Abstract
Background and Purpose
Neuroactive steroid (3β,5β,17β)‐3‐hydroxyandrostane‐17‐carbonitrile (3β‐OH) is a novel hypnotic and voltage‐dependent blocker of T‐type calcium channels. Here, we examine its potential analgesic effects and adjuvant anaesthetic properties using a post‐surgical pain model in rodents.
Experimental Approach
Analgesic properties of 3β‐OH were investigated in thermal and mechanical nociceptive tests in sham or surgically incised rats and mice, with drug injected either systemically (intraperitoneal) or locally via intrathecal or intraplantar routes. Hypnotic properties of 3β‐OH and its use as an adjuvant anaesthetic in combination with isoflurane were investigated using behavioural experiments and in vivo EEG recordings in adolescent rats.
Key Results
A combination of 1% isoflurane with 3β‐OH (60 mg·kg−1, i.p.) induced suppression of cortical EEG and stronger thermal and mechanical anti‐hyperalgesia during 3 days post‐surgery, when compared to isoflurane alone and isoflurane with morphine. 3β‐OH exerted prominent enantioselective thermal and mechanical antinociception in healthy rats and reduced T‐channel‐dependent excitability of primary sensory neurons. Intrathecal injection of 3β‐OH alleviated mechanical hyperalgesia, while repeated intraplantar application alleviated both thermal and mechanical hyperalgesia in the rats after incision. Using mouse genetics, we found that CaV3.2 T‐calcium channels are important for anti‐hyperalgesic effect of 3β‐OH and are contributing to its hypnotic effect.
Conclusion and Implications
Our study identifies 3β‐OH as a novel analgesic for surgical procedures. 3β‐OH can be used to reduce T‐channel‐dependent excitability of peripheral sensory neurons as an adjuvant for induction and maintenance of general anaesthesia while improving analgesia and lowering the amount of volatile anaesthetic needed for surgery.
Abbreviations
- 3β‐OH
(3β,5β,17β)‐3‐hydroxyandrostane‐17‐carbonitrile
- ent‐3β‐OH
ent‐[(3β,5β,17β)‐3‐hydroxyandrostane‐17‐carbonitrile]
- GABAA
γ‐aminobutyric acid receptor type A
- LORR
loss of righting reflex
- NMDA
N‐methyl‐d‐aspartate
- P25‐P70
postnatal day 25–postnatal day 70
- POD
post‐operative day
- PWL
paw withdrawal latency
- PWR
paw withdrawal response
- TTA‐P2
3,5‐dichloro‐N‐[1‐(2,2‐dimethyl‐tetrahydro‐pyran‐4‐ylmethyl)‐4‐fluoro‐piperidin‐4‐ylmethyl]‐benzamide
What is already known
Clinical post‐surgical pain management is often suboptimal, leading to both acute and chronic pain development.
What this study adds
Investigation of analgesic properties of a novel hypnotic neuroactive steroid in post‐surgical pain in rats.
What is the clinical significance
This study identifies analgesic/hypnotic neuroactive steroid as a new potential therapeutic for alleviating post‐surgical pain.
1. INTRODUCTION
Current clinical management options for pain arising after surgery are often suboptimal, which leads to the development of acute and sometimes chronic pain after surgery (Chapman, Duncan, & Lipman, 2013). One of the reasons is poor understanding of the mechanisms underlying surgical tissue injury (Honore et al., 2000).
Conventional medicines such as opiate analgesics are often associated with a plethora of adverse events (Apfelbaum, Chen, Mehta, & Gan, 2003; Pavlin, Chen, Penaloza, Polissar, & Buckley, 2002), suggesting a need for multimodal analgesia, utilizing adjuvant analgesics that could reduce the amount of opioids and volatile general anaesthetics (Mathiesen et al., 2013).
Endogenous neurosteroids and their synthetic analogues have recently emerged as promising antinociceptive agents (Mensah‐Nyagan et al., 2008; Patte‐Mensah, Meyer, Taleb, & Mensah‐Nyagan, 2014; Svensson, Persson, Fitzsimmons, & Yaksh, 2013). It is proposed that their various effects on the sensory transmission are exerted via modulation of intracellular/nuclear receptors, postsynaptic https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72, and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 membrane receptors, as well as some voltage‐gated ion channels (Baulieu & Robel, 1990; Belelli & Lambert, 2005; Majewska, Harrison, Schwartz, Barker, & Paul, 1986; Schumacher et al., 2008). Our previous studies showed that 5α‐reduced neurosteroids alleviate neuropathic pain (Pathirathna, Todorovic, Covey, & Jevtovic‐Todorovic, 2005), mostly by interacting with GABAA receptors and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=80 (T‐channels) in peripheral nociceptors (Pathirathna, Brimelow, et al., 2005). Furthermore, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4188, an endogenous 5β‐reduced neurosteroid that has no effect on GABAA‐mediated currents, exerts potent analgesia in healthy rats and mice as a result of inhibition of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=536&familyId=80&familyType=IC isoform in peripheral nociceptors (Ayoola et al., 2014).
The supportive role of CaV3.2 isoform of T‐channels in pain pathways has been relatively well established since blocking peripheral T‐channels leads to very potent antinociceptive effects in the various pain models (Choi et al., 2007; Todorovic et al., 2001; Todorovic, Meyenburg, & Jevtovic‐Todorovic, 2002; Todorovic, Rastogi, & Jevtovic‐Todorovic, 2003). Furthermore, our recent study (Joksimovic et al., 2018) has implicated CaV3.2 channels in hyperalgesia using postsurgical pain model in rodents.
Our group has already shown that synthetic analogues of 5β‐reduced neurosteroids exert potent analgesic effects in the test of thermal nociception by blocking T‐channels in peripheral nociceptors (Todorovic et al., 2004). The most potent T‐channel blocker in this family, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10537 (3β‐OH), is also a hypnotic devoid of any effects on GABAA‐gated currents in rat pups (Atluri et al., 2018). Therefore, we propose that 3β‐OH, as a relatively potent T‐channel blocker, may induce hypnosis and peripheral analgesia, both necessary to maintain adequate anaesthesia during surgery.
In order to investigate potential analgesic properties of 3β‐OH in the setting of surgically induced hyperalgesia, we used an experimental pain model described as plantar incision of the hind paw in rats and mice (Brennan, Vandermeulen, & Gebhart, 1996; Pogatzki & Raja, 2003), a rodent model thought to be relevant to the characteristics of human post‐operative pain.
2. METHODS
2.1. Animals
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Experimental protocols were approved by the Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus, as well as University of Virginia, and are in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). All animals were housed two per cage, on a 12‐hr light–dark cycle with food and water ad libitum. Weanlings and young adolescent female Sprague–Dawley (P25‐P42; Envigo, Indianapolis, IN, USA) rats were used for the EEG study. Weanlings of age range P25–P27 were implanted with electrodes and left to recuperate and heal for 2 weeks. The actual EEG experiments were performed on adolescent rats (P40–P42). Young adult female Sprague–Dawley rats (P50‐P72; Envigo, Indianapolis, IN, USA), as well as adult female C57BL/6j (RRID:IMSR_JAX:000664) and CaV3.2 (CACNA1H) knock out (KO) mice of the same background and source (The Jackson Laboratory), were used for other behavioural experiments. There is growing scientific evidence suggesting sex differences in pain perception, in both acute and chronic pain states. Some of the pain conditions, such as fibromyalgia, migraine, rheumatoid arthritis and irritable bowel syndrome are more prevalent in women than in men. Bartley and Fillingim (2013) have performed a comprehensive analysis of both clinical and non‐clinical studies of different pain models and sex differences in pain perception and concluded that females were more susceptible to chronic pain conditions than males. Yet over 70% of animal pain studies are performed in males (Mogil & Chanda, 2005). Hence, in order to better understand the underlying mechanisms of pain perception post‐surgery in understudied population, we used only female rats in our experiments.
On each experimental day, animals were randomly assigned to treatment groups with the experimenter blinded to drug treatment. All efforts were made to reduce animal suffering and the number of animals used. In in vivo experiments, all groups consisted of five or more than five animals, except a group of rats in EEG experiments and a vehicle group of wild type (WT) C57BL/6j mice. In vitro electrophysiology experiments were performed on five or more than five cells. Whenever possible, studies were designed to generate groups of equal size, using randomization and blinded analysis. In our behaviour experiments, we used the vehicle in the same fashion as previously published (Joksimovic et al., 2018) and we have previously shown that this vehicle does not exert any effect on hyperalgesia. Therefore, to be in accordance with the 3R principle (replace, reduce and refine), we minimized the use of animals for well‐established experiments, due to which a smaller group of animals received vehicle as compared to the treatment groups.
2.2. Materials
3β‐OH (3β,5β,17β)‐3‐hydroxyandrostane‐17‐carbonitrile and ent‐3β‐OH ent‐[(3β,5β,17β)‐3‐hydroxyandrostane‐17‐carbonitrile] are a synthetic neurosteroid analogues synthetized using procedures that have been described in our previous publications (Atluri et al., 2018; Han, Zorumski, & Covey, 1996; Todorovic et al., 2004). ent‐3β‐OH was also produced from ent‐testosterone (Hu et al., 1997) using the procedure reported for the preparation of 3β‐OH from testosterone (Atluri et al., 2018). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627 was purchased through University of Colorado Hospital Pharmacy (Morphine sulfate inj. USP 4 mg·ml−1, West Ward). For intrathecal and intraplantar injections, the compounds were dissolved in 15% 2‐hydroxypropyl‐β‐cyclodextrin solution in pH‐balanced saline (pH = 7.4 to avoid tissue irritation). Morphine was already provided as a solution for injection and sterile water was used to further dilute morphine. For local application, 3β‐OH was applied in rats at doses of 16, 0.16 and 0.016 μg in 50 μl, which corresponds to concentrations of 1, 0.1 and 0.01 mM. In mice, 3β‐OH was applied intrathecally in a dose of 16 μg in a volume of 10 μl, which corresponds to concentration of the solution of 5 mM. Systemic dose of 3β‐OH applied in rats was 60 mg·kg−1 of body weight, which corresponds to the concentration of 6 mg·ml−1. ent‐3β‐OH was applied in dose of 16 μg in 50 μl which corresponds to the concentration of 1 mM. The dose of morphine (10 μg in 50 μl) corresponds to the concentration of 0.7 nM. In order to eliminate any possible systemic contribution to anti‐hyperalgesic effects of intrathecal application of 3β‐OH, we assessed sensory motor abilities of injected animals. With intraplantar application, we used contralateral noninjected paw to confirm that the analgesic effects are arising from local ipsilateral intraplantar application (data not shown).
For intraperitoneal injections, the drug was dissolved in 25% 2‐hydroxypropyl‐β‐cyclodextrin solution prepared in sterile water. 2‐Hydroxypropyl‐β‐cyclodextrin (45% solution) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.3. Incisional pain model
For the purposes of studying the antinociceptive effect of 3β‐OH in acute post‐surgical pain, we used the skin and muscle incision for the induction of post‐surgical pain (Brennan et al., 1996; Pogatzki‐Zahn). Briefly, animals were anaesthetized with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2505 (2.5% for induction and for maintenance) and the plantar surface of the right paw was incised longitudinally with blade No. 11. The underlying plantaris muscle was elevated and incised also longitudinally, after which the skin was sutured with 5‐0 nylon suture with FS‐2 needle. Animals were allowed to recover in cages, and all experiments were initiated as early as 2 hr post‐incision.
2.4. Intrathecal injections
In order to study antinociceptive effects of spinally applied drugs, intrathecal injections of the study compound were performed. After briefly anaesthetizing animals with isoflurane (2.5% for induction in an induction chamber and 2.5% for maintenance), the back of each animal was shaved to expose the injection site in the region of L4–L6 of the spinal column. A 28 G needle was used for the acute intrathecal injection. After inserting the needle into the L4–L6 lumbar region, the experimental compound or vehicle was delivered in a volume of 50 μl, and the animal was left to recover in a cage before initiating experiments.
2.5. Thermal nociception testing
For assessment of thermal (heat) nociception threshold, an apparatus based upon Hargreaves method was used (custom created at UCSD University Anesthesia Research and Development Group, La Jolla, CA). In brief, after 15 min of acclimation, a radiant heat source is positioned directly underneath a plantar surface of the hind paws to deliver a thermal stimulus. When the animal withdraws the paw, an automatic timer shuts off measuring the animal's paw withdrawal latency (PWL). Each paw was tested three times and the average value of PWLs was used in further analysis. To prevent thermal injury, the light beam is automatically discontinued at 20 s if the rat fails to withdraw its paw.
2.6. Mechanical sensitivity
In order to determine mechanical sensitivity in rats, we used the electronic Von Frey apparatus (Ugo Basile, Varese, Italy). The apparatus utilizes a single rigid filament that exerts pressure to the plantar surface of the paw in a range from 0 to 50 g. Animals were placed in plastic enclosures on a wire mesh stand to habituate for 15 min. After habituation, a probe was applied to the plantar surface of the paw through the mesh floor of the stand, and constant force was applied to the mid‐plantar area of the paw. As soon as the exerted pressure of the punctate stimulus reaches the maximum force that the animal can endure, immediate brisk paw withdrawal is noticeable, and the force in grams is displayed on the apparatus representing a threshold for paw withdrawal response (PWR). Each paw was tested three times, and the average value of threshold PWRs was used in further analysis. Any other voluntary movement of the animal was not considered as a response.
2.7. Assessment of sensorimotor abilities
To eliminate the possible effect of the highest applied dose of the tested drug on sensorimotor abilities, we tested unoperated rats as previously described (Wozniak, Olney, Kettinger, Price, & Miller, 1990). In brief, an animal was tested in four different tests: incline plane, elevated platform, ledge, and walking initiation experiments. For inclined plane experiments, a rat was placed in the middle of a wire mesh (eight squares in 10 cm) tilted at 60° angle. The animal was placed with her head down, and time was measured for how long it can stay without falling down. A cut‐off value of 120 s was assumed as the maximum time an animal can stay on the inclined mesh. For elevated platform experiments, a rat was placed on a platform (7.6 × 15.2 cm) 61 cm above the ground, and the animal was timed for how long it can remain there. A mean value was calculated from two trials with a maximum of 120 s (test cut‐off value). For ledge experiments, a rat was timed for how long it stayed on a 3‐cm‐wide plank. Means for each animal were calculated over two trials, with a maximum of 60 s per trial. For walking initiation, a square 50 × 50 cm was made with the pieces of tape on the desk. The rat would be placed in the centre of the square, and time necessary for animal to leave the square with all four paws was measured (s). The highest dose of 3β‐OH that exerted an analgesic effect in healthy animals was tested 30 min after intrathecal injection.
2.8. Loss of righting reflex measurements
The righting reflex, also known as the labyrinthine righting reflex, helps correct the orientation of the body when it is taken out of its normal upright position. We used loss of righting reflex (LORR) as a surrogate measure of hypnosis induced by anaesthetics, as previously described (Orestes, Bojadzic, Chow, & Todorovic, 2009). After intraperitoneal injection of 3β‐OH, rats or mice were placed in a chamber to monitor for the onset of hypnosis. The chamber was rotated to gently place the rat on its back, and the rat was monitored for three consecutive tries, the first lasting 45 s and the second two lasting 1 min each. LORR onset was considered to be a failure of the animal to right itself on all three tries but disregarded if the animal was able to right itself on any of the three.
2.9. Electrode implantation
To monitor EEG signals, female rats aged P25–27 were implanted with screw electrodes over the cortices under https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4233 (100 mg·kg−1) and isoflurane anaesthesia (0.5–2%). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2623 (1%) was injected locally at the surgery site to minimize the incision pain. The following stereotaxic coordinates were used to place the active electrodes: anterioposterior = −2.8 mm from bregma, mediolateral = 3.0 mm from midline, and dorsoventral = below the skull surface and over the cortex. A screw electrode placed behind the lambda on each side of the midline served as ground (right) and reference (left). The electrodes were fixed to the skull using dental acrylic. The rats were treated post‐operatively with an analgesic (Banamine, 2.5 mg·kg−1, s.c.) every 24 hr for 48 hr.
2.10. Video‐EEG monitoring and drug treatment
Synchronized, time‐locked video and EEG signals were recorded from P40–P42 female rats using the Pinnacle system (Pinnacle Technology Inc., Lawrence, KS, USA). The EEG signal was acquired with the bandpass filter set at 1 to 500 Hz. The signals were digitized at 2000 Hz and stored on a hard disk for offline analysis. A 60‐min baseline recording was obtained before any manipulation was done. The rats were selected randomly for a specific treatment paradigm on the day of the experiment. The rats were first given a single dose of 25% cyclodextrin i.p. (vehicle, volume equivalent to test drug) and 1% or 2.5% isoflurane (inhalation) and then, 48 hr later, a single dose of 3β‐OH (60 mg kg−1, i.p.) and 1% isoflurane (inhalation). Baseline recordings taken during quiet awake states were analysed using power spectral analysis (fast Fourier transformation), which showed a non‐significant difference between experiments across a range of frequencies (1–100 Hz; data not shown).
2.11. Burst suppression and power analysis
The burst suppression was defined as an EEG pattern with alternative periods of marked voltage attenuation (suppression, at least 70% lower amplitude than the maximal amplitude of the burst episode), lasting for at least 500 ms and slow waves of high amplitude (burst). The burst suppression characteristics were analysed taking into account both right and left cortices. The duration of suppression episodes during a period of 5 min after the first episode was calculated and divided by the total time yielding a suppression ratio. The spectrograms (Hann window size 8192, 50% overlap) were generated using LabChart and then transferred to GraphPad Prism software for visualization. Importantly, all animals exposed to 3β‐OH (with or without isoflurane) remained euthermic during LORR, SpO2 values were stable (90–100%), as well as their heart rate (394–458 bpm), and animals resumed normal locomotor activity after effects of 3β‐OH dissipated (data not shown).
2.12. Acute dissociation of DRG neurons
DRG neurons from adult rats were prepared as we previously described (Todorovic & Lingle, 1998). Animals were first deeply anaesthetized with 5% isoflurane, after which decapitation was performed, and L4 to L6 DRGs were removed and immediately placed in ice‐cold Tyrode's solution, which contained 140‐mM NaCl, 4‐mM KCl, 2‐mM MgCl2, 10‐mM glucose, and 10‐mM HEPES, adjusted to pH 7.4 with NaOH. Next, harvested tissue was placed into Tyrode's solution containing collagenase H (Sigma‐Aldrich) and dispase II (Roche) and incubated for 50 min at 35°C. Dissociated single neuronal cell bodies were obtained by trituration in Tyrode's solution at room temperature through fire‐polished pipettes of progressively reduced sized tips. After trituration, cells were plated onto uncoated glass coverslips, placed in a culture dish, and perfused with external solution. All in vitro experiments were done at room temperature. Since it was previously shown that small‐ to medium‐sized cells (<35 mm soma diameter) are abundant with T‐channels, we used only these cells for our recordings (Nelson, Joksovic, Perez‐Reyes, & Todorovic, 2005; Todorovic & Lingle, 1998).
2.13. Cell culture
Cultured HEK cells (CLS Cat# 300192/p777_HEK293, RRID:CVCL_0045) with stable expression of CaV3.2 channels (a gift of Dr Paula Q. Barrett) were used in our study. For electrophysiology recordings, cells were typically used 1 to 3 days after plating.
2.14. Electrophysiology
Internal solution for current‐clamp recordings contained the following: 130‐mM potassium‐d‐gluconate, 5‐mM EGTA, 4‐mM NaCl, 0.5‐mM CaCl2, 10‐mM HEPES, 2‐mM Mg adenosine 5′‐triphosphate, and 0.5‐mM tris guanosine 5′‐triphosphate (pH 7.2). All current‐clamp recordings were performed in Tyrode's external solution. Tonic and rebound burst firing properties of DRG sensory neurons were characterized by injecting a family of depolarizing current pulses of 400‐ms duration in 10‐pA incremental steps through the recording pipette. Resting membrane potential was measured at the beginning of each recording and was not corrected for the liquid junction potential, which was around 10 mV in our experiments. The external solution for voltage‐clamp experiments measuring T‐currents in HEK cells contained 152‐mM tetraethylammonium (TEA)–Cl, 2‐mM CaCl2, and 10‐mM HEPES, with TEA‐OH, which was used for adjustment of pH to 7.4. The internal solution contained 135‐mM tetramethyilammonium‐OH, 40‐mM HEPES, 10‐mM EGTA, and 2‐mM MgCl2, adjusted to pH 7.2 with hydrogen fluoride. Series resistance (Rs) and membrane capacitance (Cm) were recorded directly from the amplifier after electronic subtraction of the capacitive transients. Current densities were calculated by dividing current amplitudes with cell capacitance. The membrane input resistance was calculated by dividing the end amplitude of steady‐state hyperpolarizing voltage deflection by the injected current. Current–voltage (I–V) curves were generated by voltage steps from holding potentials (Vh) of −90 mV to test potentials (Vt) from −80 to −30 mV in incremental steps of 5 mV. The voltage dependence of activation was expressed with single Boltzmann distribution:
| (1) |
2.15. Data analysis and statistics
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2015; Curtis et al., 2018). The declared group size is the number of independent values, and statistical analysis was done using these independent values. For each experiment, animals were randomly assigned to experimental groups in order to generate biological replicates, and the experimenter was blinded for the treatment until subsequent data analyses have been performed. No outliers were excluded in these experiments. For all studies, rats were litter‐matched and age‐matched to keep the treatment groups as similar as possible. All studies were predetermined to last 7 days from the incision. In order to assure stable recording conditions for the measurements of mechanical and thermal sensitivities, we determined baseline values on both paws on 2 days prior to surgery and again prior to drug application. Each data point from experiments was expressed as mean ± SEM. All data sets were tested for normality and equal variance (Mauchly's test of sphericity and Levene's test of equality of equal variances) and proper statistical analysis of the differences in effects between the treatment and the vehicle groups was performed using Student's unpaired or paired t‐test, two‐way RM ANOVA followed by Tukey's and Sidak's post hoc tests, one‐way ANOVA with Dunnett's post hoc test or Mann–Whitney test as appropriate. Mann–Whitney test was used on data set where normality and equal variance were not accommodated. In multigroup studies with parametric variables, post hoc tests (recommended by GraphPad prism, RRID:SCR_002798) were conducted only if F in ANOVA achieved the necessary level of statistical significance and there was no significant variance inhomogeneity. For in vitro experiments, data were analysed with paired or unpaired Student's t‐test, as well with two‐way RM ANOVA followed by appropriate post hoc test. Significant differences between group means are indicated when P < .05. GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA, RRID:SCR_002798) and SigmaPlot 10 (SigmaPlot Software, La Jolla, CA, USA, RRID:SCR_003210) were used for all statistical analyses.
2.16. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. 3β‐OH induces hypnosis and reduces the concentration of volatile anaesthetic isoflurane necessary to perform surgery in young adult rats
We asked if systemic administration of 3β‐OH in young female rats (P26–P42) may facilitate post‐operative analgesia and reduce requirement for potent volatile anaesthetic during surgery. In order to determine hypnotic potency in adult rats, 3β‐OH was injected intraperitoneally in escalating doses of 5, 10, 15, 20, 50, and 60 mg·kg−1, and a loss of righting reflex (LORR) was monitored for each animal. Based upon a dose–response curve, an ED50 for LORR was calculated to be about 15 mg·kg−1 (Figure S1). Next, we tested if 3β‐OH at 60 mg·kg−1, i.p., may decrease the requirement of isoflurane during surgical incision. Although 3β‐OH induced lasting hypnosis up to 3 hr in these animals, toe and paw pinch reflexes were still present (data not shown). However, when 1% isoflurane was added via nose cone, both reflexes were absent. To confirm that this drug combination could indeed serve as an anaesthetic regimen, we implanted rats with cortical screw electrodes and recorded EEG activity of sensory cortex.
First, we recorded baseline EEG activity during quiet wakefulness, which revealed typical low‐amplitude, high‐frequency EEG (Figure 1a, black trace; Figure 1c, left spectrogram). Then we injected animals with 25% cyclodextrin (vehicle), followed by exposure to 1% isoflurane, first in the chamber until LORR is achieved, and then using the nose cone. As expected, this subanaesthetic concentration of isoflurane produced a shift in cortical EEG towards high‐amplitude, low‐frequency oscillations without burst suppressions (Luo & Leung, 2009; Figure 1a, light blue trace; Figure 1c, middle spectrogram). On the other hand, when the concentration of isoflurane was increased to the full anaesthetic concentrations of 2.5%, a typical burst suppression pattern was detected (Figure 1a, dark blue trace) with the average effect of 71.3 ± 2.3% (Figure 1b). This EEG pattern represents a state of unconsciousness and profound brain inactivation (Kenny, Westover, Ching, Brown, & Solt, 2014). Interestingly, suppression of EEG activity was noticed when 3β‐OH was combined with subanaesthetic (1%) isoflurane (Figure 1b; 89.3 ± 4.4%). This distinct EEG pattern was clearly visible in the spectrogram as high‐power spectral density across a range of frequencies interspersed between periods of quiescence (Figure 1c, right panel).
Figure 1.
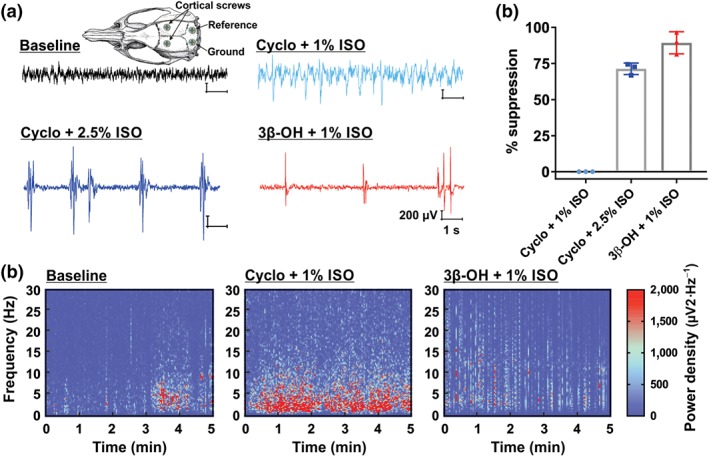
Combination of novel neurosteroid hypnotic and 1% isoflurane induces the burst suppression EEG pattern. (a) Representative raw EEG traces showing a typical baseline recording (black), a recording after 25% cyclodextrin (Cyclo) and 1% isoflurane (ISO) exposure (light blue), and a burst suppression pattern from a rodent undergoing general anaesthesia from Cyclo and 2.5% ISO (dark blue) or 3β‐OH and 1% ISO (red trace). The inset depicts the approximate placement of cortical screws, reference and ground. (b) Bar graphs displaying a higher percent of EEG suppression after exposure to 3β‐OH and 1% ISO, compared to both Cyclo +1% ISO and Cyclo +1% ISO groups (n = 3 rats). (c) Spectrograms computed from the same rat during the baseline (left), 5 min of exposure to Cyclo and 1% ISO (middle) or 3β‐OH and 1% ISO (right). Warm colours indicate frequency components with high, whereas cool colours indicate frequency components with low power density. Note a typical burst suppression pattern after exposure to 3β‐OH and 1% ISO
3.2. Comparison of the effects of isoflurane anaesthesia combined with either 3β‐OH or morphine on thermal and mechanical nociception after surgical incision performed under anaesthesia
Next, we hypothesized that combination of 3β‐OH (i.p.) with isoflurane anaesthesia may reduce hyperalgesia post‐incision (Figure 2a). We induced anaesthesia with injections of 3β‐OH (60 mg·kg−1, i.p.) and 1% isoflurane via a nose cone in order to perform surgical incision in rats. We subsequently measured paw withdrawal responses to noxious thermal (heat) and mechanical stimulus daily during the next 3 days. We found that combined anaesthesia of 3β‐OH and 1% isoflurane resulted in significantly less heat hypersensitivity, as compared to the vehicle group (25% β‐cyclodextrin, i.p.) and 2.5% isoflurane (Figure 2b). This was similarly observed or mechanical testing (Figure 2c). In contrast, 10 mg·kg−1 i.p. morphine applied pre‐emptively in the same manner before the incision performed under 2.5% isoflurane anaesthesia did not have significant effect in both thermal and mechanical tests (Figure 2d,e). In control experiments, 10 mg·kg−1 morphine injected intraperitoneally effectively reversed mechanical hyperalgesia when applied 24 hr post‐incision (data not shown). This data strongly suggests that heat and mechanical hyperalgesia in post‐operative period can be significantly reduced if surgery was performed with 3β‐OH combined with 1% isoflurane, instead of 2.5% isoflurane alone or with combined morphine/isoflurane protocol. Since nociceptive testing was performed in the days post‐surgery, it is unlikely that hypnotic effect may be a confounding factor to the noted analgesic effects.
Figure 2.
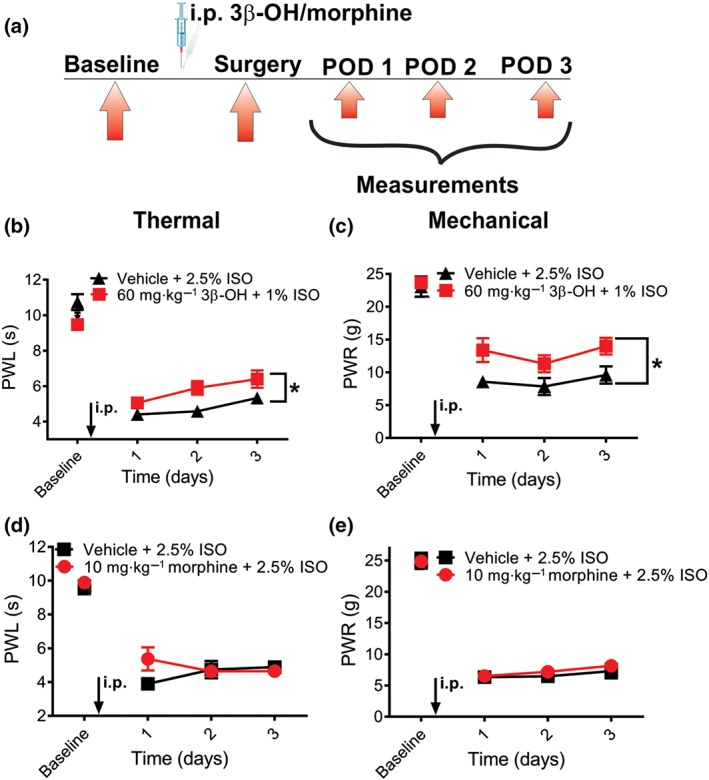
3β‐OH applied as a hypnotic reduces thermal and mechanical hyperalgesia post‐incision. (a) A time course of experimental protocol. Rats were anaesthetized with either 2.5% isoflurane, combination of 2.5% isoflurane with 10 mg·kg−1 i.p. of morphine, or a combination of 60 mg·kg−1 3β‐OH i.p. and 1% isoflurane. (b) 60 mg·kg−1 i.p. 3β‐OH significantly reduced thermal hyperalgesia when applied with 1% isoflurane, as compared to the vehicle group that received 2.5% isoflurane with 25% β‐cyclodextrin (n = 9 animals in vehicle and n = 13 animal in treatment group; P < .05, two‐way RM‐ANOVA). (c) 60 mg·kg−1 i.p. 3β‐OH significantly reduced mechanical hyperalgesia when applied with 1% isoflurane, as compared to the vehicle group that received 2.5% isoflurane with 25% β‐cyclodextrin (n = 5 animals per group, P < .05, two‐way RM‐ANOVA). (d) When 10 mg·kg−1 i.p. morphine was applied pre‐emptively, it did not exert analgesic effect in thermal nociceptive testing (n = 6 animals per group, P > .05, two‐way RM‐ANOVA). (e) When 10 mg·kg−1 i.p. morphine was applied pre‐emptively, it did not exert analgesic effect in mechanical nociceptive testing (n = 6 animals per group, P > .05, two‐way RM‐ANOVA). Data are presented as mean ± SEM
3.3. Analgesic effects of 3β‐OH after intrathecal injections on thermal and mechanical nociception in healthy rats
Next, we examined if intrathecal application of 3β‐OH may alleviate thermal and mechanical nociception in healthy rats (Figure 3a). Indeed, 3β‐OH exerted a substantial thermal and mechanical antinociceptive effect, when given intrathecally during 120 min post‐injection, period, with the peak effect at 60 min for thermal and 30 min for mechanical testing (Figure 3b,c). While the doses of 0.16 and 16 μg exerted antinociceptive effects in thermal and mechanical sensitivity tests, the lowest dose of 0.016 μg did not have a significant antinociceptive effect.
Figure 3.
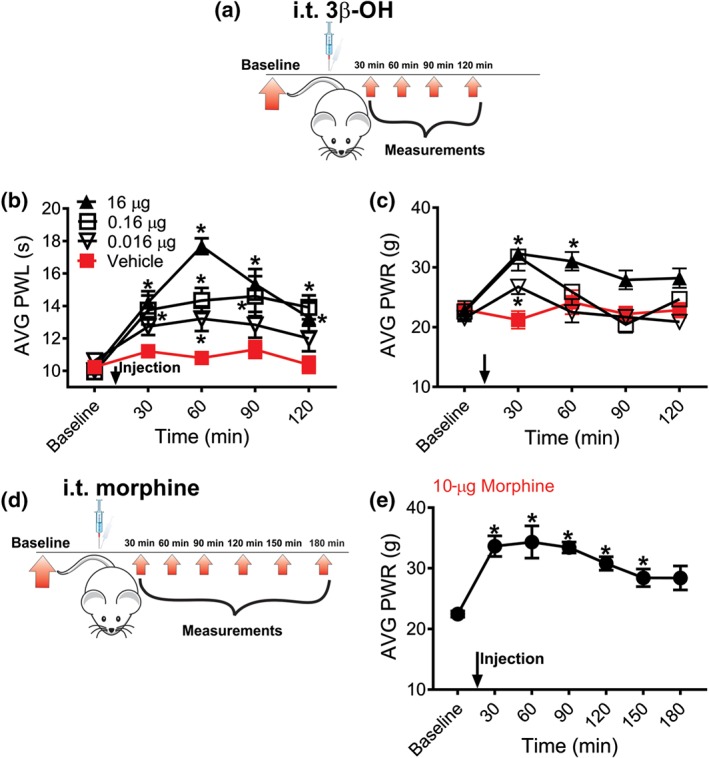
Intrathecal application of 3β‐OH reduces thermal and mechanical nociception in healthy rats. (a) Time course showing baseline measurements to heat and mechanical stimuli determined 2 days before the injection and thermal and mechanical nociception assessment post‐injection. Heat and mechanical hypersensitivity were assessed 30, 60, 90 and 120 min after 3β‐OH or vehicle was applied intrathecally. (b) 3β‐OH alleviates thermal nociception in a dose‐dependent manner (n = 11 in vehicle group, n = 9 in 0.016 μg group, n = 9 in 0.16 μg, and n = 8 in 16 μg group; P < .05, ordinary one‐way ANOVA with Dunnett's post‐hoc test). (c) Time course of antinociceptive effect of 3β‐OH on mechanical nociception (n = 6 in vehicle group, n = 8 in 0.016‐μg group, n = 7 in 0.16‐μg group, and n = 7 in 16‐μg group; P < .05, two‐way RM‐ANOVA with Tukey's post hoc test). (d) Time course showing baseline measurements to mechanical stimulus determined 2 days before the injection and mechanical nociception assessment post‐injection. Mechanical hypersensitivity was assessed 30, 60, 90, 120, 150 and 180 min after 3β‐OH was applied intrathecally. (e) Morphine in a single dose of 10 μg alleviates mechanical nociception in a dose‐dependent manner (n = 8 animals; P < .05, repeated measures one‐way ANOVA with Dunnett's post hoc test). Each data point represents the mean ± SEM
In order to compare antinociceptive effects of 3β‐OH with a common analgesic used in operating rooms we applied morphine sulphate intrathecally and we measured the responses to mechanical stimulus. As expected, morphine in a single dose of 10 μg i.t. (Wang, Pettus, Gao, Phillips, & Bowersox, 2000) effectively reduced mechanical nociception similar to 16‐μg 3β‐OH i.t. (Figure 3d,e).
3.4. Effects of 3β‐OH and morphine on sensory motor abilities of rats
Next, we investigated if the reduced responsiveness to painful stimuli is due to possible side effects such as the impairment of motor capabilities and/or sedation of the animals after injection of 3β‐OH. Therefore, we tested healthy rats injected with the highest dose of 3β‐OH (16 μg i.t.) in a series of sensory motor tests and compared with the animals that had been injected with 10 μg i.t. morphine. All tests were performed 30 min after the injection of either treatments (Figure S2A). We noticed that the time, expressed as percentage normalized from baseline, that animals spent on plank, platform and inclined screen was unchanged before and after the injection of 3β‐OH (animals reached the predetermined cut‐off; Figure S2B–E). Intrathecal morphine exhibited the same effects as 3β‐OH treatment, except in the walking initiation test (Figure S2C), where morphine significantly impaired walking initiation time as compared to 3β‐OH. Hence, we concluded that decreased responses to painful stimuli after intrathecal injections of 3β‐OH are unlikely to be secondary due to other effects of the drug.
3.5. Effects of single intrathecal application of 3β‐OH on post‐incision hyperalgesia in rats
We next injected the compound or vehicle intrathecally either 24 or 48 hr post‐surgery in rats and measured mechanical and thermal responses in incised rats up to 180 min and 120 min post‐injection (Figure 4a). The dose of 3β‐OH used in these experiments was 16 μg i.t. and it was the dose that exerted the highest antinociceptive effect from previous experiments in the healthy rats (Figure 3b,c). We found that 3β‐OH applied as a single dose after surgery, only partially alleviated thermal hyperalgesia. However, it prominently reduced mechanical hyperalgesia, either 24 or 48 hr post‐surgery (Figure 4b–e). It is noteworthy that alleviation of hyperalgesia was more pronounced at 48 hr post‐surgery, when 3β‐OH virtually “normalized” the response to mechanical stimuli, which transiently returned to the pre‐incision level (Figure 4e, dashed black line).
Figure 4.
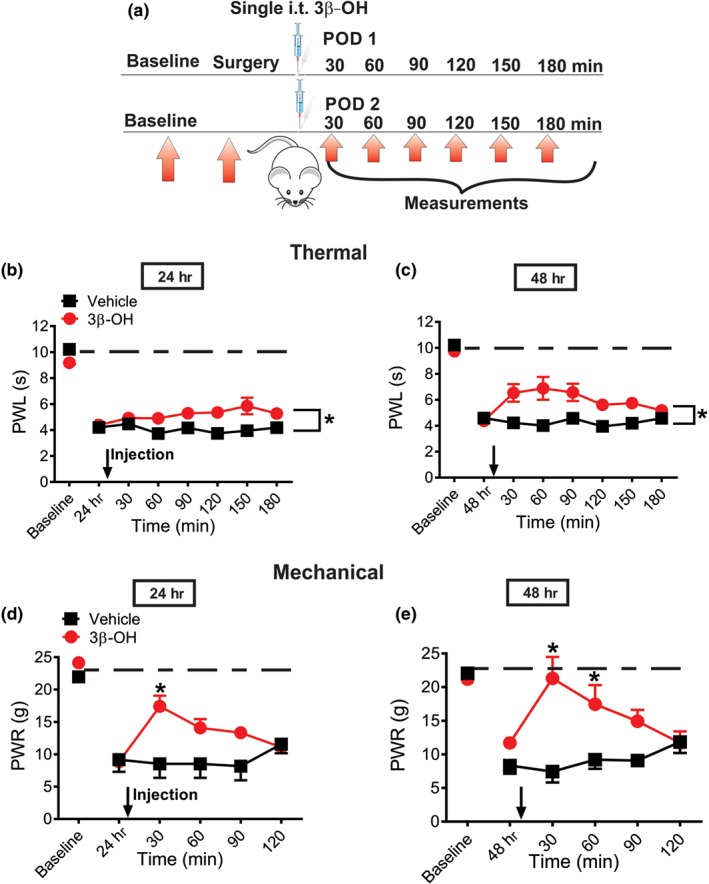
Single intrathecal application of 16‐μg 3β‐OH reduces thermal and mechanical nociception in incised rats. (a) Time course showing baseline measurements to heat and mechanical stimuli determined 2 days before the incision and thermal and mechanical nociception assessment post‐injection in incised rats. The injections of either 16 μg i.t. 3β‐OH or vehicle were performed either on post‐operative days 1 or 2 (POD 1 and POD 2). Heat hyperalgesia was assessed 30, 60, 90, 120, 150 and 180 min after 3β‐OH, or vehicle was applied intrathecally. Mechanical hyperalgesia was assessed 30, 60, 90, and 120 min post‐injection of either 3β‐OH or vehicle. (b) Time‐course of anti‐hyperalgesic effect of 3β‐OH on thermal hyperalgesia after intrathecal injection on POD 1 (n = 6 in vehicle and n = 7 in treatment groups; P < .05, two‐way RM ANOVA). (c) Time course of anti‐hyperalgesic effect of 3β‐OH on thermal hyperalgesia after intrathecal injection on POD 2 (n = 6 in vehicle and n = 8 in treatment groups; P < .05, two‐way RM ANOVA). (d) Time course of anti‐hyperalgesic effect of 3β‐OH on mechanical hyperalgesia after intrathecal injection on POD 1 (n = 6 in vehicle and n = 8 in treatment groups; P < .05, two‐way RM ANOVA with Sidak's post hoc test). (e) Time course of anti‐hyperalgesic effect of 3β‐OH on mechanical hyperalgesia after intrathecal injection on POD 2 (n = 8 animals per group; P < .05, two‐way RM ANOVA with Sidak's post hoc test). Each data point represents the mean ± SEM
3.6. Effects of repetitive intrathecal application of 3β‐OH on post‐incision hyperalgesia in rats
Next, we injected 3β‐OH intrathecally repeatedly at 2 and 24 hr post‐incision (Figure 5a). Neuroaxial application of analgesic drugs during the first 2 days of a post‐operative period is a well known and safe practice in humans (Walker & Yaksh, 2012). Either test compound or vehicle was applied in one group of animals. Although there was no effect at 2 hr post‐incision (Figure 5b), we found a partial alleviation of heat hyperalgesia when 3β‐OH was applied 24 hr post‐surgery (Figure 5c). Interestingly, repeated intrathecal application of 3β‐OH alleviated mechanical hypersensitivity at both time points, 2 and 24 hr after two consecutive applications (Figure 5d,e). Similar to the observed findings with single injection, repetitive intrathecal application of 3β‐OH effectively reversed sensitivity to mechanical stimulus close to the pre‐incision levels (dashed lines).
Figure 5.
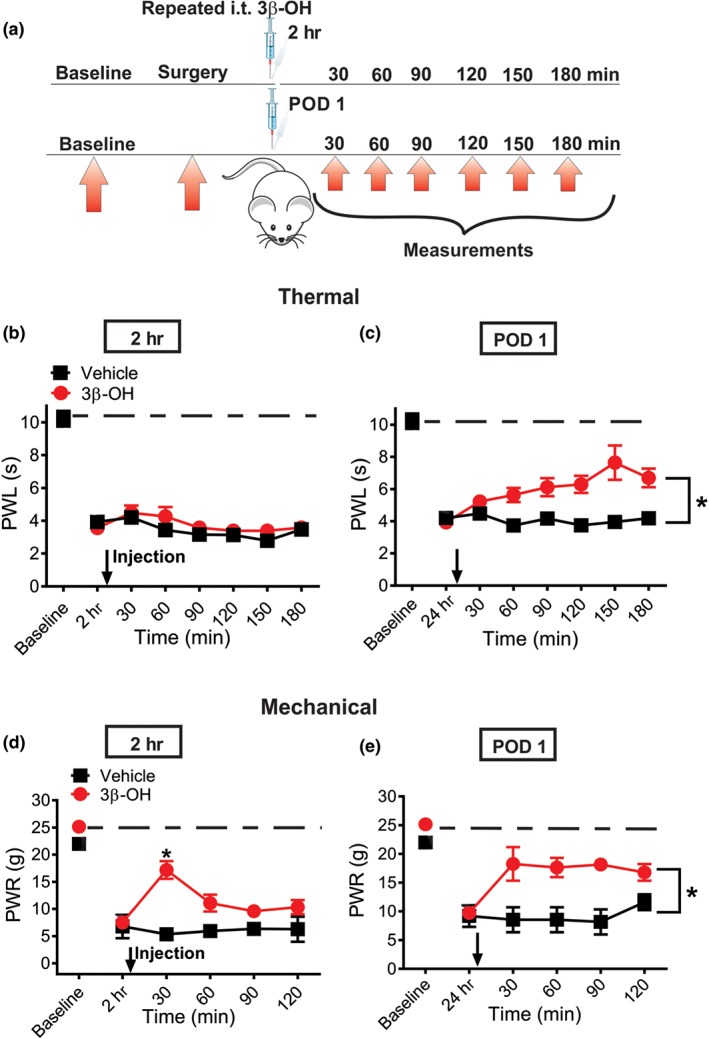
Repetitive intrathecal application of 16‐μg 3β‐OH reduces thermal and mechanical nociception in incised rats. (a) Time course showing baseline measurements to heat and mechanical stimuli determined 2 days before the incision and thermal and mechanical nociception assessment post‐injection in incised rats. The injections of either 3β‐OH or vehicle were performed 2 hr and on post‐operative day 1 (POD 1). Heat hyperalgesia was assessed 30, 60, 90, 120, 150, and 180 min after 3β‐OH or vehicle was applied intrathecally. Mechanical hyperalgesia was assessed 30, 60, 90 and 120 min post‐injection of either 3β‐OH or vehicle. (b) 3β‐OH did not exert anti‐hyperalgesic effect on thermal hyperalgesia after intrathecal injection 2 hr post‐surgery (n = 6 in vehicle and n = 13 in treatment groups; P > .05, two‐way RM ANOVA). (c) Time course of anti‐hyperalgesic effect of 3β‐OH on thermal hyperalgesia after intrathecal injection on POD 1 (n = 6 in vehicle and n = 13 in treatment groups; P < .05, Mann–Whitney test). (d) Time course of anti‐hyperalgesic effect of 3β‐OH on mechanical hyperalgesia after intrathecal injection 2 hr post‐surgery (n = 6 in vehicle and n = 8 in treatment groups; P < .05, two‐way RM ANOVA with Sidak's post hoc test). (e) Time course of anti‐hyperalgesic effect of 3β‐OH on mechanical hyperalgesia after intrathecal injection on POD 1 (n = 6 in vehicle and n = 8 in treatment groups; P < .05, Mann–Whitney test, P = .029). Each data point represents the mean ± SEM
3.7. Effects of pre‐treatment with intrathecal 3β‐OH on post‐surgical incision hyperalgesia in rats
Next, we injected 16 μg of 3β‐OH or vehicle intrathecally 5 min before performing surgical incision and consecutively measured responses to mechanical and thermal stimuli at time points of 2 hr, Day 1, Day 2 and Day 5 post‐incision (Figure 6a). We observed that pre‐treatment with 3β‐OH partially increased paw withdrawal threshold to mechanical stimuli (Figure 6b), indicating diminished hyperalgesia when compared to the vehicle group. It is noteworthy that paw withdrawal responses to mechanical stimuli almost recovered to the pre‐surgery level in 3β‐OH cohort at post‐operative Day 5, as compared to the paw withdrawal responses in the vehicle group on the same day. On the other hand, pre‐treatment with intrathecal application of 3β‐OH did not exert significant analgesic effect on thermal nociceptive testing (Figure 6c).
Figure 6.
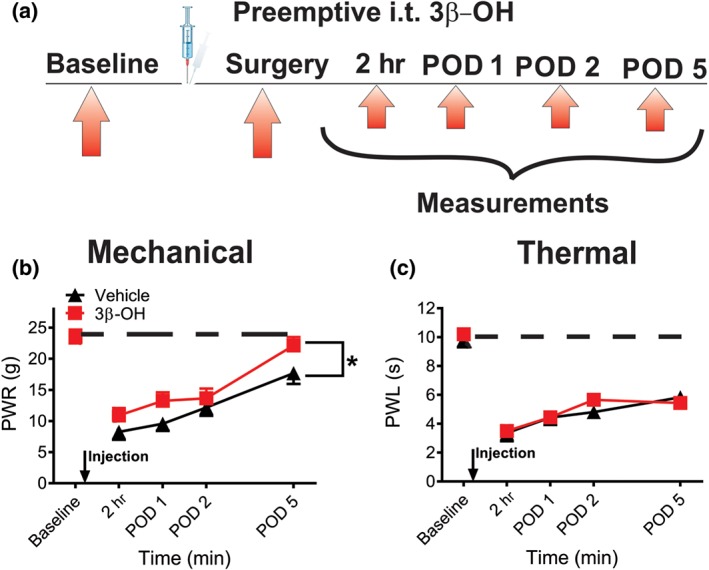
Intrathecal application of 16‐μg 3β‐OH during isoflurane anaesthesia alleviates mechanical but not thermal hyperalgesia after surgery in rats. (a) Time course showing baseline measurements to mechanical stimulus determined 2 days before the incision and mechanical nociception assessment post‐injection in incised rats. Intrathecal injections of either 3β‐OH or vehicle were performed 5 min before the incision. Mechanical hyperalgesia was assessed 2 hr, as well as on post‐operative days 1, 2, and 5 (POD 1, 2, and 5). (b) Time course of anti‐hyperalgesic effect of 3β‐OH on mechanical hyperalgesia after intrathecal injections (n = 6 per group, P < .05, two‐way RM ANOVA). (c) Time course of anti‐hyperalgesic effect of 3β‐OH on thermal hyperalgesia after intrathecal injections (n = 6 per group, P > .05, two‐way RM ANOVA). Each data point represents the mean ± SEM
3.8. Effects of repetitive local intraplantar application of 3β‐OH on post‐incision pain in rats
Next, we studied potential local peripheral analgesic effects of 3β‐OH after surgery. We injected intraplantary the drug or vehicle repeatedly (2 hr, on Day 1 and Day 2 post‐surgery) and measured paw withdrawal latencies and withdrawal responses in response to thermal (heat) and mechanical stimuli, respectively (Figure 7a). We found a significant increase in thermal PWLs in the treatment group, comparing to the vehicle group during post‐operative period of thermal hyperalgesia evaluation (Days 1 to 5). Importantly, paw withdrawal latencies of the group that received 3β‐OH recovered to the pre‐surgery baseline on Day 5, while the group that received vehicle achieved paw withdrawal latencies recovery to the pre‐surgery levels 2 days later (Figure 7b). Similar anti‐hyperalgesic effect of 3β‐OH was seen in the group tested for mechanical sensitivity post‐surgery (Figure 7c). We conclude that the post‐surgical recovery of the group that received the intraplantar 3β‐OH treatment was faster compared to the vehicle group.
Figure 7.
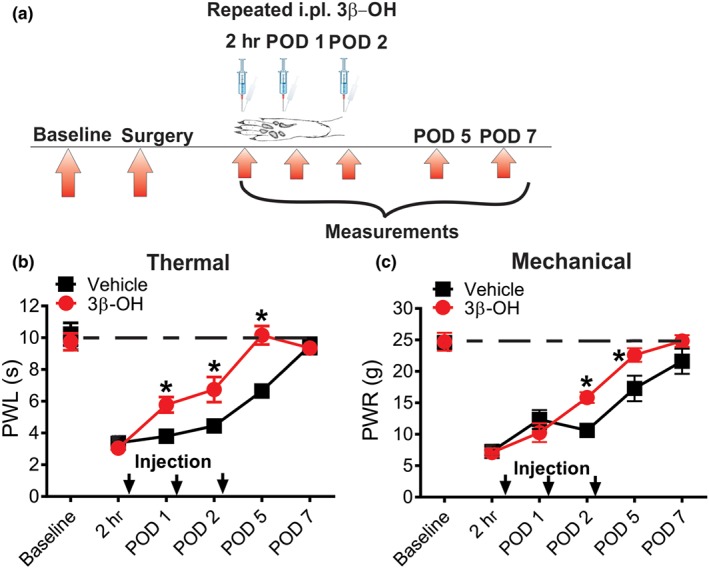
Repetitive intraplantar application of 16‐μg 3β‐OH reduces thermal and mechanical nociception in incised rats. (a) Time course showing baseline measurements to heat and mechanical stimuli determined 2 days before the incision and thermal and mechanical nociception assessment post‐injection in incised rats. Intraplantar injections of either 3β‐OH or vehicle were performed 2 hr post‐incision, Day 1 and 2 post‐incision (POD 1 and 2) and after nociceptive evaluation. Heat and mechanical hyperalgesia were assessed 2 hr, Days 1, 2, 5 and 7 post‐incision (POD 1, 2, 5, and 7). (b) Time course of anti‐hyperalgesic effect of 3β‐OH on thermal hyperalgesia after intraplantar injections (n = 6 in vehicle and n = 8 in treatment groups; P < .05, two‐way RM ANOVA with Sidak's post hoc test). (c) Time course of anti‐hyperalgesic effect of 3β‐OH on mechanical hyperalgesia after intraplantar injections (n = 6 per group, P < .05, two‐way RM ANOVA with Sidak's post hoc test). Each data point represents the mean ± SEM
3.9. Effects of 3β‐OH on excitability of dorsal root ganglia (DRG) sensory neurons in healthy rats
It is generally accepted that changes in excitability of nociceptive DRG neurons underlie pain sensation in vivo. Hence, we used current‐clamp recordings from acutely dissociated DRG neurons to investigate the mechanisms of neurosteroid‐induced analgesia. Current‐clamp stimulus waveform for the dual step protocol (brief depolarizing stimulus followed by a hyperpolarizing stimulus) is shown in the top panel of Figure 8a. Note that depolarizing pulse evoked a barrage of action potentials (APs) in uniform pattern (tonic firing) while hyperpolarization of membrane was followed by a rebound firing of APs. We next applied 3‐μM 3β‐OH and monitored spike firing of DRG neurons. We have previously shown that 3‐μM 3β‐OH blocks about 50% of isolated T‐currents in this preparation in vitro (Todorovic et al., 2004). We found that 3β‐OH completely abolished rebound firing of APs, while at the same time did not significantly affect tonic firing of DRG sensory neurons (Figure 8a–c). This inhibitory effect was reversible 4 min after application of 3β‐OH (Figure 8a last trace). Furthermore, 3β‐OH did not affect input resistance (Figure 8d) and holding resting membrane potentials (Figure 8e). Taken together, these data suggest that 3β‐OH reduces excitability of peripheral sensory neurons by blocking T‐channel dependent rebound firing in these neurons, hence reducing transmission of nociceptive stimuli.
Figure 8.
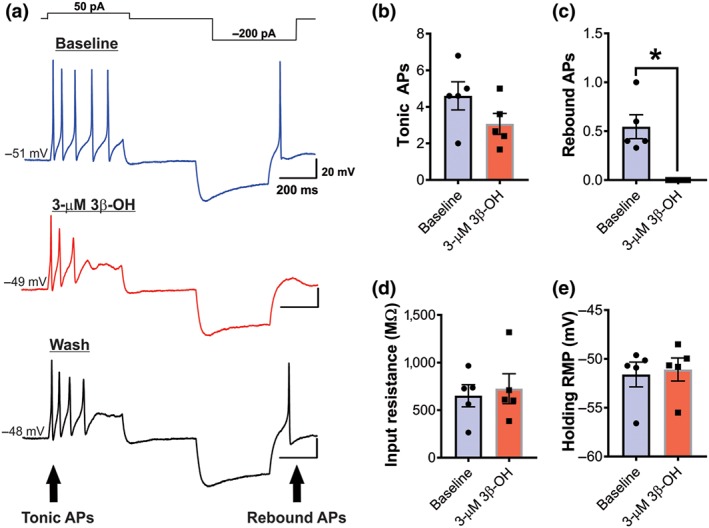
3β‐OH reduces excitability of acutely dissociated peripheral sensory neurons in healthy rats. (a) Original traces of firing patterns of dissociated dorsal root ganglia neurons generated before (blue line, baseline), after 3‐μM 3β‐OH (red line) and after wash (black line). (b) Average number of tonic action potentials before (baseline) and after application of 3‐μM 3β‐OH (n = 5 cells per group, Student's paired t‐test, P > .05). (c) Average number of rebound action potentials before (baseline) and after application of 3‐μM 3β‐OH (n = 5 cells per group, P < .05, paired t test). (d) Input resistance before (baseline) and after application of 3‐μM 3β‐OH (n = 5 cells per group, paired t test, P > .05). (e) Holding resting membrane potential before (baseline) and after application of 3‐μM 3β‐OH (n = 5 cells per group, paired t test, P > .05). Each data point represents the mean ± SEM
3.10. Effects of 3β‐OH and ent‐3β‐OH on isolated T‐currents in HEK‐293 cells expressing CaV3.2 calcium channels
In order to further study the mechanisms of inhibitory effects of 3β‐OH on T‐currents, we applied 3β‐OH in a concentration of 10 μM on HEK cells expressing CaV3.2 channel, a T‐channel subtype important for the development of incisional pain (Joksimovic et al., 2018). This concentration of 3β‐OH was used since it blocked potently T‐currents in DRG cells (Todorovic et al., 2004). In order to study the enantioselectivity of its effect on T‐currents, we applied an enantiomer ent‐3β‐OH in the same concentration as 3β‐OH (Figure 9a). Steroid enantiomers are mirror images of natural steroid compounds and have identical chemical and physical properties except for their ability to rotate plane‐polarized light (+/−). Due to its properties, we propose that the enantiomer used in our study may have a reduced affinity towards T‐channels. Indeed, we found that 10‐μM 3β‐OH effectively blocked recombinant T‐currents, while at the same time equipotent concentration of enantiomer did not exhibit such effect (Figure 9b). In summary, graphs show that the average T‐current amplitude was significantly reduced after 10‐μM 3β‐OH (Figure 9c), while ent‐3β‐OH did not significantly affect baseline currents (Figure 9d). Furthermore, ent‐3β‐OH had no significant effect on voltage‐dependent kinetics of T‐current activation (Figure 9e). Consequently, we also used ent‐3β‐OH to test the idea that anti‐nociceptive effects of this steroid in an incisional pain model are enantioselective. The dose of ent‐3β‐OH used in these experiments was 16 μg i.t. (Figure 9f), which was the dose that exerted the highest antinociceptive effect in previous experiments with 3β‐OH (Figure 3c). After injection, we measured PWRs up to 90 min. We found that unlike 3β‐OH at the same dose, ent‐3β‐OH did not exert any effect to mechanical punctate stimulus and PWRs remained stable (Figure 9g).
Figure 9.
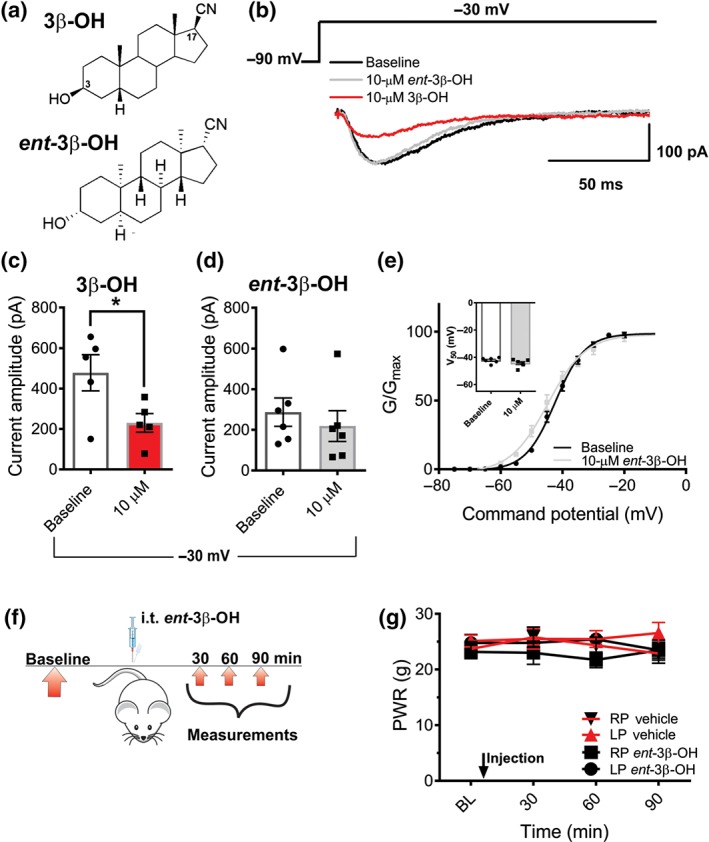
Blocking effect of 3β‐OH on T‐currents in HEK 293 expressing cells CaV3.2 T‐channels is enantioselective. (a) Images of structures of 3β‐OH and ent‐3β‐OH. (b) Original traces of generated T‐currents (Vh − 90 mV, Vt − 30 mV) at baseline (black trace) and after application of either 10‐μM 3β‐OH (red trace) or 10‐μM ent‐3β‐OH (green trace). (c) Current amplitude at baseline and after 10‐μM 3β‐OH (n = 5 cells per group, P < .05, Student's paired t‐test). (d) Current amplitude at baseline and after 10‐μM ent‐3β‐OH (n = 5 cells per group, paired t test, P > .05). (e) Normalized channel conductance on baseline and after application of 10‐μM ent‐3β‐OH calculated from I–V curves and fit with Equation (1) (Section 2). In average, V50 on baseline was −42.33 mV and after the treatment −44.69 mV (n = 5 cells per group, Student's t‐test, P > .05). Average V50 potentials of the baseline conditions and after the treatment with enantiomer are represented as inset graph (paired t test, P > .05). (f) Time course showing baseline measurements to mechanical stimulus determined 2 days before the injection, and mechanical nociception assessment post‐injection. Mechanical hypersensitivity was assessed 30, 60 and 90 min after ent‐3β‐OH or vehicle was applied intrathecally. (g) ent‐3β‐OH did not exert significant antinociception, when compared to the vehicle (n = 8 in vehicle group and n = 6 in treatment group, P > .05, two‐way RM ANOVA). Each data point represents the mean ± SEM
3.11. CaV3.2 channels are essential for anti‐hyperalgesic effect and are contributing to induction of hypnotic effect of 3β‐OH in post‐surgical pain model
We have previously shown that CaV3.2 channels are essential for the development of post‐surgical pain in rodents (Joksimovic et al., 2018). In order to investigate if CaV3.2 isoform of T‐channels represents a target for the in vivo analgesic effects of 3β‐OH post‐incision, we applied neurosteroid (16 μg i.t.) in WT mice and in mice lacking CaV3.2 calcium channels (CaV3.2 KO mice). The treatment was applied on the second day post‐incision (Joksimovic et al., 2018), and the effects of the treatment were measured at time points of 30, 60 and 90 min after the injection (Figure 10a). We found that 3β‐OH significantly reduced mechanical hyperalgesia post‐incision in WT mice, but not in KO mice (Figure 10b). Vehicle applied intrathecally in WT mice did not exert any effect on mechanical hyperalgesia after incision (Figure 10b). Similar to our previous finding (Joksimovic et al., 2018), the pre‐injection baseline comparison revealed that KO mice exhibit significantly reduced hyperalgesic response to mechanical stimulus on POD 2 (n = 9 in WT and n = 6 in KO groups; P = .025, Student's unpaired t‐test, data not shown).
Figure 10.
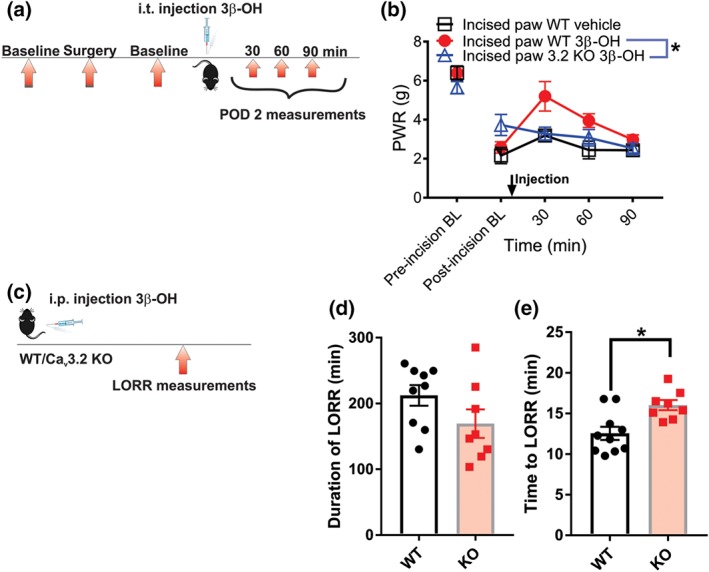
The contribution of CaV3.2 channels in anti‐hyperalgesic and hypnotic effect of 3β‐OH in postsurgical pain model in mice. (a) Time course showing baseline measurements determined before the incision, baseline on post‐operative day 2 (POD 2), injection of 3β‐OH, and assessment of mechanical hyperalgesia after injection. (b) Mechanical hyperalgesia measured after intrathecal 3β‐OH injection of 16 μg/10 μl in WT and CaV3.2 KO or vehicle in WT mice (n = 5 in 3β‐OH in WT and n = 6 in 3β‐OH in CaV3.2 KO group, P < .05, two‐way RM ANOVA; statistical analysis not performed on WT vehicle group n = 4). (c) Experimental protocol of measuring the loss of righting reflex in mice. (d) Duration of LORR in WT versus KO mice (n = 9 in WT and n = 8 in CaV3.2 KO group, P > .05, Student's unpaired t‐test). (e) Time to LORR in WT versus KO mice (n = 10 in WT and n = 8 in CaV3.2 KO group, P < .05, Student's unpaired t‐test). Each data point represents the mean ± SEM
In order to investigate the role of CaV3.2 channels in the steroid‐induced hypnosis, we applied intraperitoneally 3β‐OH in a dose that exerted loss of righting reflex (LORR) in all WT female mice (80 mg·kg−1), and we monitored the duration and onset of loss of righting reflex in WT and KO mice (Figure 10c). Our results revealed that the loss of righting reflex duration was not significantly affected (Figure 10d); however, time to loss of righting reflex was significantly delayed in KO mice, as compared with the WT mice (Figure 10e). We conclude that CaV3.2 channels are required for anti‐hyperalgesic effect of 3β‐OH in incisional pain and that these channels are important for the induction of its hypnotic effect.
4. DISCUSSION
Here, we demonstrate that neuroactive steroid 3β‐OH exerts effective analgesia following systemic, intrathecal and peripheral adminstration. A combination regimen of intraperitoneal 3β‐OH and subanaesthetic dose (1%) of isoflurane induced a profound burst suppression, a hallmark of surgical plane of anaesthesia, known for many anaesthetics (Kenny et al., 2014, 2016) and humans (Hartikainen, Rorarius, Peräkylä, Laippala, & Jäntti, 1995; Huotari et al., 2004). Application of this combination regimen allowed for surgical procedure and lasting reduction of hyperalgesia post‐incision, whereas replacing morphine for 3β‐OH in this combination failed to produce post‐surgical analgesia. Our data reveals that CaV3.2 isoform of T‐channels is relevant for the induction but not for the maintenance of 3β‐OH hypnotic effect. These channels are expressed in cortex and reticular thalamic nucleus (Talley et al., 1999), brain structures heavily involved in the induction of anaesthesia (Flores et al., 2017; Lewis et al., 2015). Furthermore, our previous finding revealed that CaV3.2 KO mice exhibited delayed onset of isoflurane anaesthesia (Orestes et al., 2009). This strongly suggests that the induction of hypnosis with different anaesthetics in mice is facilitated via CaV3.2 channels.
Intrathecal application exerts effects on both peripheral and spinal pain processing representing a common route of administration of analgesics in clinical practice (Bennett et al., 2000). In our study, we found that intrathecal 3β‐OH reduces thermal and mechanical nociception in healthy rats. Also, either as a single or repeated intrathecal injection after, or single injection before the surgery (combined application), 3β‐OH reduced both thermal and mechanical hyperalgesia, having a more potent effects on mechanical hypersensitivity. Furthermore, we found that 3,5‐dichloro‐N‐[1‐(2,2‐dimethyl‐tetrahydro‐pyran‐4‐ylmethyl)‐4‐fluoro‐piperidin‐4‐ylmethyl]‐benzamide (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7723), a structurally unrelated selective T‐channel blocker, when applied repeatedly, also alleviates mechanical but not thermal nociception 2 hr post‐incision (Joksimovic et al., 2018). This suggests different mechanisms of modulation of thermal and mechanical hyperalgesia after surgery. Finally, it appears that T‐channels seem to be more important for the maintenance rather than for the development of thermal hyperalgesia post‐surgery (Joksimovic et al., 2018), which could explain the lack of effect of 3β‐OH at time point of 2 hr post‐surgery and prominent anti‐hyperalgesia when applied at 24 or 48 hr post‐incision.
Combined administration of an analgesia with an anaesthetic represents a desirable antinociceptive intervention (Kissin, 1994). Based upon the results from the animal studies, it has been hypothesized that adding an analgesia to anaesthetic regime prevents the development of central sensitization, which in turn reduces the post‐operative hyperalgesia (Woolf & Chong, 1993). Although some clinical trials have not shown the superiority of combined analgesic with anaesthetic over the conventional post‐surgical therapy (Møiniche, Kehlet, & Dahl, 2002), Ong, Lirk, Seymour, and Jenkins (2005) have found that epidural analgesia applied under anaesthesia can improve post‐surgical pain outcomes in humans. Although combined anaesthesia with morphine reduced nociceptive behaviour in the late phase of formalin testing in mice (Abram & Yaksh.,1993), it failed to show longer lasting effects even at the dose of 30 μg i.t. post‐incision (Brennan, 1997). On the other hand, we found that both intraperitoneal and intrathecal application of 3β‐OH in the presence of anaesthesia provided noteworthy anti‐hyperalgesic effect after surgical incision. Therefore, the addition of 3β‐OH to the anaesthetic regimen could reduce post‐surgical consumption of analgesics and at the same time decrease amount of volatile agents during surgical procedure. Also, reported is the effective peripheral anti‐hyperalgesia with intraplantar application of 3β‐OH post‐surgery. This is a relatively unique property compared with other known sedative/hypnotic drugs and it may be useful in procedures where general anaesthesia is not required.
Previous studies showed the efficacy of morphine, applied intrathecally or subcutaneously, in incisional pain model (Whiteside et al., 200; Wang et al., 2000). However, clinical application of morphine is often restricted due to known problems such as tolerance, addiction and opioid‐induced hyperalgesia. Roeckel et al. (2017) have shown that morphine induces tolerance and hyperalgesia in wild type, but not in μ‐opioid receptor KO mice, suggesting that opioid induced hyperalgesia likely occurs via the similar mechanism that provides beneficial analgesic effects. In contrast, we did not notice hyperalgesia occurrence after repetitive application of 3β‐OH.
It is well established that rebound firing of APs in multiple subpopulations of sensory neurons is dependent on CaV3.2 isoform of T‐channels (Jagodic et al., 2007; Nelson et al., 2005). Here, we also demonstrate that 3β‐OH can abolished rebound bursts of small‐ to medium‐sized sensory neurons. It is well known that steroids can exert direct and indirect effects (via steroid alteration of the membrane environment) on membrane receptor function (Covey, 2009). In order to distinguish between these effects, we used an enantiomer of 3β‐OH. Enantiomers represent a mirror image of the active molecule with the same physicochemical properties, thus enabling the receptor to discriminate between ligands of different shape, while at the same time, their effects on the membrane characteristics will be unchanged (non‐enantioselective). In our study, ent‐3βOH did not exert antinociceptive effect in healthy animals and did not inhibit recombinant CaV3.2 currents. This finding suggests that 3β, 5β, and 17β configuration of a neurosteroid is necessary in order to effectively inhibit T‐channels.
Our data also demonstrated that 3β‐OH exerted significant analgesic effect in WT mice but not in CaV3.2 KO mice post incision. CaV3.2 T‐channel isoform is strongly implicated in nociception, as these channels were found on the cell bodies of sensory neurons in DRG and nociceptive nerve endings in the skin (Rose et al., 2013). Furthermore, presynaptic CaV3.2 channels facilitate excitatory pain transmission in the dorsal horn of spinal cord (Jacus, Uebele, Renger, & Todorovic, 2012). Although expression of other T‐channel isoforms (CaV3.1 and CaV3.3) is relatively low in DRG cells, intrathecal application of 3β‐OH could theoretically block CaV3.1 and CaV3.3 isoforms located in the spinal cord (Talley et al., 1999). Nevertheless, given that our recent study (Joksimovic et al., 2018) heavily implicates CaV3.2 isoform of T‐channels in hyperalgesia post‐surgery, we propose that the anti‐hyperalgesic effect of 3β‐OH in incisional pain model is most likely mediated via enantioselective inhibition of CaV3.2 channels.
The role of high voltage‐activated (HVA) calcium channels has also been studied in incisional pain model. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5483, which targets α2‐δ subunit of HVA channels, alleviated post‐surgical pain in rodents (Whiteside et al., 200). It was also described in the same pain model that N‐type calcium channel blocker https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2536 alleviated hyperalgesia post‐surgery (Wang et al., 2000). The effects of ziconotide on mechanical hyperalgesia were comparable with the observed effects of 3β‐OH in the present study. Since 3β‐OH can block HVA currents (mostly N type and L‐type) in smaller DRG neurons at concentrations 10 times higher than T‐currents (Todorovic, Covey, Zorumski, & Jevtovic‐Todorovic, 2005), the potential role of HVA channels in analgesic effect of 3β‐OH unlikely. However, this notion needs to be further investigatged in future experiments
Overall, our study has shown that 3β‐OH partially alleviates post‐surgical sensitivity to thermal and mechanical stimuli in rats, applied either with the anaesthetic or after the surgical procedure. Importantly, we have shown that when used as an adjuvant hypnotic for the surgical procedure, 3β‐OH alleviated hyperalgesia in days after surgery. We conclude that 3β‐OH could be introduced as a new class of hypnotic analgesics with CaV3.2 calcium channel blocking properties, that could provide a novel therapeutic approach for the pre‐med and supportive treatment of post‐operative pain in humans.
AUTHOR CONTRIBUTIONS
S.L.J. performed experiments, analysed the data, and wrote main draft of the manuscript; S.M.J., B.A., and Y.H.R. performed experiments and analysed the data; K.K. synthetized 3β‐OH and ent‐3β‐OH; V.J.T., D.F.C., and S.M.T. designed experiments; S.M.T. supervised the whole study. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no competing interests.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
DATA AND MATERIALS AVAILABILITY
All data needed to evaluate the conclusions in the paper are presented in the paper.
Supporting information
Fig. S1. 3β‐OH induces hypnosis in young adult rats. A dose–response curve of i.p. injected 3β‐OH in doses 5, 10, 15, 20, 50 and 60 mg/kg. ED50 is 14.6 ± 0.7 mg/kg (slope of the fit: n = 2.58 ± 0.39). The numbers in brackets represent the number of the animals in each group.
Fig. S2. Impact of intrathecal application of 3β‐OH or morphine on sensory‐motor abilities of rats. A. Time course showing baseline measurements determined before injection, and sensory‐motor assessment performed after injection. B. Time expressed as percentage relative to baseline that rats spent walking the plank before and 30 minutes after i.t. 3β‐OH injection of 16 μg /50 μl or i.t. morphine 10 μg /50 μl (n = 5 in morphine and n = 8 in 3β‐OH group). C. Time expressed as percentage relative to baseline that rats spent in placing all four paws outside the labelled square (walking initiation) before and 30 minutes after i.t. 3β‐OH injection of 16 μg /50 μl or i.t. morphine 10 μg /50 μl (n = 5 in morphine and n = 8 in 3β‐OH group; P < 0.05, paired t‐test). D. Time expressed as percentage relative to baseline that rats spent on an elevated platform before and 30 minutes after i.t. 3β‐OH injection of 16 μg /50 μl or i.t. morphine 10 μg /50 μl (n = 5 in morphine and n = 8 in 3β‐OH group). E. Time expressed as percentage relative to baseline that rats spent on an inclined screen before and 30 minutes after i.t. 3β‐OH injection of 16 μg/50 μl or i.t. morphine 10 μg/50 μl (n = 5 in morphine and n = 8 in 3β‐OH group). Data are presented as means±SEM.
ACKNOWLEDGEMENT
We thank Dr. Alex Keizer for help with statistical analysis of the data.
This work was supported by the NIH Grant 1 R01 GM123746‐01 to S.M.T. and V.J.T., the funds from the Department of Anesthesiology at UC Denver.
Joksimovic SL, Joksimovic SM, Manzella FM, et al. Novel neuroactive steroid with hypnotic and T‐type calcium channel blocking properties exerts effective analgesia in a rodent model of post‐surgical pain. Br J Pharmacol. 2020;177:1735–1753. 10.1111/bph.14930
REFERENCES
- Abram, S. E. , & Yaksh, T. L. (1993). Morphine, but not inhalation anesthesia, blocks post‐injury facilitation. Anesthesiology, 78, 713–721. 10.1097/00000542-199304000-00015 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. M. , Striessnig, J. , Kelly, E. , … CGTP collaboratories (2019). The Coincise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfelbaum, J. L. , Chen, C. , Mehta, S. S. , & Gan, T. J. (2003). Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia & Analgesia, 97(2), 534–540. 10.1213/01.ane.0000068822.10113.9e [DOI] [PubMed] [Google Scholar]
- Atluri, N. , Joksimovic, S. M. , Oklopcic, A. , Milanovic, D. , Klawitter, J. , Eggan, P. , … Jevtovic‐Todorovic, V. (2018). A neurosteroid analogue with T‐type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. British Journal of Anaesthesia, 120(4), 768–778. 10.1016/j.bja.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola, C. , Hwang, S. M. , Hong, S. J. , Rose, K. E. , Boyd, C. , Bozic, N. , … Todorovic, S. M. (2014). Inhibition of CaV3.2 T‐type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone. Psychopharmacology, 231(17), 3503–3515. 10.1007/s00213-014-3588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley, E. J. , & Fillingim, R. B. (2013). Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthesia, 11(1), 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu, E. E. , & Robel, P. (1990). Neurosteroids: A new brain function? The Journal of Steroid Biochemistry and Molecular Biology, 37(3), 395–403. 10.1016/0960-0760(90)90490-c [DOI] [PubMed] [Google Scholar]
- Belelli, D. , & Lambert, J. J. (2005). Neurosteroids: Endogenous regulators of the GABAA receptor. Nature Reviews Neuroscience, 6(7), 565–575. 10.1038/nrn1703 [DOI] [PubMed] [Google Scholar]
- Bennett, G. , Serafini, M. , Burchiel, K. , Buchser, E. , Classen, A. , Deer, T. , et al. (2000). Evidence‐based review of the literature on intrathecal delivery of pain medication. Journal of Pain and Symptom Management, 20(2), 12–36. [DOI] [PubMed] [Google Scholar]
- Brennan, T. J. (1997). Comparison of pre‐ versus post‐incision administration of intrathecal bupivacaine and intrathecal morphine in a rat model of postoperative pain. Anesthesiology, 87, 1517–1528. 10.1097/00000542-199712000-00031 [DOI] [PubMed] [Google Scholar]
- Brennan, T. J. , Vandermeulen, E. P. , & Gebhart, G. F. (1996). Characterization of a rat model of incisional pain. Pain, 64(3), 493–502. 10.1016/0304-3959(95)01441-1 [DOI] [PubMed] [Google Scholar]
- Chapman, C. R. , Duncan, A. S. , & Lipman, A. G. (2013). Quality of postoperative pain management in American versus European institutions. Journal of Pain & Palliative Care Pharmacotherapy, 27(4), 350–358. 10.3109/15360288.2013.846955 [DOI] [PubMed] [Google Scholar]
- Choi, S. , Na, H. S. , Kim, J. , Lee, J. , Lee, S. , Kim, D. , … Shin, H. S. (2007). Attenuated pain responses in mice lacking CaV3.2 T‐type channels. Genes, Brain and Behavior, 6(5), 425–431. 10.1111/j.1601-183X.2006.00268.x [DOI] [PubMed] [Google Scholar]
- Covey, D. F. (2009). ent‐Steroids: Novel tools for studies of signaling pathways. Steroids, 74(7), 577–585. 10.1016/j.steroids.2008.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Bond, R. A. , Spina, D. , Ahluwalia, A. , Alexander, S. P. A. , Giembycz, M. A. , … McGrath, J. C. (2015). Experimental design and analysis and their reporting: New guidance for publication in BJP . British Journal of Pharmacology, 172, 3461–3471. 10.1111/bph.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores, F. J. , Hartnack, K. E. , Fath, A. B. , Kim, S. E. , Wilson, M. A. , Brown, E. N. , & Purdon, P. L. (2017). Thalamocortical synchronization during induction and emergence from propofol‐induced unconsciousness. Proceedings of the National Academy of Sciences U SA, 114(32), E6660–E6668. 10.1073/pnas.1700148114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, M. , Zorumski, C. F. , & Covey, D. F. (1996). Neurosteroid analogues. 4. The effect of methyl substitution at the C‐5 and C‐10 positions of neurosteroids on electrophysiological activity at GABAA receptors. Journal of Medicinal Chemistry, 39(21), 4218–4232. 10.1021/jm960304p [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen, K. M. , Rorarius, M. , Peräkylä, J. J. , Laippala, P. J. , & Jäntti, V. (1995). Cortical reactivity during isoflurane burst‐suppression anesthesia. Anesthesia and Analgesia, 81(6), 1223–1228. 10.1097/00000539-199512000-00018 [DOI] [PubMed] [Google Scholar]
- Honore, P. , Rogers, S. D. , Schwei, M. J. , Salak‐Johnson, J. L. , Luger, N. M. , Sabino, M. C. , … Mantyh, P. W. (2000). Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience, 98(3), 585–598. 10.1016/S0306-4522(00)00110-X [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Wittmer, L. L. , Kalkbrenner, M. , Evers, A. S. , Zorumski, C. F. , & Covey, D. F. (1997). Neurosteroid analogues. Part 5. Enantiomers of neuroactive steroids and benz[e]indenes: Total synthesis, electrophysical effects on GABAA receptor function and anesthetic actions in tadpoles. Journal of the Chemical Society, Perkin Transactions, 1, 3665–3671. [Google Scholar]
- Huotari, A.‐M. , Koskinen, M. , Suominen, K. , Alahuhta, S. , Remes, R. , Hartikainen, K. M. , & Jäntti, V. (2004. Jan). Evoked EEG patterns during burst suppression with propofol. British Journal of Anaesthesia, 92(1), 18–24. 10.1093/bja/aeh022 [DOI] [PubMed] [Google Scholar]
- Jacus, M. O. , Uebele, V. N. , Renger, J. J. , & Todorovic, S. M. (2012). Presynaptic CaV3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. The Journal of Neuroscience, 32(27), 9374–9382. 10.1523/JNEUROSCI.0068-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic, M. M. , Pathirathna, S. , Nelson, M. T. , Mancuso, S. , Joksovic, P. M. , Rosenberg, E. R. , … Todorovic, S. M. (2007). Cell‐specific alterations of T‐type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. Journal of Neuroscience, 27(12), 3305–3316. 10.1523/JNEUROSCI.4866-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic, S. L. , Joksimovic, S. M. , Tesic, V. , García‐Caballero, A. , Feseha, S. , Zamponi, G. W. , … Todorovic, S. M. (2018). Selective inhibition of CaV3.2 channels reverses hyperexcitability of peripheral nociceptors and alleviates postsurgical pain. Science Signaling, 11, eaao4425‐4437. 10.1126/scisignal.aao4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, J. D. , Chemali, J. J. , Cotten, J. F. , Van Dort, C. J. , Kim, S. E. , Ba, D. , et al. (2016). Physostigmine and methylphenidate induce distinct arousal states during isoflurane general anesthesia in rats. Anesthesia and Analgesia, 123(5), 1210–1219. 10.1213/ANE.0000000000001234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, J. D. , Westover, M. B. , Ching, S. , Brown, E. N. , & Solt, K. (2014). Propofol and sevoflurane induce distinct burst suppression patterns in rats. Frontiers in Systems Neuroscience, 18(8), 237‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin, I. (1994). Preemptive analgesia: Terminology and clinical relevance. Anesthesia and Analgesia, 79(4), 809–810. [PubMed] [Google Scholar]
- Lewis, L. D. , Voigts, J. , Flores, F. J. , Schmitt, L. I. , Wilson, M. A. , Halassa, M. M. , & Brown, E. N. (2015). Thalamic reticular nucleus induces fast and local modulation of arousal state. eLife, 4, e08760 08783. 10.7554/eLife.08760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, T. , & Leung, L. S. (2009). Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology, 111(4), 725–733. 10.1097/ALN.0b013e3181b061a0 [DOI] [PubMed] [Google Scholar]
- Majewska, M. D. , Harrison, N. L. , Schwartz, R. D. , Barker, J. L. , & Paul, S. M. (1986). Steroid hormone metabolites are barbiturate‐like modulators of the GABA receptor. Science, 232(4753), 1004–1007. 10.1126/science.2422758 [DOI] [PubMed] [Google Scholar]
- Mathiesen, O. , Dahl, B. , Thomsen, B. A. , Kitter, B. , Sonne, N. , Dahl, J. B. , & Kehlet, H. (2013). A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, 22(9), 2089–2096. 10.1007/s00586-013-2826-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah‐Nyagan, A. G. , Kibaly, C. , Schaeffer, V. , Venard, C. , Meyer, L. , & Patte‐Mensah, C. (2008). Endogenous steroid production in the spinal cord and potential involvement in neuropathic pain modulation. Journal of Steroid Biochemistry and Molecular Biology, 109(3–5), 286–293. 10.1016/j.jsbmb.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Mogil, J. S. , & Chanda, M. L. (2005). The case for the inclusion of female subjects in basic science studies of pain. Pain 2005 Sep, 117(1–2), 1–5Review. 10.1016/j.pain.2005.06.020 [DOI] [PubMed] [Google Scholar]
- Møiniche, S. , Kehlet, H. , & Dahl, J. B. (2002). A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology, 96(3), 725–741. Review. 10.1097/00000542-200203000-00032 [DOI] [PubMed] [Google Scholar]
- Nelson, M. T. , Joksovic, P. M. , Perez‐Reyes, E. , & Todorovic, S. M. (2005). The endogenous redox agent l‐cysteine induces T‐type Ca2+ channel‐dependent sensitization of a novel subpopulation of rat peripheral nociceptors. The Journal of Neuroscience, 25(38), 8766–8775. 10.1523/JNEUROSCI.2527-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, C. K.‐S. , Lirk, P. , Seymour, R. A. , & Jenkins, B. J. (2005). The efficacy of preemptive analgesia for acute postoperative pain management: A meta‐analysis. Anesthesia and Analgesia, 100(3), 757–773. 10.1213/01.ANE.0000144428.98767.0E [DOI] [PubMed] [Google Scholar]
- Orestes, P. , Bojadzic, D. , Chow, R. M. , & Todorovic, S. M. (2009). Mechanisms and functional significance of inhibition of neuronal T‐type calcium channels by isoflurane. Molecular Pharmacology, 75(3), 542–554. 10.1124/mol.108.051664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirathna, S. , Brimelow, B. C. , Jagodic, M. M. , Krishnan, K. , Jiang, X. , Zorumski, C. F. , … Jevtovic‐Todorovic, V. (2005). New evidence that both T‐type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α‐reduced neuroactive steroids. Pain, 114(3), 429–443. 10.1016/j.pain.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Pathirathna, S. , Todorovic, S. M. , Covey, D. F. , & Jevtovic‐Todorovic, V. (2005). 5α‐reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain, 117(3), 326–339. 10.1016/j.pain.2005.06.019 [DOI] [PubMed] [Google Scholar]
- Patte‐Mensah, C. , Meyer, L. , Taleb, O. , & Mensah‐Nyagan, A. G. (2014). Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Progress in Neurobiology, 113, 70–78. 10.1016/j.pneurobio.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Pavlin, D. J. , Chen, C. , Penaloza, D. A. , Polissar, N. L. , & Buckley, F. P. (2002). Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesthesia and Analgesia, 95(3), 627–634. 10.1097/00000539-200209000-00025 [DOI] [PubMed] [Google Scholar]
- Pogatzki, E. M. , & Raja, S. N. (2003). A mouse model of incisional pain. Anesthesiology, 99(4), 1023–1027. 10.1097/00000542-200310000-00041 [DOI] [PubMed] [Google Scholar]
- Roeckel, L. A. , Utard, V. , Reiss, D. , Mouheiche, J. , Maurin, H. , Robé, A. , … Gaveriaux‐Ruff, C. (2017). Morphine‐induced hyperalgesia involves μ opioid receptors and the metabolite morphine‐3‐glucuronide. Scientific Reports Sep 4, 7(1), 10406‐10421. 10.1038/s41598-017-11120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, K. E. , Lunardi, N. , Boscolo, A. , Dong, X. , Erisir, A. , Jevtovic‐Todorovic, V. , & Todorovic, S. M. (2013). Immunohistological demonstration of CaV3.2 T‐type voltage‐gated calcium channel expression in soma of dorsal root ganglion neurons and peripheral axons of rat and mouse. Neuroscience, 250, 263–274. 10.1016/j.neuroscience.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, M. , Liere, P. , Akwa, Y. , Rajkowski, K. , Griffiths, W. , Bodin, K. , … Baulieu, E. E. (2008). Pregnenolone sulfate in the brain: A controversial neurosteroid. Neurochemistry International, 52(4–5), 522–540. 10.1016/j.neuint.2007.08.022 [DOI] [PubMed] [Google Scholar]
- Svensson, E. , Persson, J. , Fitzsimmons, B. , & Yaksh, T. L. (2013). Intrathecal neurosteroids and a neurosteroid antagonist: Effects on inflammation‐evoked thermal hyperalgesia and tactile allodynia. Neuroscience Letters, 548, 27–32. 10.1016/j.neulet.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley, E. M. , Cribbs, L. L. , Lee, J. H. , Daud, A. , Perez‐Reyes, E. , & Bayliss, D. A. (1999). Differential distribution of three members of a gene family encoding low voltage‐activated (T‐type) calcium channels. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(6), 1895–1911. 10.1523/JNEUROSCI.19-06-01895.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic, S. M. , Covey, D. F. , Zorumski, C. F. , & Jevtovic‐Todorovic, V. (2005). Neuroactive steroids as targets for development of novel pain therapies. Current Medicinal Chemistry: Central Nervous System Agents, 5(2), 157–164. 10.2174/1568015054022407 [DOI] [Google Scholar]
- Todorovic, S. M. , Jevtovic‐Todorovic, V. , Meyenburg, A. , Mennerick, S. , Perez‐Reyes, E. , Romano, C. , … Zorumski, C. F. (2001). Redox modulation of T‐type calcium channels in rat peripheral nociceptors. Neuron, 31(1), 75–85. 10.1016/s0896-6273(01)00338-5 [DOI] [PubMed] [Google Scholar]
- Todorovic, S. M. , & Lingle, C. J. (1998). Pharmacological properties of T‐type Ca2+ current in adult rat sensory neurons: Effects of anticonvulsant and anesthetic agents. Journal of Neurophysiology, 79(1), 240–252. 10.1152/jn.1998.79.1.240 [DOI] [PubMed] [Google Scholar]
- Todorovic, S. M. , Meyenburg, A. , & Jevtovic‐Todorovic, V. (2002). Mechanical and thermal antinociception in rats following systemic administration of mibefradil, a T‐type calcium channel blocker. Brain Research, 951(2), 336–340. 10.1016/s0006-8993(02)03350-4 [DOI] [PubMed] [Google Scholar]
- Todorovic, S. M. , Pathirathna, S. , Brimelow, B. C. , Jagodic, M. M. , Ko, S. H. , Jiang, X. , … Jevtovic‐Todorovic, V. (2004). 5β‐reduced neuroactive steroids are novel voltage‐dependent blockers of T‐type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Molecular Pharmacology, 66(5), 1223–1235. 10.1124/mol.104.002402 [DOI] [PubMed] [Google Scholar]
- Todorovic, S. M. , Rastogi, A. J. , & Jevtovic‐Todorovic, V. (2003). Potent analgesic effects of anticonvulsants on peripheral thermal nociception in rats. British Journal of Pharmacology, 140(2), 255–260. 10.1038/sj.bjp.0705434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, & Yaksh, T. L. (2012). Neuraxial analgesia in neonates and infants: A review of clinical and preclinical strategies for the development of safety and efficacy data. Anesthesia and Analgesia, 115(3), 638–662. 10.1213/ANE.0b013e31826253f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.‐X. , Pettus, M. , Gao, D. , Phillips, C. , & Bowersox, S. S. (2000). Effects of intrathecal administration of ziconotide, a selective neuronal N‐type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain, 84(2–3), 151–158. 10.1016/S0304-3959(99)00197-9 [DOI] [PubMed] [Google Scholar]
- Whiteside, G. T. , Harrison, J. , Boulet, J. , Mark, L. , Pearson, M. , Gottshall, S. , & Walker, K. (200. 4). Pharmacological characterisation of a rat model of incisional pain. British Journal of Pharmacology, 141(1), 85–91. 10.1038/sj.bjp.0705568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf, C. J. , & Chong, M. S. (1993). Preemptive analgesia—Treating postoperative pain by preventing the establishment of central sensitization. Anesthesia & Analgesia, 77, 362–379. 10.1213/00000539-199377020-00026 [DOI] [PubMed] [Google Scholar]
- Wozniak, D. F. , Olney, J. W. , Kettinger, L. III , Price, M. , & Miller, J. P. (1990). Behavioral effects of MK‐801 in the rat. Psychopharmacology, 101, 47–56. 10.1007/bf02253717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. 3β‐OH induces hypnosis in young adult rats. A dose–response curve of i.p. injected 3β‐OH in doses 5, 10, 15, 20, 50 and 60 mg/kg. ED50 is 14.6 ± 0.7 mg/kg (slope of the fit: n = 2.58 ± 0.39). The numbers in brackets represent the number of the animals in each group.
Fig. S2. Impact of intrathecal application of 3β‐OH or morphine on sensory‐motor abilities of rats. A. Time course showing baseline measurements determined before injection, and sensory‐motor assessment performed after injection. B. Time expressed as percentage relative to baseline that rats spent walking the plank before and 30 minutes after i.t. 3β‐OH injection of 16 μg /50 μl or i.t. morphine 10 μg /50 μl (n = 5 in morphine and n = 8 in 3β‐OH group). C. Time expressed as percentage relative to baseline that rats spent in placing all four paws outside the labelled square (walking initiation) before and 30 minutes after i.t. 3β‐OH injection of 16 μg /50 μl or i.t. morphine 10 μg /50 μl (n = 5 in morphine and n = 8 in 3β‐OH group; P < 0.05, paired t‐test). D. Time expressed as percentage relative to baseline that rats spent on an elevated platform before and 30 minutes after i.t. 3β‐OH injection of 16 μg /50 μl or i.t. morphine 10 μg /50 μl (n = 5 in morphine and n = 8 in 3β‐OH group). E. Time expressed as percentage relative to baseline that rats spent on an inclined screen before and 30 minutes after i.t. 3β‐OH injection of 16 μg/50 μl or i.t. morphine 10 μg/50 μl (n = 5 in morphine and n = 8 in 3β‐OH group). Data are presented as means±SEM.


