Abstract
Background and Purpose
Cannabis or cannabinoids produce characteristic tetrad effects—analgesia, hypothermia, catalepsy and suppressed locomotion, which are believed to be mediated by the activation of cannabinoid CB1 receptors. Given recent findings of CB2 and GPR55 receptors in the brain, we examined whether these receptors are also involved in cannabinoid action.
Experimental Approach
We compared Δ9‐tetrahydrocannabinol (Δ9‐THC)‐, WIN55212‐2‐, or XLR11‐induced tetrad effects between wild‐type (WT) and each genotype of CB1‐, CB2‐ or GPR55‐knockout (KO) mice and then observed the effects of antagonists of these receptors on these tetrad effects in WT mice.
Key Results
Systemic administration of Δ9‐THC, WIN55212‐2 or XLR11 produced dose‐dependent tetrad effects in WT mice. Genetic deletion or pharmacological blockade of CB1 receptors abolished the tetrad effects produced by all three cannabinoids. Unexpectedly, genetic deletion of CB2 receptor abolished analgesia and catalepsy produced by Δ9‐THC or WIN55212‐2, but not by XLR11. Microinjections of Δ9‐THC into the lateral ventricles also produced tetrad effects in WT, but not in CB1‐KO mice. CB2‐KO mice displayed a reduction in intraventricular Δ9‐THC‐induced analgesia and catalepsy. In contrast to CB1 and CB2 receptors, genetic deletion of GPR55 receptors caused enhanced responses to Δ9‐THC or WIN55212‐2. Antagonisim of CB1, CB2 or GPR55 receptors produced alterations similar to those observed in each genotype mouse line.
Conclusions and Implications
These findings suggest that in addition to CB1, both CB2 and GPR55 receptors are also involved in some pharmacological effects produced by cannabinoids. CB1/CB2, in contrast to GPR55, receptors appears to play opposite roles in cannabinoid action.
Abbreviations
- AUC
Area under curve
- ANOVA
Analysis of variance
- CB1Rs
type 1 cannabinoid receptors
- CB2Rs
type 2 cannabinoid receptors
- CB1‐KO
CB1 receptor‐knockout
- CB2‐KO
CB2 receptor‐knockout
- GPR55s
GPR55 receptors
- GPR55‐KO
GPR55 receptor‐knockout
- XLR‐11
5″‐fluoro‐UR‐144
- Δ9‐THC
Δ9‐tetrahydrocannabinol
What is already known
Cannabinoids produce characteristic tetrad effects, which are generally believed to be mediated by CB1 receptors.
What this study adds
In addition to CB1, CB2 and GPR55 receptors are involved in some of cannabinoid‐induced tetrad.
Activation of CB1/CB2 versus GPR55 receptors appears to produce opposite effects in the cannabinoid‐induced tetrad.
What is the clinical significance
These new findings increase our understanding of the receptor mechanisms underlying cannabinoid action.
These findings may lead to the discovery of new pharmacotherapies for cannabinoid use disorder.
1. INTRODUCTION
Cannabis is one of the most commonly abused substances worldwide. The principal psychoactive component in cannabis is https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424 (Δ9‐THC), which produces a wide range of effects, including pleasure, relaxation, hypothermia, depressed motor activity, catalepsy and analgesia (Compton, Johnson, Melvin, & Martin, 1992; Little, Compton, Johnson, Melvin, & Martin, 1988; Parsons & Hurd, 2015; Varvel et al., 2005). For a long time, the psychoactive and biological effects of cannabis have been thought to be mediated mainly by stimulation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 This premise was based on the findings that CB1 receptors are highly expressed in the brain (Glass, Dragunow, & Faull, 1997) and pharmacological blockade or transgenic deletion of CB1 receptors abolishes Δ9‐THC‐induced characteristic tetrad effects (analgesia, hypothermia, catalepsy and locomotor impairment; Monory et al., 2007 ; Rinaldi‐Carmona et al., 1994 ; Zimmer, Zimmer, Hohmann, Herkenham, & Bonner, 1999). However, little is known about other non‐CB1 receptor mechanisms in cannabinoid action. We and others have recently reported that functional https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57 are also expressed in the brain (see a comprehensive review by Jordan & Xi, 2019) and are involved in multiple behavioural effects of Δ9‐THC or other cannabinoids in experimental animals (DeLong, Wolf, Poklis, & Lichtman, 2010; Lesniak et al., 2019; Liu et al., 2017). Although, little is known if brain CB2 receptors are also involved in cannabinoid‐induced tetrad effects. We have recently reported that brain CB1 and CB2 receptors mediate cannabis reward versus aversion, respectively, as assessed in a brain stimulation reward paradigm in rats (Spiller et al., 2019). Selective deletion of CB2 receptors from midbrain dopamine neurons altered multiple behavioural effects in mice produced by WIN55212‐2, a synthetic cannabinoid (Liu et al., 2017), suggesting a possible involvement of brain CB2 receptors in cannabinoid‐induced tetrad effects. In addition, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=109, another putative cannabinoid receptor (Baker, Pryce, Davies, & Hiley, 2006; Moriconi, Cerbara, Maccarrone, & Topai, 2010), was also found in brain regions involved in locomotion and nociception (Martínez‐Pinilla et al., 2014; Ryberg et al., 2007; Wu et al., 2013). It has been reported that GPR55 agonists inhibited haloperidol‐induced catalepsy (Celorrio et al., 2017), whereas a GPR55 antagonist reduced locomotor activity (Rahimi, Hajizadeh Moghaddam, & Roohbakhsh, 2015). Genetic deletion of GPR55 altered nociceptive responses in multiple pain models in rodents (Bjursell et al., 2016; Rahimi et al., 2015; Staton et al., 2008). These findings suggest that GPR55 activation may also be involved in cannabinoid‐induced tetrad effects. To test these hypotheses, we first used CB2‐knockout (KO) and GPR55‐KO mice to determine whether genetic deletion of CB2 or GPR55 receptors alters cannabinoid‐induced tetrad effects. Then we used pharmacological approaches to determine whether selective blockade of CB1, CB2 or GPR55 receptors similarly alters cannabinoid‐induced tetrad effects.
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=733 are among the most commonly used cannabinoids in research, while K2, Spice and other newly synthesized cannabinoids have gained popularity on the black market as “safe” or “legal” marijuana alternatives (Vardakou, Pistos, & Spiliopoulou, 2010). However, little is known about the pharmacology and biological effects of those synthetic cannabinoids. XLR11 (5″‐fluoro‐UR‐144) is a newly synthesized cannabinoid with potent https://en.wikipedia.org/wiki/Agonist profiles for CB1 and CB2 receptors (EC50 values of 98 and 83 nM, respectively; Banister et al., 2015). XLR11 was found to produce rapid, short‐lived hypothermic effects in rats (Banister et al., 2015). Given that XLR11 was invented specifically for grey‐market recreational use, we also included XLR11 in this study to determine whether XLR11 produces Δ9‐THC‐like tetrad effects via the same cannabinoid receptor mechanisms.
2. METHODS
2.1. Subjects
Male wild‐type (WT), CB 1 −/−, CB 2 −/− and GPR55 −/− mice with C57BL/6J genetic backgrounds (https://www.jax.org/strain/000664) were bred at the National Institute on Drug Abuse Intramural Research Program. Three CB 1 −/+ breeding pairs (Zimmer et al., 1999) were generously donated by A. Zimmer (National Institute of Mental Health). Three CB 2 −/+ breeding pairs (Buckley et al., 2000) were generously donated by G. Kunos (National Institute on Alcohol Abuse and Alcoholism). GPR55−/+ mouse breeders were purchased from the Texas Institute of Genomic Medicine (Houston, TX, USA; Wu et al., 2010). Heterozygous male and female (CB 1 −/+, CB 2 −/+ and GPR55−/+) mice were used to generate homozygous (CB1 −/−, CB2 −/− and GPR55−/−) mice and their WT littermates, aged 8–16 weeks, for the experiments. Mice were maintained on a reversed 12:12 hr light/dark cycle under standard vivarium conditions with free access to food and water. The housing conditions and care of the animals were consistent with those specified by the Guide for the Care and Use of Laboratory Animals. The protocols used in the present experiments were approved by the National Institute on Drug Abuse Animal Care and Use Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
2.2. Surgery
For the mice used in Experiment 4 below, intracranial guide cannula implantations were conducted under ketamine/xylazine (100 and 10 mg·kg−1, respectively) anaesthesia. Stainless steel guide cannulae were inserted bilaterally 1.0 mm above the microinjection sites in the lateral ventricles (at co‐ordinates from bregma: AP: −0.2 mm, ML: ±1.0 mm, and DV: −2.0 mm with the skull horizontally flat) and secured to the skull with screws and dental acrylic. Obturators, extending 1 mm beyond the cannulae, were inserted into the cannulae to prevent blockage and remained there at all times, except during microinjections. More details about the intracranial surgery and post‐surgery care and monitoring are described in the approved animal research protocol (19‐BNRB‐84) at NIDA IRP.
2.3. Cannabinoid‐induced tetrad effects in different genotypes of mice
Four genotypes of mice (WT, CB1 −/−, CB2 −/− and GPR55−/−) were used in this study. Each genotype of mice was then randomly divided into three groups (n = 8–10 per group) to evaluate the tetrad effects produced by Δ9‐THC, WIN55212‐2 or XLR11, respectively. Each animal randomly received three injections of different doses of Δ9‐THC (0, 10 or 30 mg·kg−1, s.c.), WIN55212‐2 (0, 3 or 10 mg·kg−1, s.c.), or XLR11 (0, 10 or 30 mg·kg−1, s.c.). In experiments involving intraventricular microinjections, after basal levels of tetrad measures were established, Δ9‐THC (0, 30 or 100 μg/0.5 μl) was microinjected into the ventricles bilaterally over 60 s and the microinjectors were kept in place for an additional 60 s to allow the drug to diffuse. In all experiments, mice were tested sequentially for hypothermia, analgesia, catalepsy and immobility (rotarod) at 0 (before the drug injection) and 0.5, 1, 2 and 3 hr after the drug injection. The group sizes of animals used in different experiments are stated in each figure legends. A four‐scale “tetrad” behavioural test (Compton et al., 1992; Little et al., 1988) was used to assess the effects of systemic administration of Δ9‐THC, WIN55212‐2, XLR11 and intraventricular injections of Δ9‐THC on hypothermia, analgesia, catalepsy and locomotor impairment. In each instance, all ratings of behavioural tests were performed by observers blinded to the experimental treatment.
2.4. Cannabinoid‐induced tetrad effects in the presence of CB1, CB2 or GPR55 receptor antagonists in WT mice
To confirm the above findings in gene mutant mice, we further investigated whether pharmacological blockade of CB1, CB2 or GPR55 receptors, respectively, produces similar alterations in cannabinoid‐induced tetrad effects in WT mice. Due to the concern of repeated cannabinoid‐induced tolerance in tetrad effects, we used between‐subjects design in this experiment. A total of 88 WT mice were divided into 12 groups—four Δ9‐THC dose groups (n = 8 per group), four WIN55212‐2 dose groups (n = 8), and four XLR11 dose groups (n = 6–8). Each group of mice received only one cannabinoid injection pretreated with vehicle, AM251 (a selective CB1 antagonist, 3 mg·kg−1, i.p.), AM630 (a selective CB2 antagonist, 3 mg·kg−1, i.p.) or CID16020046 (a selective GPR55 antagonist, 3 mg·kg−1, i.p.), 30 min prior to Δ9‐THC (30 mg·kg−1), WIN55212‐2 (10 mg·kg−1) or XLR11 (30 mg·kg−1) injection. Then the effects of each of the antagonists on cannabinoid‐induced tetrad effects were evaluated.
2.5. Analgesia assessment
Analgesia (defined as a decrease in thermal pain sensitivity) was assessed using a hot‐plate device (Model 39, IITC Life Science Inc., CA, USA). A mouse was placed inside a transparent cage on the hot plate (55 ± 0.2°C). The latency to the first sign of thermal nociception (licking, stomping the hind paw or jumping) was measured in seconds. The cut‐off time for the test was 60 s to avoid tissue damage.
2.6. Hypothermia assessment
Hypothermia (defined as a decrease in rectal temperature; °C) was measured with a thermometer connected to a lubricated RET‐2 rectal probe (Harvard Apparatus, Holliston, MA, USA) that was inserted into the rectum to a depth of 2 cm.
2.7. Catalepsy assessment
Catalepsy, defined as an impaired capacity to initiate movements, was measured using an elevated bar test. Mice were hung by their front paws from a rubber coated metal bar (12‐cm length) that was fixed horizontally at a height allowing their hind paws to just touch the floor. The latency to when the mouse descended from the bar (i.e. when the two forepaws touched the floor) or when 3 min elapsed (i.e. cut‐off time) was measured.
2.8. Immobility assessment
Immobility was determined using a rotarod device (Harvard Apparatus) and defined as latency to fall from the rotarod rotating at increasing speed from 4 to 40 rev·min−1 over 5 min. The time (s) taken to fall off the rotarod was recorded.
2.9. Drugs
Δ9‐THC was provided by the National Institute on Drug Abuse (Baltimore, MD, USA). WIN55212‐2 was purchased from Sigma‐Aldrich (St. Louis, MO, USA) and XLR11 was purchased from Caymen Chemical (Ann Arbor, MI, USA). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3317, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=750 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6577 were purchased from Tocris Bio‐Techne Corporation (MN, USA). All cannabinoids were dissolved in 5% cremophor (Sigma‐Aldrich) and were injected subcutaneously in volumes of 1 ml·kg−1. In an experiment involving microinjections, Δ9‐THC was dissolved to achieve 30‐ and 100‐μg doses that were delivered in volumes of 0.5 μl per microinjection side.
2.10. Data and analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Animal group sizes were chosen based on a power analysis (n = 8–10 per group) and extensive previous experience with the animal models used. No data points were excluded from the analysis in any experiment. To validate the use of parametric statistics, we ensured that our data were normally distributed and met variance homogeneity criteria with group sizes n > 5. Statistical analysis was done using the independent values coming from individual animals in each group. All values were presented as mean ± SEM. Three‐way ANOVAs were used to analyse the drug effects over time, drug dose, and mouse genotype in each measure of the tetrad. Significant Dose × Genotype × Time interactions were followed by Dose × Time interaction comparisons for each genotype. The post hoc tests were conducted only if F in ANOVA achieved P < .05 and there was no significant variance inhomogeneity. We also measured the changes in the AUC (ΔAUC) to evaluate the tetrad effects of each of the cannabinoids over dose or genotype. Specifically, ΔAUC was measured as the sum of differences between each time point after the drug injection and the baseline before the injection. Group differences were analysed using separate two‐way (Genotype × Dose or Treatment × Time) ANOVAs, followed by tests of simple effect of mouse genotype for each dose using one‐way ANOVAs. The value of P < .05 was used to indicate statistically significant differences among or between groups.
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (RRID:SCR_013077; Alexander et al., 2019).
3. RESULTS
3.1. Δ9‐THC‐induced tetrad in different genotypes of mice
As shown in Figure 1, Δ9‐THC, at 10 and 30 mg·kg−1 (s.c.), produced characteristic cannabinoid‐induced tetrad effects—analgesia (Figure 1a), hypothermia (Figure 1b), catalepsy (Figure 1c) and immobility (Figure 1d) in WT mice. These effects were completely absent in CB1‐KO mice (Figure 1, left second panels). Since CB1‐KO mice did not show any response to Δ9‐THC, we further assessed their nociceptive response to opioid administration. Systemic administration of morphine (10 mg·kg−1, s.c.) produced a significant increase in pain threshold to thermal stimulation, similar to that observed in WT mice (Figure 1a), suggesting a selective loss of analgesia to Δ9‐THC in CB1‐KO mice. Unexpectedly, genetic deletion of CB2 receptors also abolished Δ9‐THC‐induced analgesia and catalepsy but had no effect on Δ9‐THC‐induced hypothermia and immobility, suggesting an involvement of CB2 receptors in Δ9‐THC‐induced analgesia and catalepsy. In contrast, genetic deletion of GPR55 produced augmented hypothermia, catalepsy and immobility responses to the low dose of Δ9‐THC (10 mg·kg−1) as compared to WT mice, suggesting an inhibitory role of GPR55 on Δ9‐THC action. Three‐way ANOVAs revealed significant Time × Dose × Genotype interactions for analgesia, hypothermia, catalepsy and locomotor impairment. Subsequent two‐way ANOVA results for each drug‐induced tetrad effect in each genotype of mice are provided in Table 1. For presentation clarity, post hoc group comparison results are shown on each figure panel in Figure 1 using different symbols (*P < .05, as compared to vehicle. # P < .05, as compared to the baseline prior to the Δ9‐THC injection).
Figure 1.
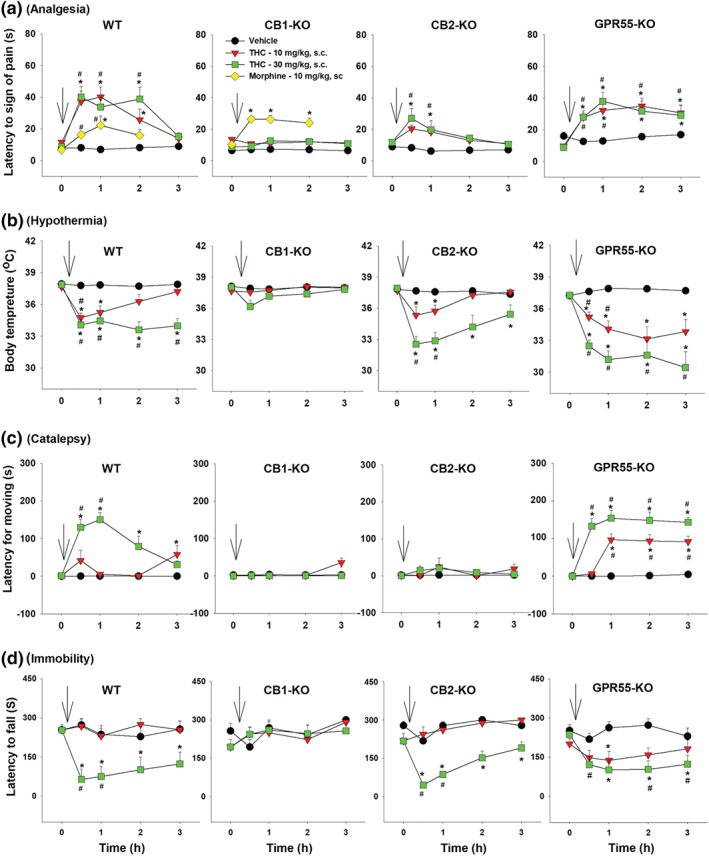
The effect of systemic administration of Δ9‐THC or morphine on (a) analgesia (as assessed by hot‐plate test), (b) hypothermia (as assessed by deep rectal temperature), (c) catalepsy (as assessed by elevated bar test) and (d) immobility (as assessed by rotarod performance) in WT, CB1‐KO, CB2‐KO and GPR55‐KO mice. *P < .05, as compared to vehicle. # P < .05, as compared to the baseline prior to the Δ9‐THC injection. Arrows indicate drug injections. Group sizes: for WT, n = 7; for CB1‐KO, n = 8; for CB2‐KO, n = 8; and for GPR55‐KO, n = 8
Table 1.
Statistical analysis results (Treatment × Time interactions) from two‐way ANOVAs for repeated measures over time and drug dose
| Figure 1 | WT Δ9‐THC (s.c.) | CB1‐KO Δ9‐THC (s.c.) | CB2‐KO Δ9‐THC (s.c.) | GPR55‐KO Δ9‐THC (s.c.) |
|---|---|---|---|---|
| Analgesia | F 8,320 = 11.17; P < .05 | F 8,320 = 0.26; P > .05 | F 8,320 = 1.84; P > .05 | F 8,320 = 5.88; P < .05 |
| Hypothermia | F 8,324 = 7.25; P < .05 | F 8,324 = 0.96; P > .05 | F 8,324 = 8.11; P < .05 | F 8,324 = 34.07; P < .05 |
| Catalepsy | F 8,324 = 19.94; P < .05 | F 8,324 = 0.93; P > .05 | F 8,324 = 0.62; P > .05 | F 8,324 = 15.76; P < .05 |
| Immobility | F 24,320 = 5.57; P < .05 | F 8,320 = 1.54; P > .05 | F 8,320 = 4.5; P < .05 | F 8,320 = 2.31; P < .05 |
| Figure 3 | WT WIN55212‐2 (s.c.) | CB1‐KO WIN55212‐2 (s.c.) | CB2‐KO WIN55212‐2 (s.c.) | GPR55‐KO WIN55212‐2 (s.c.) |
|---|---|---|---|---|
| Analgesia | F 8,348 = 2.18; P < .05 | F 8,348 = 0.16; P > .05 | F 8,348 = 1.15; P > .05 | F 8,348 = 19.04; P < .05 |
| Hypothermia | F 8,360 = 18.35; P < .05 | F 8,360 = 0.43; P > .05 | F 8,360 = 11.19; P < .05 | F 8,360 = 33.55; P < .05 |
| Catalepsy | F 8,348 = 15.93; P < .05 | F 8,348 = 0.03; P > .05 | F 8,348 = 1.72; P > .05 | F 8,348 = 37.01; P < .05 |
| Immobility | F 8,340 = 11.79; P < .05 | F 8,340 = 0.83; P > .05 | F 8,340 = 1.48; P < .05 | F 8,340 = 29.39; P < .05 |
| Figure 5 | WT XLR‐11 (s.c.) | CB1‐KO XLR‐11 (s.c.) | CB2‐KO XLR‐11 (s.c.) | GPR55‐KO XLR‐11 (s.c.) |
|---|---|---|---|---|
| Analgesia | F 8,284 = 10.29; P < .05 | F 8,284 = 0.49; P > .05 | F 8,284 = 16.09 P < .05 | F 8,284 = 12.01; P < .05 |
| Hypothermia | F 8,288 = 17.13; P < .05 | F 8,288 = 0.55; P > .05 | F 8,288 = 19.90; P < .05 | F 8,288 = 26.15; P < .05 |
| Catalepsy | F 8,292 = 14.07; P < .05 | F 8,292 = 0.008; P > .05 | F 8,292 = 9.02; P < .05 | F 8,292 = 12.21; P < .05 |
| Immobility | F 8,268 = 14.98; P < .05 | F 8,268 = 1.34; P > .05 | F 8,268 = 15.94; P < .05 | F 8,268 = 9.43; P < .05 |
| Figure 7 | WT Δ9‐THC (i.c.v.) | CB1‐KO Δ9‐THC (i.c.v.) | CB2‐KO Δ9‐THC (i.c.v.) |
|---|---|---|---|
| Analgesia | F 8,292 = 9.44; P < .05 | F 8,292 = 1.79; P > .05 | F 8,292 = 0.89; P > .05 |
| Hypothermia | F 8,292 = 11.35; P < .05 | F 8,292 = 1.12; P > .05 | F 8,292 = 6.08; P < .05 |
| Catalepsy | F 8,236 = 12.78; P < .05 | F 8,236 = 1.16; P > .05 | F 8,236 = 2.14; P > .05 |
| Immobility | F 4,156 = 5.64; P < .05 | F 4,156 = 0.84; P > .05 | F 4,146 = 6.36; P < .05 |
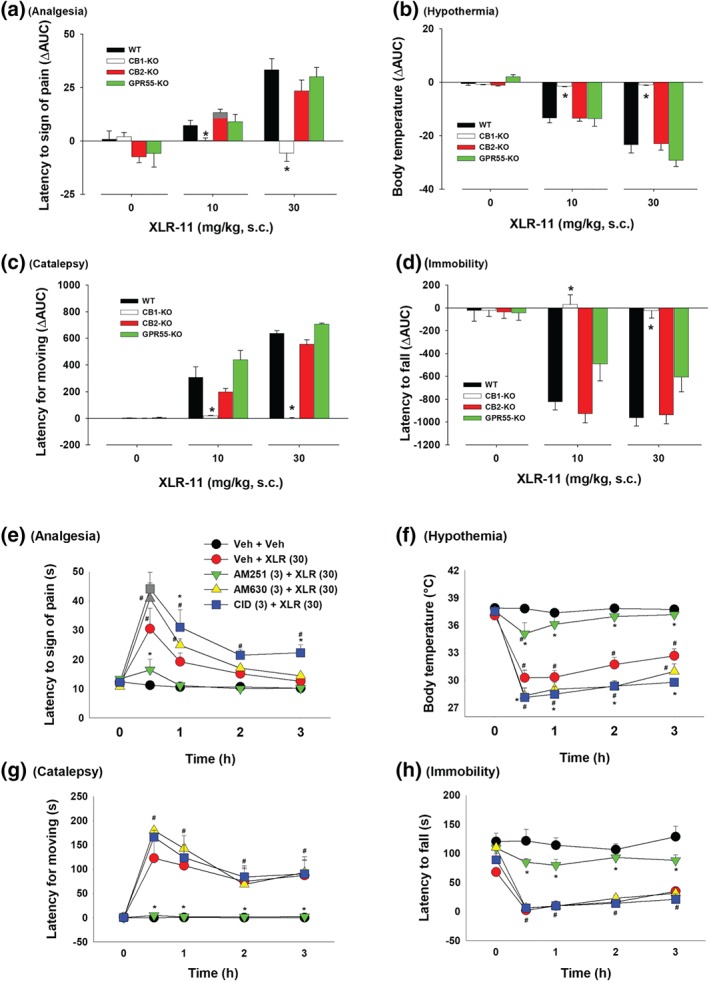
To further determine genotype differences in Δ9‐THC‐induced tetrad, we calculated changes in the AUC (ΔAUC) after each dose of Δ9‐THC (Figure 2). Two‐way ANOVAs revealed significant Dose × Genotype interactions for analgesia (Figure 2a), hypothermia (Figure 2b), catalepsy (Figure 2c) and locomotor impairment (Figure 2d). Subsequent interaction comparisons for each dose of Δ9‐THC revealed significant genotype effects for analgesia (Figure 2a, 10 and 30 mg·kg−1), hypothermia (Figure 2b, 10 and 30 mg·kg−1), catalepsy (Figure 2c, 10 and 30 mg·kg−1) and locomotor impairment (Figure 2d, 30 mg·kg−1). Post hoc individual group comparisons revealed significant reductions in each measure in CB1‐KO mice (Figure 2a–d), significant reductions in analgesia and catalepsy in CB2‐KO mice (Figure 2a–d) and significant increases in hypothermia and catalepsy in GPR55‐KO mice (Figure 2a–d, as compared to WT control mice).
Figure 2.
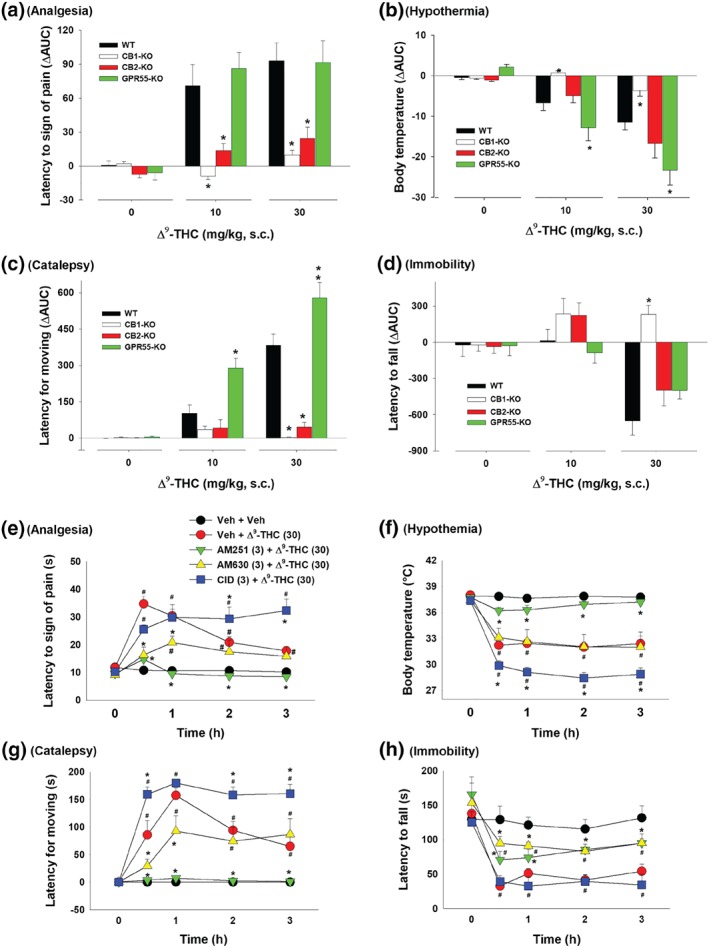
Differences in the AUC (ΔAUC) in (a) analgesia, (b) hypothermia, (c) catalepsy and (d) immobility after each dose of Δ9‐THC or vehicle injection in the four genotypes of mice. *P < .05, as compared to WT mice. (e–h) The effects of pretreatment with AM251, AM630 or CID16020046 on Δ9‐THC‐induced tetrad in WT mice (n = 8 per group). # P < .05, as compared to the baseline before Δ9‐THC injection in each group; *P < .05, as compared to “Veh + Δ9‐THC” group
3.2. Δ9‐THC‐induced tetrad effects in the presence of CB1, CB2, or GPR55 antagonist
Figure 2e–h shows that blockade of CB1 receptors by AM251 abolished 30 mg·kg−1 Δ9‐THC‐induced tetrad effects in WT mice, while pretreatment with AM630 significantly attenuated Δ9‐THC‐induced analgesia and catalepsy but had no effect on Δ9‐THC‐induced hypothermia and locomotor impairment. In contrast, pretreatment with CID16020046 produced significant enhancement in Δ9‐THC‐induced analgesia, hypothermia, and catalepsy. A two‐way ANOVA with time as a repeated‐measures factor revealed a significant main effect of antagonist treatment (Figure 2e‐h), main effect of time (Figure 2e‐h) and Treatment × Time interactions (Figure 2e‐h). Post hoc tests for multiple group comparisons revealed significant reductions or increases in Δ9‐THC‐induced tetrad effects after the AM251, AM630 or CID16020046 administration (Figure 2e–h).
3.3. WIN55212‐2‐induced tetrad effects in different genotypes of mice
As shown in Figure 3, WIN55212‐2, at 3 and 10 mg·kg−1 (s.c.), produced significant and dose‐dependent tetrad effects in WT mice similar to those produced by Δ9‐THC. Genetic deletion of CB1 receptors abolished WIN55212‐2‐induced tetrad effects (Figure 3, left second panels), while genetic deletion of CB2 receptors selectively blocked WIN55212‐2‐induced analgesia and catalepsy but had no effect on WIN55212‐2‐induced hypothermia or immobility (Figure 3, right second panels). Strikingly, genetic deletion of GPR55 produced robust enhancement in each tetrad measure in response to WIN55212‐2, particularly at the lower dose (3 mg·kg−1) of WIN55212‐2 (Figure 3, right panels). Three‐way ANOVAs revealed significant Time × Dose × Genotype interactions for analgesia, hypothermia, catalepsy and locomotor impairment. Subsequent statistical analyses using two‐way ANOVAs for each figure panel are provided in Table 1.
Figure 3.
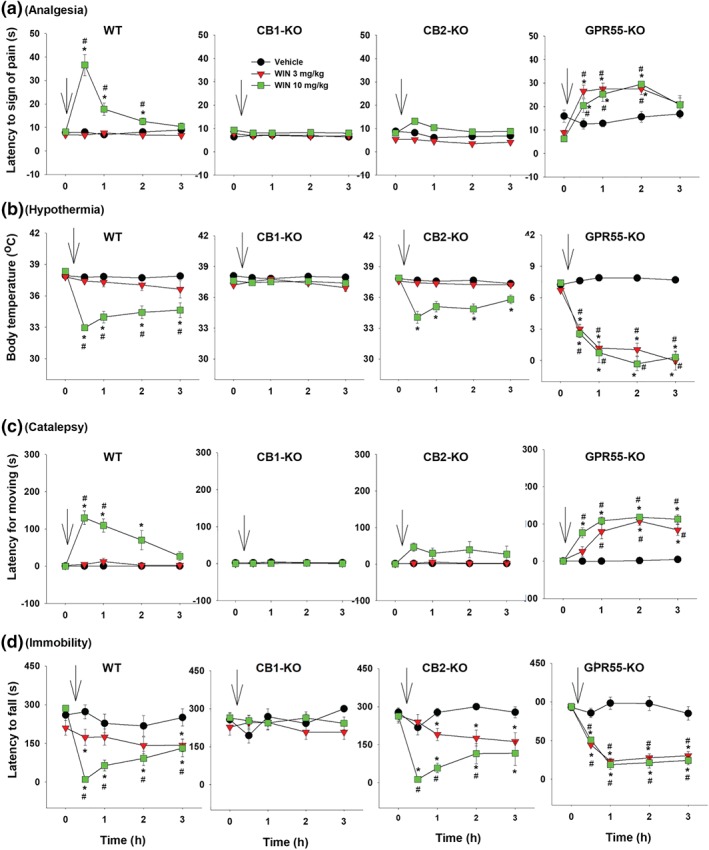
The effect of systemic administration of WIN55212‐2 on (a) analgesia, (b) hypothermia, (c) catalepsy and (d) immobility in WT, CB1‐KO, CB2‐KO, and GPR55‐KO mice. *P < .05, as compared to vehicle. # P < .05, as compared to the baseline before the WIN55212‐2 treatment. For WT, n = 10; for CB1‐KO, n = 8; for CB2‐KO, n = 9; for GPR55‐KO, n = 8
Figure 4 shows the ΔAUC data after each dose of WIN55212‐2 administration in the different mouse genotypes. Two‐way ANOVAs revealed significant Dose × Genotype interactions in analgesia (Figure 4a), hypothermia (Figure 4b), catalepsy (Figure 4c) and immobility (Figure 4d). Subsequent one‐way ANOVAs for each dose of WIN55212‐2 revealed significant genotype effects in analgesia (Figure 4a, 3 and 10 mg·kg−1), hypothermia (Figure 4b, 3 and 10mg·kg−1), catalepsy (Figure 4c, 3 and 10 mg·kg−1) and locomotor impairment (Figure 4d, 3 and 10 mg·kg−1). Post hoc tests for multiple group comparisons revealed significant reductions in each tetrad measure after CB1 receptor deletion (Figure 4a–d), significant reductions in analgesia and catalepsy after CB2 receptor deletion (Figure 4a–d) and significant increases in responses to 3 mg·kg−1 WIN55212‐2 after GPR55 deletion (Figure 4a–d).
Figure 4.
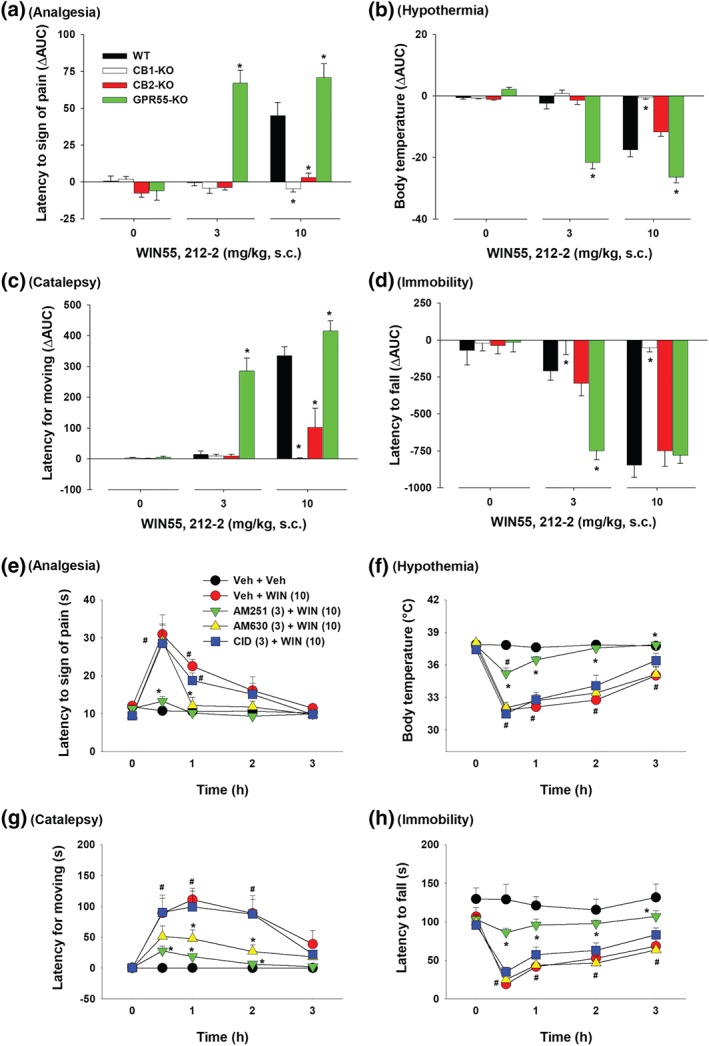
Differences in the AUC (ΔAUC) for (a) analgesia, (b) hypothermia, (c) catalepsy and (d) immobility after the WIN55212‐2 treatment in four genotypes of mice. *P < .05, as compared to WT mice. (e–h) The effects of pretreatment with AM251, AM630, or CID16020046 on WIN55212‐2‐induced tetrad in WT mice (n = 8 per group). # P < .05, as compared to the baseline before the Δ9‐THC injection in each group; *P < .05, as compared to “Veh + WIN” group
3.4. WIN55212‐2‐induced tetrad effects in the presence of CB1, CB2 or GPR55 antagonist
Figure 4e–h shows that blockade of CB1 receptors by AM251 abolished 10 mg·kg−1 WIN55212‐2‐induced tetrad effects, while pretreatment with AM630 attenuated WIN55212‐2‐induced analgesia and catalepsy. CID16020046 failed to alter WIN55212‐2‐induced tetrad effects. Two‐way ANOVAs with time as repeated‐measure factor and dose as between‐group factor revealed significant main effects of pretreatments (Figure 4e‐h), main effects of time (Figure 4e‐h) and Treatment × Time interactions (Figure 4e‐h). Post hoc tests for multiple group comparisons revealed significant reductions in WIN55212‐2‐induced tetrad after AM251 administration (Figure 4e–h)
3.5. XLR11‐induced tetrad effects in different genotypes of mice
Figure 5 shows that systemic administration of XLR11 produced cannabinoid‐like tetrad effects in WT mice (Figure 5, left panels), in a similar manner as Δ9‐THC (Figure 1) and WIN55212‐2 (Figure 3). Genetic deletion of CB1 receptors abolished XLR11‐induced tetrad effects, while genetic deletion of CB2 receptors or GPR55 receptors failed to alter XLR11‐induced tetrad effects. Three‐way ANOVAs revealed significant Time × Dose × Genotype interactions for analgesia, hypothermia, catalepsy and immobility. Interaction comparisons revealed significant XLR11 Dose × Time interactions in WT, CB2‐KO and GPR55‐KO mice, but not in CB1‐KO mice (see Table 1 for more details).
Figure 5.
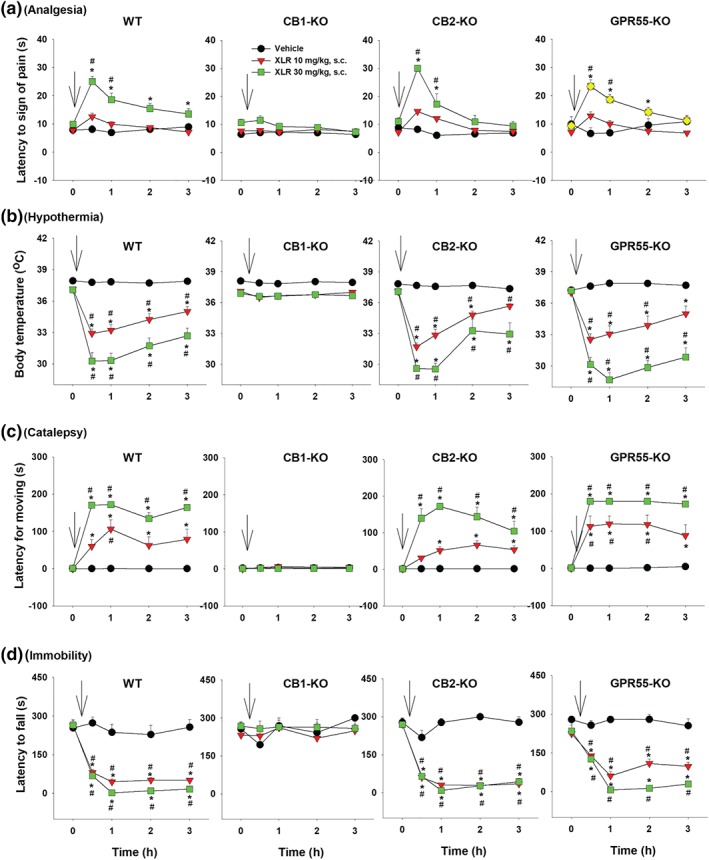
The effect of systemic administration of XLR11 on (a) analgesia, (b) hypothermia, (c) catalepsy and (d) immobility in WT, CB1‐KO, CB2‐KO, and GPR55‐KO mice. *P < .05, as compared to vehicle. # P < .05, as compared to the baseline before the XLR11 injection. For WT, n = 7; for CB1‐KO, n = 8; for CB2‐KO, n = 6; for GPR55‐KO, n = 8
Figure 6 shows the ΔAUC data after each dose of XLR11 administration, indicating that the tetrad effects of XLR11 are mediated by stimulation of CB1 but not CB2 or GPR55 receptors. Two‐way ANOVAs revealed significant Dose × Genotype interactions for analgesia (Figure 6a), hypothermia (Figure 6b), catalepsy (Figure 6c) and locomotor impairment (Figure 6d). Subsequent one‐way ANOVAs for each dose of XLR11 revealed significant genotype effects for analgesia (Figure 6a, 30 mg·kg−1), hypothermia (Figure 6b, 10 and 30 mg·kg−1), catalepsy (Figure 6c, 10 and 30 mg·kg−1), and immobility (Figure 6d, 10 and 30 mg·kg−1). Post hoc tests for multiple group comparisons revealed significant reductions in each measure after CB1 receptor deletion (Figure 6a–d).
Figure 6.
Differences in the AUC (ΔAUC) for (a) analgesia, (b) hypothermia, (c) catalepsy and (d) immobility after the XLR11treatment in four genotypes of mice. *P < .05, as compared to WT mice. (e–h) The effects of pretreatment with AM251, AM630, or CID16020046 on XLR11‐induced tetrad in WT mice (n = 6 per group). # P < .05, as compared to the baseline before the XLR11 injection in each group; *P < .05, as compared to “Veh + XLR” group
3.6. XLR11‐induced tetrad effects in the presence of CB1, CB2 or GPR55 antagonist
Figure 6e–h shows that blockade of CB1 receptors by AM251 abolished 10 mg·kg−1 XLR11‐induced tetrad effects, while pretreatment with AM630 or CID16020046 failed to alter XLR11‐induced tetrad effects or produced an enhancement in XLR11‐induced analgesia and hypothermia. Two‐way ANOVAs with time as repeated‐measure factor revealed significant main effects of pretreatments with different antagonists (Figure 6e,‐h), main effects of time (Figure 6e‐h) and Treatment × Time interactions (Figure 6e‐h). Post hoc tests for multiple group comparisons revealed significant reductions in XLR11‐induced tetrad effect after the AM251 administration (Figure 6e–h as compared to “Veh + XLR” group) but enhancement in analgesia and hypothermia after the CID16020046 administration (Figure 6e–h).
3.7. Intraventricular Δ9‐THC‐induced tetrad effects
An important finding in the above experiments is that CB2 receptor deletion significantly attenuated Δ9‐THC‐ or WIN55212‐2‐induced analgesia and catalepsy (Figures 1, 2, 3, 4). To determine whether brain or peripheral CB2 receptor mediate Δ9‐THC‐induced analgesia and catalepsy, we assessed the effects of intracerebral ventricular (i.c.v.) microinjections of Δ9‐THC‐induced tetrad in WT, CB1‐KO and CB2‐KO mice. Figure 7 shows that i.c.v. microinjections of Δ9‐THC (100 μg per side) produced significant tetrad effects in WT mice, similar to those observed with higher doses of Δ9‐THC (10–30 mg·kg−1 ≅ 300–900 μg per mouse administered systemically [30 g], Figure 1). Again, genetic deletion of CB1 receptors abolished i.c.v. Δ9‐THC‐induced tetrad effects (Figure 7, left second panels), while genetic deletion of CB2 receptors selectively blocked i.c.v. Δ9‐THC‐induced analgesia and catalepsy (Figure 7, right second panels).
Figure 7.
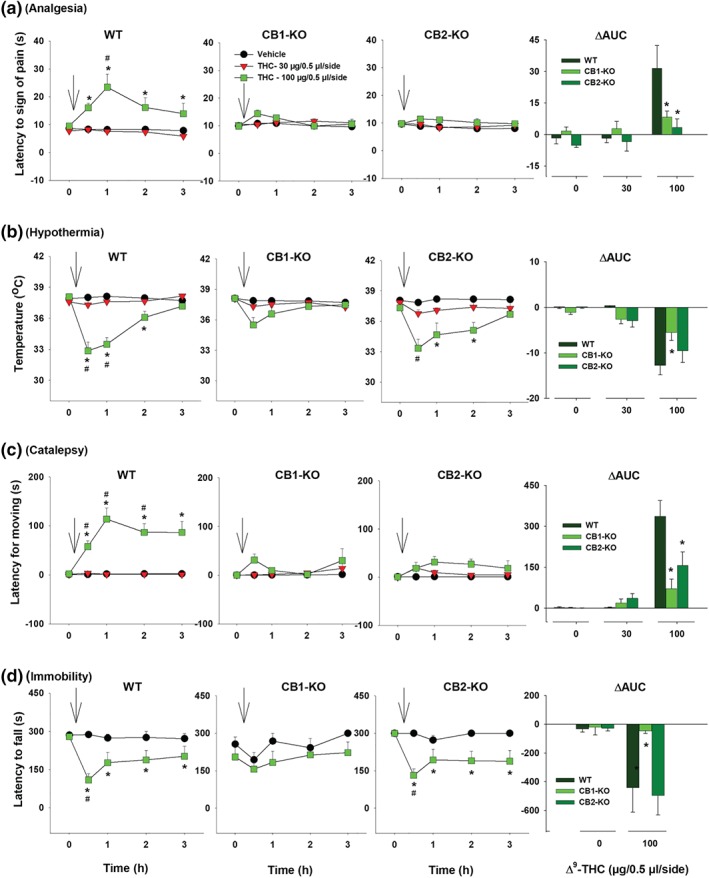
The effects of intracerebral ventricular (i.c.v.) microinjections of Δ9‐THC on (a) analgesia, (b) hypothermia, (c) catalepsy and (d) immobility in WT, CB1‐KO, and CB2‐KO mice. The right panels show the ΔAUC after each dose of i.c.v. Δ9‐THC microinjections. *P < .05, as compared to vehicle. # P < .05, as compared to the baseline before the Δ9‐THC microinjection. For WT, n = 8; for CB1‐KO, n = 10; for CB2‐KO, n = 10
Finally, we analysed the ΔAUC data after each dose of i.c.v. Δ9‐THC (Figure 7, right panels). Two‐way ANOVAs revealed significant Dose × Genotype interactions for analgesia (Figure 7a, right), hypothermia (Figure 7b, right), catalepsy (Figure 7c, right) and immobility (Figure 7d, right). Subsequent one‐way ANOVAs for each dose of Δ9‐THC revealed significant genotype effect in analgesia (Figure 7a, right, 100 μg/0.5 μl per side), hypothermia (Figure 7b, right, 100 μg/0.5 μl per side), catalepsy (Figure 7c, right, 100 μg/0.5 μl per side) and immobility (Figure 7d, right, 100 μg/0.5 μl per side). Post hoc tests for multiple group comparisons revealed significant reductions in these measures after CB1 receptor deletion.
4. DISCUSSION
The major findings of the present study include (a) all three tested cannabinoids produced the classical tetrad effects; (b) genetic deletion of CB1 receptors abolished these effects produced by all three compounds, suggesting a critical role of CB1 receptors in cannabinoid‐induced tetrad effects; (c) genetic deletion of CB2 receptors selectively blocked Δ9‐THC‐ or WIN55212‐2‐induced analgesia and catalepsy, suggesting an involvement of CB2 receptors in some but not all cannabinoid effects; (d) genetic deletion of CB1 receptors or CB2 receptors produced similar alterations on the intraventricular Δ9‐THC‐induced tetrad effects; (e) genetic deletion of GPR55 either failed to alter or produced an enhanced response to Δ9‐THC or WIN55212‐2; (f) genetic deletion of CB1 receptors, but not CB21 receptors or GPR55s, blocked XLR11‐induced tetrad effects, and finally (g) pharmacological blockade of each receptor produced alterations similar to those observed in respective mutant mice. These findings suggest that distinct receptor mechanisms, at least including CB1, CB2 and GPR55 receptors, underlie the classical pharmacological action produced by phytocannabinoids versus synthetic cannabinoids.
4.1. Role of CB1 receptors in cannabinoid‐induced tetrad effects
The first important finding in the present experiment is that CB1 receptors are critically involved in the three cannabinoid‐induced tetrad effects, that is, those produced by Δ9‐THC, WIN55212‐2 and XLR11. This is fully consistent with previous reports that pharmacological blockade or genetic deletion of CB1 receptors attenuated or abolished Δ9‐THC‐ or WIN55212‐2‐induced tetrad effects (Abalo et al., 2011; Compton, Aceto, Lowe, & Martin, 1996; Fox et al., 2001; Grim et al., 2017; Ledent et al., 1999; Varvel et al., 2005). It is well documented that CB1 receptors are broadly expressed in both the CNS and peripheral tissues (Howlett & Abood, 2017), but it is to be determined whether brain or peripheral CB1 receptors underlie cannabinoid‐induced tetrad effects. Here, we showed that i.c.v. microinjections of Δ9‐THC produced dose‐dependent tetrad effects in WT mice, but not in CB1‐KO mice, while systemic administration of low doses (<10 mg·kg−1) or the same micro‐amount of Δ9‐THC (200 μg) failed to produce any tetrad effects in WT mice (data not shown), suggesting that brain CB1 receptors mediate these effects. Our behavioural findings with these three cannabinoids are consistent with their high binding affinities to CB1 receptors (Paronis, Nikas, Shukla, & Makriyannis, 2012; Pertwee, 2010; Tai & Fantegrossi, 2017).
Given that CB1 receptors are highly expressed on both glutamatergic and GABAergic neurons in the brain, a research group in Germany used Cre‐LoxP techniques to selectively delete CB1 receptors from cortical glutamatergic or GABAergic neurons (Monory et al., 2007). They found that mice lacking CB1 receptors in GABAergic neurons responded to Δ9‐THC similarly to WT littermates, whereas mice lacking CB1 receptors in all principal (glutamatergic) neurons showed little, if any, behavioural or autonomic responses to Δ9‐THC, suggesting that CB1 receptors on cortical glutamatergic neurons play an important role in Δ9‐THC‐induced tetrad effects. More studies are needed to determine whether a cell type‐specific CB1 receptor mechanism underlies WIN55212‐2‐ or XLR11‐induced tetrad effects.
4.2. Role of CB2 receptors in cannabinoid‐induced tetrad effects
The second important finding in the present study is the involvement of brain CB2 receptors in cannabinoid‐induced analgesia and catalepsy—as indicated by a lack of Δ9‐THC‐ or WIN55212‐2‐induced analgesia and catalepsy in CB2‐KO mice. CB2 receptors were previously considered peripheral receptors modulating the immune system (Atwood & Mackie, 2010; Buckley, 2008). However, this view has been challenged by recent findings indicating that functional CB2 receptors are expressed in the brain (Jordan & Xi, 2019). Although brain CB2 receptor levels are very low in healthy subjects, the expression of CB2 receptors in the brain is dynamic and inducible and can be up‐regulated in response to various insults (Jordan & Xi, 2019; Zhang et al., 2014, 2017). Strikingly, systemic or intracranial microinjections of Δ9‐THC produced full tetrad effects in WT, while genetic deletion of CB2 receptors blocked Δ9‐THC‐induced analgesia and catalepsy but had no effect on Δ9‐THC‐induced hypothermia and immobility, suggesting an involvement of brain CB2 receptors . These findings are consistent with an early report indicating that deletion of CB2 receptors in CB2‐KO mice failed to alter Δ9‐THC‐induced hypothermia and immobility (Buckley et al., 2000). Furthermore, Onaivi et al. (2008) have recently reported that selective deletion of CB2 receptors on dopaminergic neurons reduced WIN55212‐2‐induced catalepsy and altered WIN55212‐2‐induced analgesia (as assessed in a tail flick test) but had no effect on WIN55212‐2‐induced hypothermia and hypoactivity (Liu et al., 2017), which provides additional evidence supporting brain CB2 receptor involvement in cannabinoid‐induced pharmacological effects. We note that pretreatment with AM251 almost completely blocked, while AM630 only partially attenuated Δ9‐THC‐ or WIN55212‐2‐induced analgesia and catalepsy, suggesting that CB1 receptors play a more important role than CB2 receptors in cannabinoid action. Overall, this is consistent with their distributions in the brain.
4.3. Role of GPR55 receptors in cannabinoid‐induced tetrad effects
The third important finding in the present study is that mice lacking GPR55 receptors displayed enhanced tetrad responses to low doses of Δ9‐THC or WIN55212‐2, but not to XLR11. Similarly, blockade of GPR55 by CID16020046 also enhanced behavioural response to Δ9‐THC, but not WIN55212‐2, suggesting that GPR55 stimulation produces an inhibitory effect on cannabinoid action. Thus, we propose that the tetrad effects observed in WT mice could be the final balance between two opposite actions—CB1/CB2‐mediated tetrad‐enhancing effects and GPR55‐mediated tetrad‐attenuating effects. This hypothesis is supported by the findings that GPR55 stimulation by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5526 (abn‐CBD) attenuated haloperidol‐induced catalepsy (Celorrio et al., 2017), while GPR55 deletion impaired locomotor performance (Bjursell et al., 2016; Celorrio et al., 2017; Meadows et al., 2016; Wu et al., 2013) and produced anti‐nociceptive (analgesic) effects in rat inflammatory and neuropathic https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/erethism models (Staton et al., 2008).
The molecular and cellular mechanisms underlying GPR55 response modulation of cannabinoid action are unclear. A number of functional assays suggest that Δ9‐THC (Baker et al., 2006; Paronis et al., 2012; Pertwee, 2007, 2010; Pertwee et al., 2010), but not WIN55212‐2 (Lauckner et al., 2008; Oka, Nakajima, Yamashita, Kishimoto, & Sugiura, 2007; Ryberg et al., 2007), may alter GPR55‐mediated biological effects in cultured cells, suggesting that Δ9‐THC is a GPR55 agonist with higher affinity than at CB1 or CB2 receptors , while WIN55212‐2 is not (Pertwee, 2007; Ryberg et al., 2007). These findings suggest that different receptor mechanisms may underlie the enhanced tetrad effects produced by Δ9‐THC versus WIN55212‐2. Given that CB1 and CB2 receptors are coupled to Gi/o proteins that inhibit AC and neuronal activity (Howlett et al., 2002) and that GPR55 receptors are coupled to Gq and Gα12/13 proteins that increase intracellular calcium and neuronal excitation (Lauckner et al., 2008), we propose that GPR55 stimulation by Δ9‐THC may directly antagonize CB1‐ and CB2 receptors‐mediated effects at intracellular signal molecule level. This is supported by the finding that GPR55 and CB1 receptors are co‐expressed in several brain regions such as striatum, frontal cortex, hippocampus and hypothalamus (Ryberg et al., 2007; Wu et al., 2013). In addition, GPR55 and CB1 receptors (or CB2 receptors) may form functional heterodimers in striatum (Martínez‐Pinilla et al., 2014) or immune or cancer cells (Balenga et al., 2014; Moreno et al., 2014). Thus, co‐activation of GPR55 and CB1 (or CB2) receptors by Δ9‐THC may modulate each other's signalling pathways and therefore alter each other's functional activities (Kargl et al., 2012). Alternatively, the enhanced tetrad effects to Δ9‐THC could be a compensatory response to the loss of GRP55 in GPR55‐KO mice. It is unknown how GPR55 deletion causes an enhanced response to WIN55212‐2. More studies are required to further address this issue. It is worth noting that it is the first report that genetic deletion of GPR55 caused an enhanced tetrad response to Δ9‐THC or WIN55212‐2, suggesting that GPR55 activation may have a neuroprotective function against cannabis effects or its toxicity.
4.4. CB1 receptor mechanisms underlying XLR11 tetrad effects
The last important finding in the present study is that the new synthetic cannabinoid XLR11 also produced potent tetrad effects in a way similar as Δ9‐THC (Figure 1 vs. Figure 5). Deletion of CB1 receptors abolished XLR11‐induced tetrad effects, suggesting an involvement of a CB1 receptor mechanism. This is consistent to a previous report indicating that XLR11 is a potent CB1 receptor agonist (Banister et al., 2015). Paradoxically, XLR11 also shows high affinity for CB2 receptors (Ki = 0.6–2.1 nM; Banister et al., 2015; Banister & Connor, 2018; Wiley et al., 2013). In the present study, deletion of CB2 receptors did not alter XLR11‐induced tetrad effects, suggesting that the binding profiles of XLR11 on CB2 receptors in vivo versus in vitro may differ. Finally, deletion of GPR55 also failed to significantly alter XLR11‐induced tetrad effects, while pharmacological blockade of GRP55 with CID16020046 produced an enhancement in XLR11‐induced analgesia and hypothermia, suggesting that GPR55 may also play a role in XLR11 action. This is consistent with the finding regarding the role of GPR55 in Δ9‐THC action. It is not known why genetic deletion of GPR55 failed to, while pharmacological blockade of GPR55 enhanced WIN55212‐2‐induced tetrad effects. Clearly, more studies are required to further address the role of GPR55 in cannabinoid action.
In summary, in light of the current cannabis legalization movement and the rapid increases in use of new synthetic cannabinoids, it is critically important to understand the biological effects and underlying receptor mechanisms of these agents. In the present studies, we used multiple transgenic mouse lines, combined with pharmacological approaches, to study the classical pharmacological action of three representative cannabinoids. We found that distinct receptor mechanisms may underlie the effects produced by the plant versus synthetic cannabinoids. Perhaps the most important findings are (a) multiple receptor mechanisms (CB1, CB2 receptors and GPR55) may underlie Δ9‐THC‐, WIN55212‐2‐ or XLR11‐induced tetrad effects; and (b) stimulation of CB1/CB2 receptors versus GPR55 produces opposite effects on cannabis action. Once these findings are confirmed by additional experiments, they will not only expand our understanding about receptor mechanisms underlying cannabinoid action but also lead to new discoveries in medication development for the treatment of cannabis abuse disorder.
AUTHOR CONTRIBUTIONS
M.B., E.L.G., and Z.‐X.X. designed the experiments. X.‐F.W., E.G., C.Z., Y.H., and G.‐H.B. performed the behavioural experiments. X.‐F.W, E.G., G.‐H.B., and Z.‐X.X. analysed data and prepared the figures. E.G. and Z.‐X.X. wrote the manuscript. M.B. and E.L.G. revised the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
ACKNOWLEDGEMENT
This work was supported by the Intramural Research Program (IRP) at the National Institute on Drug Abuse (NIDA; DA000620‐02), National Institutes of Health (NIH), U.S. Public Health Service, USA.
Wang X‐F, Galaj E, Bi G‐H, et al. Different receptor mechanisms underlying phytocannabinoid‐ versus synthetic cannabinoid‐induced tetrad effects: Opposite roles of CB1/CB2 versus GPR55 receptors. Br J Pharmacol. 2020;177:1865–1880. 10.1111/bph.14958
Xiao‐Fei Wang, Ewa Galaj, and Guo‐Hua Bi contributed equally to this work.
REFERENCES
- Abalo, R. , Cabezos, P. A. , Vera, G. , López‐Miranda, V. , Herradón, E. , & Martín‐Fontelles, M. I. (2011). Cannabinoid‐induced delayed gastric emptying is selectively increased upon intermittent administration in the rat: Role of CB1 receptors. Neurogastroenterology and Motility, 23, 457–467. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peter, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/2020: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood, B. K. , & Mackie, K. (2010). CB2: A cannabinoid receptor with an identity crisis. British Journal of Pharmacology, 160, 467–479. 10.1111/j.1476-5381.2010.00729.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, D. , Pryce, G. , Davies, W. L. , & Hiley, C. R. (2006). In silico patent searching reveals a new cannabinoid receptor. Trends in Pharmacological Sciences, 27, 1–4. 10.1016/j.tips.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Balenga, N. A. , Martínez‐Pinilla, E. , Kargl, J. , Schröder, R. , Peinhaupt, M. , Platzer, W. , … Franco, R. (2014). Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. British Journal of Pharmacology, 171, 5387–5406. 10.1111/bph.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banister, S. D. , & Connor, M. (2018). The chemistry and pharmacology of synthetic cannabinoid receptor agonist new psychoactive substances: Evolution. Handbook of Experimental Pharmacology, 252, 191–226. [DOI] [PubMed] [Google Scholar]
- Banister, S. D. , Stuart, J. , Kevin, R. C. , Edington, A. , Longworth, M. , Wilkinson, S. M. , … Kassiou, M. (2015). Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH‐018, AM‐2201, UR‐144, XLR11, PB‐22, 5F‐PB‐22, APICA, and STS‐135. ACS Chemical Neuroscience, 6, 1445–1458. 10.1021/acschemneuro.5b00107 [DOI] [PubMed] [Google Scholar]
- Bjursell, M. , Ryberg, E. , Wu, T. , Greasley, P. J. , Bohlooly‐Y, M. , & Hjorth, S. (2016). Deletion of Gpr55 results in subtle effects on energy metabolism, motor activity and thermal pain sensation. PLoS ONE, 11, e0167965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, N. E. (2008). The peripheral cannabinoid receptor knockout mice: An update. British Journal of Pharmacology, 153, 309–318. 10.1038/sj.bjp.0707527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, N. E. , McCoy, K. L. , Mezey, E. , Bonner, T. , Zimmer, A. , Felder, C. C. , … Zimmer, A. (2000). Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. European Journal of Pharmacology, 396, 141–149. 10.1016/s0014-2999(00)00211-9 [DOI] [PubMed] [Google Scholar]
- Celorrio, M. , Rojo‐Bustamante, E. , Fernández‐Suárez, D. , Sáez, E. , Estella‐Hermoso de Mendoza, A. , Müller, C. E. , … Aymerich, M. S. (2017). GPR55: A therapeutic target for Parkinson's disease? Neuropharmacology, 125, 319–332. 10.1016/j.neuropharm.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Compton, D. R. , Aceto, M. D. , Lowe, J. , & Martin, B. R. (1996). In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): Inhibition of delta 9‐tetrahydrocannabinol‐induced responses and apparent agonist activity. The Journal of Pharmacology and Experimental Therapeutics, 277, 586–594. [PubMed] [Google Scholar]
- Compton, D. R. , Johnson, M. R. , Melvin, L. S. , & Martin, B. R. (1992). Pharmacological profile of a series of bicyclic cannabinoid analogs: Classification as cannabimimetic agents. The Journal of Pharmacology and Experimental Therapeutics, 260, 201–209. [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, G. T. , Wolf, C. E. , Poklis, A. , & Lichtman, A. H. (2010). Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)‐tetrahydrocannabinol. Drug Alcohol Depen, 112, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, A. , Kesingland, A. , Gentry, C. , McNair, K. , Patel, S. , Urban, L. , & James, I. (2001). The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain, 92, 91–100. 10.1016/s0304-3959(00)00474-7 [DOI] [PubMed] [Google Scholar]
- Glass, M. , Dragunow, M. , & Faull, R. L. (1997). Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience, 77, 299–318. 10.1016/s0306-4522(96)00428-9 [DOI] [PubMed] [Google Scholar]
- Grim, T. W. , Morales, A. J. , Thomas, B. F. , Wiley, J. L. , Endres, G. W. , Negus, S. S. , & Lichtman, A. H. (2017). Apparent CB1 receptor rimonabant affinity estimates: Combination with THC and synthetic cannabinoids in the mouse in vivo triad model. The Journal of Pharmacology and Experimental Therapeutics, 362, 210–218. 10.1124/jpet.117.240192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, A. C. , & Abood, M. E. (2017). CB1 and CB2 receptor pharmacology. Advances in Pharmacology, 80, 169–206. 10.1016/bs.apha.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, A. C. , Barth, F. , Bonner, T. I. , Cabral, G. , Casellas, P. , Devane, W. A. , … Pertwee, R. G. (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews, 54, 161–202. 10.1124/pr.54.2.161 [DOI] [PubMed] [Google Scholar]
- Jordan, C. , & Xi, Z. X. (2019). Progress in brain cannabinoid CB2 receptors: From gene to behavior. Neuroscience and Biobehavioral Reviews, 98, 208–220. 10.1016/j.neubiorev.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargl, J. , Balenga, N. , Parzmair, G. P. , Brown, A. J. , Heinemann, A. , & Waldhoer, M. (2012). The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. Journal of Biological Chemistry, 287, 44234–44248. 10.1074/jbc.M112.364109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner, J. E. , Jensen, J. B. , Chen, H.‐Y. , Lu, H.‐C. , Hille, B. , & Mackie, K. (2008). GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proceedings of the National Academy of Sciences of the United States of America, 105, 2699–2704. 10.1073/pnas.0711278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent, C. , Valverde, O. , Cossu, G. , Petitet, F. , Aubert, J. F. , Beslot, F. , … Parmentier, M. (1999). Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science, 283, 401–404. 10.1126/science.283.5400.401 [DOI] [PubMed] [Google Scholar]
- Lesniak, A. , Chmielewska, D. , Poznanski, P. , Bujalska‐Zadrozny, M. , Strzemecka, J. , & Sacharczuk, M. (2019). Divergent response to cannabinoid receptor stimulation in high and low stress‐induced analgesia mouse lines is associated with differential G‐protein activation. Neuroscience, 404, 246–258. 10.1016/j.neuroscience.2019.02.015 [DOI] [PubMed] [Google Scholar]
- Little, P. J. , Compton, D. R. , Johnson, M. R. , Melvin, L. S. , & Martin, B. R. (1988). Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. The Journal of Pharmacology and Experimental Therapeutics, 247, 1046–1051. [PubMed] [Google Scholar]
- Liu, Q.‐R. , Canseco‐Alba, A. , Zhang, H.‐Y. , Tagliaferro, P. , Chung, M. , Dennis, E. , … Onaivi, E. S. (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Scientific Reports, 7, 17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Pinilla, E. , Reyes‐Resina, I. , Oñatibia‐Astibia, A. , Zamarbide, M. , Ricobaraza, A. , Navarro, G. , … Franco, R. (2014). CB1 and GPR55 receptors are co‐expressed and form heteromers in rat and monkey striatum. Experimental Neurology, 261, 44–52. 10.1016/j.expneurol.2014.06.017 [DOI] [PubMed] [Google Scholar]
- Meadows, A. , Lee, J. H. , Wu, C.‐S. , Wei, Q. , Pradhan, G. , Yafi, M. , … Sun, Y. (2016). Deletion of G‐protein‐coupled receptor 55 promotes obesity by reducing physical activity. International Journal of Obesity, 40, 417–424. 10.1038/ijo.2015.209 [DOI] [PubMed] [Google Scholar]
- Monory, K. , Blaudzun, H. , Massa, F. , Kaiser, N. , Lemberger, T. , Schütz, G. , … Marsicano, G. (2007). Genetic dissection of behavioural and autonomic effects of Δ9‐tetrahydrocannabinol in mice. PLoS Biology, 5, e269 10.1371/journal.pbio.0050269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, E. , Andradas, C. , Medrano, M. , Caffarel, M. M. , Pérez‐Gómez, E. , Blasco‐Benito, S. , … Sánchez, C. (2014). Targeting CB2‐GPR55 receptor heteromers modulates cancer cell signaling. The Journal of Biological Chemistry, 289, 21960–21972. 10.1074/jbc.M114.561761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriconi, A. , Cerbara, I. , Maccarrone, M. , & Topai, A. (2010). GPR55: Current knowledge and future perspectives of a purported ‘Type‐3’ cannabinoid receptor. Current Medicinal Chemistry, 17, 1411–1429. 10.2174/092986710790980069 [DOI] [PubMed] [Google Scholar]
- Oka, S. , Nakajima, K. , Yamashita, A. , Kishimoto, S. , & Sugiura, T. (2007). Identification of GPR55 as a lysophosphatidylinositol receptor. Biochemical and Biophysical Research Communications, 362, 928–934. 10.1016/j.bbrc.2007.08.078 [DOI] [PubMed] [Google Scholar]
- Onaivi, E. S. , Ishiguro, H. , Gong, J.‐P. , Patel, S. , Meozzi, P. A. , Myers, L. , … Uhl, G. R. (2008). Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Annals of the new York Academy of Sciences, 1139, 434–449. 10.1196/annals.1432.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis, C. A. , Nikas, S. P. , Shukla, V. G. , & Makriyannis, A. (2012). Δ9‐Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behavioural Pharmacology, 23, 802–805. 10.1097/FBP.0b013e32835a7c4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, L. H. , & Hurd, Y. L. (2015). Endocannabinoid signalling in reward and addiction. Nature Reviews. Neuroscience, 16, 579–594. 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee, R. G. (2007). GPR55: A new member of the cannabinoid receptor clan? British Journal of Pharmacology, 152, 984–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee, R. G. (2010). Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Current Medicinal Chemistry, 17, 1360–1381. 10.2174/092986710790980050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee, R. G. , Howlett, A. C. , Abood, M. E. , Alexander, S. P. H. , Di Marzo, V. , Elphick, M. R. , … Ross, R. A. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2 . Pharmacological Reviews, 62, 588–631. 10.1124/pr.110.003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi, A. , Hajizadeh Moghaddam, A. , & Roohbakhsh, A. (2015). Central administration of GPR55 receptor agonist and antagonist modulates anxiety‐related behaviors in rats. Fundamental & Clinical Pharmacology, 29, 185–190. 10.1111/fcp.12099 [DOI] [PubMed] [Google Scholar]
- Rinaldi‐Carmona, M. , Barth, F. , Héaulme, M. , Shire, D. , Calandra, B. , Congy, C. , … Caput, D. (1994). SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letters, 350, 240–244. 10.1016/0014-5793(94)00773-x [DOI] [PubMed] [Google Scholar]
- Ryberg, E. , Larsson, N. , Sjögren, S. , Hjorth, S. , Hermansson, N.‐O. , Leonova, J. , … Greasley, P. J. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology, 152, 1092–1101. 10.1038/sj.bjp.0707460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller, K. , Bi, G. H. , He, Y. , Galaj, E. , Garder, E. L. , & Xi, Z. X. (2019). Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. British Journal of Pharmacology. 176, 1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton, P. C. , Hatcher, J. P. , Walker, D. J. , Morrison, A. D. , Shapland, E. M. , Hughes, J. P. , … Chessell, I. P. (2008). The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain, 139, 225–236. 10.1016/j.pain.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Tai, S. , & Fantegrossi, W. E. (2017). Pharmacological and toxicological effects of synthetic cannabinoids and their metabolites. Current Topics in Behavioral Neurosciences, 32, 249–262. 10.1007/7854_2016_60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou, I. , Pistos, C. , & Spiliopoulou, C. (2010). Spice drugs as a new trend: Mode of action, identification and legislation. Toxicology Letters, 197, 157–162. 10.1016/j.toxlet.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Varvel, S. A. , Bridgen, D. T. , Tao, Q. , Thomas, B. F. , Martin, B. R. , & Lichtman, A. H. (2005). Δ9‐Tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. The Journal of Pharmacology and Experimental Therapeutics, 314, 329–337. 10.1124/jpet.104.080739 [DOI] [PubMed] [Google Scholar]
- Wiley, J. L. , Marusich, J. A. , Lefever, T. W. , Grabenauer, M. , Moore, K. N. , & Thomas, B. F. (2013). Cannabinoids in disguise: Δ9‐Tetrahydrocannabinol‐like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology, 75, 145–154. 10.1016/j.neuropharm.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C.‐S. , Chen, H. , Sun, H. , Zhu, J. , Jew, C. P. , Wager‐Miller, J. , … Lu, H. C. (2013). GPR55, a G‐protein coupled receptor for lysophosphatidylinositol, plays a role in motor coordination. PLoS ONE, 8, e60314 10.1371/journal.pone.0060314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C.‐S. , Zhu, J. , Wager‐Miller, J. , Wang, S. , O'Leary, D. , Monory, K. , … Lu, H. C. (2010). Requirement of cannabinoid CB1 receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. The European Journal of Neuroscience, 32, 693–706. 10.1111/j.1460-9568.2010.07337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.‐Y. , Gao, M. , Liu, Q.‐R. , Bi, G.‐H. , Li, X. , Yang, H.‐J. , … Xi, Z. X. (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine‐related behavior in mice. Proceedings of the National Academy of Sciences of the United States of America, 111, E5007–E5015. 10.1073/pnas.1413210111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.‐Y. , Gao, M. , Shen, H. , Bi, G.‐H. , Yang, H.‐J. , Liu, Q.‐R. , … Xi, Z. X. (2017). Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biology, 22, 752–765. 10.1111/adb.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, A. , Zimmer, A. M. , Hohmann, A. G. , Herkenham, M. , & Bonner, T. I. (1999). Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 96, 5780–5785. 10.1073/pnas.96.10.5780 [DOI] [PMC free article] [PubMed] [Google Scholar]


