Abstract
Background
The extent to which cardiovascular disease (CVD) risk factors across the menopause explain racial/ethnic differences in subclinical vascular disease in late midlife women is not well documented and was explored in a multi‐ethnic cohort.
Methods and Results
Participants (n=1357; mean age 60 years) free of clinical CVD from the Study of Women's Health Across the Nation had common carotid artery intima‐media thickness, interadventitial diameter, and carotid plaque presence assessed by ultrasonography on average 13.7 years after baseline visit. Early to late midlife time‐averaged cumulative burden of traditional CVD risk factors calculated using serial measures from baseline to the ultrasound visit were generally less favorable in black and Hispanic women compared with white and Chinese women, including education and smoking status and time‐averaged cumulative blood pressure, high‐density lipoprotein cholesterol, and fasting insulin. Independent of these risk factors, BMI, and medications, common carotid artery intima‐media thickness was thicker in black women, interadventitial diameter was wider in Chinese women, yet plaque presence was lower in black and Hispanic women compared with white women. CVD risk factor associations with subclinical vascular measures did not vary by race/ethnicity except for high‐density lipoprotein cholesterol on common carotid artery intima‐media thickness; an inverse association between high‐density lipoprotein cholesterol and common carotid artery intima‐media thickness was observed in Chinese and Hispanic but not in white or black women.
Conclusions
Race/ethnicity did not particularly moderate the association between traditional CVD risk factors measured across the menopause transition and late midlife subclinical vascular disease. Unmeasured socioeconomic, cultural, and nontraditional biological risk factors likely play a role in racial/ethnic differences in vascular health and merit further exploration.
Keywords: atherosclerosis, cardiovascular disease risk factors, menopause, race and ethnicity, women
Subject Categories: Race and Ethnicity, Women, Risk Factors, Cardiovascular Disease
Clinical Perspective
What Is New?
We identified race/ethnicity differences in subclinical CVD in late midlife women with thicker carotid walls in black women, wider arterial diameter in Chinese women and less carotid plaque in black and Hispanic women compared with white women.
Cumulative cardiometabolic risk factor burden from early to late midlife, (including higher blood pressure, obesity, and an adverse lipid profile), were associated with worse subclinical CVD in late midlife.
The relationship between midlife CVD risk factor burden and level of subclinical CVD in late midlife was similar across racial/ethnic groups.
What Are the Clinical Implications?
The midlife represents a window of increased cardiovascular risk and an opportunity for CVD prevention and early intervention in women.
CVD prevention strategies should target traditional CVD risk factors in all racial/ethnic groups in early midlife.
Unmeasured socioeconomic, cultural, and nontraditional biological risk factors likely play a role in racial/ethnic differences in vascular health and merit further exploration.
Introduction
Cardiovascular disease (CVD) remains the primary cause of mortality and a significant contributor to morbidity among women of all races/ethnicities in the United States.1 Certain racial/ethnic groups experience a greater burden of disease, with black women experiencing a higher prevalence of CVD morbidity and total and premature CVD mortality.1 The racial/ethnic distribution of CVD risk factors across a woman's life span is not uniform, with specific groups exposed to greater risk factor burden.2 For example, black and Hispanic women have a higher prevalence of hypertension and diabetes mellitus compared with non‐Hispanic white and Asian women.3, 4 Yet differences in traditional CVD risk factors do not fully explain racial/ethnic CVD disparities. Furthermore, the strength of the associations between some CVD risk factors and CVD mortality differ by race and ethnicity.5 In the National Health and Nutrition Examination Survey (NHANES III) population, Framingham risk factors predicted CVD mortality equally well in non‐Hispanic whites and blacks and Mexican Americans; however, older age was more strongly associated with CVD mortality in non‐Hispanic whites and high‐density lipoprotein cholesterol (HDL‐C) in Mexican Americans.5
Markers of subclinical vascular disease can be leveraged to assess the effects of specific exposures on the vasculature before clinical CVD events occur.6 For example, carotid ultrasound derived intima‐media thickness (IMT), interadventitial diameter (IAD), and plaque presence predict CVD events7, 8, 9, 10 and may worsen during the menopause, a time of increasing CVD risk for midlife women.11, 12 Furthermore, there are known differences in the extent of subclinical vascular disease by race/ethnicity,11, 13, 14, 15 although most studies have been limited to comparisons between black versus white women, with little data on other US racial/ethnic groups specifically among midlife women. These studies have shown that compared with white women, black women have greater IMT, particularly in the common carotid artery (CCA).13, 14, 16 More recently the MESA (Multi‐Ethnic Study of Atherosclerosis) reported thicker CCA‐IMT in middle‐to‐older‐age black women relative to white, Hispanic, and Chinese women.17 Wider carotid adventitial diameter, a measure of vascular remodeling, has also been observed among black women,18, 19 although not consistently across studies.20, 21 In contrast, and not consistent with their increased cardiometabolic risk burden, some population‐based studies have reported lower prevalence of carotid plaque in black and Hispanic women as compared with white women.12, 22
While the data suggest racial/ethnic differences in the extent of subclinical vascular disease, results have not been consistent and certain racial/ethnic groups remain understudied. Moreover, observed racial/ethnic differences in these subclinical vascular disease markers have not necessarily aligned with corresponding patterns in CVD risk factor burden, suggesting that specific CVD risk factors may differentially affect the vasculature across racial/ethnic groups.18, 22, 23, 24, 25 Furthermore, most studies have not specifically focused on midlife, typically the period directly before the onset of clinical CVD in women increases, which presents an opportunity for prevention and early intervention. We took advantage of one of the more comprehensive cohort studies of midlife women, the SWAN (Study of Women's Health Across the Nation), to assess (1) racial/ethnic differences in indices of vascular health measured at late midlife in 4 racial/ethnic groups (white, black, Hispanic, and Chinese) and (2) whether the association between early‐to‐late midlife time‐averaged cumulative CVD risk factor burden and indices of vascular health varies by race/ethnicity. We hypothesized that in all women a greater burden of CVD risk factors from early to late midlife would be associated with worse vascular health in late midlife, specifically thicker CCA‐IMT, wider IAD, and greater plaque burden in the carotid artery. We further hypothesized that the association between blood pressure and measures of arterial remodeling (CCA‐IMT and IAD) would be stronger in black women compared with white women.26
Methods
Transparency and Reproducibility
SWAN provides access to public use data sets that extend through the 10th annual follow‐up visit. Some, but not all, of the data used for this article are contained in the public use data sets. Members of the scientific community who are interested in working with the SWAN data that are not contained in the public use data sets may submit an application to become a SWAN Investigator. Links to each of the public use data sets, as well as instructions for how to apply for SWAN Investigator status, are located on the SWAN web site: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use data set or applying for SWAN investigator status may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.
Study Population
SWAN is a multi‐ethnic, community‐based, longitudinal study of the natural history of the menopausal transition. It enrolled 3302 women at 7 field sites throughout the United States (Boston, MA; Chicago, IL; Detroit, MI; Los Angeles, CA; Oakland, CA; Newark, NJ; and Pittsburgh, PA). Each site recruited white women plus 1 other racial‐ethnic group; black women (Pittsburgh, Chicago, Michigan, Boston), Chinese women (Oakland), Japanese women (Los Angeles), and Hispanic women (Newark). The study design has been previously described.27 At the baseline visit, the cohort consisted of nonpregnant women, aged 42 to 52 years, with an intact uterus and at least 1 ovary, who reported having at least 1 menstrual period in the preceding 3 months, and not using hormone therapy in the preceding 3 months. Women were enrolled in 1996 to 1997 and have been followed approximately annually. The institutional review board at each participating site and at the Data Coordinating Center (University of Pittsburgh) approved the study protocol, and all participants provided a written informed consent before enrollment.
The sample for the current analysis consists of participants who had a carotid ultrasound examination during either the 12th or 13th follow‐up clinic visit. The Los Angeles, CA site did not participate in the carotid ultrasound protocol, and therefore white and Japanese women from this site were not included. Of the 1984 women who participated in carotid examination visits (SWAN visits 12 or 13), 1704 attended an in‐person clinic visit (280 women did not because of home/phone interview visits, scheduling problems, and refusals). A carotid examination was completed on 1620 of these women. Carotid data on 14 (0.9%) women were not usable because of poor quality or missing images, resulting in 1606 women with any carotid measures. Women reporting a history of CVD (myocardial infarction, stroke, revascularization, or angina) at the time of the carotid examination, or bilateral oophorectomy or indeterminate menopausal status because of hormone therapy use or hysterectomy, before the final menstrual period were excluded from the current analyses (n=249). Thus, the present analysis includes 1357 SWAN women.
Data Collection
Study participants underwent annual study visits consisting of (1) interviewer‐administered questionnaires to ascertain sociodemographic information, medication use (including antihypertensive agents, lipid‐lowering and diabetes mellitus medications and hormone therapy), current smoking status, and medical history including self‐reported CVD status, (2) physical measures (height, weight, blood pressure), and (3) a morning blood draw following an overnight fast (minimum 10 hours). Menopausal status was based on self‐reported frequency and regularity of menstrual bleeding and classified into the following categories: (1) premenopausal (a menstrual period within past 3 months and no change in regularity), (2) early perimenopausal (at least 1 menstrual period within past 3 months and change in regularity), (3) late perimenopausal (3 consecutive months of amenorrhea), (4) postmenopausal (≥12 months of amenorrhea), (5) surgical menopausal (bilateral oophorectomy with or without hysterectomy), and (6) indeterminate menopausal status (hormone therapy use or hysterectomy before final menstrual period). Because the majority of women were postmenopausal, for analyses, pre‐ and perimenopausal status categories were combined. Race/ethnicity was self‐reported at baseline and categorized as black, non‐Hispanic white, Hispanic, Chinese, and Japanese. Education was assessed at baseline and classified as high school or less, some college/vocational, and college or more.
Systolic blood pressure (SBP) and diastolic blood pressure measurements were taken in the right arm while participants were seated with feet flat on floor following at least 5 minutes of sitting quietly. The average of 2 sequential readings was used in the analyses. Height and weight were measured without shoes. Height was measured by a stadiometer. Weight was measured with light indoor clothing using calibrated scales. Body mass index (BMI; kg/m2) was calculated based on these measures. Hypertension was defined as having a SBP reading ≥130 mm Hg, or a diastolic blood pressure ≥85 mm Hg at the carotid examination visit, or ever reporting use of antihypertensive treatment at any visit. Women were considered to have diabetes mellitus if they reported diabetes mellitus or had fasting glucose levels ≥126 mg/dL or reported any use of insulin/antidiabetic agents at ≥70% of the visits or for ≥3 consecutive visits. Hormone therapy use was assessed using longitudinal data from baseline to the study visit corresponding to the carotid scan and coded as never, past, or current.
Blood was separated, frozen, and sent on dry ice to the Medical Research Laboratory, Lexington, KY, for study visits 0 to 7, and to the University of Michigan for study visits 12 and 13. Because of limited resources, biomarker assays were only available at visits 0 to 7 and visits 12 or 13. All laboratories are Clinical Laboratory Improvement Act certified and accredited by the College of American Pathologists. Visit 0 to 7 plasma lipids were measured using a Hitachi 747‐200 clinical analyzer; cholesterol using an automated cholesterol oxidase assay, triglycerides using an automated glycerol kinase enzymatic assay, and HDL‐C following precipitation of low‐density lipoprotein (LDL) and very low‐density lipoprotein with heparin and manganese chloride by the modified Lipid Research Clinics procedure.28 For visits 0 to 7, serum glucose was measured by automated enzymatic assay on a Hitachi 747‐200 chemistry analyzer using the hexokinase reaction and serum insulin in duplicate by competitive binding radioimmunoassay.28 Coefficient of variation ranged from 0.8% to 8% for the visit 0 to 7 analytes. A Siemens ADVIA 2400 automated chemistry analyzer (Siemens Healthcare Diagnostics, Deerfield, IL) was used to measure visit 12 and 13 lipids and glucose at the University of Michigan Pathology Laboratory, Ann Arbor, MI. EDTA‐treated plasma was used to determine lipid fractions. Total cholesterol and triglycerides were determined using a coupled enzymatic method.29, 30 The ADVIA Direct‐HDL Cholesterol method measured HDL‐C in serum and plasma without prior separation, based on procedures developed by Izawa, Okada, and Matsui.31 Serum glucose was measured using a 2‐step enzymatic reaction that utilizes hexokinase and glucose‐6‐phosphate dehydrogenase enzymes.32 At Visit 12/13, serum insulin was measured at the University of Michigan, CLASS laboratory using the ADVIA Centaur Insulin assay, a 2‐site sandwich immunoassay using direct chemiluminescent technology that uses constant amounts of 2 antibodies.33 Intra‐ and interassay precision expressed as coefficient of variation ranged from 0.2% to 4.7% for the visit 12/13 analytes. For all visits, LDL cholesterol (LDL‐C) was calculated using the Friedewald formula (LDL‐C=(Cholesterol)−(HDL‐C)−(Triglycerides/5); LDL‐C was not calculated when triglycerides were ≥400 mg/dL.34
Time‐averaged cumulative exposure to traditional cardiovascular risk factors
Continuous traditional CVD risk factor variables were selected a priori (SBP, diastolic blood pressure, BMI, waist, LDL‐C, HDL‐C, triglycerides, and fasting insulin and glucose) based on prior literature. A measure of time‐averaged cumulative CVD risk factor burden was calculated by computing the area under the curve of serial values from baseline to carotid visit and dividing by follow‐up years. The area under the curve was computed using the trapezoidal rule by first computing the area of each time interval and summing the results. Because LDL‐C was determined only when triglyceride levels were <400 mg/dL, person‐visit observations with triglyceride levels ≥400 mg/dL were excluded from area under the curve calculations for LDL‐C.
For the time‐averaged cumulative CVD risk factors of interest, data from at least 80% of visits were available for 67% to 79% of participants. Individual person‐time observations that were missing for cumulative risk factor levels averaged over time were not included. With an area under the curve algorithm, this effectively is the same process as imputing each missing value by interpolation based on the observed values before and after the missing measurement. The categorical variables were assessed using longitudinal data from baseline to the study visit corresponding to the carotid scan and coded as (1) never or ever use (lipid lowering, antihypertensive and diabetes mellitus medication use) or (2) never, past, or current (smoking status).
Subclinical vascular measures
At each site, centrally trained and certified sonographers obtained ultrasound images of the left and right carotid arteries using a Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA) equipped with a variable frequency 5 to 12 MHz linear array transducer. Two digitized images for later reading were obtained of the left and right distal CCA at end‐diastole. From each of these 4 images, using a semi‐automated edge detection software,35 near and far wall CCA‐IMT measures were obtained by electronically tracing the lumen‐intima interface and the media‐adventitia interface across a 1‐cm segment proximal to the carotid bulb. CCA IAD was measured directly as the distance from the adventitial‐medial interface on the near wall to the medial‐adventitial interface on the far wall across the same CCA segments used for CCA‐IMT measurement. For both CCA‐IMT and IAD measures, the mean of the average measurements across the 4 images were used in analyses. Images were read centrally at the SWAN Ultrasound Reading Center (University of Pittsburgh Ultrasound Research Lab). Reproducibility was evaluated at each site. Reproducibility of IMT measures was good to excellent with an intraclass correlation coefficient between sonographers of ≥0.77 and between readers of >0.90. Sonographers at each site evaluated the presence of plaque in each of 5 segments of the left and right carotid artery (distal and proximal CCA, carotid bulb, and proximal internal and external carotid arteries) as previously described.36 Consistent with the Mannheim consensus statement,37 plaque was defined as a distinct area protruding into the vessel lumen that was at least 50% thicker than the adjacent IMT. The SWAN Ultrasound Reading Center assessed centrally the quality of all carotid images obtained at each site and using images and clips provided by the sites confirmed the presence and extent of plaque. The presence (yes/no) of any plaque was used for analysis. Identical scanning and reading protocols have been used previously.12, 38
Statistical Analyses
Descriptive statistics in the overall population as well as by race/ethnicity were calculated to summarize study variables at baseline and at the carotid visit. Continuous variables were examined for departure from normality and outliers and transformation was applied to these variables as appropriate. In these analyses, triglycerides, insulin, and glucose were log‐transformed. To initially compare individual risk factors and vascular measures by race/ethnicity, ANOVA was used for continuous variables, and χ2 test for categorical variables.
Multivariable linear regression (CCA‐IMT, IAD) and logistic regression (plaque presence) were used to assess relationships between CVD risk factors and the vascular measures. Spearman correlation coefficients were calculated to identify CVD risk factors that were correlated with each of the continuous subclinical vascular outcomes. Logistic regression was used to identify individual risk factors associated with plaque presence. Variables having a significant association with a P≤0.1 were included in multivariable analyses. Additionally, all primary multivariable models included the following variables: site, race/ethnicity, age at carotid visit, education, smoking status, and use of antihypertensive, lipid‐lowering, and diabetes mellitus medications.
Interactions between race/ethnicity and the 10 a priori selected CVD risk factors (BMI, SBP, HDL‐C, LDL‐C, glucose, insulin, smoking status, and use of diabetes mellitus, antihypertensive, and lipid‐lowering medications) were examined individually to test whether the relations of risk factors found to be relevant to a vascular measure varied by race/ethnicity. These race/ethnicity and CVD risk factor interactions were determined a priori. To account for the large number of tests of interactions with race/ethnicity, a more conservative threshold (P<0.01) was used to assess statistical significance. Non‐Hispanic white (henceforth referred to as white) women were the reference group for all analyses. To further investigate whether predictors of each subclinical vascular measure vary by race/ethnicity, exploratory analyses of racial/ethnic‐specific models were carried out for each vascular outcome with statistically significant race/ethnicity interactions. For these exploratory analyses, to address the small sample size in the Chinese and Hispanic groups, backward elimination was used to derive a parsimonious model. All models included variables found to be significant at P<0.1. For some racial/ethnic‐specific models, levels of categorical covariates were collapsed because of small numbers; past and current smoking status and hormone use were collapsed into ever smoking and hormone use, respectively, for Chinese and Hispanic women. Finally, because different laboratories were used for assaying biomarkers in visits 0 to 7 compared with visits 12 and 13, we ran sensitivity analyses excluding visits 12 and 13 data from the estimates of time‐averaged cumulative exposure. For these sensitivity analyses, only baseline to visit 7 data were used for time‐averaged cumulative exposure variables and covariates. All data analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
Study Population at Carotid Examination Visit
SWAN women included in these analyses attended their carotid examination visit, on average, 13.7 years after their baseline visit (range: 12.3–15.4 years). The majority were postmenopausal with an average age of 60 (range: 54–67) years. Nearly half reported at least a college degree, and the majority were never smokers at the time of the carotid examination visit (Table 1). White, black, Chinese, and Hispanic women represented 51%, 29%, 13%, and 7% of the study sample, respectively.
Table 1.
Characteristics, CVD Risk Factors, and Subclinical Vascular Measures of SWAN Study Population at Carotid Exam Visit by Race/Ethnicity
| Overall (N=1357) | White (N=697) | Black (N=393) | Chinese (N=179) | Hispanic (N=88) | P Valuea | |
|---|---|---|---|---|---|---|
| Age, y | 59.7 (2.7) | 59.7 (2.7) | 59.4 (2.6) | 60.1 (2.6) | 59.9 (2.9) | 0.0105 |
| Education, n (%) | ||||||
| < HS | 303 (22.6) | 99 (14.3) | 95 (24.7) | 52 (29.1) | 57 (67.1) | <0.0001 |
| Some college | 408 (30.4) | 199 (28.7) | 157 (40.8) | 33 (18.4) | 19 (22.4) | |
| ≥ College | 631 (47.0) | 395 (57.0) | 133 (34.5) | 94 (52.5) | 9 (10.6) | |
| Smoking status, n (%) | ||||||
| Never smoker | 822 (60.6) | 366 (52.5) | 232 (59.0) | 167 (93.3) | 57 (64.8) | <0.0001 |
| Past smoker | 408 (30.1) | 275 (39.5) | 103 (26.2) | 10 (5.6) | 20 (22.7) | |
| Current smoker | 127 (9.4) | 56 (8.0) | 58 (14.8) | 2 (1.1) | 11 (12.5) | |
| Menopausal status, n (%) | ||||||
| Pre/perimenopause | 38 (2.89) | 19 (2.7) | 12 (3.0) | 3 (1.7) | 4 (4.5) | 0.6051 |
| Postmenopause | 1319 (97.2) | 678 (97.3) | 381 (97.0) | 176 (98.3) | 84 (95.5) | |
| Hypertension, n (%) | 726 (54.1) | 323 (46.9) | 285 (72.9) | 55 (30.7) | 63 (75.0) | <0.0001 |
| Diabetes mellitus, n (%) | 180 (13.3) | 73 (10.5) | 69 (17.6) | 16 (8.9) | 22 (25.0) | <0.0001 |
| Medication ever use, n (%) | ||||||
| Antihypertensive | 648 (47.8) | 278 (39.9) | 257 (65.4) | 57 (31.8) | 56 (63.6) | <0.0001 |
| Lipid lowering | 436 (32.2) | 210 (30.1) | 143 (36.4) | 48 (26.8) | 37 (40.7) | 0.0314 |
| Diabetes mellitus | 169 (12.5) | 70 (10.0) | 64 (16.3) | 15 (8.4) | 20 (22.7) | <0.0001 |
| Hormone use, n (%) | ||||||
| Never | 823 (60.7) | 366 (52.5) | 268 (68.2) | 123 (68.7) | 66 (75.0) | <0.0001 |
| Past | 456(33.6) | 274 (39.3) | 110 (28.0) | 52 (29.1) | 20 (22.7) | |
| Current | 78 (5.8) | 57 (8.2) | 15 (3.8) | 4 (2.2) | 2 (2.3) | |
| Subclinical vascular measures | ||||||
| CCA IMT, mm | 0.79 (0.12) | 0.78 (0.11) | 0.84 (0.13) | 0.76 (0.12) | 0.80 (0.11) | <0.0001 |
| CCA AD, mm | 7.19 (0.66) | 7.10 (0.62) | 7.39 (0.71) | 7.23 (0.60) | 7.03 (0.64) | <0.0001 |
| Any plaque, n (%) | 599 (44.2) | 329 (47.2) | 156 (39.9) | 88 (49.2) | 26 (29.6) | 0.0019 |
Data are presented as mean (SD) for continuous variables and frequency (percentages) for categorical variables. AD indicates interadventitial diameter; CCA, common carotid artery; CVD, cardiovascular disease; HS, high school; IMT, intima‐media thickness; SWAN, Study of Women's Health Across the Nation.
P value is for the comparison across racial/ethnic groups; χ2 or Fisher exact tests were used for the categorical variables, and ANOVA was used for the continuous variables.
CVD Risk Factors and Subclinical Vascular Measures by Race/Ethnicity
Age varied modestly by race/ethnicity, with Chinese women being older on average than the other groups. Hispanic women were less educated, and black and Hispanic women were more likely to be current smokers (Table 1). As compared with white women (the reference group for all race/ethnicity comparisons in subsequent analyses), Hispanic and black women were more likely to report use of medications for cardiometabolic conditions. Time‐averaged cumulative CVD risk factors selected a priori differed across racial/ethnic groups with the exception of LDL‐C (Figure 1; Table S1). In general, CVD risk factors appeared to be less favorable in black and Hispanic women and more favorable in Chinese women including measures of obesity, blood pressure, HDL‐C, and fasting insulin. All subclinical vascular measures varied by race/ethnicity (Table 1). In unadjusted analyses, compared with white women, black women had significantly thicker CCA‐IMT and both black and Chinese women had significantly wider IAD (P<0.05 for all comparisons). Black and Hispanic women had significantly lower plaque prevalence than white women (P<0.05 for both comparisons).
Figure 1.
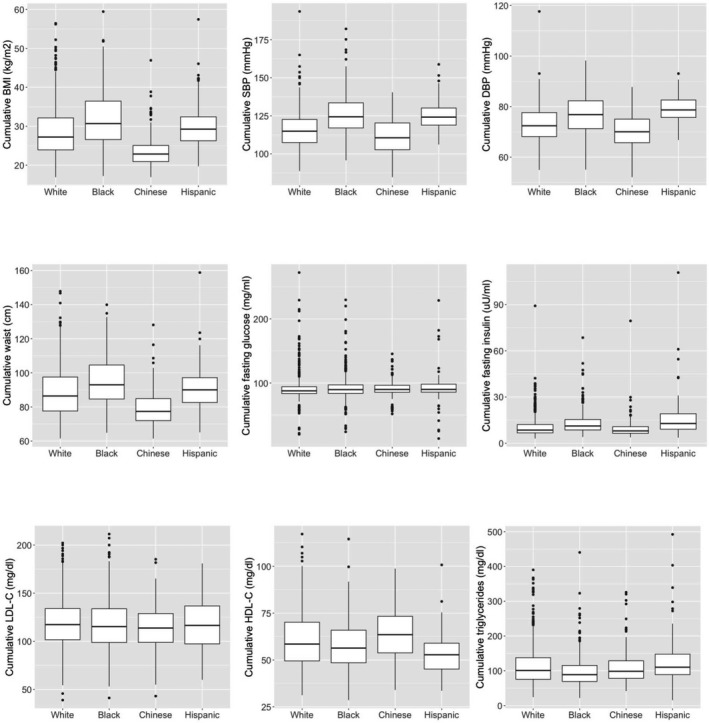
Boxplots of time‐averaged cumulative CVD risk factors by race/ethnicity in late midlife women (n=1357); with exception of LDL‐C, all risk factors varied by race/ethnicity, overall P<0.05. BMI indicates body mass index; CVD,cardiovascular disease; DBP, diastolic blood pressure; LDL‐C, low density lipoprotein cholesterol; SBP, systolic blood pressure.
Time‐Averaged Cumulative CVD Risk Factor Exposure and Race/Ethnicity as Predictors of Subclinical Vascular Measures
In multivariable models, race/ethnicity was a significant predictor of CCA‐IMT, IAD, and plaque presence (Table 2).
Table 2.
Multivariable Regression Models of the Relationship Between Race/Ethnicity and CVD Risk Factors With Subclinical Vascular Diseasea
| Parameter | CCA‐IMT, mm (n=1307) | AD, mm (n=1309) | Plaque Presence (n=1336) | |||
|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | OR (95% CI) | P Value | |
| Race/ethnicity | <0.001 | <0.001 | <0.001 | |||
| White (reference) | ··· | ··· | ··· | ··· | ··· | ··· |
| Black | 0.0317 (0.008) | <0.001 | 0.0632 (0.0438) | 0.149 | 0.52 (0.38, 0.71) | <0.001 |
| Chinese | 0.0168 (0.013) | 0.198 | 0.318 (0.0709) | <0.001 | 1.07 (0.65, 1.76) | 0.790 |
| Hispanic | −0.0114 (0.0207) | 0.581 | −0.1834 (0.1131) | 0.105 | 0.18 (0.08, 0.40) | <0.001 |
| Age | 0.0045 (0.0012) | <0.001 | 0.0107 (0.0064) | 0.093 | 1.06 (1.01, 1.11) | 0.010 |
| Education | 0.153 | 0.359 | 0.018 | |||
| < HS (reference) | ··· | ··· | ··· | ··· | ||
| Some college | 0.0012 (0.0086) | 0.892 | −0.0177 (0.0469) | 0.705 | 1.03 (0.74, 1.44) | 0.841 |
| ≥ College | −0.0117 (0.0083) | 0.162 | −0.0593 (0.0455) | 0.193 | 0.72 (0.52, 0.99) | 0.041 |
| Smoking status | 0.489 | 0.038 | 0.017 | |||
| Never smoker (reference) | ··· | ··· | ··· | ··· | ||
| Past smoker | 0.0058 (0.0069) | 0.399 | 0.0672 (0.0376) | 0.074 | 0.92 (0.71, 1.21) | 0.578 |
| Current smoker | −0.007 (0.0109) | 0.522 | 0.1304 (0.0597) | 0.029 | 1.75 (1.15, 2.66) | 0.009 |
| Cumulative BMIb | 0.0015 (0.0006) | 0.011 | 0.0213 (0.0033) | <0.001 | 1.02 (0.99, 1.04) | 0.192 |
| Cumulative SBPb | 0.0022 (0.0003) | <0.001 | 0.0162 (0.0016) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| Cumulative LDL‐Cb | 0.0003 (0.0001) | 0.013 | −0.001 (0.0007) | 0.125 | 1.01 (1.00, 1.01) | 0.007 |
| Cumulative HDL‐Cb | −0.0003 (0.0002) | 0.161 | −0.0029 (0.0013) | 0.029 | 1.00 (0.99, 1.01) | 0.656 |
| Cumulative fasting glucoseb, c | 0.0408 (0.017) | 0.017 | 0.1782 (0.0929) | 0.055 | 1.66 (0.84, 3.29) | 0.142 |
| Cumulative fasting insulinb, c | −0.0062 (0.0096) | 0.518 | −0.0741 (0.0521) | 0.155 | 0.91 (0.63, 1.31) | 0.593 |
| Diabetes mellitus medicationb, d | 0.0261 (0.0114) | 0.022 | 0.0584 (0.0621) | 0.347 | 1.31 (0.85, 2.03) | 0.219 |
| Antihypertensive medicationb, d | 0.001 (0.0074) | 0.893 | −0.0039 (0.0405) | 0.922 | 1.30 (0.98, 1.74) | 0.071 |
| Lipid‐lowering medicationb, d | −0.0015 (0.0074) | 0.842 | −0.0561 (0.0405) | 0.166 | 0.94 (0.70, 1.24) | 0.645 |
| Hormone use | 0.553 | 0.046 | 0.274 | |||
| Never (reference) | ··· | ··· | ··· | ··· | ··· | ··· |
| Past | 0.0047 (0.0066) | 0.469 | 0.0888 (0.0357) | 0.013 | 0.88 (0.69, 1.14) | 0.329 |
| Current | 0.0124 (0.0131) | 0.344 | 0.0339 (0.0715) | 0.636 | 0.68 (0.41, 1.14) | 0.146 |
| Menopausal status | ||||||
| Pre/peri (reference) | ··· | ··· | ··· | ··· | ··· | ··· |
| Post | −0.0048 (0.0183) | 0.795 | 0.06 (0.1001) | 0.549 | 1.35 (0.65, 2.80) | 0.425 |
AD indicates interadventitial diameter; BMI, body mass index; CCA, common carotid artery; CVD, cardiovascular disease; HS, high school; IMT, intima media thickness; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure.
Linear regression was used for CCA‐IMT and AD and logistic regression for plaque presence. In addition to all the variables listed in the table, which were considered together in the model, these models also adjusted for site.
“Cumulative” refers to continuous traditional CVD risk factor variables proposed a priori as a measure of time‐averaged cumulative CVD risk burden and calculated by computing the area under the curve of serial values from baseline to carotid visit.
Log‐transformed.
Ever users compared with never users.
Common carotid artery‐intima media thickness
Predictors
Black race, age, time‐averaged cumulative BMI, SBP, LDL‐C, and fasting glucose and ever use of diabetes mellitus medications were significantly associated with thicker CCA‐IMT in multivariable analyses (Table 2).
Effect modification by race/ethnicity
Race/ethnicity modified the association between time‐averaged cumulative HDL‐C and CCA‐IMT (P=0.008). Specifically, the inverse association between time‐averaged cumulative HDL‐C and CCA‐IMT was observed in Chinese and Hispanic women but not in white or black women. The estimated associations between HDL‐C and CCA‐IMT by race/ethnicity are presented in Figure 2 using the mean value for the other continuous covariates in the model. No other race/ethnicity moderation was noted.
Figure 2.
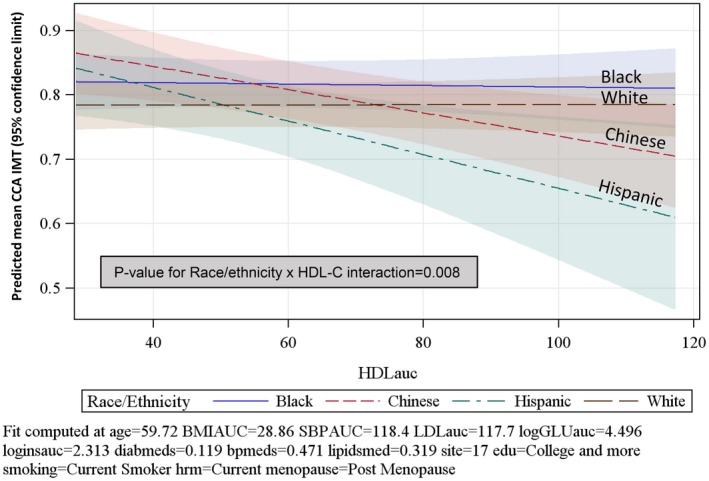
Predicted mean and 95% confidence limits for CCA‐IMT using mean values of covariates by race/ethnicity (n=1307). AUC indicates area under the curve; BMI, body mass index; CCA‐IMT, common carotid artery– intima media thickness; GLU, glucose; HDL‐C HDL cholesterol; hrm, hormone use.
Interadventitial diameter
Predictors
In the overall population, Chinese ethnicity, current smoking, past hormone use, and time‐averaged cumulative BMI, SBP, and lower HDL‐C were significantly associated with wider IAD (Table 2).
Effect modification by race/ethnicity
No statistically significant race/ethnicity and risk factor interactions were observed for models predicting IAD.
Plaque
Predictors
As compared with white women, black and Hispanic women were less likely to have plaque. Significant predictors of plaque presence were age, lower educational attainment, current smoking, and time‐averaged cumulative SBP and LDL‐C (Table 2).
Effect modification by race/ethnicity
The only significant race/ethnicity interaction observed was with ever use of diabetes mellitus medications (P=0.004, Figure 3). The odds of plaque were statistically significantly higher in women reporting ever use of diabetes mellitus medication compared with never users but only in white (odds ratio=2.20, 95% CI=1.16–4.16) and Hispanic women (odds ratio=3.26, 95% CI=1.07–9.96; Figure 3). These data should be interpreted with caution since only 15 Chinese and 20 Hispanic women in these analyses reported ever using diabetes mellitus medication.
Figure 3.
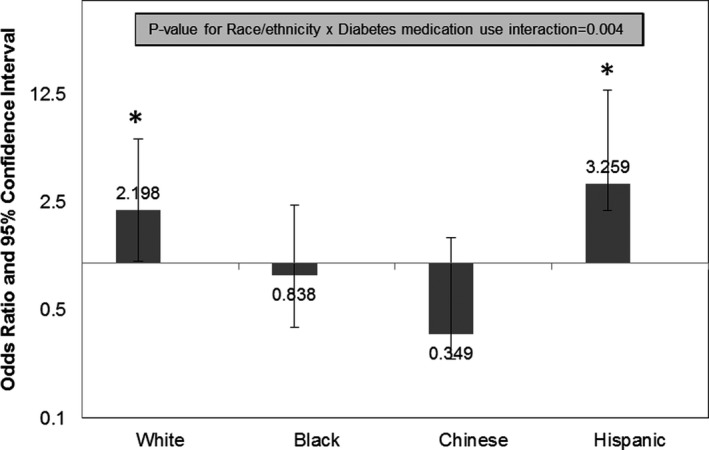
Odds ratio and 95% CI for plaque presence in ever users of diabetes mellitus medication compared with never users by race/ethnicity (n=1336), *P<0.05.
Exploratory Racial/Ethnic‐Specific Models of Time‐Averaged Cumulative CVD Risk Factors as Predictors of Subclinical Vascular Measures
Common carotid artery‐intima media thickness
In all racial/ethnic groups, age and either higher time‐averaged cumulative SBP or ever use of antihypertensive medications predicted thicker CCA‐IMT (Table 3). In addition, time‐averaged cumulative BMI in white and black women, and ever use of diabetes mellitus medication in black women predicted thicker IMT. Consistent with the race/ethnicity and HDL‐C interaction detected in the overall multivariable model, lower HDL‐C predicted thicker CCA‐IMT in Chinese women and Hispanic women.
Table 3.
Race/Ethnicity–Specific Multivariable Linear Regression Models of CVD Risk Factors and CCA‐IMT
| Parameter | White (n=691)a | Black (n=370)a | Chinese (n=178) | Hispanic (n=85) | ||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |
| Age | 0.0039 (0.0014) | 0.006 | 0.0048 (0.0025) | 0.057 | 0.007 (0.0032) | 0.038 | 0.0084 (0.0038) | 0.03 |
| Cumulative BMIb | 0.0016 (0.0007) | 0.020 | 0.0016 (0.0009) | 0.086 | ||||
| Cumulative SBPb | 0.0022 (0.0004) | <0.0001 | 0.0019 (0.0005) | <0.001 | 0.0038 (0.0012) | 0.002 | ||
| Cumulative LDL‐Cb | 0.0003 (0.0002) | 0.047 | ||||||
| Cumulative HDL‐Cb | −0.0023 (0.0006) | <0.001 | −0.0029 (0.001) | 0.005 | ||||
| Cumulative fasting glucosec | 0.041 (0.0193) | 0.034 | ||||||
| Diabetes mellitus medicationd | 0.0619 (0.0183) | <0.001 | ||||||
| Antihypertensive medicationd | 0.051 (0.018) | 0.005 | ||||||
BMI indicates body mass index; CCA, common carotid artery; CVD, cardiovascular disease; HDL‐C, high density lipoprotein cholesterol; IMT, intima‐media thickness; LDL‐C, low density lipoprotein cholesterol; SBP, systolic blood pressure.
In addition to all the variables listed in the table, the model for white and black women also includes site.
“Cumulative” refers to continuous traditional CVD risk factor variables proposed a priori as a measure of time‐averaged cumulative CVD risk burden and calculated by computing the area under the curve of serial values from baseline to carotid visit.
Log‐transformed.
Ever users compared with never users.
Plaque
Time‐averaged cumulative SBP predicted greater plaque prevalence in all groups except Hispanic women (Table 4). Higher time‐averaged cumulative fasting glucose in white women and current smoking in white and black women were associated with greater plaque presence. Although only marginally statistically significant, use of diabetes mellitus medication was associated with greater plaque prevalence in Hispanic women, consistent with the race/ethnicity interaction in the overall model.
Table 4.
Race/Ethnicity‐Specific Multivariable Logistic Regression Models of CVD Risk Factors and Carotid Plaque Presence
| Risk Factor | OR (95% CI) | |||
|---|---|---|---|---|
| White (n=696)a | Black (n=391)a | Chinese (n=179) | Hispanic (n=88) | |
| Age | 1.20 (1.01, 1.42) | |||
| Smoking status | ||||
| Never smoker (reference) | ··· | ··· | ||
| Past smoker | 1.01 (0.72, 1.40) | 1.01 (0.62, 1.66) | ||
| Current smoker | 2.61 (1.41, 4.86) | 1.85 (1.00, 3.43) | ||
| Cumulative SBPb | 1.03 (1.01, 1.05) | 1.03 (1.01, 1.04) | 1.03 (1.01, 1.06) | |
| Cumulative LDL‐Cb | 1.01 (1.00, 1.02) | |||
| Cumulative fasting glucoseb, c | 3.46 (1.40, 8.57) | |||
| Diabetes mellitus medicationd | 2.69 (0.92, 7.92) | |||
| Antihypertensive medicationd | 1.53 (1.06, 2.20) | |||
CVD indicates cardiovascular disease; LDL‐C, low density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure.
In addition to all the variables listed in the table, the model for white and black women also includes site.
“Cumulative” refers to continuous traditional CVD risk factor variables proposed a priori as a measure of time‐averaged cumulative CVD risk burden and calculated by computing the area under the curve of serial values from baseline to carotid visit.
Log‐transformed,
Ever users compared with never users.
Sensitivity Analyses
In sensitivity analyses, excluding visit 12/13 CVD risk factor data and limiting determination of risk factors to data from baseline to visit 7, predictors of vascular measures in the overall population were largely similar to those observed in the main analyses with some attenuation of the association estimates (Table S2).
Discussion
This is one of the first studies to examine racial/ethnic differences in the association between time‐averaged cumulative midlife CVD risk factor levels and various indices of vascular health in a large population‐based multiethnic cohort of women in late midlife, a period of increasing CVD risk.39 Consistent with prior studies, we found generally a worse risk factor profile in black and Hispanic women and more favorable profile in Chinese and white women. However, the relative burden of subclinical vascular disease across racial/ethnic groups did not consistently align with their CVD risk factor profiles. Compared with white women, measures of carotid arterial remodeling (CCA‐IMT and IAD) were worse in black women, similar in Hispanic women, and worse or similar in Chinese women. However, carotid plaque was less prevalent in black and Hispanic women and of similar prevalence in Chinese women. Despite the racial/ethnic inconsistency between subclinical vascular disease burden and CVD risk factors assessed across the menopause transition, there was no strong evidence that the associations between traditional CVD risk factors and individual subclinical vascular measures differed by race/ethnicity. One novel and notable exception was the inverse association between time‐averaged cumulative HDL‐C and CCA‐IMT, which was only apparent in Chinese and Hispanic women. Other pathways beyond traditional CVD risk factors, involving unmeasured socioeconomic, sociocultural, and nontraditional biological risk factors likely play a role and merit further exploration.
It is well documented that black and Hispanic women have higher rates of major risk factors for CVD compared with white women, including in our population of late‐midlife women.1, 2, 3 Our findings of thicker CCA‐IMT and wider IAD in black women are consistent with their worse cardiometabolic risk profile, including higher rates of hypertension, and are similar to other studies,16, 40, 41 including more recent data from MESA, the largest multiethnic population‐based study of subclinical CVD in the United States.17, 41 In contrast we found no difference in measures of vascular remodeling between Hispanic and white women, also consistent with MESA findings of similar CCA‐IMT (0.86 and 0.87 mm, in Hispanic and white women, respectively).17 However, MESA did report wider IAD in Hispanic women as compared with white women, which may be because of their inclusion of a wider age range (45–84 years) with older women past the midlife stage.
Despite their higher CVD risk factor burden, in black and Hispanic women we found a lower prevalence of carotid plaque, focal thickening more likely to reflect atherosclerotic lesions. The literature on racial/ethnic differences in carotid plaque is less consistent and more sparse. Most studies have reported no difference16 or less carotid plaque in black populations42 and less carotid plaques in Hispanic populations22, 42 as compared with their white counterparts. However, these studies have examined younger populations16 or have not focused on midlife women.22, 42 In a younger and smaller subgroup of SWAN, there was no difference in carotid plaque presence between black and white women, but women were on average 10 years younger than the present cohort when burden of subclinical atherosclerosis is considerably less.16 Racial differences in arterial geometry and atherogenesis have been implicated as a potential explanation for lower prevalence of plaque in black individuals.43 A narrower internal carotid artery and a wider external carotid artery have been reported in greater proportions in blacks as compared with whites or Caribbean Hispanics.43 Moreover, a higher prevalence of intracranial versus extracranial atherosclerosis has been reported in individuals of African, Hispanic, and Asian ancestries as compared with whites44 and implicated as the more prevalent cause of ischemic stroke in blacks and Hispanics compared with whites.45
In line with our findings, in the NOMAS (Northern Manhattan Study), one of the earliest studies examining subclinical CVD in US Hispanics, internal carotid artery plaque thickness and prevalence was significantly lower in older Hispanic women compared with white women.22, 46 The lower prevalence of carotid plaque, and no difference in vascular remodeling, in Hispanic women compared with their white counterparts in our study of late midlife is consistent with the “Hispanic paradox.” This phenomenon refers to Hispanic and Latino Americans tending to have certain health outcomes, including CVD and overall mortality, comparable to or better than those of US whites, despite generally worse risk factors including lower socioeconomic status (SES) and less healthcare access.47, 48 A recent paper examining CVD mortality across Hispanic subgroups suggests that current classification of Hispanics into 1 group may mask heterogeneity in CVD47 and is consistent with SWAN findings of significant heterogeneity in CVD risk factors across Hispanic subgroups.49 We were unable to explore similar heterogeneity in measures of vascular aging, given the smaller numbers in the Hispanic ethnic group. Future studies with greater number of individuals across Hispanic subgroups are needed to address this question.
Subclinical vascular data are even sparser in Chinese midlife women living in the United States. Chinese women in SWAN had a generally healthier CVD risk profile, namely, fewer smokers, hypertensives, lower BMI/waist, and higher HDL‐C, yet they had a wider arterial diameter and did not differ in burden of subclinical atherosclerosis as compared with white women. One potential explanation for these findings is substantiated by studies reporting a higher risk of diabetes mellitus and CVD risk factors at lower levels of BMI in Asian ancestry populations.50 Chinese women in our study were also older than the other groups, which may partially account for our findings. Consistent with our findings, Chinese women in MESA had significantly wider IAD as compared with white women (adjusted β: MESA 0.36 mm and SWAN 0.32 mm).41 Less consistent were MESA findings that Chinese women had thinner CCA‐IMT51 and were less likely to develop new carotid plaques at follow‐up, as compared with their white counterparts.42 Nonetheless, our finding of wider carotid arterial diameter in late midlife Chinese women is notable and merits exploration given the predictive value of IAD for future events8 and positive associations with left ventricular mass.41
Greater early to late midlife burden of CVD risk factors, including higher blood pressure and BMI and an adverse lipid profile, were associated with greater subclinical vascular disease in late midlife women. Our findings build on prior studies in younger52 and older populations25 and in midlife women around the menopause transition.53, 54 Contrary to our hypotheses, based on our assessment of several indices of carotid vascular health, the association between time‐averaged cumulative traditional CVD risk factor levels and the vasculature of late midlife women did not appear to vary by race/ethnicity. One novel departure from this pattern was the inverse association between HDL‐C and CCA‐IMT, which was only apparent in Chinese and Hispanic women. These findings are intriguing in light of recent research on the conflicting protective effects of HDL‐C on the vasculature, in particular in women and during the menopause transition.55 Largely consistent with our findings, in the recent USE‐IMT individual participant meta‐analysis of >60 000 individuals, the direction of associations between traditional CVD risk factors and CCA‐IMT were similar across racial/ethnic groups, although the magnitudes of the associations in some cases varied.25
There are several potential explanations for our findings. Measures of vascular health, such as CCA‐IMT, in certain groups (eg, blacks) may represent a different phenotype, less reflective of atherosclerosis and more of blood pressure–mediated arterial remodeling.26 This is in line with our findings of thicker CCA‐IMT but lower carotid plaque prevalence in black women and with MESA findings of weak association between carotid artery IMT and coronary artery calcification in blacks versus other racial/ethnic groups.17 Surprisingly, we did not find evidence for a stronger association between blood pressure and CCA‐IMT in black women, although a much greater proportion of black women were on antihypertensive medication at the time of the vascular assessment.
Our findings may also point to the importance of unmeasured and nontraditional CVD risk factors. In the multi‐ethnic NOMAS study, traditional CVD risk factors explained only 11% of the variance in IMT.56 Similarly, most traditional CVD risk scores have not consistently performed well in predicting events in different racial/ethnic groups,5, 57 although the recent ASCVD pooled risk score shows evidence of improved prediction for subclinical CVD in black populations.58 Furthermore, adjusting for behavioral factors that differ by racial/ethnic groups, and thus, are potential pathway factors, such as diet, physical activity, and smoking, have not explained away a significant proportion of the differential CVD risk across racial/ethnic groups.59, 60, 61 In our analyses, adjusting for education and smoking status did not explain the racial/ethnic differences in subclinical vascular disease burden in late midlife women.
Other CVD risk factors not considered in our study, such as markers of inflammation and coagulation, which vary by race/ethnicity,62 may exert stronger vascular effects in certain racial/ethnic groups of women.63, 64 Future studies examining the vascular effect of more specific markers of inflammation and coagulation in population‐based studies with a sufficient number of midlife women from multiple racial/ethnic groups may be warranted. Finally, genetic factors may explain some of the racial/ethnic differences in vascular outcomes and are of great interest in precision cardiovascular medicine.65 For example, recent evidence from a whole genome sequencing study suggests that certain variants of the LPA gene may confer greater relative risk for subclinical atherosclerosis in blacks as compared with the directly measured biomarker Lp(a).66 However, current evidence of the genetic contribution to CVD racial disparities at the population level remains controversial.67
It is well established that low SES and multilevel social determinants are associated with increased CVD risk68 and subclinical vascular measures,36, 69 although not consistently in the same direction across racial/ethnic groups.40, 70 In the Healthy Aging in Neighborhoods of Diversity Across the Life Span study, thicker CCA‐IMT in blacks versus whites appeared to be particularly evident in the high SES group.70 These findings support the diminishing returns hypothesis that the benefits of higher SES may not be experienced equally across racial/ethnic groups and in particular among black populations. However, in late midlife women, we did not observe a race and education interaction for any of the subclinical vascular disease measures.
Our findings should be interpreted with several limitations in mind. The Chinese and Hispanic race/ethnicity groups had small numbers, which potentially limited the power to detect important group differences and risk factor associations. We did not measure IMT of the full carotid segment, which may mask or attenuate certain risk factor associations given that IMT in the common carotid segment may represent more adaptive diffuse wall thickening of the arterial medial layer as opposed to focal atherosclerotic lesions more prone to develop in arterial segments with increased turbulent flow.71 However, we did ascertain the presence of atherosclerotic plaque in all visible carotid arterial segments. Because carotid measures were not obtained on the overall SWAN cohort at an earlier time point, we only have assessment of carotid atherosclerosis in late midlife and thus cannot rule out existing subclinical CVD at the baseline visit. Inherent in observational study designs, causality cannot be inferred for relationships examined. We do not have measures of most CVD risk factors before midlife and cannot exclude the possibility that CVD risk factors accumulating since childhood and young adulthood may be as important in predicting subclinical vascular burden in late midlife.72 Finally, race/ethnicity in the United States is confounded by SES, and the construct of ethnicity includes individuals of any race.73 Nonetheless, despite their limitations, the constructs of race and ethnicity remain important for exploring and addressing inequities in health and health care.
Our study has significant strengths that bolster the findings and add to the existing literature. Our current work extends previous findings of racial/ethnic differences in subclinical vascular disease burden to women in the late midlife period, a significant period of increasing CVD risk for women, because of CVD risk factor changes attributable to both chronological and ovarian aging.28, 54, 74 Few studies in midlife women have focused on racial/ethnic groups other than white and black women. The SWAN cohort is a large, well‐characterized multiethnic cohort prospectively followed with extensive biological, behavioral, and SES data collected through the menopause transition. Finally, we used several well‐established indices of subclinical vascular disease that have been linked to future CHD, myocardial infarction, and stroke events, making them ideal for studying women in late midlife, a time when significant events have not accrued yet CVD risk is increasing.
In conclusion, in late midlife women carotid arterial measures of vascular remodeling and atherosclerosis vary by race/ethnicity, but these differences do not consistently align with racial/ethnic differences in traditional CVD risk factors assessed from early to late midlife. Furthermore, we provide evidence that, in general, the association between traditional CVD risk factors and vascular health does not appear to vary by race/ethnicity. Studies with larger cohorts, including greater numbers of heterogeneous racial/ethnic groups, and multilevel assessments of sociocultural, socioeconomic, and novel biological and genetic factors are needed to shed light on the mechanisms explaining racial/ethnic disparities in subclinical and clinical CVD.
Sources of Funding
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI grant number UL1 RR024131. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Disclosures
None.
Supporting information
Table S1. CVD Risk Factors at Carotid Exam Visit by Race/Ethnicity
Table S2. Multivariable Regression Models of the Relationship Between Race, CVD Risk Factors, and Subclinical Vascular Measures (Sensitivity Analyses)*
Acknowledgments
We thank the study staff at each site, the Ultrasound Research Laboratory, and all the women who participated in SWAN; Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016‐ present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers. Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). SWAN Repository: University of Michigan, Ann Arbor—Siobán Harlow 2013–Present; Dan McConnell 2011–2013; MaryFran Sowers 2000–2011. Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton‐Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
(J Am Heart Assoc. 2020;9:e013876 DOI: 10.1161/JAHA.119.013876.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Matthews KA, Sowers MF, Derby CA, Stein E, Miracle‐McMahill H, Crawford SL, Pasternak RC. Ethnic differences in cardiovascular risk factor burden among middle‐aged women: Study of Women's Health Across the Nation (SWAN). Am Heart J. 2005;149:1066–1073. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Sutton‐Tyrrell K, Patel AS, Matthews KA, Pasternak RC, Everson‐Rose SA, Scuteri A, Chae CU. Ethnic variation in hypertension among premenopausal and perimenopausal women: Study of Women's Health Across the Nation. Hypertension. 2005;46:689–695. [DOI] [PubMed] [Google Scholar]
- 4. Golden SH, Brown A, Cauley JA, Chin MH, Gary‐Webb TL, Kim C, Sosa JA, Sumner AE, Anton B. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97:E1579–E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurley LP, Dickinson LM, Estacio RO, Steiner JF, Havranek EP. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes. 2010;3:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuller L, Borhani N, Furberg C, Gardin J, Manolio T, O'Leary D, Psaty B, Robbins J. Prevalence of subclinical atherosclerosis and cardiovascular disease and association with risk factors in the Cardiovascular Health Study. Am J Epidemiol. 1994;139:1164–1179. [DOI] [PubMed] [Google Scholar]
- 7. Polak JF, Sacco RL, Post WS, Vaidya D, Arnan MK, O'Leary DH. Incident stroke is associated with common carotid artery diameter and not common carotid artery intima‐media thickness. Stroke. 2014;45:1442–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sedaghat S, van Sloten TT, Laurent S, London GM, Pannier B, Kavousi M, Mattace‐Raso F, Franco OH, Boutouyrie P, Ikram MA, Stehouwer CDA. Common carotid artery diameter and risk of cardiovascular events and mortality: pooled analyses of four cohort studies. Hypertension. 2018;72:85–92. [DOI] [PubMed] [Google Scholar]
- 9. Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit AJ, Giral P, Kurl S, Rauramaa R, Mannarino E, Grossi E, Paoletti R, Tremoli E. Measurements of carotid intima‐media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT‐Progression as Predictors of Vascular Events in a High Risk European Population) study. J Am Coll Cardiol. 2012;60:1489–1499. [DOI] [PubMed] [Google Scholar]
- 10. Naqvi TZ, Lee MS. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. [DOI] [PubMed] [Google Scholar]
- 11. Sutton‐Tyrrell K, Lassila HC, Meilahn E, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29:1116–1121. [DOI] [PubMed] [Google Scholar]
- 12. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton‐Tyrrell K. Progression rates of carotid intima‐media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Agostino RB Jr, Burke G, O'Leary D, Rewers M, Selby J, Savage PJ, Saad MF, Bergman RN, Howard G, Wagenknecht L, Haffner SM. Ethnic differences in carotid wall thickness. The Insulin Resistance Atherosclerosis Study. Stroke. 1996;27:1744–1749. [DOI] [PubMed] [Google Scholar]
- 14. Manolio TA, Burke GL, Psaty BM, Newman AB, Haan M, Powe N, Tracy RP, O'Leary DH. Black‐white differences in subclinical cardiovascular disease among older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. J Clin Epidemiol. 1995;48:1141–1152. [DOI] [PubMed] [Google Scholar]
- 15. Kalra L, Rambaran C, Chowienczyk P, Goss D, Hambleton I, Ritter J, Shah A, Wilks R, Forrester T. Ethnic differences in arterial responses and inflammatory markers in Afro‐Caribbean and Caucasian subjects. Arterioscler Thromb Vasc Biol. 2005;25:2362–2367. [DOI] [PubMed] [Google Scholar]
- 16. Everson‐Rose SA, Lewis TT, Karavolos K, Matthews KA, Sutton‐Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: the Study of Women's Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006;152:982.e7–13. [DOI] [PubMed] [Google Scholar]
- 17. Manolio TA, Arnold AM, Post W, Bertoni AG, Schreiner PJ, Sacco RL, Saad MF, Detrano RL, Szklo M. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: the Multi‐Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;197:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruan L, Chen W, Srinivasan SR, Sun M, Wang H, Toprak A, Berenson GS. Correlates of common carotid artery lumen diameter in black and white younger adults: the Bogalusa Heart Study. Stroke. 2009;40:702–707. [DOI] [PubMed] [Google Scholar]
- 19. Eigenbrodt ML, Bursac Z, Rose KM, Couper DJ, Tracy RE, Evans GW, Brancati FL, Mehta JL. Common carotid arterial interadventitial distance (diameter) as an indicator of the damaging effects of age and atherosclerosis, a cross‐sectional study of the Atherosclerosis Risk in Community Cohort Limited Access Data (ARICLAD), 1987–89. Cardiovasc Ultrasound. 2006;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markert MS, Della‐Morte D, Cabral D, Roberts EL Jr, Gardener H, Dong C, Wright CB, Elkind MS, Sacco RL, Rundek T. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis. 2011;219:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lloyd KD, Barinas‐Mitchell E, Kuller LH, Mackey RH, Wong EA, Sutton‐Tyrrell K. Common carotid artery diameter and cardiovascular risk factors in overweight or obese postmenopausal women. Int J Vasc Med. 2012;2012:169323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sacco RL, Roberts JK, Boden‐Albala B, Gu Q, Lin IF, Kargman DE, Berglund L, Hauser WA, Shea S, Paik MC. Race‐ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929–935. [DOI] [PubMed] [Google Scholar]
- 23. Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, Jacobs DR Jr, Wu C, Saad MF, Szklo M. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2007;30:2951–2956. [DOI] [PubMed] [Google Scholar]
- 24. Li S, Chen W, Srinivasan SR, Tang R, Bond MG, Berenson GS. Race (black‐white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima‐media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis. 2007;194:421–425. [DOI] [PubMed] [Google Scholar]
- 25. Gijsberts CM, Groenewegen KA, Hoefer IE, Eijkemans MJ, Asselbergs FW, Anderson TJ, Britton AR, Dekker JM, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Holewijn S, Ikeda A, Kitagawa K, Kitamura A, de Kleijn DP, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'Leary DH, Pasterkamp G, Peters SA, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Bots ML, den Ruijter HM. Race/ethnic differences in the associations of the Framingham risk factors with carotid IMT and cardiovascular events. PLoS One. 2015;10:e0132321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mackinnon AD, Jerrard‐Dunne P, Porteous L, Markus HS. Carotid intima‐media thickness is greater but carotid plaque prevalence is lower in black compared with white subjects. AJNR Am J Neuroradiol. 2010;31:1951–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multi‐center, multi‐ethnic, community‐based cohort study of women and the menopausal transition In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000:175–188. [Google Scholar]
- 28. Matthews KA, Crawford SL, Chae CU, Everson‐Rose SA, Sowers MF, Sternfeld B, Sutton‐Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 30. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 31. Okada M, Matsui H, Ito Y, Fujiwara A. Direct measurement of HDL cholesterol: method eliminating apolipoprotein E‐rich particles. J Clin Lab Anal. 2001;15:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bondar RJ, Mead DC. Evaluation of glucose‐6‐phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20:586–590. [PubMed] [Google Scholar]
- 33. El Kenz H, Bergmann P. Evaluation of immunochemiluminometric assays for the measurement of insulin and C‐peptide using the ADVIA Centaur. Clin Lab. 2004;50:171–174. [PubMed] [Google Scholar]
- 34. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 35. Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima‐media thickness. Stroke. 1997;28:2195–2200. [DOI] [PubMed] [Google Scholar]
- 36. Thurston RC, El Khoudary SR, Derby CA, Barinas‐Mitchell E, Lewis TT, McClure CK, Matthews KA. Low socioeconomic status over 12 years and subclinical cardiovascular disease: the Study of Women's Health Across the Nation. Stroke. 2014;45:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima‐media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sutton‐Tyrrell K, Kuller LH, Matthews KA, Holubkov R, Patel A, Edmundowicz D, Newman A. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160:407–416. [DOI] [PubMed] [Google Scholar]
- 39. El Khoudary SR, Thurston RC. Cardiovascular implications of the menopause transition: endogenous sex hormones and vasomotor symptoms. Obstet Gynecol Clin North Am. 2018;45:641–661. [DOI] [PubMed] [Google Scholar]
- 40. Ranjit N, Diez‐Roux AV, Chambless L, Jacobs DR Jr, Nieto FJ, Szklo M. Socioeconomic differences in progression of carotid intima‐media thickness in the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2006;26:411–416. [DOI] [PubMed] [Google Scholar]
- 41. Polak JF, Wong Q, Johnson WC, Bluemke DA, Harrington A, O'Leary DH, Yanez ND. Associations of cardiovascular risk factors, carotid intima‐media thickness and left ventricular mass with inter‐adventitial diameters of the common carotid artery: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2011;218:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA, Stein JH. Predictors of carotid thickness and plaque progression during a decade: the Multi‐Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koch S, Nelson D, Rundek T, Mandrekar J, Rabinstein A. Race‐ethnic variation in carotid bifurcation geometry. J Stroke Cerebrovasc Dis. 2009;18:349–353. [DOI] [PubMed] [Google Scholar]
- 44. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. [DOI] [PubMed] [Google Scholar]
- 45. White H, Boden‐Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 46. Rundek T, Arif H, Boden‐Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borrell LN, Lancet EA. Race/ethnicity and all‐cause mortality in US adults: revisiting the Hispanic paradox. Am J Public Health. 2012;102:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Pina IL, Ramirez SM, Rodriguez B, Sims M; American Heart Association Council on E, Prevention, American Heart Association Council on Clinical C, American Heart Association Council on C and Stroke N . Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Derby CA, Wildman RP, McGinn AP, Green RR, Polotsky AJ, Ram KT, Barnhart J, Weiss G, Santoro N. Cardiovascular risk factor variation within a Hispanic cohort: SWAN, the Study of Women's Health Across the Nation. Ethn Dis. 2010;20:396–402. [PMC free article] [PubMed] [Google Scholar]
- 50. Fang J, Zhang Z, Ayala C, Thompson‐Paul AM, Loustalot F. Cardiovascular health among non‐Hispanic Asian Americans: NHANES, 2011–2016. J Am Heart Assoc. 2019;8:e011324 DOI: 10.1161/JAHA.118.011324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones MR, Diez‐Roux AV, O'Neill MS, Guallar E, Sharrett AR, Post W, Kaufman JD, Navas‐Acien A. Ambient air pollution and racial/ethnic differences in carotid intima‐media thickness in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Epidemiol Community Health. 2015;69:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhuiyan AR, Srinivasan SR, Chen W, Paul TK, Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189:1–7. [DOI] [PubMed] [Google Scholar]
- 53. El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL‐C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol. 2016;10:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matthews KA, El Khoudary SR, Brooks MM, Derby CA, Harlow SD, Barinas‐Mitchell EJ, Thurston RC. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke. 2017;48:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. El Khoudary SR. HDL and the menopause. Curr Opin Lipidol. 2017;28:328–336. [DOI] [PubMed] [Google Scholar]
- 56. Rundek T, Blanton SH, Bartels S, Dong C, Raval A, Demmer RT, Cabral D, Elkind MS, Sacco RL, Desvarieux M. Traditional risk factors are not major contributors to the variance in carotid intima‐media thickness. Stroke. 2013;44:2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tillin T, Hughes AD, Whincup P, Mayet J, Sattar N, McKeigue PM, Chaturvedi N; Group SS . Ethnicity and prediction of cardiovascular disease: performance of QRISK2 and Framingham scores in a U.K. tri‐ethnic prospective cohort study (SABRE–Southall And Brent REvisited). Heart. 2014;100:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Topel ML, Shen J, Morris AA, Al Mheid I, Sher S, Dunbar SB, Vaccarino V, Sperling LS, Gibbons GH, Martin GS, Quyyumi AA. Comparisons of the Framingham and pooled cohort equation risk scores for detecting subclinical vascular disease in blacks versus whites. Am J Cardiol. 2018;121:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Markus H, Kapozsta Z, Ditrich R, Wolfe C, Ali N, Powell J, Mendell M, Cullinane M. Increased common carotid intima‐media thickness in UK African Caribbeans and its relation to chronic inflammation and vascular candidate gene polymorphisms. Stroke. 2001;32:2465–2471. [DOI] [PubMed] [Google Scholar]
- 60. Redmond N, Baer HJ, Hicks LS. Health behaviors and racial disparity in blood pressure control in the National Health and Nutrition Examination Survey. Hypertension. 2011;57:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, Howard VJ; REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators . Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half‐full (empty?) glass. Stroke. 2011;42:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Albert MA, Glynn RJ, Buring JE, Ridker PM. Relation between soluble intercellular adhesion molecule‐1, homocysteine, and fibrinogen levels and race/ethnicity in women without cardiovascular disease. Am J Cardiol. 2007;99:1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Green D, Foiles N, Chan C, Schreiner PJ, Liu K. Elevated fibrinogen levels and subsequent subclinical atherosclerosis: the CARDIA Study. Atherosclerosis. 2009;202:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang NC, Matthews KA, Barinas‐Mitchell EJ, Chang CC, El Khoudary SR. Inflammatory/hemostatic biomarkers and coronary artery calcification in midlife women of African‐American and White race/ethnicity: the Study of Women's Health Across the Nation (SWAN) heart study. Menopause. 2016;23:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–1336. [DOI] [PubMed] [Google Scholar]
- 66. Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, Peloso GM, Manichaikul A, Yang C, Ryan KA, Fu M, Johnson WC, Tsai M, Budoff M, Vasan RS, Cupples LA, Rotter JI, Rich SS, Post W, Mitchell BD, Correa A, Metspalu A, Wilson JG, Salomaa V, Kellis M, Daly MJ, Neale BM, McCarroll S, Surakka I, Esko T, Ganna A, Ripatti S, Kathiresan S, Natarajan P; NHLBI TOPMed Lipids Working Group . Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaufman JS, Dolman L, Rushani D, Cooper RS. The contribution of genomic research to explaining racial disparities in cardiovascular disease: a systematic review. Am J Epidemiol. 2015;181:464–472. [DOI] [PubMed] [Google Scholar]
- 68. Martinez‐Garcia M, Salinas‐Ortega M, Estrada‐Arriaga I, Hernandez‐Lemus E, Garcia‐Herrera R, Vallejo M. A systematic approach to analyze the social determinants of cardiovascular disease. PLoS One. 2018;13:e0190960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wendell CR, Waldstein SR, Evans MK, Zonderman AB. Distributions of subclinical cardiovascular disease in a socioeconomically and racially diverse sample. Stroke. 2017;48:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dalager S, Paaske WP, Kristensen IB, Laurberg JM, Falk E. Artery‐related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima‐media thickness. Stroke. 2007;38:2698–2705. [DOI] [PubMed] [Google Scholar]
- 72. Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. [DOI] [PubMed] [Google Scholar]
- 73. Williams DR. Race and health: basic questions, emerging directions. Ann Epidemiol. 1997;7:322–333. [DOI] [PubMed] [Google Scholar]
- 74. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009;169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CVD Risk Factors at Carotid Exam Visit by Race/Ethnicity
Table S2. Multivariable Regression Models of the Relationship Between Race, CVD Risk Factors, and Subclinical Vascular Measures (Sensitivity Analyses)*


