Abstract
Background
Non‐alcoholic fatty liver disease (NAFLD) is associated with high cardiovascular morbidity/mortality, including heart failure. Abnormalities in left ventricular (LV) structure/function are associated with heart failure risk.
Methods and Results
Participants from the population‐based CARDIA (Coronary Artery Risk Development in Young Adults) study year 25 exam (2010–2011, aged 43–55 years, 61% women, 48% black) with computed tomography measured liver fat and comprehensive echocardiography were included. Echocardiography was repeated at year 30 follow‐up (aged 47–62 years, N=1827). NAFLD was defined as liver attenuation ≤40 HU after exclusions. LV geometry was classified into normal and abnormal by integrating relative wall thickness and LV mass index. Diastolic function was defined using Doppler and tissue Doppler imaging. Systolic function was assessed with myocardial strain measured by speckle tracking. NAFLD prevalence was 8.7% (n=159). NAFLD participants had higher LV mass, relative wall thickness, incident LV hypertrophy and abnormal LV geometry versus non‐NAFLD (P<0.02). NAFLD participants had impaired LV relaxation (E/A ratio 1.1 versus 1.2), higher LV filling pressures (E/e′ ratio 7.9 versus 7.2), worse longitudinal strain (−13.9% versus −15.3%), and lower LV ejection fraction (58.9% versus 60.2%, P<0.01). In multivariable analyses adjusted for heart failure risk factors, NAFLD was independently associated with incident LV hypertrophy (odds ratio: 1.9, 95% CI: 1.1–3.4), abnormal LV geometry (odds ratio: 1.9, 1.1–3.3) and greater change in strain (odds ratio: 2.2, 1.1–4.7). Adjustment for body mass index attenuated associations to non‐significance.
Conclusions
NAFLD is associated with subclinical changes over time in LV structure/function and obesity explains much of the association. Presence of obesity in mid‐life may identify an important at‐risk population in whom to focus preventive heart failure strategies.
Keywords: heart failure, metabolic syndrome, NAFLD, NASH, obesity
Subject Categories: Epidemiology, Metabolic Syndrome, Heart Failure, Echocardiography, Obesity
Clinical Perspective
What Is New?
Non‐alcoholic fatty liver disease (NAFLD) is cross‐sectionally associated with subclinical myocardial dysfunction.
Whether NAFLD is prospectively associated with short‐term changes in myocardial dysfunction is unknown.
This study investigates whether computed tomography‐defined NAFLD is associated with 5‐year changes in echocardiographic markers of subclinical heart failure among middle aged adults in the CARDIA (Coronary Artery Risk Development in Young Adults) study.
What Are the Clinical Implications?
Study findings indicate that NAFLD is associated with incident left ventricular hypertrophy, abnormal left ventricular geometry and worsening myocardial strain over a 5‐year period independent of traditional heart failure risk factors and change in body mass index.
Obesity explains much of the association between NAFLD and changes in myocardial structure/function.
Diagnosis of asymptomatic NAFLD in middle age may be a previously unrecognized risk marker for left ventricular remodeling over time.
Introduction
Heart failure (HF) and non‐alcoholic fatty liver disease (NAFLD) are obesity‐related conditions with high cardiovascular disease (CVD) morbidity and mortality that have reached epidemic proportions.1, 2, 3 Evidence suggests that NAFLD is an independent risk factor for CVD.4, 5, 6, 7, 8, 9, 10 In fact, patients with NAFLD are more likely to die from complications of CVD than from liver disease.11 We, and others, have demonstrated a cross‐sectional association between NAFLD and subclinical myocardial remodeling and function independent of established HF risk factors, providing important insight into the relationship between NAFLD and HF.12, 13, 14
Adverse structural remodeling of the heart is a pivotal process in the progression of HF.15 However, the longitudinal associations of NAFLD with changes in myocardial structure and function are unknown. Thus, the primary objective of the current study was to prospectively examine whether NAFLD is associated with short‐term changes (5 years) in echocardiographic measures of left ventricular (LV) structure and function. We hypothesized that NAFLD is prospectively associated with LV remodeling, and change in LV remodeling, even after adjustment for traditional HF risk factors.
Methods
The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the procedure from the CARDIA (Coronary Artery Risk Development in Young Adults) Coordinating Center.16 CARDIA complies with data‐sharing requirements of the National Institutes of Health by providing limited‐access data sets from various CARDIA examinations to the National Heart, Lung and Blood Institute bioLINCC.17
Study Sample
The CARDIA (Coronary Artery Risk Development in Young Adults) study is a multicenter longitudinal cohort study of the determinants of CVD in 5115 black and white young adults recruited between 1985 and 1986 at 18 to 30 years of age across 4 US cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). The study design has been published previously.18 Participants were balanced by sex, race (white or black), age (18–24 years or 25–30 years), and education level (≤ high school or >high school). Follow‐up visits were conducted at years 2, 5, 7, 10, 15, 25 with retention of 72% of surviving participants attending, and at year 30 with 71% retention (Y30, 2015–2016). Informed consent was obtained at each follow‐up examination and the study was approved by the Institutional Review Boards at each CARDIA site (University of Alabama Birmingham; Northwestern University; University of Minneapolis; Kaiser Permanente).
The present study includes participants who underwent comprehensive echocardiography and cross‐sectional imaging with non‐contrast computed tomography (CT) scanning of the abdomen as part of the Y25 follow‐up exam (2010–2011) and who underwent repeat echocardiography at the Y30 exam ≈5 years later. Figure demonstrates the sample inclusion criteria. Participants were excluded if they were pregnant, weighed >450 pounds or were unable to fit in the CT scanner, or had missing liver fat or echocardiogram data (n=327). Participants with prevalent liver disease or self‐reported causes of liver fat, including heavy alcohol use (>14 standard drinks per week for women or >21 standard drinks per week for men), human immunodeficiency virus, hepatitis C virus and medications that cause hepatic steatosis (eg, amiodarone, diltiazem, methotrexate, valproate, tamoxifen) were excluded (n=560). Finally, we excluded participants with heart disease (n=112) and those missing key echocardiogram covariates for analysis at Y30 (n=671). The final study sample included 1827 participants.
Figure 1.
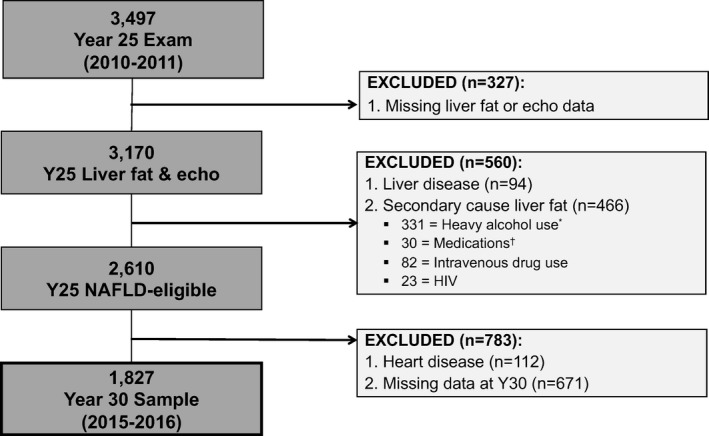
Sample population. *Heavy alcohol use was defined as >14 standard drinks per week in women, >21 standard drinks per week in men. †Medications=valproic acid, methotrexate, tamoxifen, and amiodarone. NAFLD indicates non‐alcoholic fatty liver disease.
Measurements and Imaging Protocols
Standardized protocols for data and image collection were used across centers, and measurements and protocols have previously been described.10, 18, 19, 20 The CT protocol included the heart and abdomen using a non‐contrast multidetector CT scan from either General Electric (GE 750HD 64 and GE LightSpeed VCT 64 Birmingham and Oakland Centers, respectively; GE Healthcare, Waukesha, WI) or Siemens (Sensation 64, Chicago and Minneapolis Centers; Siemens Medical Solutions, Erlangen, Germany).10 Quality control and image analysis was performed at a core reading center (Wake Forest University Health Sciences, Winston‐Salem, NC). Liver attenuation was reported as the average of 9 measurements. The interclass correlation coefficient between different readers on a random selected sample of 156 participants was 0.975 for liver attenuation, indicating high reproducibility of CT‐measured liver attenuation in this study. Volume of adipose tissue was measured within a 10‐mm block of slices centered between the fourth and fifth (L4–L5) lumbar vertebrae. Medical Image Processing, Analysis, and Visualization software (http://mipav.cit.nih.gov/index.php) was used to segment and quantify volume, in milliliters, of visceral (VAT) and subcutaneous adipose tissue within each compartment. The interclass correlation coefficient for inter‐reader comparisons was 0.989 for VAT, and intra‐ and inter‐reader error were 2.4% and 6.7%, respectively, in 156 scans that were blinded and reevaluated.
Comprehensive echocardiography, including Doppler and tissue Doppler imaging, was performed by trained sonographers who made measurements from digitized images using a standard software offline image analysis system (Digisonics, TX). The echocardiography protocol followed existing American Society of Echocardiography guidelines for study acquisition and measurement.19, 20 Quality control and image analysis was performed at a core reading center (Johns Hopkins University, Baltimore, MD). The speckle tracking echocardiography images for myocardial strain and strain rate measurements were analyzed in a 16‐segment basis for LV mid‐wall layer, using Wall Motion 2‐dimensional Tracking software (Toshiba Medical Systems). Three cardiac cycles from each view were recorded for offline analyses. Strain was calculated as the change in segment length relative to its end‐diastolic length, and the peak systolic value was recorded21 .
Demographic and medical characteristics of the participants were obtained, self‐reported, and interviewed using administered questionnaires. Body weight was measured to the nearest 0.2 kg with a calibrated balance‐beam scale. Height was measured with a vertical ruler to the nearest 0.5 cm. Waist circumference was measured midway between the iliac crest and bottom of the rib cage. Seated blood pressure was measured 3 times at 1‐minute intervals after 5 minutes of resting and the second and third measurements were averaged. Fasting blood was drawn in the seated position, separated and plasma was frozen to −70°C before analysis in a central laboratory.18 Glucose was assayed using the hexokinase method and insulin by the Elecsys sandwich immunoassay. Total cholesterol, high‐density lipoprotein cholesterol and triglycerides were measured enzymatically by the Northwest Lipid Laboratory.22 Low‐density lipoprotein cholesterol was calculated using the Friedewald equation. Creatinine was measured in serum and urine by the Roche enzymatic method on a Roche Modular P Chemistry Analyzer.
Definitions
NAFLD was defined as mean liver attenuation ≤40 HU, which is indicative of at least moderate‐to‐severe NAFLD, after exclusion of other causes of liver fat (Figure).23 Left ventricular hypertrophy (LVH) was defined as LV mass (LVM) indexed to body surface area (LVMi) >115 g/m2 (men) or >95 g/m2 (women). LV geometry was classified into normal and abnormal geometry by integrating relative wall thickness (RWT) and LVMi: Normal was defined as RWT ≤0.42 and no LVH; concentric remodeling was defined as RWT >0.42 and no LVH; concentric hypertrophy was defined as RWT >0.42 and LVH, and eccentric hypertrophy was defined as RWT ≤0.42 and LVH.24 BMI was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥30 kg/m2 . 25 Hypercholesterolemia was defined as a total cholesterol level of ≥240 mg/dL or statin use. Hypertension was defined as antihypertensive medication use and/or systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL, treatment with insulin or hypoglycemic agent, 2‐hour post‐challenge glucose ≥200 mg/dL, and/or HbA1c ≥6.5%. The modified National Cholesterol Education Program Adult Treatment Panel III criteria were used to define metabolic syndrome.26 Kidney function estimated as glomerular filtration rate was assessed via the modified CKD‐EPI equation. To quantify physical activity (reported as exercise units), the CARDIA physical activity history questionnaire was used, which was an interviewer‐based self‐report of duration and intensity of participation in 13 categories of exercise over the previous 12 months.27 For reference, 300 exercise units ≈150 minutes of moderate‐intensity activity per week.27
Statistical Analysis
Binary and multinomial logistic regression models were used to quantify associations between presence of Y25 NAFLD and Y30 echocardiographic measures of LV structure. Linear regression models were used to quantify associations between Y25 NAFLD and Y30 echocardiographic measures of LV function. To address the impact of change in weight we included percentage change (%Δ) in BMI relative to baseline Y25 BMI (%Δ BMI=Y30 BMI‐Y25 BMI)/BMI X100) in the final multivariable models (Model 5 and 7). Covariates in the multivariable models were chosen a priori for clinical importance. Seven models were fitted: Model 1 (base model): adjusted for center only; Model 2 (multivariable model): age, race, sex, center, education, income level, alcohol intake (drinks/week), smoking status (current versus former/never), physical activity score; Model 3: Multivariable model+HF risk factors (systolic blood pressure, antihypertensive and antihyperlipidemic medication use, total and high‐density lipoprotein cholesterol, diabetes mellitus status), estimated as glomerular filtration rate, and Y25 echocardiogram measures (eg, LVMi [LVH model] or RWT [LV remodeling models]); Model 4: Model 3+Y25 BMI; Model 5: Model 3+%ΔBMI; Model 6: Model 3+Y25 visceral adipose tissue (VAT); Model 7: Model 3+Y25 BMI+%ΔBMI Multiplicative interaction terms were generated to assess for interactions between NAFLD and race, sex, VAT volume, or BMI with outcomes of LVH, any abnormal LV geometry, E/e′ ratio, E/A ratio, and longitudinal strain. A P<0.05 was considered statistically significant. Analyses were performed using SAS 9.4 (SAS institute, Cary, NC).
Results
Study Sample
Characteristics of the 1827 participants (60.7% women, 48.4% black) stratified by presence of moderate‐to‐severe NAFLD at Y25 are summarized in Table 1. Moderate‐to‐severe NAFLD prevalence was 8.7% (n=159). Participants with NAFLD were of similar age (mean [SD] 50.4 [3.6] years] compared with those without NAFLD, but were more likely to be male (54.7% versus 37.9%), and have metabolic syndrome (66.0% versus 17.5%) and its components. Participants with NAFLD were also more likely to be obese (80.5% versus 39.8%) and had higher BMI (36.0 [7.4] versus 29.6 [6.9]) kg/m2), waist to hip ratio (0.93 [0.08] versus 0.83 [0.08]), and VAT volume (216.8 [75.2] versus 116.8 [62.7]), and had a higher prevalence of insulin resistance (mean hemoglobin A1c 6.4% [1.4%] versus 5.6% [0.76%]) than those without NAFLD.
Table 1.
Characteristics of the Overall Study Sample and Participants With and Without NAFLD, the CARDIA Study, Year 25 Exam, 2010 to 2011
| Overall Sample (n=1827) | No NAFLD (n=1668) | NAFLD (n=159) | P Value* | |
|---|---|---|---|---|
| Age, y | 50.0±3.6 | 49.9±3.6 | 50.4 ±3.6 | 0.11 |
| Women | 1108 (60.7) | 1036 (62.1) | 72 (45.3) | <0.0001† |
| Black | 885 (48.4) | 818 (49.0) | 67 (42.1) | 0.10 |
| Grade of school completed | 15.2±2.6 | 15.2±2.6 | 15.3±2.4 | 0.57 |
| Income <$50 000/y | 593 (32.5) | 537 (32.3) | 56 (35.2) | 0.45 |
| Current smokers | 212 (11.8) | 196 (11.9) | 16 (10.2) | 0.52 |
| Any alcohol use | 907 (50.0) | 839 (50.6) | 68 (43.3) | 0.08 |
| Alcohol use among drinkers, mL/wk | 11.7±8.4 | 11.7±8.3 | 12.0±10.2 | 0.73 |
| Physical activity (exercise units/wk) | 337.6±273.9 | 342.2±275.7 | 290.4±250.1 | 0.02† |
| Comorbidities | ||||
| Hyperlipidemia | 400 (21.9) | 347 (20.9) | 53 (33.3) | 0.0003† |
| Hypertension | 573 (31.4) | 481 (28.9) | 92 (57.9) | <0.0001† |
| Chronic kidney disease | 3 (0.16) | 3 (0.18) | 0 (0) | 1.0‡ |
| Diabetes mellitus | 196 (10.8) | 132 (7.9) | 64 (40.3) | <0.0001† |
| Obstructive sleep apnea | 160 (8.8) | 124 (7.4) | 36 (22.6) | <0.0001† |
| Metabolic syndrome§ | 397 (21.7) | 292 (17.5) | 105 (66.0) | <0.0001† |
| Systolic blood pressure, mm Hg | 118.5±15.6 | 118.0±15.7 | 124.2±13.5 | <0.0001† |
| BMI, kg/m2 | 30.2±7.2 | 29.6±6.9 | 36.0±7.4 | <0.0001† |
| BMI ≥30 | 790 (43.3) | 662 (39.8) | 128 (80.5) | <0.0001† |
| Waist‐to‐hip ratio | 0.84±0.09 | 0.83±0.08 | 0.93±0.08 | <0.0001† |
| Body surface area, m2 | 2.0±0.28 | 1.99±0.27 | 2.26±0.27 | <0.0001† |
| CT fat measures | ||||
| SAT, cm3 | 340.0±170.0 | 331.8±169.5 | 426.1±150.8 | <0.0001† |
| VAT, cm3 | 125.5±69.8 | 116.8±62.7 | 216.8±75.2 | <0.0001† |
| Liver attenuation, HU | 56.4±11.1 | 58.9±7.2 | 29.6±8.4 | <0.0001† |
| Metabolic variables | ||||
| Fasting glucose, mg/dL | 98.0±25.2 | 95.7±20.9 | 122.1±46.0 | <0.0001† |
| Hemoglobin A1c, % | 5.7±0.87 | 5.6±0.76 | 6.4±1.4 | <0.0001† |
| Total cholesterol, mg/dL | 192.8±36.1 | 193.2±35.9 | 188.0±38.5 | 0.09 |
| LDL cholesterol, mg/dL | 113.3±31.8 | 113.7±31.6 | 108.6±33.4 | 0.06 |
| HDL cholesterol, mg/dL | 58.0±17.0 | 59.1±17.0 | 46.4±12.0 | <0.0001† |
| Triglycerides, mg/dL | 109.7±84.3 | 102.5±63.2 | 185.3±183.3 | <0.0001† |
| Creatinine, mg/dL | 0.87±0.40 | 0.87±0.42 | 0.87±0.18 | 0.95 |
| eGFR, mL/min per 1.73 m2 | 96.0±19.5 | 95.8±19.3 | 98.3±21.4 | 0.12 |
| C‐reactive protein, mg/L | 3.00±4.67 | 2.83±4.56 | 4.78±5.35 | <0.0001† |
NAFLD=liver attenuation ≤40 HU. BMI indicates body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CT, computed tomography; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; NAFLD, non‐alcoholic fatty liver disease; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; VLDL, very‐low‐density lipoprotein.
Results are expressed as mean±SD or number (%); t test for continuous variables, Chi‐square, or Fisher exact for categorical variables for the difference between NAFLD and no NAFLD.
Statistically significant.
Fisher exact test.
Defined using ATPIII criteria.
Longitudinal Association of NAFLD With Abnormal Cardiac Geometry and Remodeling
In unadjusted analyses, participants with NAFLD at Y25 exhibited LV remodeling at Y30, manifested by higher LVMi, RWT, LV dimensions, and left atrial dimensions (Table 2). Those with NAFLD at Y25 had higher Y30 prevalence of LVH (46.5% with NAFLD versus 24.3% without NAFLD) and abnormal structural remodeling (59.7% versus 38.2%). Participants with NAFLD at Y25 also had higher rates of incident LVH (17.6% versus 11.3%) and incident abnormal LV geometry (24.5% versus 20.4%, P<0.0001 for both).
Table 2.
Univariate Analyses of the Longitudinal Association of Non‐Alcoholic Fatty Liver Disease and Echocardiographic Markers of Cardiac Geometry and Remodeling and Left Ventricular Function
| Prevalent Y30 Cardiac Dimensions | Incident Y25 to Y30* Cardiac Dimensions | |||||
|---|---|---|---|---|---|---|
| No NAFLD (n=1668) | NAFLD (n=159) | P Value | No NAFLD (n=1668) | NAFLD (n=159) | P Value | |
| Longitudinal association of NAFLD with cardiac geometry and remodeling | ||||||
| Cardiac dimensions | ||||||
| LV mass, g | 166.7±52.7 | 200.7±67.4 | <0.0001† | 3.4±41.3 | 4.3±53.2 | 0.80 |
| LV mass index to height2.7, g/m2.7 ‡ | 40.1±11.8 | 45.9±13.2 | <0.0001† | 1.0±10.0 | 1.2±12.2 | 0.83 |
| LV mass index to BSA, g/m2 | 79.0±20.0 | 87.3±24.0 | <0.0001† | −2.3±19.8 | −0.17±21.9 | 0.23 |
| LV hypertrophy, n (%) | 405 (24.3) | 74 (46.5) | <0.0001† | 188 (11.3) | 28 (17.6) | <0.0001† |
| LV internal diameter, systole, cm§ | 1.9±0.25 | 2.0±0.30 | 0.004† | 0.10±0.26 | 0.12±0.28 | 0.27 |
| LV internal diameter, diastole, cm§ | 2.9±0.27 | 3.0±0.29 | 0.0002† | −0.09±0.24 | −0.08±0.25 | 0.76 |
| LV posterior wall diameter, cm | 0.94±0.16 | 1.02±0.18 | <0.0001† | 0.06±0.18 | 0.06±0.21 | 0.79 |
| Interventricular septum, diastole, cm | 0.93±0.19 | 1.0±0.20 | <0.0001† | 0.04±0.20 | 0.03±0.21 | 0.88 |
| LV relative wall thickness | 0.38±0.08 | 0.40±0.08 | 0.03† | 0.04±0.09 | 0.03±0.09 | 0.65 |
| Concentric LV geometry | 405 (24.3) | 52 (32.7) | 0.02† | 297 (17.8) | 34 (21.4) | 0.06 |
| LV chamber characteristics | ||||||
| Normal geometry, n (%) | 1029 (61.7) | 64 (40.3) | Reference† | 1329 (46.0) | 120 (75.4) | Reference† |
| Any abnormal geometry, n (%) | 638 (38.2) | 95 (59.7) | <0.0001† | 340 (20.4) | 39 (24.5) | <0.0001† , ∥ |
| Concentric remodeling | 233 (14.0) | 21 (13.2) | ||||
| Concentric hypertrophy | 172 (10.3) | 31 (19.5) | ||||
| Eccentric hypertrophy | 233 (14.0) | 43 (27.0) | ||||
| Left atrial diameter, cm | 3.9±0.49 | 4.2±0.49 | <0.0001† | 0.25±0.41 | 0.29±0.48 | 0.24 |
| Left atrial volume, mL | 50.7±16.2 | 56.2±18.5 | <0.0001† | 1.1±9.4 | 1.6±10.5 | 0.54 |
| Left atrial volume index, mL/m§ | 29.9±9.2 | 32.6±10.2 | 0.0008† | 1.7±15.9 | 2.6±18.0 | 0.51 |
| Longitudinal association of NAFLD with LV function | ||||||
| LV systolic function | ||||||
| LV ejection fraction, % | 60.2±5.4 | 58.9±6.5 | 0.005† | −1.5±7.5 | −3.0±7.9 | 0.02† |
| Abnormal ejection fraction <50% | 64 (3.8) | 11 (6.9) | 0.04† | 46 (2.8) | 4 (2.5) | 0.91 |
| Longitudinal strain, % | −15.3±2.8 | −13.9±2.7 | <0.0001† | 0.001±3.0 | −0.04±3.2 | 0.88 |
| Circumferential strain, % | −14.7±3.7 | −13.3±3.4 | <0.0001† | 0.74±4.1 | 1.4±4.1 | 0.13 |
| LV diastolic function | ||||||
| E/A ratio | 1.2±0.34 | 1.1±0.33 | <0.0001† | −0.13±0.35 | −0.10±0.31 | 0.47 |
| Isovolumic relaxation time, ms | 67.8±15.6 | 67.2±16.3 | 0.68 | −5.5±17.6 | −7.3±17.3 | 0.26 |
| E deceleration time, ms | 176.1±38.9 | 180.4±38.4 | 0.19 | 41.9±59.6 | 36.6±51.5 | 0.29 |
| Lateral tissue Doppler e′ velocity, cm/s | 12.0±2.8 | 10.8±2.6 | <0.0001† | −0.51±2.7 | −0.55±2.6 | 0.89 |
| E/e′ ratio | 7.2±2.3 | 7.9±2.6 | 0.0004† | 0.25±2.2 | 0.25±2.6 | 1.0 |
| Hemodynamic variables | ||||||
| Cardiac output, L/min | 4.7±1.2 | 5.5±1.5 | <0.0001† | −0.91±1.4 | −1.1±1.7 | 0.25 |
| Cardiac index, L/min per m2 | 2.4±0.54 | 2.5±0.59 | 0.01† | −0.47±0.71 | −0.47±0.74 | 1.0 |
| Heart rate, bpm | 64.6±10.3 | 68.3±10.8 | <0.0001† | −0.35±9.5 | −0.08±9.5 | 0.74 |
Non‐alcoholic fatty liver disease=liver attenuation ≤40 HU after exclusions for secondary causes of liver fat. Left ventricular hypertrophy was defined as left ventricular mass indexed to body surface area >115 g/m2 (men) or >95 g/m2 (women). Concentric left ventricular geometry was defined as relative wall thickness >0.42. Concentric remodeling was defined as relative wall thickness >0.42 and left ventricular hypertrophy. Concentric hypertrophy was defined as relative wall thickness >0.42 and left ventricular hypertrophy. Eccentric hypertrophy was defined as relative wall thickness ≤0.42 and left ventricular hypertrophy.24 Results are expressed as mean±SD for continuous variable and n (%) for categorical variables, t test for continuous variables, Chi‐square for categorical variables. LV indicates left ventricular; NAFLD, non‐alcoholic fatty liver disease.
Incident defined as Y25 measurement−Y30 measurement.
Statistically significant.
In secondary analysis with left ventricular mass indexed to height, results were similar.
Indexed to height.
Result from multinomial model with normal as referent,24 n=700 participants were missing measurements for calculation of left ventricular geometry at Y30.
In multivariable analyses adjusted for demographics, health behaviors, HF risk factors, and %ΔBMI, NAFLD at Y25 was prospectively associated with prevalent LVH [odds ratio (OR): 1.57, 95% CI: 1.01, 2.45] and eccentric hypertrophy (OR: 2.24, 95% CI: 1.38, 3.58) at Y30 (Table 3). The association between NAFLD and prevalent LVH was attenuated to non‐significance after adjustment for either Y25 BMI or VAT (Table 3). NAFLD remained associated with prevalent eccentric hypertrophy when adjusted for Y25 VAT (OR: 1.74, 95% CI: 1.00, 3.02) or %ΔBMI (OR: 2.24, 95% CI: 1.38, 3.58), but not when adjusted for Y25 BMI (OR: 1.56, 95% CI: 0.91, 2.67). NAFLD at Y25 was also associated with incident abnormal LV geometry (OR: 1.93, 95% CI: 1.12, 3.34) and incident LVH (OR: 1.90, 95% CI: 1.05, 3.43) at Y30 when adjusted for HF risk factors and %ΔBMI, but non‐significant when adjusted for baseline Y25 BMI. Incident abnormal LV geometry remained significant when adjusted for Y25 VAT (OR: 1.86, 95% CI: 1.04, 3.30).
Table 3.
Odds Ratios (95% CI) for the Longitudinal Association of NAFLD with Prevalent and Incident Abnormal Left Ventricular Geometry and Remodeling, The CARDIA Study
| Prevalent Abnormal LV Geometry and Remodeling | Incident Abnormal LV Geometry and Remodeling | |||||
|---|---|---|---|---|---|---|
| Prevalent LVH | Concentric Remodeling* | Concentric Hypertrophy* | Eccentric Hypertrophy* | Incident LVH | Incident Abnormal LV Geometry | |
| Base model† | 2.74 (1.97–3.82)‡ | 1.48 (0.88–2.47) | 3.00 (1.89–4.75)‡ | 2.97 (1.96–4.48)‡ | 2.88 (1.78–4.66)‡ | 2.77 (1.70–4.50)‡ |
| Multivariable§ | 2.86 (2.02–4.05)‡ | 1.41 (0.83–2.40) | 2.89 (1.79–4.67)‡ | 3.26 (2.11–5.04)‡ | 2.91 (1.75–4.86)‡ | 2.78 (1.68–4.61)‡ |
| +HF risk factors∥ | 1.56 (1.00–2.43)‡ | 1.19 (0.65–2.17) | 1.27 (0.70–2.28) | 1.98 (1.16–3.37)‡ | 1.90 (1.06–3.40)‡ | 1.91 (1.11–3.29)‡ |
| Multivariable§ +HF RFs+ BMI | 1.27 (0.81–2.00) | 1.12 (0.61–2.05) | 1.05 (0.58–1.90) | 1.56 (0.91–2.67) | 1.43 (0.78–2.60) | 1.57 (0.90–2.73) |
| Multivariable§ +HF RFs+ %change BMI¶ | 1.57 (1.01–2.45)‡ | 1.13 (0.64–2.00) | 1.51 (0.88–2.59) | 2.24 (1.38–3.58)‡ | 1.90 (1.05–3.43)‡ | 1.93 (1.12–3.34)‡ |
| Multivariable§ +HF RFs+ VAT | 1.39 (0.88–2.20) | 1.10 (0.59–2.04) | 1.11 (0.61–2.04) | 1.74 (1.00–3.02)‡ | 1.67 (0.91–3.11) | 1.86 (1.04–3.30)‡ |
| Multivariable§ +HF RFs+ BMI+%change BMI¶ | 1.28 (0.81–2.01) | 1.11 (0.62–1.99) | 1.06 (0.61–1.84) | 1.41 (0.86–2.33) | 1.42 (0.78–2.61) | 1.57 (0.90–2.74) |
Left ventricular hypertrophy was defined as left ventricular mass indexed to body surface area >115 g/m2 (men) or >95 g/m2.7 (women). Concentric remodeling was defined as relative wall thickness >0.42 and left ventricular hypertrophy. Concentric hypertrophy was defined as relative wall thickness >0.42 and left ventricular hypertrophy. Eccentric hypertrophy was defined as relative wall thickness ≤0.42 and left ventricular hypertrophy. Any abnormal left ventricular geometry was defined as either concentric hypertrophy or concentric remodeling or eccentric hypertrophy.24 BMI indicates body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; HF, heart failure; LV, left ventricle; LVH, left ventricular hypertrophy; LVM, left ventricular mass; NAFLD, non‐alcoholic fatty liver disease; OR, odds ratio; RWT, relative wall thickness; VAT, visceral adipose tissue.
Result from multinomial model with normal geometry as referent.24
Adjusted for center only.
Statistically significant.
Multivariable model: adjusted for Y25 age, race, sex, study center, education, income level, alcohol intake (drinks/week), smoking status (current vs former/never), physical activity score.
Heart failure risk factors: Y25 systolic blood pressure, antihypertensive medication use, anti‐hyperlipidemic medication use, total cholesterol, high‐density lipoprotein cholesterol, diabetes mellitus status, glomerular filtration rate, and Y25 echocardiogram measures (eg, Y25 left ventricular mass/body surface area [left ventricular hypertrophy model] or Y25 left ventricular relative wall thickness [left ventricular remodeling models]).
%change body mass index=(Y30 body mass index−Y25 body mass index)/Y25 body mass index×100.
Longitudinal Association of NAFLD With LV Function
Among the systolic function parameters, Y30 myocardial strain and LV ejection fraction (LVEF), were significantly worse in participants with NAFLD at Y25 (Table 2). Those with NAFLD at Y25 were more likely to have abnormal LVEF <50% at Y30 than those without NAFLD (7.2% versus 3.9%). Participants with NAFLD at Y25 also had a greater decrease in LVEF over time than participants without NAFLD (−3.0 [7.9]% versus −1.5 [7.5]%). Several diastolic function parameters were worse at Y30 among participants with NAFLD at Y25, including lower E/A ratio (1.1 [0.34] versus 1.2 [0.33]) and e′ velocity (10.8 [2.6] versus 12.0 [2.8] cm/s), and a higher E/e′ ratio (7.9 [2.6] versus 7.2 [2.3]) (Table 2). Y30 cardiac output was also higher (5.5 [1.5] versus 4.7 [1.2] L/min) in the participants with NAFLD at Y25 even after accounting for body surface area (2.5 [0.59] versus 2.4 [0.54] L/min/m2). There was no prospective association between NAFLD at Y25 and the magnitude of change in diastolic function parameters or cardiac output at Y30.
In multivariable linear regression analyses adjusted for demographics, health behaviors, HF risk factors and %ΔBMI the presence of NAFLD at Y25 was associated with worse longitudinal strain at Y30 (Table 4, β [SE], 0.61 [0.25], P=0.02). However, the association between NAFLD at Y25 and Y30 longitudinal strain was attenuated in models with HF risk factors and either Y25 BMI or VAT. Likewise, NAFLD at Y25 remained independently associated with greater change/impairment (quartile 4) in longitudinal strain over time when adjusted for HF risk factors (OR: 2.24; 95% CI: 1.06, 4.73). However, this association was attenuated to non‐significance when adiposity measures were added to the HF risk factor model (Table S1). NAFLD at Y25 was also associated with several markers of diastolic dysfunction at Y30, including e′ velocity, E/A ratio and E/e′ ratio (a marker of LV filling pressure) when adjusted for demographics and health behaviors (Table 4). These associations persisted after adjustment for HF risk factors and %ΔBMI but were attenuated to non‐significance when VAT was added to the model (Table 4). Finally, NAFLD at Y25 was associated with increased cardiac output and LV filling pressures at Y30 independent of HF risk factors and either baseline or %Δ BMI (Table 4). There were no interactions between NAFLD and race, sex, BMI, or VAT in the multivariable models thus all results are shown as aggregate data and not stratified by race or sex. Associations were similar when continuous liver attenuation (rather than dichotomous NAFLD <40 HU) was used as the main exposure variable (Tables S2 and S3).
Table 4.
Linear Regression Analyses for the Longitudinal Association of NAFLD With Prevalent Left Ventricular Function, The CARDIA Study, 2015 to 2016
| Y30 Markers of LV Function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LVEF | E/A Ratio | E/e′ Ratio | Longitudinal Strain | Cardiac Output | ||||||
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |
| Base Model* | −1.3 (0.47)† | 0.006† | −0.12 (0.03)† | <0.0001† | 0.68 (0.20)† | 0.0006† | 1.4 (0.24)† | <0.0001† | 0.80 (0.10)† | <0.0001† |
| Multivariable‡ | −1.1 (0.46)† | 0.02† | −0.12 (0.03)† | <0.0001† | 0.86 (0.19)† | <0.0001† | 1.2 (0.24)† | <0.0001† | 0.70 (0.10)† | <0.0001† |
| +HF risk factors§ | −0.96 (0.50) | 0.05 | −0.07 (0.03)† | 0.02† | 0.48 (0.20)† | 0.02† | 0.59 (0.25)† | 0.02† | 0.50 (0.11)† | <0.0001† |
| Multivariable‡ +HF RFs+BMI | −0.89 (0.50) | 0.08 | −0.05 (0.03) | 0.07 | 0.41 (0.20)† | 0.04† | 0.49 (0.26) | 0.06 | 0.22 (0.10)† | 0.03† |
| Multivariable‡ +HF RFs+ %change BMI | −1.0 (0.50)† | 0.04† | −0.07 (0.001)† | 0.01† | 0.50 (0.20)† | 0.01† | 0.61 (0.25)† | 0.02† | 0.52 (0.11)† | <0.0001† |
| Multivariable‡ +HF RFs + VAT | −0.95 (0.52) | 0.07 | −0.03 (0.03) | 0.34 | 0.35 (0.21) | 0.10 | 0.41 (0.26) | 0.12 | 0.12 (0.11) | 0.30 |
| Multivariable‡ + HF RFs+ BMI + %change BMI∥ | −0.02 (0.01) | 0.19 | −0.003 (0.0009)† | 0.005† | 0.10 (0.006) | 0.07 | 0.20 (0.008) | 0.08 | 0.10 (0.003)† | 0.0005† |
BMI indicates body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; HF, heart failure; LVEF, left ventricular ejection fraction; NAFLD, non‐alcoholic fatty liver disease; VAT, visceral adipose tissue.
Adjusted for center only.
Statistically significant.
Multivariable model: adjusted for age, race, sex, study center, education, income level, alcohol intake (drinks/week), smoking status (current/former vs never), physical activity score.
Heart failure risk factors: systolic blood pressure, antihypertensive medication use, anti‐hyperlipidemic medication use, total cholesterol, high‐density lipoprotein cholesterol, diabetes mellitus status, and glomerular filtration rate.
%change BMI=(Y30 BMI−Y25 BMI)/Y25 BMI×100.
Discussion
In a large population‐based prospective study of black and white middle‐aged adults with asymptomatic NAFLD followed for 5 years, we have shown that NAFLD is longitudinally associated with subclinical LV remodeling, abnormal geometry, and impaired LV function, which are important precursors to HF. However, obesity in NAFLD explains much of the association between NAFLD and LV structure/function. Thus, presence of obesity in mid‐life may identify an important at‐risk population in whom to focus preventive HF strategies.
LV remodeling represents a global biomarker of systemic effects, such as that of hypertension and neurohormonal activation, on the entire cardiovascular system, with a likely association between LV remodeling and vascular changes responsible for cardiovascular events.28 For example, in the Cardiovascular Health Study investigators observed that compared with patients without LV remodeling, patients with LV remodeling had a greater risk of incident HF and all‐cause mortality.29 Cardiac remodeling is thus an important aspect of CVD progression and is, therefore, emerging as a therapeutic target in HF prevention.28 In the current study we demonstrate that not only is NAFLD associated with prevalent LV remodeling, but that presence of asymptomatic NAFLD in mid‐life is associated with incident abnormal LV geometry and remodeling. Better understanding of potential mediators in the developmental pathways towards abnormal LV geometry, such as NAFLD, may offer important potential therapeutic targets to prevent and treat the HF epidemic. Current NAFLD therapy includes a combination of lifestyle modification and surgical weight loss strategies.30 However, in the near future treatment is likely to include several drugs, which are in the development pipeline and target multiple pathways for this complex metabolic disease.31, 32
Consistent with previous research,13, 14 CARDIA participants with NAFLD were characterized by markers of underlying subclinical diastolic dysfunction, including lower early diastolic relaxation (e′) tissue velocity, lower E/A ratio, and higher estimated LV filling pressures (E/e′ ratio). Early diastolic dysfunction is a strong predictor of future cardiovascular morbidity,33 in particular HF with preserved ejection fraction (HFpEF). Several studies have demonstrated that HFpEF is highly prevalent in patients with underlying NAFLD. Furthermore, severe NAFLD, as assessed by increased liver stiffness, is associated with worse clinical outcomes among patients with HFpEF.34, 35 Thus, identification of predisposing factors for diastolic cardiac dysfunction is a pivotal first step toward implementation of effective prevention strategies and screening programs for early detection of HF (stage A HF). A major finding of our study is that NAFLD was associated with markers of early diastolic dysfunction independent of traditional HF risk factors. However, markers of either baseline overall adiposity (BMI) or visceral adiposity (VAT) attenuated these relationships, suggesting that obesity accounts for a significant proportion of the observed association between NAFLD and subclinical diastolic dysfunction among black and white adults.
We also prospectively demonstrate on a population level that participants with NAFLD have greater reduction in LV systolic function compared with participants without NAFLD over a relatively short follow‐up period. We, and others, have previously demonstrated a cross‐sectional relationship between imaging‐diagnosed NAFLD and impaired LV systolic function.12, 36, 37 To the best of our knowledge, this is the first study to demonstrate that NAFLD is potentially associated with greater progression of subclinical LV systolic dysfunction independent of HF risk factors. The association between NAFLD and change in LV systolic function was attenuated by markers of baseline adiposity, again highlighting the important role of obesity in the progression of HF. A few small cross‐sectional studies that used liver biopsy to diagnose NAFLD have demonstrated a significant, graded relationship between LV systolic dysfunction and the severity of NAFLD histology in both children and adults, suggesting that hepatic fibrosis and/or hepatic inflammation may be an additional risk factor in the development and progression of myocardial dysfunction.38, 39 Future prospective studies in biopsy‐proven NAFLD are needed to further assess the putative mechanisms, including obesogenic pathways, linking hepatic histology to progression of myocardial dysfunction.
The strengths of our study include the large, well‐characterized population‐based cohort of black and white adults, a moderate‐to‐severe NAFLD prevalence that is consistent with published population estimates,40 and the measurement of a comprehensive set of metabolic covariates, particularly VAT. The main limitation is the inability to assess for severity of NAFLD. Contemporaneous laboratory data on hepatic function is not available to us in CARDIA, and CT is unable to assess for the presence of hepatic fibrosis. In addition, given the high prevalence of obesity in CARDIA (>30%) we are underpowered to perform stratified analyses among obese versus non‐obese CARDIA participants with and without NAFLD. However, although obesity attenuated many of the observed relationships between NAFLD and myocardial structure/function, there were no significant interactions noted between obesity measures and NAFLD. In addition, those with morbid obesity in whom NAFLD is highly prevalent were excluded if they were unable to fit in the CT scanner or their weight exceeded the scanner limit. Thus, our results may underestimate the true association of NAFLD with LV remodeling and dysfunction. We also acknowledge the possibility of multiple testing error given the many outcomes examined in this study. However, all observed associations between NAFLD and markers of LV structure/function were consistent in both direction and magnitude. Finally, although we observed statistical differences in markers of LV structure/function by NAFLD status, these differences are not necessarily clinically meaningful. Future studies with longer follow‐up (>5 years) are needed to determine whether NAFLD results in clinically meaningful changes in cardiac function and links to HF outcomes.
Conclusions
NAFLD is prospectively associated with incident LVH, abnormal LV geometry, and impaired myocardial strain independent of established HF risk factors. However, obesity explains much of the observed association between NAFLD and changes in myocardial structure/function. Obesity plays an important role in the development of HF, and presence of NAFLD in mid‐life may identify an at‐risk population for HF prevention.
Sources of Funding
The CARDIA study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). Dr VanWagner is supported by the National Institutes of Health's National Center for Advancing Translational Sciences (KL2 TR001424) and the National Heart, Lung, and Blood Institute (K23 HL136891). Dr Carr is supported by the National Institutes of Health (R01 HL098445). Dr Shah is supported by the National Institutes of Health (R01 HL107577 and R01 HL127028). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Table S1. Logistic Regression Analyses for the Longitudinal Association of NAFLD With Quartiles of Change in Longitudinal Strain From Y25 (2010–2011) to Y30 (2015–2016) in CARDIA
Table S2. Odds Ratios (95% CI) for the Longitudinal Association of Continuous Liver Attenuation With Prevalent and Incident Abnormal Left Ventricular Geometry and Remodeling, The CARDIA Study
Table S3. Linear Regression Analyses for the Longitudinal Association of Continuous Liver Attenuation With Prevalent Left Ventricular Function, The CARDIA Study, 2015 to 2016
Acknowledgments
The authors thank the participants of the CARDIA study for their long‐term commitment and important contributions to the study. This article has been reviewed by CARDIA for scientific content.
(J Am Heart Assoc. 2020;9:e014279 DOI: 10.1161/JAHA.119.014279.)
References
- 1. Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non‐alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez‐Sendon JL, Nieminen MS, Pierard L, Remme WJ. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. [DOI] [PubMed] [Google Scholar]
- 3. Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non‐alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–80. [DOI] [PubMed] [Google Scholar]
- 5. Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi‐Takenaka Y, Nakano K, Oda Y, Yoshikawa T. Evaluation of vulnerable coronary plaques and non‐alcoholic fatty liver disease (NAFLD) by 64‐detector multislice computed tomography (MSCT). Circ J. 2008;72:618–25. [DOI] [PubMed] [Google Scholar]
- 6. Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. [DOI] [PubMed] [Google Scholar]
- 7. Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA, Fox CS. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, Lee HS, Larson J, Therneau TM, Kim WR. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, Terry JG, Lloyd‐Jones DM, Jacobs DR Jr, Carnethon MR. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle‐aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver‐related mortality in non‐alcoholic fatty liver disease. J Hepatol. 2008;49:608–12. [DOI] [PubMed] [Google Scholar]
- 12. VanWagner LB, Wilcox JE, Colangelo LA, Lloyd‐Jones DM, Carr JJ, Lima JA, Lewis CE, Rinella ME, Shah SJ. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population‐based study. Hepatology. 2015;62:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simon TG, Bamira DG, Chung RT, Weiner RB, Corey KE. Nonalcoholic steatohepatitis is associated with cardiac remodeling and dysfunction. Obesity (Silver Spring). 2017;25:1313–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wijarnpreecha K, Lou S, Panjawatanan P, Cheungpasitporn W, Pungpapong S, Lukens FJ, Ungprasert P. Association between diastolic cardiac dysfunction and nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Dig Liver Dis. 2018;50:1166–1175. [DOI] [PubMed] [Google Scholar]
- 15. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. [DOI] [PubMed] [Google Scholar]
- 16. University of Alabama at Birmingham . CARDIA Study contact information. Available at: http://www.cardia.dopm.uab.edu/contact-cardia. Accessed October 27, 2019.
- 17. National Institutes of Health . NHLBI Biologic Specimen and Data Repository Information Coordinating Center. Available at: https://biolincc-nhlbi-nih-gov.ezproxy.galter.northwestern.edu/home/. Accessed October 27, 2019.
- 18. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 19. Gidding SS, Liu K, Colangelo LA, Cook NL, Goff DC, Glasser SP, Gardin JM, Lima JA. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging. 2013;6:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR Jr, Carr JJ, Terry JG, Liu K, Goff DC Jr, Lima JA. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail. 2014;2:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, Stengel E, Sidney S, Lewis CE, Schreiner PJ, Shikany JM, Keck K, Merlo J, Gidding SS, Lima JA. Quality control and reproducibility in m‐mode, two‐dimensional, and speckle tracking echocardiography acquisition and analysis: the CARDIA study, year 25 examination experience. Echocardiography. 2015;32:1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–23. [DOI] [PubMed] [Google Scholar]
- 23. Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, Vauthey JN, Charnsangavej C. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–12. [DOI] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 25. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–8. [DOI] [PubMed] [Google Scholar]
- 27. Parker ED, Schmitz KH, Jacobs DR, Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow‐up: the CARDIA study. Am J Public Health. 2007;97:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. [DOI] [PubMed] [Google Scholar]
- 29. Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community‐dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail. 2014;2:512–22. [DOI] [PubMed] [Google Scholar]
- 30. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 31. Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander‐Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Chalasani NP, Anstee QM, Kowdley KV, George J, Goodman ZD, Lindor K. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, Goodman Z, Bedossa P, MacConell L, Shringarpure R, Shah A, Younossi Z. REGENERATE: design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials. 2019;84:105803. [DOI] [PubMed] [Google Scholar]
- 33. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- 34. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Hearzuht Fail. 2018;5:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan MS, Siddiqi TJ, Khan SU, Shah SJ, VanWagner LB, Khan SS. Association of liver stiffness and cardiovascular outcomes in patients with heart failure: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2018. Available at: https://journals.sagepub.com/doi/10.1177/2047487318810013. Accessed January 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, Kurtoglu U, Dindar I. Impairment of the left ventricular systolic and diastolic function in patients with non‐alcoholic fatty liver disease. Cardiol J. 2010;17:457–63. [PubMed] [Google Scholar]
- 37. Karabay CY, Kocabay G, Kalayci A, Colak Y, Oduncu V, Akgun T, Kalkan S, Guler A, Kirma C. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle‐tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325–31. [DOI] [PubMed] [Google Scholar]
- 38. Petta S, Argano C, Colomba D, Camma C, Di Marco V, Cabibi D, Tuttolomondo A, Marchesini G, Pinto A, Licata G, Craxi A. Epicardial fat, cardiac geometry and cardiac function in patients with non‐alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928–33. [DOI] [PubMed] [Google Scholar]
- 39. Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, Chiesa C. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59:461–70. [DOI] [PubMed] [Google Scholar]
- 40. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Logistic Regression Analyses for the Longitudinal Association of NAFLD With Quartiles of Change in Longitudinal Strain From Y25 (2010–2011) to Y30 (2015–2016) in CARDIA
Table S2. Odds Ratios (95% CI) for the Longitudinal Association of Continuous Liver Attenuation With Prevalent and Incident Abnormal Left Ventricular Geometry and Remodeling, The CARDIA Study
Table S3. Linear Regression Analyses for the Longitudinal Association of Continuous Liver Attenuation With Prevalent Left Ventricular Function, The CARDIA Study, 2015 to 2016


