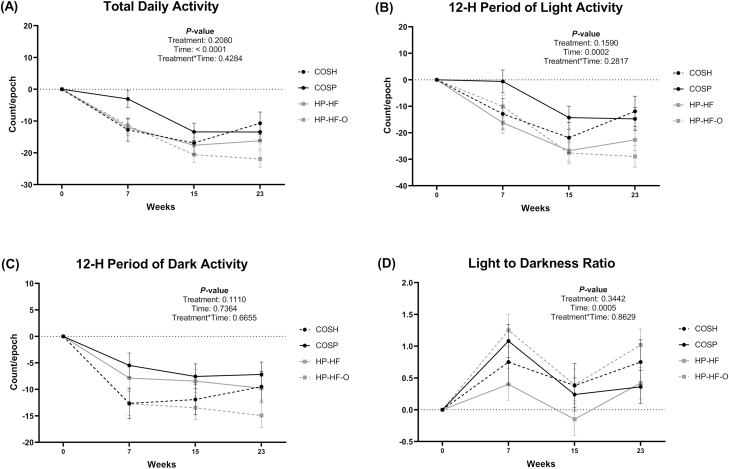
Treatment × time interactions were observed for total body fat and body fat percentage (Figure 3). The body fat increase in dogs fed COSP was greater (P < 0.01) than that of sham-operated dogs at week 24, with dogs fed HP-HF and HP-HF-O being intermediate. A treatment effect was observed for lean mass, with dogs fed HP-HF having greater (P < 0.05) lean mass than sham-operated dogs. Bone mineral content tended to be different due to treatment (P = 0.10). Bone mineral content and lean mass were increased (P < 0.0001) with time. None of the voluntary physical activity variables measured were affected by treatment (Figure 4), but mean daily activity and mean activity during the light period were decreased (P < 0.001) over time. The light:dark activity ratio was also affected by time (Figure 4).
Figure 3.
Change from baseline total body fat, fat percentage, lean muscle mass, and bone mineral content of adult female dogs fed different diets under restricted or ad libitum feeding regimens after spay surgery. Total body fat (A), body fat percentage (B), total lean muscle mass (C), and total bone mineral content (D) of dogs from four treatment groups: COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber (black circles discontinued line); COSP, spayed dogs fed the control diet (black circles solid line); HP-HF, spayed dogs fed a high-protein, high-fiber diet (gray squares solid line); HP-HF-O, spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids (gray squares discontinued line). Data are presented as the change from baseline (week 0) least square means ± SEM.
Figure 4.
Change from baseline voluntary physical activity levels of adult female dogs fed different diets under restricted or ad libitum feeding regimens after spay surgery. Total daily activity (A), 12-h period of light (B), 12-h period of dark (C), and the light to darkness ratio (D) of activity counts of voluntary physical activity of dogs from four treatment groups: COSH, sham-operated dogs fed a control diet containing a moderate amount of crude protein and dietary fiber (black circles discontinued line); COSP, spayed dogs fed the control diet (black circles solid line); HP-HF, spayed dogs fed a high-protein, high-fiber diet (gray squares solid line); HP-HF-O, spayed dogs fed a high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids (gray squares discontinued line). Data are presented as the change from baseline (week 0) least square means ± SEM. Mean activity is represented as activity counts per epoch (epoch duration, 15 s).
Discussion
Pet overpopulation not only increases the risk of animal-to-human diseases but also causes millions of unwanted healthy dogs and cats in shelters to be euthanized in the United States each year, creating a moral and ethical problem for society. The surgery to remove the reproductive organs, particularly in female animals, helps to solve pet overpopulation. Additionally, this surgical procedure also is a way to eliminate the risk of some diseases. Thus, pet owners request this preventive operation for their pets because they consider pets to be precious family members (McNeil and Constandy, 2006). Those reasons have led to a large neutered pet population that are at high risk of becoming overweight or obese.
In a recent retrospective study, the data records of 50,787 middle-aged neutered dogs that attended 900 pet hospitals in the United States were analyzed. In that study, researchers reported a negative correlation (P < 0.001) between having an overweight body condition and life span (Salt et al., 2019). Studying the effects of spay surgery may identify proper preventive and management strategies to minimize adverse effects including obesity.
Various factors affect maintenance energy requirements (MER) in dogs, such as age, breed, sex, neuter status, husbandry, activity level, owner behaviors, feeding patterns, and nutrient content of food (Gross et al., 2010; Heuberger and Wakshlag, 2011). One study (Heuberger and Wakshlag, 2011) reported a significant correlation (P < 0.02) between feeding table scraps to overweight or obese dogs compared with nonobese dogs. Furthermore, the misperception of the dog’s body condition by pet owners leads to overfeeding practice and is a risk factor of obesity (Yam et al., 2017).
The National Research Council (NRC, 2006) suggests several MER equations depending on dog activity level, breed, and age. For inactive and/or old dogs, an MER of 95 × BW kg0.75 to 105 × BW kg0.75 is recommended. For active pet dogs and kennel dogs, an MER of 130 × BW kg0.75 is recommended. For some breeds, the factors used in these equations are even higher. Neuter status, however, was not a consideration when developing these equations. A meta-analysis study was conducted to determine the MER of adult dogs by analyzing the data from 29 publications (from 1982 to 2013). In that study, the average MER of pet dogs was 124.1 ± 38.0 kcal/BW kg0.75/d, with a predicted allometric equation of 62.5 kcal/BW kg0.97/d (Bermingham et al., 2014). Those researchers claimed that this equation is more accurate than the equations recommended by the NRC (2006).
In the current study, MER for intact dogs fed to maintain BW at baseline was approximately 115 × BW kg0.75, which is 11.5% lower than the NRC recommendation for active dogs (130 × BW kg0.75), but a bit higher than that for inactive and old dogs. Neutered pets are known to require less energy compared with those that are intact. During the first 12 wk after spay (weeks 1 to 12), the mean MER was reduced to 109 × BW kg0.75. The reduction in MER after neutering in this study was not as drastic as that observed in previous studies such as Bermingham et al. (2014) who reported that neutered dogs had an MER of 146.4 ± 21.5 kcal/BW kg0.75/d and intact dogs had an MER of 195.7 ± 23.4 kcal/BW kg0.75/d. In cats, Belsito et al. (2009) reported that a 30% reduction was needed to avoid BW gain after spay. Another study (Mitsuhashi et al., 2011) reported that the MER of cats was 25% lower than the NRC recommendation after spay surgery. Collectively, our data and that of others suggest that the MER recommendations for spayed and neutered dogs and cats are lower than that of intact animals. Given the high percentage of neutered pets in developed nations, these recommendations should be listed by NRC and other advisory groups so that proper feeding recommendations can be developed and followed.
One of the beneficial effects of increased fiber consumption is that it may reduce the amount of energy absorbed from the diet by reducing fat and protein digestibility (Weber et al., 2007; Adam et al., 2014; Kröger et al., 2017; Davis, 2018; Hervik and Svihus, 2019). The fermentation of fiber by microbiota in the large intestine also leads to greater heat loss (Hervik and Svihus, 2019). Another advantage of feeding a high-fiber diet to dogs was presented in the current study. The fiber-supplemented diets dramatically decreased serum cholesterol and triglycerides, particularly during the first 12 wk. These results are similar to previous experiments studying the impact of short-chain fructooligosaccharides and β-glucans on the blood lipid-lowering effects in dogs (Diez et al., 1997; Beylot, 2005; Surampudi et al., 2016).
Based on a couple of studies that reported that EPA and DHA help attenuate muscle protein breakdown and increase muscle protein synthesis (Kamolrat and Gray, 2013; Wang et al., 2013), we hypothesized that dogs fed HP-HF-O diet would have the highest increase in muscle mass. Unexpectedly, lean body mass increases in HP-HF-O dogs were lower than dogs in the COSP and HP-HF groups. This area needs further investigation to determine the amount of EPA and DHA in blood cells needed to maximize concentrations and bioavailability for use by tissues (Cholewski et al., 2018). Although the lean body mass data did not confirm our hypothesis, serum CRP concentrations of dogs fed HP-HF-O were lower than that of COSP dogs, which was likely due to the anti-inflammatory properties of EPA and DHA. As expected, plasma myristic acid concentrations were the highest in the HP-HF-O group due to the coconut oil included in the diet.
Jeusette et al. (2005) conducted a similar study by comparing lean dogs to obese dogs. In that study, dogs were allowed to gain weight for up to 15 mo, and until dogs were grossly obese (BCS 8 to 9) and had a stable BW for at least 1 yr. Those researchers measured several obesity-related parameters (i.e., serum ghrelin, leptin, insulin, cholesterol, and triglycerides) and reported differences in those parameters between lean and obese dogs. In the present study, blood samples were collected from dogs over time for the measurement of serum metabolites and hormones of dogs at an ideal BCS and during weight gain. Interestingly, serum leptin concentrations in HP-HF-O dogs were not different from COSH dogs during the weight gain phase. This aligns with changes to BCS, with dogs fed COSP having the highest BCS, increasing by approximately 3 BCS units compared with COSH (increased by 0.88), HP-HF (increased by 2.34), and HP-HF-O (increased by 2.34) dogs.
Blood cholesterol and triglyceride concentrations were significantly decreased in dogs fed the HP-HF diets during the first 12 wk after spay. These changes were not quite as dramatic during the second 12 wk during weight gain but were still numerically lower in those groups at week 24. Respondek et al. (2008) reported that differences in blood lipid profiles were noticed in dogs after at least 20 wk of consuming a high-caloric diet. Therefore, if the ad libitum phase in the current study was extended to more than 12 wk, a greater separation among groups may have been observed. Also, because the TDF concentration of the three diets was not greatly different from one another (TDF of a control diet = 12.9%; HP-HF diet = 20.9%; HP-HF-O diet = 21.0%) and contained β-glucans from barley inclusion, all animals may have had some protection against blood lipids that may have not occurred with a different basal diet.
Based on evidence from several studies (Courcier et al., 2010; Chauvet et al., 2011; Warren et al., 2011; Morrison et al., 2013; German et al., 2017), not only does low physical activity lead to obesity but obesity may also promote low physical activity. In animals, sex hormones play a role in regulating physical activity (Lightfoot, 2008). Neutered dogs have lower activity and are at a high risk to become obese. Physical activity was not affected by treatments in this study, but mean daily activity and mean activity during the light period were decreased after spay surgery. Surprisingly, sham-operated dogs also had lower activity compared with baseline. This may have been due to the relatively small space allowed for housing in each cage and exercise area that limited the activity. Additionally, sham-operated dogs were socialized with spayed dogs in groups of three to four at a time, so the lower activity of spayed dogs may have indirectly resulted in the lower activity of sham-operated dogs.
Overall, the results of this study suggest that permanently removing the ovaries of dogs creates massive changes to their physiology, notably increasing the risk of obesity. Because the obese condition generates many comorbidities, such as chronic inflammation, insulin resistance, and metabolic syndrome, researching the pathophysiological changes that occur after spaying is needed so that the proper prevention and treatment strategies can be developed. In this study, several changes were observed after spaying in dogs consuming HP-HF diets with or without omega-3 and medium-chain fatty acids. Our results indicate that these diets can limit weight gain and body fat increase and attenuate serum cholesterol, triglyceride, ALP, CALP and leptin concentrations in dogs after spay surgery. The benefits of HP-HF diets were most evident during ad libitum feeding. While many pet owners feed their dogs in this manner, they should be reminded that a controlled feeding strategy is recommended. Long-term investigation of changes after spay may be useful to expand our knowledge in this area and develop better preventive strategies of obesity and comorbidities.
Supplementary Material
Acknowledgments
This paper was presented as a poster presentation at the 2019 American Academy of Veterinary Nutrition Clinical Nutrition and Research Symposium, Phoenix, AZ, June 2019. This study was funded by Perfect Companion Group Co., Ltd., Thailand.
Glossary
Abbreviations
- AAFCO
Association of the American Feed Control Officials
- ALP
alkaline phosphatase
- BCS
body condition score
- BW
body weight
- CALP
corticosteroid isoenzyme of alkaline phosphatase
- COSH
sham-operated dogs consuming control diet
- COSP
spayed dogs consuming control diet
- CRP
C-reactive protein
- DEXA
dual-energy X-ray absorptiometry
- DHA
docosahexaenoic acid
- DM
dry matter
- EPA
eicosapentaenoic acid
- HP-HF
spayed dogs consuming high-protein, high-fiber diet
- HP-HF-O
spayed dogs consuming high-protein, high-fiber diet containing additional omega-3 and medium-chain fatty acids
- MDA
malondialdehyde
- ME
metabolizable energy
- MER
maintenance energy requirement
- NRC
National Research Council
- SEM
standard error of the means
- SOD
superoxide dismutase
- TDF
total dietary fiber
Conflicts of interest statement
T.P. is an employee of Perfect Companion Group Co., Ltd., Thailand. All other authors have no conflict of interest.
Literature Cited
- AAFCO 2016. Official publication. Oxford (IN):Association of American Feed Control Officials, Inc. [Google Scholar]
- Adam C. L., Williams P. A., Dalby M. J., Garden K., Thomson L. M., Richardson A. J., Gratz S. W., and Ross A. W.. . 2014. Different types of soluble fermentable dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr. Metab. (Lond). 11:36. doi: 10.1186/1743-7075-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Association of Cereal Chemists (AACC) 1983. Approved methods. 8th ed.St. Paul (MN: ): American Association of Cereal Chemists. [Google Scholar]
- Asarian L., and Geary N.. . 2006. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361:1251–1263. doi: 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2006. Official methods of analysis. 17th ed Gaithersburg (MD):Association Of Analytical Chemists. [Google Scholar]
- Association for Pet Obesity Prevention 2007–2018 Pet obesity survey results: U.S. pet obesity rates plateau and nutritional confusion grows Available at: https://petobesityprevention.org/2018 [accessed March 12, 2019].
- Belsito K. R., Vester B. M., Keel T., Graves T. K., and Swanson K. S.. . 2009. Impact of ovariohysterectomy and food intake on body composition, physical activity, and adipose gene expression in cats. J. Anim. Sci. 87:594–602. doi: 10.2527/jas.2008-0887 [DOI] [PubMed] [Google Scholar]
- Bermingham E. N., Thomas D. G., Cave N. J., Morris P. J., Butterwick R. F., and German A. J.. . 2014. Energy requirements of adult dogs: a meta-analysis. PLoS One 9:e109681. doi: 10.1371/journal.pone.0109681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylot M. 2005. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br. J. Nutr. 93(Suppl 1):S163–S168. doi: 10.1079/bjn20041339 [DOI] [PubMed] [Google Scholar]
- Budde E. F. 1952. The determination of fat in baked biscuit type of dog foods. J. Assoc. Off. Agric. Chem. 35:799–805. [Google Scholar]
- Chauvet A., Laclair J., Elliott D. A., and German A. J.. . 2011. Incorporation of exercise, using an underwater treadmill, and active client education into a weight management program for obese dogs. Can. Vet. J. 52:491–496. [PMC free article] [PubMed] [Google Scholar]
- Chen B., Lam K. S. L., Wang Y., Wu D., Lam M. C., Shen J., Wong L., Hoo R. L. C., Zhang J., and Xu A.. . 2006. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem. Biophys. Res. Commun. 341:549–556. doi: 10.1016/j.bbrc.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Cholewski M., Tomczykowa M., and Tomczyk M.. . 2018. A comprehensive review of chemistry, sources and biovailability of omega-3 fatty acids. Nutrients 10:1–33. doi: 10.3390/nu10111662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcier E. A., Thomson R. M., Mellor D. J., and Yam P. S.. . 2010. An epidemiological study of environmental factors associated with canine obesity. J. Small Anim. Pract. 51:362–367. doi: 10.1111/j.1748-5827.2010.00933.x [DOI] [PubMed] [Google Scholar]
- Davis H. C. 2018. Can the gastrointestinal microbiota be modulated by dietary fibre to treat obesity? Ir. J. Med. Sci. 187:393–402. doi: 10.1007/s11845-017-1686-9 [DOI] [PubMed] [Google Scholar]
- Diez M., Hornick J. L., Baldwin P., and Istasse L.. . 1997. Influence of a blend of fructo-oligosaccharides and sugar beet fiber on nutrient digestibility and plasma metabolite concentrations in healthy beagles. Am. J. Vet. Res. 58:1238–1242. [PubMed] [Google Scholar]
- Gayet C., Bailhache E., Dumon H., Martin L., Siliart B., and Nguyen P.. . 2004. Insulin resistance and changes in plasma concentration of TNF alpha, IGF1, and NEFA in dogs during weight gain and obesity. J. Anim. Physiol. Anim. Nutr. 88:157–165. doi: 10.1111/j.1439-0396.2003.00473.x [DOI] [PubMed] [Google Scholar]
- German A. J. 2006. The growing problem of obesity in dogs and cats. J. Nutr. 136:1940S–1946S. doi: 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- German A. J., Blackwell E., Evans M., and Westgarth C.. . 2017. Overweight dogs exercise less frequently and for shorter periods: results of a large online survey of dog owners from the UK. J. Nutr. Sci. 6:e11. doi: 10.1017/jns.2017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. L., Yamka R. M., Khoo C., Friesen K. G., Jewell D. E., Schoenherr W. D., Debraekeleer J., and Zicker S. C.. . 2010. Macronutrients. In: Hand M. S., Thatcher C. D., Remillard R. L., Roundebush P., and Novotny B. J., editors. Small animal clinical nutrition. 5th ed Topeka, (KS):Mark Morris Institute; p. 49–105. [Google Scholar]
- Hervik A. K., and Svihus B.. . 2019. The role of fiber in energy balance. J. Nutr. Metab. 4983657:1–11. doi: 10.1155/2019/4983657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger R., and Wakshlag J.. . 2011. The relationship of feeding patterns and obesity in dogs. J. Anim. Physiol. Anim. Nutr. (Berl). 95:98–105. doi: 10.1111/j.1439-0396.2010.01024.x [DOI] [PubMed] [Google Scholar]
- Jeusette I. C., Lhoest E. T., Istasse L. P., and Diez M. O.. . 2005. Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am. J. Vet. Res. 66:81–86. doi: 10.2460/ajvr.2005.66.81 [DOI] [PubMed] [Google Scholar]
- Kamolrat T., and Gray S. R.. . 2013. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 432:593–598. doi: 10.1016/j.bbrc.2013.02.041 [DOI] [PubMed] [Google Scholar]
- Kröger S., Vahjen W., and Zentek J.. . 2017. Influence of lignocellulose and low or high levels of sugar beet pulp on nutrient digestibility and the fecal microbiota in dogs. J. Anim. Sci. 95:1598–1605. doi: 10.2527/jas.2016.0873 [DOI] [PubMed] [Google Scholar]
- Laflamme D. 1997. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract. 25:13–18. [Google Scholar]
- Lepage G., and Roy C. C.. . 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27:114–120. [PubMed] [Google Scholar]
- Li G., Kawasumi K., Okada Y., Ishikawa S., Yamamoto I., Arai T., and Mori N.. . 2014. Comparison of plasma lipoprotein profiles and malondialdehyde between hyperlipidemia dogs with/without treatment. BMC Vet. Res. 10:67. doi: 10.1186/1746-6148-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot J. T. 2008. Sex hormones’ regulation of rodent physical activity: a review. Int. J. Biol. Sci. 4:126–132. doi: 10.7150/ijbs.4.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder D., and Mueller M.. . 2014. Pet obesity management: beyond nutrition. Vet. Clin. North Am. Small Anim. Pract. 44:789–806, vii. doi: 10.1016/j.cvsm.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Masood A., Stark K. D., and Salem N. Jr. 2005. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J. Lipid Res. 46:2299–2305. doi: 10.1194/jlr.D500022-JLR200 [DOI] [PubMed] [Google Scholar]
- McNeil J., and Constandy E.. . 2006. Addressing the problem of pet overpopulation: the experience of New Hanover County Animal Control Services. J. Public Health Manag. Pract. 12:452–455. doi: 10.1097/00124784-200609000-00008 [DOI] [PubMed] [Google Scholar]
- Mitsuhashi Y., Chamberlin A. J., Bigley K. E., and Bauer J. E.. . 2011. Maintenance energy requirement determination of cats after spaying. Br. J. Nutr. 106(Suppl 1):S135–S138. doi: 10.1017/S0007114511001899 [DOI] [PubMed] [Google Scholar]
- Morrison R., Penpraze V., Beber A., Reilly J. J., and Yam P. S.. . 2013. Associations between obesity and physical activity in dogs: a preliminary investigation. J. Small Anim. Pract. 54:570–574. doi: 10.1111/jsap.12142 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 2006. Energy. In: Nutrient requirements of dogs and cats. Washington (DC):National Academies Press; p. 28–48. [Google Scholar]
- Park H. J., Lee S. E., Oh J. H., Seo K. W., and Song K. H.. . 2014. Leptin, adiponectin and serotonin levels in lean and obese dogs. BMC Vet. Res. 10:113. doi: 10.1186/1746-6148-10-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pet Product News 2019. Pet obesity-related insurance claims increase for eighth consecutive year Available from http://www.petproductnews.com/News/Pet-obesity-related-insurance-claims-increase-for-eighth-consecutive-year. [accessed January 2, 2019].
- Prosky L., Asp N. G., Furda I., De Vries J. W., Schweizer T. F., and Harland B. F.. . 1985. Determination of total dietary fiber in foods and food products: collaborative study. J. AOAC. 68:677–679. [PubMed] [Google Scholar]
- Reichler I. M. 2009. Gonadectomy in cats and dogs: a review of risks and benefits. Reprod. Domest. Anim. 44(Suppl 2):29–35. doi: 10.1111/j.1439-0531.2009.01437.x [DOI] [PubMed] [Google Scholar]
- Respondek F., Swanson K. S., Belsito K. R., Vester B. M., Wagner A., Istasse L., and Diez M.. . 2008. Short-chain fructooligosaccharides influence insulin sensitivity and gene expression of fat tissue in obese dogs. J. Nutr. 138:1712–1718. doi: 10.1093/jn/138.9.1712 [DOI] [PubMed] [Google Scholar]
- Salt C., Morris P. J., Wilson D., Lund E. M., and German A. J.. . 2019. Association between life span and body condition in neutered client-owned dogs. J. Vet. Intern. Med. 33:89–99. doi: 10.1111/jvim.15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surampudi P., Enkhmaa B., Anuurad E., and Berglund L.. . 2016. Lipid-lowering with soluble dietary fibers. Curr. Atheroscler. Rep. 18:1–13. doi: 10.1007/s11883-016-0624-z [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Wang B., and Wood I. S.. . 2008. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 100:227–235. doi: 10.1017/S0007114508971282 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin Q-W., Zheng P-P., Zhang J-S., and Huang F-R.. . 2013. DHA inhibits protein degradation more efficiently than EPA by regulating the PPARγ/NFκB pathway in C2C12 myotubes. Biomed. Res. Int. 318981:1–9. doi: 10.1155/2013/318981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B. S., Wakshlag J. J., Maley M., Farrell T. J., Struble A. M., Panasevich M. R., and Wells M. T.. . 2011. Use of pedometers to measure the relationship of dog walking to body condition score in obese and non-obese dogs. Br. J. Nutr. 106(Suppl 1):S85–S89. doi: 10.1017/S0007114511001814 [DOI] [PubMed] [Google Scholar]
- Webb C. B., and Falkowski L.. . 2009. Oxidative stress and innate immunity in feline patients with diabetes mellitus: the role of nutrition. J. Feline Med. Surg. 11:271–276. doi: 10.1016/j.jfms.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Bissot T., Servet E., Sergheraert R., Biourge V., and German A. J.. . 2007. A high-protein, high-fiber diet designed for weight loss improves satiety in dogs. J. Vet. Intern. Med. 21:1203–1208. doi: 10.1892/07-016.1 [DOI] [PubMed] [Google Scholar]
- Yam P. S., Naughton G., Butowski C. F., and Root A. L.. . 2017. Inaccurate assessment of canine body condition score, body weight, and pet food labels: a potential cause of inaccurate feeding. Vet. Sci. 4:1–8. doi: 10.3390/vetsci4020030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X. 2017. Brain estrogens and feeding behavior. In: Mauvais-Jarvis F., editor. Sex and gender factors affecting metabolic homeostasis, diabetes and obesity, advances in experimental medicine and biology. Gewerbestrasse (Switzerland):Springer International Publishing AG; pp. 337–358. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.