Abstract
Fibrosis is a pathophysiological hallmark of cardiorenal disease. In the heart, fibrosis leads to contractile dysfunction and arrhythmias; in the kidney, it is the final common pathway for many diseases and predicts end‐stage renal failure. Despite this, there are currently no specific anti‐fibrotic treatments available for cardiac or renal disease. Recently and unexpectedly, IL‐11 was found to be of major importance for cardiorenal fibroblast activation and fibrosis. In mouse models, IL‐11 overexpression caused fibrosis of the heart and kidney while genetic deletion of Il11ra1 protected against fibrosis and preserved organ function. Neutralizing antibodies against IL‐11 or IL‐11RA have been developed that have anti‐fibrotic activity in human fibroblasts and protect against fibrosis in murine models of disease. While IL‐11 biology has been little studied and, we suggest, largely misunderstood, its autocrine activity in myofibroblasts appears non‐redundant for fibrosis, which offers new opportunities to better understand and potentially target cardiorenal fibrosis.
Abbreviations
- OSM
oncostatin M
- LIF
leukaemia inhibitory factor
- CNTF
ciliary neurotrophic factor
- CLCF
cardiotrophin‐like cytokine
- RSK
40S ribosomal protein S6 kinase
- ACTA2
α‐smooth muscle actin
- ECM
extra‐cellular matrix
- PCTS
precision cut tissue slices
1. FIBROSIS: A FINAL COMMON PATHWAY UNDERLYING CARDIAC AND RENAL FAILURE
Fibrosis occurs in response to tissue injury. While this may be adaptive in the short term, prolonged or uncontrolled fibrogenesis leads to parenchymal disruption and loss of tissue function, eventually resulting in organ failure (Rockey, Bell, & Hill, 2015a). Two organs notably affected by fibrosis are the heart and the kidney, where the resulting cardiac and renal failure are significant contributors to global morbidity and mortality (Rockey et al., 2015a; Rosenbloom, Macarak, Piera‐Velazquez, & Jimenez, 2017).
Myocardial fibrosis contributes to both systolic and, particularly, diastolic ventricular impairment, resulting in increased myocardial stiffness, impaired relaxation and eventually contractile dysfunction (González, Schelbert, Díez, & Butler, 2018; Moreo et al., 2009). A collagenous scar can also slow conduction, form micro‐reentrant circuits and produce triggered activity (Rockey et al., 2015a) to promote malignant ventricular arrhythmias (Chen et al., 2015; Iles et al., 2011). Consequently, the presence of ventricular fibrosis is a major predictor of sudden cardiac death (Gulati et al., 2013; Halliday et al., 2017; Musa et al., 2018). Fibrosis is also a pathophysiological hallmark of atrial fibrillation (Gal & Marrouche, 2017; Kottkamp, 2012), which has a prevalence of ~9% in those >65 years old and is a major risk factor for stroke (Piccini et al., 2012; Staerk, Sherer, Ko, Benjamin, & Helm, 2017). In the conduction system, fibrosis causes bradyarrhythmia and heart block, a substantial cause of morbidity in the elderly (Csepe, Kalyanasundaram, Hansen, Zhao, & Fedorov, 2015; Kerola et al., 2019). Risk factors for cardiac fibrosis are diverse and include hypertension (Cuspidi, Ciulla, & Zanchetti, 2006), ischaemic heart disease (Hinderer & Schenke‐Layland, 2019), aortic stenosis (Bing et al., 2019; Katbeh et al., 2018), inherited cardiomyopathy (Gulati et al., 2013; Ho et al., 2010), diabetes (Russo & Frangogiannis, 2016), and ageing (Lu et al., 2017).
Chronic kidney disease is defined as a persistent loss of renal function and is a growing global health problem, affecting ~13% of the world's population (Jager & Fraser, 2017). Renal fibrosis occurs in both the glomerulus (glomerulosclerosis) and the tubulointerstitium and is the final common pathway for a diverse range of aetiologies which lead to chronic kidney disease (including infection, ischaemia, diabetes, autoimmune disease, physical obstruction of the urinary tract and toxic or drug insults; Djudjaj & Boor, 2019; Knoppert, Valentijn, Nguyen, Goldschmeding, & Falke, 2019). Fibrosis of the interstitium predicts the progression of chronic kidney disease to end‐stage renal failure and may play a role in the transition of acute to chronic renal failure (Hewitson, Holt, & Smith, 2017; Rodríguez‐Iturbe, Johnson, & Herrera‐Acosta, 2005).
Despite its importance for disease, there are currently no treatments that specifically target cardiac or renal fibrosis. Of note, the majority of cardio‐renal fibrotic diseases are more common with increasing age—in particular, chronic kidney disease, diastolic heart failure, atrial fibrillation, aortic stenosis, and cardiac conduction system disease (Figure 1; Chiao, Lakatta, Ungvari, Dai, & Rabinovitch, 2016; O'Sullivan, Hughes, & Ferenbach, 2017). In an increasingly ageing population, the use of effective anti‐fibrotic treatments will be important for increasing healthy lifespan.
Figure 1.
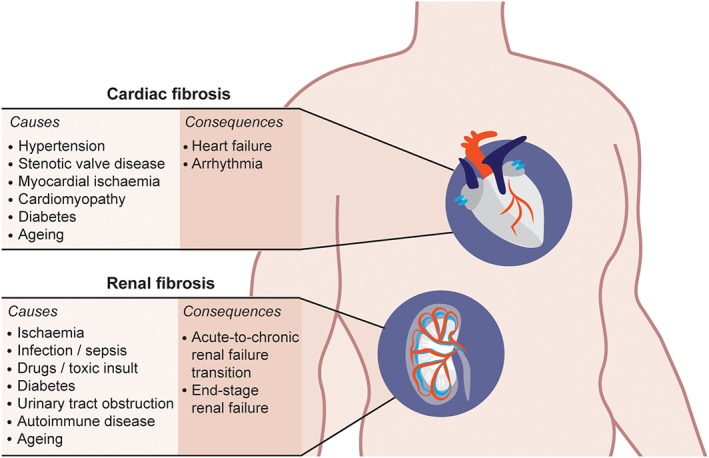
Overview of heart and kidney diseases defined by fibrosis and the consequent effects on organ function
2. CELLULAR AND MOLECULAR MECHANISMS OF FIBROSIS
The mechanisms for fibrosis consist of a complex medley of interacting cellular and molecular systems, but a key point of convergence for all forms of fibrosis is the transdifferentiation of fibroblasts into myofibroblasts (Rockey, Bell, & Hill, 2015b; Rosenbloom et al., 2017). Myofibroblasts display two specific features: Firstly, they secrete extracellular matrix which constitutes fibrotic scar (predominantly type I and type III collagen and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6754). Secondly, they are contractile—via the expression of α‐smooth muscle actin (ACTA2)—thus causing tissue contraction, increased tissue stiffness, and the parenchymal distortion characteristic of fibrotic organs (Rosenbloom et al., 2017; Wynn, 2008).
The molecular factors involved in fibrosis are wide ranging, including https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1803, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8927, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2504 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=989 (Rockey et al., 2015b; Wynn, 2008). But the core pathway, involved in virtually all types of fibrosis, is the https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5060 signalling cascade (Meng, Nikolic‐Paterson, & Lan, 2016; Rockey et al., 2015b). However, inhibition of TGF‐β—either directly or indirectly—is associated with side effects due to its pleiotropic role across various cell types (Bierie et al., 2009; Shull et al., 1992). Repeated efforts to target TGF‐β family members in clinical trials have failed either due to lack of efficacy, likely reflecting dose‐limiting toxicities, or due to on‐target toxicity itself (Group, C. A. T. Trabeculectomy Study et al., 2007; Voelker et al., 2017). More recently, trials targeting the processing of the latency‐associated peptide—as an indirect means of inhibiting TGF‐β with suggested less toxicity—were discontinued due to, yet again, safety issues (Keown, 2019).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4976 is a less frequently studied cytokine which has only recently been shown to be an important downstream regulator of TGF‐β in cardiorenal fibrosis (Schafer et al., 2017). Here, we will suggest that treatments targeting IL‐11 have the potential to more safely prevent, treat, and perhaps even reverse fibrotic cardiorenal disease.
3. IL‐11 IS AN IL‐6 FAMILY CYTOKINE WITH DISTINCT BIOLOGICAL FUNCTIONS
The IL‐6 family of cytokines includes https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998, IL‐11, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6151, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5035 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5016 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4906‐1, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4895 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4897; Murakami, Kamimura, & Hirano, 2019; Rose‐John, 2018). These cytokines have traditionally been grouped together as they all signal via the ubiquitously expressed signalling receptor subunit glycoprotein 130 kDa (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2317). IL‐6 and IL‐11 form a complex with a gp130 heterodimer, whereas other members of the family form homodimers (Garbers & Scheller, 2013; Taga & Kishimoto, 1997). This has led to a belief that the functions of some of these cytokines are partly overlapping and redundant. However, specificity is provided by unique, high‐affinity receptor subunits leading to a diversity in biological function which is apparent from genetics: mutations affecting the function of the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1714 lead to familial primary localized cutaneous amyloidosis (Arita et al., 2008), whereas mutations in the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=307#1713 cause Stüve‐Wiedemann syndrome, a severe form of bent‐bone dysplasia which typically causes death in early life (Dagoneau et al., 2004). Null mutations in CNTF cause motor neuron degeneration in mice (Masu et al., 1993) but do not cause disease in humans (Takahashi et al., 1994). While IL‐6 has been very extensively studied, the functions of the other IL‐6 family cytokines remain less well understood.
IL‐11 is most often compared to IL‐6 as both initiate signalling by forming ostensibly similar hexameric complexes with their cognate receptors and gp130. In both cases, the cytokine first binds with its “α” receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1708 or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1709) either at the membrane (classical signalling) or, at least for IL‐6, in solution (trans‐signalling). This complex then interacts with gp130 molecules, triggering their dimerization and subsequent downstream signalling (Garbers & Scheller, 2013; Taga & Kishimoto, 1997). However, important structural differences have been found between IL‐6 and IL‐11, suggesting that IL‐11 may engage gp130 differently to IL‐6, with potential consequences for downstream effects (Putoczki, Dobson, & Griffin, 2014). Indeed, IL‐6 is known to signal predominantly via the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=581/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=990 pathway (Taga & Kishimoto, 1997). However, recent work in fibroblasts found that IL‐11 causes sustained https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 activation, without physiologically relevant activation of STAT (Ng et al., 2019; Schafer et al., 2017; Widjaja, Sing, et al., 2019). It has also been shown that IL‐11 at high concentrations can activate STAT in transformed cell lines (Lu et al., 1994) and some primary cells, although some of these cell lines do not appear to express the IL‐11RA, which requires further study.
Importantly, IL‐6RA and IL‐11RA differ in their expression pattern across cell types, indeed almost mutually exclusively so in the FANTOM datasets, in support of the concept that IL‐11 and IL‐6 activate distinct target cell populations (Schafer et al., 2017). IL‐6RA is most highly expressed on immune cells and as such has become an important target for treating immune‐mediated diseases, such as rheumatoid arthritis. IL‐6RA has also been detected in transformed hepatic cells such as HepG2. In contrast, IL‐11RA is most highly expressed on stromal cells including fibroblasts, vascular smooth muscle cells, adipocytes, hepatic/pancreatic stellate cells, and pericytes, as wells as on polarized cells such as hepatocytes, alveolar epithelial cells, and kidney tubular epithelial cells (Ng et al., 2019; Schafer et al., 2017; Widjaja, Sing, et al., 2019).
In keeping with these differences, the effect of loss‐of‐function mutations in the IL‐6 pathway varies greatly from those in the IL‐11 pathway. Humans with homozygous loss‐of‐function mutations in IL‐6R suffer from severe immune dysregulation (Spencer et al., 2019), and predicted loss‐of‐function mutations in IL‐6 or IL‐6R are selected against in the general population. Accordingly, one of the most frequent on‐target side effects of anti‐IL‐6 therapy is infection (Khanna et al., 2016). In contrast, predicted loss‐of‐function mutations in IL‐11 or IL‐11RA in the general population are not selected against and are as common as expected by chance (Karczewski et al., 2019). Individuals with biallelic (homozygous or compound heterozygous) null mutations in IL‐11RA sometimes have abnormalities of delayed tooth eruption, mild craniosynostosis, scoliosis, and joint laxity but are otherwise healthy, with no increased risk of cancer, wound healing, cardiovascular disease, immune dysfunction or infection (Keupp et al., 2013; Miller et al., 2017; Nieminen et al., 2011; Papachristoforou, Petrou, Sawyer, Williams, & Drousiotou, 2014). It is also notable that while IL‐6 is expressed in healthy adults, IL‐11 is not, suggesting that IL‐11 plays a limited role in healthy adult humans.
4. DISCOVERY OF IL‐11 AND DEVELOPMENT OF RECOMBINANT HUMAN IL‐11 TO TREAT THROMBOCYTOPAENIA
IL‐11 was initially characterized as a stromal‐derived cytokine able to stimulate megakaryocyte colony formation and also to exert an inhibitory action on adipogenesis in the bone marrow micro‐environment (Kawashima et al., 1991; Kawashima & Takiguchi, 1992; Keller, Du, Srour, Hoffman, & Williams, 1993; Paul et al., 1990). The initial characterization as a haematopoietic cytokine inspired studies of IL‐11 on platelet production, which it was found to increase in vivo. This chance finding led to the development of recombinant human IL‐11 (rhIL‐11) for the treatment of chemotherapy‐induced thrombocytopenia (Isaacs et al., 1997).
However, it was later found that IL11ra1 knockout mice and humans with a null mutation in the IL11RA gene display normal blood cell counts (Brischoux‐Boucher et al., 2018; Nandurkar et al., 1997), indicating that IL‐11 signalling is, in fact, redundant for normal haematopoiesis. IL‐11 alone was found in some studies not to directly stimulate megakaryocytes (Teramura, Kobayashi, Hoshino, Oshimi, & Mizoguchi, 1992), and long‐term anti‐IL‐11 therapy in mice has no effect on platelet counts (Widjaja, Singh, et al., 2019). Following its development as a therapeutic agent in the 1990s, rhIL‐11 became readily available as a tool for the scientific community and was used extensively to study the effects of rhIL‐11 in preclinical rodent models. Unfortunately, as we discuss in detail below, recombinant IL‐11 has species‐specific activity (Schafer et al., 2017; Widjaja, Dong, et al., 2019), and the use of rhIL‐11 in mice and rats has led to misunderstanding as to the true biological function of IL‐11.
5. REDISCOVERY OF IL‐11 IN HUMAN CARDIAC FIBROBLASTS: A MASTER SWITCH FOR FIBROSIS
As discussed, TGF‐β1 is a major driver of fibrosis across organs and disease aetiologies, inducing the transition of fibroblasts into activated myofibroblasts which express ACTA2 and deposit ECM (Akhurst & Hata, 2012; Rockey et al., 2015b). However, the ability to target TGF‐β1 to reduce fibrosis is limited by on‐target side effects (Bierie et al., 2009; Shull et al., 1992). Therefore, to look for potential drug targets downstream of TGF‐β1 with the potential to block fibrosis while avoiding toxicities outside of the fibroblast, Schafer et al. (2017) combined quantification of fibroblast activation and ECM production with RNA sequencing of TGF‐β1‐stimulated, versus unstimulated, primary human cardiac fibroblast cultures. Unexpectedly, IL‐11 up‐regulation by more than eightfold was the dominant genome‐wide response to TGF‐β1 stimulation, and its expression was strongly correlated with fibroblast activation. By comparison, IL‐6 expression in fibroblasts was largely unchanged by TGF‐β1. Stimulating primary human fibroblasts directly with rhIL‐11 was strongly pro‐fibrotic—increasing ECM production and myofibroblast numbers, motility, contraction, and invasion. Further in vitro studies showed that IL‐11 protein secretion from primary cardiac fibroblasts is a common feature of stimulation by diverse pro‐fibrotic stimuli, including PDGF, OSM, angiotensin II, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4924, endothelin‐1, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4980, and connective tissue growth factor (Figure 2). Finally, the ability of all of these factors (and TGF‐β1) to activate fibroblasts and ECM secretion can be blocked by antibodies against IL‐11/IL‐11RA.
Figure 2.

In fibroblasts, IL‐11 acts at a point of signalling convergence downstream of multiple and diverse stimuli and is the nexus between pro‐fibrotic initiating factors and organ fibrosis. ANG II, angiotensin II; bFGF, basic FGF; CTGF, connective tissue growth factor; ET‐1, endothelin 1; OSM, oncostatin M
6. IL‐11 SIGNALS VIA THE ERK PATHWAY IN FIBROBLASTS TO ACTIVATE PRO‐FIBROTIC COMPONENTS POST‐TRANSCRIPTIONALLY
In contrast to TGF‐β, which has profound effects on transcription, RNA‐seq data have consistently shown negligible differences in mRNA levels following IL‐11 stimulation of fibroblasts (Ng et al., 2019; Schafer et al., 2017). This is somewhat unexpected, as both IL‐6 and IL‐11 signal via a gp130 homodimer, which results in canonical JAK/STAT activation downstream of IL‐6 to initiate a pro‐inflammatory RNA expression profile. In contrast to this, in fibroblasts IL‐11 was found to drive pro‐fibrotic gene expression at the post‐transcriptional level, which results in myofibroblast activation and secretion of ECM proteins. This effect appears to be dependent on the phosphorylation of ERK and its downstream targets—including https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=541 and eurkaryotic translation initiation factor 4E (eIF4E)—which are involved in activation of protein translation (Schafer et al., 2017). MEK/ERK inhibitors (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5282 and PD98509) block the pro‐fibrotic effect of IL‐11 on fibroblasts, and TGF‐β stimulation is unable to activate ERK in fibroblasts isolated from IL‐11ra1−/− mice (Ng et al., 2019; Schafer et al., 2017). Interestingly, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2994—considered the canonical signalling pathway for IL‐11—is only mildly and transiently phosphorylated after IL‐11 stimulation in primary fibroblasts, and this has little measurable transcriptional consequences. Further insights into IL‐11 signalling mechanisms have recently been elucidated by a study suggesting that IL‐11 may mediate translation of proline‐rich proteins (such as collagen or IL‐11 itself) via activation of glutamyl‐prolyl‐tRNA synthetase (EPRS) (Wu, Subbaiah, Xie, Jiang, & Mickelsen, 2019).
In primary human cardiac fibroblasts, the post‐transcriptional gene expression programme dependent on autocrine IL‐11 signalling is a necessary requirement for fibrogenesis, downstream of multiple pro‐fibrotic stimuli (Figure 2). Previous studies suggest that IL‐11 activates myofibroblasts and results in ECM secretion post‐transcriptionally via ERK (Figure 3), but other non‐canonical pathways may also play a role. It is also unclear whether signalling downstream of IL‐11 is different across cell types and states. In addition to stromal cells, many polarized and epithelial cells—such as cardiac myocytes, hepatocytes, alveolar, and tubular epithelial cells—also express IL‐11RA and pathways of activation following IL‐11 binding may vary.
Figure 3.

Putative IL‐11 signalling pathways in fibroblasts. In contrast to TGFβ (and other upstream pro‐fibrotic stimuli), IL‐11 has a negligible effect on transcription in fibroblasts. Instead, IL‐11 signals via ERK to enhance translation of pro‐fibrotic proteins while also possibly employing EPRS activation to translate proline rich proteins where ribosomes can stall on proline repeat regions (PRRs). ACTA2, actin α2 (aka smooth muscle actin); COL1A2, collagen type 1 α2; eIF4E, eurkaryotic translation initiation factor 4E; EPRS, glutamyl‐prolyl‐tRNA synthetase; gp130, glycoprotein 130; IL‐11RA: IL‐11 receptor α; MNK, MAPK‐interacting serine/threonine‐protein kinase 1; p90RSK, p90 ribosomal S6 kinase; POSTN, periostin; TGFβ Rec, TGF β receptor
7. ROLE OF IL‐11 IN EPITHELIAL VERSUS STROMAL CELLS
In this review, we concentrate mainly on the pro‐fibrotic role of IL‐11 in stromal cells (fibroblasts). However, it should be noted that there is evidence of IL‐11 release from and signalling within epithelial cells, typically in response to acute injury. For example, respiratory syncytial virus is a potent stimulus for IL‐11 secretion from lung epithelium (Einarsson, Geba, Zhu, Landry, & Elias, 1996; Elias et al., 1994). Similarly, IL‐11 is released from hepatocytes after injury with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239) and can then act in an autocrine manner to induce ROS and cell death (Widjaja, Dong, et al., 2019). In epithelial cells isolated from lung organoid models of idiopathic pulmonary fibrosis, IL‐11 expression is increased and is hypothesized to have a role in epithelial‐to‐mesenchymal transition and the early initiation of fibrosis (Strikoudis et al., 2019). In the kidney, the response of tubular epithelial cells to injury are now thought to have a crucial role in the initiation of fibrosis and the transition from acute to chronic renal failure (Qi & Yang, 2018). Given the large and relatively early increases in IL‐11 expression observed in whole kidney tissue following injury (Table 1 and discussed below), there may be an important role for IL‐11 signalling in tubular epithelial cells. Exploring this should be a priority for future study.
Table 1.
Summary of studies with data on the role of IL‐11 in cardiac or renal fibrosis
| Reference | Species | Experimental design | Findings related to IL‐11 | Study used recombinant human IL‐11 in a rodent model |
|---|---|---|---|---|
| RENAL—Observational studies of IL‐11 expression/secretion in animal models and in human disease | ||||
| Menendez‐Castro et al., 2019 | Rats, strain unspecified | Renovascular hypertension was induced by clipping of one renal artery (1 clip, 2 kidney model). The kidney of rats developing malignant hypertensive nephrosclerosis were compared with those of rats which were hypertensive but did not develop malignant nephrosclerosis. Control animals received a sham operation. |
IL‐11 mRNA was up‐regulated 28.3‐fold in the kidneys of rats with malignant hypertension and 11.9‐fold in non‐malignant hypertension. This was confirmed at the protein level by Western blot. IL‐11 expression correlated with collagen deposition, myofibroblast activation and with TGF‐ß1, TIMP‐1, and Col1a expression. |
NA |
| Bigaeva et al., 2019 | Humans and mice (C57BL/6) | Cultured precision cut tissue slices (PCTS) were prepared from the explanted kidneys of healthy mice, healthy humans and diseased humans. RNA‐seq compared the transcriptomic response after 48 hr of culture (by which time pro‐fibrotic changes have occurred) to that pre‐culture. | IL‐11 was one of the few genes strongly up‐regulated in cultured kidney PCTS across species and disease states (5 to 10‐fold increase). | NA |
| Harlan et al., 2018 |
Mice, C57BKLS db/db |
Chronic kidney disease was induced by the combined effects of hypertension (AAV‐mediated renin expression plus uni‐nephrectomy) and Type 2 diabetes (db/db mouse). | Microarray analysis showed significant up‐regulation of IL‐11 expression in mice with chronic kidney disease. | NA |
| Schafer et al., 2017 | Mice, C57BL/6J background | Kidney injury induced by a single i.p. injection of folic acid (FA) 180 mg·kg−1 in wild‐type or IL‐11ra1 KO mice; kidneys assessed at Day 28 | Kidney injury from FA resulted in expression of IL‐11 protein and significant renal fibrosis in wild‐type mice. IL‐11ra1 KO prevented FA‐induced kidney fibrosis. | NA |
| Grgic et al., 2014 | Mice, C57BL/6J background | Time course of IL‐11 mRNA levels assessed in two models: Model 1: UUO for up to 5 days. Model 2: 35 min of unilateral IRI (UIRI) for up to 28 days |
In UUO, IL‐11 expression was up‐regulated 80‐fold by 48 hr and remained elevated at 20‐fold up to experiment conclusion at Day 5. In UIRI, IL‐11 expression was >200‐fold up‐regulated by 24 hr, remaining elevated to at least Day 28. |
NA |
| Xu, Podok, Xie, & Lu, 2014 | Crucian carp | Carp were infected with Cyprinid herpesvirus 2. Virus specific host gene activation in the head kidneys of the carp was assessed in moribund versus surviving fish. The kidney is a principal immune organ of the fish. | IL11 is the most highly expressed gene in the head kidney of moribund versus surviving fish, being up‐regulated 200‐fold. | NA |
| https://paperpile.com/c/PRszFm/tcs8 | Mice, C57BL/6 background | Mice were subjected to 20 or 30 min unilateral IRI with contralateral nephrectomy. The effect of a selective adenosine A1 receptor agonist (CCPA) on renal injury was assessed in wild‐type mice versus IL‐11ra1 KO mice and in wild‐type mice with or without pretreatment with a neutralizing IL‐11 antibody. |
1. Administration of a selective A1 adenosine receptor agonist CCPA induces IL‐11 in mouse kidneys 2. IL‐11ra1 KO mice had worse renal function at 24 hr after IRI compared to wild‐type mice 3. Pretreatment with CCPA protects against renal IRI in wild‐type but not IL‐11ra1 KO mice 4. Neutralizing IL‐11 antibody abolishes the renal protection provided by CCPA |
NA |
| Mitazaki, Kato, Suto, Hiraiwa, & Abe, 2009 | Mice, C57BL/6J | Acute renal failure was induced by a single high‐dose injection of cisplatin 30 mg·kg−1, i.p. | IL‐11 expression is up‐regulated ~80‐fold at 72 hr after cisplatin injection | NA |
| Chien et al., 2006 | Humans | 24‐hr urine collected from patients with IgA nephropathy, lupus nephritis, or idiopathic nephrotic syndrome and assessed for total protein, IL‐11 protein, and IL‐11 mRNA. | Urinary IL‐11 protein and urinary IL‐11 mRNA significantly correlated with total proteinuria in patients with IgA nephropathy and lupus nephritis. | NA |
| Lemay, Rabb, Postler, & Singh, 2000 | Mice, NIH Swiss | Mice underwent 30‐min bilateral IRI. Kidneys were harvested at 1, 4, 12 or 24 hr after ischaemia. | IL‐11 mRNA was raised at 4, 12, and 24 hr, with the peak at 12 hr | NA |
| RENAL—Interventional studies of the effects of recombinant IL‐11 in animal models | ||||
| Schafer et al., 2017 | Mice, C57BL/6J background |
Exp 1: Recombinant mouse IL‐11 (rmIL‐11) injected (100 μg·kg−1·day−1, 3 weeks) into Col1a‐GFP reporter mouse. Exp 2: Inducible rmIL‐11 mouse created by crossing rmIL‐11‐Tg mice with Col1a2–Cre mice. |
Both exogenous rmIL‐11 injection and induced rmIL‐11 expression produced activation of fibroblasts in the renal interstitium, caused renal fibrosis and impaired renal function. | X |
| Lee et al., 2012 | Mice, C57BL/6 background | Mice underwent 30+min unilateral IRI with contralateral nephrectomy. Recombinant human IL‐11 or vehicle was given, either pre‐ or 30–60 min post‐IRI. | Mice receiving rhIL‐11 had lower serum creatinine and reduced renal apoptosis, necrosis, and inflammation. | ✓ |
| Lai et al., 2005 | Mice, C57BL/6J | Recombinant human IL‐11 injected in a mouse model of crescentic glomerulonephritis induced by injection of sheep anti‐mouse nephrotoxic serum | Human IL‐11 treatment decreased albuminuria, glomerular macrophage number, and glomerular fibrin deposition in mice | ✓ |
| Lai et al., 2001 | Rats, Wistar Kyoto | Recombinant human IL‐11 injected in a rat model of necrotizing glomerulonephritis induced by an injection of anti‐glomerular basement membrane antibody | Human IL‐11 reduced proteinuria, fibrinoid necrosis, and macrophage activation in rats | ✓ |
| CARDIAC—Observational studies of IL‐11 expression/secretion in animal models and in human disease | ||||
| Ye et al., 2019 | Humans | Plasma concentrations of IL‐11 were measured in 240 patients with chronic heart failure and compared to 80 patients without signs of heart disease. Patients were followed up for the occurrence of cardiac events. |
Plasma IL‐11 level was ~1.3‐fold higher in the heart failure patients than in the control group. In patients, IL‐11 levels correlated with symptoms, with NT‐pro BNP level and predicted cardiac events. |
NA |
| Xu et al., 2018 | Humans | Aortic tissue samples were collected from patients with acute thoracic aortic dissection. Blood samples were collected from patients with thoracic aortic dissection patients and compared to patients with chest pain but no dissection. |
IL‐11 was increased greater than twofold in the aortic tissue of acute thoracic aortic dissection patients. Plasma IL‐11 was 1.7‐fold higher in the dissection patients. |
NA |
| Schafer et al., 2017 | Mice, C57BL/6J background |
Exp 1: Angiotensin (2 mg·kg−1·day−1) was infused via s.c. pump for 28 days to induce hypertension and pressure overload in wild‐type and IL‐11ra KO mice Exp 2: Wild‐type and IL‐11ra1 KO mice underwent transverse aortic constriction to produce pressure overload |
Both models of hypertension and pressure overload result in IL‐11 expression and fibrosis in wild‐type mice. These effects are significantly reduced in the IL‐11ra1 KO mice. | NA |
| Liu et al., 2015 | Humans | Serum cytokine levels were assessed in patients undergoing invasive coronary angiography: patients found to have coronary artery disease were compared to those completely free of coronary atherosclerosis |
Serum IL‐11 was significantly higher in patients with coronary artery disease compared to those without. However, IL‐11 level did not correlate with the degree of coronary disease assessed by Gensini score. |
NA |
| Smith, 2000 | Humans | Review of safety data from randomized trials in patients receiving rhIL‐11 for treatment of chemotherapy‐induced thrombocytopaenia. | Common side effects of rhIL‐11 in human cancer patients include oedema, dyspnoea, pleural effusions, and atrial arrhythmia. | NA |
| CARDIAC—Interventional studies of the effects of recombinant IL‐11 in animal models and human patients | ||||
| Liu et al., 2019 | Humans | The cardiovascular side effects of rhIL‐11 were assessed in 24 leukaemia patients receiving rhILL for treatment of chemotherapy‐induced thrombocytopaenia. |
During rhIL‐11 treatment, patients' brain natriuretic peptide levels rose from 22 to 215 pg·ml−1. 38% of patients had oedema and weight gain. 17% experienced acute left ventricular failure. 8% had an episode of paroxysmal atrial fibrillation. |
X |
| Tamura, Kohno, Mohri, Fujio, & Matsumiya, 2018 | Rats, Sprague–Dawley | Recombinant human IL‐11 was given i.v. 10 min before harvesting the rat heart. The hearts were preserved in cold buffer for 6 hr before being reperfused with solution containing either rhIL‐11 or saline. | rhIL‐11 improved myocardial function (LV developed pressure and change in LV pressure) after 6 hr of cold ischaemia. The number of apoptotic cardiomyocytes was also reduced approximately fourfold. |
✓ |
| Schafer et al., 2017 | Mice, C57BL/6J background |
Exp 1: Recombinant mouse IL‐11 (rmIL‐11, 100 μg·kg−1·day−1) or PBS was administered to mice for 6 days following ligation of the left coronary artery. Exp 2: Inducible rmIL‐11 mouse created by crossing rmIL‐11‐Tg mice with Col1a2–Cre mice. |
Exogenous or induced rmIL‐11 resulted in greater epicardial fibrosis and worse cardiac function after myocardial infarction In addition, while rmIL‐11 activated mouse cardiac fibroblasts in vitro (EC50, 2 ng·ml−1), rhIL‐11 did not activate mouse cardiac fibroblasts |
X |
| Obana et al., 2012 | Mice, C57BL/6 | Mice received 30 min of cardiac ischaemia followed by 24‐hr reperfusion. Recombinant human IL‐11 (20 μg·kg−1), or PBS, was given at the start of reperfusion. | rhIL‐11 reduced myocardial damage 1.6‐fold, reduced myocardial apoptosis, and resulted in better LV function on echocardiography. | ✓ |
| Obana et al., 2010 | Mice, C57BL/6 | Myocardial infarction (MI) was induced by ligation of the left coronary artery. Recombinant human IL‐11 (8 μg·kg−1, i.v.) was administered daily for 5 days by i.v. Mouse hearts were harvested at 14 days. |
IL‐11 mRNA was up‐regulated >50‐fold in the infarct zone, and 20‐fold in the remote zone, 24 hr after MI, maintained for ≥7 days. Intravenous rhIL‐11 up‐regulated p‐STAT3 in explanted mouse myocardium, reduced infarct area by 33%, reduced cardiomyocyte apoptosis, and improved LV function. |
✓ |
| Kimura et al., 2007 | Mice, C57BL/6 |
Recombinant human IL‐11 (8 μg·kg−1) or PBS was injected into mice 15 hr before cardiac IRI was induced via 60‐min left coronary artery ligation. Hearts were harvested after 60 min of reperfusion. Cultured rat cardiomyocytes were stimulated with rhIL‐11 |
rhIL‐11 reduced infarct size by 63%. In vitro, high‐dose rhIL‐11 (20 ng·ml−1) activated STAT3 and ERK in rat cultured cardiomyocytes. |
✓ |
Abbreviations: AAV, adeno‐associaed virus; BNP, brain (or b‐type) natriuretic peptide; CCPA, 2‐chloro‐N 6‐cyclopentyladenosine; Col1a, collagen type I α; FA, folic acid; IL‐11ra1: IL‐11 receptor subunit α; IRI, ischaemia reperfusion injury; KO, knock out; LV, left ventricle; MI, myocardial infarction; NT‐pro BNP, N‐terminal pro b‐type natriuretic peptide; PCTS, precision cut tissue slices; p‐STAT3, phosphorylated STAT3; rhIL‐11, recombinant human IL11; rmIL‐11, recombinant mouse IL‐11; Tg, transgenic; TIMP1, metallopeptidase inhibitor 1; UUO: unilateral ureteric obstruction.
8. IL‐11 IS ASSOCIATED WITH FIBROTIC CARDIOVASCULAR DISEASE IN MOUSE MODELS AND HUMAN PATIENTS
In the heart, IL‐11RA is expressed on both fibroblasts and cardiomyocytes (Kimura et al., 2007; FANTOM Consortium and the RIKEN PMI and CLST [DGT] et al., 2014). As mentioned, the dominant transcriptional response to TGF‐β1 stimulation of human atrial fibroblasts is IL‐11 expression (Schafer et al., 2017). Several studies have explored the association of IL‐11 and cardiovascular disease across species. These are summarized in Table 1 and discussed below.
Following myocardial infarction in mice, IL‐11 expression is up‐regulated >50‐fold after 24 hr, and increased levels are maintained for at least 14 days (Obana et al., 2010). Similarly, in rats fed a high‐salt diet—resulting in hypertension, cardiac fibrosis, and heart failure with preserved ejection fraction—cardiac IL‐11 expression is significantly increased (Zhou et al., 2019). In two other models of fibrotic heart disease—continuous angiotensin II infusion and transverse aortic constriction—increased levels of IL‐11 protein are seen in the heart (Schafer et al., 2017).
In humans, serum IL‐11 is increased in patients with congestive cardiac failure, correlates with the severity of heart failure symptoms, and predicts cardiac events including cardiovascular death and heart failure rehospitalization (Ye et al., 2019). Similarly, serum IL‐11 is raised in patients with coronary heart disease (Liu et al., 2015), and both serum and aortic IL‐11 are increased in patients with thoracic aortic dissection (Xu et al., 2018). Intriguingly, common side effects of rhIL‐11 in patients being treated for thrombocytopenia include atrial arrhythmia, pulmonary congestion, raised brain natriuretic peptide levels, and, in some cases, left ventricular failure (Liu et al., 2019; Smith, 2000), though this is at least partly related to acute sodium and water retention (Dykstra et al., 2000).
In summary, increased IL‐11 levels are found in primary human cells subjected to pro‐fibrotic stimuli, in the hearts of mice with fibrotic heart disease and in the serum and tissue of patients with cardiovascular disease. These associations suggest that IL‐11 may have a pathogenic role in fibrotic cardiac disease. An alternative explanation—which is prevalent in the literature—is that IL‐11 is protective against fibrotic heart disease and is induced as a natural suppressor of fibrosis. In the next section, we discuss how earlier work studying the effect of recombinant human IL‐11 (rhIL‐11) on the mouse or rat heart suggested the latter conclusion, whereas more recent work using species‐matched IL‐11 has revealed the true pathophysiological role of IL‐11 as a pro‐fibrotic cytokine.
9. SPECIES‐SPECIFIC RECOMBINANT IL‐11 HAS A PRO‐FIBROTIC EFFECT ON THE HEART
In 2007, Kimura et al. reported that pretreatment with an IL‐11 infusion had a protective and anti‐fibrotic effect in mice subjected to 60 min of cardiac ischaemia followed by reperfusion. Subsequent work found similar results in permanent left coronary artery ligation (Obana et al., 2010), in post‐reperfusion treatment (Obana et al., 2012) and in a cold ischaemia model designed to mimic the conditions of cardiac transplant (Tamura et al., 2018). However, in all cases, mice or rats received recombinant human—rather than mouse or rat—IL‐11 at high doses.
These earlier data directly contrast the more recent results discussed in the previous section, which showed a strong pro‐fibrotic effect of recombinant human IL‐11 when applied to primary human myofibroblasts (Schafer et al., 2017). To explore this apparent contradiction, Schafer et al. (2017) generated recombinant mouse IL‐11 (rmIL‐11) and compared the effects of this to rhIL‐11 on mouse fibroblasts—rmIL‐11 produced a strong pro‐fibrotic effect at a physiological dose, whereas rhIL‐11 had little effect, even at very high doses. Importantly, species‐matched rhIL‐11 on human fibroblasts or rmIL‐11 on mouse fibroblasts resulted in activation of non‐canonical signalling pathways, predominantly via the phosphorylation of ERK. Conversely, STAT3 activation, although transiently mildly elevated, had no downstream effects on transcription. This is in contrast to the signalling seen in previous studies where species‐discordant rhIL‐11 was administered to mice.
Recent work in hepatocytes has shed further light on the molecular underpinning of species‐specific IL‐11 effects (Widjaja, Dong, et al., 2019). Similar to myofibroblasts, rmIL‐11 stimulated the ERK (and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=518) pathway in mouse hepatocytes whereas rhIL‐11 had no effect in mouse cells. The activation of non‐canonical signalling pathways (i.e., not STAT) was also observed when species‐matched rhIL‐11 was used with primary human cells. Interestingly, despite not activating mouse signalling pathways, surface plasmon resonance experiments demonstrated that rhIL‐11 binds to the mouse IL‐11ra1 with a slightly higher affinity than rmIL‐11. Competition ELISA showed that rhIL‐11 is a highly effective blocker of mouse IL‐11 binding to IL‐11ra1 and that rhIL‐11 actually inhibits rmIL‐11 activity in murine hepatocytes. Thus—paradoxically—rhIL‐11 acts as an inhibitor of endogenous IL‐11 in mice, a result which may explain the protective effect of rhIL‐11 in rodent models of IL‐11‐mediated disease (Widjaja, Dong, et al., 2019).
Expanding on the in vitro results, Schafer et al. (2017) tested the effect of injecting recombinant mouse IL‐11 into healthy mice. In contrast to the earlier studies that had used human IL‐11 in the mouse, this resulted in cardiac and renal fibrosis. Injection of rmIL‐11 to the mouse increased epicardial fibroblast activation, a hallmark of fibrosis in myocardial infarction, and resulted in worsening of left ventricular function (Schafer et al., 2017).
Next, the in vivo effects of fibroblast‐specific IL‐11 expression were investigated by generating murine Il11‐transgenic mice crossed with Tam‐inducible Col1a2–Cre mice: Within 2 weeks, there was widespread activation of cardiac and renal fibroblasts and accumulation of collagen. This was accompanied by a reduction in cardiac and renal function and increased serum TGF‐β1 (Schafer et al., 2017).
Together, these results show that IL‐11 biology is conserved across species, but the role of endogenous IL‐11 cannot be inferred from the use of recombinant human IL‐11 on rodent cells or tissues. The effect of species‐matched IL‐11, either administered exogenously or overexpressed endogenously, is pro‐fibrotic in the heart. Combined with the observational data of increased IL‐11 in multiple animal models and human cardiac disease, this suggests a causative role for IL‐11 in fibrotic heart disease, rather than the previously assumed protective, anti‐inflammatory, and anti‐fibrotic role.
10. IL‐11 IS ASSOCIATED WITH RENAL INJURY IN DIVERSE ANIMAL MODELS AND IN HUMAN PATIENTS
Fibrotic renal injury in patients can result from diverse insults. Experiments with animal models across different species (mouse, rat, and carp) have found an association between IL‐11 and the varied renal insults, including ischaemia (Grgic et al., 2014; Lemay et al., 2000), drug or chemical toxicity (Mitazaki et al., 2009; Schafer et al., 2017), hypertension (Harlan et al., 2018; Menendez‐Castro et al., 2019), diabetes (Harlan et al., 2018), infection (Xu et al., 2014), and physical obstruction of the urinary tract (Grgic et al., 2014). These studies are summarized in Table 1.
In humans, few studies to date have investigated the role of IL‐11 in renal disease. However, a study in paediatric patients found a highly significant correlation between urinary IL‐11 protein and mRNA levels and proteinuria in patients with IgA nephropathy or lupus nephritis (Chien et al., 2006). In primary human renal epithelial cells, adenovirus‐mediated expression of GADD45γ (a protein highly up‐regulated in renal tissue injured by urinary tract obstruction) results in a 6.7‐fold up‐regulation of IL‐11 expression (Shin, Kim, Lim, Yim, & Kim, 2008). A recent study by Bigaeva et al. (2019) used RNA‐seq in cultured precision cut tissue slices (PCTS)—an in vitro model of fibrosis which maintains the complex three‐dimensional structures of organs—to assess the fibrosis‐associated transcriptomic response in healthy mouse, healthy human, and diseased human tissues. They found that IL‐11 was one of the few genes to be consistently up‐regulated in PCTS across species and organs (including kidney, liver, ileum, and colon; cardiac tissue was not assessed). In particular, IL‐11 was one of the most up‐regulated genes (5‐ to 10‐fold) in kidney PCTS from healthy mouse, healthy human, and diseased human (end‐stage renal failure) donors.
11. SPECIES‐MATCHED EXOGENOUS IL‐11 CAUSES KIDNEY FIBROSIS AND DYSFUNCTION
Similar to work on cardiac fibrosis, the role of IL‐11 in kidney disease has likely been confounded and confused by the use of recombinant human IL‐11 (rhIL‐11) in rodent models. For example, it has been shown that rhIL‐11 protects from renal‐ischaemia reperfusion injury (Lee et al., 2012) and suppresses extracellular matrix deposition and glomerular injury in experimental glomerulonephritis (Lai et al., 2001, 2005).
In contrast, more recent work has shown that exogenous administration or overexpression of mouse IL‐11 in the mouse causes, rather than inhibits, renal fibrosis. Three weeks of daily rmIL‐11 treatment in mice activated fibroblasts in the renal interstitium, resulted in deposition of collagen and caused impairment of renal function (Schafer et al., 2017). Similarly, 2 weeks of inducible IL‐11 overexpression in a fibroblast‐specific manner in murine Il11‐transgenic mice crossed with inducible Col1a2–Cre mice caused renal fibrosis and an increase in serum urea and creatinine, indicating renal dysfunction (Schafer et al., 2017).
12. GENETIC DELETION OF THE IL‐11 RECEPTOR HAS NO MAJOR ADVERSE EFFECTS AND PROTECTS AGAINST CARDIAC AND RENAL FIBROSIS IN MICE
Individuals with biallelic null mutations in IL‐11RA have delayed tooth eruption, mild craniosynostosis, and variable joint laxity but are otherwise well (Brischoux‐Boucher et al., 2018; Keupp et al., 2013; Nieminen et al., 2011). IL‐11ra1−/− mice are a notable phenocopy of the human null phenotype, having slight developmental abnormalities of the skull and teeth but being otherwise healthy with a normal life span, although females (unlike human female nulls) are infertile (Nandurkar et al., 1997).
The similarities in the human and mouse IL‐11RA null phenotype suggest strong conservation of IL‐11 function across mammalian species and that proof‐of‐concept studies targeting IL‐11 in mice could provide valuable data that translate to humans. Furthermore, the lack of overt adverse post‐developmental consequences of IL‐11RA deletion in either mice or humans suggests that targeting IL‐11 signalling could be a safe means to treat fibrosis in adult mammals.
To investigate this further, IL‐11ra1−/− mice were compared to wild‐type controls in three independent models of cardiac and renal fibrosis (Schafer et al., 2017). Following angiotensin II infusion or transverse aortic constriction, IL‐11ra1−/− mice had less cardiac fibrosis than wild‐type mice, an effect which was independent of loading conditions. Similarly, after folic acid‐induced kidney injury, IL‐11ra1−/− mice had reduced renal fibrosis. Deletion of IL‐11ra1 signalling resulted in reduced ERK signalling across all models tested.
13. NEUTRALIZING IL‐11 ANTIBODIES ARE ANTI‐FIBROTIC
Our group genetically immunized mice with IL‐11 to generate neutralizing IL‐11 antibodies. Clones were then screened for neutralization activity using fibroblast transformation as a readout. Clones X203 (anti‐IL‐11) and X209 (anti‐IL‐11ra1) effectively blocked the fibrotic response in mice and human cells and were chosen for preclinical testing in mouse models (Ng et al., 2019; Widjaja, Singh, et al., 2019). Neutralizing anti‐IL‐11 antibodies markedly reduced the pro‐fibrotic effect of TGF‐β stimulation on atrial fibroblasts across a wide range of assays, including myofibroblast activation, ECM production, and gel contraction and cell migration (Ng et al., 2019; Schafer et al., 2017).
In animal models, clones X203 and/or X209 have been shown to prevent or reverse fibrosis in the lung and liver (Ng et al., 2019; Widjaja, Singh, et al., 2019). Studies investigating the effects of anti‐IL‐11 therapy on cardiac or renal fibrosis are ongoing and are expected to be reported in the near future.
14. CONCLUSIONS
Fibrosis is a key and common driver of cardiac and renal failure, which account for a large proportion of global morbidity and mortality. As fibrotic diseases are more prevalent in older individuals, this burden will increase as populations age. Therefore, new treatments that safely and specifically target cardiorenal fibrosis are needed, and this represents an unmet medical need. This is especially so, given that recent trials have now clearly shown that targeting TGF‐β directly, or indirectly via integrins, is toxic and ineffective and new drug targets are needed.
IL‐11 up‐regulation—acting downstream of TGF‐β and other pro‐fibrotic factors—appears to be a unifying feature underlying cardiorenal fibrotic diseases. Earlier work using human IL‐11 in murine models led to the conclusion that its up‐regulation in fibroinflammatory diseases was a protective response, a compensatory mechanism to prevent runaway fibrosis. More recent studies have questioned this conclusion: The true endogenous role of IL‐11 appears pro‐fibrotic, resulting in the activation of myofibroblasts, inflammation, the deposition of ECM and ultimately organ dysfunction and failure.
From a therapeutic point of view, biallelic IL‐11RA null mutations in mice and humans are tolerated in adults, which suggests inhibiting IL‐11 signalling may be safe, but this remains to be proven. Future work exploring the effect of anti‐IL‐11 therapies in animal models and, ultimately, in human clinical trials will determine if its potential can be realized to help the millions of patients worldwide suffering with fibrotic diseases of the kidney or heart.
14.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Fabbro et al., 2019a, b; Alexander, Kelly et al., 2019).
CONFLICT OF INTEREST
S.A.C. and S.S. are co‐inventors of the patent applications (WO/2017/103108: TREATMENT OF FIBROSIS, WO/2018/109174: IL‐11 ANTIBODIES, WO/2018/109170: IL‐11RA ANTIBODIES). S.A.C. and S.S. are co‐founders and shareholders of Enleofen Bio PTE LTD, a company that develops anti‐IL‐11 therapeutics.
Corden B, Adami E, Sweeney M, Schafer S, Cook SA. IL‐11 in cardiac and renal fibrosis: Late to the party but a central player. Br J Pharmacol. 2020;177:1695–1708. 10.1111/bph.15013
REFERENCES
- Akhurst, R. J. , & Hata, A. (2012). Targeting the TGFβ signalling pathway in disease. Nature Reviews. Drug Discovery, 11, 790–811. 10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019a). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019b). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Faccenda, E. , … CGTP Collaborators (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita, K. , South, A. P. , Hans‐Filho, G. , Sakuma, T. H. , Lai‐Cheong, J. , Clements, S. , … McGrath, J. (2008). Oncostatin M receptor‐β mutations underlie familial primary localized cutaneous amyloidosis. American Journal of Human Genetics, 82, 73–80. 10.1016/j.ajhg.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie, B. , Chung, C. H. , Parker, J. S. , Stover, D. G. , Cheng, N. , Chytil, A. , … Moses, H. L. (2009). Abrogation of TGF‐β signaling enhances chemokine production and correlates with prognosis in human breast cancer. The Journal of Clinical Investigation, 119, 1571–1582. 10.1172/JCI37480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaeva, E. , Gore, E. , Simon, E. , Zwick, M. , Oldenburger, A. , de Jong, K. P. , … Olinga, P. (2019). Transcriptomic characterization of culture‐associated changes in murine and human precision‐cut tissue slices. Archives of Toxicology, 93, 3549–3583. 10.1007/s00204-019-02611-6 [DOI] [PubMed] [Google Scholar]
- Bing, R. , Cavalcante, J. L. , Everett, R. J. , Clavel, M.‐A. , Newby, D. E. , & Dweck, M. R. (2019). Imaging and impact of myocardial fibrosis in aortic stenosis. JACC: Cardiovascular Imaging, 12, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux‐Boucher, E. , Trimouille, A. , Baujat, G. , Goldenberg, A. , Schaefer, E. , Guichard, B. , … van Maldergem, L. (2018). IL11RA‐related Crouzon‐like autosomal recessive craniosynostosis in 10 new patients: Resemblances and differences. Clinical Genetics, 94, 373–380. 10.1111/cge.13409 [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Sohal, M. , Voigt, T. , Sammut, E. , Tobon‐Gomez, C. , Child, N. , … Rinaldi, C. A. (2015). Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non‐ischemic cardiomyopathy patients with implantable cardioverter‐defibrillators. Heart Rhythm, 12, 792–801. 10.1016/j.hrthm.2014.12.020 [DOI] [PubMed] [Google Scholar]
- Chiao, Y. A. , Lakatta, E. , Ungvari, Z. , Dai, D.‐F. , & Rabinovitch, P. (2016). Cardiovascular disease and aging In Sierra F., & Kohanski R. (Eds.), Advances in Geroscience (pp. 121–160). Cham: Springer International Publishing. [Google Scholar]
- Chien, J.‐W. , Chen, W.‐L. , Tsui, Y.‐G. , Lee, M.‐C. , Lin, A.‐Y. , & Lin, C.‐Y. (2006). Daily urinary interleukin‐11 excretion correlated with proteinuria in IgA nephropathy and lupus nephritis. Pediatric Nephrology, 21, 490–496. 10.1007/s00467-006-0016-7 [DOI] [PubMed] [Google Scholar]
- Csepe, T. A. , Kalyanasundaram, A. , Hansen, B. J. , Zhao, J. , & Fedorov, V. V. (2015). Fibrosis: A structural modulator of sinoatrial node physiology and dysfunction. Frontiers in Physiology, 6, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi, C. , Ciulla, M. , & Zanchetti, A. (2006). Hypertensive myocardial fibrosis. Nephrology, Dialysis, Transplantation, 21, 20–23. [DOI] [PubMed] [Google Scholar]
- Dagoneau, N. , Scheffer, D. , Huber, C. , Al‐Gazali, L. I. , Di Rocco, M. , Godard, A. , … Nicole, S. (2004). Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve‐Wiedemann/Schwartz‐Jampel type 2 syndrome. American Journal of Human Genetics, 74, 298–305. 10.1086/381715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djudjaj, S. , & Boor, P. (2019). Cellular and molecular mechanisms of kidney fibrosis. Molecular Aspects of Medicine, 65, 16–36. 10.1016/j.mam.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Dykstra, K. H. , Rogge, H. , Stone, A. , Loewy, J. , Keith, J. C. Jr. , & Schwertschlag, U. S. (2000). Mechanism and amelioration of recombinant human interleukin‐11 (rhIL‐11)‐induced anemia in healthy subjects. Journal of Clinical Pharmacology, 40, 880–888. 10.1177/00912700022009521 [DOI] [PubMed] [Google Scholar]
- Einarsson, O. , Geba, G. P. , Zhu, Z. , Landry, M. , & Elias, J. A. (1996). Interleukin‐11: Stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. The Journal of Clinical Investigation, 97, 915–924. 10.1172/JCI118514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias, J. A. , Zheng, T. , Einarsson, O. , Landry, M. , Trow, T. , Rebert, N. , & Panuska, J. (1994). Epithelial interleukin‐11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. The Journal of Biological Chemistry, 269, 22261–22268. [PubMed] [Google Scholar]
- FANTOM Consortium and the RIKEN PMI and CLST (DGT) , Forrest, A. R. R. , Kawaji, H. , Rehli, M. , Baillie, J. K. , de Hoon, M. J. L. , … Andersson, R. (2014). A promoter‐level mammalian expression atlas. Nature, 507, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal, P. , & Marrouche, N. F. (2017). Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. European Heart Journal, 38, 14–19. 10.1093/eurheartj/ehv514 [DOI] [PubMed] [Google Scholar]
- Garbers, C. , & Scheller, J. (2013). Interleukin‐6 and interleukin‐11: Same but different. Biological Chemistry, 394, 1145–1161. 10.1515/hsz-2013-0166 [DOI] [PubMed] [Google Scholar]
- González, A. , Schelbert, E. B. , Díez, J. , & Butler, J. (2018). Myocardial interstitial fibrosis in heart failure: Biological and translational perspectives. Journal of the American College of Cardiology, 71, 1696–1706. 10.1016/j.jacc.2018.02.021 [DOI] [PubMed] [Google Scholar]
- Grgic, I. , Krautzberger, A. M. , Hofmeister, A. , Lalli, M. , DiRocco, D. P. , Fleig, S. V. , … Humphreys, B. D. (2014). Translational profiles of medullary myofibroblasts during kidney fibrosis. Journal of the American Society of Nephrology, 25, 1979–1990. 10.1681/ASN.2013101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, C. A. T. Trabeculectomy Study , Khaw, P. , Grehn, F. , Hollo, G. , Overton, B. , Wilson, R. , et al. (2007). A phase III study of subconjunctival human anti‐transforming growth factor β2 monoclonal antibody (CAT‐152) to prevent scarring after first‐time trabeculectomy. Ophthalmology, 114, 1822–1830. [DOI] [PubMed] [Google Scholar]
- Gulati, A. , Jabbour, A. , Ismail, T. F. , Guha, K. , Khwaja, J. , Raza, S. , … Prasad, S. K. (2013). Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA, 309, 896–908. 10.1001/jama.2013.1363 [DOI] [PubMed] [Google Scholar]
- Halliday, B. P. , Gulati, A. , Ali, A. , Guha, K. , Newsome, S. , Arzanauskaite, M. , … Prasad, S. K. (2017). Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation, 135, 2106–2115. 10.1161/CIRCULATIONAHA.116.026910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan, S. M. , Heinz‐Taheny, K. M. , Sullivan, J. M. , Wei, T. , Baker, H. E. , Jaqua, D. L. , … Heuer, J. G. (2018). Progressive renal disease established by renin‐coding adeno‐associated virus‐driven hypertension in diverse diabetic models. Journal of the American Society of Nephrology, 29, 477–491. 10.1681/ASN.2017040385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson, T. D. , Holt, S. G. , & Smith, E. R. (2017). Progression of tubulointerstitial fibrosis and the chronic kidney disease phenotype—Role of risk factors and epigenetics. Frontiers in Pharmacology, 8, 520–536. 10.3389/fphar.2017.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer, S. , & Schenke‐Layland, K. (2019). Cardiac fibrosis—A short review of causes and therapeutic strategies. Advanced Drug Delivery Reviews, 146, 77–82. 10.1016/j.addr.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Ho, C. Y. , López, B. , Coelho‐Filho, O. R. , Lakdawala, N. K. , Cirino, A. L. , Jarolim, P. , … Seidman, C. E. (2010). Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. The New England Journal of Medicine, 363, 552–563. 10.1056/NEJMoa1002659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles, L. , Pfluger, H. , Lefkovits, L. , Butler, M. J. , Kistler, P. M. , Kaye, D. M. , & Taylor, A. J. (2011). Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter‐defibrillators for primary prevention of sudden cardiac death. Journal of the American College of Cardiology, 57, 821–828. 10.1016/j.jacc.2010.06.062 [DOI] [PubMed] [Google Scholar]
- Isaacs, C. , Robert, N. J. , Bailey, F. A. , Schuster, M. W. , Overmoyer, B. , Graham, M. , … Kaye, J. A. (1997). Randomized placebo‐controlled study of recombinant human interleukin‐11 to prevent chemotherapy‐induced thrombocytopenia in patients with breast cancer receiving dose‐intensive cyclophosphamide and doxorubicin. Journal of Clinical Oncology, 15, 3368–3377. 10.1200/JCO.1997.15.11.3368 [DOI] [PubMed] [Google Scholar]
- Jager, K. J. , & Fraser, S. D. S. (2017). The ascending rank of chronic kidney disease in the global burden of disease study. Nephrology, Dialysis, Transplantation, 32, ii121–ii128. 10.1093/ndt/gfw330 [DOI] [PubMed] [Google Scholar]
- Karczewski, K. J. , Francioli, L. C. , Tiao, G. , Cummings, B. B. , Alföldi, J. , Wang, Q. , … Gauthier, L. D. (2019). Variation across 141,456 human exomes and genomes reveals the spectrum of loss‐of‐function intolerance across human protein‐coding genes. BioRxiv. 531210 [Google Scholar]
- Katbeh, A. , Ondrus, T. , Barbato, E. , Galderisi, M. , Trimarco, B. , Van Camp, G. , … Penicka, M. (2018). Imaging of myocardial fibrosis and its functional correlates in aortic stenosis: A review and clinical potential. Cardiology, 141, 141–149. 10.1159/000493164 [DOI] [PubMed] [Google Scholar]
- Kawashima, I. , Ohsumi, J. , Mita‐Honjo, K. , Shimoda‐Takano, K. , Ishikawa, H. , Sakakibara, S. , … Takiguchi, Y. (1991). Molecular cloning of cDNA encoding adipogenesis inhibitory factor and identity with interleukin‐11. FEBS Letters, 283, 199–202. 10.1016/0014-5793(91)80587-s [DOI] [PubMed] [Google Scholar]
- Kawashima, I. , & Takiguchi, Y. (1992). Interleukin‐11: A novel stroma‐derived cytokine. Progress in Growth Factor Research, 4, 191–206. 10.1016/0955-2235(92)90019-e [DOI] [PubMed] [Google Scholar]
- Keller, D. C. , Du, X. X. , Srour, E. F. , Hoffman, R. , & Williams, D. A. (1993). Interleukin‐11 inhibits adipogenesis and stimulates myelopoiesis in human long‐term marrow cultures. Blood, 82, 1428–1435. 10.1182/blood.V82.5.1428.1428 [DOI] [PubMed] [Google Scholar]
- Keown, A. (2019). Biogen halts mid‐stage IPF drug trial due to safety concerns | BioSpace (BioSpace).
- Kerola, T. , Eranti, A. , Aro, A. L. , Haukilahti, M. A. , Holkeri, A. , Junttila, M. J. , … Marcus, G. M. (2019). Risk Factors Associated With Atrioventricular Block. JAMA Network Open, 2, e194176 10.1001/jamanetworkopen.2019.4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keupp, K. , Li, Y. , Vargel, I. , Hoischen, A. , Richardson, R. , Neveling, K. , … Wollnik, B. (2013). Mutations in the interleukin receptor IL11RA cause autosomal recessive Crouzon‐like craniosynostosis. Molecular Genetics & Genomic Medicine, 1, 223–237. 10.1002/mgg3.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, D. , Denton, C. P. , Jahreis, A. , van Laar, J. M. , Frech, T. M. , Anderson, M. E. , … Furst, D. E. (2016). Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet, 387, 2630–2640. 10.1016/S0140-6736(16)00232-4 [DOI] [PubMed] [Google Scholar]
- Kimura, R. , Maeda, M. , Arita, A. , Oshima, Y. , Obana, M. , Ito, T. , … Azuma, J. (2007). Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine, 38, 107–115. 10.1016/j.cyto.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Knoppert, S. N. , Valentijn, F. A. , Nguyen, T. Q. , Goldschmeding, R. , & Falke, L. L. (2019). Cellular senescence and the kidney: Potential therapeutic targets and tools. Frontiers in Pharmacology, 10, 770 10.3389/fphar.2019.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottkamp, H. (2012). Fibrotic atrial cardiomyopathy: A specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. Journal of Cardiovascular Electrophysiology, 23, 797–799. 10.1111/j.1540-8167.2012.02341.x [DOI] [PubMed] [Google Scholar]
- Lai, P. C. , Cook, H. T. , Smith, J. , Keith, J. C. Jr. , Pusey, C. D. , & Tam, F. W. (2001). Interleukin‐11 attenuates nephrotoxic nephritis in Wistar Kyoto rats. Journal of the American Society of Nephrology, 12, 2310–2320. [DOI] [PubMed] [Google Scholar]
- Lai, P. C. , Smith, J. , Bhangal, G. , Chaudhry, K. A. , Chaudhry, A. N. , Keith, J. C. Jr. , … Cook, H. T. (2005). Interleukin‐11 reduces renal injury and glomerular NF‐κB activity in murine experimental glomerulonephritis. Nephron. Experimental Nephrology, 101, e146–e154. 10.1159/000087938 [DOI] [PubMed] [Google Scholar]
- Lee, H. T. , Park, S. W. , Kim, M. , Ham, A. , Anderson, L. J. , Brown, K. M. , … Cox, G. N. (2012). Interleukin‐11 protects against renal ischemia and reperfusion injury. American Journal of Physiology. Renal Physiology, 303, F1216–F1224. 10.1152/ajprenal.00220.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay, S. , Rabb, H. , Postler, G. , & Singh, A. K. (2000). Prominent and sustained up‐regulation of gp130‐signaling cytokines and the chemokine MIP‐2 in murine renal ischemia‐reperfusion injury. Transplantation, 69, 959–963. 10.1097/00007890-200003150-00049 [DOI] [PubMed] [Google Scholar]
- Liu, N.‐W. , Huang, X. , Liu, S. , Liu, W.‐J. , Wang, H. , Wang, W. , & Lu, Y. (2019). Elevated BNP caused by recombinant human interleukin‐11 treatment in patients with chemotherapy‐induced thrombocytopenia. Supportive Care in Cancer, 27(11), 4293–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Zhang, M. , Wu, J. , Zhou, P. , Liu, Y. , Wu, Y. , … Lu, X. (2015). Serum CD121a (Interleukin 1 receptor, Type I): A potential novel inflammatory marker for coronary heart disease. PLoS ONE, 10, e0131086 10.1371/journal.pone.0131086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L. , Guo, J. , Hua, Y. , Huang, K. , Magaye, R. , Cornell, J. , … Wang, B. H. (2017). Cardiac fibrosis in the ageing heart: Contributors and mechanisms. Clinical and Experimental Pharmacology & Physiology, 44(44 Suppl 1), 55–63. 10.1111/1440-1681.12753 [DOI] [PubMed] [Google Scholar]
- Lu, Z. Y. , Zhang, X. G. , Gu, Z. J. , Yasukawa, K. , Amiot, M. , Etrillard, M. , … Klein, B. (1994). A highly sensitive quantitative bioassay for human interleukin‐11. Journal of Immunological Methods, 173, 19–26. 10.1016/0022-1759(94)90278-X [DOI] [PubMed] [Google Scholar]
- Masu, Y. , Wolf, E. , Holtmann, B. , Sendtner, M. , Brem, G. , & Thoenen, H. (1993). Disruption of the CNTF gene results in motor neuron degeneration. Nature, 365, 27–32. [DOI] [PubMed] [Google Scholar]
- Menendez‐Castro, C. , Cordasic, N. , Dambietz, T. , Veelken, R. , Amann, K. , Hartner, A. , & Hilgers, K. F. (2019). Correlations between Interleukin‐11 expression and hypertensive kidney injury in a rat model of renovascular hypertension. American Journal of Hypertension. 10.1093/ajh/hpz194 [DOI] [PubMed] [Google Scholar]
- Meng, X.‐M. , Nikolic‐Paterson, D. J. , & Lan, H. Y. (2016). TGF‐β: the master regulator of fibrosis. Nature Reviews. Nephrology, 12, 325–338. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- Miller, K. A. , Twigg, S. R. F. , McGowan, S. J. , Phipps, J. M. , Fenwick, A. L. , Johnson, D. , … Wilkie, A. O. M. (2017). Diagnostic value of exome and whole genome sequencing in craniosynostosis. Journal of Medical Genetics, 54, 260–268. 10.1136/jmedgenet-2016-104215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitazaki, S. , Kato, N. , Suto, M. , Hiraiwa, K. , & Abe, S. (2009). Interleukin‐6 deficiency accelerates cisplatin‐induced acute renal failure but not systemic injury. Toxicology, 265, 115–121. 10.1016/j.tox.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Moreo, A. , Ambrosio, G. , De Chiara, B. , Pu, M. , Tran, T. , Mauri, F. , & Raman, S. V. (2009). Influence of myocardial fibrosis on left ventricular diastolic function. Circulation. Cardiovascular Imaging, 2(6), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, M. , Kamimura, D. , & Hirano, T. (2019). Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity, 50, 812–831. 10.1016/j.immuni.2019.03.027 [DOI] [PubMed] [Google Scholar]
- Musa, T. A. , Treibel, T. A. , Vassiliou, V. S. , Captur, G. , Singh, A. , Chin, C. , … Greenwood, J. P. (2018). Myocardial scar and mortality in severe aortic stenosis. Circulation, 138, 1935–1947. 10.1161/CIRCULATIONAHA.117.032839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandurkar, H. H. , Robb, L. , Tarlinton, D. , Barnett, L. , Köntgen, F. , & Begley, C. G. (1997). Adult mice with targeted mutation of the interleukin‐11 receptor (IL11Ra) display normal hematopoiesis. Blood, 90, 2148–2159. 10.1182/blood.V90.6.2148 [DOI] [PubMed] [Google Scholar]
- Ng, B. , Dong, J. , D'Agostino, G. , Viswanathan, S. , Widjaja, A. A. , Lim, W.‐W. , … Cook, S. A. (2019). Interleukin‐11 is a therapeutic target in idiopathic pulmonary fibrosis. Science Translational Medicine, 11, eaaw1237 10.1126/scitranslmed.aaw1237 [DOI] [PubMed] [Google Scholar]
- Nieminen, P. , Morgan, N. V. , Fenwick, A. L. , Parmanen, S. , Veistinen, L. , Mikkola, M. L. , … Thesleff, I. (2011). Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. American Journal of Human Genetics, 89, 67–81. 10.1016/j.ajhg.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obana, M. , Maeda, M. , Takeda, K. , Hayama, A. , Mohri, T. , Yamashita, T. , … Fujio, Y. (2010). Therapeutic activation of signal transducer and activator of transcription 3 by interleukin‐11 ameliorates cardiac fibrosis after myocardial infarction. Circulation, 121, 684–691. 10.1161/CIRCULATIONAHA.109.893677 [DOI] [PubMed] [Google Scholar]
- Obana, M. , Miyamoto, K. , Murasawa, S. , Iwakura, T. , Hayama, A. , Yamashita, T. , … Fujio, Y. (2012). Therapeutic administration of IL‐11 exhibits the postconditioning effects against ischemia‐reperfusion injury via STAT3 in the heart. American Journal of Physiology. Heart and Circulatory Physiology, 303, H569–H577. 10.1152/ajpheart.00060.2012 [DOI] [PubMed] [Google Scholar]
- O'Sullivan, E. D. , Hughes, J. , & Ferenbach, D. A. (2017). Renal aging: Causes and consequences. Journal of the American Society of Nephrology, 28, 407–420. 10.1681/ASN.2015121308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristoforou, R. , Petrou, P. P. , Sawyer, H. , Williams, M. , & Drousiotou, A. (2014). A novel large deletion encompassing the whole of the galactose‐1‐phosphate uridyltransferase (GALT) gene and extending into the adjacent interleukin 11 receptor alpha (IL11RA) gene causes classic galactosemia associated with additional phenotypic abnormalities. JIMD Reports, 12, 91–98. 10.1007/8904_2013_249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, S. R. , Bennett, F. , Calvetti, J. A. , Kelleher, K. , Wood, C. R. , O'Hara, R. M. Jr. , … Williams, D. A. (1990). Molecular cloning of a cDNA encoding interleukin 11, a stromal cell‐derived lymphopoietic and hematopoietic cytokine. Proceedings of the National Academy of Sciences of the United States of America, 87, 7512–7516. 10.1073/pnas.87.19.7512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini, J. P. , Hammill, B. G. , Sinner, M. F. , Jensen, P. N. , Hernandez, A. F. , Heckbert, S. R. , … Curtis, L. H. (2012). Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993‐2007. Circulation. Cardiovascular Quality and Outcomes, 5, 85–93. 10.1161/CIRCOUTCOMES.111.962688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putoczki, T. L. , Dobson, R. C. J. , & Griffin, M. D. W. (2014). The structure of human interleukin‐11 reveals receptor‐binding site features and structural differences from interleukin‐6. Acta Crystallographica. Section D, Biological Crystallography, 70, 2277–2285. [DOI] [PubMed] [Google Scholar]
- Qi, R. , & Yang, C. (2018). Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death & Disease, 9, 1126 10.1038/s41419-018-1157-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey, D. C. , Bell, P. D. , & Hill, J. A. (2015a). Fibrosis—A common pathway to organ injury and failure. The New England Journal of Medicine, 372, 1138–1149. 10.1056/NEJMra1300575 [DOI] [PubMed] [Google Scholar]
- Rockey, D. C. , Bell, P. D. , & Hill, J. A. (2015b). Fibrosis—A common pathway to organ injury and failure. The New England Journal of Medicine, 373, 96. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Iturbe, B. , Johnson, R. J. , & Herrera‐Acosta, J. (2005). Tubulointerstitial damage and progression of renal failure. Kidney International. Supplement, 68, S82–S86. 10.1111/j.1523-1755.2005.09915.x [DOI] [PubMed] [Google Scholar]
- Rose‐John, S. (2018). Interleukin‐6 family cytokines. Cold Spring Harbor Perspectives in Biology, 10, a028415 10.1101/cshperspect.a028415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom, J. , Macarak, E. , Piera‐Velazquez, S. , & Jimenez, S. A. (2017). Human fibrotic diseases: Current challenges in fibrosis research. Methods in Molecular Biology, 1627, 1–23. 10.1007/978-1-4939-7113-8_1 [DOI] [PubMed] [Google Scholar]
- Russo, I. , & Frangogiannis, N. G. (2016). Diabetes‐associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. Journal of Molecular and Cellular Cardiology, 90, 84–93. 10.1016/j.yjmcc.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, S. , Viswanathan, S. , Widjaja, A. A. , Lim, W.‐W. , Moreno‐Moral, A. , DeLaughter, D. M. , … Cook, S. A. (2017). IL‐11 is a crucial determinant of cardiovascular fibrosis. Nature, 552, 110–115. 10.1038/nature24676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, G.‐T. , Kim, D. R. , Lim, J.‐E. , Yim, H. , & Kim, H. (2008). Upregulation and function of GADD45γ in unilateral ureteral obstruction. Kidney International, 73, 1251–1265. [DOI] [PubMed] [Google Scholar]
- Shull, M. M. , Ormsby, I. , Kier, A. B. , Pawlowski, S. , Diebold, R. J. , Yin, M. , … Calvin, D. (1992). Targeted disruption of the mouse transforming growth factor‐β1 gene results in multifocal inflammatory disease. Nature, 359, 693–699. 10.1038/359693a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. W. 2nd (2000). Tolerability and side‐effect profile of rhIL‐11. Oncology, 14, 41–47. [PubMed] [Google Scholar]
- Spencer, S. , Köstel Bal, S. , Egner, W. , Lango Allen, H. , Raza, S. I. , Ma, C. A. , … Thaventhiran, J. E. D. (2019). Loss of the interleukin‐6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. The Journal of Experimental Medicine, 216, 1986–1998. 10.1084/jem.20190344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerk, L. , Sherer, J. A. , Ko, D. , Benjamin, E. J. , & Helm, R. H. (2017). Atrial fibrillation: Epidemiology, pathophysiology, and clinical outcomes. Circulation Research, 120, 1501–1517. 10.1161/CIRCRESAHA.117.309732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikoudis, A. , Cieślak, A. , Loffredo, L. , Chen, Y.‐W. , Patel, N. , Saqi, A. , … Snoeck, H. W. (2019). Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Reports, 27, 3709, e5–3723. 10.1016/j.celrep.2019.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga, T. , & Kishimoto, T. (1997). Gp130 and the interleukin‐6 family of cytokines. Annual Review of Immunology, 15, 797–819. [DOI] [PubMed] [Google Scholar]
- Takahashi, R. , Yokoji, H. , Misawa, H. , Hayashi, M. , Hu, J. , & Deguchi, T. (1994). A null mutation in the human CNTF gene is not causally related to neurological diseases. Nature Genetics, 7, 79–84. 10.1038/ng0594-79 [DOI] [PubMed] [Google Scholar]
- Tamura, Y. , Kohno, H. , Mohri, T. , Fujio, Y. , & Matsumiya, G. (2018). The cardioprotective effect of interleukin‐11 against ischemia‐reperfusion injury in a heart donor model. Ann Cardiothorac Surg, 7, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramura, M. , Kobayashi, S. , Hoshino, S. , Oshimi, K. , & Mizoguchi, H. (1992). Interleukin‐11 enhances human megakaryocytopoiesis in vitro. Blood, 79, 327–331. [PubMed] [Google Scholar]
- Voelker, J. , Berg, P. H. , Sheetz, M. , Duffin, K. , Shen, T. , Moser, B. , … Lewis, J. B. (2017). Anti‐TGF‐β1 antibody therapy in patients with diabetic nephropathy. Journal of the American Society of Nephrology, 28, 953–962. 10.1681/ASN.2015111230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja, A. A. , Dong, J. , Adami, E. , Viswanathan, S. , Ng, B. , Singh, B. K. , … Tan, J. (2019). Redefining Interleukin 11 as a regeneration‐limiting hepatotoxin. bioRxiv. 830018 [Google Scholar]
- Widjaja, A. A. , Singh, B. K. , Adami, E. , Viswanathan, S. , Dong, J. , D'Agostino, G. A. , … Tripathi, M. (2019). Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology, 157, 777, e14–792. [DOI] [PubMed] [Google Scholar]
- Wu, J. , Subbaiah, K. C. V. , Xie, L. H. , Jiang, F. , & Mickelsen, D. (2019). EPRS regulates proline‐rich pro‐fibrotic protein synthesis during cardiac fibrosis. bioRxiv, 82931263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn, T. A. (2008). Cellular and molecular mechanisms of fibrosis. The Journal of Pathology, 214, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Podok, P. , Xie, J. , & Lu, L. (2014). Comparative analysis of differential gene expression in kidney tissues of moribund and surviving crucian carp (Carassius auratus gibelio) in response to cyprinid herpesvirus 2 infection. Archives of Virology, 159, 1961–1974. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Ye, J. , Wang, M. , Wang, Y. , Ji, Q. , Huang, Y. , … Wan, J. (2018). Increased interleukin‐11 levels in thoracic aorta and plasma from patients with acute thoracic aortic dissection. Clinica Chimica Acta, 481, 193–199. 10.1016/j.cca.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Ye, J. , Wang, Z. , Ye, D. , Wang, Y. , Wang, M. , Ji, Q. , … Wan, J. (2019). Increased interleukin‐11 levels are correlated with cardiac events in patients with chronic heart failure. Mediators of Inflammation, 20191575410, 1–8. 10.1155/2019/1575410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , Filiberti, A. , Humphrey, M. B. , Fleming, C. D. , Scherlag, B. J. , Po, S. S. , & Stavrakis, S. (2019). Low‐level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Experimental Physiology, 104, 28–38. 10.1113/EP087351 [DOI] [PMC free article] [PubMed] [Google Scholar]


