Abstract
Expression of particular genes in hypothami of ewes was measured across the natural pubertal transition by in situ hybridization. The ewes were allocated to three groups (n = 4); prepubertal, postpubertal and postpubertally gonadectomized (GDX). Prepubertal sheep were euthanized at 20 weeks of age and postpubertal animals at 32 weeks. GDX sheep were also euthanized at 32 weeks, 1 week after surgery. Expression of KISS1, TAC3, PDYN in the arcuate nucleus (ARC), RFRP in the dorsomedial hypothalamus and GNRH1 in the preoptic area was quantified on a cellular basis. KISS1R expression by GNRH1 cells was quantified by double‐label in situ hybridization. Across puberty, detectable KISS1 cell number increased in the caudal ARC and whilst PDYN cell numbers were low, numbers increased in the rostral ARC. TAC3 expression did not change but RFRP expression/cell was reduced across puberty. There was no change across puberty in the number of GNRH1 cells that expressed the kisspeptin receptor (KISS1R). GDX shortly after puberty did not increase expression of any of the genes of interest. We conclude that KISS1 expression in the ARC increases during puberty in ewes and this may be a causative factor in the pubertal activation of the reproductive axis. A reduction in expression of RFRP may be a factor in the onset of puberty, removing negative tone on GNRH1 cells. The lack of changes in expression of genes following GDX suggest that the effects of gonadal hormones may differ in young and mature animals.
Keywords: gonadotropin releasing hormone, gonadotropins, hypothalamus
Kisspeptin is a major regulator of GnRH secretion and its function has been shown to be mandatory for pubertal transition. In this article, we examined the expression of KISS1, TAC3, PDYN, RFRP, GNRH1 and KISS1R in specific areas of hypothalamus across puberty in ewe. We conclude that KISS1 expression in the ARC increases during puberty in ewes and this may be a causative factor in the pubertal activation of the reproductive axis and a reduction in expression of RFRP may be a factor in the onset of puberty, removing negative tone on GNRH1 cells.

1. INTRODUCTION
Puberty is typified by an increase in the secretion of gonadotropin releasing hormone (GnRH) which drives an increase in gonadotropins secretion from the pituitary gland (Clarke & Pompolo, 2005; Ojeda, Roth, et al., 2006), leading to activation of the gonads. GnRH neurons are controlled by a number of interactive neuronal pathways which are regulated by internal signals and external cues (Clarke, 2015; Clarke & Arbabi, 2015; Clarke, Campbell, Smith, Prevot, & Wray, 2011; Ojeda, Lomniczi, et al., 2006; Tena‐Sempere, 2012; Terasawa & Fernandez, 2001), many of which may be involved in the process of puberty. Although much is known about neuronal systems that regulate GnRH secretion, the neurochemical basis of integrated control of puberty remains only partially understood. In higher primates, there is a “brake” on the secretion of GnRH and gonadotropins prior to puberty that is not due to feedback effects of steroids from the gonads (Plant, 2015; Plant & Shahab, 2002). In species such as the sheep, however, pulsatile GnRH secretion and gonadotropin secretion is apparent in the prepubertal period (I'Anson et al., 2000) but is held in check by an enhanced negative feedback action of estrogen (Foster & Ryan, 1979).
Since realization that kisspeptin is a major regulator of GnRH secretion (de Roux et al., 2003; Gottsch et al., 2004; Irwig et al., 2004; Messager et al., 2005; Seminara et al., 2003), its function was shown to be mandatory for pubertal transition (de Roux et al., 2003; Seminara et al., 2003). Nevertheless, the question as to whether altered function of kisspeptin is the primary and essential driver of the increase in GnRH that occurs at the time of puberty remains a matter of debate. There are two populations of kisspeptin neurons in hypothalamus which are differentially regulated by sex steroids. One population in the anteroventral periventricular nucleus (AVPV) in rodent or preoptic area (POA) in species such as sheep is involved in the positive feedback action of estrogen that causes the GnRH/luteinizing hormone (LH) surge in females (Hoffman, Le, Franceschini, Caraty, & Advis, 2011; Robertson, Clifton, Iglesia, Steiner, & Kauffman, 2009; Smith, Li, Pereira, & Clarke, 2009; Smith, Popa, Clifton, Hoffman, & Steiner, 2006). A second group of kisspeptin cells is located in the arcuate nucleus (ARC) and relays the negative feedback effects of sex steroid on GnRH secretion in both sexes (Franceschini et al., 2006; Smith, Cunningham, Rissman, Clifton, & Steiner, 2005). In sheep, kisspeptin neurons in caudal ARC also initiate the positive feedback effect of estrogen on GnRH secretion (Estrada, Clay, Pompolo, Smith, & Clarke, 2006; Smith, 2008; Smith et al., 2009), which is potentiated by activation of the preoptic kisspeptin neurons at the time of the surge (Hoffman et al., 2011).
Mutations in either the kisspeptin gene (KISS1) or the kisspeptin receptor (KISS1R) in humans and gene knockout in mice causes pubertal failure (d'Anglemont de Tassigny & Colledge, 2010; Dungan Lemko & Elias, 2012; de Roux et al., 2003; Seminara et al., 2003; Topaloglu et al., 2012). Nevertheless, a range of other gene mutations can also cause failure of puberty (Silveira, Trarbach, & Latronico, 2010). Developmental changes in KISS1 expression have been described in many species, with variable patterns within and between species. In mice and rats, KISS1 is expressed in the ARC before birth but some studies did not detect significant changes in expression in the ARC at the time of puberty in either species (Gill et al., 2010; Han et al., 2005; Navarro et al., 2012). Others (Molnar et al., 2016) have reported an increase in expression of KISS1 in male mice at the time of puberty. Increased KISS1 expression in the early stages of puberty has been observed in both male and female monkeys and rats (Bentsen et al., 2010; Shahab et al., 2005; Takase et al., 2009). No change in KISS1 expression was observed in the ARC of female pigs across puberty (Ieda et al., 2014).
In female sheep, the enhanced negative feedback of estradiol‐17β (E2) in the prepubertal period is lost at puberty onset (Foster & Ryan, 1979). One study showed that, in ovariectomized (OVX)‐E2‐treated ewes, escape from the estrogenic “clamp” occurs at 35 weeks, typified by a marked increase in pulsatile LH secretion (Redmond et al., 2011). There was, however, no change in KISS1 expression in the ARC of these animals, although an increase was observed in the POA by 30 weeks. In another study, immunohistochemistry showed the presence of more kisspeptin cells in ARC in adult ewes (>9 month) than in prepubertal ewes (5–6 months) (Nestor et al., 2012), consistent with the notion that kisspeptin may facilitate if not initiate puberty. No studies have been performed on gonad‐intact ewes across the pubertal transition to determine any potential change in KISS1 gene expression.
Kisspeptin cells of the ARC also express PDYN and TAC3, the genes encoding dynorphin (DYN) and neurokinin B (NKB) (Goodman et al., 2007), leading to nomenclature of “KNDY” cells (Lehman, Coolen, & Goodman, 2010; Maeda et al., 2010; Navarro et al., 2009; Rance, Krajewski, Smith, Cholanian, & Dacks, 2010). NKB may act in an “autocrine” manner to stimulate kisspeptin secretion, whereas DYN inhibits KNDY cell function (Goodman, Coolen, & Lehman, 2014; Grachev et al., 2014; Sakamoto et al., 2012). Mutations in TAC3 or the NKB receptor cause hypogonadotropic hypogonadism in humans (Gianetti et al., 2010; Topaloglu et al., 2009). NKB agonists can stimulate LH secretion prior to puberty in rats (Ruiz‐Pino et al., 2012), ewes (Nestor et al., 2012) and juvenile primates (Ramaswamy et al., 2010). In rats, developmental changes of TAC3 expression show a steady increase from birth until the juvenile stage, but there is little change in the transition through puberty (Navarro et al., 2012). Most recently, it has been proposed that NKB/kisspeptin interaction is enhanced during the pubertal transition in female, but not male rhesus monkeys (Garcia, Keen, Kenealy, Seminara, & Terasawa, 2018). Immunoreactive NKB cell numbers were seen to be similar in prepubertal (5–6 months) and postpubertal ewes (>9 months) (Nestor et al., 2012).
DYN has an inhibitory effect on GnRH neurons as do other opioid peptides (Goodman et al., 2004; Navarro et al., 2009; Yen, Quigley, Reid, Ropert, & Cetel, 1985). This may be due to its action on kisspeptin cells or directly upon GnRH cells, which express the relevant receptor (Weems et al., 2016). In sheep, DYN neurons in the ARC appear to mediate the negative feedback effect of progesterone on GnRH/LH pulse secretion (Goodman et al., 2004, 2011; Goodman, Parfitt, Evans, Dahl, & Karsch, 1995). Thus, the numbers of DYN neurons are reduced in OVX ewes and restored by progesterone treatment (Foradori, Goodman, Adams, Valent, & Lehman, 2005). This is consistent withresults in human studies (Rometo & Rance, 2008), but not with data on manipulation by sex steroids in rats and nonhuman primates (Eghlidi, Haley, Noriega, Kohama, & Urbanski, 2010; Navarro et al., 2009). There are few studies of the role that DYN might play during puberty, but administration of a kappa receptor antagonist did advance puberty in female rats (Nakahara et al., 2013).
Gonadotropin inhibitory hormone (GnIH), also known as RF‐amide‐related peptide (RFRP) is a negative regulator of GnRH/gonadotropin function in various species, including sheep (Clarke et al., 2008, 2011, 2012; Clarke & Parkington, 2014; Clarke & Smith, 2010; Gibson et al., 2008; Johnson, Tsutsui, & Fraley, 2007; Kadokawa et al., 2009; Kriegsfeld et al., 2006; Murakami et al., 2008; Tsutsui et al., 2000). Administration of GnIH antisense oligonucleotide to juvenile male rats increased plasma LH levels and testicular weight (Johnson & Fraley, 2008) and RFRP receptor knock‐out mice have elevated plasma LH levels (Leon et al., 2014). In both studies, animals were fertile and had normal pubertal onset, providing no strong evidence that a change in GnIH function is important in reproductive development. On the other hand, RFRP expression changes with development in rodents, with a postnatal rise, a peak in expression at the time of puberty, and a decline in adulthood in both rats and mice (Iwasa et al., 2012; Poling, Kim, Dhamija, & Kauffman, 2012; Quennell, Rizwan, Relf, & Anderson, 2010). Strangely, RFRP‐3 stimulates LH secretion in male mice but is inhibitory in females (Ancel, Inglis, & Anderson, 2017). How this relates to control of the onset of puberty in either sex is not clear.
This study aimed to clarify the role of kisspeptin, NKB, DYN in the ARC, and RFRP in the DMH across puberty in ewes by measuring expression of the genes for the peptides. In addition, we examined if removal of sex steroid feedback by GDX shortly after puberty indicated whether the feedback loops evident in adult animals are fully developed at this time.
2. MATERIAL AND METHODS
All sheep were maintained at the Monash University Sheep Facility (Werribee) and the experiments were carried out in accordance with the Code of Practice for the Care and Use of Animals for Experimental Purposes provided by the National Health and Medical Research Council/Commonwealth Scientific and Industrial Research Organisation/Australian Animal Commission. The work was approved by the Monash University, School of Biomedical Sciences Animal Ethics Committee.
2.1. Animals and experimental design
Corriedale ewes were born in September in the Southern Hemisphere and were allocated into three groups (n = 4), being prepubertal, postpubertal and postpubertally gonadectomized (GDX). Prepubertal sheep were euthanized at 20 weeks (February). The estrous cycles of postpubertal intact ewes were synchronized by an injection (i.m.) of a synthetic prostaglandin (Estrumate, 125 µg; Pitman‐Moore) and were euthanized 10 days later during luteal phase at 32 weeks of age (April). The ovaries were examined and corpora lutea and large follicles were observed in all postpubertal ewes. Gonadectomies were performed one week prior to euthanizing at 32 weeks. Prior to perfusion, three jugular venous blood samples were collected and LH was measured as described previously (Lee et al., 1976). Body weights and plasma LH levels are shown in Table 1.
TABLE 1.
Mean (±SEM) body weights and plasma LH concentrations of ewes prior to and postpuberty and after GDX
| Prepuberty | Postpuberty | Postpuberty GDX | |
|---|---|---|---|
| Bodyweight (kg) | 27.25 ± 0.66 | 28.38 ± 0.80 | 30.88 ± 0.43 |
| LH (ng/ml) | 0.18 ± 0.09 | 0.33 ± 0.07 | 1.1 ± 0.41 |
2.2. Tissue processing
The sheep were euthanized with an IV overdose of sodium pentobarbital (Lethabarb; Virbarc) and the brains were perfused with paraformaldehyde as described previously (Smith et al., 2009). Hypothalami were dissected out of the brains and postfixed for 24 hr at 4°C, and then placed in phosphate buffer containing 30% sucrose for 7 days. The hypothalamic blocks were frozen and stored at −20°C. Cryostat sections were cut at 30 µm and stored in cryoprotectant solution at −20°C.
2.3. In situ hybridization
Antisense riboprobes for KISS1, TAC3, PDYN, RFRP, GNRH1, and KISS1R were synthesized with SP6 or T7 transcription kits (Ambion), using corresponding DNA fragments as templates. A 262bp antisense riboprobe for TAC3 (Genbank accession number XM_004006562.1, bases 31–292) was prepared as described previously (Li, Millar, Clarke, & Smith, 2015). For PDYN, a 200 bp fragment of the bovine gene in pCRII (Genbank accession number U58500.1) was provided as a gift by Dr. Hong Jiang, University of Missouri); the use of this probe in sheep has been reported previously (Iqbal, Henry, Pompolo, Rao, & Clarke, 2003). The KISS1‐specific template spanned bases 1–357 of the partial ovine cDNA sequence (GenBank accession no. DQ059506) and a 460 bp cDNA sequence of the ovine RFRP (bases 43–502, GenBank accession no.NM_001127268) was cloned as previously described (Clarke et al., 2008). The KISS1R‐specific template spanned bases 6–636 of ovine cDNA sequence (GenBank accession no. EU272411). The GNRH1‐specific template spanned bases 18–169 of the ovine partial cDNA sequence (GenBank accession No. U02517).
In situ hybridization was performed as described previously (Simmons, Arriza, & Swanson, 1989) using 35S‐labeled probes. For each sheep and each gene, three sections were selected for analysis. ARC sections were selected to represent the rostral, middle, and caudal regions. The caudal section was 150–300 µm from mammillary recess of the third ventricle and the middle and rostral sections are 600 µm apart. For sections used in RFRP detection, rostrocaudal sections of PVN/DMH were chosen: the caudal section was 300–450 µm from mammillary recess of the third ventricle and the middle and rostral sections are 600 µm apart. These were mounted onto SuperFrost plus slides and air‐dried overnight. Following 0.001% proteinase K treatment for 30 min at 37°C, sections were acetylated with 0.0025% acetic anhydride in 0.1 M TEA for 10 min. After rinsing in 2 × SSC, the sections were dehydrated through ascending series of ethanol, delipidated in chloroform, rinsed in absolute ethanol, and air‐dried. The hybridization solutions containing 35S labeled antisense probe (5 × 106 cpm/ml) in a cocktail solution of 50% formamide, 5 × SSC, pH 7.0, 250 µg/ml herring sperm DNA, 100 µg/ml yeast tRNA, 5% dextran sulfate, 1× Denhardt's solution, 0.1% Tween‐20 was applied to sections and hybridized overnight at 53°C. After hybridization, sections were washed in decreasing concentrations of SSC, dehydrated, and coated with emulsion (Ilford Imaging). Exposure was 1 week in the dark at 4°C.
Double label in situ hybridization using DIG‐labeled GNRH1 and 35S‐labeled KISS1R riboprobes was performed as described previously (Li, Goodchild, Seyedabadi, & Pilowsky, 2005; Li, Rao, Pereira, Clarke, & Smith, 2011; Smith et al., 2011). Rostral, medial, and caudal regions were hybridized with the DIG‐GnRH1 riboprobe and the 35S‐labeled KISS1R riboprobes (5 × 106 cpm/ml) at 53°C overnight. After posthybridization washes with descending concentrations of citrate acid and NaCl (SSC), sections were rinsed twice in Tris‐buffered saline (TBS) (0.1 M Tris–HCl, 0.9% NaCl, pH 7.4). The GNRH1 expressing neurons were revealed with an alkaline phosphatase conjugated goat anti‐digoxigenin antibody (dilution 1:1,000; Roche) and a colorimetric solution of nitro‐blue tetrazolium and 5‐bromo‐4‐chloro‐3‐indolyl phosphate salts (Roche). The 35S signal for KISS1R was revealed on GnRH neurons, as silver grain staining. The sections were coated with 3% parlodion in isoamyl acetate, dried, dipped in photographic emulsion (Ilford Imaging), and left at 4°C for 5 weeks. Grain‐counting software (Image‐Pro plus; Media Cybernetics) was used to count the number of KISS1R mRNA silver grains over each GnRH cell under darkfield illumination. The signal‐to‐noise ratio was set at the 3× background.
For each gene, a sense probe, using the same template as antisense, was used as a negative control to assess nonspecific hybridization.
2.4. Microscopy
Image analysis was carried out using randomly coded slides under dark‐field illumination with software designed to count the total number of cells and the number of silver grains per cell (ImagePro plus, Media Cybernetics, Inc.). Cells were counted when silver grain density was three times greater than the background. Data are expressed as the mean number of identifiable cells/section and the mean number of silver grains/cell (a semiquantitative index of mRNA expression/cell). Densitometry (expression/cell) for PDYN was not performed as there were very few cells detected in the ARC.
2.5. LH Radioimmunoassay
Plasma LH concentrations were measured in duplicate, using the method of Lee et al. (1976). Assay sensitivity was 0.1 ng/ml and the intra‐assay coefficient of variation (CV) was less than 10% over the range of 0.6–15 ng/ml.
2.6. Statistical analysis
All grouped data are presented as the means (±SEM). Statistical analyses were conducted after checking for heterogeneity of variance, by one‐way ANOVA. Differences were considered significant at p < .05.
3. RESULTS
Plasma LH levels increased from 0.33 ± 0.07 ng/ml to 1.1 ± 0.41 ng/ml following GDX (p < .05) (Table 1).
Examples of the in situ hybridization signal for KISS1, TAC3, PDYN, and RFRP are shown in Figure 1. These images indicate high signal‐to‐noise and clear silver grain concentration over the relevant cells. For all probes, no signal was observed after the application of radioactive‐labeled sense probes.
FIGURE 1.
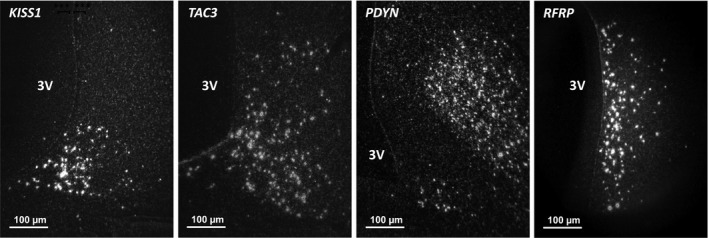
Representative microphotographs of the in situ hybridization signal for KISS1, TAC3, PDYN in arcuate nucleus (ARC) and RFRP in the dorsomedial hypothalamus
3.1. KISS1, TAC3, PDYN, and RFRP expression
KISS1 cell number in the caudal ARC was higher (p < .05) in postpubertal ewes than in prepubertal ewes (Figures 2 and 3a), with no change in expression/cell (Figure 3a). GDX did not change KISS1 expression in postpubertal females (Figure 3a).
FIGURE 2.
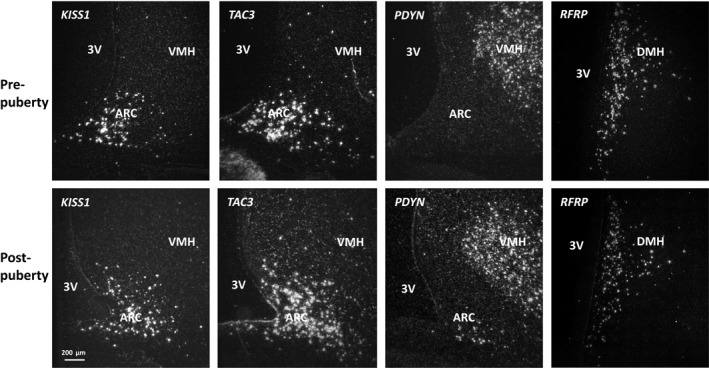
Examples of KISS1, TAC3, PDYN gene expression at the same level in arcuate nucleus and RFRP in the dorsalmedial nucleus prior to and after puberty in ewes. Note the fewer number of PDYN expressing cells. ARC, arcuate nucleus; VMH, ventromedial hypothalamus; DMH, dorsalmedial hypothalamus; 3V, third ventricle
FIGURE 3.
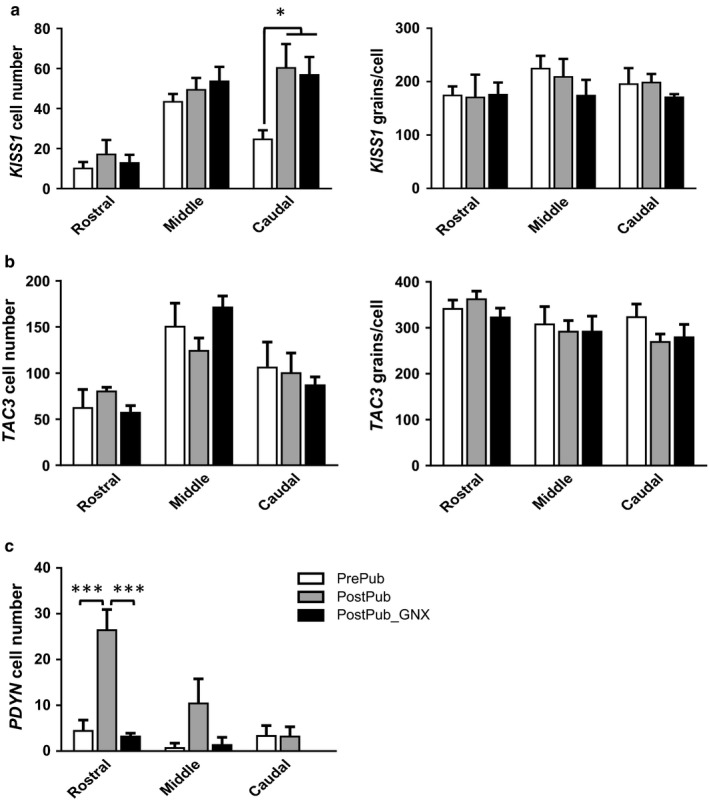
Mean (±SEM) expression of KISS1 (a), TAC3 (b), and PDYN (c) in the ARC of ewes prior to and postpuberty and following GDX. Panels on the left show cell number and those on the right show expression/cell (silver grains/cell) in rostral mid and caudal sections of the ARC. *p < .05, ***p < .001
The number of TAC3 cells and the expression of TAC3/cell was similar in prepubertal, postpubertal intact and GDX ewes (Figures 2 and 3b).
PDYN mRNA was highly expressed in supraoptic nucleus (SOR), dorsal medial hypothalamus (DMH) (data not shown), and the ventromedial hypothalamus (Figure 2). PDYN expression was low in the ARC, with the number of cells being less than observed for KISS1 and TAC3 cells (Figures 2 and 3). PDYN cell number increased (p < .01) in the rostral ARC across puberty and GDX reduced cell numbers (Figure 3c, p < .001).
The number of RFRP‐expressing cells was similar in prepubertal, postpubertal intact, and GDX females, but RFRP expression/cell was lower (p < .01) after puberty at all levels of the dorsomedial nucleus (Figure 4). The level of expression after puberty was not affected by GDX (Figure 4).
FIGURE 4.
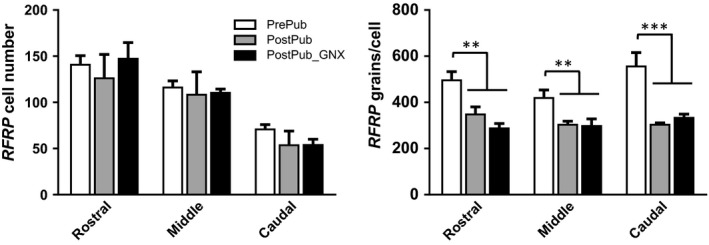
Mean (±SEM) RFRP gene expression in the DMH of ewes prior to and postpuberty and following GDX. Panels on the left show cell number and those on the right show expression/cell (silver grains/cell) in rostral mid and caudal sections of the dorsomedial nucleus. **p < .01, ***p < .001
3.2. KISS1R expression in GNRH1 cells
The number of GNRH1 expressing neurons was similar in pre and postpubertal sheep (intact or GDX (data not shown). Here 35S labeled KISS1R expression was distinctively visible on digoxigenin‐labeled GnRH neurons (Figure 5a). KISS1R expression/cell and the percentage of GnRH neurons that expressed KISS1R were similar before and after puberty (Figure 5b).
FIGURE 5.
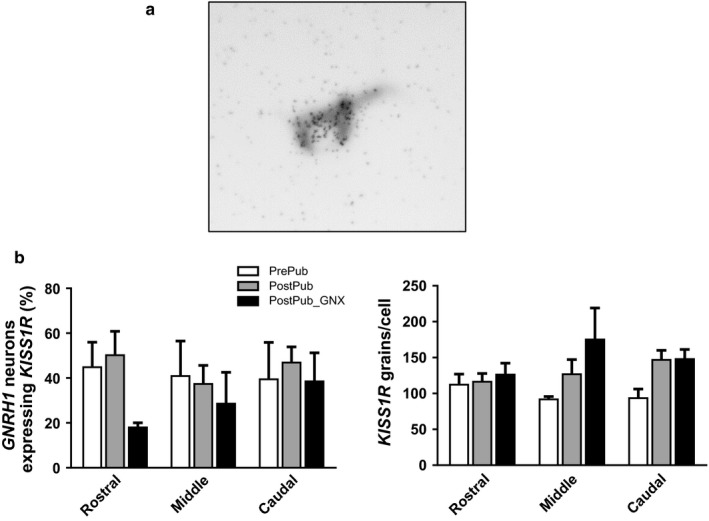
KISS1R expression in GnRH1 cells. Panel A shows co‐localization of KISS1R (silver grains; 35S‐labeled riboprobe) in a Digoxigenin‐labeled GNRH1 neuron visualized in gray‐scale. Panel B shows mean (±SEM) % KISSR expression by GNRH1 cells (left panel) and silver grain density per cell (right panel)
4. DISCUSSION
It is generally accepted that kisspeptin signaling is mandatory for puberty (d'Anglemont de Tassigny & Colledge, 2010; Dungan Lemko & Elias, 2012; de Roux et al., 2003; Seminara et al., 2003; Topaloglu et al., 2012), but as to whether an increase in expression of KISS1 or KISS1R is seen at this time across all species is questionable. In this study, we focused on the KISS1 cells of the ARC of the sheep, because an increase in expression.in the POA was recorded in earlier work (Redmond et al., 2011). The present data show that the number of cells expressing KISS1 in the caudal ARC is higher shortly after puberty in female sheep. The increased number of KISS1 expression in the ARC of gonad‐intact ewes after puberty is consistent with the increased expression seen in the POA of OVX ewes‐bearing estrogen implants (Redmond et al., 2011). The rise in KISS1 function in the POA at the time of puberty seems most likely dependent on the action of estrogen on KISS1 neurons in this location, based on studies in mice (Clarkson, Han, Liu, Lee, & Herbison, 2010). On the other hand, some studies report no change in expression of KISS1 was seen across the pubertal period in female pigs or in mice and rats of either sex (see Introduction).
We found no change of expression of KISS1 in the ARC of ewes after GDX soon after puberty, which contrasts with our earlier results obtained with mature ewes (Smith, Clay, Caraty, & Clarke, 2007). This may be because the animals of this study were euthanized one week after GDX or it could be due to there being relatively less influence of gonadal steroids on KISS1 expression in the early postpubertal stage of development. Certainly there is overwhelming evidence that KISS1 expression in the ARC increases with GDX of either sex in adult rodents (Irwig et al., 2004; Kauffman et al., 2007; Navarro et al., 2009; Smith, Cunningham, et al., 2005; Smith, Dungan, et al., 2005) and an increase is also seen in female primates after GDX or menopause (Kim, Jessen, Auger, & Terasawa, 2009; Rometo & Rance, 2008). Despite there being no increase in KISS1 after GDX, the plasma levels of LH increased in both males and females. This suggests that the negative feedback effects of gonadal steroids on GnRH secretion may be mediated by neural elements other than KNDY cells. Certainly, a number of neuronal systems express estrogen receptors (Clarke & Tilbrook, 2009) and could be involved in the suppression of GnRH/LH secretion at this time. In addition, the gonadotropes express sex steroid receptors and negative feedback is effected at that level (Clarke, Cummins, Crowder, & Nett, 1989).
The percentage of GNRH1 cells expressing KISS1R did not change across puberty. The lack of effect of GDX is consistent with results in male rhesus monkeys at the time of expected puberty (Shahab et al., 2005), with similar results in male mice (Molnar et al., 2016) and female rats (Adachi et al., 2007). Neither does KISS1R expression change in female rhesus monkeys in the transition to menopause (Kim et al., 2009). On balance, it seems most likely that the transition through puberty is associated with upregulation of kisspeptin activity rather than that of its receptor.
Regarding TAC3 expression we found no change across puberty or after GDX, consistent with immunohistochemical data on female sheep (Nestor et al., 2012). Our data also concur with data obtained from rats (Navarro et al., 2012). Others (Gill et al., 2012) presented data from female mice and showed that TAC3 and its receptor (TAC3R) were most likely markers of pubertal activation but were not triggers for puberty while data from male mice showed no change in TAC3R (Molnar et al., 2016). Overall, there seems to be no significant role for NKB in the initiation of puberty in either sex, even though mutations in TAC3 and TAC3R genes lead to reproductive failure (Silveira et al., 2010).
Around the time of puberty, the number of detectable PDYN neurons was lower than for KISS1 and TAC3 neurons, which is consistent with other data obtained in female sheep showing very few PDYN cells in the prepubertal ewe (Lopez et al., 2016). The striking difference in the number of neurons expressing TAC3, KISS1, and PDYN at this stage of development is interesting, considering that the three peptides co‐localized in the KNDy neurons in the adult sheep (Goodman et al., 2007). This suggests that the neuroendocrine axis governing reproductive function is different in young and adult animals and, as suggested earlier (Lopez et al., 2016), a rise in progesterone levels may be necessary for induction of PDYN expression, perhaps explaining why the number of cells increased markedly in the rostral ARC, following puberty. In spite of the low number of cells, we showed that GDX reduced the number of PDYN cells. These data are consistent with a fundamental role for this peptide in the negative control of pulsatile GnRH secretion. The low cell number precluded meaningful quantification of the level of expression/cell.
A steady increase in GnIH levels in the hypothalami of male and female rats has been seen across development (Iwasa et al., 2012). Likewise, Poling et al (Poling & Kauffman, 2012) showed a steady increase of GnIH levels in a subpopulation of GnIH neurons in mice of both sexes, whereas Quennell et al (Quennell et al., 2010) showed that RFRP expression in the dorsomedial nucleus of male mice peaked at 4 weeks. Nevertheless, this maximal level of expression at 4 weeks was followed by a progressive decline up to 8 weeks, although this was not statistically significant. In another study of mice, a marked increase in RFRP cell number was observed at 3 weeks of age in males, with a progressive decline occurring between 7 and 13 weeks of age (Sethi, Tsutsui, & Chaturvedi, 2010). We observed a reduction in the level of expression of RFRP/cell in females after puberty which is not consistent with the aforementioned data obtained in rats and mice but is consistent with there being a release from inhibitory influence on GnRH cells at the time of puberty. When considering the role of GnIH in control of reproduction, it should be noted that this peptide is stimulatory in males but inhibitory in females (Ancel et al., 2017). The lack of effect of ovariectomy in females is similar to results obtained in mice (Iwasa et al., 2012; Quennell et al., 2010).
In conclusion, our data from this study indicate an increase in the level of expression of KISS1 and a reduction in expression of RFRP around the time of puberty in ewes. PDYN expression is increased across the pubertal transition with no change in expression of TAC3. Because of the prominent role that kisspeptin is thought to play in puberty, we also measured KISS1R expression in GnRH cells prior to and after puberty, but there were no significant changes in the percentage of cells expressing the receptor and the expression of mRNA. In addition to the role that the genes investigated in this study play in the pubertal transition, other work strongly suggests that a fundamental switch in expression of transcriptional regulators are the drivers of puberty (Lomniczi et al., 2013). Further studies of such regulators would be warranted in a range of species if a comprehensive understanding of puberty is to be elucidated; these could include neuronal systems involved in brain sensing of bodyweight, which are also involved in regulation of reproduction. Expression of relevant receptors by Kisspeptin neurons and the level of input from modulatory afferents would also be informative. Finally, GDX had little effect on the expression of the genes of interest, suggesting that feedback effects of gonadal steroids at this time are different to those seen in the mature animal.
CONFLICT OF INTEREST
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGMENTS
We thank Bruce Doughton, Lynda Morrish, and Alexandra Rao for their technical assistance.
Li Q, Smith JT, Henry B, Rao A, Pereira A, Clarke IJ. Expression of genes for Kisspeptin (KISS1), Neurokinin B (TAC3), Prodynorphin (PDYN), and gonadotropin inhibitory hormone (RFRP) across natural puberty in ewes. Physiol Rep. 2020;8:e14399 10.14814/phy2.14399
REFERENCES
- Adachi, S. , Yamada, S. , Takatsu, Y. , Matsui, H. , Kinoshita, M. , Takase, K. , … Maeda, K. (2007). Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. Journal of Reproduction and Development, 53, 367–378. [DOI] [PubMed] [Google Scholar]
- Ancel, C. , Inglis, M. A. , & Anderson, G. M. (2017). Central RFRP‐3 stimulates LH secretion in male mice and has cycle stage‐dependent inhibitory effects in females. Endocrinology, 158, 2873–2883. [DOI] [PubMed] [Google Scholar]
- Bentsen, A. H. , Ansel, L. , Simonneaux, V. , Tena‐Sempere, M. , Juul, A. , & Mikkelsen, J. D. (2010). Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides, 31, 275–283. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. (2015). Hypothalamus as an endocrine organ. Comprehensive Physiology, 5, 217–253. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , & Arbabi, L. (2015). New concepts of the central control of reproduction, integrating influence of stress, metabolic state and season. Domestic Animal Endocrinology, 56, S165–S179. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , Campbell, R. , Smith, J. T. , Prevot, V. , & Wray, S. (2011). Neuroendocrine control of reproduction In Fink G., Pfaff D., & Levine J. (Eds.), Handbook of neuroendocrinology (pp. 198–235). London, UK: Elsevier. [Google Scholar]
- Clarke, I. J. , Cummins, J. T. , Crowder, M. E. , & Nett, T. M. (1989). Long‐term negative feedback effects of oestrogen and progesterone on the pituitary gland of the long‐term ovariectomized ewe. Journal of Endocrinology, 120, 207–214. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , & Parkington, H. C. (2014). Gonadotropin inhibitory hormone (GnIH) as a regulator of gonadotropes. Molecular and Cellular Endocrinology, 385, 36–44. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , & Pompolo, S. (2005). Synthesis and secretion of GnRH. Animal Reproduction Science, 88, 29–55. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , Sari, I. P. , Qi, Y. , Smith, J. T. , Parkington, H. C. , Ubuka, T. , … Bentley, G. E. (2008). Potent action of RFamide‐related peptide‐3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology, 149, 5811–5821. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , & Smith, J. T. (2010). The role of kisspeptin and gonadotropin inhibitory hormone (GnIH) in the seasonality of reproduction in sheep. Society for Reproduction and Fertility Supplement, 67, 159–169. [PubMed] [Google Scholar]
- Clarke, I. J. , Smith, J. T. , Henry, B. A. , Oldfield, B. J. , Stefanidis, A. , Millar, R. P. , … Fraley, G. S. (2012). Gonadotropin‐inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology, 95, 305–316. [DOI] [PubMed] [Google Scholar]
- Clarke, I. J. , & Tilbrook, A. J. (2009). Gonadotropin, neural and hormonal control. Oxford: Academic Press, Elsevier. [Google Scholar]
- Clarkson, J. , Han, S. K. , Liu, X. , Lee, K. , & Herbison, A. E. (2010). Neurobiological mechanisms underlying kisspeptin activation of gonadotropin‐releasing hormone (GnRH) neurons at puberty. Molecular and Cellular Endocrinology, 324, 45–50. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny, X. , Colledge, W. H. (2010). The role of kisspeptin signaling in reproduction. Physiology, 25, 207–217. [DOI] [PubMed] [Google Scholar]
- de Roux, N. , Genin, E. , Carel, J. C. , Matsuda, F. , Chaussain, J. L. , & Milgrom, E. (2003). Hypogonadotropic hypogonadism due to loss of function of the KiSS1‐derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America, 100, 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan Lemko, H. M. , & Elias, C. F. (2012). Kiss of the mutant mouse: How genetically altered mice advanced our understanding of kisspeptin's role in reproductive physiology. Endocrinology, 153, 5119–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghlidi, D. H. , Haley, G. E. , Noriega, N. C. , Kohama, S. G. , & Urbanski, H. F. (2010). Influence of age and 17beta‐estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate‐median eminence of female rhesus macaques. Endocrinology, 151, 3783–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, K. M. , Clay, C. M. , Pompolo, S. , Smith, J. T. , & Clarke, I. J. (2006). Elevated KiSS‐1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin‐releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen‐positive feedback. Journal of Neuroendocrinology, 18, 806–809. [DOI] [PubMed] [Google Scholar]
- Foradori, C. D. , Goodman, R. L. , Adams, V. L. , Valent, M. , & Lehman, M. N. (2005). Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology, 146, 1835–1842. [DOI] [PubMed] [Google Scholar]
- Foster, D. L. , & Ryan, K. D. (1979). Endocrine mechanisms governing transition into adulthood: A marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology, 105, 896–904. [DOI] [PubMed] [Google Scholar]
- Franceschini, I. , Lomet, D. , Cateau, M. , Delsol, G. , Tillet, Y. , & Caraty, A. (2006). Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co‐express estrogen receptor alpha. Neuroscience Letters, 401, 225–230. [DOI] [PubMed] [Google Scholar]
- Garcia, J. P. , Keen, K. L. , Kenealy, B. P. , Seminara, S. B. , & Terasawa, E. (2018). Role of kisspeptin and neurokinin B signaling in male rhesus monkey puberty. Endocrinology, 159, 3048–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianetti, E. , Tusset, C. , Noel, S. D. , Au, M. G. , Dwyer, A. A. , Hughes, V. A. , … Seminara, S. B. (2010). TAC3/TACR3 mutations reveal preferential activation of gonadotropin‐releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. Journal of Clinical Endocrinology and Metabolism, 95, 2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, E. M. , Humber, S. A. , Jain, S. , Williams III, W. P. , Zhao, S. , Bentley, G. E. , … Kriegsfeld, L. J. (2008). Alterations in RFamide‐related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology, 149, 4958–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, J. C. , Navarro, V. M. , Kwong, C. , Noel, S. D. , Martin, C. , Xu, S. , … Kaiser, U. B. (2012). Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid‐negative feedback than Kiss1. Endocrinology, 153, 4883–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, J. C. , Wang, O. , Kakar, S. , Martinelli, E. , Carroll, R. S. , & Kaiser, U. B. (2010). Reproductive hormone‐dependent and ‐independent contributions to developmental changes in kisspeptin in GnRH‐deficient hypogonadal mice. PLoS ONE, 5, e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R. L. , Coolen, L. M. , Anderson, G. M. , Hardy, S. L. , Valent, M. , Connors, J. M. , … Lehman, M. N. (2004). Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin‐releasing hormone neurons in sheep. Endocrinology, 145, 2959–2967. [DOI] [PubMed] [Google Scholar]
- Goodman, R. L. , Coolen, L. M. , & Lehman, M. N. (2014). A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology, 99, 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R. L. , Holaskova, I. , Nestor, C. C. , Connors, J. M. , Billings, H. J. , Valent, M. , … Hileman, S. M. (2011). Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology, 152, 3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R. L. , Lehman, M. N. , Smith, J. T. , Coolen, L. M. , de Oliveira, C. V. , Jafarzadehshirazi, M. R. , … Clarke, I. J. (2007). Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology, 148, 5752–5760. [DOI] [PubMed] [Google Scholar]
- Goodman, R. L. , Parfitt, D. B. , Evans, N. P. , Dahl, G. E. , & Karsch, F. J. (1995). Endogenous opioid peptides control the amplitude and shape of gonadotropin‐releasing hormone pulses in the ewe. Endocrinology, 136, 2412–2420. [DOI] [PubMed] [Google Scholar]
- Gottsch, M. L. , Cunningham, M. J. , Smith, J. T. , Popa, S. M. , Acohido, B. V. , Crowley, W. F. , … Steiner, R. A. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology, 145, 4073–4077. [DOI] [PubMed] [Google Scholar]
- Grachev, P. , Li, X. F. , Hu, M. H. , Li, S. Y. , Millar, R. P. , Lightman, S. L. , & O'Byrne, K. T. (2014). Neurokinin B signaling in the female rat: A novel link between stress and reproduction. Endocrinology, 155, 2589–2601. [DOI] [PubMed] [Google Scholar]
- Han, S. K. , Gottsch, M. L. , Lee, K. J. , Popa, S. M. , Smith, J. T. , Jakawich, S. K. , … Herbison, A. E. (2005). Activation of gonadotropin‐releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. Journal of Neuroscience, 25, 11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, G. E. , Le, W. W. , Franceschini, I. , Caraty, A. , & Advis, J. P. (2011). Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology, 152, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I'Anson, H. , Manning, J. M. , Herbosa, C. G. , Pelt, J. , Friedman, C. R. , Wood, R. I. , … Foster, D. L. (2000). Central inhibition of gonadotropin‐releasing hormone secretion in the growth‐restricted hypogonadotropic female sheep. Endocrinology, 141, 520–527. [DOI] [PubMed] [Google Scholar]
- Ieda, N. , Uenoyama, Y. , Tajima, Y. , Nakata, T. , Kano, M. , Naniwa, Y. , … Tsukamura, H. (2014). KISS1 gene expression in the developing brain of female pigs in pre‐ and peripubertal periods. Journal of Reproduction and Development, 60, 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, J. , Henry, B. A. , Pompolo, S. , Rao, A. , & Clarke, I. J. (2003). Long‐term alteration in bodyweight and food restriction does not affect the gene expression of either preproorexin or prodynorphin in the sheep. Neuroscience, 118, 217–226. [DOI] [PubMed] [Google Scholar]
- Irwig, M. S. , Fraley, G. S. , Smith, J. T. , Acohido, B. V. , Popa, S. M. , Cunningham, M. J. , … Steiner, R. A. (2004). Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS‐1 mRNA in the male rat. Neuroendocrinology, 80, 264–272. [DOI] [PubMed] [Google Scholar]
- Iwasa, T. , Matsuzaki, T. , Murakami, M. , Kinouchi, R. , Osugi, T. , Gereltsetseg, G. , … Tsutsui, K. (2012). Developmental changes in the mammalian gonadotropin‐inhibitory hormone (GnIH) ortholog RFamide‐related peptide (RFRP) and its cognate receptor GPR147 in the rat hypothalamus. International Journal of Developmental Neuroscience, 30, 31–37. [DOI] [PubMed] [Google Scholar]
- Johnson, M. A. , & Fraley, G. S. (2008). Rat RFRP‐3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS‐1 gene expression or the onset of puberty in male rats. Neuroendocrinology, 88, 305–315. [DOI] [PubMed] [Google Scholar]
- Johnson, M. A. , Tsutsui, K. , & Fraley, G. S. (2007). Rat RFamide‐related peptide‐3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Hormones and Behavior, 51, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadokawa, H. , Shibata, M. , Tanaka, Y. , Kojima, T. , Matsumoto, K. , Oshima, K. , & Yamamoto, N. (2009). Bovine C‐terminal octapeptide of RFamide‐related peptide‐3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domestic Animal Endocrinology, 36, 219–224. [DOI] [PubMed] [Google Scholar]
- Kauffman, A. S. , Gottsch, M. L. , Roa, J. , Byquist, A. C. , Crown, A. , Clifton, D. K. , … Tena‐Sempere, M. (2007). Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology, 148, 1774–1783. [DOI] [PubMed] [Google Scholar]
- Kim, W. , Jessen, H. M. , Auger, A. P. , & Terasawa, E. (2009). Postmenopausal increase in KiSS‐1, GPR54, and luteinizing hormone releasing hormone (LHRH‐1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides, 30, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld, L. J. , Mei, D. F. , Bentley, G. E. , Ubuka, T. , Mason, A. O. , Inoue, K. , … Silver, R. (2006). Identification and characterization of a gonadotropin‐inhibitory system in the brains of mammals. Proceedings of the National Academy of Sciences of the United States of America, 103, 2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, V. W. , Cumming, I. A. , de Kretser, D. M. , Findlay, J. K. , Hudson, B. , & Keogh, E. J. (1976). Regulation of gonadotrophin secretion in rams from birth to sexual maturity. I. Plasma LH, FSH and testosterone levels. Journal of Reproduction and Fertility, 46, 1–6. [DOI] [PubMed] [Google Scholar]
- Lehman, M. N. , Coolen, L. M. , & Goodman, R. L. (2010). Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin‐releasing hormone secretion. Endocrinology, 151, 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon, S. , Garcia‐Galiano, D. , Ruiz‐Pino, F. , Barroso, A. , Manfredi‐Lozano, M. , Romero‐Ruiz, A. , … Tena‐Sempere, M. (2014). Physiological roles of gonadotropin‐inhibitory hormone signaling in the control of mammalian reproductive axis: Studies in the NPFF1 receptor null mouse. Endocrinology, 155, 2953–2965. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Goodchild, A. K. , Seyedabadi, M. , & Pilowsky, P. M. (2005). Pre‐protachykinin A mRNA is colocalized with tyrosine hydroxylase‐immunoreactivity in bulbospinal neurons. Neuroscience, 136, 205–216. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Millar, R. P. , Clarke, I. J. , & Smith, J. T. (2015). Evidence that neurokinin B controls basal gonadotropin‐releasing hormone secretion but is not critical for estrogen‐positive feedback in sheep. Neuroendocrinology, 101, 161–174. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Rao, A. , Pereira, A. , Clarke, I. J. , & Smith, J. T. (2011). Kisspeptin cells in the ovine arcuate nucleus express prolactin receptor but not melatonin receptor. Journal of Neuroendocrinology, 23, 871–882. [DOI] [PubMed] [Google Scholar]
- Lomniczi, A. , Loche, A. , Castellano, J. M. , Ronnekleiv, O. K. , Bosch, M. , Kaidar, G. , … Ojeda, S. R. (2013). Epigenetic control of female puberty. Nature Neuroscience, 16, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, J. A. , Bedenbaugh, M. N. , McCosh, R. B. , Weems, P. W. , Meadows, L. J. , Wisman, B. , … Hileman, S. M. (2016). Does dynorphin play a role in the onset of puberty in female sheep? Journal of Neuroendocrinology, 28 10.1111/jne.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, K. , Ohkura, S. , Uenoyama, Y. , Wakabayashi, Y. , Oka, Y. , Tsukamura, H. , & Okamura, H. (2010). Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Research, 1364, 103–115. [DOI] [PubMed] [Google Scholar]
- Messager, S. , Chatzidaki, E. E. , Ma, D. , Hendrick, A. G. , Zahn, D. , Dixon, J. , … Aparicio, S. A. (2005). Kisspeptin directly stimulates gonadotropin‐releasing hormone release via G protein‐coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America, 102, 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, C. S. , Sarvari, M. , Vastagh, C. , Maurnyi, C. , Fekete, C. , Liposits, Z. , & Hrabovszky, E. (2016). Altered gene expression profiles of the hypothalamic arcuate nucleus of male mice suggest profound developmental changes in peptidergic signaling. Neuroendocrinology, 103, 369–382. [DOI] [PubMed] [Google Scholar]
- Murakami, M. , Matsuzaki, T. , Iwasa, T. , Yasui, T. , Irahara, M. , Osugi, T. , & Tsutsui, K. (2008). Hypophysiotropic role of RFamide‐related peptide‐3 in the inhibition of LH secretion in female rats. Journal of Endocrinology, 199, 105–112. [DOI] [PubMed] [Google Scholar]
- Nakahara, T. , Uenoyama, Y. , Iwase, A. , Oishi, S. , Nakamura, S. , Minabe, S. , … Tsukamura, H. (2013). Chronic peripheral administration of kappa‐opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. Journal of Reproduction and Development, 59, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, V. M. , Gottsch, M. L. , Chavkin, C. , Okamura, H. , Clifton, D. K. , & Steiner, R. A. (2009). Regulation of gonadotropin‐releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. Journal of Neuroscience, 29, 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, V. M. , Ruiz‐Pino, F. , Sanchez‐Garrido, M. A. , Garcia‐Galiano, D. , Hobbs, S. J. , Manfredi‐Lozano, M. , … Tena‐Sempere, M. (2012). Role of neurokinin B in the control of female puberty and its modulation by metabolic status. Journal of Neuroscience, 32, 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor, C. C. , Briscoe, A. M. , Davis, S. M. , Valent, M. , Goodman, R. L. , & Hileman, S. M. (2012). Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology, 153, 2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda, S. R. , Lomniczi, A. , Mastronardi, C. , Heger, S. , Roth, C. , Parent, A. S. , … Mungenast, A. E. (2006). Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology, 147, 1166–1174. [DOI] [PubMed] [Google Scholar]
- Ojeda, S. R. , Roth, C. , Mungenast, A. , Heger, S. , Mastronardi, C. , Parent, A. S. , … Jung, H. (2006). Neuroendocrine mechanisms controlling female puberty: New approaches, new concepts. International Journal of Andrology, 29, 256–263; discussion 286–90. [DOI] [PubMed] [Google Scholar]
- Plant, T. M. (2015). Neuroendocrine control of the onset of puberty. Frontiers in Neuroendocrinology, 38, 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant, T. M. , & Shahab, M. (2002). Neuroendocrine mechanisms that delay and initiate puberty in higher primates. Physiology & Behavior, 77, 717–722. [DOI] [PubMed] [Google Scholar]
- Poling, M. C. , & Kauffman, A. S. (2012). Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin‐Kiss1r and GnRH signaling. Endocrinology, 153, 782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling, M. C. , Kim, J. , Dhamija, S. , & Kauffman, A. S. (2012). Development, sex steroid regulation, and phenotypic characterization of RFamide‐related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology, 153, 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell, J. H. , Rizwan, M. Z. , Relf, H. L. , & Anderson, G. M. (2010). Developmental and steroidogenic effects on the gene expression of RFamide related peptides and their receptor in the rat brain and pituitary gland. Journal of Neuroendocrinology, 22, 309–316. [DOI] [PubMed] [Google Scholar]
- Ramaswamy, S. , Seminara, S. B. , Ali, B. , Ciofi, P. , Amin, N. A. , & Plant, T. M. (2010). Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology, 151, 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance, N. E. , Krajewski, S. J. , Smith, M. A. , Cholanian, M. , & Dacks, P. A. (2010). Neurokinin B and the hypothalamic regulation of reproduction. Brain Research, 1364, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond, J. S. , Baez‐Sandoval, G. M. , Spell, K. M. , Spencer, T. E. , Lents, C. A. , Williams, G. L. , & Amstalden, M. (2011). Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. Journal of Neuroendocrinology, 23, 815–822. [DOI] [PubMed] [Google Scholar]
- Robertson, J. L. , Clifton, D. K. , de la Iglesia, H. O. , Steiner, R. A. , & Kauffman, A. S. (2009). Circadian regulation of Kiss1 neurons: Implications for timing the preovulatory gonadotropin‐releasing hormone/luteinizing hormone surge. Endocrinology, 150, 3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo, A. M. , & Rance, N. E. (2008). Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. Journal of Neuroendocrinology, 20, 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Pino, F. , Navarro, V. M. , Bentsen, A. H. , Garcia‐Galiano, D. , Sanchez‐Garrido, M. A. , Ciofi, P. , … Tena‐Sempere, M. (2012). Neurokinin B and the control of the gonadotropic axis in the rat: Developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology, 153, 4818–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, K. , Murata, K. , Wakabayashi, Y. , Yayou, K. , Ohkura, S. , Takeuchi, Y. , … Okamura, H. (2012). Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non‐breeding season. Journal of Reproduction and Development, 58, 700–706. [DOI] [PubMed] [Google Scholar]
- Seminara, S. B. , Messager, S. , Chatzidaki, E. E. , Thresher, R. R. , Acierno Jr, J. S. , Shagoury, J. K. , … Colledge Jr, W. H. (2003). The GPR54 gene as a regulator of puberty. New England Journal of Medicine, 349, 1614–1627. [DOI] [PubMed] [Google Scholar]
- Sethi, S. , Tsutsui, K. , & Chaturvedi, C. M. (2010). Age‐dependent variation in the RFRP‐3 neurons is inversely correlated with gonadal activity of mice. General and Comparative Endocrinology, 168, 326–332. [DOI] [PubMed] [Google Scholar]
- Shahab, M. , Mastronardi, C. , Seminara, S. B. , Crowley, W. F. , Ojeda, S. R. , & Plant, T. M. (2005). Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America, 102, 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira, L. F. , Trarbach, E. B. , & Latronico, A. C. (2010). Genetics basis for GnRH‐dependent pubertal disorders in humans. Molecular and Cellular Endocrinology, 324, 30–38. [DOI] [PubMed] [Google Scholar]
- Simmons, D. M. , Arriza, J. L. , & Swanson, L. W. (1989). A complete protocol for in situ hybridization of messager RNA in brain and other tissue with radiolabeled single‐stranded RNA probes. Journal of Histotechnology, 12, 169–181. [Google Scholar]
- Smith, J. T. (2008). Kisspeptin signalling in the brain: Steroid regulation in the rodent and ewe. Brain Research Reviews, 57, 288–298. [DOI] [PubMed] [Google Scholar]
- Smith, J. T. , Clay, C. M. , Caraty, A. , & Clarke, I. J. (2007). KiSS‐1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology, 148, 1150–1157. [DOI] [PubMed] [Google Scholar]
- Smith, J. T. , Cunningham, M. J. , Rissman, E. F. , Clifton, D. K. , & Steiner, R. A. (2005). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology, 146, 3686–3692. [DOI] [PubMed] [Google Scholar]
- Smith, J. T. , Dungan, H. M. , Stoll, E. A. , Gottsch, M. L. , Braun, R. E. , Eacker, S. M. , … Steiner, R. A. (2005). Differential regulation of KiSS‐1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology, 146, 2976–2984. [DOI] [PubMed] [Google Scholar]
- Smith, J. T. , Li, Q. , Pereira, A. , & Clarke, I. J. (2009). Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology, 150, 5530–5538. [DOI] [PubMed] [Google Scholar]
- Smith, J. T. , Li, Q. , Yap, K. S. , Shahab, M. , Roseweir, A. K. , Millar, R. P. , & Clarke, I. J. (2011). Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology, 152, 1001–1012. [DOI] [PubMed] [Google Scholar]
- Smith, J. T. , Popa, S. M. , Clifton, D. K. , Hoffman, G. E. , & Steiner, R. A. (2006). Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. Journal of Neuroscience, 26, 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase, K. , Uenoyama, Y. , Inoue, N. , Matsui, H. , Yamada, S. , Shimizu, M. , … Maeda, K. I. (2009). Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. Journal of Neuroendocrinology, 21, 527–537. [DOI] [PubMed] [Google Scholar]
- Tena‐Sempere, M. (2012). Deciphering puberty: Novel partners, novel mechanisms. European Journal of Endocrinology, 167, 733–747. [DOI] [PubMed] [Google Scholar]
- Terasawa, E. , & Fernandez, D. L. (2001). Neurobiological mechanisms of the onset of puberty in primates. Endocrine Reviews, 22, 111–151. [DOI] [PubMed] [Google Scholar]
- Topaloglu, A. K. , Reimann, F. , Guclu, M. , Yalin, A. S. , Kotan, L. D. , Porter, K. M. , … Semple, R. K. (2009). TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nature Genetics, 41, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu, A. K. , Tello, J. A. , Kotan, L. D. , Ozbek, M. N. , Yilmaz, M. B. , Erdogan, S. , … Yuksel, B. (2012). Inactivating KISS1 mutation and hypogonadotropic hypogonadism. New England Journal of Medicine, 366, 629–635. [DOI] [PubMed] [Google Scholar]
- Tsutsui, K. , Saigoh, E. , Ukena, K. , Teranishi, H. , Fujisawa, Y. , Kikuchi, M. , … Sharp, P. J. (2000). A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochemical and Biophysical Research Communications, 275, 661–667. [DOI] [PubMed] [Google Scholar]
- Weems, P. W. , Witty, C. F. , Amstalden, M. , Coolen, L. M. , Goodman, R. L. , & Lehman, M. N. (2016). kappa‐Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology, 157, 2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, S. S. , Quigley, M. E. , Reid, R. L. , Ropert, J. F. , & Cetel, N. S. (1985). Neuroendocrinology of opioid peptides and their role in the control of gonadotropin and prolactin secretion. American Journal of Obstetrics and Gynecology, 152, 485–493. [DOI] [PubMed] [Google Scholar]


